Exploring Binder–Ionic Liquid Electrolyte Systems in Silicon Oxycarbide Negative Electrodes for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Active Material and Electrode Preparation
2.2. Synthesis of Ionic Liquids
2.3. Electrolyte and Material Characterization
3. Results
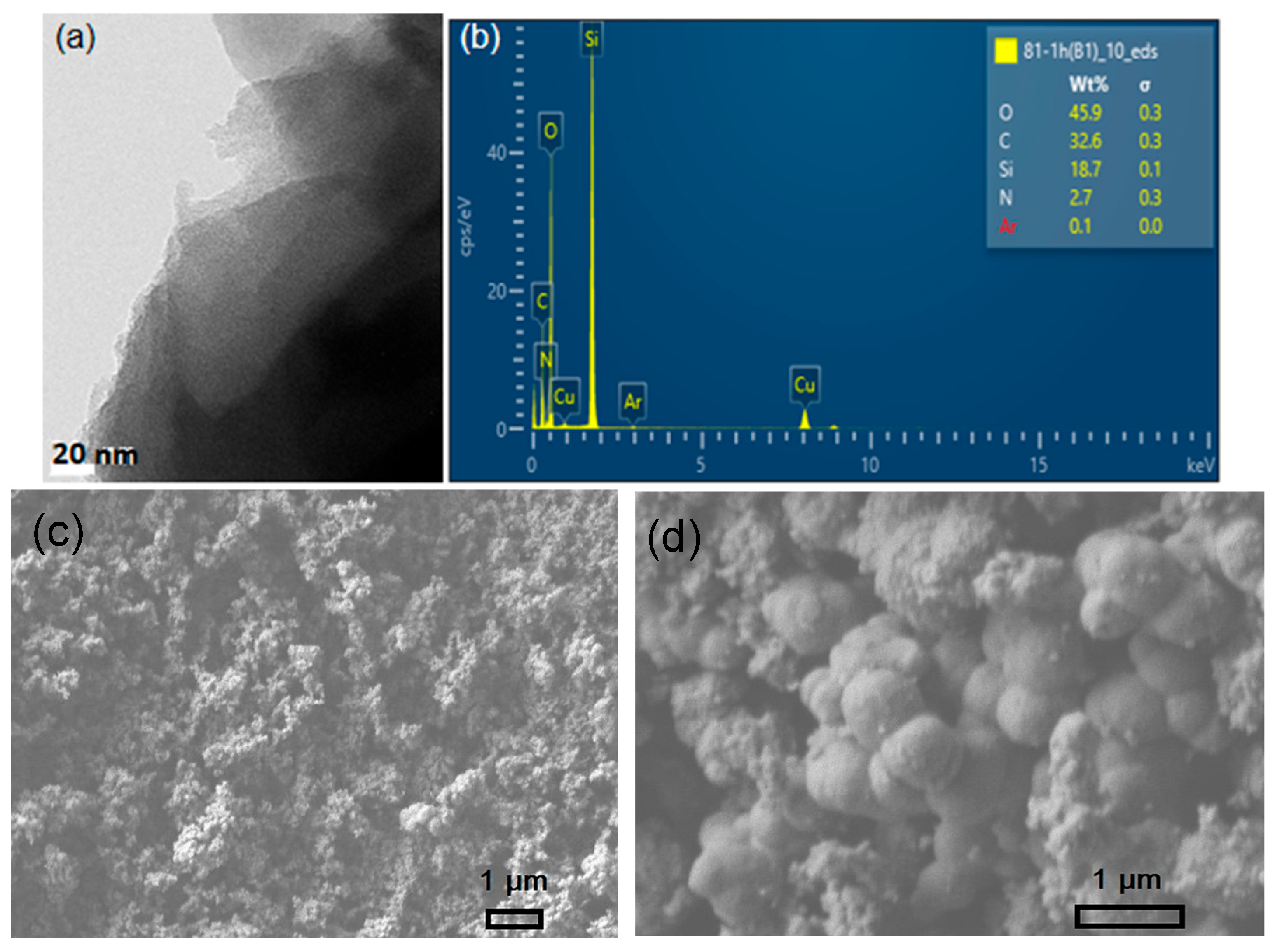
3.1. Composite Electrodes and Electrolyte Characterization
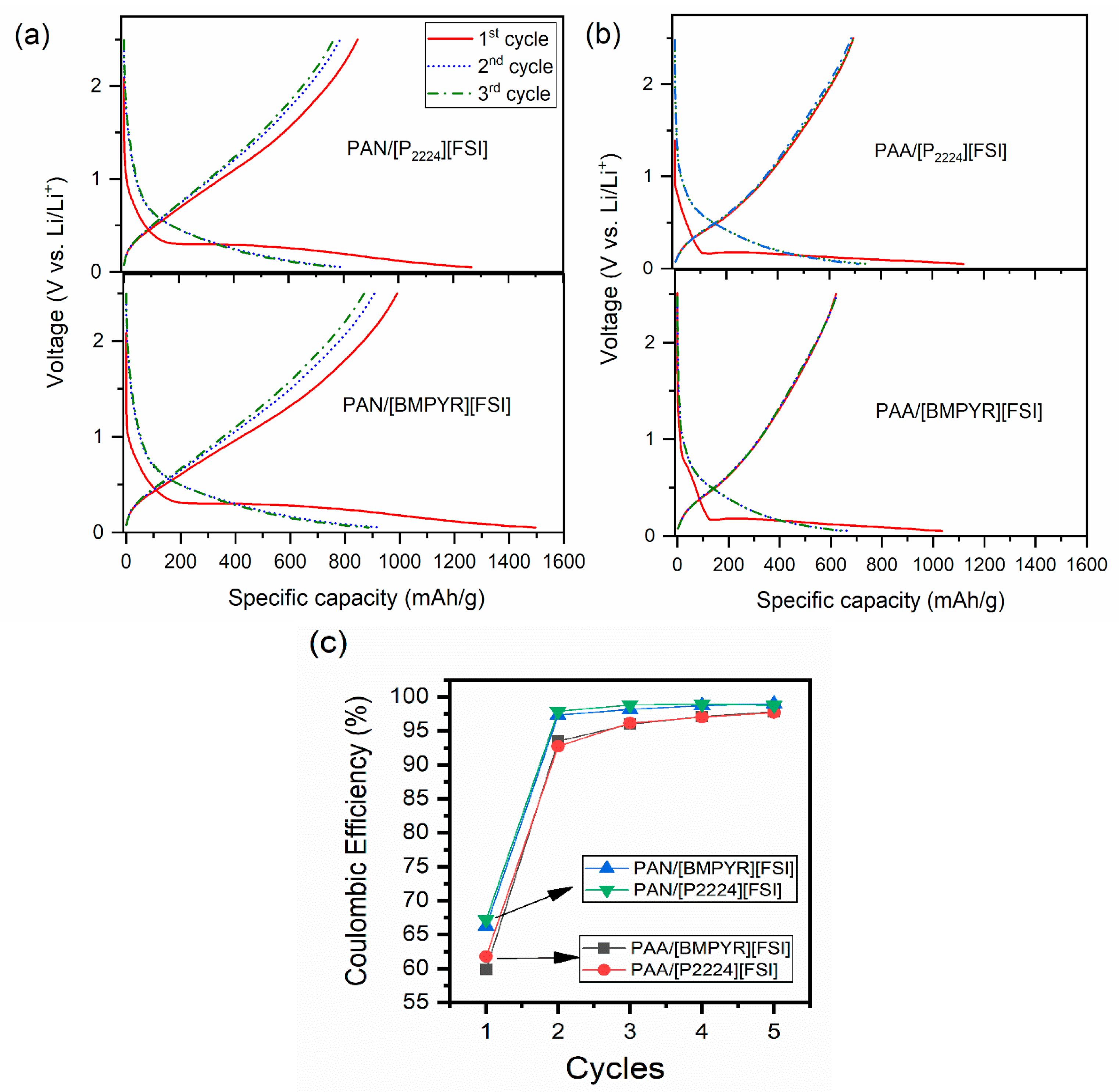
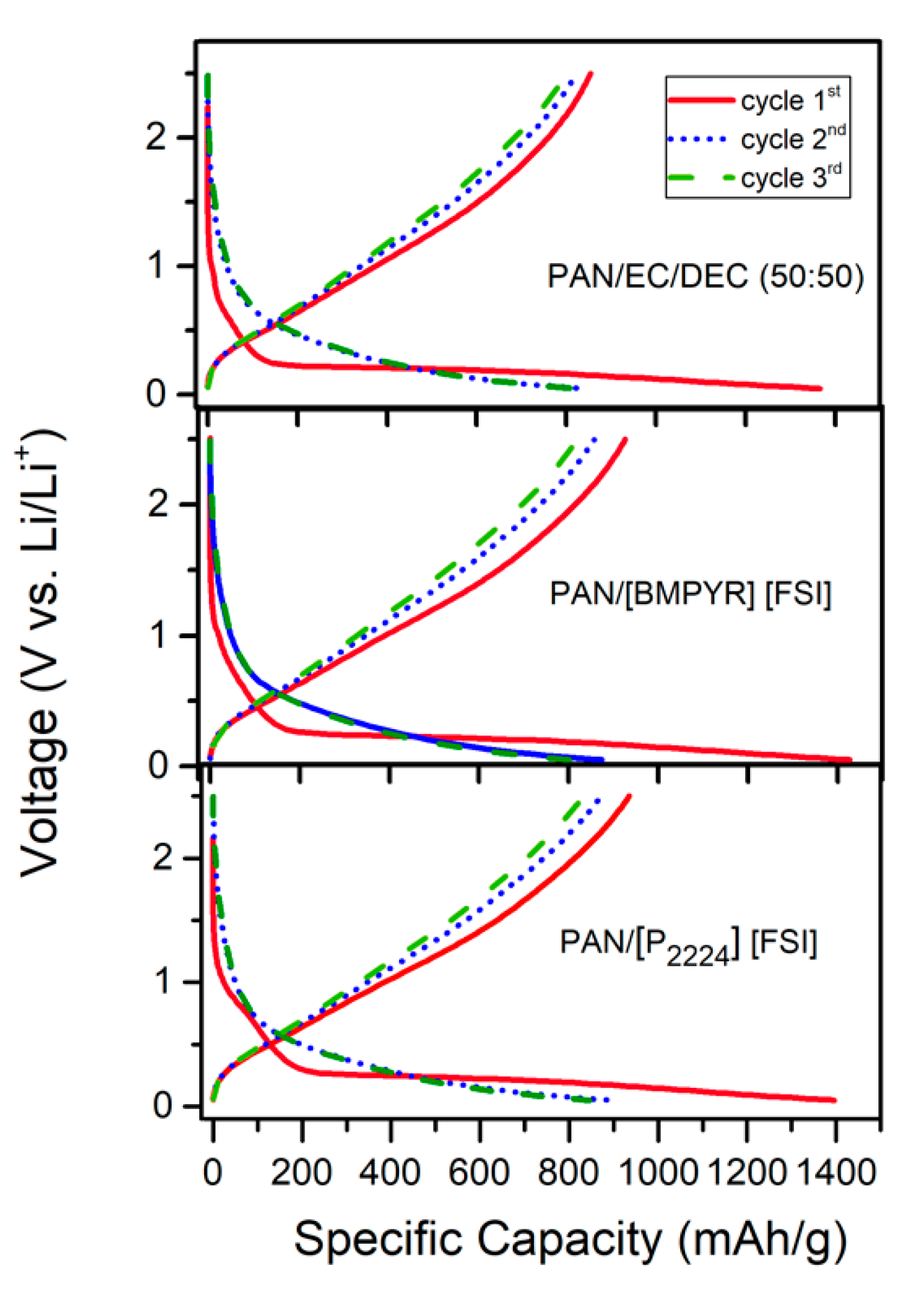
3.2. Electrochemical Performance
3.3. Effect of Binder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LIBs | Lithium-ion batteries |
| ILs | Ionic liquids |
| NC-SiOC | Nitrogen-doped carbon silicon oxycarbide |
| PAA | Poly(acrylic acid) |
| PAN | Poly(acrylonitrile) |
| SEI | Solid electrolyte interphase |
| FSI | bis(fluoromethylsulfonyl) imide |
| NPs | Nanoparticles |
| PVDF | Polyvylidene fluoride |
| CMC | Carboxymethyl cellulose |
| DEC | Diethly carbonate |
| EC | Ethylene carbonate |
| EIS | Electrochemical impedance spectroscopy |
| P2224 | triethyl-n-butylphosphonium bis(fluoromethylsulfonyl)imide |
| BMPYR | N-propyl-N–methylpyrrolidinium bis(fluoromethylsulfonyl)imide |
| PFG-NMR | Pulsed-field gradient nuclear magnetic resonance |
| FEG-TEM | Field-emission gun transmission electron microscope |
| APTES | 3-aminopropyltriethoxysilane |
Appendix A
| Active Material Mass (mg) | Thickness (µm) | ICE (%) | 1st Discharge mAh/g | 1st Charge mAh/g | |
|---|---|---|---|---|---|
| NC-SiOC/PAA | |||||
| [BMPYR][FSI] | 0.81 | 27 | 60 | 1035.3 | 619.8 |
| [P2224][FSI] | 0.64 | 24 | 61.8 | 1122.4 | 693.6 |
| NC-SiOC/PAN | |||||
| [BMPYR][FSI] | 0.84 | 28 | 66.2 | 1495.6 | 990.8 |
| [P2224][FSI] | 0.84 | 24 | 67.2 | 1266.1 | 851.1 |
| Active Material | Binder, Electrolyte (Li Salt) | First Delithiation Capacity mAh/g (Current Density) | Initial Coulombic Efficiency % | Ref. |
|---|---|---|---|---|
| NC/SiOC | PAN, EC/DEC (1 M LiPF6) PAN, [BMPYR][FSI] (1 M LiFSI) PAN, [P2224][FSI] (1 M LiFSI) | 854 (0.1) 926 (0.1) 934 (0.1) | 62 64 67 | This work |
| NC/SiOC | PAA, EC/DEC (1 M LiPF6) | 622 (0.1) 367 (0.1) | 63 55 | [32] |
| SiOC/N-doped C fibers | Free-standing, PC:EC:DMC (1 M LiPF6) | 518 | 73 | [21] |
| SiOC/ Graphene (60:40) | Free-standing, DMC:EC (1 M LiPF6) | 702 (0.1) | 70 | [20] |
| SiOC/graphene | CMC, EC:DMC:EMC (1 M LiPF6) | 608 (0.05) | 63 | [22] |
| SiOC/CNT | PAA, EC:DMC (1 M LiPF6) | 842 (at C/10~0.1) | 67 | [31] |
| SiOC-10-HF | PVDF, EC:DMC (1 M LiPF6) | 272 (0.018) | 60 | [24] |
| SiOC/C rich | PVDF, EC:DMC (1 M LiPF6) | 700 (0.018) | --- | [28] |
| SiOC | PVDF, EC:DMC (1 M LiPF6) | 562 (0.019) | 61.5 | [18] |
References
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, Z.; Kumar, P.; Liang, H.; Cao, Z.; Xie, H.; Cavallo, L.; Li, Q.; Ming, J. Non-Flammable Electrolyte Mediated by Solvation Chemistry toward High-Voltage Lithium-Ion Batteries. ACS Energy Lett. 2024, 9, 1604–1616. [Google Scholar] [CrossRef]
- Chawla, N.; Bharti, N.; Singh, S. Recent advances in non-flammable electrolytes for safer lithium-ion batteries. Batteries 2019, 5, 19. [Google Scholar] [CrossRef]
- Wang, Y.; Zaghib, K.; Guerfi, A.; Bazito, F.F.C.; Torresi, R.M.; Dahn, J.R. Accelerating rate calorimetry studies of the reactions between ionic liquids and charged lithium ion battery electrode materials. Electrochim. Acta 2007, 52, 6346–6352. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, N.; Assresahegn, B.D.; Torresi, R.M.; Bélanger, D. Producing high-performing silicon anodes by tailoring ionic liquids as electrolytes. Energy Storage Mater. 2020, 25, 477–486. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, N.; Monje, I.E.; Bélanger, D.; Camargo, P.H.C.; Torresi, R.M. High rate and long-term cycling of silicon anodes with phosphonium-based ionic liquids as electrolytes for lithium-ion batteries. Electrochim. Acta 2023, 439, 141680. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, N.; Monje, I.E.; Martins, V.L.; Bélanger, D.; Camargo, P.H.C.; Torresi, R.M. Four Phosphonium-based Ionic Liquids. Synthesis, Characterization and Electrochemical Performance as Electrolytes for Silicon Anodes. ChemistrySelect 2022, 7, e202104430. [Google Scholar] [CrossRef]
- Pomerantseva, E.; Bonaccorso, F.; Feng, X.; Cui, Y.; Gogotsi, Y. Energy storage: The future enabled by nanomaterials. Science 2019, 366, eaan8285. [Google Scholar] [CrossRef]
- Piper, D.M.; Evans, T.; Xu, S.; Kim, S.C.; Han, S.S.; Liu, K.L.; Oh, K.H.; Yang, R.; Lee, S. Optimized Silicon Electrode Architecture, Interface, and Microgeometry for Next-Generation Lithium-Ion Batteries. Adv. Mater. 2016, 28, 188–193. [Google Scholar] [CrossRef]
- Andersen, H.F.; Foss, C.E.L.; Voje, J.; Tronstad, R.; Mokkelbost, T.; Vullum, P.E.; Ulvestad, A.; Kirkengen, M.; Mæhlen, J.P. Silicon-Carbon composite anodes from industrial battery grade silicon. Sci. Rep. 2019, 9, 14814. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Halim, M.; Hudaya, C.; Kim, A.Y.; Lee, J.K. Phenyl-rich silicone oil as a precursor for SiOC anode materials for long-cycle and high-rate lithium ion batteries. J. Mater. Chem. A 2016, 4, 2651–2656. [Google Scholar] [CrossRef]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: ‘All-Rounder’ materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Shi, H.; Yuan, A.; Xu, J. Tailored synthesis of monodispersed nano/submicron porous silicon oxycarbide (SiOC) spheres with improved Li-storage performance as an anode material for Li-ion batteries. J. Power Sources 2017, 364, 288–298. [Google Scholar] [CrossRef]
- Fukui, H.; Harimoto, Y.; Akasaka, M.; Eguchi, K. Lithium species in electrochemically lithiated and delithiated silicon oxycarbides. ACS Appl. Mater. Interfaces 2014, 6, 12827–12836. [Google Scholar] [CrossRef] [PubMed]
- Wilamowska-Zawlocka, M.; Puczkarski, P.; Grabowska, Z.; Kaspar, J.; Graczyk-Zajac, M.; Riedel, R.; Sorarù, G.D. Silicon oxycarbide ceramics as anodes for lithium ion batteries: Influence of carbon content on lithium storage capacity. RSC Adv. 2016, 6, 104597–104607. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Vrankovic, D.; Waleska, P.; Hess, C.; Sasikumar, P.V.; Lauterbach, S.; Kleebe, H.-J.; Sorarù, G.D. The Li-storage capacity of SiOC glasses with and without mixed silicon oxycarbide bonds. J. Mater. Chem. A 2017, 6, 93–103. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Silicon oxycarbide glass-graphene composite paper electrode for long-cycle lithium-ion batteries. Nat. Commun. 2016, 7, 10998. [Google Scholar] [CrossRef]
- Ma, M.; Wang, H.; Li, X.; Peng, K.; Xiong, L.; Du, X. Free-standing SiOC/nitrogen-doped carbon fibers with highly capacitive Li storage. J. Eur. Ceram. Soc. 2020, 40, 5238–5246. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, B.; Huang, X.; Chu, F.; Qiu, J.; Ding, J. Intercalated SiOC/graphene composites as anode material for li-ion batteries. Solid State Ion. 2015, 278, 198–202. [Google Scholar] [CrossRef]
- Xia, K.; Wu, Z.; Xuan, C.; Xiao, W.; Wang, J.; Wang, D. Effect of KOH etching on the structure and electrochemical performance of SiOC anodes for lithium-ion batteries. Electrochim. Acta 2017, 245, 287–295. [Google Scholar] [CrossRef]
- Dibandjo, P.; Graczyk-Zajac, M.; Riedel, R.; Pradeep, V.S.; Soraru, G.D. Lithium insertion into dense and porous carbon-rich polymer-derived SiOC ceramics. J. Eur. Ceram. Soc. 2012, 32, 2495–2503. [Google Scholar] [CrossRef]
- Sujith, R.; Gangadhar, J.; Greenough, M.; Bordia, R.K.; Panda, D.K. A review of silicon oxycarbide ceramics as next generation anode materials for lithium-ion batteries and other electrochemical applications. J. Mater. Chem. A 2023, 11, 20324–20348. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Carbon-Rich Silicon Oxycarbide (SiOC) and Silicon Oxycarbide / Element (SiOC/X, X = Si, Sn) Nano-Composites as New Anode Materials for Li-Ion Battery Application. Ph.D. Thesis, Technische Universität Darmstadt, Darmstadt, Germany, 2014; pp. 1–117. [Google Scholar]
- Wilamowska, M.; Pradeep, V.S.; Graczyk-Zajac, M.; Riedel, R.; Sorarù, G.D. Tailoring of SiOC composition as a way to better performing anodes for Li-ion batteries. Solid State Ion. 2014, 260, 94–100. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Toma, L.; Fasel, C.; Riedel, R. Carbon-rich SiOC anodes for lithium-ion batteries: Part I. Influence of material UV-pre-treatment on high power properties. Solid State Ion. 2012, 225, 522–526. [Google Scholar] [CrossRef]
- Tao, A.; Ren, J.; Liu, B.; Yang, M.; Kong, Y.; Zhao, X.; Shen, X. Hierarchical porous silicon oxycarbide as a stable anode material for lithium-ion batteries. J. Energy Storage 2024, 104, 114617. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, C.; Do, K.; Ahn, H. Maximizing the utilization of active sites through the formation of native nanovoids of silicon oxycarbide as anode materials in lithium-ion batteries. Energy Storage Mater. 2021, 35, 130–141. [Google Scholar] [CrossRef]
- Bhandavat, R.; Singh, G. Stable and Efficient Li-Ion Battery Anodes Prepared from Polymer- Derived Silicon Oxycarbide–Carbon Nanotube Shell/Core Composites. J. Phys. Chem. C 2013, 117, 11899–11905. [Google Scholar] [CrossRef]
- Monje, I.; Sanchez-Ramirez, N.; Santagneli, S.H.; Camargo, P.H.; Bélanger, D.; Schougaard, S.B.; Torresi, R.M. In-situ formed Nitrogen-doped carbon/silicon-based materials as negative electrodes for lithium ion batteries. J. Electroanal. Chem. 2021, 901, 115732. [Google Scholar] [CrossRef]
- Piper, D.M.; Yersak, T.A.; Son, S.; Kim, S.C.; Kang, C.S.; Oh, K.H.; Ban, C.; Dillon, A.C.; Lee, S. Conformal coatings of cyclized-PAN for mechanically resilient Si nano-composite anodes. Adv. Energy Mater. 2013, 3, 697–702. [Google Scholar] [CrossRef]
- Piper, D.M.; Evans, T.; Leung, K.; Watkins, T.; Olson, J.; Kim, S.C.; Han, S.S.; Bhat, V.; Oh, K.H.; Buttry, D.A.; et al. Stable silicon-ionic liquid interface for next-generation lithium-ion batteries. Nat. Commun. 2015, 6, 6230. [Google Scholar] [CrossRef]
- Martins, V.L.; Sanchez-Ramirez, N.; Ribeiro, M.C.C.; Torresi, R.M. Two phosphonium ionic liquids with high Li+ transport number. Phys. Chem. Chem. Phys. 2015, 17, 23041–23051. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, N.; Assresahegn, B.D.; Bélanger, D.; Torresi, R.M. A Comparison among Viscosity, Density, Conductivity, and Electrochemical Windows of N-n-Butyl-N-methylpyrrolidinium and Triethyl-n-pentylphosphonium Bis(fluorosulfonyl imide) Ionic Liquids and Their Analogues Containing Bis(trifluoromethylsulfonyl) Imide Anion. J. Chem. Eng. Data 2017, 62, 3437–3444. [Google Scholar] [CrossRef]
- Rennie, A.J.R.; Sanchez-Ramirez, N.; Torresi, R.M.; Hall, P.J. Ether-Bond-Containing Ionic Liquids as Supercapacitor Electrolytes. J. Phys. Chem. Lett. 2013, 4, 2970–2974. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
- Wei, L.; Chen, C.; Hou, Z.; Wei, H. Poly (acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries. Sci. Rep. 2016, 6, 19583. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T.; Golmohammadi, B. Thermodynamic and transport properties of ionic liquids, 1-alkyl-3-methylimidazolium thiocyanate in the aqueous lithium halides solutions. J. Chem. Thermodyn. 2020, 141, 105953. [Google Scholar] [CrossRef]
- Bazito, F.F.C.; Kawano, Y.; Torresi, R.M. Synthesis and characterization of two ionic liquids with emphasis on their chemical stability towards metallic lithium. Electrochim. Acta 2007, 52, 6427–6437. [Google Scholar] [CrossRef]
- Yuan, W.; Yang, X.; He, L.; Xue, Y.; Qin, S.; Tao, G. Viscosity, Conductivity, and Electrochemical Property of Dicyanamide Ionic Liquids. Front. Chem. 2018, 6, 59. [Google Scholar] [CrossRef]
- Sanchez-Ramirez, N.; Martins, V.L.; Ando, R.A.; Camilo, F.F.; Urahata, S.M.; Ribeiro, M.C.C.; Torresi, R.M. Physicochemical Properties of Three Ionic Liquids Containing a Tetracyanoborate Anion and Their Lithium Salt Mixtures. J. Phys. Chem. B 2014, 118, 8772–8781. [Google Scholar] [CrossRef]
- Hayamizu, K. Temperature dependence of self-diffusion coefficients of ions and solvents in ethylene carbonate, propylene carbonate, and diethyl carbonate single solutions and ethylene carbonate + diethyl carbonate binary solutions of LiPF6 studied by NMR. J. Chem. Eng. Data 2012, 57, 2012–2017. [Google Scholar] [CrossRef]
- Lundgren, H.; Behm, M.; Lindbergh, G. Electrochemical Characterization and Temperature Dependency of Mass-Transport Properties of LiPF6 in EC:DEC. J. Electrochem. Soc. 2015, 162, A413–A420. [Google Scholar] [CrossRef]
- Tsimpanogiannis, I.N.; Jamali, S.H.; Economou, I.G.; Vlugt, T.J.H.; Moultos, O.A. On the validity of the Stokes—Einstein relation for various water force fields. Mol. Phys. 2019, 118, e1702729. [Google Scholar] [CrossRef]
- Shao, Y.; Shigenobu, K.; Watanabe, M.; Zhang, C. Role of Viscosity in Deviations from the Nernst-Einstein Relation. J. Phys. Chem. B 2020, 124, 4774–4780. [Google Scholar] [CrossRef]
- Hess, S.; Wohlfahrt-Mehrens, M.; Wachtler, M. Flammability of Li-Ion Battery Electrolytes: Flash Point and Self-Extinguishing Time Measurements. J. Electrochem. Soc. 2015, 162, A3084–A3097. [Google Scholar] [CrossRef]
- Dai, Y.; Panahi, A. Thermal runaway process in lithium-ion batteries: A review. Next Energy 2025, 6, 100186. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Crosthwaite, J.M.; Aki, S.N.V.K.; Brennecke, J.F. Thermal stability of ionic liquids in nitrogen and air environments. J. Chem. Thermodyn. 2021, 161, 106560. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, H.; Gao, C.; Du, Z.; Wang, J.; Liu, X. SiOx-C dual-phase glass for lithium ion battery anode with high capacity and stable cycling performance. J. Power Sources 2015, 274, 542–550. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Determination of the chemical diffusion coefficient of Li-ions in carbon-rich silicon oxycarbide anodes by electro-analytical methods. Electrochim. Acta 2014, 115, 665–670. [Google Scholar] [CrossRef]
- Dahn, J.R.; Zheng, T.; Liu, Y.; Xue, J.S. Mechanisms for lithium insertion in carbonaceous materials. Science 1995, 270, 590–593. [Google Scholar] [CrossRef]
- Gauthier, M.; Carney, T.J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H.-H.; Fenning, D.P.; Lux, S.F.; Paschos, O.; Bauer, C.; et al. Electrode–Electrolyte Interface in Li-Ion Batteries: Current Understanding and New Insights. J. Phys. Chem. Lett. 2015, 6, 4653–4672. [Google Scholar] [CrossRef]
- Fukui, H.; Ohsuka, H.; Hino, T.; Kanamura, K. A Si-O-C composite anode: High capability and proposed mechanism of lithium storage associated with microstructural characteristics. ACS Appl. Mater. Interfaces 2010, 2, 998–1008. [Google Scholar] [CrossRef]
- Nita, C.; Fullenwarth, J.; Monconduit, L.; Le Meins, J.-M.; Fioux, P.; Parmentier, J.; Ghimbeu, C.M. Eco-friendly synthesis of SiO2 nanoparticles confined in hard carbon: A promising material with unexpected mechanism for Li-ion batteries. Carbon 2019, 143, 598–609. [Google Scholar] [CrossRef]
- Kammoun, H.; Ossonon, B.D.; Tavares, A.C. Nitrogen-Doped Graphene Materials with High Electrical Conductivity Produced by Electrochemical Exfoliation of Graphite Foil. Nanomaterials 2024, 14, 123. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; Ramirez-Rico, J. An electrochemical evaluation of nitrogen-doped carbons as anodes for lithium ion batteries. Carbon 2020, 164, 261–271. [Google Scholar] [CrossRef]
- Naveenkumar, P.; Maniyazagan, M.; Yang, H.W.; Kang, W.S.; Kim, S.J. Nitrogen-doped graphene/silicon-oxycarbide nanosphere as composite anode for high-performance lithium-ion batteries. J. Energy Storage 2023, 59, 106572. [Google Scholar] [CrossRef]
- Bhattacharjya, D.; Park, H.Y.; Kim, M.S.; Choi, H.S.; Inamdar, S.N.; Yu, J.S. Nitrogen-doped carbon nanoparticles by flame synthesis as anode material for rechargeable lithium-ion batteries. Langmuir 2014, 30, 318–324. [Google Scholar] [CrossRef]
- Ou, J.; Zhang, Y.; Chen, L.; Zhao, Q.; Meng, Y.; Guo, Y.; Xiao, D. Nitrogen-rich porous carbon derived from biomass as a high performance anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 6534–6541. [Google Scholar] [CrossRef]
- Chae, S.; Ko, M.; Kim, K.; Ahn, K.; Cho, J. Confronting Issues of the Practical Implementation of Si Anode in High-Energy Lithium-Ion Batteries. Joule 2017, 1, 47–60. [Google Scholar] [CrossRef]
- Komaba, S.; Shimomura, K.; Yabuuchi, N.; Ozeki, T.; Yui, H.; Konno, K. Study on polymer binders for high-capacity SiO negative electrode of Li-Ion batteries. J. Phys. Chem. C 2011, 115, 13487–13495. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and improving the initial Coulombic efficiency of high-capacity anode materials for practical sodium ion batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Leung, K. Electronic structure modeling of electrochemical reactions at electrode/electrolyte interfaces in lithium ion batteries. J. Phys. Chem. C 2013, 117, 1539–1547. [Google Scholar] [CrossRef]
- Lewandowski, A.; Świderska-Mocek, A. Ionic liquids as electrolytes for Li-ion batteries—An overview of electrochemical studies. J. Power Sources 2009, 194, 601–609. [Google Scholar] [CrossRef]
- Kopeć, M.; Lamson, M.; Yuan, R.; Tang, C.; Kruk, M.; Zhong, M.; Matyjaszewski, K.; Kowalewski, T. Polyacrylonitrile-derived nanostructured carbon materials. Prog. Polym. Sci. 2019, 92, 89–134. [Google Scholar] [CrossRef]
- Komaba, S.; Okushi, K.; Ozeki, T.; Yui, H.; Katayama, Y.; Miura, T.; Saito, T.; Groult, H. Polyacrylate modifier for graphite anode of lithium-ion batteries. Electrochem. Solid-State Lett. 2009, 12, 107–110. [Google Scholar] [CrossRef]
- Komaba, S.; Yabuuchi, N.; Ozeki, T.; Okushi, K.; Yui, H.; Konno, K.; Katayama, Y.; Miura, T. Functional binders for reversible lithium intercalation into graphite in propylene carbonate and ionic liquid media. J. Power Sources 2010, 195, 6069–6074. [Google Scholar] [CrossRef]
- Martin, T.R.; Pekarek, R.T.; Coyle, J.E.; Schulze, M.C.; Neale, N.R. Understanding Why Poly(Acrylic Acid) Works: Decarbonylation and Cross-Linking Provide an Ionically Conductive Passivation Layer in Silicon Anodes. J. Mater. Chem. A 2021, 9, 21929–21938. [Google Scholar] [CrossRef]
- Assresahegn, B.D.; Bélanger, D. Effects of the Formulations of Silicon-Based Composite Anodes on their Mechanical, Storage, and Electrochemical Properties. ChemSusChem 2017, 10, 4080–4089. [Google Scholar] [CrossRef]
- Jin, B.; Dolocan, A.; Liu, C.; Cui, Z.; Manthiram, A. Regulating Anode-Electrolyte Interphasial Reactions by Zwitterionic Binder Chemistry in Lithium-Ion Batteries with High-Nickel Layered Oxide Cathodes and Silicon-Graphite Anodes. Angew. Chem. Int. Ed. 2024, 63, e202408021. [Google Scholar] [CrossRef]
- Jin, B.; Wang, D.; Song, L.; Cai, Y.; Ali, A.; Hou, Y.; Chen, J.; Zhang, Q.; Zhan, X. Biomass-derived fluorinated corn starch emulsion as binder for silicon and silicon oxide based anodes in lithium-ion batteries. Electrochim. Acta 2021, 365, 137359. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, K. Atomistic Origins of High Capacity and High Structural Stability of Polymer-Derived SiOC Anode Materials. ACS Appl. Mater. Interfaces 2017, 9, 35001–35009. [Google Scholar] [CrossRef]



| Ionic Liquid | Cation | Anion | Lithium Salt (1 mol L−1) |
|---|---|---|---|
| [P2224][FSI] Triethyl-n-butylphosphonium bis(fluoromethylsulfonyl)imide | 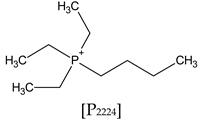 |  | LiFSI |
| [BMPYR][FSI] N-propyl-N-methylpyrrolidinium bis(fluoromethylsulfonyl)imide | 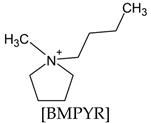 |  | LiFSI |
| Alkyl carbonate | |||
| EC/DEC 50:50 Ethylene carbonate/ Diethyl carbonate |  | LiPF6 | |
| Electrolyte | DLi+ NMR (10−7 cm2s−1) | η (mPa.s) | σ (mS.cm−1) |
|---|---|---|---|
| EC/DEC | 17.0 [44] | 4.7 [45] | 7.8 [45] |
| [BMPYR][FSI] | 0.18 ± 0.01 | 87 [7] | 3.3 [7] |
| [P2224][FSI] | 0.16 ± 0.01 | 97.3 | 2.5 |
| NC-SiOC/PAN | Active Material Mass (mg) | Thickness (µm) | 1st Discharge mAh/g | 1st Charge mAh/g | ICE (%) |
|---|---|---|---|---|---|
| EC/DEC | 1.58 | 38 | 1367 | 854 | 62.5 |
| [BMPYR][FSI] | 1.52 | 35 | 1427 | 926 | 64.9 |
| [P2224][FSI] | 1.4 | 33 | 1395 | 934 | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monje, I.E.; Sanchez-Ramírez, N.; Savignac, L.; Camargo, P.H.; Schougaard, S.B.; Bélanger, D.; Torresi, R.M. Exploring Binder–Ionic Liquid Electrolyte Systems in Silicon Oxycarbide Negative Electrodes for Lithium-Ion Batteries. Electrochem 2025, 6, 34. https://doi.org/10.3390/electrochem6030034
Monje IE, Sanchez-Ramírez N, Savignac L, Camargo PH, Schougaard SB, Bélanger D, Torresi RM. Exploring Binder–Ionic Liquid Electrolyte Systems in Silicon Oxycarbide Negative Electrodes for Lithium-Ion Batteries. Electrochem. 2025; 6(3):34. https://doi.org/10.3390/electrochem6030034
Chicago/Turabian StyleMonje, Ivonne E., Nedher Sanchez-Ramírez, Laurence Savignac, Pedro H. Camargo, Steen B. Schougaard, Daniel Bélanger, and Roberto M. Torresi. 2025. "Exploring Binder–Ionic Liquid Electrolyte Systems in Silicon Oxycarbide Negative Electrodes for Lithium-Ion Batteries" Electrochem 6, no. 3: 34. https://doi.org/10.3390/electrochem6030034
APA StyleMonje, I. E., Sanchez-Ramírez, N., Savignac, L., Camargo, P. H., Schougaard, S. B., Bélanger, D., & Torresi, R. M. (2025). Exploring Binder–Ionic Liquid Electrolyte Systems in Silicon Oxycarbide Negative Electrodes for Lithium-Ion Batteries. Electrochem, 6(3), 34. https://doi.org/10.3390/electrochem6030034








