Alternative Supports for Electrocatalysis of the Oxygen Evolution Reaction in Alkaline Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterisation
2.3. Electrochemical Measurements
2.4. The Methodology for Measuring the OER Activity and Selectivity
3. Results and Discussion
3.1. Morphology and Structure of Synthesized Materials
3.2. Electrochemical Characterisation
3.2.1. Anodic Behavior of Vulcan XC72, BDD, WC, and Fe3O4
3.2.2. Electrochemical Properties of Composite Materials
3.2.3. Electrochemical Activity of Composite Materials
3.2.4. Stability Tests of ISAC Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDD | Boron-doped diamond |
| Vulcan | Vulcan XC72 carbon |
| OER | oxygen evolution reaction |
| COR | carbon oxidation reaction |
| ORR | oxygen evolution reaction |
| HER | hydrogen evolution reaction |
| RRDE | rotating ring disc electrode |
| RDE | rotation disc electrode |
| XRD | X-ray diffraction |
| TGA | thermogravimetric |
| TEM | transmission electron microscopy |
| EELS | electron energy loss spectroscopy |
| XPS | X-ray photoelectron spectroscopy |
| BET | Brunauer–Emmett–Teller approximation |
| BJH | Barrett–Joyner–Halenda |
| AC | autocombustion |
| ISAC | in situ autocombustion |
| calc | calcined |
| TOC | total organic carbon |
| UHV | ultrahigh vacuum |
| SBET | specific surface area |
| GC | glassy carbon |
| RHE | reversible hydrogen electrode |
| F.E. | faradaic efficiency |
| ICDD | International Centre for Diffraction Data |
| COD | crystal open database |
| ECSA | electrochemically active surface area |
| CV(s) | cyclic voltammogram(s) |
References
- Kumar, R.S.; Austeria, P.M.; Neethinathan, C.S.S.; Ramakrishnan, S.; Sekar, K.; Kim, A.R.; Kim, D.H.; Yoo, P.J.; Yoo, D.J. Highly mixed high-energy d-orbital states enhance oxygen evolution reactions in spinel catalysts. Appl. Surf. Sci. 2023, 641, 158469. [Google Scholar] [CrossRef]
- Sun, T.; Liu, P.; Zhang, Y.; Chen, Z.; Zhang, C.; Guo, X.; Ma, C.; Gao, Y.; Zhang, S. Boosting the electrochemical water splitting on Co3O4 through surface decoration of epitaxial S-doped CoO layers. Chem. Eng. J. 2020, 390, 124591. [Google Scholar] [CrossRef]
- Chou, S.-C.; Tso, K.-C.; Hsieh, Y.-C.; Sun, B.-Y.; Lee, J.-F.; Wu, P.-W. Facile synthesis of Co3O4@CoO@Co gradient Core@Shell nanoparticles and their applications for oxygen evolution and reduction in alkaline electrolytes. Materials 2020, 13, 2703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.; Dai, K.; Zhao, L.; Wei, Q.; Zhang, B.; Xiang, X. Ultrafine Co3O4 nanolayer-shelled CoWP nanowire array: A bifunctional electrocatalyst for overall water splitting. RSC Adv. 2020, 10, 29326. [Google Scholar] [CrossRef] [PubMed]
- Filimonenkov, I.S.; Bouillet, C.; Kéranguéven, G.; Simonov, P.A.; Tsirlina, G.A.; Savinova, E.R. Carbon materials as additives to the OER catalysts: RRDE study of carbon corrosion at high anodic potentials. Electrochim. Acta 2019, 321, 134657. [Google Scholar] [CrossRef]
- Kéranguéven, G.; Royer, S.; Savinova, E. Synthesis of efficient Vulcan–LaMnO3 perovskite nanocomposite for the oxygen reduction reaction. Electrochem. Commun. 2015, 50, 28–31. [Google Scholar] [CrossRef]
- Kéranguéven, G.; Bouillet, C.; Papaefthymiou, V.; Simonov, P.A.; Savinova, E.R. How key characteristics of carbon materials influence the ORR activity of LaMnO3- and Mn3O4-carbon composites prepared by in situ autocombustion method. Electrochim. Acta 2020, 353, 136557. [Google Scholar] [CrossRef]
- Panich, A.M.; I Shames, A.; Goren, S.; Froumin, N.; Shenderova, O. Magnetic resonance study of lightly boron-doped diamond. Mater. Res. Express 2019, 6, 75612. [Google Scholar] [CrossRef]
- Einaga, Y. Development of electrochemical applications of boron-doped diamond electrodes. Bull. Chem. Soc. Jpn. 2018, 91, 1752–1762. [Google Scholar] [CrossRef]
- McLaughlin, M.H.S.; Corcoran, E.; Pakpour-Tabrizi, A.C.; de Faria, D.C.; Jackman, R.B. Influence of temperature on the electrochemical window of boron doped diamond: A comparison of commercially available electrodes. Nat. Sci. Rep. 2020, 10, 15707. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-Like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ignaszak, A.; Song, C.; Baker, R.; Hui, R.; Zhang, J.; Nan, F. Nanocrystalline tungsten carbide (WC) synthesis/characterization and its possible application as a PEM fuel cell catalyst support. Electrochim. Acta 2012, 61, 198–206. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, X.; Yang, Z. First-principles study on the Cu-Au alloy monolayer supported on WC for hydrogen evolution. Appl. Surf. Sci. 2021, 565, 150568. [Google Scholar] [CrossRef]
- Koverga, A.A.; Flórez, E.; Rodriguez, J.A. Pushing Cu uphill of the volcano curve: Impact of a WC support on the catalytic activity of copper toward the hydrogen evolution reaction. Int. J. Hydrogen Energy 2021, 49, 25092–25102. [Google Scholar] [CrossRef]
- Liu, Y.; You, H.; Kimmel, Y.C.; Esposito, D.V.; Chen, J.G.; Moffat, T.P. Self-terminating electrodeposition of Pt on WC electrocatalysts. Appl. Surf. Sci. 2020, 504, 144472. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Dong, L.; Zhao, P.; Zhang, Y.; Wang, W.; Jia, S.; Zang, J. A novel hybrid of Ni and WC on new-diamond supported Pt electrocatalyst for methanol oxidation and oxygen reduction reactions. ChemCatChem 2017, 9, 3982–3988. [Google Scholar] [CrossRef]
- Weidman, M.C.; Esposito, D.V.; Hsu, I.J.; Chen, J.G. Electrochemical Stability of Tungsten and Tungsten Monocarbide (WC) Over Wide pH and Potential Ranges. J. Electrochem. Soc. 2010, 157, F179–F188. [Google Scholar] [CrossRef]
- Göhl, D.; Mingers, A.M.; Geiger, S.; Schalenbach, M.; Cherevko, S.; Knossalla, J.; Jalalpoor, D.; Schüth, F.; Mayrhofer, K.J.; Ledendecker, M. Electrochemical stability of hexagonal tungsten carbide in the potential window of fuel cells and water electrolyzers investigated in a half-cell configuration. Electrochim. Acta 2018, 270, 70–76. [Google Scholar] [CrossRef]
- Itai, R.; Shibuya, M.; Matsumura, T.; Ishi, G. Electrical resistivity of Magnetite anodes. J. Electrochem. Soc. 1971, 118, 1709. [Google Scholar] [CrossRef]
- Royer, L.; Makarchuk, I.; Hettler, S.; Arenal, R.; Asset, T.; Rotonnelli, B.; Bonnefont, A.; Savinova, E.; Pichon, B.P. Core–shell Fe3O4@CoFe2O4 nanoparticles as high-performance anode catalysts for enhanced oxygen evolution reaction. Sustain. Energy Fuels 2023, 7, 3239–3243. [Google Scholar] [CrossRef]
- Royer, L.; Bonnefont, A.; Asset, T.; Rotonnelli, B.; Velasco-Vélez, J.-J.; Holdcroft, S.; Hettler, S.; Arenal, R.; Pichon, B.; Savinova, E. Cooperative Redox Transitions Drive Electrocatalysis of the Oxygen Evolution Reaction on Cobalt–Iron Core–Shell Nanoparticles. ACS Catal. 2023, 13, 280–286. [Google Scholar] [CrossRef]
- Kéranguéven, G.; Filimonenkov, I.S.; Savinova, E.R. Investigation of the stability of the boron-doped diamond support for Co3O4-based oxygen evolution reaction catalysts synthesized through in situ autocombustion method. J. Electroanal. Chem. 2022, 916, 116367. [Google Scholar] [CrossRef]
- Filimonenkov, I.S.; Istomin, S.Y.; Antipov, E.V.; Tsirlina, G.A.; Savinova, E.R. Rotating ring-disk electrode as a quantitative tool for the investigation of the oxygen evolution reaction. Electrochim. Acta 2018, 286, 304–312. [Google Scholar] [CrossRef]
- Costa, G.C.; Shenderova, O.; Mochalin, V.; Gogotsi, Y.; Navrotsky, A. Thermochemistry of nanodiamond terminated by oxygen containing functional groups. Carbon 2014, 80, 544–550. [Google Scholar] [CrossRef]
- Shenderova, O.A.; McGuire, G.E. Science and engineering of nanodiamond particle surfaces for biological applications. Biointerphases 2015, 10, 030802. [Google Scholar] [CrossRef]
- Kondo, T.; Kato, T.; Miyashita, K.; Aikawa, T.; Tojo, T.; Yuasa, M. Boron-Doped Diamond Powders for Aqueous Supercapacitors with High Energy and High Power Density. J. Electrochem. Soc. 2019, 166, A1425–A1431. [Google Scholar] [CrossRef]
- Poux, T.; Napolskiy, F.; Dintzer, T.; Kéranguéven, G.; Istomin, S.Y.; Tsirlina, G.; Antipov, E.; Savinova, E. Dual role of carbon in the catalytic layers of perovskite/carbon composites for the electrocatalytic oxygen reduction reaction. Catal. Today 2012, 189, 83–92. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods—Fundamentals and Applications, 2nd ed.; Wiley: Phoenix, TX, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Schmidt, T.J.; Gasteiger, H.A.; Stäb, G.D.; Urban, P.M.; Kolb, D.M.; Behm, R.J. Characterization of High-Surface-Area Electrocatalysts Using a Rotating Disk Electrode Configuration. J. Electrochem. Soc. 1998, 145, 2354–2358. [Google Scholar] [CrossRef]
- Suntivich, J.; Gasteiger, H.A.; Yabuuchi, N.; Shao-Horn, Y. Electrocatalytic Measurement Methodology of Oxide Catalysts Using a Thin-Film Rotating Disk Electrode. J. Electrochem. Soc. 2010, 157, B1263. [Google Scholar] [CrossRef]
- Savinova, E.R.; Oshchepkov, A.G. Benchmarking in electrocatalysis. In Comprehensive Inorganic Chemistry III, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 492–550. [Google Scholar]
- Hatel, R.; Baitoul, M. Nanostructured Tungsten Trioxide (WO3): Synthesis, structural and morphological investigations. J. Phys. Conf. Ser. 2019, 1292, 012014. [Google Scholar] [CrossRef]
- Fleischer, K.; Mauit, O.; Shvets, I.V. Stability and capping of magnetite ultra-thin films. Appl. Phys. Lett. 2014, 104, 192401. [Google Scholar] [CrossRef]
- Grumelli, D.E.; Wiegmann, T.; Barja, S.; Reikowski, F.; Maroun, F.; Allongue, P.; Balajka, J.; Parkinson, G.S.; Diebold, U.; Kern, K.; et al. Electrochemical Stability of the Reconstructed Fe3O4(001) Surface. Angew. Chem. Int. Ed. 2020, 59, 21904–21908. [Google Scholar] [CrossRef] [PubMed]
- Baaziz, W.; Pichon, B.P.; Fleutot, S.; Liu, Y.; Lefevre, C.; Greneche, J.-M.; Toumi, M.; Mhiri, T.; Begin-Colin, S. Magnetic Iron Oxide Nanoparticles: Reproducible Tuning of the Size and Nanosized-Dependent Composition, Defects, and Spin Canting. J. Phys. Chem. C 2014, 118, 3795–3810. [Google Scholar] [CrossRef]
- Weidman, M.C.; Esposito, D.V.; Hsu, Y.-C.; Chen, J.G. Comparison of electrochemical stability of transition metal carbides (WC, W2C, Mo2C) over a wide pH range. J. Power Sources 2012, 202, 11–17. [Google Scholar] [CrossRef]
- Acedera, R.A.E.; Gupta, G.; Mamlouk, M.; Balela, M.D.L. Solution combustion synthesis of porous Co3O4 nanoparticles as oxygen evolution reaction (OER) electrocatalysts in alkaline medium. J. Alloys Compd. 2020, 836, 154919. [Google Scholar] [CrossRef]
- Reikowski, F.; Maroun, F.; Pacheco, I.; Wiegmann, T.; Allongue, P.; Stettner, J.; Magnussen, O.M. Operando Surface X-Ray Diffraction Studies of Structurally Defined Co3O4 and CoOOH Thin Films During Oxygen Evolution. ACS Catal. 2019, 9, 3811–3821. [Google Scholar] [CrossRef]
- Bergmann, A.; Martinez-Moreno, E.; Teschner, D.; Chernev, P.; Gliech, M.; de Araújo, J.F.; Reier, T.; Dau, H.; Strasser, P. Unified structural motifs of the catalytically active state of Co(oxyhydr)oxides during the electrochemical oxygen evolution reaction. Nat. Catal. 2018, 1, 711–719. [Google Scholar] [CrossRef]
- Koza, J.A.; He, Z.; Miller, A.S.; Switzer, J.A. Electrodeposition of Crystalline Co3O4—A Catalyst for the Oxygen Evolution Reaction. Chem. Mater. 2012, 24, 3567–3573. [Google Scholar] [CrossRef]
- Faisal, F.; Bertram, M.; Stumm, C.; Cherevko, S.; Geiger, S.; Kasian, O.; Lykhach, Y.; Lytken, O.; Mayrhofer, K.J.J.; Brummel, O.; et al. Atomically Defined Co3O4(111) Thin Films Prepared in Ultrahigh Vacuum: Stability Under Electrochemical Conditions. J. Phys. Chem. C 2018, 122, 7236–7248. [Google Scholar] [CrossRef]
- Alexander, C.T.; Abakumov, A.M.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Role of the Carbon Support on the Oxygen Reduction and Evolution Activities in LaNiO3 Composite Electrodes in Alkaline Solution. ACS Appl. Energy Mater. 2018, 1, 1549–1558. [Google Scholar] [CrossRef]
- Yang, J.; Park, S.; Choi, K.Y.; Park, H.-S.; Cho, Y.-G.; Ko, H.; Song, H.-K. Activity-Durability Coincidence of Oxygen Evolution Reaction in the Presence of Carbon Corrosion: Case Study of MnCo2O4 Spinel with Carbon Black. ACS Sustain. Chem. Eng. 2018, 6, 9566–9571. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, Y.; Peng, H.-Q.; Ji, B.; Yang, Y.; Tang, Y.; Lee, C.-S.; Zhang, W. Nanostructured and Boron-Doped Diamond as an Electrocatalyst for Nitrogen Fixation. ACS Energy Lett. 2020, 5, 2590–2596. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324. [Google Scholar] [CrossRef]
- Yang, J.; Liu, H.; Martens, W.N.; Frost, R.L. Synthesis and Characterization of Cobalt Hydroxide, Cobalt Oxyhydroxide and Cobalt Oxide Nanodiscs. J. Phys. Chem. C 2010, 114, 111–119. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yin, J.S.; Jiang, Y.D. EELS analysis of cation valence states and oxygen vacancies in magnetic oxides. Micron 2000, 31, 571–580. [Google Scholar] [CrossRef]




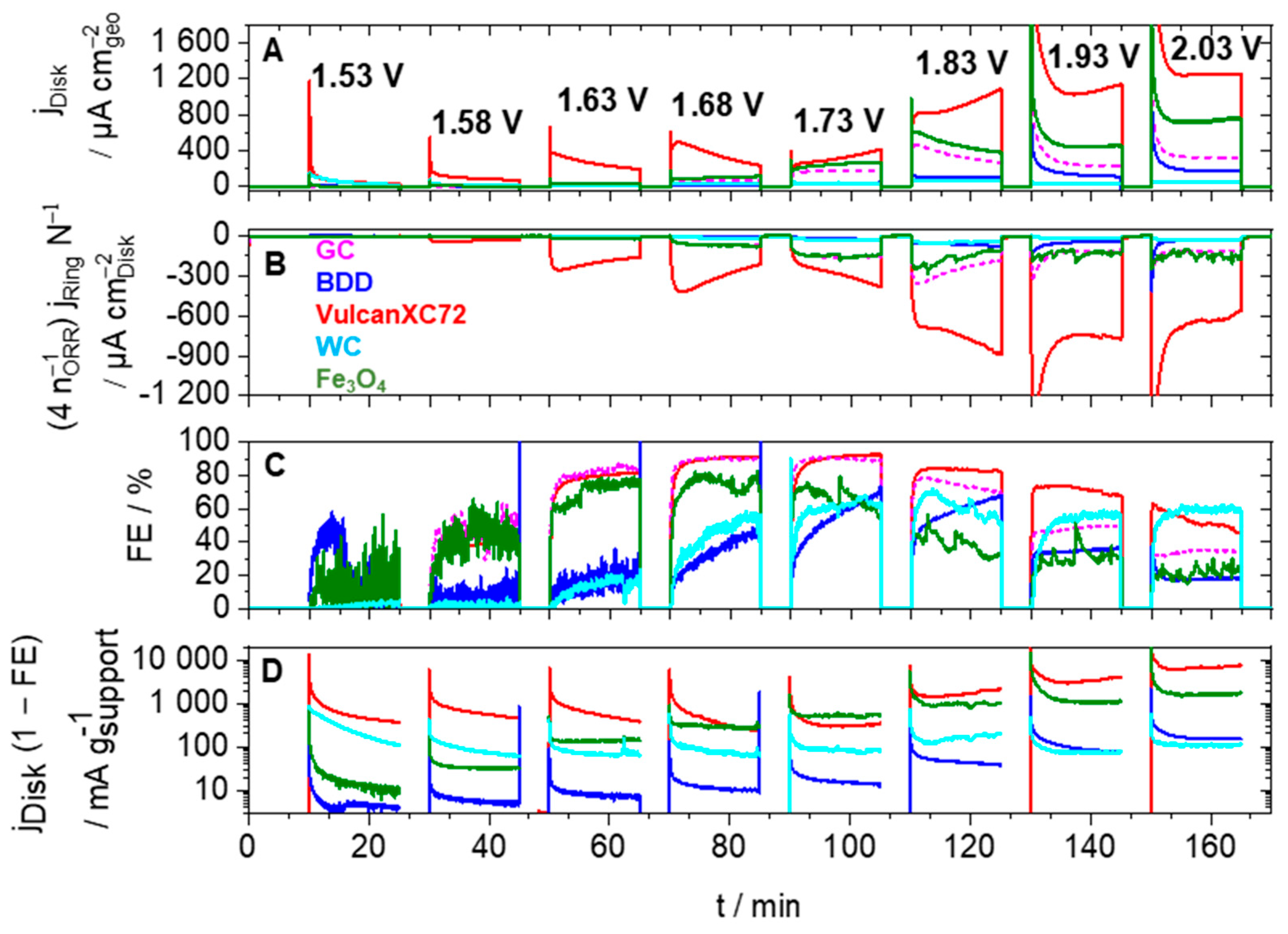


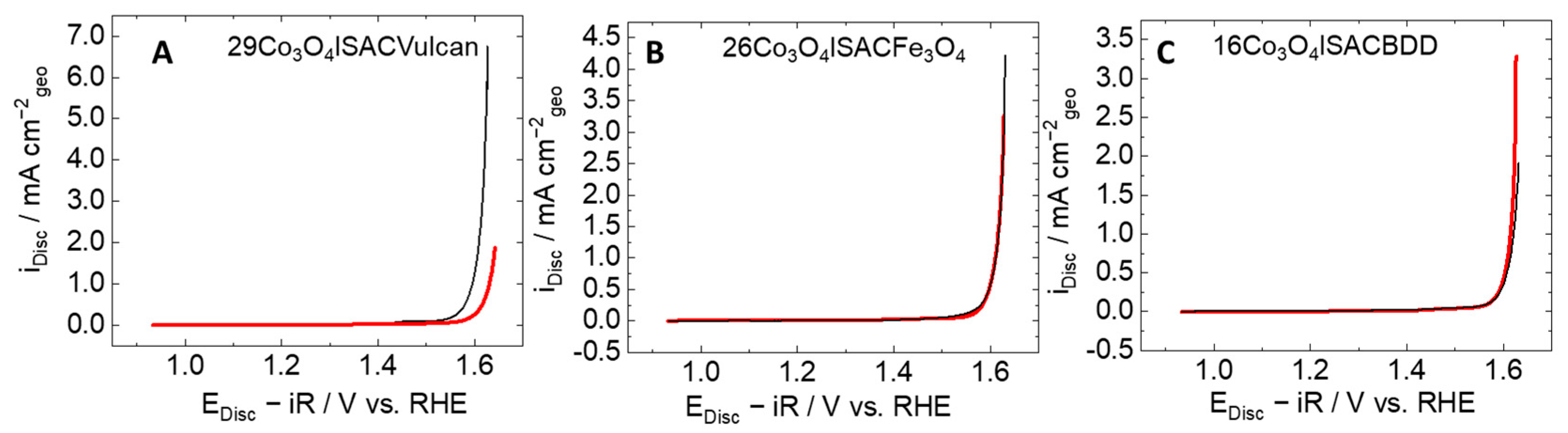
| Support or Composite | SBET/m2 g−1 | SBJH/ m2 g−1 | VBJH/cm3 g−1 | Smicroa/m2 g−1 | Vmicro a/cm3 g−1 | Mean Pore Size/nm | DDRX b/nm | Q c/ C g−1 |
|---|---|---|---|---|---|---|---|---|
| WC commercial | 4 | 4.9 | 0.010 | - | - | 8.5 | - | - |
| Fe3O4 commercial | 36 | 43.4 | 0.045 | - | - | 4.2 | - | - |
| Vulcan XC-72 | 194 | 117 | 0.3 | 118 | 59 | 11 | - | - |
| BDD | 181 | 184 | 0.4 | - | - | 8.5 | - | - |
| 16Co3O4ISACBDD | 43.1 | 42.5 | 0.1 | 4 | 0.0003 | - | 21 | 24.40 |
| 29Co3O4ISACVulcan | 80 | 46.7 | 0.33 | 34.8 | 0.0146 | 28.5 | 9 | 4.80 |
| 26Co3O4ISACFe3O4 | 7 | 11.4 | 0.04 | 10 | - | 14 | 23 | 10.00 |
| 32Co3O4ISACWCcalc | 19 | 20.6 | 0.052 | 21.3 | - | 10.1 | 22 | - |
| Co3O4AC | 1.47 | 0.56 | 0.0067 | 1.18 | 0.0006 | - | 24 | 0.01 |
| Material | Designation (Oxide: Support wt Ratio) | Oxide Loading/µg cm−2geo | Media | ECSA */m2 g−1 | Tafel Slope/ mV dec−1 | Mass-Normalized OER Activity at 1.58 V RHE/ A g−1oxide | Ref. |
|---|---|---|---|---|---|---|---|
| Co3O4AC | Unsupported Co3O4 nanoparticles | 15 | 1 M NaOH | ~5 × 10−3 * | 119 | 1.3 | this work and [22] |
| 16Co3O4ISACBDD | BDD-supported Co3O4 nanoparticles (16:84) | 15 | 1 M NaOH | 13 * | 65 | 14.2 [12.6] | this work and [22] |
| 29Co3O4ISACVulcan | Vulcan XC72 carbon-supported Co3O4 nanoparticles (29:71) | 15 | 1 M NaOH | 2.4 * | 59 | 55.7 [54.6] | this work and [22] |
| 26Co3O4ISACFe3O4 | Fe3O4-supported Co3O4 nanoparticles (26:74) | 15 | 1 M NaOH | 4.9 * | 48 | 24.8 [23.3] | this work |
| 32Co3O4ISACWCcalc | WC-supported Co3O4 nanoparticles (32:68) | 15 | 1 M NaOH | - | 134 | 19.7 [13.4] | this work |
| Fe3O4@CoFe2O4 | Core-shell Fe3O4@CoFe2O4 nanoparticles | 1.43 | 0.1 M NaOH | - | 63 ± 2 | 28.8 | [20] |
| 16Co3O4ISACSibunit152 | Sibunit-supported Co3O4 nanoparticles (16:84) | 15 | 1 M NaOH | 24 * | 80 | 28.1 [18.5] | [22] |
| Co3O4 | Acetylene Black- supported mesoporous Co3O4 | 380 | 1 M NaOH | 161 | 3.5 | [2] | |
| Co3O4 (a) | Carbon fiber paper-supportedCo3O4 nanoparticles | 300 | 1 M KOH | 61.8 | 11 | [37] | |
| Co3O4 (b) | Co3O4 impregnated on the graphene | 127.4 | 0.1 M KOH | 147 | 19.6 | [3] | |
| Co3O4 (c) | Co3O4 post coated on carbon cloth | 8100 | 1M KOH | 90 | 0.4 | [4] | |
| Co3O4(111) (d) | Co3O4 film electrodeposited on Au(111) | 15 | 0.1 M NaOH | 65 | - | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kéranguéven, G.; Filimonenkov, I.; Dintzer, T.; Picher, M. Alternative Supports for Electrocatalysis of the Oxygen Evolution Reaction in Alkaline Media. Electrochem 2025, 6, 23. https://doi.org/10.3390/electrochem6030023
Kéranguéven G, Filimonenkov I, Dintzer T, Picher M. Alternative Supports for Electrocatalysis of the Oxygen Evolution Reaction in Alkaline Media. Electrochem. 2025; 6(3):23. https://doi.org/10.3390/electrochem6030023
Chicago/Turabian StyleKéranguéven, Gwénaëlle, Ivan Filimonenkov, Thierry Dintzer, and Matthieu Picher. 2025. "Alternative Supports for Electrocatalysis of the Oxygen Evolution Reaction in Alkaline Media" Electrochem 6, no. 3: 23. https://doi.org/10.3390/electrochem6030023
APA StyleKéranguéven, G., Filimonenkov, I., Dintzer, T., & Picher, M. (2025). Alternative Supports for Electrocatalysis of the Oxygen Evolution Reaction in Alkaline Media. Electrochem, 6(3), 23. https://doi.org/10.3390/electrochem6030023







