Abstract
In recent years, clean energy technologies that meet ever-increasing energy demands without the risk of environmental contamination has been a major interest. One approach is the utilization of plant leaves, which release redox-active NADPH as a result of photosynthesis, to generate photocurrent. In this work, we show for the first time that photocurrent can be harvested directly from the fruit of a cherry tree when associated with a bio-electrochemical cell. Furthermore, we apply electrochemical and spectroscopic methods to show that NADH in the fruit plays a major role in electric current production.
1. Introduction
Concerns about climate change and the increasing global energy demand have urged scientists to invent creative clean energy technologies to replace the traditional use of fossil fuels. One approach is the utilization of live bacterial cells as electron donors in microbial fuel cells (MFCs) [1]. Direct electron transfer between bacteria and the anode may occur through conductive proteins such as metal respiratory complexes [2,3] or pili [4,5,6,7]. Among the bacterial species that exhibit high direct exoelectrogenic activity are Shewanella oneidensis [5] and Geobacter sulfurreducens [8]. Electron transfer can also be mediated by cellular release and diffusion to the anode of low-molecular weight metabolites such as quinones and phenazines. Solar illumination [9] and/or addition of artificial electron mediators such as cysteine, neutral red, thionine, sulfides, ferric chelated complexes, quinones, phenazines, and humic acids [10,11,12,13,14,15] have been used to enhance current generation. Current production in bioelectrochemical cells (BECs) may also be catalyzed by activity of enzymatic cascade assemblies on carbon matrices [16,17,18,19]. An advancement of MFCs is the bio-photo electrochemical cells (BPECs) that use photosynthetic organisms to generate photocurrent. Most reports on BPECs to date have studied microorganisms such as cyanobacteria [20,21,22,23] and microalgae [24,25]. A unique BPEC configuration that enhances photocurrent production uses a biofilm, in which cells are arranged at a higher density on the electrode surface than within a suspension [25]. This approach has been successfully implemented in non-photosynthetic MFCs [5,26,27]. Recent studies showed that BPECs are not limited to photosynthetic microorganisms and can be expanded to macroalgae [28] and terrestrial plants [29,30,31,32] Photocurrent production from these plants is about 1000 times greater than reported for microorganisms [33]. Plants’ roots secrete reducing molecules [34,35] that can also be utilized for electrical current production in BECs [36]. Rather than direct electricity production, organic exudate from plant roots feeds bacteria in soil MFCs [37]. Recently, the variety of species that can be used in BECs was broadened to include marine animals, whereby Nematostella vectensis and Artemia salina were applied as an electron source [38].
An efficient electroanalytical system that is extensively used in recent years to study these systems is screen-printed electrodes (SPEs) [39,40]. SPEs have several advantages over traditional bulk electrochemical systems. The analyte volume required for each measurement (50–100 µL) is ~3 orders of magnitude smaller than traditional systems. This is advantagous for studying expensive or limited quantities of analyte. The distance between electrodes, which is known to influence electrochemical measurement, is also fixed with an SPE. Furthmore, the advantage of disposable SPEs can avoid persistent issues with electrode fouling.
For most organisms studied thus far, the major redox species that engage in electron transfer between the cell and the anode are NADH and NADPH, which are products of glycolysis [22] and photosynthesis [33], respectively. These moleules can be released from cells upon association with the anode [21]. Yet, the identity of all major redox contributors for different organisms is still elusive. When probing the complex internal electrolyte solution of a bulk organism, such as the inside of a succulent leaf [32] or yeast extract [16,17,41], constituent reducing molecules can be applied as electron donors in BECs to produce current and photocurrent. Similarly, the internal environment of plant by-products such as fruit may pose an interesting route for harvesting electricity due to their abundance of redox-active pigments and vitamins [42]. In this work, we broaden the classes of organisms that can be used in BPECs showing for the first time that photocurrent can be harvested directly from a cherry fruit.
2. Materials and Methods
2.1. Materials
NaCl was purchased from Merck. Commercially available ripe and overripe bing cherries (Prunus avium) were purchased from Smart and Final store (Goleta, CA, USA), and pre-rinsed with DI water. Unless otherwise stated, fresh cherry juice was collected by crushing 3–5 cherries with a mortar and pestle for 60 s, and the supernatant as collected for measurement.
2.2. Fluorescence Measurements
Fluorescence measurements were conducted using a Cary Eclipse fluorimeter (Varian) with excitation and emission slit bands of 5 nm, applying a voltage of 700 V on the photomultiplier detector.
2.3. Electrochemical Apparatus
All electrochemical measurements were performed using Palmsens4 potentiostat with SPEs (BASi), with a graphite-working electrode (1 mm in diameter), graphite counter electrode, and silver coated with a silver chloride reference electrode. In the case of a direct measurements from the cherry under illumination, the SPE was inserted into the center of the cherry 2 mm below it top. Illumination of the cherry was conducted from the top using a white LED. For experiments utilizing cherry juice, a solution of 50 μm was added to fully submerge the electrodes. Illumination of the cherry was conducted from the top using a white LED. The light intensity was measured using a “Lux” app light meter, placing it at the same height of the electrode. The top of the cherry (5 mm) that was above the SPE in the chronoamperometry measurement was cut using a blade and placed on top of the light meter for measurement.
2.4. Chronoamperometry
Chronoamperometry measurements inside the cherry were conducted by applying an electrical potential of 0.9 V on the working electrode (the anode).
2.5. Cyclic Voltammetry
Cyclic voltammetry of cherry juice was measured in the range from 0 to 1.2 V, and a scan rate of 0.1 V/s.
2.6. Electrochemical Impedance Spectroscopy (EIS)
EIS of filtered cherry juice (0.45 μm PES syringe filter) was conducted by applying an AC potential of 0.01 V, a DC potential of 0.055 V, minimal frequency of 0.01 Hz, and a maximal frequency of 10,000 Hz.
3. Results and Discussion
3.1. Identification of Redox-Active Molecules in a Cherry
Previous studies of succulent-based BPECs [33] reported the advantage of using leaf structures enclosed by a protective cuticle; this cuticle acts as an envelope for a BEC, whereby its inner electrolyte gel consists of reducing molecules. We wished to study the redox activity of the inner content of a cherry. A cherry was homogenized and 50 µL of its inner juice was placed on an SPE, with a graphite-working electrode (1 mm in diameter), graphite counter electrode, and silver coated with a silver chloride reference electrode (Figure 1a). The cyclic voltammetry (CV) of the juice was measured (0–1.2 V, scan rate = 0.1 V/s). A solution of 0.1 M NaCl that should not show any redox peaks in the CV measurements was applied as a control (Figure 1b). A positive peak with a maximum of around 0.8 V was obtained for the cherry juice only, corresponding to the voltametric fingerprint of NADH [43], ascorbic acid [44] and/or anthocyanins [45]. We note that NADH was previously reported to be a major electron mediator in BECs using various organisms [21,28,38].
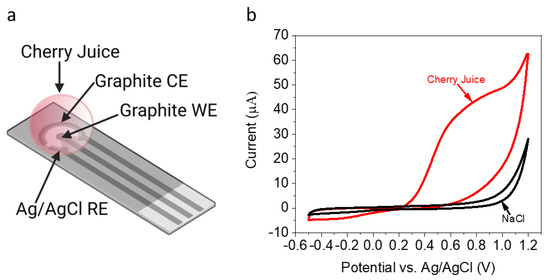
Figure 1.
Identification of redox-active molecules in a cherry. A cherry was homogenized and 50 µL of its internal solution was placed on a SPE. CV of the solution and 0.1 M NaCl (as a control) was measured (0–1.2 V, scan rate = 0.1 V/s). (a) Schematic description of the system consisted of SPE with graphite-working and counter electrodes (WE and CE respectively), and Ag coated with an AgCl Reference electrode (RE). A drop of collected cherry juice was placed on top of the electrodes. (b) CV of 0.1 M NaCl (black) and cherry juice (red).
3.2. Analysis of the Internal Electrolyte in Ripe and Overripe Cherries
The composition of the internal juice electrolyte of fruits, along with their moisture content [42], is known to change with aging. Among these changes are the levels of anthocyanin pigments and ascorbic acid [46]. Based on observation of a voltametric fingerprint from CV that overlaps with signals for anthocyanins and/or NADH, we evaluated their relative current intensities at different stages of aging (Figure 2a). Absorption spectra of the internal juice of ripe and overripe cherries were evaluated for relative differences in anthocyanin concentration at 550 nm. We observe higher anthocyanins levels with overripe cherries (Figure 2b), which agrees with the darker overall coloration. The measured pH values of juices of the ripe and overripe were 3.73 and 3.86, respectively. To explore the presence of NADH in each juice, fluorescence was measured (λExcitation = 340 nm, λEmission = 400–600 nm). Spectra revealed an emission peak around 450 nm that correspond to NADH [21] (Figure 2d). These results agree with the absorption (Figure 2a) and CV (Figure 1b) measurements, supporting the hypothesis that NADH plays a major role in current generation. To further differentiate between the maturation stages in cherries, without the impact of variable solid and moisture content, electrochemical impedance spectroscopy (EIS) of the filtered juice was measured. Higher impendence values at low frequency, corresponding to decreased water content [47], were observed for overripe cherry juice (Figure 2c,d). Nyquist plots reveal a significant shift in both the real (−Z″) and imaginary (Z’) impedance data for overripe cherries, which agrees with previous studies on orange fruit maturation [47]. Based on absorption, EIS, and fluorescence measurements, we suggest that cherries at different levels of maturation will produce altered electricity production in a BEC as a result of different moisture content and concentration of redox-active molecules.
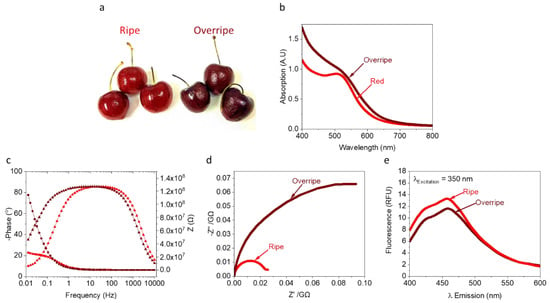
Figure 2.
Analysis of the internal electrolyte in ripe, and overripe red cherries. Absorption, EIS, and fluorescence measurements were conducted for the internal solution of ripe and overripe cherries. (a) Photograph of ripe (left) and overripe (right) cherries. (b) Absorption spectra of the ripe and overripe cherries’ juices. (c,d) EIS of ripe and overripe cherry (e) Fluorescence spectra of ripe and overripe cherry juice (λExcitation = 350 nm, λEmission = 400–600 nm).
3.3. Direct Photocurrent Production from a Cherry
Based on detection of NADH and other known redox-active molecules such as anthocyanins [46] and ascorbic acid [48,49], we explored whether electrical and photoelectrical current can be harvested from an intact cherry. To do that, an SPE was inserted directly into the center of a cherry (horizontally 5 mm below its top). Illumination was conducted from the top of the cherry by a white LED (Figure 3a). To quantify the light intensity at the electrode surface, a simultaneous experiment was conducted in which the top portion of the cherry (directly above the SPE) was sectioned and placed over a light meter. Illumination intensity was measured as 1000 W/m2. As a control experiment, a drop of aqueous NaCl (0.1 M) was placed below the top cut of the cherry with a transparent thin nylon sheet between the NaCl solution and cherry flesh to prevent mixing of solutions. Chronoamperometry (CA) measurements were conducted in dark/light intervals of 100 s. Results showed a relative current density of ~0.8 µA/cm2 for the NaCl solution independent of light illumination. We suspect that this current originates from the oxygen evolution reaction at 0.9 V. CA of the cherry, which showed a dark current density of ~1.7 µA/cm2 that was enhanced by ~0.3 µA/cm2 in light. Furthermore, the overall trend of current density increased gradually over a period of 1200 s (Figure 3b,c). Under continuous illumination for 1 h, the ripe and overripe cherries produce photocurrents of 2.2 and 2.6 µA/cm2, respectively. (Figure S1) These data suggest a favorable increase in electricity production, and therefore practical use, for fruit flesh that approaches what consumers traditionally consider expired and worthy of discarding. Simultaneous thermocouple measurements of the flesh and SPE show constant temperature readings of 17.5 °C, confirming the absence of thermal effects on the BEC. These current densities are higher than previously obtained from intact non-photosynthetic bacteria, similar to photosynthetic microorganisms, and lower than plant leaves and seaweeds (Table S1). We suggest that the dark current originates mostly from NADH in the cherry while the light-dependent current derives from ascorbic acid, whose levels in cherries are enhanced in the presence of light [49]. Thus, the overall trend in increased photocurrent may be coupled with light-activated ascorbic acid production at a higher rate than its oxidation rate at the anode. We note that overripe cherries outperform ripe cherries in both transient (Figure 3) and continuous (Figure S1) illumination experiments, indicating enhanced performance due to higher redox-active flavonoid levels (e.g., anthocyanins). We note that light-mediated energy transfer (e.g., FRET) from these chromophores may further enhance performance [50].
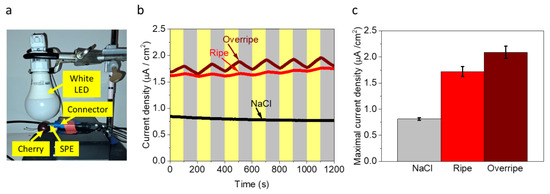
Figure 3.
Direct photocurrent production from a Cherry. CA of a cherry and 0.1 M NaCl solution (control) was measured in dark/light intervals of 100 s applying a potential of 0.9 V on the anode. (a) Photograph of the experimental setup. An SPE was inserted into the center of the cherry (5 mm below its top). Illumination was conducted from the top using a white LED. Intensity at the SPE surface below the cherry was 1000 W/m2. The SPE was inserted into a connector for the potentiostat. Yellow labels with arrows indicate each system elements. (b) CA of 0.1 M NaCl (black), ripe (red) and overripe (wine) cherry under dark/light intervals of 100 s. Gray and yellow rectangular shapes represent the dark and light during the measurement, respectively. (c) Maximal current density of NaCl (gray), ripe cherry (red), and overripe (wine). Error bars represent the standard deviation over three independent repetitions.
3.4. Proposed Mechanism for Electrical Current and Photocurrent Production
Based on this proof-of-concept study and previous supporting studies [46,48,49], we suggest possible mechanisms for our observed electrical and photoelectrical current generation (Figure 4). NADH, which exists in the internal solution of the cherry, can donate electrons at the anode. Another electron donor is ascorbic acid, whose concentration is elevated upon illumination enhancing photocurrent production. Electron donation at the anode may also originate from anthocyanins, which exists as a native antioxidant that increases in concentration with cherry maturation. As a pigment, we also suggest a possible role in conducting Förster energy transfer (FRET) to the anode.
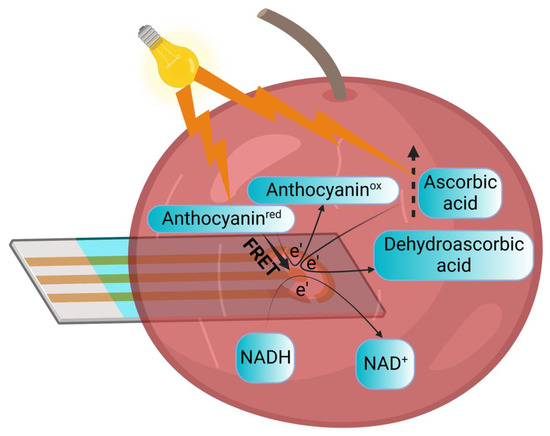
Figure 4.
Suggesting a mechanism for the electrical current and photocurrent production. NADH that exists in the cherry can be oxidized at the anode to produce current. The current formation may also originate from ascorbic acid whose formation is induced by light illumination. Another electron donor may be the pigment anthocyanins, which may also conduct Förster energy transfer in light. The light bulb icon and lightning shapes represent the illumination. The round arrows marked with e’ represent the electron transfer between the electron donors and the anode. The dashed arrow represents the elevation of ascorbic acid levels that occur in the light. The solid arrow represents the Förster energy transfer from anthocyanins to the anode.
4. Conclusions
In this work, we showed for the first time that cherries can be used for direct photocurrent production in a BEC. Based on spectrophotometric and electrochemical measurements, we identified likely electron donors that play a role in this current production as NADH, anthocyanins, and ascorbic acid. We identified separate routes of current generation that rely on constituent metabolites and photo-illumination. Future studies aimed at identification and quantification of the primary redox contributors for electricity production will be performed as a function of fruit maturation.
The idea of BECs was invented more than 100 years ago by Potter et al. [51], who established the concept of making bio-electricity from bacteria. Since then, most studies of live-based BECs have focused on microorganisms and their improvements. Demonstrating that fruit can become an electron source instead of bacteria may open an entire new field of research. Without the need to maintain living organisms and their microenvironments in a BEC, utilization of fruit may pose a simple and large-scale use for agricultural surplus products. This field could explore the benefits of making electricity from different fruits and improving their output with adding electron mediators, altered environmental conditions, and genetic engineering.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/electrochem4010005/s1, Figure S1: Continuous photocurrent production from ripe and overripe cherries; Table S1: Comparison of maximal current production from BECs based on intact organisms.
Author Contributions
Y.S. conceived the idea and designed and performed the main experiments. A.S.C., N.S.N. and K.C.R. aided in additional experiments. Y.S. and A.S.C. wrote the paper. A.S.C. supervised the research project. All authors have read and agreed to the published version of the manuscript.
Funding
The University of California Santa Barbara. This research was funded with an Otis Williams Fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Some of the results reported in this work were obtained using central facilities at the Materials Research Laboratory (MRL). We thank Jaya Nolt for her technical support. Some of the figures were created using Biorender.com (accessed 1 February 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial Fuel Cells, a Renewable Energy Technology for Bio-Electricity Generation: A Mini-Review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Hartshorne, R.S.; Jepson, B.N.; Clarke, T.A.; Field, S.J.; Fredrickson, J.; Zachara, J.; Shi, L.; Butt, J.N.; Richardson, D.J. Characterization of Shewanella Oneidensis MtrC: A Cell-Surface Decaheme Cytochrome Involved in Respiratory Electron Transport to Extracellular Electron Acceptors. JBIC J. Biol. Inorg. Chem. 2007, 12, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Rosso, K.M.; Zachara, J.M.; Fredrickson, J.K. Mtr Extracellular Electron-Transfer Pathways in Fe(III)-Reducing or Fe(II)-Oxidizing Bacteria: A Genomic Perspective. Biochem. Soc. Trans. 2012, 40, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Heidary, N.; Kornienko, N.; Kalathil, S.; Fang, X.; Ly, K.H.; Greer, H.F.; Reisner, E. Disparity of Cytochrome Utilization in Anodic and Cathodic Extracellular Electron Transfer Pathways of Geobacter Sulfurreducens Biofilms. J. Am. Chem. Soc. 2020, 142, 5194–5203. [Google Scholar] [CrossRef]
- Lovley, D.R. Electromicrobiology. Annu. Rev. Microbiol. 2012, 66, 391–409. [Google Scholar] [CrossRef]
- Nevin, K.P.; Richter, H.; Covalla, S.F.; Johnson, J.P.; Woodard, T.L.; Orloff, A.L.; Jia, H.; Zhang, M.; Lovley, D.R. Power Output and Columbic Efficiencies from Biofilms of Geobacter Sulfurreducens Comparable to Mixed Community Microbial Fuel Cells. Environ. Microbiol. 2008, 10, 2505–2514. [Google Scholar] [CrossRef]
- Yi, H.; Nevin, K.P.; Kim, B.-C.; Franks, A.E.; Klimes, A.; Tender, L.M.; Lovley, D.R. Selection of a Variant of Geobacter Sulfurreducens with Enhanced Capacity for Current Production in Microbial Fuel Cells. Biosens. Bioelectron. 2009, 24, 3498–3503. [Google Scholar] [CrossRef]
- Neu, J.; Shipps, C.C.; Guberman-Pfeffer, M.J.; Shen, C.; Srikanth, V.; Spies, J.A.; Kirchhofer, N.D.; Yalcin, S.E.; Brudvig, G.W.; Batista, V.S.; et al. Microbial Biofilms as Living Photoconductors Due to Ultrafast Electron Transfer in Cytochrome OmcS Nanowires. Nat. Commun. 2022, 13, 5150. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Limwongyut, J.; Moreland, A.S.; Bazan, G.C. Non-Photosynthetic Bacteria Produce Photocurrent Mediated by NADH. bioRxiv 2023. [Google Scholar] [CrossRef]
- Simoska, O.; Sans, M.; Eberlin, L.S.; Shear, J.B.; Stevenson, K.J. Electrochemical Monitoring of the Impact of Polymicrobial Infections on Pseudomonas Aeruginosa and Growth Dependent Medium. Biosens. Bioelectron. 2019, 142, 111538. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C.; Hart, J. Comparative Study of Three Types of Microbial Fuel Cell. Enzyme Microb. Technol. 2005, 37, 238–245. [Google Scholar] [CrossRef]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel Cells Select for Microbial Consortia That Self-Mediate Electron Transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial Phenazine Production Enhances Electron Transfer in Biofuel Cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Coates, J.D.; Blunt-Harris, E.L.; Phillips, E.J.P.; Woodward, J.C. Humic Substances as Electron Acceptors for Microbial Respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction; Academic Press: Cambridge, MA, USA, 2004; Volume 49, pp. 219–286. ISBN 0065-2911. [Google Scholar]
- Herkendell, K. Status Update on Bioelectrochemical Systems: Prospects for Carbon Electrode Design and Scale-Up. Catalysts 2021, 11, 278. [Google Scholar] [CrossRef]
- Herkendell, K.; Tel-Vered, R.; Stemmer, A. Switchable Aerobic/Anaerobic Multi-Substrate Biofuel Cell Operating on Anodic and Cathodic Enzymatic Cascade Assemblies. Nanoscale 2017, 9, 14118–14126. [Google Scholar] [CrossRef]
- Herkendell, K.; Stemmer, A.; Tel-Vered, R. Extending the Operational Lifetimes of All-Direct Electron Transfer Enzymatic Biofuel Cells by Magnetically Assembling and Exchanging the Active Biocatalyst Layers on Stationary Electrodes. Nano Res. 2019, 12, 767–775. [Google Scholar] [CrossRef]
- Herkendell, K.; Stemmer, A.; Tel-Vered, R. Magnetically Induced Enzymatic Cascades—Advancing towards Multi-Fuel Direct/Mediated Bioelectrocatalysis. Nanoscale Adv. 2019, 1, 1686–1692. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Spungin, D.; Schuster, G.; Frank, I.-B.; Adir, N. Trichodesmium Erythraeum Produces a Higher Photocurrent than Other Cyanobacterial Species in Bio-Photo Electrochemical Cells. Biochim. Biophys. Acta Bioenerg. 2022, 1863, 148910. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Eichenbaum, B.; Tóth, T.N.; Levin, G.; Liveanu, V.; Schuster, G.; Adir, N. NADPH Performs Mediated Electron Transfer in Cyanobacterial-Driven Bio-Photoelectrochemical Cells. iScience 2020, 24, 101892. [Google Scholar] [CrossRef]
- Saper, G.; Kallmann, D.; Conzuelo, F.; Zhao, F.; Tóth, T.N.; Liveanu, V.; Meir, S.; Szymanski, J.; Aharoni, A.; Schuhmann, W.; et al. Live Cyanobacteria Produce Photocurrent and Hydrogen Using Both the Respiratory and Photosynthetic Systems. Nat. Commun. 2018, 9, 2168. [Google Scholar] [CrossRef] [PubMed]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in Clinical Practice: Evidence-Based Human Applications. Evid.-Based Complement. Altern. Med. 2011, 2011, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, Y.; Tóth, T.N.; Eichenbaum, B.; Keysar, L.; Schuster, G.; Adir, N. Electron Mediation and Photocurrent Enhancement in Dunalliela Salina Driven Bio-Photo Electrochemical Cells. Catalysts 2021, 11, 1220. [Google Scholar] [CrossRef]
- McCormick, A.J.J.; Bombelli, P.; Scott, A.M.M.; Philips, A.J.J.; Smith, A.G.G.; Fisher, A.C.C.; Howe, C.J.J. Photosynthetic Biofilms in Pure Culture Harness Solar Energy in a Mediatorless Bio-Photovoltaic Cell (BPV) System. Energy Environ. Sci. 2011, 4, 4699–4709. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial Fuel Cells: From Fundamentals to Applications. A Review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.L.; Phang, S.M.; Periasamy, V.; Yunus, K.; Fisher, A.C. Enhancement of Power Output by Using Alginate Immobilized Algae in Biophotovoltaic Devices. Sci. Rep. 2017, 7, 16237. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Krupnik, N.; Tóth, T.N.; Eichenbaum, B.; Meirovich, M.; Meiri, D.; Yehezkeli, O.; Schuster, G.; Israel, Á.; Adir, N. Bioelectricity Generation from Live Marine Photosynthetic Macroalgae. Biosens. Bioelectron. 2021, 198, 113824. [Google Scholar] [CrossRef]
- Hubenova, Y.; Mitov, M. Conversion of Solar Energy into Electricity by Using Duckweed in Direct Photosynthetic Plant Fuel Cell. Bioelectrochemistry 2012, 87, 185–191. [Google Scholar] [CrossRef]
- Hubenova, Y.; Mitov, M. Enhanced Metabolic and Redox Activity of Vascular Aquatic Plant Lemna Valdiviana under Polarization in Direct Photosynthetic Plant Fuel Cell. Bioelectrochemistry 2015, 106, 226–231. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Meirovich, M.; Yehezkeli, O.; Schuster, G.; Adir, N. Production of Photocurrent and Hydrogen Gas from Intact Plant Leaves. Biosens. Bioelectron. 2022, 215, 114558. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Schuster, G.; Adir, N. Self-Enclosed Bio-Photoelectrochemical Cell in Succulent Plants. ACS Appl. Mater. Interfaces 2022, 14, 53761–53766. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, Y.; Schuster, G.; Adir, N. Harnessing Photosynthesis to Produce Electricity Using Cyanobacteria, Green Algae, Seaweeds and Plants. Front. Plant Sci. 2022, 13, 955843. [Google Scholar] [CrossRef] [PubMed]
- Gvamichava, N.E. Vitamin Secretion by Roots. Fiziol. Drev. Rastenii 1966, 2, 5–16. [Google Scholar]
- Pardha-Saradhi, P.; Yamal, G.; Peddisetty, T.; Sharmila, P.; Nagar, S.; Singh, J.; Nagarajan, R.; Rao, K.S. Reducing Strength Prevailing at Root Surface of Plants Promotes Reduction of Ag+ and Generation of Ag0/Ag2O Nanoparticles Exogenously in Aqueous Phase. PLoS ONE 2014, 9, e106715. [Google Scholar] [CrossRef]
- Shlosberg, Y. Direct Electricity Production from Green Onion’s Roots. bioRxiv 2022. [Google Scholar] [CrossRef]
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.-K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Aranda-Ruíz, J. Progress and Recent Trends in Photosynthetic Assisted Microbial Fuel Cells: A Review. Biomass Bioenergy 2021, 148, 106028. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Brekhman, V.; Lotan, T.; Sepunaru, L. Direct Electricity Production from Nematostella and Arthemia’s Eggs in a Bio-Electrochemical Cell. Int. J. Mol. Sci. 2022, 23, 15001. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Sepunaru, L. Advantages of Imprinted Polymer Electrodes for Electrochemical Pathogen Detection. Curr. Opin. Electrochem. 2022, 36, 101123. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent Developments and Applications of Screen-Printed Electrodes in Environmental Assays—A Review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Shlosberg, Y.; Smith, M.; Carlini, A. Redox-Active Molecules in Bacterial Cultivation Media Produce Photocurrent. bioRxiv 2022. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Bogdanović, S.; Voća, S.; Skendrović Babojelić, M. Biological Potential of Fruit and Leaves of Strawberry Tree (Arbutus Unedo L.) from Croatia. Molecules 2020, 25, 5102. [Google Scholar] [CrossRef] [PubMed]
- Blandón-Naranjo, L.; Della Pelle, F.; Vázquez, M.V.; Gallego, J.; Santamaría, A.; Alzate-Tobón, M.; Compagnone, D. Electrochemical Behaviour of Microwave-Assisted Oxidized MWCNTs Based Disposable Electrodes: Proposal of a NADH Electrochemical Sensor. Electroanalysis 2018, 30, 509–516. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Danet, A.F.; Kalinowski, S. Ascorbic Acid Determination in Commercial Fruit Juice Samples by Cyclic Voltammetry. J. Autom. Methods Manag. Chem. 2008, 2008, 937651. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, M.; Torabi, S.; Tavan, M.; Azizi, A.; Khazalpour, S. Electrochemical Behavior and LC-MS Analysis of Anthocyanin’s in Vaccinium Arctostaphylos L. Extract: The Molecular Modelling of Potential Inhibition to COVID-19 and ROS Generation Receptors. J. Electrochem. Soc. 2020, 167, 155505. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Singh, P.; Bera, T.K.; Ghoshal, D.; Chakraborty, B. Electrical Impedance Spectroscopic Study of Mandarin Orange during Ripening. J. Food Meas. Charact. 2017, 11, 1654–1664. [Google Scholar] [CrossRef]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G. Antioxidant Capacity, Phenol, Anthocyanin and Ascorbic Acid Contents in Raspberries, Blackberries, Red Currants, Gooseberries and Cornelian Cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Wang, S.Y. Effect of Pre-Harvest Conditions on Antioxidant Capicity in Fruits. In Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 30 June 2006; pp. 299–306. [Google Scholar]
- Singh, V.; Mishra, A.K. White Light Emission from Vegetable Extracts. Sci. Rep. 2015, 5, 11118. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical Effects Accompanying the Decomposition of Organic Compounds. Proc. R. Soc. Lond. B 1911, 84, 260–286. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).