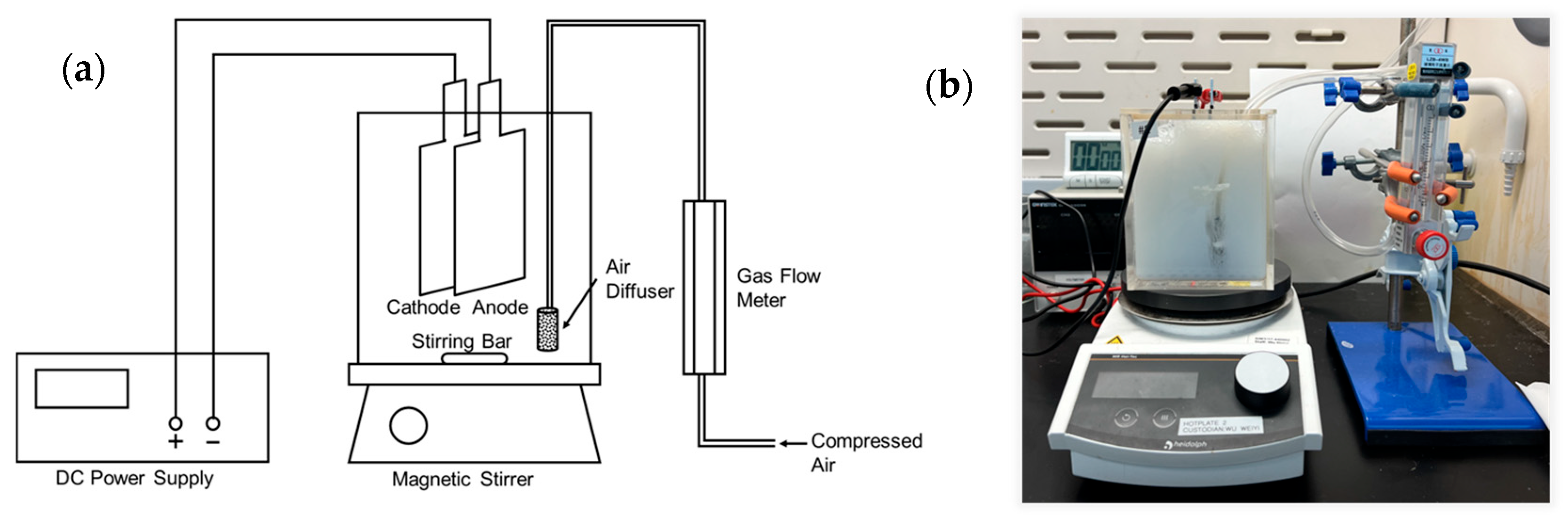
3.1. Effect of Electrode Materials
The anode plays a crucial role in electrocoagulation as the sacrificial electrode by releasing metal ions that neutralise the surface charge of the pollutants. This neutralisation allows the pollutants to agglomerate and form flocculants, also known as flocs. The process repeats until the agglomerated flocs become heavier, eventually settling as sludge at the bottom of the wastewater.
During electrocoagulation with SS-304 electrodes, ferrous iron (Fe
2+) was initially generated. However, Fe
2+ was less stable than Fe
3+ in the presence of dissolved oxygen and other potential oxidants, leading to its gradual conversion to Fe
3+-treated spent coolant wastewater. As shown in
Figure 3c, the top layer of the sludge appeared as orange–brown, indicating the presence of Fe
3+, while the dark greenish sludge at the bottom was due to the oxidants in the treated wastewater being consumed and forming stable ferrous iron (Fe
2+).
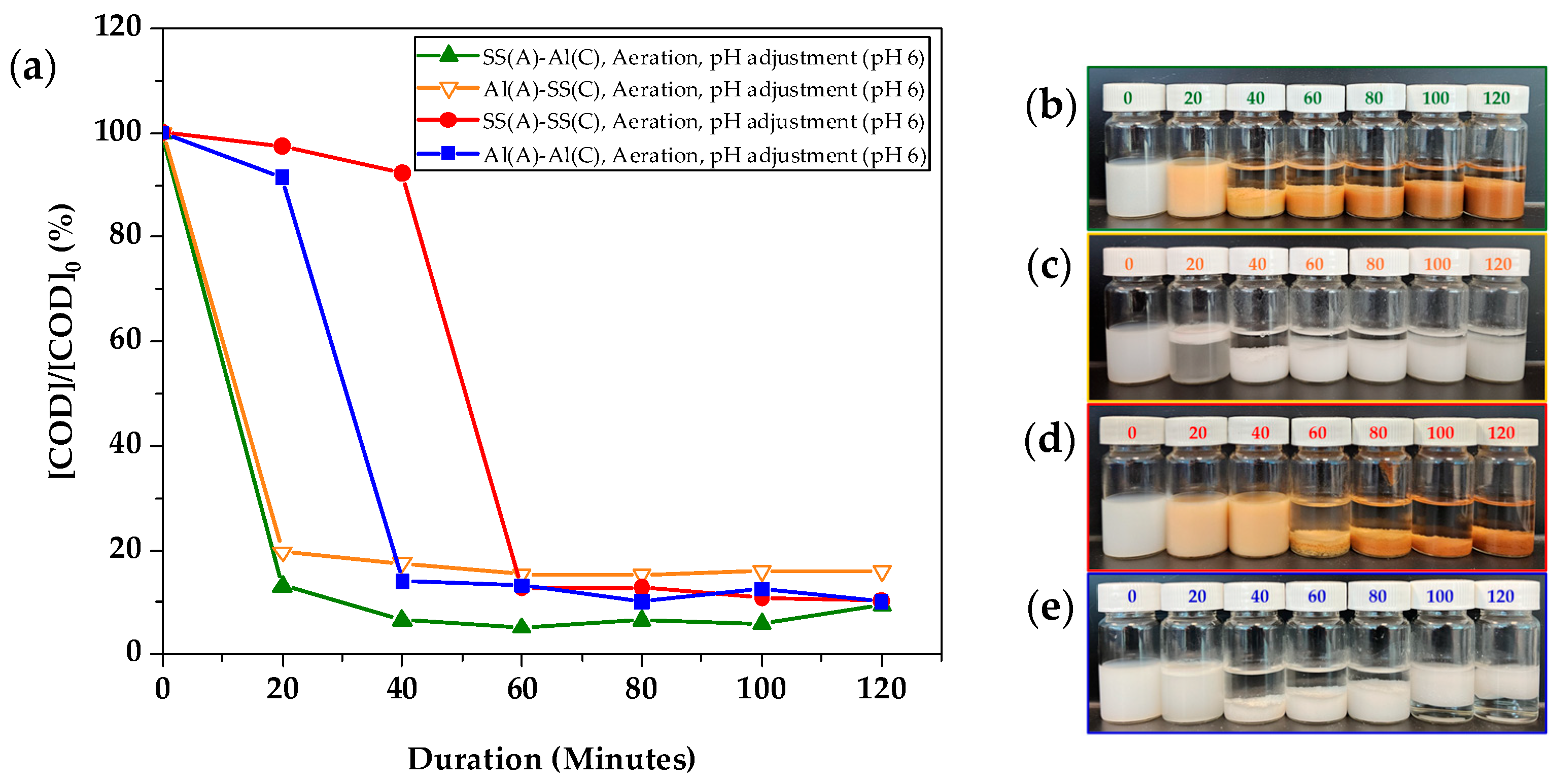
Among the three groups of anode–cathode combinations, the combination of SS(A)-SS(C), referring to SS-304 as both the anode and cathode, resulted in the slowest COD reduction rate compared to the combination of Al(A)-Al(C) where Al-6061 was used as both the anode and cathode, as shown in
Figure 3a. The observation of the COD value surpassing 100% may result from the dissolved Fe
2+ ions from the anode, together with the inherent margin of error in COD testing vials. SS(A)-Al(C) achieved a medium COD removal rate of 88.0% which was achieved after 60 min. Although Al(A)-Al(C) exhibited the fastest COD removal rate, it produced a significantly larger amount of sludge than the other two experimental setups. Notably, after 100 min, a portion of the sludge began to form flocs and floated to the top of the wastewater, creating a thin, clear separation between the flocs and the sludge. These flocs were likely formed due to the entrapment of hydrogen gas bubbles generated by the cathode during electrocoagulation. Consistent with the findings of Mahmad et al., who demonstrated that aluminium electrodes were more effective in removing turbidity (
Table S1) and colour than stainless steel, our study further confirmed that aluminium achieved a faster separation rate than stainless steel [
7].
The use of SS-304 as the anode in a dissimilar electrode material combination effectively mitigated the observed phenomenon. Sludge separation occurred much faster with this combination, reducing the time from 100 min to 60 min, compared to using SS(A)-SS(C). Moreover, the amount of sludge formed was significantly lower when SS(A) was paired with Al(C), compared to Al(A)-Al(C). When Al-6061 was used as the cathode, it also enhanced the COD reduction rate and accelerated the speed of achieving effective sludge and wastewater separation. The performance of Al(A)-SS(C) is close to that of Al(A)-Al(C), as showed in
Figure S1.
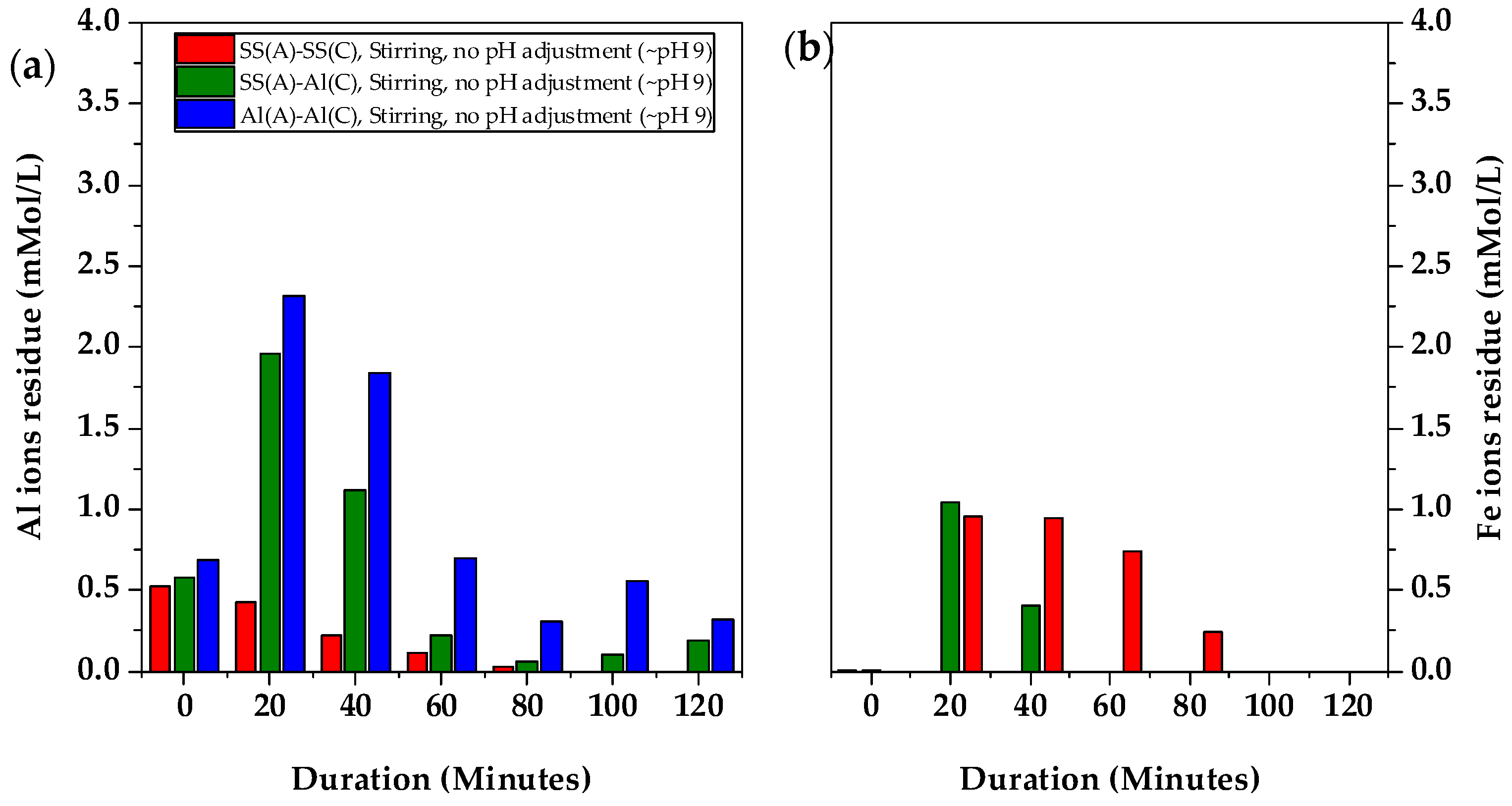
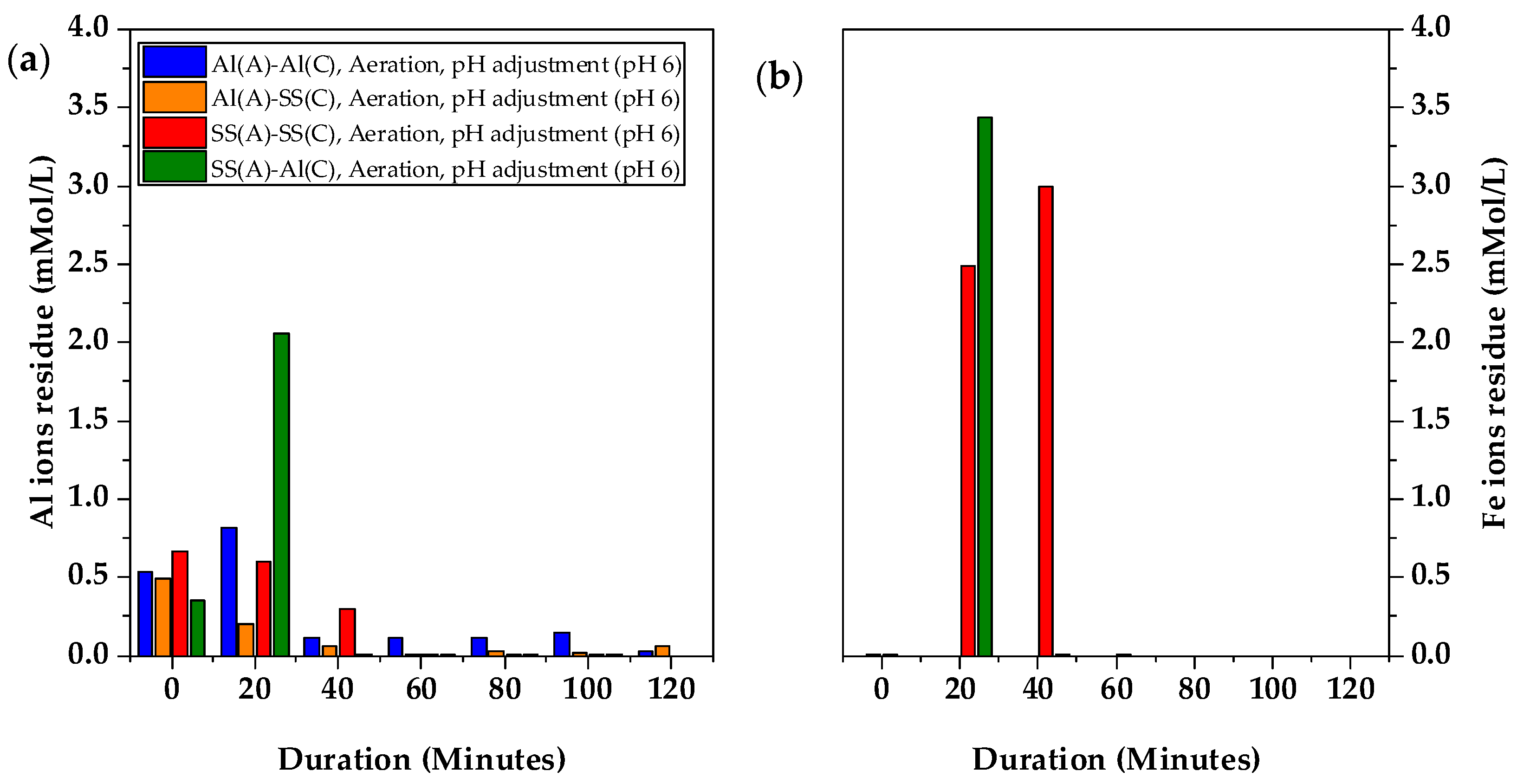
Figure 4a showed the concentration of the Al and Fe elements over the treatment. The Al concentrations were detected in the initial spent coolant wastewater prior to the electrocoagulation treatment. This provided valuable insight into the real application of the spent coolant, highlighting the presence of Al ions, which indicates the use of aluminium materials in precision engineering. The anode materials played a significant role in the electrocoagulation process. When SS-304 was used as the anode in the SS(A)-SS(C) arrangement, the releasing of Fe ions from the anode surface led to an increase in the Fe concentration in the wastewater, with a maximum concentration of 0.95 mMol/L observed at 20 min. After that, the Fe concentration went down and was totally removed when the full sludge separation was achieved after 100 min. In addition, the Al concentration decreased from an initial 0.52 mMol/L to 0 mMol/L at 100 min, demonstrating that the Al pollutants in the initial spent coolant wastewater were also removed effectively by electrocoagulation. In contrast, when Al-6061 was used as both the anode and cathode in the Al(A)-Al(C) arrangement, a sharp increase in Al ions was observed, peaking at 2.32 mMol/L at 20 min before gradually decreasing to 0.32 mMol/L by 120 min. For the dissimilar electrode material arrangement of SS(A)-Al(C), the Al concentration exhibited a similar trend to that observed with Al(A)-Al(C). At 20 min, the Al concentration spiked to 1.96 mMol/L, while 1.05 mMol/L of Fe elements were also observed in
Figure 4b. However, the Fe concentration quickly reduced to 0 mMol/L at 60 min when full sludge separation was achieved, without further Fe elements detected on wastewater after 60 min. This indicated that it was easier for Fe to form precipitation instead of dissolved Fe ions. However, the trace amount of the Al concentration persisted throughout the entire electrocoagulation process, which may be due to the broader species of Al ions which can be stable at a wider pH range. The change of Al ions residue in the solution by using Al(A)-SS(C) electrodes is similar to that of Al(A)-Al(C) with less residue from 80 min, as showed in
Figure S4.
Al electrodes produced Al ions more readily during electrocoagulation, releasing Al ions (Al3+), which then reacted with water to form aluminium hydroxide (Al(OH)3). These hydroxides formed larger, lighter flocs with less density due to the entrapment of air pockets. In contrast, SS electrodes, which contained Fe ions, were less reactive in electrocoagulation. They released Fe ions (Fe2+ or Fe3+), which reacted with water to form iron hydroxide, resulting in denser, heavier flocs due to the electrochemical differences between the materials. These electrochemical differences explained why Al ions remain detectable throughout the process, while Fe ions became undetectable by 60 min.
The changes in the electrode weight, wastewater pH, wastewater conductivity and applied voltage are summarised in
Table 2. Al-Marris et al. [
8] compared the experimentally measured dissolved Al concentrations with predictions based on Faraday’s law. When the specific electrical charge (Q) exceeded 0.5 Ah/L, the experimental values exceeded those predicted by Faraday’s law. This discrepancy in the Al concentration is likely attributed to the dissolution of the Al anode via chemical corrosion. In the present study, a constant specific electrical charge of 4.0 Ah/L was employed across all experiments, regardless of the electrode material combination. Notably, when Al-6061 was utilised as the anode, the current efficiencies consistently surpassed 100%. Ingelsson et al. [
9] stated that Al(A) has “super-faradaic” behaviour due to the chemical corrosion of both the anode and cathode. The weight loss of the SS(A) in the dissimilar electrode material configuration SS(A)-Al(C) was 2877.45 mg, significantly higher than that of SS(A) in the SS(A)-SS(C) configuration. It demonstrated that the current efficiency was greatly improved by a dissimilar configuration.
Unlike SS(C), weight losses of Al(C) were observed during electrocoagulation. Although the cathode was not the sacrificial electrode during electrocoagulation, it had been reported that aluminium could be dissolved by chemical dissolution due to its high pH area near the cathode surface during H
2 generation. The weight loss of the Al(C) in the SS(A)-Al(C) was 486.92 mg, which was also marginally higher than in the similar electrode material configuration. Although the Al(C) tended to generate lighter flocs, the SS(A) produced heavier iron hydroxides, which helped mitigate the flotation effect. As depicted in
Figure 3, after 60 min, SS-Al and Al-Al achieved COD removal of 88.0% and 76.0%, respectively. In contrast, SS(A)-SS(C) resulted in a much lower COD removal rate of 11.2%. These results demonstrated the significant influence of the Al-6061 cathode on the rate of COD reduction. The combination of SS(A) and Al(C) in the dissimilar electrode material configuration exhibited a synergistic effect through the electrogenerated Fe ions in the anode and chemically dissolved Al ions in the cathode, improving both COD removal and reducing flotation.
During the initial stage of electrocoagulation, the stable protective passive oxide layer on stainless steel electrodes prevented the excessive dissolution of SS(A). This oxide layer may further oxidise at a high current density. Increasing the oxide layer’s thickness could potentially reduce the release of Fe ions, thereby diminishing the efficiency of electrocoagulation [
9]. On the other hand, aluminium could oxidise more easily and formed Al
3+. As a result, aluminium anodes were more effective in generating coagulants, which destabilised pollutants and aided in the aggregation of suspended solids. This accounts for the quicker formation of clear separation in treated spent coolant with the use of Al(A).
3.2. Effect of Initial pH
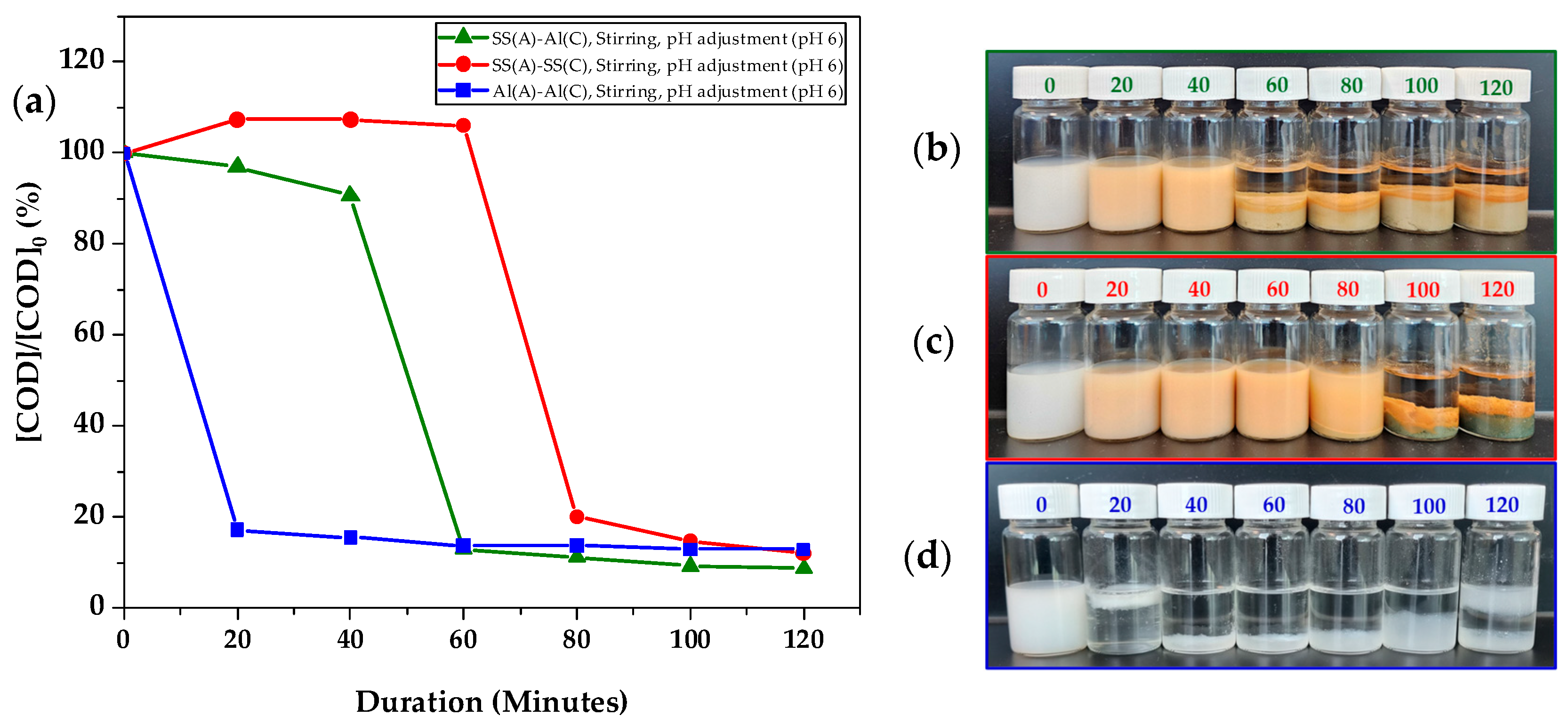
The initial pH of the wastewater played a crucial role in the ionisation of the pollutant and coagulant, thus affecting the pollutant removal efficiency. As machinery coolant usually required the surfactant to achieve a homogenous mixture of coolant compounds with water, a change in the pH may significantly affect the stability of the coolant. In our study, spent coolant wastewater with an initial pH of 9 was adjusted to pH 6 using 0.5 M HCl. As shown in
Figure 5, the spent coolant wastewater became significantly more turbid after pH adjustment, indicating the change in the surfactant and coolant interaction. We also observed that pH adjustment to a slightly acidic level accelerated the COD removal rate by 20 min, independent of the electrode materials, in comparison to the results presented in
Figure 3. Boinpally et al. [
10] reported that the pH of wastewater fluctuated throughout the electrocoagulation process, with different pollutants coagulating and settling at different pH ranges. In general, acidic pH levels tended to promote the formation of larger flocs in wastewater, as the production of amphoteric metal hydroxides becomes more dominant compared to basic pH levels.
Among the three electrode combinations, Al(A)-Al(C) demonstrated the fastest COD removal of 82.8% at 20 min, surpassing SS(A)-SS(C), as shown in
Figure 5a. Although Al-6061 yielded the quickest COD removal in a similar electrode material configuration, it resulted in a significant amount of sludge by 100 min. At 120 min, a mixture of sludge and flocs formed with a thin and clear separation between them. This indicated the entrapment of H
2 gas within the sludge, partially converting it into flocs. In comparison, the dissimilar electrode material configuration of SS(A)-Al(C) showed a moderate COD removal of 87.0% at 60 min and 91.3% at 120 min. Unlike the dark green and orange sludge formed with SS(A)-SS(C), SS(A)-Al(C) produced lighter beige and orange sludge, signalling a blend of aluminium and iron hydroxides. Additionally, the dissimilar electrode configuration facilitated faster separation, with clearer sludge separation observed from 60 min onwards, in contrast to the slower separation in the similar electrode material configuration of SS(A)-SS(C). Under this reaction condition, the COD removal rate of Al(A)-SS(C) electrodes is also same as that of Al(A)-Al(C), as in
Figure S2.
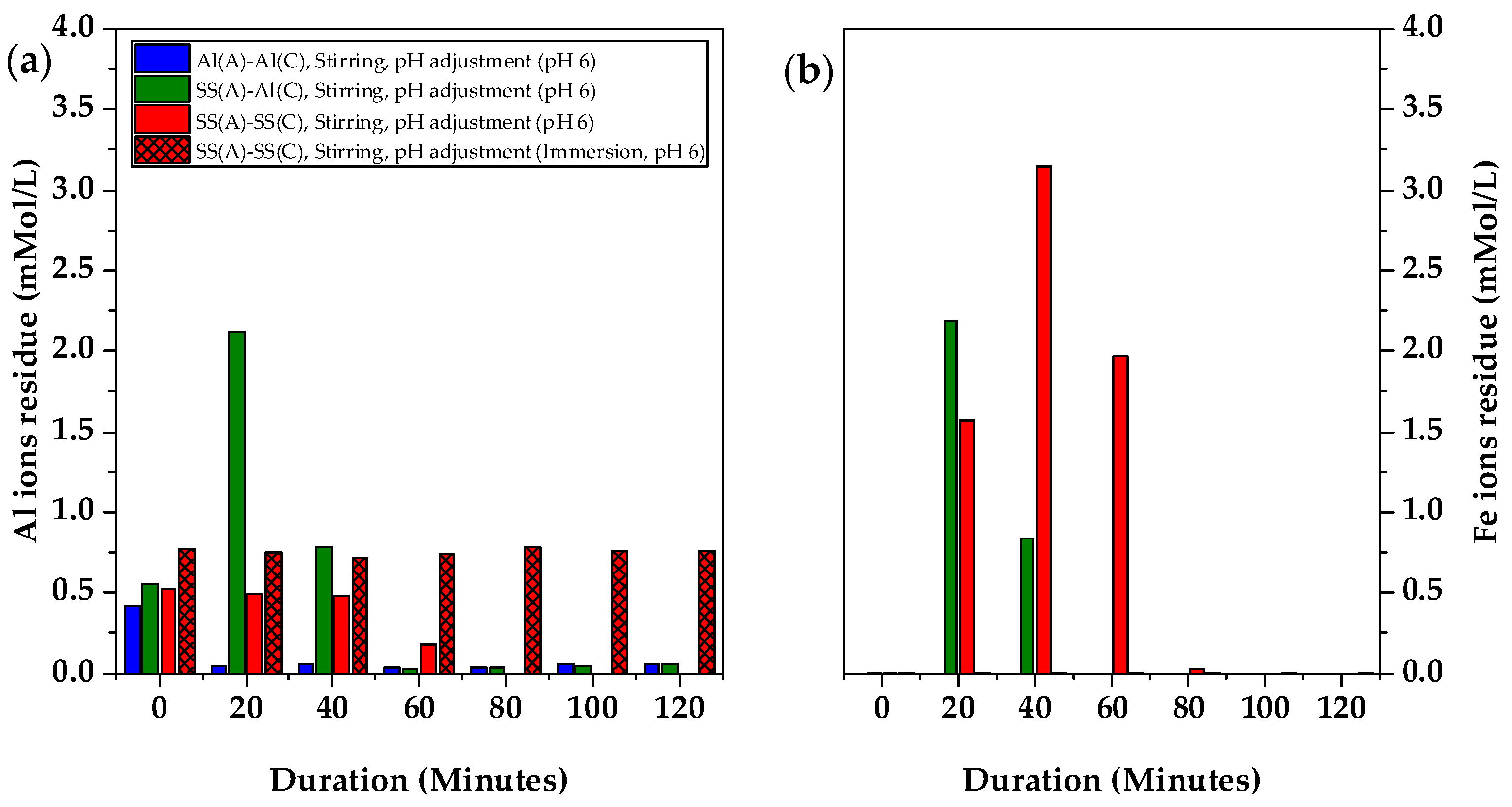
Figure 6a shows the evolution of the Al and Fe concentration during the 120 min electrocoagulation. In the Al(A)-Al(C) configuration, the Al concentration significantly decreased from 0.42 mMol/L to 0.05 mMol/L after 20 min, suggesting its separation from wastewater into the flotation layer above the clear liquid layer (
Figure 5d). As electrocoagulation continued, the low Al concentration remained relatively stable. However, in the SS(A)-SS(C) configuration, a gradual reduction in the Al concentration was observed, with complete removal only achieved at 80 min. At 20 min, the Fe concentration was detected at 1.6 mMol/L and increased to 3.2 mMol/L at 40 min. By 60 min, the Fe concentration decreased to 1.8 mMol/L, indicating the effectiveness of electrocoagulation. No Fe concentration was detected at 100 min when the good sludge precipitation was achieved, confirming no residual Fe in the liquid. With pH adjusted, the change of Al ions residue in the solution by using Al(A)-SS(C) electrodes is close to that of Al(A)-Al(C) electrodes, as showed in
Figure S5.
Similarly, in the SS(A)-Al(C) configuration, 2.1 mMol/L Al concentration and 2.2 mMol/L Fe concentration were detected in the early stage of electrocoagulation at 20 min, before both Al and Fe concentrations decreased to 0.8 mMol/L at 40 min, reflecting the efficiency of electrocoagulation. At 60 min, both Al and Fe were completely removed after sludge precipitation, suggesting that the electrocoagulation process was completed.
When SS-304 electrodes in the SS(A)-SS(C)(immersion) configuration were immersed in pH pre-adjusted spent coolant without any electrical current applied, no decrease in the Al ion concentration and no presence of an Fe concentration were observed. This indicated that the Fe ions did not readily release from the SS-304 electrodes without the application of an electrical current, confirming that electrocoagulation was not occurring in the absence of electricity.
The changes in the electrode weight, wastewater pH, wastewater conductivity and applied voltage are summarised in
Table 3. From
Table 3, the weight loss of the SS(A) in the dissimilar electrode material setup SS(A)-Al(C) was 2856.38 mg, which was 30.05% higher than that of SS(A) in the similar electrode material configuration SS(A)-SS(C). Likewise, the weight loss of the Al(C) in the dissimilar electrode material setup SS(A)-Al(C) was 416.36 mg, also greater than in the similar electrode material configuration Al(A)-Al(C). Although the Al(C) may produce lighter flocs, the SS(A) generated heavier iron hydroxide, which could mitigate the flotation effect and lead to better water–sludge separation. Based on the COD reduction and the images of the electrocoagulation-treated spent coolant, as shown in
Figure 5a–d, the Al(C) had a notable influence on the speed of COD reduction. In the dissimilar electrode material combination, SS(A) and Al(C) worked synergistically, enhancing both the rate of COD reduction and the reduction of flotation.
3.3. Effect of Agitation Method
Agitation improved the liquid flow around the electrodes in the reactor, optimising the interaction between the coagulant and pollutants, which enhanced the pollutant removal efficiency. It also prevented a localised high pH and temperature near the electrodes, promoting a uniform distribution of these changes throughout the wastewater. This helped to avoid electrode passivation, which could otherwise hinder the coagulation efficiency. Additionally, aeration outperformed stirring in accelerating coagulation, promoting the faster formation of larger flocs.
In comparison to stirring-agitated electrocoagulation, as shown in
Figure 5a, aeration-induced electrocoagulation, as shown in
Figure 7a, exhibited faster separation when SS-304 was used as the anode, regardless of the cathode material. Trinh et al. [
11] noted that aeration increased the dissolved oxygen, which converted the generated Fe
2+ into Fe
3+ and facilitated the formation of Fe(OH)
3, an effective coagulant in electrocoagulation. However, in our study, the increase in dissolved oxygen due to aeration did not significantly enhance electrocoagulation with aluminium electrodes. Aluminium flocs were generally less dense and aeration may disrupt their formation, reducing the pollutant removal efficiency [
11]. As a result, using Al-6061 as both the anode and cathode in the Al(A)-Al(C) configuration did not lead to an improvement in the COD removal efficiency.
Chezeau et al. [
12] suggested that the chemical dissolution of the aluminium cathode can occur alongside the electrochemical dissolution of iron anodes, with both metallic electrodes acting as coagulants simultaneously. In our study, the spent coolant was adjusted to pH 6 prior to the experiment. During electrocoagulation, the pH of the spent coolant gradually increased due to the release of metallic ions. As the pH rose, the Al species, which were more reactive at a lower pH, were released from the electrode, while the Fe species became more effective at a higher pH. This explained why the combination of an Fe anode and an Al cathode enhanced the separation efficiency during electrocoagulation.
The combination of SS-304 as the anode and Al-6061 as the cathode in the SS(A)-Al(C) configuration achieved the highest COD removal rate, as shown in
Figure 7a, with reductions of 86.9% at 20 min and 93.4% at 40 min, indicating a synergistic effect from pH adjustment and aeration. However, despite having an 86.9% COD removal rate at 20 min, clear separation was not observed, as shown in
Figure 7b. Instead, clear separation occurred at 40 min, and by 60 min, the COD removal rate was 94.9%, much higher than in the other three sets. Although SS(A)-Al(C) resulted in the highest COD removal rate, the amount of generated sludge was significantly higher compared to SS(A)-SS(C). This suggested that Al(C) promoted greater sludge formation, likely because the aluminium electrodes released Al ions more readily than iron ions, resulting in thicker, less dense sludge. By replacing Al cathode to SS, the removing of COD is less effective at 20 min and becomes similar after that (
Figure S3.)
The Al concentration was initially detected in the spent coolant at the initial stage of electrocoagulation (
Figure 8a), indicating the recovery of coolant from the aluminium processing. When Al-6061 was used as both the anode and cathode in the Al(A)-Al(C) configuration, there was a significant decrease in the Al concentration from 0.82 mMol/L to 0.12 mMol/L between 20 and 40 min, suggesting the occurrence of liquid–sludge/flocs separation.
Figure 7e showed the presence of a flotation layer with a relatively clear liquid beneath. Despite this, traces of the Al concentration remained detectable at 120 min. Under aeration, the residue from Al(A)-SS(C) becomes more concentrated from 100 min, as showed in
Figure S6.
SS(A)-SS(C) showed a gradual decrease in its Al concentration, with almost complete removal (<0.1 mMol/L) at 60 min. An Fe concentration of 2.5 mMol/L was detected at 20 min, increasing to 3.0 mMol/L at 40 min, before being fully removed by 60 min, indicating that electrocoagulation was taking place.
In the combination of Al(A) and SS(C) in the Al(A)-SS(C) configuration, the Al concentration gradually decreased to trace levels between 60 and 120 min. No Fe concentration was detected throughout the experiment, indicating that SS(C) did not release Fe ions.
SS(A)-Al(C) configuration had an Al concentration of 2.1 mMol/L and Fe concentration of 3.4 mMol/L detected at 20 min, marking the initiation of the electrocoagulation process. At 40 min, trace residues of both Al and Fe concentrations of 0.8 mMol/L each were detected, indicating the strong efficiency of electrocoagulation. At 60 min, both the Al and Fe ion concentrations dropped to zero, signalling the completion of electrocoagulation.
In short, SS(A)-Al(C) with aeration-agitated electrocoagulation demonstrated the most efficient removal of metal elements, with all pollutants in the spent coolant being electro-coagulated in 40 min.
From
Table 4, the weight loss of SS(A) in the dissimilar electrode material configuration SS(A)-Al(C) was 3256.63 mg, higher than the weight loss of SS(A) in the similar electrode configuration SS(A)-SS(C). Meanwhile, it was found that when SS was used as the anode, regardless of a similar or dissimilar electrode configuration, higher Fe dissolution is accompanied with faster COD removal. The Al(C) in this SS(A)-Al(C) setup experienced a weight loss of 375.71 mg, slightly lower than that observed in Al(C) in Al(A)-Al(C). In the dissimilar configuration of Al(A)-SS(C), Al(A) had a weight loss of 1568.13 mg, marginally lower than the loss in Al(A) in the similar arrangement Al(A)-Al(C). The SS(C) in this Al(A)-SS(C) setup showed no weight loss, consistent with the SS(C) in the similar arrangement of SS(A)-SS(C). Overall, the combined weight loss of the Al(A) and SS(C) in the dissimilar electrode configuration was notably lower than in the similar electrode configurations of Al(A)-Al(C) and SS(A)-SS(C). Despite the lower release of metal elements, the flocs in this arrangement, as shown in
Figure 7c, were thicker compared to the others, likely due to the lighter flocs produced by the Al(A). This may explain why the dissimilar electrode material configuration SS(A)-Al(C) exhibited the fastest separation, as indicated by the highest concentration of combined metal ions detected.
3.4. Electrochemical Analysis
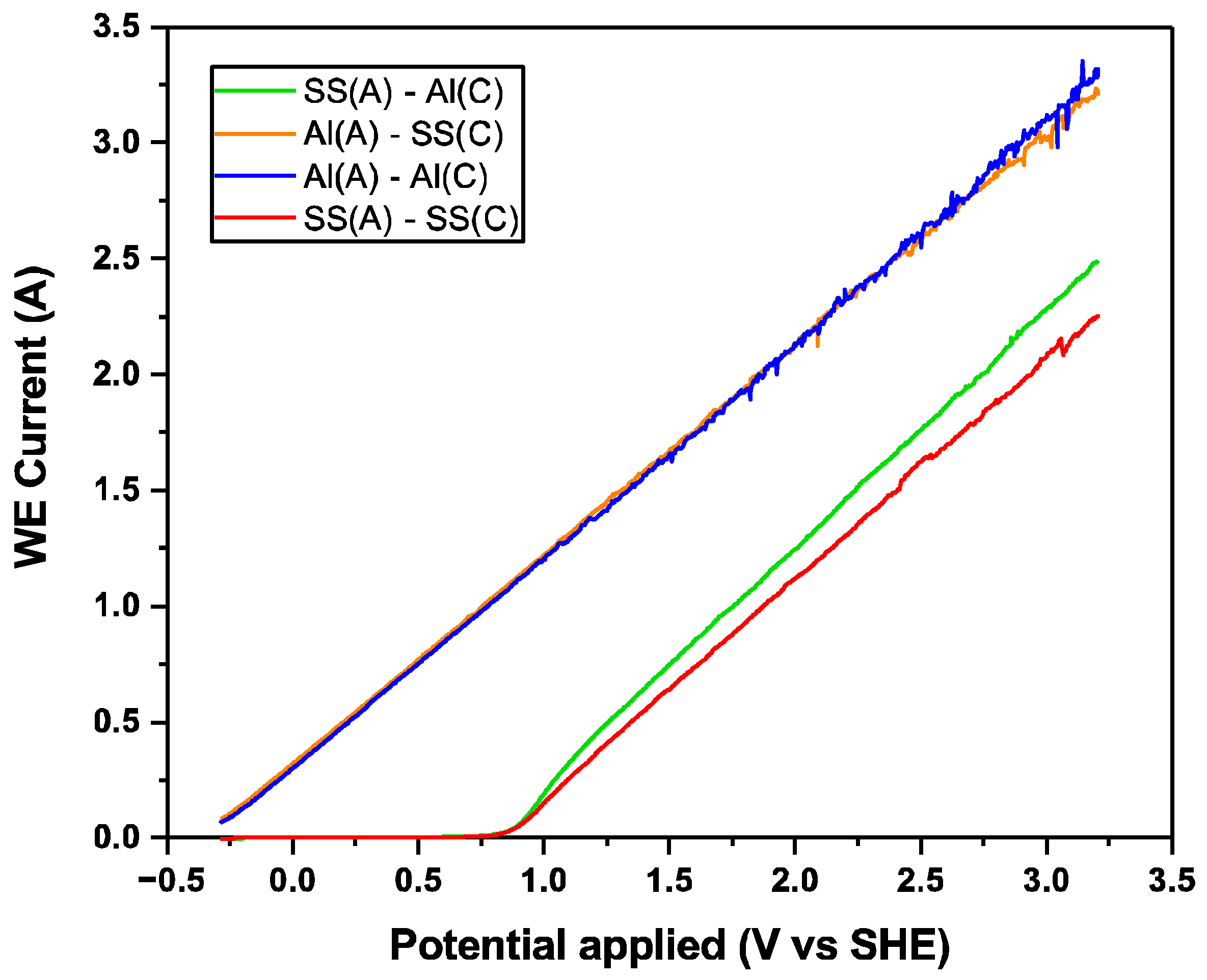
Linear Sweep Voltammetry (LSV) was employed to further examine the electrochemical behaviour of the electrodes during electro-coagulation, as shown in
Figure 9. Regardless of the cathode material, SS(A) exhibited an onset potential of 0.85 to 0.90 V (vs. SHE), beyond which the current began to rise significantly in response to the applied potential. The onset potential indicated the potential at which the energy barrier was overcome, allowing the oxidation of Fe to Fe
2+ to occur. Compared to the SS(A)-SS(C) configuration, the dissimilar electrode pairing of SS(A)-Al(C) exhibited a steeper linear sweep voltammetry (LSV) response, indicating a faster electrochemical reaction of SS(A) under the applied potential. This behaviour correlates with the improved current efficiencies observed for SS(A)-Al(C) over SS(A)-SS(C), as shown in
Table 2,
Table 3 and
Table 4, with increases from 49.27% to 68.86%, 52.56% to 68.35% and 66.27% to 77.93%, respectively. Furthermore, the shift in onset potential contributes to even greater improvements in the current efficiency, reaching 71.55%, 76.90% and 85.04% in
Table 2,
Table 3 and
Table 4, respectively. The higher applied voltage required for SS(A) compared to Al(A) may also play a role. However, oxygen evolution due to water splitting can negatively impact the current efficiency.
However, when using Al-6061 as the anode, the two LSV plots of Al(A)-Al(C) and Al(A)-SS(C) configurations were quite similar and exhibited a linear response to the applied potential. The current was around 0.3 A at 0 V (vs. SHE) and the corresponding onset potential for both Al(A)-Al(C) and Al(A)-SS(C) was −0.35 V (vs. SHE) by extending the LSV plots. As a result, less applied voltage was required when using the Al anode compared to the SS anode, demonstrating an advantage in energy consumption. In addition, the similar electrochemical behaviour of Al(A)-Al(C) and Al(A)-SS(C) in the LSV experiment also aligned with the similar dissolution weight of Al anodes (
Table 4), indicating that the contribution of the electrochemical dissolution of Al anodes was not affected by the cathode material.
The LSV graph provided valuable insights into the electrocoagulation process. Initially, during the anodic sweep, stainless steel formed a protective passive oxide layer, which was evident in the LSV graph as minimal electrochemical activity. This behaviour mirrored the electrocoagulation process, where the oxide layer remained stable at low potentials, preventing the excessive dissolution of the SS-304 anode. This explained why SS-304 anodes exhibited slower separation compared to Al-6061 anodes.
As the applied potential increased, the oxide layer thickened and the current rose, reflecting the anodic dissolution of Fe ions (Fe2+ or Fe3+) into the wastewater. These Fe ions act as coagulants, facilitating the aggregation of suspended solids. At higher potentials, the passive oxide layer on SS-304 began to break down, as seen in the LSV graph with a sharp increase in the current, known as the onset potential. In electrocoagulation, exceeding the breakdown potential could lead to the excessive dissolution of the electrodes, resulting in a shorter electrode lifespan.