Abstract
Echinometra lucunter, the most abundant sea urchin in Brazil, causes numerous accidents by puncture wounds, primarily on hands and feet. Beyond mechanical trauma, recent research has identified bioactive molecules in spine extracts and coelomic fluid contributing to these inflammatory reactions. This study investigated spine morphology to better understand the envenomation and defense processes for the animal. Using various microscopy techniques, the spines were revealed to be mineral structures with longitudinal canals and a sponge-like central mesh rich in granular cells. These cells extend from the spine’s center to its edges, terminating in fimbriae-like structures, likely involved in molecular exchange with the environment. The spine tip is more cellular than the base, suggesting a defensive role, while the base provides structural support. Several cell types were identified, including granulocytes, red spherulocytes, and phagocytic amoebocytes, also found in the coelomic fluid. Other cells displayed prominent Golgi apparatuses and secretory granules, indicating specialized secretory functions, likely the source of bioactive molecules involved in chemical defense and spine regeneration. Understanding this cellular structure is crucial for comprehending the urchin’s envenomation and defense mechanisms.
1. Introduction
The phylum Echinodermata encompasses a diverse group of strictly marine organisms characterized by their distinct pentaradial symmetry. Their body wall contains a skeleton of calcareous spicules and spines (from Greek: echinos = spiky; dermata = skin) that function in defense against predators, filter particles from seawater, and aid in locomotion [1,2].
Echinoderms possess an integument comprising the epidermis and dermis, which are virtually inseparable. The epidermis, consisting of uninucleate cells, covers all body parts and is usually single-layered, although stratification can be found in the iridophore zone—a layer with cells that produce iridescence or metallic structural color [3,4].
Sea urchin spines are not fixed structures; they can actively move due to the presence of muscles and collagen fibers inserted at their base, forming a tendon. These spines are symmetrically distributed around the animal’s body [5]. Generally, spines are calcified structures immersed in dermis and covered by epidermis, which can be hollow or solid depending on the species. They consist of a three-dimensional framework of mineralized trabeculae similar to a sponge, termed stereom, which encircles an organic core filled with connective tissue—the stroma [6,7].
The stereom is composed of calcium carbonate and/or magnesium carbonate [8]. Morphological characterization of the organic structures present in sea urchin spines is scarce, and these organic components account for approximately 0.1% of the entire spine. Such components include the extracellular matrix, proteins, glycoproteins, and skeletogenic cells (known as sclerocytes and phagocytes) [9]. These macromolecules are synthesized during skeletal tissue formation and are believed to regulate mineral deposition [10].
Although sea urchins are not sessile and their spines act as a defense mechanism, these animals rely on a passive defensive response. Human incidents are typically unintended encounters involving bathers, divers, surfers, and fishermen [11]. While painful to humans [12,13], spine injuries from echinoderms generally do not constitute a public health issue. Nevertheless, inflammatory reactions are frequent, and granuloma formation is often reported as a chronic manifestation of sea urchin injuries [14].
Echinometra lucunter is a common sea urchin found along Brazilian oceanic rock shores [15]. Despite its prevalence, to the best of our knowledge, there are few published studies describing the structure of E. lucunter spines. In this regard, the present work provides a morphological study of these structures, focusing on their organic aspects.
As we have previously demonstrated, E. lucunter spines are more than mere puncturing calcified structures; they are living tissues filled with granular cells [16] that release molecules capable of inducing inflammation in mice [12,13]. These molecules include low molecular mass compounds, peptides, proteins, and enzymes [16,17,18].
To provide a more comprehensive understanding of the toxinology involved in E. lucunter incidents, we now describe the morphology of the spines and their relation to the biological, pharmacological, and medical effects previously reported. This deeper knowledge of the animal’s biology and human reactions aims to enhance our understanding of marine envenomation.
2. Materials and Methods
E. lucunter individuals were collected in São Sebastião, São Paulo, Brazil, without distinction of sex, age, or size, with ~10 cm diameter, under the permanent license number 13852-1 (issued by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA), last expedited 25 March 2024), in partnership with the Center for Marine Biology (CEBIMar/USP). Spines were removed in situ, immediately after collection, using scissors to cut the connective tissue. Samples approximately 3 cm in length were selected and immediately fixed in appropriate buffers, as described below.
2.1. Light Microscopy
Spines were immersed in Karnovsky’s fixative solution for 48 h. They were then decalcified with 4% EDTA, pH 7.2, under constant agitation for 4–6 h, dehydrated through an ethanol series (70–100%), and embedded in glycol methacrylate (Leica Microsystems, Wetzlar, Germany). Transverse sections, 2 µm thick, were obtained using a Microm® HM 340E microtome (Microm, Walldorf, Germany) and stained with toluidine blue-fuchsin, periodic acid-Schiff (PAS), or bromophenol blue. Photomicrographs were captured using an Olympus BX51 microscope coupled with an Olympus QColor 5 camera (Olympus Corporation, Hachioji, Japan), utilizing Image Pro Express 5.0 software (Media Cybernetics, Bethesda, MD, USA) for image acquisition.
2.2. Transmission Electron Microscopy (TEM)
Transverse fragments of the decalcified spines, as described above, were post-fixed in 1% osmium tetroxide for 40 min, dehydrated in ethanol (70%, 95%, and 100%), and embedded in epoxy resin (Electron Microscopy Sciences, Hatfield, PA, USA). Ultrathin sections (~60 nm) were obtained using a Sorvall MT 6000 ultramicrotome, collected on copper grids, and contrasted with aqueous 2% uranyl acetate and lead citrate. Sections were examined using a LEO 906E transmission electron microscope (Carl Zeiss Microscopy, Jena, Germany) operating at 80 kV. Images were captured with a MegaView III camera using the iTEM Universal TEM Imaging Platform (Olympus Soft Imaging Solutions GmbH, Munster, Germany).
2.3. Scanning Electron Microscopy (SEM)
A spine preserved in the same fixative cited above was washed with 70% ethanol, air-dried naturally, manually fractured into several pieces, and examined using low-vacuum scanning electron microscopy at 60 Pa, operating at 10 kV (Quanta 250, FEI, Eindhoven, The Netherlands).
3. Results
A typical 3 cm primary spine of E. lucunter is presented in Figure 1a. Figure 1b shows a scanning electron micrograph of a manually fractured spine. In this figure, the spine’s exterior is composed of longitudinal columns running along its entire length. These columns radially penetrate towards—or radiate from—the spine’s core. Pores are present both between (Figure 1c) and within (Figure 1d) the columns, longitudinally and radially, making the spine an organized structure with a sponge-like core (Figure 1e,f).
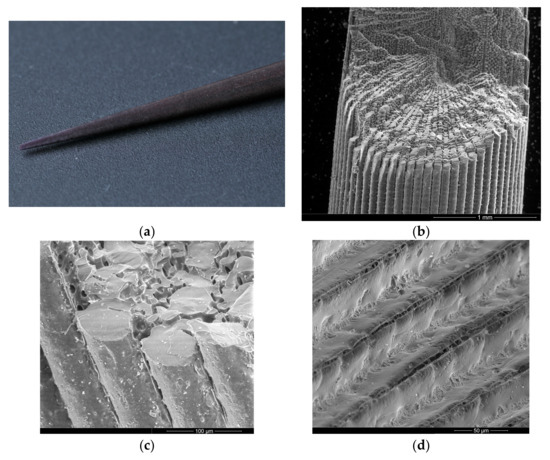
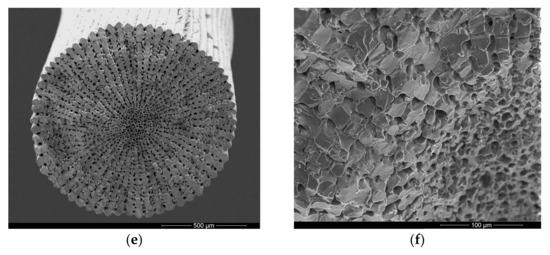
Figure 1.
General morphology of an Echinometra lucunter spine. (a) Macroscopic view of a typical primary spine. (b) Scanning electron micrograph (SEM) microscopy of a fractured spine reveals both external and internal features, including the longitudinal columns that run along the spine. (c) SEM detail of the external and internal surface. (d) Detail of external canals, with arrows indicating pores between the longitudinal columns. (e) Transverse SEM view of the spine, illustrating the sponge-like meshwork of the center and border regions. (f) SEM of the central region of the spine, highlighting the internal porous structure.
To verify the presence of cells between the mineralized structures, spines were subjected to light microscopy analyses. In a transverse section, cells are arranged in the same regular pattern (Figure 2a) as the mineral structures observed in SEM (Figure 1e), occupying the spaces between the mineral columns and radiating from a poorly organized central cellular region (Figure 2b). Moreover, the tip of the spine contains more cells, while at the base, the calcified portion is predominant.
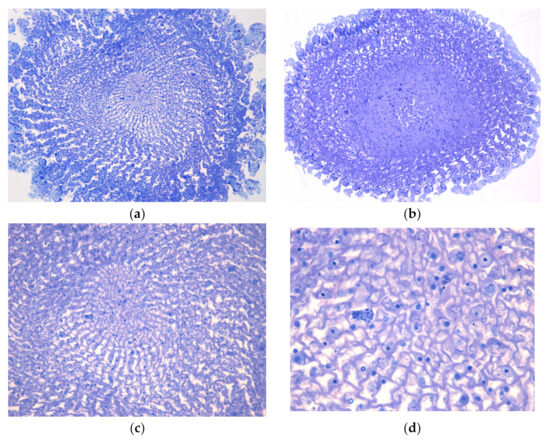
Figure 2.
Light microscopy of transverse sections of decalcified Echinometra lucunter spine, stained with toluidine blue-fuchsin. (a) General view displaying the radial arrangement of cells within the mineral matrix, from the middle of the spine. 20× (b) General view from the tip of the spine. 20×. (c) Detailed view of the central cellular region, which is less organized compared to the peripheral areas. 40×. (d) Cellular portion and presence of granular cells. 100×.
Among the cells in the channels, we observed the presence of typical cells from the coelomic fluid: granulocytes and red spherulocytes, as shown in Figure 2c,d.
Complementary histochemical analyses indicate that these migrated cells, as well as the regular parenchymal cells, contain granules rich in proteins, as revealed by bromophenol blue staining (Figure 3a). Additionally, PAS staining indicates that these granules also contain lipopolysaccharides (Figure 3b).
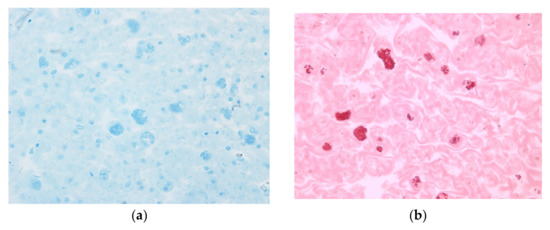
Figure 3.
Histochemical analysis of transverse sections of Echinometra lucunter spine, observed by light microscopy. (a) Bromophenol blue staining revealing protein-rich granules within granular cells. 20×. (b) Periodic acid-Shiff (PAS) staining indicating the presence of polysaccharides or glycoproteins in the granules. 20×.
To explore in detail the morphological characteristics of these cells, transmission electron microscopy was performed, as shown in Figure 4. Cells presenting electron-dense granules are clearly observed (Figure 4a,b), corresponding to granulocytes (previously observed by light microscopy, Figure 2d), the most abundant cell type in the E. lucunter spine structure. These cells exhibit a prominent Golgi apparatus (Figure 4b), typical of secretory cells.
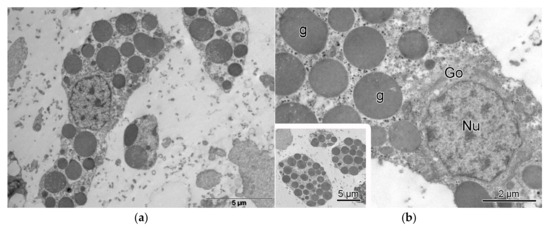
Figure 4.
Transmission electron microscopy (TEM) of a cell containing electro-dense granules, in Echinometra lucunter spines. (a) Cell profile with numerous electron-dense granules dispersed throughout the cytoplasm. (b) Detailed view highlighting cellular structures characteristic of secretory cells, including the Golgi apparatus (Go), granules (g), and nucleus (Nu).
Other cell types, not previously described, were also observed in the E. lucunter spines (Figure 5a,b). In general, these cells are rich in Golgi apparatus and secretory granules, indicating specialization in the secretion of molecules.
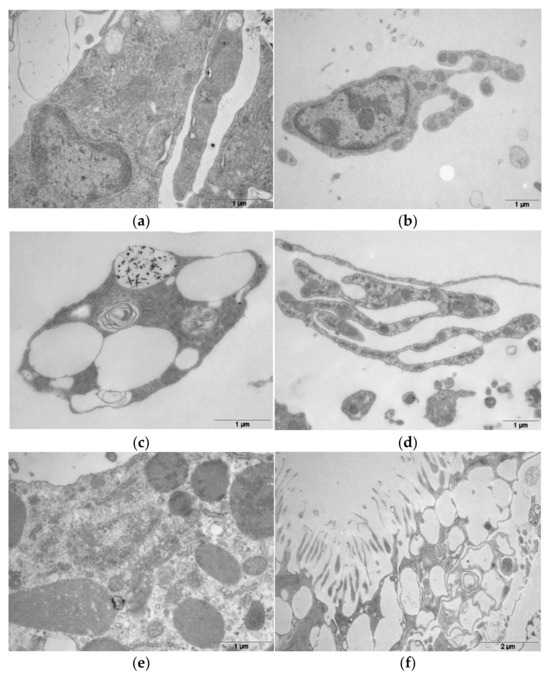
Figure 5.
Transmission electron microscopy (TEM) of different cell types found in Echinometra lucunter spines. (a) Cell rich in Golgi apparatus and secretory granules, indicating specialization in molecule secretion. (b) Secretory cell exhibiting prominent Golgi apparatus and numerous secretory granules. (c) Phagocytic amoebocyte containing specialized compartments with reflective iron bodies. (d) Fusiform cell characterized by an elongated shape and a prominent nucleus. (e) Colorless spherule cell, identified by the presence of electron-dense granules within the cytoplasm. (f) Fimbriae-like structures protruding from the pores on the spine surface, suggesting roles in environmental interaction through capture or excretion.
Conversely, cells that have been described in other sea urchin species were also observed in the spines of E. lucunter. Figure 5c presents a phagocytic amoebocyte, a coelomocyte that stores specular iron bodies in a specialized compartment, as observed by TEM. Figure 5d shows a spread cell containing a nucleus, typical of fusiform cells. Colorless spherule cells were observed, as shown in Figure 5e.
On the surface, in a transverse section, it was possible to observe that the cellular portion presents structures similar to fimbriae, which seem to function in capturing or excreting elements to and from the environment (Figure 5f).
Analyses of the collected TEM images allowed us to assemble a composite model presented in Figure 6a. By integrating multiple individual TEMs, we propose the presence of a secretory gland or structure within the central porous region of the E. lucunter spine. This composite image illustrates how secretory cells could be organized within the internal porous framework, fitting into the mineral matrix of the spine. The model highlights an entire “functional unit”, suggesting that the central region plays a crucial role in secretion and possibly in the spine’s interaction with the environment.
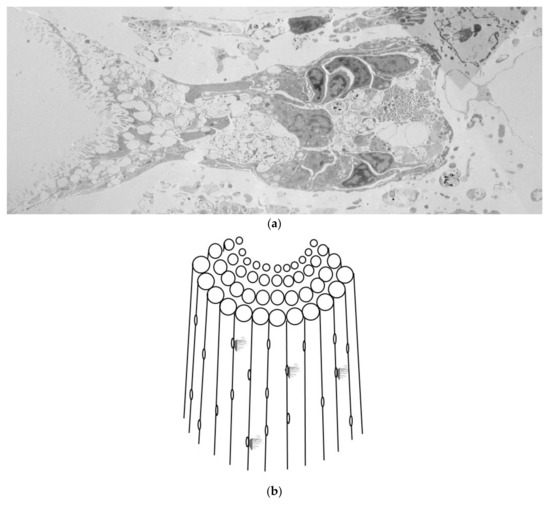
Figure 6.
Composite model proposing the presence of a secretory structure within the central region of Echinometra lucunter spine. (a) Reconstituted image created from several individual transmission electron micrographs (TEMs) to form a functional unit from the spine, in a transversal view, showing the secretory cells on the right and fimbria arrangement on the left. (b) The composite illustration synthesizes our observations into a proposed structure and lateral externalization of the functional unit.
To illustrate this proposal, Figure 6b shows a schematic of the spine and how this functional unit externalizes through its fimbriae. This proposed structure emphasizes the potential role of the central spine region in secretion and interaction with the environment, contributing to the spine’s biological functions.
4. Discussion
In this work, we describe for the first time that E. lucunter spines are organized structures composed of a porous mineral framework filled with different types of cells that ultimately combine to form complex, environment-interacting structures present at the pores. Moreover, this tissue is enveloped by an epidermis, which likely protects the cells and prevents direct contact with the mineralized parts.
Externally, E. lucunter spines are characterized by the presence of longitudinal channels, similar to those observed in Arbacia lixula [10]; these structures are composed of rods arranged longitudinally and exhibiting pores. A radial disposition of the mineral part was also observed in Strongylocentrotus purpuratus spines, termed ‘circles polycyclic spine’ [19].
Regular echinoids from Atlantic shallow waters were included in an Atlas to show, by SEM, the primary and secondary larger spine structures. Eucidaris tribuloides, Stylocidaris affinis, Tretocidaris bartletti, Arbacia punctulate and Lytechinus variegatus have the same pattern of Echinometra lucunter and Echinometra viridis, with internal meshwork and radial pores, as we showed here. Other species, such as Diadema antillarum, have spines with a central lumen, except near the tip [20]. In this species, there is a central longitudinal duct containing nerves and canals for the transport of venom synthesized in glands at the base of the spine up to the tip [21]. E. lucunter spines, on the other hand, are tougher but not massive; rather, they contain a myriad of secretory cells likely to be the source of toxic molecules released upon spine fracture.
This pattern was also observed in Lytechinus variegatus spines in a micro-CT scan. A similar structure was observed in primary and secondary spines [22].
The core of the spine contains the majority of cells but is poorly organized. This contrasts with the orderly radiating cells observed outward from the spine. The calcium/magnesium carbonate scaffold provides a porous mold that the cells orderly fill. The inorganic matrix facilitates the organization of cells and probably protects the tissue, which has a hydrovascular function specialized in transporting micronutrients from the base of the spine to the tip (or vice versa) via cellular communication [20].
Typical coelomic fluid cells, previously described for E. lucunter and other species, were found in the spines (Figure 4). There are four known types of coelomocytes in coelomic fluid: phagocytic amoebocytes (60–70%), vibratile cells (15–20%), red spherule cells (5–10%), and colorless spherule cells (5–10%) [23]. These cells play mainly immunological roles that include cellular recognition, phagocytosis, reactive oxygen intermediate production, cytotoxicity, antibacterial activity, inflammatory reactions (including C3-like expression after activation with lipopolysaccharides), prophenoloxidase activity, capsule formation, and graft reaction [24,25]. Although the presence of such cells has already been described in E. lucunter coelomic fluid, this is, to our knowledge, the first report of their presence in the spines.
Heatfiled and Travis have demonstrated the presence of spherulocytes in regenerating tips of spines from Strongylocentrotus purpuratus. They observed some structures in dermis and epidermis that we observed here, but in a non-regenerated spine of E. lucunter [26].
Fusiform cells observed here have previously been identified among coelomocytes of sea cucumbers, which belong to the same phylum, Echinodermata, as sea urchins. These cells are related to vibratile cells common in sea urchins [27].
Phagocytic amoebocytes have been identified in E. lucunter spines and correspond to cells with spicules and a concentric spiral. These cells contain intranuclear iron bodies, which may be considered their typical feature [23]. The presence of intracellular iron bodies in the digestive tube indicates an excretory pathway. These particles may act in the proliferation of cells to phagocytose foreign particles and excrete these contents, and in the spines may have a similar function [25].
In sea urchins, phagocytes are differentiated cells in the axial complex that collect and transform coelomocytes into phagocytes, degrading and excreting cellular content via the water vascular system [28]. As presented in Figure 2 and Figure 6, the spines exhibit a longitudinal, central, loose, less organized core that may function as a channel—a vascular system for cell nutrition.
Colorless spherule cells, another cell type found in the spines (Figure 5e), belong to the urchin immune system and release their granular content, causing inflammation and protecting the animal against foreign bodies [29]. Here, we identified this cell type due to electron-dense granules in the cytoplasm.
Granulocytes were observed through both conventional light microscopy and TEM, and we verified that these cells are distributed throughout the spine. This cell type is known for its immune role in chemical defense via toxin secretion and activation of the immune system [30].
Moreover, our results show that not only immune system cells are present in the spine. We also observed cells with a prominent Golgi apparatus, which is typical of secretory cells. Sea urchins possess many types of secretory cells besides spherulocytes that differ in the kind of secretory granules, the most frequent being spherical or oval with diameters of approximately 1 µm [4].
One characteristic of the spines is the presence of pigments, produced by pigment cells, which in the purple sea urchin have their developmental origins from mesodermal cells, the non-skeletogenic mesoderm (NSM). In the coelomic fluid, they are produced by pigmented coelomocytes (named red spherule cells), found in the spine of E. lucunter in the present study. The pigments have several functions in the spine, such as energy synthesis and innate immunity, and are based on naphthoquinone structures, and are named spinochromes [31,32].
Perhaps the most interesting structure observed in the sea urchin spine is depicted in Figure 6. The composite image shows a complex cellular organization with structures embedded in the calcified matrix along the whole spine, within the pores that run between the rods. There is a portion that lies outside the spine, ending in structures similar to fimbriae. This anatomical arrangement suggests an active role in capturing molecules from the environment and/or secreting toxins, eliminating metabolites, and foreign bodies. The abundance of these structures throughout the spine would guarantee nourishment, justifying the widespread presence of cells. Moreover, it could be considered an autonomous structure, as there is no communication with organs or coelomic fluid. In addition, the fimbriae may also play a sensory role [33].
In previous works, we isolated and biochemically characterized toxins from the spine of E. lucunter by immersing the spines in an isotonic ammonium acetate buffer solution. This extract (not a lysate) was able to induce inflammation in rodents [12]. From this extract, we purified a molecule responsible for the inflammatory (paw edema and leukocyte recruitment) and nociceptive effects [13]. Moreover, our group characterized a cathepsin B/X from this spine extract, which might be involved in the regeneration process of broken spines, similar to cathepsin K, as well as promoting toxic effects against predators, like other hydrolases [16].
The origin of the extracted cathepsin B/X from E. lucunter spines can now be attributed to the numerous granulocytes observed in the histochemical analyses (Figure 3), performed specifically to identify the chemical composition of such granules. Moreover, Figure 3 indicates that these granules also contain sugars. Albeck et al. [34] characterized glycoproteins in sea urchin spines and associated them with the organization of mineral reticulate growth during spine development. Other authors have characterized the presence of lectins in Paracentrotus lividus spines [35] and Arbacia lixula, which contained other monosaccharides [10].
5. Conclusions
In light of the previously described secreted sea urchin molecules (toxins) and the unequivocal anatomical correlation with the secretory structures described in the present work, we understand that we have identified the tissue source of these molecules, which are essential for homeostasis maintenance and participate in the animal’s defense.
Author Contributions
Conceptualization, J.M.S., J.R.M.C.d.S. and D.C.P.; methodology, M.M.A., J.R.M.C.d.S. and C.J.; formal analysis, J.M.S., M.M.A., C.J., J.R.M.C.d.S. and D.C.P.; investigation, J.M.S. and M.M.A.; resources, M.M.A., C.J. and D.C.P.; writing—original draft preparation, J.M.S.; writing—review and editing, D.C.P. All authors have read and agreed to the published version of the manuscript.
Funding
J.M.S.,D.C.P. and C.J. are National Council for Scientific and Technological Development (CNPq) fellow (313402/2023-0, 305525/2023-9 and 31629/2023-6, respectively). D.C.P. is a Butantan Foundation fellow.
Institutional Review Board Statement
The sea urchin collection was approved by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA), under protocol number 13852-1, expedited on 25 March 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smith, A.B.; Peterson, K.J.; Wray, G.; Littlewood, D.T.J. From bilateral symmetry to pentaradiality. In Assembling the Tree of Life; Cracraft, J., Donoghue, M.J., Eds.; Oxford University Press: New York, NY, USA, 2004; pp. 365–383. [Google Scholar]
- Swalla, B.J.; Smith, A.B. Deciphering deuterostome phylogeny: Molecular, morphological and palaeontological perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Cloney, R.A.; Brocco, S.L. Chromatophore organs, reflector cells, iridocytes and leucophores in cephalopods. Am. Zool. 1983, 23, 581–592. [Google Scholar] [CrossRef]
- Holland, N.D. Epidermal cells. In Biology of the Integument—Invertebrates; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 756–774. [Google Scholar]
- Hidaka, M.; Takahashi, K. Fine structure and mechanical properties of the catch apparatus of the sea urchin spine, a collagenous connective tissue with muscle-like holding capacity. J. Exp. Biol. 1983, 103, 1–14. [Google Scholar] [CrossRef]
- Dubois, P.; Ameye, L. Regeneration of spines and pedicellariae in Echinoderms: A review. Microsc. Res. Tech. 2001, 55, 427–437. [Google Scholar] [CrossRef]
- Moureaux, C.; Pérez-Huerta, A.; Compère, P.; Zhu, W.; Leloup, T.; Cusack, M.; Dubois, P. Structure, composition and mechanical relations to function in sea urchin spine. J. Struct. Biol. 2010, 170, 41–49. [Google Scholar] [CrossRef]
- Berman, A.; Addadi, L.; Kvick, A.; Leiserowitz, L.; Nelson, M.; Weiner, S. Intercalation of sea urchin proteins in calcite: Study of a crystalline composite material. Science 1990, 250, 664–667. [Google Scholar] [CrossRef]
- Weiner, S. Organic matrix-like macromolecules associated with the mineral phase of sea urchin skeletal plates and teeth. J. Exp. Zool. 1985, 234, 7–15. [Google Scholar] [CrossRef]
- Kanold, J.M.; Guichard, N.; Immel, F.; Plasseraud, L.; Corneillat, M.; Alcaraz, G.; Brümmer, F.; Marin, F. Spine and test skeletal matrices of the Mediterranean sea urchin Arbacia lixula—A comparative characterization of their sugar signature. FEBS J. 2015, 282, 1891–18905. [Google Scholar] [CrossRef]
- Morocco, A. Sea urchin envenomation. Clin. Toxicol. 2005, 43, 119–120. [Google Scholar] [CrossRef]
- Sciani, J.M.; Zychar, B.C.; Gonçalves, L.R.; Nogueira, T.O.; Giorgi, R.; Pimenta, D.C. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp. Biol. Med. 2011, 236, 277–280. [Google Scholar] [CrossRef]
- Sciani, J.M.; Zychar, B.C.; Gonçalves, L.R.; Giorgi, R.; Nogueira, T.; Pimenta, D.C. Preliminary molecular characterization of a proinflammatory and nociceptive molecule from the Echinometra lucunter spines extracts. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Rosseto, A.L.; Mora, J.M.; Haddad Junior, V. Sea urchin granuloma. Rev. Inst. Med. Trop. São Paulo 2006, 48, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.J.B.; Gomes, P.B.; Souza, J.R.B. Reproductive biology of Echinometra lucunter (Echinodermata: Echinoidea) in a northeast Brazilian sandstone reef. Na. Acad. Bras. Cienc. 2009, 81, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sciani, J.M.; Antoniazzi, M.M.; Neves, A.C.; Pimenta, D.C. Cathepsin B/X is secreted by Echinometra lucunter sea urchin spines, a structure rich in granular cells and toxins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 33. [Google Scholar] [CrossRef]
- Sciani, J.M.; Emerenciano, A.K.; Cunha da Silva, J.R.; Pimenta, D.C. Initial peptidomic profiling of Brazilian sea urchins: Arbacia lixula, Lytechinus variegatus and Echinometra lucunter. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 17. [Google Scholar] [CrossRef]
- da Silva, A.G.; Alves, M.D.M.; da Cunha, A.A.; Caires, G.A.; Kerkis, I.; Vigerelli, H.; Sciani, J.M. Echinometra lucunter molecules reduce Aβ42-induced neurotoxicity in SH-SY5Y neuron-like cells: Effects on disaggregation and oxidative stress. J. Venom. Anim. Toxins Incl. Trop. Dis. 2023, 29, e20230031. [Google Scholar] [CrossRef]
- Ebert, T.A. Growth and Repair of Spines in the Sea Urchin Strongylocentrotus purpuratus (Stimpson). Biol. Bull. 1967, 133, 141–149. [Google Scholar] [CrossRef]
- Donovan, S.K. The internal morphology of primary spines of extant regular echinoids in the tropical western Atlantic: A SEM atlas. Swiss J. Palaeontol. 2018, 137, 363–377. [Google Scholar] [CrossRef]
- Smith, D.S.; Brink, D.; Del Castillo, J. Nerves in the spine of a sea urchin: A neglected division of the echinoderm nervous system. Proc. Natl. Acad. Sci. USA 1985, 82, 1555–1557. [Google Scholar] [CrossRef]
- Hebert, E.; Silvia, M.; Wessel, G.M. Structural and molecular distinctions of primary and secondary spines in the sea urchin Lytechinus variegatus. Sci. Rep. 2024, 14, 28525. [Google Scholar] [CrossRef]
- Borges, J.C.S.; Jensch-Junior, B.E.; Garrido, P.A.G.; Cepellos, M.B.B.; Mangiaterra, D.; Silva, J.R.M.C. Phagocytic Amoebocyte Sub Populations in the Perivisceral Coelom of the Sea Urchin Lytechinus variegatus (Lamarck, 1816). J. Exp. Zool. 2005, 303, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Arizza, V.; Giaramita, F.T.; Parrinello, D.; Cammarata, M.; Parrinello, N. Cell cooperation in coelomocyte cytotoxic activity of Paracentrotus lividus coelomocytes. Comp. Biochem. Physiol. Part A 2007, 147, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.M.C. Immunology in sea urchins. In Sea Urchins: Biology and Ecology; Lawrence, J.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Heatfield, B.M.; Travis, D.F. Ultrastructural studies of regenerating spines of the sea urchin Strongylocentrotus purpuratus I. Cell types without spherules. J. Morphol. 1975, 145, 13–49. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Yang, H.S.; Chen, M.Y. Morphological and ultrastructural characterization of the coelomocytes in Apostichopus japonicus. Aquat. Biol. 2008, 2, 85–92. [Google Scholar] [CrossRef]
- Bachmann, S.; Pohla, H.; Goldschmid, A. Phagocytes in the Axial Complex of the Sea Urchin, Sphaerechinus granularis. Cell Tissue Res. 1980, 213, 109–120. [Google Scholar] [CrossRef]
- Faria, M.T.; Silva, J.R.M.C. Innate immune response in the sea urchin Echinometra lucunter (Echinodermata). J. Invert. Pathol. 2008, 98, 58–62. [Google Scholar] [CrossRef]
- Gross, P.S.; Al-Sharif, W.A.; Clow, L.A.; Smith, L.C. Echinoderm immunity and the evolution of the complement system. Dev. Comp. Immunol. 1999, 23, 429–442. [Google Scholar] [CrossRef]
- Spurrell, M.; Oulhen, N.; Foster, S.; Perillo, M.; Wessel, G. Gene regulatory divergence amongst echinoderms underlies appearance of pigment cells in sea urchin development. Dev. Biol. 2023, 494, 13–25. [Google Scholar] [CrossRef]
- Perillo, M.; Oulhen, N.; Foster, S.; Spurrell, M.; Calestani, C.; Wessel, G. Regulation of dynamic pigment cell states at single-cell resolution. eLife 2020, 9, e60388. [Google Scholar] [CrossRef]
- Del Castillo, J.; Smith, D.S.; Vidal, A.M.; Sierra, C. Catch in the primary spines of the sea urchin Eucidaris tribuloides: A brief review and a new interpretation. Biol. Bull. 1995, 188, 120–127. [Google Scholar] [CrossRef]
- Albeck, S.; Weiner, S.; Addadi, L. Polysaccharides of intracrystalline glycoproteins modulate calcite crystal growth in vitro. Chem. A Eur. J. 1996, 2, 278–284. [Google Scholar] [CrossRef]
- MacKenzie, C.R.; Wilbanks, S.M.; McGrath, K.M. Superimposed effect of kinetics and echinoderm glycoproteins on hierarchical growth of calcium carbonate. J. Mat. Chem. 2004, 14, 1238–1244. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).