Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia, largely associated to morbidity and mortality. Over the past decades, research in appearance and progression of this arrhythmia have turned into significant advances in its management. However, the incidence of AF continues to increase with the aging of the population and many important fundamental and translational underlaying mechanisms remain elusive. Here, we review recent advances in molecular and cellular basis for AF initiation, maintenance and progression. We first provide an overview of the basic molecular and electrophysiological mechanisms that lead and characterize AF. Next, we discuss the upstream regulatory factors conducting the underlying mechanisms which drive electrical and structural AF-associated remodeling, including genetic factors (risk variants associated to AF as transcriptional regulators and genetic changes associated to AF), neurohormonal regulation (i.e., cAMP) and oxidative stress imbalance (cGMP and mitochondrial dysfunction). Finally, we discuss the potential therapeutic implications of those findings, the knowledge gaps and consider future approaches to improve clinical management.
1. Introduction
Atrial fibrillation (AF) is a growing epidemic associated to increased morbidity and mortality [1]. Over the past decades, research in appearance and progression of this arrhythmia have turned into significant advances in its management [2]. Although the management of AF has drastically improved, it still has limited efficacy. Complete prevention, medium/long-term sinus rhythm maintenance after cardioversion or ablation of AF is not yet possible, and the progressive nature of AF is limiting the long-term success of current management therapies [3].
For AF to initiate and develop into a maintenance state, it requires a trigger and a vulnerable substrate [4]. Several mechanisms have been identified to serve as these triggers and substrates, including ectopic activity and conduction abnormalities, and several molecular upstream regulatory networks have been proposed to promote these mechanisms. This review highlights the recent advances in understanding the potential mediators and the molecular basis underlying initiation, maintenance and progression of AF, as well as highlighting resulting therapeutical implications and knowledge gaps.
2. Electrophysiological Mechanisms Associated with AF
A number of pathophysiological mechanisms have been proposed to underly initiation and maintenance of AF (Figure 1).
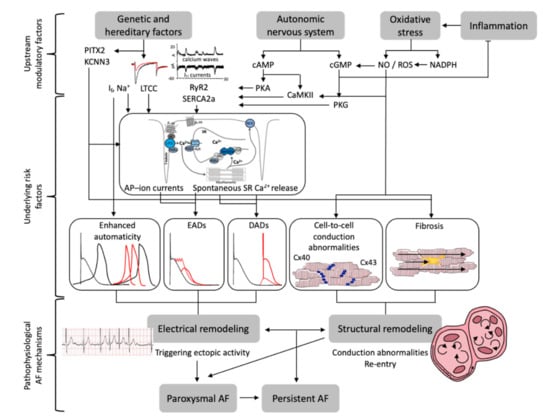
Figure 1.
Pathophysiological mechanisms underlying initiation and maintenance of atrial fibrillation (AF). Summary of upstream modulatory factors, underlying risk factors and pathophysiological AF-associated mechanisms. Abbreviations: PITX2 = paired-like homeodomain transcription factor 2, KCNN3 = Potassium Ca2+-Activated Channel, If = funny current, LTCC = L-type Ca2+ channel, RyR2 = ryanodine receptor 2, SERCA2a = SR Ca2+ ATPase, cAMP = 3′,5′-cyclic adenosine monophosphate, PKA and PKG = protein kinase A and G, CaMKII = Ca2+-calmodulin dependent protein-kinase type-II, NO = nitric oxide, ROS = reactive oxygen species, NADPH (Nicotinamide adenine dinucleotide phosphate), cGMP = cyclic guanosine monophosphate, AP = action potential, SR = sarcoplasmic reticulum, EADs/DADs = early/delayed afterdepolarizations, Cx40/43 = connexin 40/43, AF = atrial fibrillation.
2.1. Ectopic Activity
Abnormal pro-arrhythmic depolarizations, also called ectopic triggered activity, have been shown to originate mainly in the pulmonary vein (PV), where they have been linked to onset and maintenance of AF [4,5,6,7]. Another source of ectopic beats has been found in atrial muscle [6,8,9]. In both cases, anatomical variations and ion-channels properties predispose to ectopic triggered activity. Two types of abnormal depolarizations have been characterized: delayed (DADs) and early (EADs) afterdepolarizations [10]. EADs are favored by low heart rates, prolonged action potential durations (APD), partial inhibition of K+ channels, or by an increase in the inward Ca2+ currents during the plateau phase [11,12,13] or as a downstream effect in response to cellular stress [14]. DADs are favored by high heart rates and sarcoplasmic reticulum (SR) Ca2+ overloading and have been linked more directly to initiation of AF. Basically, a DAD occurs in response to a spontaneous SR Ca2+ release event, expanding as a Ca2+ wave through the cytosol and depolarizing the membrane, due to part of Ca2+ being expelled from the myocytes via Na+/Ca2+ exchanger (NCX) [15,16]. Repetitive ectopic activity initiates atrial arrhythmias in myocytes [17] and in whole hearts [18]. Thus, the most prominent channels causing ectopic activity are the ones responsible of the magnitude of the SR Ca2+ loading and release, the SR Ca2+ ATPase (SERCA2a) and ryanodine receptor 2 (RyR2) [14]. Phosphorylation of RyR2 has been shown to impair its interaction with one stabilizing agent, FKBP12.6, resulting in a “leaky” channel in canine ventricular myocytes, and RyR2 mutations linked to exercise induced arrhythmias have also been shown to decrease FKBP12.6 binding [19,20]. Additionally, reducing the open duration of RyR2 in HEK293 cells and mice ventricular myocytes with carvedilol, effectively suppressed store-overload-induced Ca2+ release [21]. SERCA2a function is regulated by phospholamban (PLB) and in atrial cells additionally by sarcolipin (SLN) [14]). In myocytes of a canine heart failure model PLB’s regulatory effect on SERCA2a was impaired due to an increased phosphorylation by type 1 phosphatase protein [22]. SLN mediates β-adrenergic response in the atrium and was significantly reduced in AF patients, resulting in an increased SR Ca2+ uptake through SERCA2a [23]. Rescuing SERCA2a function in failing rabbit myocytes by adenoviral gene transfer reversed the contractile dysfunctions, further highlighting the importance of SERCA2a function in preventing arrhythmias [24]. Overall, Ca2+ handling abnormalities reduce cellular Ca2+ stores and decrease contractile function, as well as causing beat-to-beat response irregularities [14,25]. Irregular beat-to-beat responses in human atrial myocytes (HAMs) are known to be caused by impaired L-type Ca2+ currents (ICa,L) in combination with RyR2 upregulation. Large numbers of myocytes with irregular beat-to-beat patterns could hamper the synchronized intercellular propagation of Ca2+-signaling between adjacent myocytes [25] and tissues.
2.2. Conduction Abnormalities
Fibrosis, resulting in a disorganized myocardial architecture, has been shown to support arrhythmia in multiple ways; however, mainly promoting conduction abnormalities or re-entry [26]. Myofibroblasts are able to effect electrical properties by heterocellular coupling to myocytes, shown in murine ventricular myocyte cultures, causing major conduction delays, partial cardiomyocyte depolarization or decreased contraction durations, all of which can increase the risk of fibrillation [27,28,29,30,31]. Importantly, the extended fibrotic extracellular matrix disrupts the unidirectional block which increase the likelihood of re-entry [32]. Although the role of atrial fibrosis in AF maintenance is well known, there are still sizeable knowledge gaps to enable optimal targeting of fibrotic areas during catheter ablation [26]. Cardiac stress, due to surgery or fibrillation, can activate quiescent fibroblasts [33], therefore fibrosis promoting arrhythmias can result in more fibrosis, making treatment improvements even more important. A recent study found calcitonin, a hormone product so far mainly linked to the thyroid gland, to also be produced by atrial myocytes and have protective effects on fibrosis. Patients with persistent AF, showed sixfold lower levels of calcitonin compared to controls with sinus rhythm and disruption of calcitonin signaling in mice triggered fibrogenesis and arrhythmias. Additionally, atrial specific overexpression of calcitonin in mice protected from fibrosis, highlighting a potential new treatment option for fibrosis [34]. Variations in connexin expression and function have been identified as another substrate causing conduction abnormalities. A study of patients with AF compared to those with sinus rhythm showed lateralization of connexin 40 (Cx40), Cx43 and N-cadherin, as well as significantly decreased levels of Cx43 in right atrial (RA) appendages and RA free wall only for AF patients [35]. A mutation in the Cx40 gene identified in AF patients lead to impaired gap junction formation and reduced intercellular electrical coupling, highlighting its role in coordinating atrial electrical coupling [36]. A review by Sanchez-Quintana et al. The authors of [4] highlighted one issue with the current state of knowledge linking connexin expression to AF, this being that decreased, increased and no change in expression levels of both Cx40 and Cx43 have been identified in AF patients, although these studies included a wide range of clinical sub-sets and would probably need to be investigated in more detail.
2.3. Enhanced Automaticity
The sinoatrial node (SAN) as primary pacemaker generates spontaneous APs (action potentials), the rate of which is controlled by a coupled-clock system involving the surface membrane ion channel M-clock and the SR Ca2+ clock [37]. A canine model of pacing induced atrial fibrillation with SAN dysfunction showed alterations in Ca2+ clock responsiveness to adrenergic stimulation, as well as a downregulation of RyR2 [38]. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, carrying the funny current (If), have also been linked to abnormal enhanced automaticity and atrial HCN shows increased expression in end-stage failing human hearts [39]. The degree to which enhanced automaticity causes AF is unclear and would require further investigation.
3. Molecular Regulatory Networks of Proarrhythmic Mechanisms in the Atria
Multiple potential mediators have been described to cause pathologic changes leading to the atrial substrate and electrical remodeling associated to AF (Figure 2), including hereditable genes, inflammation, fibrosis, oxidative stress, pressure and/or volume overload and autonomic changes.
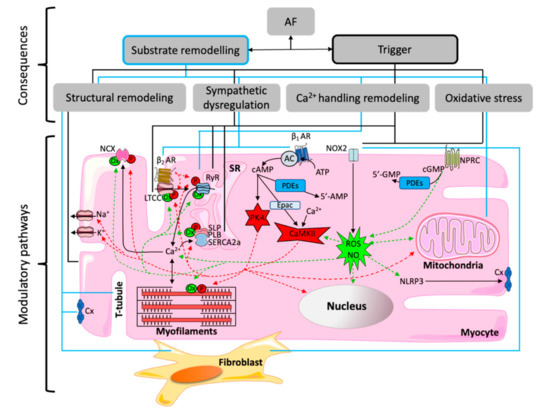
Figure 2.
Molecular regulatory networks: Many different microdomains can be modulated by oxidation (Ox) via cGMP, reactive oxygen species (ROS) and nitric oxide (NO), or by phosphorylation (P) via cAMP, protein kinase A (PKA) and Ca2+-calmodulin dependent protein-kinase type-II (CaMKII). Regulated Ca2+-handling microdomains include Na+/Ca2+ exchanger (NCX) and L-type Ca2+ channel (LTCC) at the cell membrane, ryanodine receptor (RyR) and sarcoplasmic reticulum Ca2+ ATPase (SERCA2a), which is inhibited by sarcolipin (SLP) and phospholamban (PLB), at the sarcoplasmic reticulum (SR) membrane, the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3), and contractile proteins at the myofilaments. PKA/CaMKII are activated by β-adrenoreceptor (βAR) signaling via adenyl cyclase (AC), phosphodiesterases (PDEs) and exchange protein directly activated by cAMP (Epac). ROS/NO is produced/increased by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX2) and natriuretic peptide clearance receptor (NPRC). Other modulations of atrial fibrillation-related remodeling are ion channels, such as Na+ and K+ channels, activation of fibroblasts or structural changes such as dilation or stretch affecting the tubular network, as well as connexins (Cx) function.
3.1. Genetic Regulation
There is growing evidence for a genetic involvement in the development and maintenance of AF, with heritable mutations linked to 5% of all AF cases and approximately 15% of early-onset cases without concomitant disease [40]. Understanding of these genetic modifications and their effect on therapeutic interventions are crucial to increase the effectiveness of rhythm control therapies. Genome-wide association studies (GWASs) identified over 100 independent risk variants, many of which lying close to genes and transcription factors linked to AF, for example the paired-like homeodomain transcription factor 2 (PITX2) and the T-Box Transcription Factor 5 (TBX5) [41,42,43,44]. Van Ouwerkerk et al. [45] described the networks of transcription factors linked to AF, but many other gene-regulatory networks are also known to promote AF.
Right and left atrial samples from patients with sustained AF, displayed a significant decrease in PITX2c expression [46]. A reduced Pitx2c expression in mice shortened atrial APD and increased susceptibility to induced AF [47], and a double knockout (atrial-specific (NppaCre+Pitx2−/−) and ventricular (Mlc2vCre+Pitx2−/−)) Pitx2c mutant mice resulted in a significantly more polarized resting membrane potential (RMP) and a smaller AP amplitude [46,48]. It is known that in embryonic stage PITX2 is responsible for right-left differentiation and in adult hearts it is primarily confined to the left atrium (LA) [47,49]. Current studies suggest that, within the LA, PITX2 controls the expression and/or function of ion channels controlling RMP. A recent in silico study supports these results showing shortened APD, elevated RMP and a slow conduction in LA human cells with PITX2 downregulation. They identified several targets of PITX2, including IKS (slow delayed rectifier potassium current), ICa,L, RyR and SERCA2a. PITX2 deficiency led to an enhanced SERCA2a function resulting in increased SR Ca2+ load, as well as increased IKS and decreased ICa,L currents, both resulting in reduced APD [50]. Another study looking at partial or complete loss of function (LoF) models of Pitx2 in mice found multiple calcium-handling genes (Atp2a2, Casq2 and Plb) to be altered in a dose-dependent manner. They also found Wnt8a upregulation in the complete LoF model, correlating with spontaneous AF onset compared to only triggered AF in the partial model [51]. Additionally, PITX2 inhibits SAN formation through repression of Shox2 and Tbx3 and a loss of this function has been suggested to result in the ectopic formation of SAN like cells in LA [47]. However, other studies found a significantly higher PITX2c mRNA expression in human right atrial myocytes from patients with persistent AF compared to those with sinus rhythm [52,53]. As both increased and decreased levels of PITX2 have been observed in atrial fibrillation, it could be suggested that any change in expression promotes AF related remodeling or that the location of the expression change matters. A number of single nucleotide polymorphisms (SNPs) have been identified on chromosome 4q25 proximal to PITX2 through GWAS, although they did not correlate with changes in PITX2 expression [52,54], therefore the underlying mechanisms for increasing AF risk could be PITX2 independent. However, it would be interesting to repeat these measurements only in cardiomyocytes, or to measure PITX2 dependent biomolecules, or to study proarrhythmogenic effects of up- and down-regulation of PITX2 expression in human atrial myocytes (HAMs), in order to confirm or to disprove direct PITX2 and AF risk link. One of these SNPs (rs13143308T) clearly links AF to defective Ca2+ homeostasis in HAMs. This risk variant has been associated with excessive Ca2+ releases, spontaneous electrical activity linked to increased SERCA2a expression and RyR2 phosphorylation, which likely causes the high incidence of Ca2+ induced Iti (transient inward currents) and spontaneous membrane polarizations seen in these patients. A single risk allele resulted in a five-fold increase in spontaneous DADs [54]. The exact mechanisms, by which this risk variant causes these modifications, have not been fully identified and require further investigation.
Recently, a mutation in the T-box region of TBX5 has been identified within a family of AF patients, reducing its transcriptional activity [55]. TBX5 has been shown to be crucial for cardiac development by in situ hybridization studies on human embryotic tissue [56], and its therefore believed that this mutation increased AF susceptibility due to hypoplasia of the conduction system [55]. Adult-specific removal of Tbx5 caused AF in mice and cardiomyocytes from this model revealed a multilevel gene regulatory network activated by TBX5 and repressed by PITX2 linking seven GWAS AF loci, including Scn5a, Atp2a2 and RyR2 [57]. A similar interaction has been identified through mouse genetics between Tbx5 and Gata4, with their interaction being crucial in atrial rhythm control via regulating the expression of Atp2a2 and RyR2 [58].
A GWAS study based on lone AF patients with increased heritability of AF had identified another risk locus on chromosome 1q21, with its most significant SNP being intronic to KCNN3, a potassium channel involved in atrial repolarization [41]. A screen for genetic variation in the KCNN3 gene of lone AF patients found no mutations but identified a synonymous SNP exonic to KCNN3, supporting the hypothesis of KCNN3 being involved in AF pathogenesis [59]. Even though no mutations were identified, atrial KCNN3 expression was reduced in AF with concomitant heart failure (HF) patients who also displayed a significant downregulation of histone deacetylate (HDAC) and atrial myocytes from a corresponding pig model revealed a direct regulation of KCNN3 mRNA by HDAC [60].
In addition to heritable risk factors, expression changes in many non-heritable AF genes and their products, like RyR2, NCX or SERCA2a, have been identified; for a detailed review see Heijman et al. [61]. The above changes are mostly linked to Ca2+-handling abnormalities, yet other pathways are also modulated by non-heritable expression changes. The Notch signaling pathway is dysregulated in an AF-dependent manner in end-stage HF patients, with over a quarter of its downstream transcripts being directly linked to atrial arrhythmias [62]. Several genes from the autonomic signaling cascade, including some phosphodiesterase (PDE) families, also showed pathology specific expression changes in atrial samples from AF patients [63]. Although many other genes have been linked to heritable and/or non-heritable predisposition to AF, mutations in the cardiac Na+ channel gene (SCN5A) deserve special mention because have not only been associated with AF, importantly they also increase susceptibility to ventricular arrhythmias, by promoting ectopic activity and conduction abnormalities [64,65,66].
3.2. Autonomic Remodeling and Upregulation of Sympathetic Signaling
Sympathetic neurotransmission results from the excitation of sympathetic nerves promoting the release of a neurotransmitter at the presynaptic terminal, by the membrane depolarization of the neurons, nearby G protein-coupled receptors (GPCRs). Neurotransmitters bind to GPCRs promoting the G protein dissociation activating the corresponding second messengers, mostly via adenylyl cyclase (AC), and a signaling cascade, which modulates enzymes that in turn control the phosphorylation state of important proteins for cardiomyocyte function. Thus, adrenaline-binding to β-adrenoreceptors (βAR) leads to AC activation, increasing 3′,5′-cyclic adenosine monophosphate (cAMP) which activates protein-kinase A (PKA). PKA phosphorylates a plethora of proteins, including Ca2+-handling proteins and ion channels. L-type Ca2+ channel (LTCC) phosphorylation increases ICa,L, RyR2 phosphorylation increases SR Ca2+-release and phosphorylated PLB loses inhibitory effects on SERCA2a. At the same time, the increase of Ca2+ due to cAMP-PKA-dependent phosphorylation of LTCC and/or RyR2 activates Ca2+-calmodulin dependent protein-kinase type-II (CaMKII), which may amplify phosphorylation effects of PKA on Ca2+-handling proteins. Furthermore, cAMP also activates CaMKII via the exchange protein directly activated by cAMP (Epac). Importantly, cAMP acts within discrete subcellular microdomains to fine-tune ion channel and transporter activities controlling excitation-contraction coupling due to specific isoforms of PDEs. These degrade cAMP, terminating and spatially restricting cAMP signals, thus also providing special and temporal control of the cAMP effectors, like kinases, that regulate phosphorylation of key Ca2+-handling proteins.
Several neurohormonal factors have been studied for their involvement in the initiation and perpetuation of AF. The main focus was always placed on the adrenergic (sympathetic) nervous system (ANS). Even though in AF the βAR transcription is unchanged [63,67], PKACA, IPP2, PDE4B, PDE3A, AKAP9, PKIA and AC9 are downregulated [63]. By contrast, PIK3G is upregulated in left human atria and PDE8A, PP2CA and EPAC2 are upregulated in human RA [63]. Regarding the functional and structural remodeling, increased atrial sympathetic innervation has been associated with AF in both animal models and humans [68,69,70]. Generalized sympathetic hyperactivation plays an important role in AF initiation and maintenance of the arrhythmia, as well as in its related comorbidities. In line, cAMP seem to be increased in AF [71]. Both the sympathetic and parasympathetic activation have been shown to promote downstream relevant effects in the onset of AF by increasing Ca2+ transient and diastolic Ca2+, promoting ectopic depolarizations due to spontaneous release of Ca2+ from SR, thereby facilitating spontaneous ectopic firing and increasing heart rate variability [72,73]. Indeed, common mechanisms proposed to underlie Ca2+-handling remodeling, including increased phosphorylation of the RyR2 at ser2808 via PKA [17,71,74,75] and ser2814 via CaMKII [71,76], increased PLB phosphorylation at ser16 via PKA [77] and reduced phosphorylation state of the LTCC via phosphatases [78] and PDEs [79,80]. In human atria, cAMP hydrolytic activity is mainly controlled by PDE3 [79,81]. However, reduced PDE4 activity in AF was suggested to be, at least in part, responsible for the enhanced frequency of spontaneous SR Ca2+ release in AF [74,79]. PDE4 inhibition increases the incidence of arrhythmias in human atrial strips during β-adrenergic stimulation [79]. On the other hand, although the knock-in mouse model with constitutively phosphorylated RyR2 at ser2814 showed a higher incidence of Ca2+ sparks and increased susceptibility to pacing-induced AF [71], and although CaMKII inhibitors were found to reduce Ca2+ sparks frequency as well as to increase RyR2 open probability in HAMs [71,82], it has never been shown that CaMKII inhibition reduces Ca2+ waves or arrhythmias in AF human atrial myocytes or tissues. Thereby, it is not clear whether the role of CaMKII-dependent RyR2 phosphorylation is protective maintaining SR Ca2+ load by allowing small releases of Ca2+ from the SR without promoting membrane spontaneous depolarizations and contractions [21], or proarrhythmogenic as suggested during the last years. Several spatio-temporal specific signaling alterations have been linked to the development and maintenance of AF. One of them is a time-dependent switch in β1-AR signaling from PKA to CaMKII which leads to myocyte apoptosis and maladaptive remodeling, as well as activation of LTCC increasing intracellular Ca2+ loading [83]. This mechanism has been linked to the induction of ectopic activity in rat PVs [84]. Additionally, a switch in the associated Gs subunit to Gi in response to prolonged agonist stimulation has been identified in β2-AR signaling in HEK293 cells and HAMs [85,86], as having a cardioprotective effect via PI3K/Akt, MEK/ERK and PDE4 signaling cascades [83,87,88]. Additionally, PDE subtype specific inhibitors have been shown to have different effects on electrical activity in isolated rabbit PV and SAN tissue. The increase in SAN and PV spontaneous electrical activity upon PDE3 inhibition was abolished by protein kinase G (PKG) and PKA inhibition, suggesting that PDE3 links to PKG and PKA signaling. PDE4 inhibition led to an increase in intracellular cAMP levels and increased ICaL but had no significant effect on spontaneous activity [89]. CaMKII has also been shown to autophosphorylate independently of Epac2 in atrial appendage samples of AF patients. Thus, CaMKII autophosphorylation increased Ca2+ sensitivity of apamin sensitive small-conductance Ca2+-activated K+ current (IKAS) in AF [90].
Serotonin (5-hydroxytryptamine, 5-HT) receptor mRNA transcripts are reduced by 36% in AF patients [67]. The maximum inotropic responses to 5-HT are also reduced in trabeculae of patients with AF [91] due to diminished cytosolic cAMP responses upon 5-HT in these patients regulated via PDE3 and to a lesser extend PDE4 [92]. Nevertheless, PDE3 or PDE4 regulation of 5HT-dependent cAMP levels seems to be highly compartmentalized. Thus, contrary to what happens with contraction, the PDE3 or PDE4 control on propensity of 5-HT-evoked arrhythmias on human atrial trabeculae from patients in sinus rhythm or with paroxysmal AF, is lost in persistent AF [93]. 5-HT stimulation has been shown to increase If [94] and ICa,L [91,94] in HAMs, but less than β-adrenergic stimulation [91,95] and without regulation of PDE3 or PDE4 [90].
Muscarinic receptor (M2R) activation by acetylcholine induces negative chronotropy via G-protein-activated inwardly rectifying K+ current (IK,ACh) [96,97]. Elevated IK,ACh promotes proarrhythmic effects by hyperpolarizing RMP and shortening APD and refractory period. However, muscarinic receptor-mediated IK,ACh activation is reduced in AF [98]. Conversely, agonist-independent constitutive IK,ACh activation has been implicated in AF [99,100] via adenosine A1 receptors [101].
Adenosine exerts dose-dependent antiarrhythmic and proarrhythmic actions. A1 and A3 are Gi-coupled receptors predominantly expressed in atria and sinus node [102,103]. A1 receptors promote anti-adrenergic effects by reducing isoproterenol-induced cAMP-PKA-dependent phosphorylation of Ca2+, K+ channels and contractile proteins within others [104]. A1 receptors show higher expression in the RA versus LA [8]. A3, like A2B receptors, need high concentrations of adenosine to be activated due to their low affinity. A2A and A2B are Gs-coupled receptors also expressed in human atria. A2A receptor activation increases the incidence of spontaneous SR Ca2+ releases via RyR2 phosphorylation [105], promotes beat-to-beat irregularities [106] and activates K+ channels [107]. Importantly, A2A receptors are upregulated in AF [108]. Anti-adrenergic actions of A1A are counteracted by A2A receptors [109]. However, A1 and A3 stimulation would neutralize A2A receptors signaling [101] during ischemia or hypoxia, where A1 and A3 receptors are also activated by high levels of accumulated inosine [110]. A3 activates phospholipase C (PLC) stimulating antioxidant cardio protection [111]. A2B activation inhibits endothelin-1 (ET-1) induced fibroblast proliferation, as well as angiotensin II (AngII)-mediated fibrosis via cAMP, Epac, PI3K-dependent pathways in neonatal and adult rat cardiac fibroblasts [112,113,114]. In contrast, A2B knockdown or using a selective A2B antagonist attenuated the effects of myocardial infarction on fibrosis and ventricular fibrillation in mice and rat [115,116]. The failing myocardium from chronic heart failure patients showed decreased gene expressions for A2A, A2B and A3 receptors, as well as for adenosine deaminase (ADA). The decreased receptor expression likely impaired the cardioprotective signaling effects of adenosine. However, this could have been attenuated by the increased cardiac adenosine levels due to the reduced ADA expression and resulting decreased activity [117].
Mineralocorticoid receptors mRNA expression is increased in AF [118]. Aldosterone binding and subsequent cardiac fibrosis formation has been associated with AF [119]. Furthermore, aldosterone could contribute to increased intracellular Ca2+, activating phospholipase C (PLC) and protein kinase C (PKC). Thus, inhibition of the renin-angiotensin-aldosterone system (RAAS) with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARB) has been proposed for the treatment of AF to prevent oxidative stress and structural and electrical remodeling [120].
3.3. Oxidative Stress and Inflammation
Inflammatory states have been frequently associated with AF due to inducing oxidative stress, which plays an important role in atrial structural remodeling [121]. Inflammation can readily promote AF through oxidative stress and Ca2+-dependent triggered activity. Long-term inflammation processes may promote not only electrical but also structural remodeling [122,123]. Steroidal anti-inflammatory agents can suppress atrial tachycardia induced electrophysiological changes in dogs through significantly reducing C-reactive protein (CRP) and attenuating the tachycardia induced activation of endothelial nitric oxide synthases (eNOS) [124]. Patients developing AF after coronary bypass surgery have been shown to have significantly higher preoperative CRP levels [125]. Additionally, increased CRP baseline levels have been linked to an increased risk of developing AF in the general population in a Korean health screening program spanning 11 years and a stepwise CRP elevation was seen with an increasing AF burden [126,127].
The NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome, which can be activated by oxidative stress, can in turn activate cardiac fibroblasts [128], displaying a link between inflammation and cardiac fibrosis. Importantly, increased NLRP3 inflammasome activity has been shown in patients with paroxysmal AF and chronic AF [129], and suppressing NLRP3 inflammasome activity in a mouse model of left ventricular hypertrophy attenuated cardiac fibrosis [130]. Neutrophil derived inflammation has also been shown to create an AF substrate, via myeloperoxidase (MPO) activity resulting in conduction abnormalities in a canine model [131]. RA samples of AF patients also displayed higher MPO plasma levels and larger MPO burden compared to sinus rhythm [131]. Additionally, MPO-deficient mice were protected from AF and atrial fibrosis [132]. Cell-to-cell conduction abnormalities, like those caused by MPO activity, are often due to stress induced atrial remodeling modulating Cx40 and Cx43, interfering with gap junctions and intercellular transmission among atrial myocytes [133]. In a sheep coronary infarct model, Cx43 was redistributed from the intercalated discs to the lateral surface, increasing fibroblast-cardiomyocyte coupling and resulting in an increased associated AF risk, likely due to impaired signal transduction between myocytes [134,135]. Another study showed Cx43 mediates the induction and maintenance of AF in a canine model [136]. Connexins can be regulated through phosphorylation by multiple kinases like CaMKII, PKA or PKC [137,138,139]. CaMKII can be activated by oxidative stress [140], additionally resulting in phosphorylation of RyR2 and NaV1.5 [141,142], as well as causing CaMKII independent oxidation of RyR2 leading to calstabin dissociation and a Ca2+ leak [131,143]. Phosphorylation of NaV1.5 leads to an increase in late sodium current (INaL) and APD, as well as increased DADs expression levels in mice atrial myocytes, and increased Nav1.5 phosphorylation levels in atrial samples from AF patients [133].
In response to oxidative stress, NOS function can become “uncoupled”, shifting the electron transfer to molecular oxygen, which causes superoxide anion formation, a reactive oxygen species (ROS) [144]. During AF, but not sinus rhythm, NOS contribute significantly to atrial ROS production. This is one possible mechanism leading to atrial oxidative injury and electrophysiological remodeling observed in AF patients [145]. In addition to uncoupled NOS, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX2) has been identified as main source of ROS production during AF and sinus rhythm [145]. These two different pathways of ROS production during AF have been shown to change overtime, highlighting important implications for the prevention and management of AF. In postoperative AF patients, NOX2 and inducible NOS (iNOS) upregulation was responsible for ROS production, and statins inhibiting NOX2 and iNOS were effective at preventing AF onset. In contrast, statins were not effective in managing long-term AF, which correlated with a switch to uncoupled NOS and myocardial oxidases causing the majority of ROS production [146]. A recent study, looking at independent effects of NOX2 on AF, suggested NOX2 to mostly be a biomarker for AF-induced remodeling. This work showed that NOX2 overexpression only led to a modest increase in AF susceptibility, which was associated with a modest RyR2 dysregulation, but had no impact on AF duration [147,148].
ROS can increase the open probability of mammalian RyR2 by increasing channel sensitivity to cytosolic Ca2+ and ATP, due to an altered redox state of sulfhydryl groups [149]. Additionally, they can trigger depolarization of the mitochondrial inner membrane potential in guinea pig ventricular myocytes, which can cause oscillations in APD and lead to arrhythmias [150]. There is also evidence that ROS are able to activate TGF-β signaling, which has been linked to a protective effect via attenuation of oxidative stress and reducing pro-inflammatory cytokine release [151,152].
NOS-derived nitric oxide (NO) can regulate myocardial Ca2+ flux in multiple ways, depending on NOS type. In mice left ventricular (LV) myocytes, neuronal NOS (nNOS)-derived NO inhibits Ca2+ influx via LTCC, increases SR Ca2+ reuptake by increasing phospholamban phosphorylation and regulates Ca2+ release from SR by changes to RyR2 S-nitrosylation [153,154]. Whereas eNOS-derived NO reduces myofilament Ca2+ sensitivity via PKG modulating cardiac relaxation in rat ventricular myocytes/guinea pig myocytes [155], reduces ICa,L in mice ventricular myocytes [156], and inhibits myocardial oxygen consumption in mice left ventricular myocytes [157]. Activation of eNOS due to mechanical stress has been shown to increase the open probability of RyR2 in porcine left atrial myocytes [158]. On the other hand, a link was observed between decrease in eNOS expression and NO bioavailability and AF, resulting from the loss of a protective effect against lesion and thrombus formation [159]. Another study found no change in eNOS expression in human left atrial appendages and also showed that NO inhibits IKv4.3, a main current of the Ca2+ independent component of the transient outward K+ current in humans, which could prolong the plateau phase of the chronic AF-remodeled AP [160]. These results suggest that restoring a physiological level of NO in human atria could play a part in preventing and/or terminating AF, by affecting the above mechanisms as well as possibly preventing the uncoupling of NOS function.
Other ways in which NO signaling can cause aberrant Ca2+ signals and abnormal excitation-contraction coupling have been described. NO activates guanylate cyclase (GC), which increased intracellular 3′,5′-cyclic guanosine monophosphate (cGMP), in turn inhibiting Ca2+ signals. In ventricular myocytes NO has a biphasic effect on sarcolemmal Na+/H+ exchanger (NHE1). Active NHE1 results in Na+ driven cellular Ca2+ loading (via NCX) and increases the possibility of spontaneous SR Ca2+ signals. NO modulates NHE1 through GC/cGMP, which activates NHE1 via PKG. High levels of cGMP then inhibit PDE3, resulting in an increase of cAMP and NHE1 inactivation by PKA, causing the biphasic effect of NO and major implications for targeting NHE1 as treatment for fibrillations [161]. The impact of cGMP/cAMP crosstalk via NO signaling has also been observed on the Ca2+ dynamics in HAMs [83,162]. ICa,L density increased with increasing concentrations of NO-donor SNAP and PDE3 inhibition enhanced ICa,L to similar extent as SNAP. Conversely, SNAP reduced ICa,L when applied on top of PDE3 inhibition or βAR stimulation, when cAMP was previously elevated, pointing to cGMP-cAMP cross-regulation. However, in atrial myocytes from patient with AF, basal stimulatory effects of SNAP were lost, while its inhibitory effect when cAMP was previously elevated by PDE3 inhibition or βAR stimulation was preserved [77]. Importantly, cGMP levels were found to be significantly higher in patients with AF [163,164].
Besides the dual substrate PDEs (PDE2 and PDE3) leading to a crosstalk between cGMP and cAMP, one important effector target of cGMP is PKG. NO activates GC, which increase intracellular cGMP that stimulates PKG. PKG in turn phosphorylates the muscarinic receptor-activated IK,Ach channel in sinus rhythm patients, but does not seem to be involved in the muscarinic-dependent activation of the constitutively active channel in cAF patients. This constitutive activation is due to abnormal phosphorylation by PKC, the expression of which is upregulated in right atrial appendage samples of chronic AF patients [100].
Natriuretic peptides (NP) can also activate the cGMP pathway. The atrial natriuretic peptide, which is secreted by atrial myocytes, promotes epicardial progenitor cells differentiation in adipocytes, favoring fibrosis substrate from the epicardial adipose tissue and promoting AF [165]. B-type natriuretic peptide antagonizes the pro-fibrotic effects of TGF-β1, AngII and ET-1 [166]. In accordance with this, a naturally 17-fold higher expression of natriuretic peptide clearance receptor (NPR-C) in the atria compared to ventricles of wildtype mice, has been linked to the increased atrial susceptibility to TGF-β1-mediated fibrosis [167,168].
4. Therapeutic Implications and Future Directions
Despite the significant advances in AF management, especially regarding stroke prevention with antithrombotics and maintaining sinus rhythm with invasive strategies, current treatment options still have significant limitations. Catheter ablation has limited efficacy, particularly in patients with long standing persistent AF [169]. Early rhythm control therapy may lower the risk of AF-associated complications and ameliorate outcomes [170]. On the other hand, the complex nature of AF pathophysiology hinders drug-based AF-management of both rate and rhythm control. Although recent advances in our understanding of the underlying mechanisms promoting and maintaining AF have led to implementing novel antiarrhythmic drug therapies, the current available treatments has limited efficacy, extracardiac toxicity and ventricular proarrhythmogenic effects [171,172]. These weaknesses emphasize the need of (1) better understanding of all pathomechanisms underlying each type of AF, and (2) major conceptual changes in our approaches looking for pharmacological targets. Thus, multiple regulatory networks have been identified to promote and/or maintain AF (i.e., inflammation, oxidative stress, fibrosis, genes and autonomic changes), but not many studies give information about the role of specific targets on the different steps and progression of AF. Furthermore, early identification of abnormalities may lead to early interventions to prevent AF, or AF progression. Next, the biggest dare would be to develop new therapeutic approaches to treat AF without side effects. Full understanding of AF mechanisms for defining patient-specific treatments depending on the progression stage of the arrhythmia and the specific driven mechanisms, achieving atrial selectivity on the new molecular therapeutic targets, and identifying new circulating biomarkers to detect and classify AF, may be the next potential major challenges in AF research.
Author Contributions
K.B. and C.E.M. drafted and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deutsche Forschungsgemeinschaft, grant number ES 569/2-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colilla, S.; Crow, A.; Petkun, W.; Singer, D.E.; Simon, T.; Liu, X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am. J. Cardiol. 2013, 112, 1142–1147. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef]
- Nattel, S.; Guasch, E.; Savelieva, I.; Cosio, F.G.; Valverde, I.; Halperin, J.L.; Conroy, J.M.; Al-Khatib, S.M.; Hess, P.L.; Kirchhof, P.; et al. Early management of atrial fibrillation to prevent cardiovascular complications. Eur. Heart J. 2014, 35, 1448–1456. [Google Scholar] [CrossRef]
- Sanchez-Quintana, D.; Ramon Lopez-Mínguez, J.; Pizarro, G.; Murillo, M.; Cabrera, J.A. Triggers and Anatomical Substrates in the Genesis and Perpetuation of Atrial Fibrillation. Curr. Cardiol. Rev. 2012, 8, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, J.R.; Cha, T.J.; Zhang, L.; Chartier, D.; Melnyk, P.; Hohnloser, S.H.; Nattel, S. Cellular electrophysiology of canine pulmonary vein cardiomyocytes: Action potential and ionic current properties. J. Physiol. 2003, 551, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Kholova, I.; Kautzner, J. Anatomic Characteristics of Extensions of Atrial Myocardium into the Pulmonary Veins in Subjects With and Without Atrial Fibrillation. Pacing Clin. Electrophysiol. 2003, 26, 1348–1355. [Google Scholar] [CrossRef]
- Li, N.; Csepe, T.A.; Hansen, B.J.; Sul, L.V.; Kalyanasundaram, A.; Zakharkin, S.O.; Zhao, J.; Guha, A.; Van Wagoner, D.R.; Kilic, A.; et al. Adenosine-Induced Atrial Fibrillation. Circulation 2016, 134, 486–498. [Google Scholar] [CrossRef]
- Hocini, M.; Nault, I.; Wright, M.; Veenhuyzen, G.; Narayan, S.M.; Jaïs, P.; Lim, K.T.; Knecht, S.; Matsuo, S.; Forclaz, A.; et al. Disparate Evolution of Right and Left Atrial Rate During Ablation of Long-Lasting Persistent Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.J. Cardiac adrenergic control and atrial fibrillation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 381, 235–249. [Google Scholar] [CrossRef]
- Hiraoka, M.; Sunami, A.; Fan, Z.; Sawanobori, T. Multiple Ionic Mechanisms of Early Afterdepolarizations in Isolated Ventricular Myocytes from Guinea-pig Hearts. Ann. N. Y. Acad. Sci. 1992, 644, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.M.; Alekseev, A.E.; Liu, X.K.; Park, S.; Zingman, L.V.; Bienengraeber, M.; Sattiraju, S.; Ballew, J.D.; Jahangir, A.; Terzic, A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006, 15, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Volders, P. Similarities between early and delayed afterdepolarizations induced by isoproterenol in canine ventricular myocytes. Cardiovasc. Res. 1997, 34, 348–359. [Google Scholar] [CrossRef]
- Nattel, S.; Maguy, A.; le Bouter, S.; Yeh, Y.H. Arrhythmogenic Ion-Channel Remodeling in the Heart: Heart Failure, Myocardial Infarction, and Atrial Fibrillation. Physiol. Rev. 2007, 87, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Hüser, J.; Blatter, L.A.; Lipsius, S.L. Intracellular Ca2+ release contributes to automaticity in cat atrial pacemaker cells. J. Physiol. 2000, 524, 415–422. [Google Scholar] [CrossRef]
- Kockskämper, J.; Sheehan, K.A.; Bare, D.J.; Lipsius, S.L.; Mignery, G.A.; Blatter, L.A. Activation and Propagation of Ca2+ Release during Excitation-Contraction Coupling in Atrial Myocytes. Biophys. J. 2001, 81, 2590–2605. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Bayes-Genís, A.; Roura, S.; Rodriguez Font, E.; Arís, A.; Cinca, J. Atrial Fibrillation Is Associated With Increased Spontaneous Calcium Release From the Sarcoplasmic Reticulum in Human Atrial Myocytes. Circulation 2004, 110, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Bögeholz, N.; Pauls, P.; Dechering, D.G.; Frommeyer, G.; Goldhaber, J.I.; Pott, C.; Eckhardt, L.; Müller, F.U.; Schulte, J.S. Distinct Occurrence of Proarrhythmic Afterdepolarizations in Atrial Versus Ventricular Cardiomyocytes: Implications for Translational Research on Atrial Arrhythmia. Front. Pharmacol. 2018, 9, 933. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.T.; Marks, A.R. Calstabin deficiency, ryanodine receptors, and sudden cardiac death. Biochem. Biophys. Res. Commun. 2004, 322, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Kobayashi, S.; Kohno, M.; Doi, M.; Tokuhisa, T.; Okuda, S.; Suetsugu, M.; Hisaoka, T.; Obayashi, M.; Ohkusa, T.; et al. FKBP12.6-Mediated Stabilization of Calcium-Release Channel (Ryanodine Receptor) as a Novel Therapeutic Strategy Against Heart Failure. Circulation 2003, 107, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xiao, J.; Jiang, D.; Wang, R.; Vembaiyan, K.; Wang, A.; Smith, C.D.; Xie, C.; Chen, W.; Zhang, J.; et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat. Med. 2011, 17, 1003–1009. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mishra, S.; Rastogi, S.; Imai, M.; Habib, O.; Sabbah, H.N. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2373–H2381. [Google Scholar] [CrossRef]
- Shanmugam, M.; Molina, C.E.; Gao, S.; Severac-Bastide, R.; Fischmeister, R.; Babu, G.J. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2+ uptake in human atrial fibrillation. Biochem. Biophys. Res. Commun. 2011, 410, 97–101. [Google Scholar] [CrossRef]
- Ziolo, M.T.; Martin, J.L.; Bossuyt, J.; Bers, D.M.; Pogwizd, S.M. Adenoviral Gene Transfer of Mutant Phospholamban Rescues Contractile Dysfunction in Failing Rabbit Myocytes With Relatively Preserved SERCA Function. Circ. Res. 2005, 96, 815–817. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Fernandes, J.; Padró, J.; Cinca, J.; Hove-Madsen, L. Sarcoplasmic reticulum and L-type Ca2+ channel activity regulate the beat-to-beat stability of calcium handling in human atrial myocytes. J. Physiol. 2011, 589, 3247–3262. [Google Scholar] [CrossRef] [PubMed]
- Tzeis, S.; Asvestas, D.; Vardas, P. Atrial Fibrosis: Translational Considerations for the Management of AF Patients. Arrhythmia Electrophysiol. Rev. 2019, 8, 37–41. [Google Scholar] [CrossRef]
- Gaudesius, G.; Miragoli, M.; Thomas, S.P.; Rohr, S. Coupling of Cardiac Electrical Activity Over Extended Distances by Fibroblasts of Cardiac Origin. Circ. Res. 2003, 93, 421–428. [Google Scholar] [CrossRef] [PubMed]
- McArthur, L.; Riddell, A.; Chilton, L.; Smith, G.L.; Nicklin, S.A. Regulation of connexin 43 by interleukin 1β in adult rat cardiac fibroblasts and effects in an adult rat cardiac myocyte: Fibroblast co-culture model. Heliyon 2020, 6, e03031. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Gaudesius, G.; Rohr, S. Electrotonic Modulation of Cardiac Impulse Conduction by Myofibroblasts. Circ. Res. 2006, 98, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, M.; Salvarani, N.; Rohr, S. Myofibroblasts Induce Ectopic Activity in Cardiac Tissue. Circ. Res. 2007, 101, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.A.; Camelliti, P.; Rog-Zielinska, E.A.; Siedlecka, U.; Poggioli, T.; O’Toole, E.T.; Knöpfel, T.; Kohl, P. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc. Natl. Acad. Sci. USA 2016, 113, 14852–14857. [Google Scholar] [CrossRef]
- De Bakker, J.M.T.; Stein, M.; van Rijen, H.V.M. Three-dimensional anatomic structure as substrate for ventricular tachycardia/ventricular fibrillation. Heart Rhythm 2005, 2, 777–779. [Google Scholar] [CrossRef]
- Delaunay, M.; Osman, H.; Kaiser, S.; Diviani, D. The Role of Cyclic AMP Signaling in Cardiac Fibrosis. Cells 2019, 9, 69. [Google Scholar] [CrossRef]
- Moreira, L.M.; Takawale, A.; Hulsurkar, M.; Menassa, D.A.; Antanaviciute, A.; Lahiri, S.K.; Mehta, N.; Evans, N.; Psarros, C.; Robinson, P.; et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 2020, 587, 460–465. [Google Scholar] [CrossRef]
- Kostin, S. Structural correlate of atrial fibrillation in human patients. Cardiovasc. Res. 2002, 54, 361–379. [Google Scholar] [CrossRef]
- Gollob, M.H.; Jones, D.L.; Krahn, A.D.; Danis, L.; Gong, X.Q.; Shao, Q.; Liu, X.; Veinot, J.P.; Tang, A.S.; Stewart, A.F.; et al. Somatic Mutations in the Connexin 40 Gene (GJA5) in Atrial Fibrillation. N. Engl. J. Med. 2006, 354, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Joung, B.; Chen, P.-S.; Lin, S.-F. The Role of the Calcium and the Voltage Clocks in Sinoatrial Node Dysfunction. Yonsei Med. J. 2011, 52, 211. [Google Scholar] [CrossRef]
- Stillitano, F.; Lonardo, G.; Zicha, S.; Varro, A.; Cerbai, E.; Mugelli, A.; Nattel, S. Molecular basis of funny current (If) in normal and failing human heart. J. Mol. Cell. Cardiol. 2008, 45, 289–299. [Google Scholar] [CrossRef]
- Darbar, D.; Herron, K.J.; Ballew, J.D.; Jahangir, A.; Gersh, B.J.; Shen, W.K.; Hammill, S.C.; Packer, D.L.; Olson, T.M. Familial atrial fibrillation is a genetically heterogeneous disorder. J. Am. Coll. Cardiol. 2003, 41, 2185–2192. [Google Scholar] [CrossRef]
- Ellinor, P.T.; Lunetta, K.L.; Glazer, N.L.; Pfeufer, A.; Alonso, A.; Chung, M.K.; Sinner, M.F.; de Bakker, P.I.; Mueller, M.; Lubitz, S.A.; et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat. Genet. 2010, 42, 240–244. [Google Scholar] [CrossRef]
- Husser, D.; Büttner, P.; Ueberham, L.; Dinov, B.; Sommer, P.; Arya, A.; Hindricks, G.; Bollmann, A. Association of atrial fibrillation susceptibility genes, atrial fibrillation phenotypes and response to catheter ablation: A gene-based analysis of GWAS data. J. Transl. Med. 2017, 15, 71. [Google Scholar] [CrossRef]
- Husser, D.; Büttner, P.; Stübner, D.; Ueberham, L.; Platonov, P.G.; Dinov, B.; Arya, A.; Hindricks, G.; Bollmann, A. PR Interval Associated Genes, Atrial Remodeling and Rhythm Outcome of Catheter Ablation of Atrial Fibrillation—A Gene-Based Analysis of GWAS Data. Front. Genet. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Thorolfsdottir, R.B.; Fritsche, L.G.; Zhou, W.; Skov, M.W.; Graham, S.E.; Herron, T.J.; McCarthy, S.; Schmidt, E.M.; Sveinbjornsson, G.; et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018, 50, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Van Ouwerkerk, A.F.; Hall, A.W.; Kadow, Z.A.; Lazarevic, S.; Reyat, J.S.; Tucker, N.R.; Nadadur, R.D.; Bosada, F.M.; Bianchi, V.; Ellinor, P.T.; et al. Epigenetic and Transcriptional Networks Underlying Atrial Fibrillation. Circ. Res. 2020, 127, 34–50. [Google Scholar] [CrossRef]
- Chinchilla, A.; Daimi, H.; Lozano-Velasco, E.; Dominguez, J.N.; Caballero, R.; Delpón, E.; Tamargo, J.; Cinca, J.; Hove-Madsen, L.; Aranega, A.E.; et al. PITX2 Insufficiency Leads to Atrial Electrical and Structural Remodeling Linked to Arrhythmogenesis. Circ. Cardiovasc. Genet. 2011, 4, 269–279. [Google Scholar] [CrossRef]
- Kirchhof, P.; Kahr, P.C.; Kaese, S.; Piccini, I.; Vokshi, I.; Scheld, H.H.; Rotering, H.; Fortmueller, L.; Laakmann, S.; Verheule, S.; et al. PITX2c Is Expressed in the Adult Left Atrium, and Reducing Pitx2c Expression Promotes Atrial Fibrillation Inducibility and Complex Changes in Gene Expression. Circ. Cardiovasc. Genet. 2011, 4, 123–133. [Google Scholar] [CrossRef]
- Syeda, F.; Holmes, A.P.; Yu, T.Y.; Tull, S.; Kuhlmann, S.M.; Pavlovic, D.; Betney, D.; Riley, G.; Kucera, J.P.; Jousset, F.; et al. PITX2 Modulates Atrial Membrane Potential and the Antiarrhythmic Effects of Sodium-Channel Blockers. J. Am. Coll. Cardiol. 2016, 68, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Syeda, F.; Kirchhof, P.; Fabritz, L. PITX2 -dependent gene regulation in atrial fibrillation and rhythm control. J. Physiol. 2017, 595, 4019–4026. [Google Scholar] [CrossRef]
- Bai, J.; Lo, A.; Gladding, P.A.; Stiles, M.K.; Federov, V.V.; Zhao, J. In silico investigation of the mechanisms underlying atrial fibrillation due to impaired Pitx2. PLoS Comput. Biol. 2020, 16, e1007678. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Hernández-Torres, F.; Daimi, H.; Serra, S.A.; Herraiz, A.; Hove-Madsen, L.; Aránega, A.; Franco, D. Pitx2 impairs calcium handling in a dose-dependent manner by modulating Wnt signalling. Cardiovasc. Res. 2016, 109, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Gore-Panter, S.R.; Hsu, J.; Hanna, P.; Gillinov, A.M.; Pettersson, G.; Newton, D.W.; Moravec, C.S.; Van Wagoner, D.R.; Chung, M.K.; Barnard, J.; et al. Atrial Fibrillation Associated Chromosome 4q25 Variants Are Not Associated with PITX2c Expression in Human Adult Left Atrial Appendages. PLoS ONE 2014, 9, e86245. [Google Scholar] [CrossRef]
- Pérez-Hernández, M.; Matamoros, M.; Barana, A.; Amorós, I.; Gómez, R.; Núñez, M.; Sacristán, S.; Pinto, Á.; Fernández-Avilés, F.; Tamargo, J.; et al. Pitx2c increases in atrial myocytes from chronic atrial fibrillation patients enhancing I Ks and decreasing I Ca,L. Cardiovasc. Res. 2016, 109, 431–441. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Llach, A.; Tarifa, C.; Gandía, J.; Jiménez-Sabado, V.; Lozano-Velasco, E.; Serra, S.A.; Vallmitjana, A.; Vázquez Ruiz de Castroviejo, E.; Benítez, R.; et al. The 4q25 variant rs13143308T links risk of atrial fibrillation to defective calcium homoeostasis. Cardiovasc. Res. 2019, 115, 578–589. [Google Scholar] [CrossRef]
- Guo, D.-F.; Li, R.-G.; Yuan, F.; Shi, H.Y.; Hou, X.M.; Qu, X.K.; Xu, Y.J.; Zhang, M.; Liu, X.; Jiang, J.Q.; et al. TBX5 loss-of-function mutation contributes to atrial fibrillation and atypical Holt-Oram syndrome. Mol. Med. Rep. 2016, 13, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Yi Li, Q.; Newbury-Ecob, R.A.; Terrett, J.A.; Wilson, D.I.; Curtis, A.R.; Yi, C.H.; Gebuhr, T.; Bullen, P.J.; Robson, S.C.; Strachan, T.; et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat. Genet. 1997, 15, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Nadadur, R.D.; Broman, M.T.; Boukens, B.; Maturek, S.R.; Yang, X.; van den Boogaard, M.; Bekeny, J.; Gadek, M.; Ward, T.; Zhang, M.; et al. Pitx2 modulates a Tbx5 -dependent gene regulatory network to maintain atrial rhythm. Sci. Transl. Med. 2016, 8, 354ra115. [Google Scholar] [CrossRef]
- Laforest, B.; Dai, W.; Tyan, L.; Lazarevic, S.; Shen, K.M.; Gadek, M.; Broman, M.T.; Weber, C.R.; Moskowitz, I.P. Atrial fibrillation risk loci interact to modulate Ca2+-dependent atrial rhythm homeostasis. J. Clin. Investig. 2019, 129, 4937–4950. [Google Scholar] [CrossRef]
- Olesen, M.S.; Jabbari, J.; Holst, A.G.; Nielsen, J.B.; Steinbrüchel, D.A.; Jespersen, T.; Haunsø, S.; Svendsen, J.H. Screening of KCNN3 in patients with early-onset lone atrial fibrillation. Europace 2011, 13, 963–967. [Google Scholar] [CrossRef]
- Rahm, A.-K.; Wieder, T.; Gramlich, D.; Müller, M.E.; Wunsch, M.N.; El Tahry, F.A.; Heimberger, T.; Weis, T.; Most, P.; Katus, H.A.; et al. HDAC2-dependent remodeling of KCa2.2 (KCNN2) and KCa2.3 (KCNN3) K+ channels in atrial fibrillation with concomitant heart failure. Life Sci. 2021, 266, 118892. [Google Scholar] [CrossRef]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef]
- Lipovsky, C.E.; Jimenez, J.; Guo, Q.; Li, G.; Yin, T.; Hicks, S.C.; Bhatnagar, S.; Takahashi, K.; Zhang, D.M.; Brumback, B.D.; et al. Chamber-specific transcriptional responses in atrial fibrillation. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Garnier, A.; Bork, N.I.; Jacquet, E.; Zipfel, S.; Muñoz-Guijosa, C.; Baczkó, I.; Reichenspurner, H.; Donzeau-Gouge, P.; Maier, L.S.; Dobrev, D.; et al. Mapping genetic changes in the cAMP-signaling cascade in human atria. J. Mol. Cell. Cardiol. 2021, 155, 10–20. [Google Scholar] [CrossRef]
- Darbar, D.; Kannankeril, P.J.; Donahue, B.S.; Kucera, G.; Stubblefield, T.; Haines, J.L.; George, A.L., Jr.; Roden, D.M. Cardiac Sodium Channel (SCN5A) Variants Associated with Atrial Fibrillation. Circulation 2008, 117, 1927–1935. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.M. Sodium Channel Mutations and Susceptibility to Heart Failure and Atrial Fibrillation. JAMA 2005, 293, 447. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Amin, A.S.; Remme, C.A. Disease Modifiers of Inherited SCN5A Channelopathy. Front. Cardiovasc. Med. 2018, 5. [Google Scholar] [CrossRef]
- Grammer, J.B.; Zeng, X.; Bosch, R.-F.; Kühlkamp, V. Atrial L-type Ca2+ -channel, β-adrenoreceptor, and 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res. Cardiol. 2001, 96, 82–90. [Google Scholar] [CrossRef]
- Arora, R. Recent Insights into the Role of the Autonomic Nervous System in the Creation of Substrate for Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2012, 5, 850–859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyauchi, Y.; Zhou, S.; Okuyama, Y.; Miyauchi, M.; Hayashi, H.; Hamabe, A.; Fishbein, M.C.; Mandel, W.J.; Chen, L.S.; Chen, P.S.; et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: Implications for atrial fibrillation. Circulation 2003, 108, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.L.; Fishbein, M.C.; Chen, L.S.; Chen, P.S.; Masroor, S. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm 2009, 6, 454–460. [Google Scholar] [CrossRef]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Amar, D.; Zhang, H.; Miodownik, S.; Kadish, A.H. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J. Am. Coll. Cardiol. 2003, 42, 1262–1268. [Google Scholar] [CrossRef]
- Patterson, E.; Po, S.S.; Scherlag, B.J.; Lazzara, R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm 2005, 2, 624–631. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.T.; Reiken, S.; Warrier, S.; Belevych, A.E.; Harvey, R.D.; Richter, W.; Jin, S.L.; Conti, M.; Marks, A.R. Phosphodiesterase 4D Deficiency in the Ryanodine-Receptor Complex Promotes Heart Failure and Arrhythmias. Cell 2005, 123, 25–35. [Google Scholar] [CrossRef]
- Vest, J.A.; Wehrens, X.H.T.; Reiken, S.R.; Lehnart, S.E.; Dobrev, D.; Chandra, P.; Danilo, P.; Ravens, U.; Rosen, M.R.; Marks, A.R. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 2005, 111, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients With Atrial Fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karack, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef]
- Christ, T.; Boknik, P.; Wöhrl, S.; Wettwer, E.; Graf, E.M.; Bosch, R.F.; Knaut, M.; Schmitz, W.; Ravens, U.; Dobrev, D. L-Type Ca2+ Current Downregulation in Chronic Human Atrial Fibrillation Is Associated With Increased Activity of Protein Phosphatases. Circulation 2004, 110, 2651–2657. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.E.; Leroy, J.; Richter, W.; Xie, M.; Scheitrum, C.; Lee, I.O.; Maack, C.; Rucker-Martin, C.; Donzeau-Gouge, P.; Verde, I.; et al. Cyclic Adenosine Monophosphate Phosphodiesterase Type 4 Protects Against Atrial Arrhythmias. J. Am. Coll. Cardiol. 2012, 59, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Rozmaritsa, N.; Christ, T.; van Wagoner, D.R.; Haase, H.; Stasch, J.P.; Matschke, K.; Ravens, U. Attenuated response of L-type calcium current to nitric oxide in atrial fibrillation. Cardiovasc. Res. 2014, 101, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Tovar, A.; Vargas, M.L.; Escudero, E.; Kaumann, A.J. Ontogenic changes of the control by phosphodiesterase-3 and -4 of 5-HT responses in porcine heart and relevance to human atrial 5-HT 4 receptors. Br. J. Pharmacol. 2009, 156, 237–249. [Google Scholar] [CrossRef]
- Pabel, S.; Mustroph, J.; Stehle, T.; Lebek, S.; Dybkova, N.; Keyser, A.; Rupprecht, L.; Wagner, S.; Neef, S.; Maier, L.S.; et al. Dantrolene reduces CaMKIIδC-mediated atrial arrhythmias. Europace 2020, 22, 1111–1118. [Google Scholar] [CrossRef]
- Xiao, R.-P.; Zhu, W.; Zheng, M.; Cao, C.; Zhang, Y.; Lakatta, E.G.; Han, Q. Subtype-specific α1- and β-adrenoceptor signaling in the heart. Trends Pharmacol. Sci. 2006, 27, 330–337. [Google Scholar] [CrossRef]
- Maupoil, V.; Bronquard, C.; Freslon, J.L.; Cosnay, P.; Findlay, I. Ectopic activity in the rat pulmonary vein can arise from simultaneous activation of α 1- and β 1-adrenoceptors. Br. J. Pharmacol. 2007, 150, 899–905. [Google Scholar] [CrossRef]
- Daaka, Y.; Luttrell, L.M.; Lefkowitz, R.J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 1997, 390, 88–91. [Google Scholar] [CrossRef]
- Kilts, J.D.; Gerhardt, M.A.; Richardson, M.D.; Sreeram, G.; Machensen, G.B.; Grocott, H.P.; White, W.D.; Davis, R.D.; Newman, M.F.; Reves, J.G.; et al. β 2 -Adrenergic and Several Other G Protein-Coupled Receptors in Human Atrial Membranes Activate Both G s and G i. Circ. Res. 2000, 87, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-H.; Leblais, V.; Wang, P.H.; Crow, M.T.; Xiao, R.P. Phosphatidylinositol 3-Kinase Functionally Compartmentalizes the Concurrent G s Signaling During β 2 -Adrenergic Stimulation. Circ. Res. 2002, 91, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Naro, F.; Zoudilova, M.; Jin, S.L.; Conti, M.; Kobilka, B. Phosphodiesterase 4D is required for 2 adrenoceptor subtype-specific signaling in cardiac myocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cheng, C.; Huang, J.; Chen, Y.A.; Lu, Y.Y.; Chen, Y.C.; Chen, S.A.; Chen, Y.J. Various subtypes of phosphodiesterase inhibitors differentially regulate pulmonary vein and sinoatrial node electrical activities. Exp. Ther. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yu, Y.; Lan, H.; Ou, X.; Yang, L.; Li, T.; Cao, J.; Zeng, X.; Li, M. Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) Increases Small-Conductance Ca2+-Activated K+ Current in Patients with Chronic Atrial Fibrillation. Med. Sci. Monit. 2018, 24, 3011–3023. [Google Scholar] [CrossRef]
- Christ, T.; Rozmaritsa, N.; Engel, A.; Berk, E.; Knaut, M.; Metzner, K.; Canteras, M.; Ravens, U.; Kaumann, A. Arrhythmias, elicited by catecholamines and serotonin, vanish in human chronic atrial fibrillation. Proc. Natl. Acad. Sci. USA 2014, 111, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Dolce, B.; Christ, T.; Grammatika Pavlidou, N.; Yildirim, Y.; Reichenspurner, H.; Eschenhagen, T.; Nikolaev, V.O.; Kaumann, A.J.; Molina, C.E. Impact of phosphodiesterases PDE3 and PDE4 on 5-hydroxytryptamine receptor4-mediated increase of cAMP in human atrial fibrillation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Berk, E.; Christ, T.; Schwarz, S.; Ravens, U.; Knaut, M.; Kaumann, A.J. In permanent atrial fibrillation, PDE3 reduces force responses to 5-HT, but PDE3 and PDE4 do not cause the blunting of atrial arrhythmias. Br. J. Pharmacol. 2016, 173, 2478–2489. [Google Scholar] [CrossRef]
- Pino, R.; Cerbai, E.; Calamai, G.; Alajmo, F.; Borgioli, A.; Braconi, L.; Cassai, M.; Montesi, G.F.; Mugelli, A. Effect of 5-HT4 receptor stimulation on the pacemaker current If in human isolated atrial myocytes. Cardiovasc. Res. 1998, 40, 516–522. [Google Scholar] [CrossRef]
- Scala, E.D.; Findlay, I.; Rose, S.; Aupart, M.; Argibay, J.; Cosnay, P.; Bozon, V. High Efficiency Activation of L-Type Ca2+ Current by 5-HT in Human Atrial Myocytes. Recept. Channels 2004, 10, 159–165. [Google Scholar] [CrossRef]
- Pfaffinger, P.J.; Martin, J.M.; Hunter, D.D.; Nathanson, N.M.; Hille, B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature 1985, 317, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Hisatome, I.; Wasserstrom, J.A.; Arentzen, C.E.; Singer, D.H. Acetylcholine-sensitive potassium channels in human atrial myocytes. Am. J. Physiol. Heart Circ. Physiol. 1990, 259, H1730–H1735. [Google Scholar] [CrossRef]
- Christ, T.; Wettwer, E.; Voigt, N.; Hála, O.; Radicke, S.; Matschke, K.; Várro, A.; Dobrev, D.; Ravens, U. Pathology-specific effects of the I Kur/I to/I K,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br. J. Pharmacol. 2008, 154, 1619–1630. [Google Scholar] [CrossRef]
- Dobrev, D.; Friedrich, A.; Voigt, N.; Jost, N.; Wettwer, E.; Christ, T.; Knaut, M.; Ravens, U. The G Protein–Gated Potassium Current I K,ACh Is Constitutively Active in Patients With Chronic Atrial Fibrillation. Circulation 2005, 112, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Friedrich, A.; Bock, M.; Wettwer, E.; Christ, T.; Knaut, M.; Strasser, R.H.; Ravens, U.; Dobrev, D. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK,ACh channels in patients with chronic atrial fibrillation. Cardiovasc. Res. 2007, 74, 426–437. [Google Scholar] [CrossRef]
- Soattin, L.; Lubberding, A.F.; Bentzen, B.H.; Christ, T.; Jespersen, T. Inhibition of Adenosine Pathway Alters Atrial Electrophysiology and Prevents Atrial Fibrillation. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Song, Y.; Wang, D.; Srinivas, M. Ionic basis of the electrophysiological actions of adenosine on cardiomyocytes. FASEB J. 1995, 9, 359–365. [Google Scholar] [CrossRef]
- Pelleg, A.; Mitsuoka, T.; Michelson, E.L.; Menduke, H. Adenosine mediates the negative chronotropic action of adenosine 5′-triphosphate in the canine sinus node. J. Pharmacol. Exp. Ther. 1987, 242, 791–795. [Google Scholar]
- George, E. Adenosine and acetylcholine reduce isoproterenol-induced protein phosphorylation of rat myocytes. J. Mol. Cell. Cardiol. 1991, 23, 749–764. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Molina, C.E.; Prat-Vidal, C.; Farré, J.; Roura, S.; Cinca, J. The proarrhythmic antihistaminic drug terfenadine increases spontaneous calcium release in human atrial myocytes. Eur. J. Pharmacol. 2006, 553, 215–221. [Google Scholar] [CrossRef]
- Molina, C.E.; Llach, A.; Herraiz-Martínez, A.; Tarifa, C.; Barriga, M.; Wiegerinck, R.F.; Fernandes, J.; Cabello, N.; Vallmitjana, A.; Benitéz, R.; et al. Prevention of adenosine A2A receptor activation diminishes beat-to-beat alternation in human atrial myocytes. Basic Res. Cardiol. 2016, 111, 5. [Google Scholar] [CrossRef]
- Furukawa, S.; Satoh, K.; Taira, N. Opening of ATP-sensitive K+ channels responsible for adenosine A2 receptor-mediated vasodepression does not involve a pertussis toxin-sensitive G protein. Eur. J. Pharmacol. 1993, 236, 255–262. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Prat-Vidal, C.; Fernandes, J.; Casadó, V.; Ciruela, F.; Lluís, C.; Francro, R.; Cianca, J.; Hove-Madsen, L. Abnormal calcium handling in atrial fibrillation is linked to up-regulation of adenosine A2A receptors. Eur. Heart J. 2011, 32, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Tikh, E.I.; Fenton, R.A.; Dobson, J.G. Contractile effects of adenosine A 1 and A 2A receptors in isolated murine hearts. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H348–H356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Headrick, J.P.; Peart, J.N.; Reichelt, M.E.; Haseler, L.J. Adenosine and its receptors in the heart: Regulation, retaliation and adaptation. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 1413–1428. [Google Scholar] [CrossRef]
- Maggirwar, S.B.; Dhanraj, D.N.; Somani, S.M.; Ramkumar, V. Adenosine Acts as an Endogenous Activator of the Cellular Antioxidant Defense System. Biochem. Biophys. Res. Commun. 1994, 201, 508–515. [Google Scholar] [CrossRef]
- Phosri, S.; Arieyawong, A.; Bunrukchai, K.; Parichatikanond, W.; Nishimura, A.; Nishida, M.; Mangmool, S. Stimulation of Adenosine A2B Receptor Inhibits Endothelin-1-Induced Cardiac Fibroblast Proliferation and α-Smooth Muscle Actin Synthesis Through the cAMP/Epac/PI3K/Akt-Signaling Pathway. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Phosri, S.; Bunrukchai, K.; Parichatikanond, W.; Sato, V.H.; Mangmool, S. Epac is required for exogenous and endogenous stimulation of adenosine A2B receptor for inhibition of angiotensin II-induced collagen synthesis and myofibroblast differentiation. Purinergic Signal. 2018, 14, 141–156. [Google Scholar] [CrossRef]
- Villarreal, F.; Epperson, S.A.; Ramirez-Sanchez, I.; Yamazaki, K.G.; Brunton, L.L. Regulation of cardiac fibroblast collagen synthesis by adenosine: Roles for Epac and PI3K. Am. J. Physiol. Cell Physiol. 2009, 296, C1178–C1184. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Zhong, H.; Mezzaroma, E.; Van Tassell, B.W.; Kannan, H.; Zeng, D.; Belardinelli, L.; Voelkel, N.F.; Abbate, A. GS-6201, a Selective Blocker of the A 2B Adenosine Receptor, Attenuates Cardiac Remodeling after Acute Myocardial Infarction in the Mouse. J. Pharmacol. Exp. Ther. 2012, 343, 587–595. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, H.; Everett, T.H.; Wilson, E.; Chang, R.; Zeng, D.; Belardinelli, L.; Olgin, J.E. Blockade of A2B adenosine receptor reduces left ventricular dysfunction and ventricular arrhythmias 1 week after myocardial infarction in the rat model. Heart Rhythm 2014, 11, 101–109. [Google Scholar] [CrossRef]
- Asakura, M.; Asanuma, H.; Kim, J.; Liao, Y.; Nakamaru, K.; Fujita, M.; Komamura, K.; Isomura, T.; Furukawa, H.; Tomoike, H.; et al. Impact of Adenosine Receptor Signaling and Metabolism on Pathophysiology in Patients with Chronic Heart Failure. Hypertens. Res. 2007, 30, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-T.; Chiang, F.-T.; Tseng, C.-D.; Hwang, J.J.; Kuo, K.T.; Wu, C.K.; Yu, C.; Wang, Y.C.; Lai, L.P.; Lin, J.L. Increased Expression of Mineralocorticoid Receptor in Human Atrial Fibrillation and a Cellular Model of Atrial Fibrillation. J. Am. Coll. Cardiol. 2010, 55, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Mayyas, F.; Alzoubi, K.H.; van Wagoner, D.R. Impact of aldosterone antagonists on the substrate for atrial fibrillation: Aldosterone promotes oxidative stress and atrial structural/electrical remodeling. Int. J. Cardiol. 2013, 168, 5135–5142. [Google Scholar] [CrossRef]
- Neefs, J.; van den Berg, N.W.E.; Limpens, J.; Berger, W.R.; Boekholdt, S.M.; Sanders, P.; de Groot, J.R. Aldosterone Pathway Blockade to Prevent Atrial Fibrillation: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 231, 155–161. [Google Scholar] [CrossRef]
- Van Wagoner, D.R. Oxidative Stress and Inflammation in Atrial Fibrillation: Role in Pathogenesis and Potential as a Therapeutic Target. J. Cardiovasc. Pharmacol. 2008, 52, 306–313. [Google Scholar] [CrossRef]
- Ishii, Y.; Schuessler, R.B.; Gaynor, S.L.; Hames, K.; Damiano, R.J. Postoperative atrial fibrillation: The role of the inflammatory response. J. Thorac. Cardiovasc. Surg. 2017, 153, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Scott, L.; Veleva, T.; Müller, F.U.; Nattel, S.; Dobrev, D.; Wehrens, X.H.T.; Li, N. Enhanced Activation Inflammasome Promotes Atrial Fibrillation. J. Mol. Cell. Cardiol. 2017, 112, 147. [Google Scholar] [CrossRef]
- Shiroshitatakeshita, A.; Brundel, B.; Lavoie, J.; Nattel, S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc. Res. 2006, 69, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Karabacak, K.; Kubat, E.; Akyol, F.B.; Kadan, M.; Erol, G.; Doğanci, S.; Yildirim, V.; Bolcal, C. The C-reactive protein/albumin ratio as a new predictor for postoperative atrial fibrillation after coronary artery bypass graft surgery. J. Card. Surg. 2020, 35, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Martin, D.O.; Sprecher, D.; Wazni, O.; Kanderian, A.; Carnes, C.A.; Bauer, J.A.; Tchou, P.J.; Niebauer, M.J.; Natale, A.; et al. C-Reactive Protein Elevation in Patients With Atrial Arrhythmias. Circulation 2001, 104, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Kwon, C.H.; Kang, J.G.; Lee, H.J.; Kim, N.H.; Sung, J.W.; Cheong, E.; Sung, K.C. C-reactive protein and risk of atrial fibrillation in East Asians. Europace 2017, 19, 1643–1649. [Google Scholar] [CrossRef]
- Minutoli, L.; Puzzolo, D.; Rinaldi, M.; Irrera, N.; Marini, H.; Arcoraci, V.; Bitto, A.; Crea, G.; Pisani, A.; Squadrito, F.; et al. ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Alsina, K.M.; Abu-Taha, I.; Ghezelbash, S. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Chen, J.; Zhao, S.; Li, H. Pirfenidone Attenuates Cardiac Fibrosis in a Mouse Model of TAC-Induced Left Ventricular Remodeling by Suppressing NLRP3 Inflammasome Formation. Cardiology 2013, 126, 1–11. [Google Scholar] [CrossRef]
- Pinho-Gomes, A.C.; Reilly, S.; Brandes, R.P.; Casadei, B. Targeting Inflammation and Oxidative Stress in Atrial Fibrillation: Role of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase Inhibition with Statins. Antioxid. Redox Signal. 2014, 20, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, V.; Andrié, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K.; et al. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474. [Google Scholar] [CrossRef]
- Simon, J.N.; Vrellaku, B.; Monterisi, S.; Chu, S.M.; Rawlings, N.; Lomas, O.; Marchal, G.A.; Waithe, D.; Syeda, F.; Gajendragadkar, P.R.; et al. Oxidation of Protein Kinase A Regulatory Subunit PKARIα Protects Against Myocardial Ischemia-Reperfusion Injury by Inhibiting Lysosomal-Triggered Calcium Release. Circulation 2020. [Google Scholar] [CrossRef]
- Camelliti, P. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc. Res. 2004, 62, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Schultz, F.; Swiatlowska, P.; Alvarez-Laviada, A.; Sanchez-Alonso, J.L.; Song, Q.; de Vries, A.A.F.; Pijnappels, D.A.; Ongstad, E.; Braga, V.M.M.; Entcheva, E.; et al. Cardiomyocyte–myofibroblast contact dynamism is modulated by connexin-43. FASEB J. 2019, 33, 10453–10468. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Yan, Y.; Zeng, Z.; Zhang, Z.; Liu, H.; Liu, H.; Li, J.; Huang, W.; Wu, J.; He, Y. Connexin 43 reduces susceptibility to sympathetic atrial fibrillation. Int. J. Mol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Laing, J.G.; Kanter, E.M.; Berthoud, V.M.; Bao, M.; Rohrs, H.W.; Townsend, R.R.; Yamada, K.A. Identification of CaMKII Phosphorylation Sites in Connexin43 by High-Resolution Mass Spectrometry. J. Proteome Res. 2011, 10, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Kiemer, F.; Mannell, H.; Beck, H.; Pohl, U.; Pogoda, K. PKA negatively modulates the migration enhancing effect of Connexin 43. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2019, 1866, 828–838. [Google Scholar] [CrossRef]
- Lampe, P.D.; TenBroek, E.M.; Burt, J.M.; Kurata, W.E.; Johnson, R.G.; Lauf, A.F. Phosphorylation of Connexin43 on Serine368 by Protein Kinase C Regulates Gap Junctional Communication. J. Cell Biol. 2000, 149, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Xie, W.; Betzenhauser, M.; Reiken, S.; Chen, B.X.; Wronska, A.; Marks, A.R. Calcium Leak Through Ryanodine Receptors Leads to Atrial Fibrillation in 3 Mouse Models of Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. Res. 2012, 111, 708–717. [Google Scholar] [CrossRef]
- Greer-Short, A.; Musa, H.; Alsina, K.M.; Ni, L.; Word, T.A.; Reynolds, J.O.; Gratz, D.; Lane, C.; El-Refaey, M.; Unudurthi, S.; et al. Calmodulin kinase II regulates atrial myocyte late sodium current, calcium handling, and atrial arrhythmia. Heart Rhythm 2020, 17, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Toischer, K.; Hartmann, N.; Wagner, S.; Fischer, T.H.; Herting, J.; Danner, B.C.; Sag, C.M.; Hund, T.J.; Mohler, P.J.; Belardinelli, L.; et al. Role of late sodium current as a potential arrhythmogenic mechanism in the progression of pressure-induced heart disease. J. Mol. Cell. Cardiol. 2013, 61, 111–122. [Google Scholar] [CrossRef]
- Van der Velden, H. Gap junctional remodeling in relation to stabilization of atrial fibrillation in the goat. Cardiovasc. Res. 2000, 46, 476–486. [Google Scholar] [CrossRef]
- Bonilla, I.M.; Sridhar, A.; Györke, S.; Cardounel, A.J.; Carnes, C.A. Nitric Oxide Synthases and Atrial Fibrillation. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Guzik, T.J.; Zhang, Y.H.; Zhang, M.H.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. A Myocardial Nox2 Containing NAD(P)H Oxidase Contributes to Oxidative Stress in Human Atrial Fibrillation. Circ. Res. 2005, 97, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.N.; Jayaram, R.; Nahar, K.; Antoniades, C.; Verheule, S.; Channon, K.M.; Alp, N.J.; Schotten, U.; Casadei, B. Atrial Sources of Reactive Oxygen Species Vary With the Duration and Substrate of Atrial Fibrillation. Circulation 2011, 124, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Eager, K.R.; Dulhunty, A.F. Activation of the Cardiac Ryanodine Receptor by Sulfhydryl Oxidation is Modified by Mg 2+ and ATP. J. Membr. Biol. 1998, 163, 9–18. [Google Scholar] [CrossRef]
- Marengo, J.J.; Hidalgo, C.; Bull, R. Sulfhydryl Oxidation Modifies the Calcium Dependence of Ryanodine-Sensitive Calcium Channels of Excitable Cells. Biophys. J. 1998, 74, 1263–1277. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; Marbán, E.; O’Rourke, B. Synchronized Whole Cell Oscillations in Mitochondrial Metabolism Triggered by a Local Release of Reactive Oxygen Species in Cardiac Myocytes. J. Biol. Chem. 2003, 278, 44735–44744. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6. [Google Scholar] [CrossRef]
- Burger, D.E.; Lu, X.; Lei, M.; Xiang, F.L.; Hammoud, L.; Jiang, M.; Wang, H.; Jones, D.L.; Sims, S.M.; Feng, Q. Neuronal Nitric Oxide Synthase Protects Against Myocardial Infarction-Induced Ventricular Arrhythmia and Mortality in Mice. Circulation 2009, 120, 1345–1354. [Google Scholar] [CrossRef]
- Sears, C.E.; Bryant, S.M.; Ashley, E.A.; Lygate, C.A.; Rakovic, S.; Wallis, H.L.; Neubauer, S.; Terrar, D.A.; Casadei, B. Cardiac Neuronal Nitric Oxide Synthase Isoform Regulates Myocardial Contraction and Calcium Handling. Circ. Res. 2003, 92. [Google Scholar] [CrossRef] [PubMed]
- Layland, J.; Li, J.; Shah, A.M. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002, 540, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.D.; Sagach, V.F.; Shah, A.M. Basal Release of Nitric Oxide Augments the Frank-Starling Response in the Isolated Heart. Circulation 1997, 96, 1320–1329. [Google Scholar] [CrossRef]
- Wang, H.; Kohr, M.J.; Wheeler, D.G.; Ziolo, M.T. Endothelial nitric oxide synthase decreases β-adrenergic responsiveness via inhibition of the L-type Ca2+ current. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1473–H1480. [Google Scholar] [CrossRef] [PubMed]
- Loke, K.E.; McConnell, P.I.; Tuzman, J.M.; Shesely, E.G.; Smith, C.J.; Stackpole, C.J.; Thompson, C.I.; Kaley, G.; Wolin, M.S.; Hintze, T.H. Endogenous Endothelial Nitric Oxide Synthase–Derived Nitric Oxide Is a Physiological Regulator of Myocardial Oxygen Consumption. Circ. Res. 1999, 84, 840–845. [Google Scholar] [CrossRef]
- Petroff, M.G.V.; Kim, S.H.; Pepe, S.; Dessy, C.; Marbán, E.; Balligand, J.L.; Sollott, S.J. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat. Cell Biol. 2001, 3, 867–873. [Google Scholar] [CrossRef]
- Cai, H.; Li, Z.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C.; Harrison, D.G.; Langberg, J.J. Downregulation of Endocardial Nitric Oxide Synthase Expression and Nitric Oxide Production in Atrial Fibrillation. Circulation 2002, 106, 2854–2858. [Google Scholar] [CrossRef]
- Gomez, R.; Núñez, L.; Vaquero, M.; Amorós, I.; Barana, A.; de Prada, T.; Macaya, C.; Moroto, L.; Rodrígues, E.; Caballero, R.; et al. Nitric oxide inhibits Kv4.3 and human cardiac transient outward potassium current (Ito1). Cardiovasc. Res. 2008, 80, 375–384. [Google Scholar] [CrossRef]
- Richards, M.A.; Simon, J.N.; Ma, R.; Loonat, A.A.; Crabtree, M.J.; Paterson, D.J.; Fahlman, R.P.; Casadei, B.; Fliegerl, L.; Swietach, P. Nitric oxide modulates cardiomyocyte pH control through a biphasic effect on sodium/hydrogen exchanger-1. Cardiovasc. Res. 2020, 116, 1958–1971. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, M.; Rivet-Bastide, M.; Hatem, S.; Bénardeau, A.; Mercadier, J.J.; Fischmeister, R. Nitric oxide regulates the calcium current in isolated human atrial myocytes. J. Clin. Investig. 1995, 95, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Mizuno, K. Increased Concentration of Plasma Cyclic GMP from Aconitine-Induced Atrial Fibrillation in Dogs and Patients with Paroxysmal Atrial Fibrillation. Advances in Myocardiology; Springer US: Boston, MA, USA, 1982; pp. 177–183. [Google Scholar] [CrossRef]
- Mizuno, K.; Ogawa, K. Increased Concentration of Plasma Cyclic GMP During Aconitine-Induced Atrial Fibrillation in Dogs and Paroxysmal Atrial Fibrillation in Patients. J. Cardiovasc. Pharmacol. 1981, 3, 1211–1220. [Google Scholar] [CrossRef]
- Suffee, N.; Moore-Morris, T.; Farahmand, P.; Rücker-Martin, C.; Dilanian, G.; Fradet, M.; Sawaki, D.; Derumeaux, G.; LePrince, P.; Clément, K.; et al. Atrial natriuretic peptide regulates adipose tissue accumulation in adult atria. Proc. Natl. Acad. Sci. USA 2017, 114, E771–E780. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R.; Joiner, M.A.; Guan, X.; Kutschke, W.; Yang, J.; Oddis, C.V.; Bartlett, R.K.; Lowe, J.S.; O’Donnell, S.E.; Aykin-Burns, N.; et al. A Dynamic Pathway for Calcium-Independent Activation of CaMKII by Methionine Oxidation. Cell 2008, 133, 462–474. [Google Scholar] [CrossRef]
- Rahmutula, D.; Zhang, H.; Wilson, E.E.; Olgin, J.E. Absence of natriuretic peptide clearance receptor attenuates TGF-β1-induced selective atrial fibrosis and atrial fibrillation. Cardiovasc. Res. 2019, 115, 357–372. [Google Scholar] [CrossRef]
- Yoo, S.; Aistrup, G.; Shiferaw, Y.; Ng, J.; Mohler, P.J.; Hund, T.J.; Waugh, T.; Browne, S.; Gussak, G.; Gilani, M.; et al. Oxidative stress creates a unique, CaMKII-mediated substrate for atrial fibrillation in heart failure. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Verma, A.; Jiang, C.; Betts, T.R.; Chen, J.; Deisenhofer, I.; Mantovan, R.; Macle, L.; Morillo, C.A.; Haverkamp, W.; Weerasooriya, R.; et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N. Engl. J. Med. 2015, 372, 1812–1822. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Lafuente, C.; Valembois, L.; Bergmann, J.-F.; Belmin, J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed]
- Reimold, F.R.; Reynolds, M.R. Proarrhythmia and death with antiarrhythmic drugs for atrial fibrillation, and the unfulfilled promise of comparative effectiveness research. Am. Heart J. 2018, 205, 128–130. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).