Abstract
The present article aims to describe the management of a malpractice dental implant case in a patient with a history of oral bisphosphonates (BF) intake (alendronic acid every 15 days for 20 years) and to perform a narrative review of recently published articles (2019–2023) on the topic. A female patient rehabilitated with 18 nails in the mandible 20 years ago underwent two surgeries; the first one included the explantation of the nails; the second one included the insertion of two implants in the anterior region. At the last follow-up (21 months from the first surgery and 15 months from the second one) no complications nor episodes of bisphosphonate-related osteonecrosis of the jaw (BRONJ) were highlighted. Furthermore, 12 recent articles on the topic were reported and a narrative review was performed. Based on the narrative analysis, the topic related to dental implants in patients with BF intake seems to remain controversial. Most of the findings highlight how the evidence on both the safety of the treatment and the possibility to foresee the risk of onset based on preoperative factors seem to be scarce. The case described in the present article did not report any complications nor episodes of BRONJ. However, evidence from a single case report is scarce and more clinical trials are required to deepen the knowledge on the topic.
1. Introduction
Implant therapy has revolutionized the field of dentistry, offering patients a reliable and aesthetically pleasing solution for replacing missing teeth [1,2]. With its high success rates and long-term benefits [3,4], dental implant treatment has become increasingly popular [5] and research is continuing to focus on investigating novel techniques and materials [6,7,8]. However, like any medical procedure, implant therapy is not without risks [9,10,11]. In cases where errors, negligence, or breaches of professional standards occur, malpractice in implant therapy can have significant consequences for both patients and dental professionals [12].
Malpractice refers to any act or omission by a healthcare provider that deviates from accepted standards of care and results in harm to the patient [13]. In the context of implant therapy, malpractice can encompass a range of issues, including surgical errors, improper treatment planning, material failures, inadequate informed consent, and postoperative complications [14,15,16,17]. Such instances of malpractice can lead to patient dissatisfaction, physical pain, emotional distress, functional impairment, and even the loss of natural teeth or implants [18,19]. Furthermore, in the case of extensive rehabilitation, the result of previously made damages can lead to complex situations to manage.
Oral bisphosphonates (BF), a class of medications known for their ability to inhibit bone resorption, have been associated with a rare but serious complication called bisphosphonate-related osteonecrosis of the jaw (BRONJ) following oral surgery treatments [20,21]. Depending on the BF assumed, administration type, duration of treatment and presence of comorbidities, several levels of risk for BRONJ development can be outlined [22]. This condition is characterized by jawbone necrosis, often leading to pain, bone exposure, and infection. Furthermore, after ablative surgeries, those patients are often ineligible for mandibular reconstruction using autologous bone harvest, leading to highly complex cases to rehabilitate [23]. Recently, customized Computer Assisted Design/Computer Assisted Manufacturing plates were proposed for mandibular reconstruction in patients who presented with BRONJ [23,24] as an alternative to bone harvest, representing an interesting and feasible technique.
However, given those potential risks, clinicians have been cautious about performing dental implant therapy in patients who have undergone BF treatment [25,26]. Currently, the topic of performing dental implants in patients with oral BF intake is controversial in the field of dentistry. Some studies suggest that the risk of developing BRONJ may be higher in patients who have undergone oral BF treatment [26,27]. However, more recently other studies argue that the risk is relatively low and that dental implant therapy can still be performed successfully with appropriate precautions and careful case selection [28,29,30]. Therefore, final evidence is currently absent and the topic remains actual. Indeed, the majority of the studies agree that more studies on the topic are required to deepen the knowledge on the topic.
The aims of the present article are: (1) to describe the management of a malpractice dental implant case in a patient with a history of oral BF assumption; (2) to perform a narrative review on the last evidence of safety performing implants therapy in patients with a history of oral BF assumption in order to provide readers with the most recent evidence over the last 5 years.
2. Materials and Methods
2.1. Case Report
The present case report was described following the CARE Checklist (https://www.care-statement.org/checklist, accessed on 16 July 2023).
A female patient, 66 years old, non-smoker was referred from a private clinic to the Prosthodontic and Implant Department, C.I.R. Dental School, University of Turin, in July 2021 for the evaluation of the lower jaw.
Her chief complaint was to solve the constant pain and swelling that she had been experiencing in the lower jaw.
Her anamnesis reported osteoporosis and her pharmacologic anamnesis reported a history of oral intake of alendronic acid every 15 days for 20 years suspended 12 months ago and changed with Vitamin D3.
At the clinical examination, the patient presented a removable upper prostheses and a fixed prosthesis in the lower jaw (Figure 1).
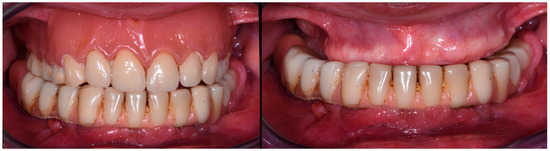
Figure 1.
Images showing the initial clinical condition (July 2021).
The patient brought an orthopantomography (OPT) X-ray previously acquired 3 years before (2018) (Figure 2A).
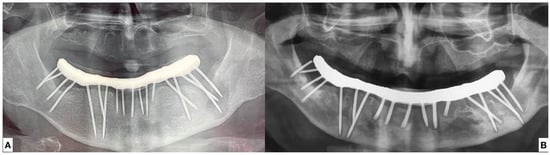
Figure 2.
(A) Ortopantomography acquired in 2018. (B) Ortopantomography acquired in July 2021.
The patient said that the lower treatment was carried out 20 years ago. No verbal information nor any written details about the material, brand or nature of the inserted nails were released to the patient. Since then, she reported to have experienced complete paresthesia of the lower left lip and she affirmed that she was told by the dentist who did the treatment that it was a natural and correct consequence of the dental implant treatment. In the last years, she reported continuous episodes of abscess in different areas of the lower jaw, constant pain, and swelling. Furthermore, she had been constantly forced to ingest painkillers to manage the pain and often antibiotics. At the clinical examination, the lower prostheses seemed firm without any movement. A neoformation in the area of the first left lower molar was noticed (Figure 3) and described as follows: 5 × 3 mm red mandibular gingival exophytic neoformation in contiguous relationship with implant-prosthetic elements III posterior quadrant. Pain symptoms reported by the patient.
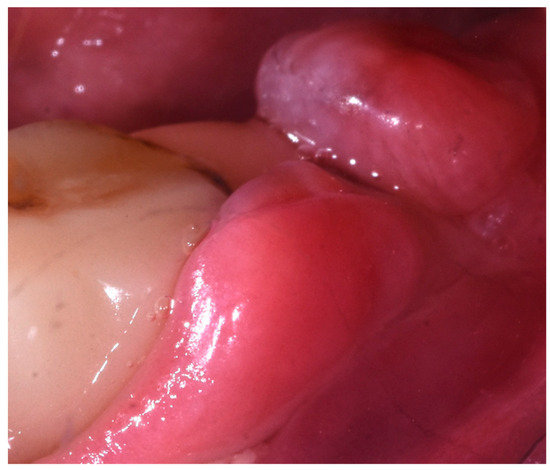
Figure 3.
Clinical image of intraoral neoformation.
A new OPT X-ray was acquired (Figure 2B).
After the clinical and radiographic examination, the diagnosis was the following:
- Irreversible and permanent damage of the left inferior alveolar nerve.
- The widespread and constant presence of infections of various sizes inside the mandible, presumably attributable to the endo-osseous presence of nails of unknown nature.
- Left lower neoformation in the area of the first left lower molar
Assuming the risk factors related to the history of BF intake, the clinical situation required intervention.
The treatment plan was the following:
- Explantation of all the nails
- Excision of the neoformation near the first lower left molar and subsequent histological analysis
- Platelet-Rich Growth Factors (PRGF) were inserted in the residual empty area left from the explantation of the nails and of the related infections.
- Re- evaluation of the healing after 1 week, 1, 3, and 6 months.
- Rehabilitation with a removable lower denture
- Re-evaluation of the denture stability after 3 months and evaluation of performing mandibular implant-supported overdenture (OVD) prostheses.
The patient was informed about the risks of the surgery: (1) risk of episodes of BRONJ due to previous oral BF intake; (2) In case of any osseointegrated nails, the possible necessity to drill the bone around the nails and leverage with the consequent risk of mandibular fracture due to the already present weakening caused by the large infections.
The patient accepted to undergo the surgery and signed an extensive and detailed informed consent.
2.1.1. Surgery Appointment (September 2021)
A maxillofacial surgeon (S.R.) performed the surgery.
0.2% chlorhexidine digluconate (Corsodyl, GlaxoSmithKline, Verona, Italy) mouth rinse twice a day was prescribed starting 1 week prior to the surgery to lower the bacteria load in the mouth. Preoperative antibiotic coverage with Amoxicillin 875 mg + Clavulanic acid 125 mg every 12 h for 6 days was prescribed starting 1 day prior to the surgery.
The patient underwent blood collection prior to the surgery to prepare for the PRGF.
The surgery was performed under local anesthesia (4% articaine with 1:10,000 adrenaline; Alfacaina SP; Dentsply Italy, Rome, Italy).
Based on the different inclinations of the nails, the prosthesis was separated between the first premolar and the canine at the right aspect of the prosthesis. After the procedure, the right side of the prostheses was easily extracted with the corresponding nails using the crown and bridge extractor (bridge and crown extractor, Bader, Porto do Molle Rúa Madanela, 36350 Nigrán, Spain) (Figure 4A). Due to some residual resistance in the left nails, a second division with burs was made between the molar and second premolar. The residual prosthesis was then removed and all the 18 pins were extracted (Figure 4B).
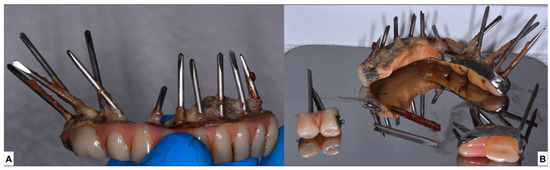
Figure 4.
(A) Left side of the extracted prostheses (B) All the extracted nails with the prostheses.
None of the nails was osseointegrated in the bone and no need for excision of the soft tissue nor drilling of the bone was required.
The neoformation was excised and immediately placed in 8% w/v formalin (20% v/v). It was then sent to the histological examination.
PRGF was then inserted in the area with a major lesion, and sutures were made where needed (Figure 5).
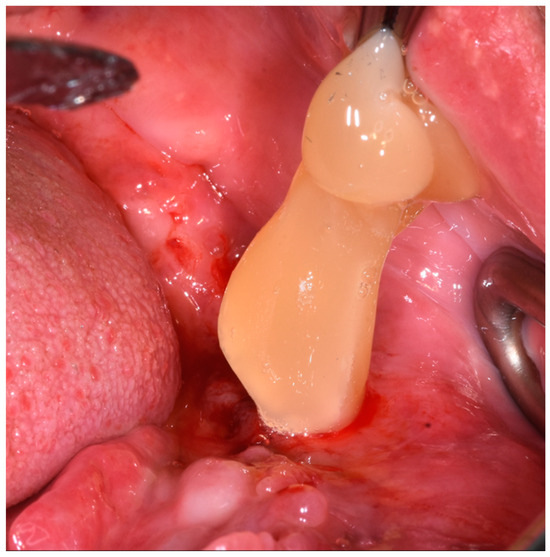
Figure 5.
PRGF insertion into the wound.
The patient was then given postoperative instructions, including the continuation of 0.2% chlorhexidine digluconate (Corsodyl, GlaxoSmithKline, Verona, Italy) mouth rinse twice a day for two weeks starting the day after surgery, painkillers if needed. Due to the absence of teeth/prostheses in the lower jaw, the patient was forced to eat liquid/soft food starting from the day after the surgery.
After one week the patient returned for the suture removal. No adverse effects were noticed and the residual wound from the nails was seen to be closed (Figure 6).
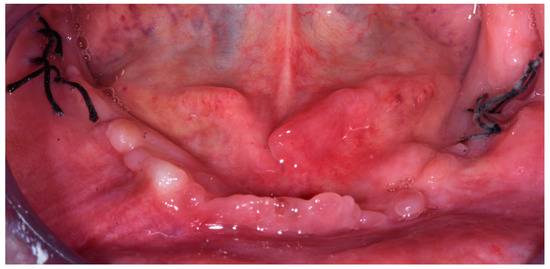
Figure 6.
Image showing the healing one week after the surgery (September 2021).
2.1.2. Histological Analysis
Two weeks after surgery, the histological report of the neoformation in the lower left mandibular area was received with the following macroscopic description: two fragments, one whitish globose 0.7 × 0.6 × 0.3 cm and the other whitish 0.7 cm laminar, both collated when cut and the following diagnosis: fragments of fibromatous and plasma cell epulides.
2.1.3. Follow Ups and Implants Insertion
One month after the surgery (October 2021) the patient returned to the clinic and was subsequently rehabilitated with complete mandibular full removable dentures made by resin [31]. Due to the discontinuous presence of bone and soft tissue support in the residual mandibular jaw (Figure 7), the denture had very low retention and stability. Therefore, the patient wore the mandibular denture with reduced comfort and only used adhesive denture pastes to increase stability.
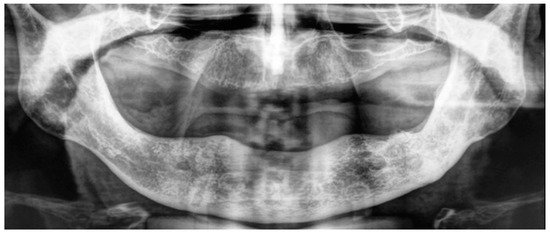
Figure 7.
Orthopantomography acquired six months from the initial surgery (March 2022).
Follow-up visits were then planned after 2 weeks, one, three and five months.
At the last follow-up (6 months from the surgery) the patient stated that it was impossible for her to wear the prostheses due to the constant instability.
New OPT (Figure 7) and Cone Beam Computed Tomography (Figure 8) were acquired to evaluate bone healing 6 months after the initial surgery.
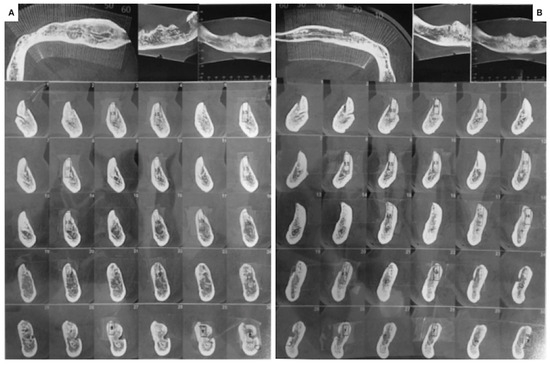
Figure 8.
Cone Beam Computed Tomography acquired six months from the initial surgery (March 2022). (A) Left side; (B) right side.
After the radiological examination, the patient was found eligible for a mandibular implant-supported OVD. The patient was extensively informed about the risk of BJORN due to the previous BF intake. However, due to the unfeasibility of wearing the complete mandibular denture, the patient clearly expressed her willingness to undergo the surgery.
The treatment planning was the following:
- surgical insertion of two anterior implants in the region of the mandibular canines
- rehabilitation with implant-supported OVD adapting the current complete removable denture, 3 months after implant surgery.
Preoperative instructions were the same as the first surgery, including a 0.2% chlorhexidine digluconate (Corsodyl, GlaxoSmithKline, Verona, Italy) mouth rinse twice a day starting 1 week prior to the surgery that was prescribed to lower the bacteria load in the mouth and preoperative antibiotic coverage with Amoxicillin 875 mg + Clavulanic acid 125 mg every 12 h for 6 days was prescribed starting 1 day prior to the surgery [32].
Two implants (Branemark System Mk III TiU RP 4 × 10 mm, Nobel Biocare, Viale Monza, 347, 20126 Milano, Italy) were inserted in February 2023 (Figure 9) following a two-stage technique.
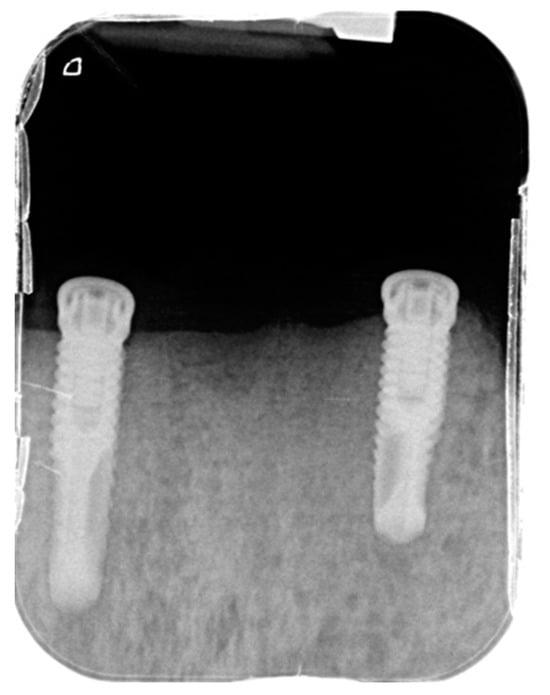
Figure 9.
Periapical X-ray showing the two anterior implants after the surgery.
Three months after surgery, the implants were uncovered and connected to the locator (Locator Abutment for 4.0 Branemark, Nobel Biocare, Viale Monza, 347, 20126 Milano, Italy) (Figure 10A). The prostheses were modified with the attachment (Figure 10B) and were delivered to the patient (June 2022) (Figure 10C).
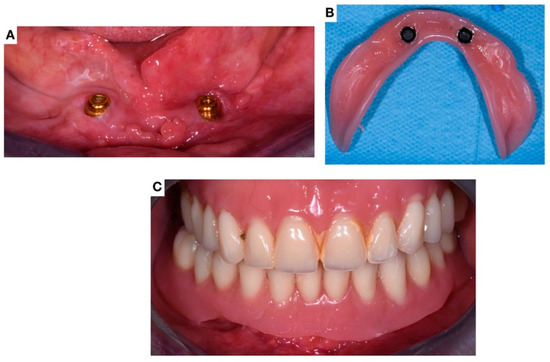
Figure 10.
Images showing the delivery of the implant-supported prostheses (June 2022). (A) Clinical image of the locators connected to the implants; (B) Image of the modified prostheses with the attachment; (C) Clinical image after the prostheses delivering.
The patient was then followed after 1, 3, 6 and 12 months (Figure 11).
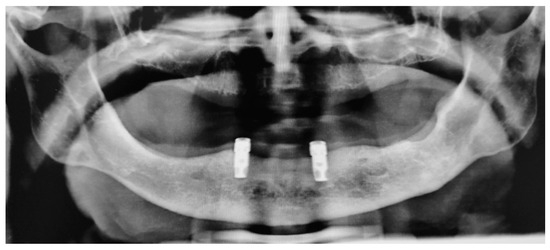
Figure 11.
OPT X-ray acquired at the last follow-up in June 2023 (12 months after the implant-supported OVD delivery, 15 months from the second surgery and 21 months from the first surgery).
2.2. Narrative Review
To investigate the latest evidence on dental implant treatment in patients with a history of BF intake, a Pubmed, Scopus, and Web of Science search was performed. The review was performed following the scale for the quality assessment of narrative review articles (SANRA) [33]. The following search string was adopted (Pubmed):
(“Dental Implantation”[Mesh] OR “Dental Implants”[Mesh] OR “Dental Prosthesis, Implant-Supported”[Mesh]) AND (“Diphosphonates”[Mesh] OR “Bisphosphonates”[Mesh]) AND (“Osteonecrosis of the Jaw”[Mesh] OR “Osteonecrosis”[Mesh] OR “Jaw Diseases”[Mesh] OR “Bisphosphonate-Associated Osteonecrosis of the Jaw”[Mesh] OR “BRONJ”[Mesh] OR “ONJ”[Mesh] OR “MRONJ”[Mesh])
The inclusion criteria were:
- articles published in the last 5 years (2019–2023)
- in vivo studies
The exclusion criteria were:
- articles written in other languages than English;
- articles with no abstract or when full text is not available;
- articles on animals;
- articles that investigated the effects of BF on dental implants already present in the mouth prior to the assumption.
Articles were initially screened by analyzing their abstract and then the full texts were analyzed for articles that matched the inclusion criteria.
3. Results
3.1. Case Report
At the last follow-up (June 2023, 12 months after the implant-supported OVD delivery, 15 months from the second surgery and 21 months from the first surgery) the clinical situation appeared to be stable. The two dental implants placed 15 months before did not show any sign of probing depth or mucositis/perimplantitis. No sign of BRONJ nor complications were highlighted at any follow-ups. The patient referred to be very satisfied with the final treatment thanks to the improved retention and stability of the prostheses.
3.2. Search Results
In total, 20 articles were found from the initial research. Of these, eight articles were excluded after the initial screening. One article was an animal study [34]; one was an article written in Chinese [35]; six articles focused on interventions that differ from the implant insertion [36,37,38,39,40,41]. Therefore, 12 articles were selected for the narrative review (Table 1)

Table 1.
Articles that met the inclusion/exclusion criteria and were analyzed for the narrative review.
4. Discussion and Narrative Review
The present article aims to report and describe the management of a singular malpractice case of dental implant therapy in a patient with a history of oral BF intake and to provide a narrative review of the latest evidence of safely performing implant therapy in patients with this type of pharmacological history. In the present case, a female patient was rehabilitated 20 years ago with 18 nails inserted in the mandibular jaw. It was described as a malpractice case following the definition provided by the American Dental Association [10], referring to any act or omission by a healthcare provider that deviates from accepted standards of care and results in harm to the patient. Indeed, the patient presented with irreversible damage to the left alveolar nerve that resulted in permanent paresthesia of the left part of the tongue and lower lip, besides recurrent episodes of abscess and constant swelling. Furthermore, the present case was of particular interest in light of the 20-year history of oral BF intake for the patient. Patients with a history of oral BF intake are at an increased risk for complications after oral surgery procedures [53]. Oral BF can affect bone metabolism and impair the normal healing process [54]. This can lead to delayed or impaired wound healing, increased susceptibility to infections, and a higher likelihood of developing BRONJ [21]. The presence of BF in the bone can interfere with the remodeling and repair mechanisms necessary for successful surgical outcomes [55]. Due to the clinical conditions, the patient underwent two different surgeries; the first one consisted of the explantation of all the 18 nails; the second one consisted of the insertion of two anterior implants to rehabilitate the edentulous lower jaw with an implant-supported OVD. Due to the possible risk of BRONJ, the first option to prosthetically rehabilitate the edentulous mandibular arch was a traditional removable complete denture. However, due to the very poor retention and stability, and following the willingness of the patient, alternative rehabilitation treatment was required. In this particular case, good closure of the previous extensive wounds of both the soft tissue and bone was observed without any episodes of BRONJ after the first surgery. Therefore, based on the positive outcome of the initial surgery, the treatment plan included a second surgery with the insertion of two implants. Regarding the surgery, PRGF was adopted to decrease the risk of BRONJ and over-infections. The use of PRGF during oral surgery procedures in patients with a history of oral BF intake may offer potential benefits. PRGF is a concentrated solution of autologous platelets obtained from the patient’s own blood, rich in growth factors that promote tissue healing and regeneration [56,57]. Applying PRGF locally to the surgical site may help enhance wound healing, improve vascularization, and stimulate bone formation, which are crucial factors for successful outcomes in patients with compromised bone healing due to BF use [58,59]. Additionally, PRGF has shown promise in reducing the risk of postoperative complications, such as infection and BRONJ, by boosting the immune response and supporting tissue repair processes [44,60]. While further research is needed to establish the efficacy and optimal protocols for PRGF application in this specific patient population, its use holds potential as an adjunctive therapy to enhance healing and minimize complications in oral surgery procedures for patients with a history of oral BF intake.
At the last follow-up (21 months from the first surgery and 15 months from the second one), the patient appeared successfully rehabilitated and no signs nor episodes of BRONJ were highlighted at any time point. Therefore, both surgeries were considered successful.
However, in regard to implant therapy in patients with a history of oral BF intake, the literature is controversial and clinicians continue to ask whether performing implant therapy in patients with this type of drug intake could be considered safe. The results obtained in the present case are limited to the inherent limitations of a single case report. However, case reports are important to collect both possible factors and clinical procedures that may help further research and clinical treatments. This is also more important in regard to the surgical treatment of patients who underwent BF, as randomized control trials are currently scarce in the literature due to the risk of inducing BRONJ in in-vivo studies as well as ethical concerns. In addition to the use of PRGF to decrease the risk of BRONJ, the absence of complications in the present case may be related to the stoppage of BF intake treatment 12 months prior to the first surgery and 18 months prior to the second one. This is in agreement with the study of Ruocco-Vertucci et al. [45] who suspended the drug intake 12 months prior to the surgery and observed no post-surgical complications. However, different articles highlighted how due to the long BF recycling process, BF may survive in the organism for up to 10 years after treatment discontinuation [61,62]. To overcome this possible risk, bone metabolism tests were proposed in order to measure the carboxy terminal telopeptide of collagen type 1 (CTX-I) and amino terminal propeptide of procollagen type 1 (P1NP) markers and to evaluate the risk of BRONJ according to their level [21,63]. The first represents a resorption marker while the second represents a bone formation marker. However, their use as a certain predictor of the development risk of BRONJ is currently controversial [63].
From the analysis of the articles published in the last 5 years (2019–2023), 12 articles were analyzed. Following the results of recent systematic reviews, the fact that patients with a history of BF intakes could be at risk of BRONJ onset seems clear. Mendes et al. [27] published an overview of systematic reviews. The authors’ findings showed how the intake of BF doesn’t present any correlation with the final implant therapy outcome (in terms of marginal bone loss and survival rate). However, the authors highlighted how patients with a history of BF intake are at a greater risk of manifesting BRONJ and that there are no validated methods to analyze the possible risk of BRONJ onset prior to the surgery. In agreement, the systematic review by Granate-Marques et al. [43] showed how the risk of BRONJ in these types of patients who seek to undergo dental implant therapy cannot be underestimated and clinicians should evaluate case by case considering alternative treatment options. However, in contrast to Mendes et al. [27] who found a greater risk of BRONJ onset, the authors’ findings showed a low risk of BRONJ onset in the case of benign bone disease. Furthermore, the authors also highlighted how evidence on the topic is currently scarce and more studies are required. In the third systematic review, Sher et al. [49] agreed with Mendes et al. [27] that implant outcomes are not influenced by the assumption of BF. Regarding the safety of performing dental implant therapy in this type of patient, assuming that the risk of BRONJ is present, the authors concluded that there is not enough evidence to state whether placing dental implants could be considered safe or not. Therefore, from the analysis of the recent systematic reviews, it seems clear that the risk of BRONJ is always present and the decision to perform dental implant therapy in this type of patient should be decided on a case-by-case basis by discussing the possible risks with the patient. In the last five years, two observational studies have been published. Pichardo et al. [47] estimated the onset of BRONJ following four patients who had a history of BF prior to or at the moment of the implant insertion and developed BRONJ. The authors’ findings highlighted an increased risk of BRONJ onset in patients with dental implants and an average onset time of 6 months after implant placement.
Ryu et al. [50] performed a national cohort study analyzing the safety of performing implant therapy in patients with osteoporosis. Among the variables considered, the authors also analyzed the assumption or not of BF. The authors’ findings showed how BP users had an estimated four times higher ratio of ONJ risks than the non-users. Furthermore, the authors also highlighted a correlation between complications after teeth extraction and an increased risk of BRONJ after dental implant therapy. Therefore, the authors suggested that patients assuming BF who experienced complications during extractions should be considered ineligible for the implant treatment.
In contrast with the above-mentioned article, a case series by Otto et al. [51] who followed 16 patients under BF therapy for a total implant number of insertions of 39 did not show any episode of BRONJ. The authors concluded that as long as the correct procedures are followed, implant therapy seems to be safe.
Lastly, five case reports and one case report with review were published. Of these, three case reports [42,44,46] reported cases where the dental implant treatment led to the onset of BRONJ. The other three described cases where the implant therapy was successful and tried to highlight possible strategies for it. Ruocco-Vertucci et al. [45] reported an interesting case report where a patient who had been under therapy with BF for 7-year follow-up underwent two teeth extractions and five implant insertions. The authors suspended the BF therapy one year before the treatments. Furthermore, they analyzed the CTX-I and P1NP serum levels prior to starting the surgeries, finding a level of 150 pg/mL and 27.3 μg/L, respectively. Lee et al. [52] reported a case of new implant insertion in a previously BRONJ-affected site reporting a 7-year follow-up with treatment success. Ferreira et al. [48] reported a successful dental implant treatment of a patient with BF intake who underwent two implant insertions in the anterior area. The protocol adopted by the authors included the suspension of BF intake starting 3 months prior to the surgery until 3 months after surgery. Furthermore, the authors analyzed the level of CTX-I with a result of 304 pg/mL.
5. Conclusions
In conclusion, based on the narrative analysis of recently published articles, the topic related to dental implants in patients with BF intake seems to remain controversial. Most of the findings highlight how the evidence on both the safety of the treatment and the possibility to foresee the risk of onset based on preoperative factors seem to be scarce. Different case reports try to describe and report different clinical situations and outcomes to encourage further research. The present article described a patient who underwent two different surgeries and, at the last follow-up, presented with no complication or episode of BRONJ. However, the evidence from a single case report is scarce and more clinical trials are required to deepen the knowledge on the topic.
Author Contributions
Conceptualization, M.C. (Massimo Carossa); methodology, N.S., M.A., F.G., S.C. and M.C. (Massimo Corsalini); formal analysis, N.S., M.A., F.G., S.C. and M.C. (Massimo Corsalini); investigation, M.C. (Massimo Carossa), S.R. and F.P.; data curation, M.C. (Massimo Carossa) and F.P.; writing—original draft preparation, M.C. (Massimo Carossa); writing—review and editing, M.C. (Massimo Carossa), F.G. and S.C.; supervision, F.P.; project administration, M.C. (Massimo Carossa) and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The paper is a case report in which the best possible treatment was delivered, and no prospective research was carried out. In accordance with the following European and international guidelines, no ethical concern was present. Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Med Etika Bioet. 2002;9(1-2):12–9. European Commission-European Medicines Agency. Report on the conference on the Operation of the Clinical Trials Di-rective (Directive 2001/20/EC) and Perspectives for the Future, Conference held on 3 October 2007 at the EMEA, London (Report issued on November 30, 2007; Doc. ref.: EMEA/565466/2007).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Institutional Review Board Statement. This change does not affect the scientific content of the article.
References
- Pera, F.; Pesce, P.; Menini, M.; Fanelli, F.; Kim, B.C.; Zhurakivska, K.; Mayer, Y.; Isola, G.; Cianciotta, G.; Crupi, A.; et al. Immediate loading full-arch rehabilitation using transmucosal tissue-level implants with different variables associated: A one-year observational study. Minerva Dent. Oral. Sci. 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Pesce, P.; Delucchi, F.; Ambrogio, G.; Canepa, C.; Carossa, M.; Pera, F. One-stage versus two-stage technique using two splinted extra-short implants: A multicentric split-mouth study with a one-year follow-up. Clin. Implant. Dent. Relat. Res. 2022, 24, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Pesce, P.; Tronchi, M.; Fiorellini, J.; Amari, Y.; Penarrocha, D. Marginal soft tissue stability around conical abutments inserted with the one abutment-one time protocol after 5 years of prosthetic loading. Clin. Implant. Dent. Relat. Res. 2018, 20, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Omori, Y.; Amari, Y.; Iannello, G.; Pesce, P. Five-year cohort prospective study on single implants in the esthetic area restored using one-abutment/one-time prosthetic approach. Clin. Implant. Dent. Relat. Res. 2018, 20, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ortensi, L.; Ortensi, M.; Minghelli, A.; Grande, F. Implant-Supported Prosthetic Therapy of an Edentulous Patient: Clinical and Technical Aspects. Prosthesis 2020, 2, 140–152. [Google Scholar] [CrossRef]
- Pera, F.; Menini, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Asero, S.; Marchetti, C.; Canepa, C.; Merlini, L.; Pesce, P.; et al. Can Abutment with Novel Superlattice CrN/NbN Coatings Influence Peri-Implant Tissue Health and Implant Survival Rate Compared to Machined Abutment? 6-Month Results from a Multi-Center Split-Mouth Randomized Control Trial. Materials 2022, 16, 246. [Google Scholar] [CrossRef]
- Pozzan, M.C.; Grande, F.; Mochi Zamperoli, E.; Tesini, F.; Carossa, M.; Catapano, S. Assessment of Preload Loss after Cyclic Loading in the OT Bridge System in an "All-on-Four" Rehabilitation Model in the Absence of One and Two Prosthesis Screws. Materials 2022, 15, 1582. [Google Scholar] [CrossRef]
- Grande, F.; Pozzan, M.C.; Marconato, R.; Mollica, F.; Catapano, S. Evaluation of Load Distribution in a Mandibular Model with Four Implants Depending on the Number of Prosthetic Screws Used for OT-Bridge System: A Finite Element Analysis (FEA). Materials 2022, 15, 7963. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur. J. Oral. Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Pera, P.; Menini, M.; Bevilacqua, M.; Pesce, P.; Pera, F.; Signori, A.; Tealdo, T. Factors affecting the outcome in the immediate loading rehabilitation of the maxilla: A 6-year prospective study. Int. J. Periodontics Restor. Dent. 2014, 34, 657–665. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Sanchez-Pérez, A.; Moya-Villaescusa, M.J.; Rodríguez-Lozano, A.; Sáez-Yuguero, M.R. Neuropathic orofacial pain after dental implant placement: Review of the literature and case report. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2010, 109, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Guabello, G.; Zuffetti, F.; Ravidà, A.; Deflorian, M.; Carta, G.; Saleh, M.H.A.; Serroni, M.; Pommer, B.; Watzek, G.; Francetti, L.; et al. Avoiding implant-related complications in medically compromised patients with or without unhealthy lifestyle/Elevated oxidative stress. Periodontol 2000 2023, 92, 329–349. [Google Scholar] [CrossRef] [PubMed]
- American Dental Association. Definition of Dental Malpractice. Available online: https://www.ada.org/en/member-center/oral-health-topics/dental-malpractice (accessed on 29 June 2023).
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K.; Kan, J.Y. Clinical complications with implants and implant prostheses. J. Prosthet. Dent. 2003, 90, 121–132. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral. Maxillofac. Implants. 1986, 1, 11–25. [Google Scholar]
- Carlsson, G.E.; Bergman, B.; Hedegård, B. Changes in contour of the maxillary alveolar process under immediate dentures. A longitudinal clinical and x-ray cephalometric study covering 5 years. Acta Odontol. Scand. 1967, 25, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Pol, R.; Camisassa, D.; Bezzi, M.; Savoldi, L.; Punzi, F.; Carossa, M.; Ruggiero, T. Evaluation of the correlation between oral infections and systemic complications in kidney transplant patients: A retrospective pilot study. BMC Oral. Health 2022, 22, 530. [Google Scholar] [CrossRef]
- Green, M.A.; Resnick, C.M.; Mercuri, L.G. Characteristics of Medical Malpractice Claims Involving Temporomandibular Joint Surgery in the United States. J. Oral. Maxillofac. Surg. 2022, 80, 1153–1157. [Google Scholar] [CrossRef]
- Diakonoff, H.; Moreau, N. Inferior alveolar nerve injury following dental implant placement: A medicolegal analysis of French liability lawsuits. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, 158–162. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral. Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw-2009 update. J. Oral. Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Ricotta, F.; Crimi, S.; Mineo, R.; Michelon, F.; Tarsitano, A.; Marchetti, C.; Bianchi, A. Mandibular Reconstruction with Bridging Customized Plate after Ablative Surgery for ONJ: A Multi-Centric Case Series. Appl. Sci. 2021, 11, 11069. [Google Scholar] [CrossRef]
- Bolognesi, F.; Tarsitano, A.; Cicciù, M.; Marchetti, C.; Bianchi, A.; Crimi, S. Surgical Management of Primary Chronic Osteomyelitis of the Jaws: The Use of Computer-Aided-Design/Computer-Aided Manufacturing Technology for Segmental Mandibular Resection. J. Craniofac Surg. 2020, 31, e156–e161. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L. Bisphosphonate-related osteonecrosis of the jaw: An overview. Ann. N. Y Acad. Sci. 2011, 1218, 38–46. [Google Scholar] [CrossRef]
- Wang, H.L.; Weber, D.; McCauley, L.K. Effect of long-term oral bisphosphonates on implant wound healing: Literature review and a case report. J. Periodontol. 2007, 78, 584–594. [Google Scholar] [CrossRef]
- Mendes, V.; Dos Santos, G.O.; Calasans-Maia, M.D.; Granjeiro, J.M.; Moraschini, V. Impact of bisphosphonate therapy on dental implant outcomes: An overview of systematic review evidence. Int. J. Oral. Maxillofac. Surg. 2019, 48, 373–381. [Google Scholar] [CrossRef]
- Lazarovici, T.S.; Yahalom, R.; Taicher, S.; Elad, S.; Hardan, I.; Yarom, N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J. Oral. Maxillofac. Surg. 2010, 68, 790–796. [Google Scholar] [CrossRef]
- Tanna, N.; Steel, C.; Stagnell, S.; Bailey, E. Awareness of medication related osteonecrosis of the jaws (MRONJ) amongst general dental practitioners. Br. Dent. J. 2017, 222, 121–125. [Google Scholar] [CrossRef]
- Yamashita, J.; McCauley, L.K. Antiresorptives and osteonecrosis of the jaw. J. Evid. Based Dent. Pract. 2012, 12, 233–247. [Google Scholar] [CrossRef]
- Grande, F.; Tesini, F.; Pozzan, M.C.; Zamperoli, E.M.; Carossa, M.; Catapano, S. Comparison of the Accuracy between Denture Bases Produced by Subtractive and Additive Manufacturing Methods: A Pilot Study. Prosthesis 2022, 4, 151–159. [Google Scholar] [CrossRef]
- Canullo, L.; Troiano, G.; Sbricoli, L.; Guazzo, R.; Laino, L.; Caiazzo, A.; Pesce, P. The Use of Antibiotics in Implant Therapy: A Systematic Review and Meta-Analysis with Trial Sequential Analysis on Early Implant Failure. Int. J. Oral. Maxillofac. Implants 2020, 35, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Davison, M.R.; Lyardet, L.; Preliasco, M.; Yaful, G.; Torres, P.; Bonanno, M.S.; Pellegrini, G.G.; Zeni, S.N. Aminobisphosphonate-treated ewes as a model of osteonecrosis of the jaw and of dental implant failure. J. Periodontol. 2020, 91, 628–637. [Google Scholar] [CrossRef]
- Wu, P.F.; Li, Y.; Lei, Z.G.; Chen, L.L. Bisphosphonate-related osteonecrosis of the jaw caused by implant: A case report. Hua Xi Kou Qiang Yi Xue Za Zhi 2020, 38, 460–463. (In Chinese) [Google Scholar] [CrossRef]
- Yamamoto, S.; Maeda, K.; Kouchi, I.; Hirai, Y.; Taniike, N.; Yamashita, D.; Imai, Y.; Takenobu, T. Development of Antiresorptive Agent-Related Osteonecrosis of the Jaw After Dental Implant Removal: A Case Report. J. Oral. Implantol. 2018, 44, 359–364. [Google Scholar] [CrossRef]
- Jung, R.E.; Al-Nawas, B.; Araujo, M.; Avila-Ortiz, G.; Barter, S.; Brodala, N.; Chappuis, V.; Chen, B.; De Souza, A.; Almeida, R.F.; et al. Group 1 ITI Consensus Report: The influence of implant length and design and medications on clinical and patient-reported outcomes. Clin. Oral. Implants Res. 2018, 16, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Campanella, V.; Carosi, P.; Casella, S.; Pinto, A.; Di Girolamo, M. Clinical fitting of a cast metal post and core obtained by means of an intraoral optical scanning (IOS) and digital workflow. J. Biol. Regul. Homeost. Agents 2019, 33, 43–50. [Google Scholar] [PubMed]
- Park, W.B.; Herr, Y.; Kwon, Y.D.; Shin, S.I.; Lim, H.C. Advanced Peri-Implantitis and Implant Removal as Risk Factors for Osteonecrosis of the Jaw in Patients on Oral Bisphosphonate Therapy. J. Oral. Implantol. 2021, 47, 420–426. [Google Scholar] [CrossRef]
- Seki, K.; Namaki, S.; Kamimoto, A.; Hagiwara, Y. Medication-Related Osteonecrosis of the Jaw Subsequent to Peri-Implantitis: A Case Report and Literature Review. J. Oral. Implantol. 2021, 47, 502–510. [Google Scholar] [CrossRef]
- Ueda, N.; Imada, M.; Kato, Y.; Okuda, N.; Nakaue, K.; Horita, S.; Kinoshita, S.; Kasahara, K.; Kirita, T. Bevacizumab-Associated Implant Presence-Triggered Osteonecrosis: A Case Report and Literature Review. J. Oral. Implantol. 2022, 48, 325–331. [Google Scholar] [CrossRef]
- Storelli, S.; Palandrani, G.; Dondi, C.; Tagliatesta, L.; Rossi, A. Severe Case of Osteonecrosis Following Implant Placement in a Patient in Therapy With Bisphosphonates: A Case Report. J. Oral. Implantol. 2019, 45, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Granate-Marques, A.; Polis-Yanes, C.; Seminario-Amez, M.; Jané-Salas, E.; López-López, J. Medication-related osteonecrosis of the jaw associated with implant and regenerative treatments: Systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2019, 24, e195–e203. [Google Scholar] [CrossRef]
- Gil, I.G.; Ponte, B.M.; Mateo, S.T.; García, J.J. Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw With Plasma Rich in Growth Factors After Dental Implant Surgery: A Case Report. J. Oral. Implantol. 2019, 45, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ruocco-Vetucci, V.; de Souza Faloni, A.P.; Faeda, R.S. Follow-Up of an Implant-Supported Rehabilitation After Long-Term Use of Alendronate. J. Craniofac Surg. 2019, 30, e793–e796. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.Y.; Hilal, G. Osteonecrosis and spontaneous exfoliation of dental implants associated with oral bisphosphonate therapy: A case report. Aust. Dent. J. 2020, 65, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.E.C.; van der Hee, J.G.; Fiocco, M.; Appelman-Dijkstra, N.M.; van Merkesteyn, J.P.R. Dental implants as risk factors for patients with medication-related osteonecrosis of the jaws (MRONJ). Br. J. Oral. Maxillofac. Surg. 2020, 58, 771–776. [Google Scholar] [CrossRef]
- Ferreira, G.Z.; Bachesk, A.B.; Bachesk, A.B.; Farah, G.J.; Filho, L.I.; Dos Santos Silva, R.; Poluha, R.L.; Danieletto-Zanna, C.F.; Gonçales, E.S. Oral Rehabilitation With Dental Implants and the Importance of a Preventive Evaluation for Osteonecrosis of the Jaws Associated With Medications. J. Oral. Implantol. 2020, 46, 431–437. [Google Scholar] [CrossRef]
- Sher, J.; Kirkham-Ali, K.; Luo, J.D.; Miller, C.; Sharma, D. Dental Implant Placement in Patients With a History of Medications Related to Osteonecrosis of the Jaws: A Systematic Review. J. Oral. Implantol. 2021, 47, 249–268. [Google Scholar] [CrossRef]
- Ryu, J.I.; Kim, H.Y.; Kwon, Y.D. Is implant surgery a risk factor for osteonecrosis of the jaw in older adult patients with osteoporosis? A national cohort propensity score-matched study. Clin. Oral. Implants Res. 2021, 32, 437–447. [Google Scholar] [CrossRef]
- Otto, S.; Schnoedt, E.M.; Troeltzsch, M.; Kaeppler, G.; Aljohani, S.; Liebermann, A.; Fliefel, R. Clinical and Radiographic Outcomes of Dental Implants in Patients Treated With Antiresorptive Drugs: A Consecutive Case Series. J. Oral. Implantol. 2023, 49, 39–45. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.U.; Lee, S.Y. Successful New Dental Implant Installation in a Healed Site of Medication-Related Osteonecrosis of the Jaw: A Case Report. J. Oral. Implantol. 2023, 49, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Badros, A.; Weikel, D.; Salama, A.; Goloubeva, O.; Schneider, A.; Rapoport, A.; Fenton, R.; Gahres, N.; Sausville, E.; Ord, R.; et al. Osteonecrosis of the jaw in multiple myeloma patients: Clinical features and risk factors. J. Clin. Oncol. 2006, 24, 945–952. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral. Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Dodson, T.B. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J. Oral. Maxillofac. Surg. 2009, 67, 44–52. [Google Scholar] [CrossRef]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. North. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef]
- Feigin, K.; Shope, B. Use of Platelet-Rich Plasma and Platelet-Rich Fibrin in Dentistry and Oral Surgery: Introduction and Review of the Literature. J. Vet. Dent. 2019, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O.; Peter, J.U. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2022, 7, CD012432. [Google Scholar] [CrossRef]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Klinger, M.; Hakim, S.G. Impacts of platelet-rich fibrin and platelet-rich plasma on primary osteoblast adhesion onto titanium implants in a bisphosphonate in vitro model. J. Oral. Pathol. Med. 2019, 48, 943–950. [Google Scholar] [CrossRef]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef]
- Rodan, G.A.; Fleisch, H.A. Bisphosphonates: Mechanisms of action. J. Clin. Investig. 1996, 97, 26922696. [Google Scholar] [CrossRef]
- Rodan, G.A. Mechanism of action of bisphosphonates. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 375388. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.; Ikeagwani, O.; Kearns, G. A role for C-terminal cross-linking telopeptide (CTX) level to predict the development of bisphosphonate-related osteonecrosis of the jaws (BRONJ) following oral surgery? J. Med. Sci. 2012, 181, 16. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).