Isolation, Synthesis, and Use of Natural Photosensitizers in the Treatment of Central Nervous System Tumors
Abstract
1. Introduction
1.1. History of Photodynamic Therapy (PDT)
1.2. Mechanism of Photodynamic Therapy (PDT)
1.3. Photosensitizers (PSs)
2. Natural Photosensitizers
2.1. Quinonoids
2.2. Curcumin
2.3. Chlorophyll Derivatives and Pheophorbide A
2.4. Alkaloids and Berberine
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wu, X.; Long, G.; Yu, J.; He, W.; Zhang, H.; Wang, D.; Ye, Z.; Tian, J. Revolutionizing cancer treatment: Nanotechnology-enabled photodynamic therapy and immunotherapy with advanced photosensitizers. Front. Immunol. 2023, 14, 1219785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawauchi, K.; Urano, R.; Kinoshita, N.; Kuwamoto, S.; Torii, T.; Hashimoto, Y.; Taniguchi, S.; Tsuruta, M.; Miyoshi, D. Photosensitizers Based on G-Quadruplex Ligand for Cancer Photodynamic Therapy. Genes 2020, 11, 1340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Staudinger, J.N.; Mindt, T.L.; Gasser, G. Theranostics with photodynamic therapy for personalized medicine: To see and to treat. Theranostics. Theranostics 2023, 13, 5501–5544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lima, E.; Reis, L.V. Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers. Molecules. Molecules 2023, 28, 5092. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H. Photodynamic therapy: Innovative approaches for antibacterial and anticancer treatments. Med. Res. Rev. 2023, 43, 717–774. [Google Scholar] [CrossRef] [PubMed]
- Aires-Fernandes, M.; Botelho Costa, R.; Rochetti do Amaral, S.; Mussagy, C.U.; Santos-Ebinuma, V.C.; Primo, F.L. Development of Biotechnological Photosensitizers for Photodynamic Therapy: Cancer Research and Treatment-From Benchtop to Clinical Practice. Molecules 2022, 27, 6848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, M.; Li, C. Recent Advances in Activatable Organic Photosensitizers for Specific Photodynamic Therapy. Chempluschem 2020, 85, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M.M.; Giram, P.; Foster, B.A.; You, Y. Photodynamic Therapy for Bladder Cancers, A Focused Review. Photochem. Photobiol. 2023, 99, 420–436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartusik-Aebisher, D.; Serafin, I.; Dynarowicz, K.; Aebisher, D. Photodynamic therapy and associated targeting methods for treatment of brain cancer. Front. Pharmacol. 2023, 14, 1250699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gustalik, J.; Aebisher, D.; Bartusik-Aebisher, D. Photodynamic therapy in breast cancer treatment. J. Appl. Biomed. 2022, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhou, L.; Lu, J.; Jiang, B.; Liu, C.; Guo, J. Photodynamic therapy of pancreatic cancer: Where have we come from and where are we going? Photodiagnosis Photodyn. Ther. 2020, 31, 101876. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.; Bartusik-Aebisher, D.; Osuchowski, F.; Aebisher, D. Photodynamic therapy for prostate cancer—A narrative review. Photodiagnosis Photodyn. Ther. 2021, 33, 102158. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Mahootchi, P.; Rastegar, Z.; Abbasi, B.; Alam, M.; Abbasi, K.; Fani-Hanifeh, S.; Amookhteh, S.; Sadeghi, S.; Soufdoost, R.S.; et al. Photodynamic Therapy in Oral Cancer: A Narrative Review. Photobiomodulation Photomed. Laser Surg. 2023, 41, 248–264. [Google Scholar] [CrossRef] [PubMed]
- El-Hussein, A.; Manoto, S.L.; Ombinda-Lemboumba, S.; Alrowaili, Z.A.; Mthunzi-Kufa, P. A Review of Chemotherapy and Photodynamic Therapy for Lung Cancer Treatment. Anti Cancer Agents Med. Chem. 2020, 21, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Adnane, F.; El-Zayat, E.; Fahmy, H.M. The combinational application of photodynamic therapy and nanotechnology in skin cancer treatment: A review. Tissue Cell 2022, 77, 101856. [Google Scholar] [CrossRef] [PubMed]
- Oskroba, A.; Bartusik-Aebisher, D.; Myśliwiec, A.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Photodynamic Therapy and Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 2974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, J.; Chen, Z.; Li, X.; Yang, M.; Lv, J.; Li, H.; Yuan, Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int. J. Mol. Sci. 2022, 23, 12556. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verger, A.; Brandhonneur, N.; Molard, Y.; Cordier, S.; Kowouvi, K.; Amela-Cortes, M.; Dollo, G. From molecules to nanovectors: Current state of the art and applications of photosensitizers in photodynamic therapy. Int. J. Pharm. 2021, 604, 120763. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, L.; Tang, H.; Cao, D. Recent advances in type I organic photosensitizers for efficient photodynamic therapy for overcoming tumor hypoxia. J. Mater. Chem. B 2023, 11, 4600–4618. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Tang, S.; Hong, G.; Chen, W.; Chen, M.; Song, J.; Li, Z.; Peng, X.; Song, F.; Zheng, W.H. An unexpected strategy to alleviate hypoxia limitation of photodynamic therapy by biotinylation of photosensitizers. Nat. Commun. 2022, 13, 2225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Ying, X.; Wu, L.; Liu, L.; Wang, Y.; He, Y.; Han, M. Research Progress of Natural Product Photosensitizers in Photodynamic Therapy. Planta Med. 2024, 90, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Montoya, S.C.N.; Comini, L.R.; Sarmiento, M.; Becerra, C.; Albesa, I.; Argüello, G.A.; Cabrera, J.L. Natural anthraquinones probed as Type I and Type II photosensitizers: Singlet oxygen and superoxide anion production. J. Photochem. Photobiol. B Biol. 2005, 78, 77–83. [Google Scholar] [CrossRef]
- Walter, A.B.; Simpson, J.; Jenkins, J.L.; Skaar, E.P.; Jansen, E.D. Optimization of optical parameters for improved photodynamic therapy of Staphylococcus aureus using endogenous coproporphyrin III. Photodiagnosis Photodyn. Ther. 2020, 29, 101624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Geng, S.; Wang, X.; Cui, Z.; Ma, W.; Yuan, M.; Liu, C.; Ji, Y. Aloe-emodin-mediated antimicrobial photodynamic therapy against multidrug-resistant Acinetobacter baumannii: An in vivo study. Photodiagnosis Photodyn. Ther. 2021, 34, 102311. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, C.; Li, J.; Hao, M.; Ji, Y.; Zeng, X. The effects of aloe emodin-mediated antimicrobial photodynamic therapy on drug-sensitive and resistant Candida albicans. Photobiol. Sci. 2020, 19, 485–494. [Google Scholar] [CrossRef]
- Comini, L.R.; Fernandez, I.M.; Vittar, N.B.R.; Nunez Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Photodynamic activity of anthraquinones isolated from Heterophyllaea pustulata Hook f.(Rubiaceae) on MCF-7c3 breast cancer cells. Phytomedicine 2011, 18, 1093–1095. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Zhang, Z.; Fu, J.; You, L.; He, Y.; Hao, Y.; Gu, Z.; Yu, Z.; Qu, C.; et al. Hypericin-mediated photodynamic therapy for the treatment of cancer: A review. J. Pharm. Pharmacol. 2021, 73, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, C.; Li, Y.; Liu, W.; Yao, N.; Gao, M.; Ji, Y.; Huang, D.; Yin, Z.; Sun, Z.; et al. Evaluation of hypericin: Effect of aggregation on targeting biodistribution. J. Pharm. Sci. 2015, 104, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Motoyoshiya, J.; Masue, Y.; Nishi, Y.; Aoyama, H. Synthesis of Hypericin via Emodin Anthrone Derived from a Two-fold Diels-Alder Reaction of 1,4-Benzoquinone. Nat. Prod. Commun. 2007, 2, 67–70. [Google Scholar] [CrossRef]
- Huang, L.F.; Wang, Z.H.; Chen, S.L. Hypericin: Chemical synthesis and biosynthesis. Chin. J. Nat. Med. 2014, 12, 81–88. [Google Scholar] [CrossRef]
- Tobia, A.J.; Cabana, B.E.; Vadlapatla, V.; Connolly, R.H. US Patent Methods for Preparing Hypericin. U.S. Patent WO2011034922A1, 24 March 2011. [Google Scholar]
- Pillai, P.P.; Nair, A.R. Hypericin biosynthesis in Hypericum hookerianum Wight and Arn: Investigation on biochemical pathways using metabolite inhibitors and suppression subtractive hybridization. Comptes Rendus Biol. 2014, 337, 571–580. [Google Scholar] [CrossRef]
- Priyadarshini, M.; S, K.; P, T.; Murugesan, S.; S, V.; Nayak, S.; Roopan, S.M.; Raj, N.A.N. Green synthesis of hypericin from Hypericum perforatum (St. John’s Wort) for photodynamic Antibacterial treatment against Staphylococcus aureus and Escherichia coli. Nat. Prod. Res. 2025, 1–8. [Google Scholar] [CrossRef]
- Nobakht, S.Z.; Akaberi, M.; Mohammadpour, A.H.; Tafazoli Moghadam, A.; Emami, S.A. Hypericum perforatum: Traditional uses, clinical trials, and drug interactions. Iran. J. Basic Med. Sci. 2022, 25, 1045–1058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miccoli, L.; Beurdeley-Thomas, A.; De Pinieux, G.; Sureau, F.; Oudard, S.; Dutrillaux, B.; Poupon, M.F. Light-induced Photoactivation of Hypericin Affects the Energy Metabolism of Human Glioma Cells by Inhibiting Hexokinase Bound to Mitochondria. Cancer Res. 1998, 58, 5777–5786. [Google Scholar] [PubMed]
- Ritz, R.; Wein, H.T.; Dietz, K.; Schenk, M.; Roser, F.; Tatagiba, M.; Strauss, W.S. Photodynamic therapy of malignant glioma with hypericin: Comprehensive in vitro study in human glioblastoma cell lines. Int. J. Oncol. 2007, 30, 659–667. [Google Scholar] [CrossRef] [PubMed]
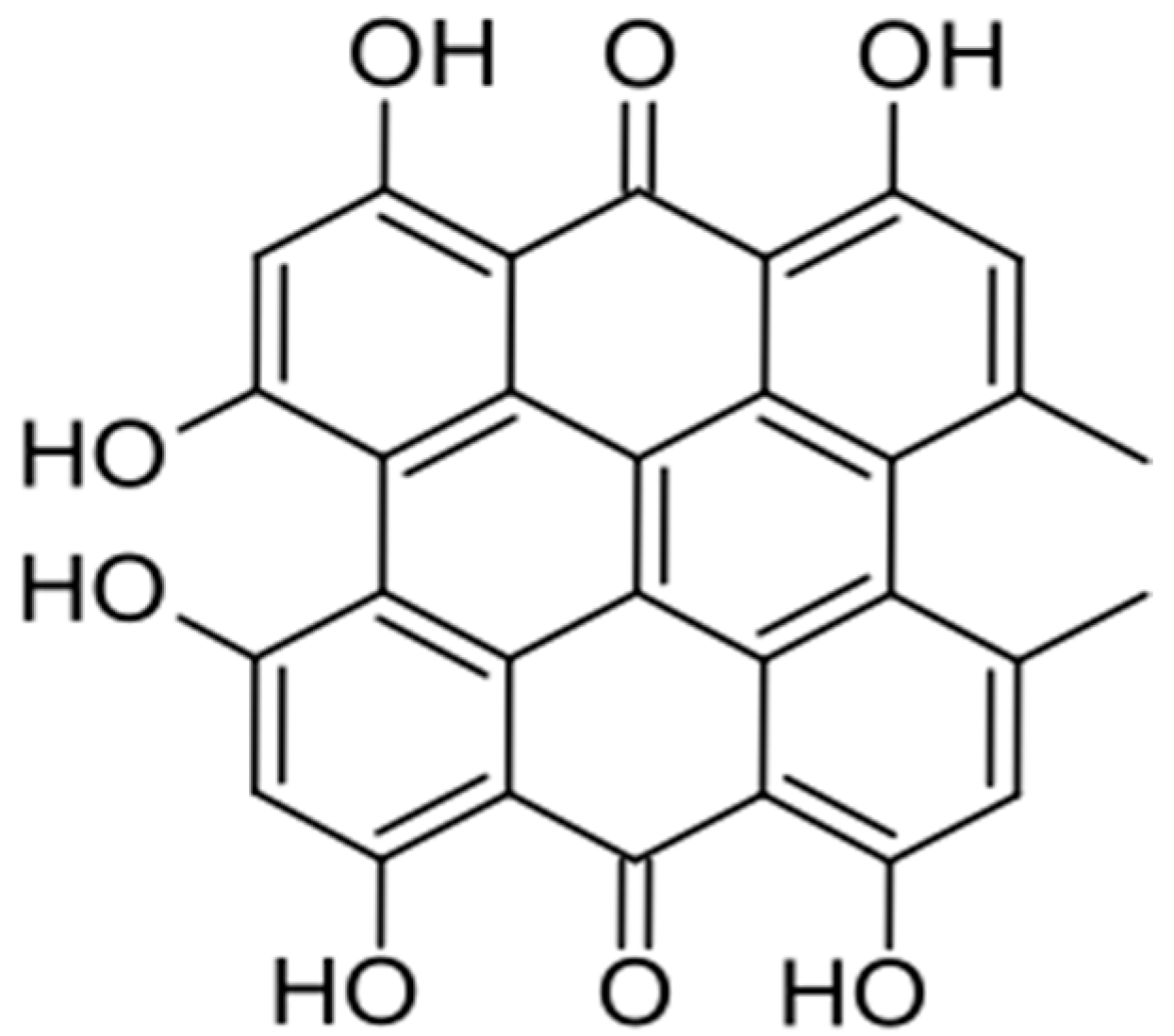
- Mulrooney, C.A.; O’BRien, E.M.; Morgan, B.J.; Kozlowski, M.C. Perylenequinones: Isolation, Synthesis, and Biological Activity. Eur. J. Org. Chem. 2012, 2012, 3887–3904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, J.M.; Murray, J. Isolation of 4,9-dihydroxyperylene-3,10-quinone from a fungus. In book Chemistry and Industry Society of Chemical Industry. May 12 1956. Available online: https://books.google.pl/books/about/Chemistry_and_Industry.html?id=HpFhdYTE7P4C&redir_esc=y (accessed on 6 February 2025).
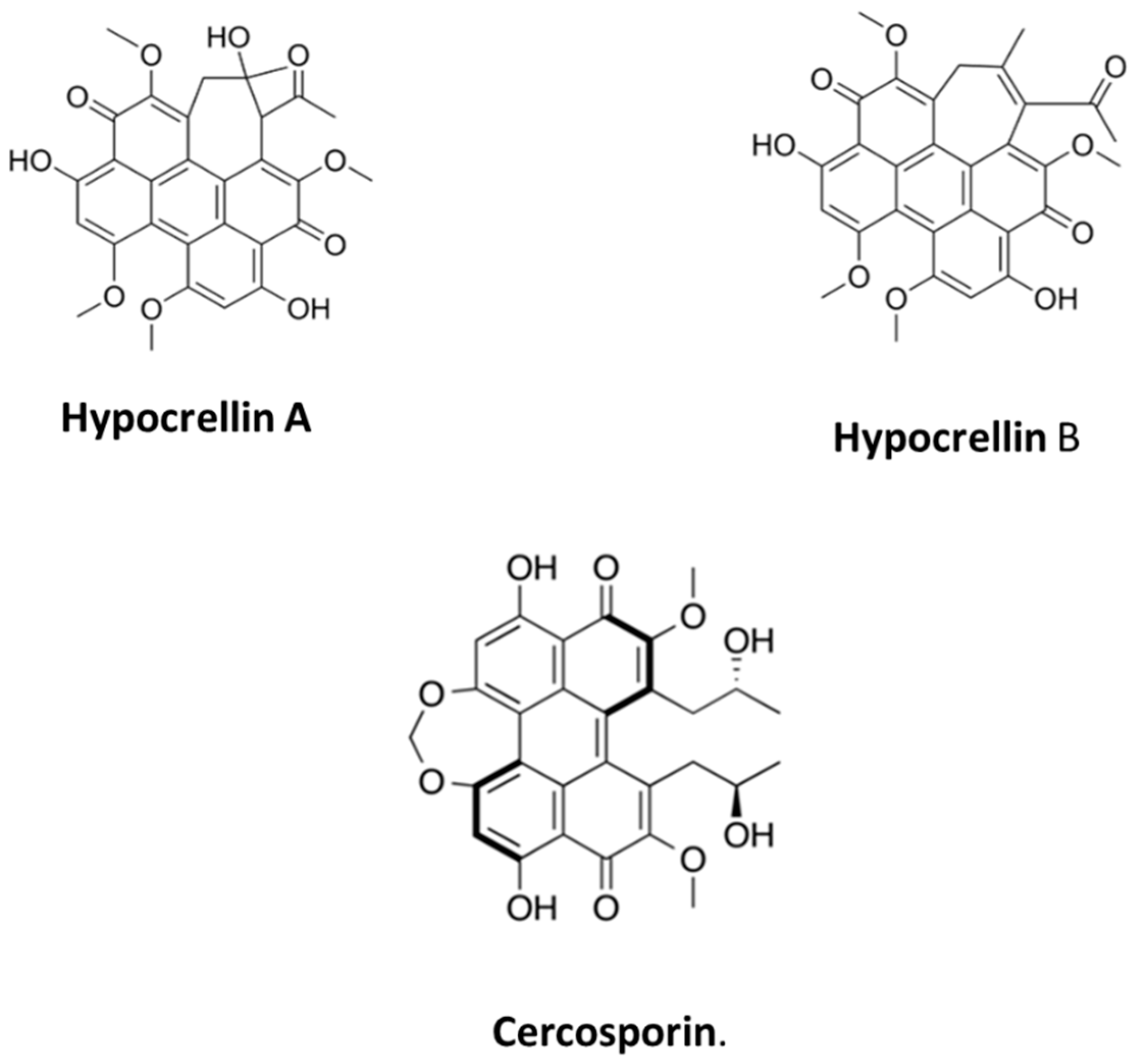
- Cai, Y.; Liang, X.; Liao, X.; Ding, Y.; Sun, J.; Li, X. High-yield hypocrellin A production in solid-state fermentation by Shiraia sp. SUPER-H168. Appl. Biochem. Biotechnol. 2010, 160, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Nenghui, W.; Zhiyi, Z. Relationship between photosensitizing activities and chemical structure of hypocrellin A and B. J. Photochem. Photobiol. B Biol. 1992, 14, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, C.A.; Li, X.; DiVirgilio, E.S.; Kozlowski, M.C. General approach for the synthesis of chiral perylenequinones via catalytic enantioselective oxidative biaryl coupling. J. Am. Chem. Soc. 2003, 125, 6856–6857. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.M.; Morgan, B.J.; Kozlowski, M.C. Dynamic Sterochemistry Transfer in a Transannular Aldol Reaction: Total Synthesis of Hypocrellin A. Angew. Chem. Int. Ed. 2008, 47, 6877–6880. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, C.A.; Morgan, B.J.; Li, X.; Kozlowski, M.C. Perylenequinone natural products: Enantioselective synthesis of the oxidized pentacyclic core. J. Org. Chem. 2010, 75, 16–29. [Google Scholar] [CrossRef]
- O’Brien, E.M.; Morgan, B.J.; Carroll, P.J.; Kozlowski, M.C. Perylenequinone natural products: Total synthesis of hypocrellin A. J. Org. Chem. 2010, 75, 57–68. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Zhuge, Y.; Zhang, J.; Xu, K.; Zhang, Q.; Zhang, H.; Chen, H.; Chu, M.; Jia, C. Photodynamic antifungal activity of hypocrellin A against Candida albicans. Front. Microbiol. 2019, 10, 1810. [Google Scholar] [CrossRef]
- Xiao, Q.; Wu, J.; Pang, X.; Jiang, Y.; Wang, P.; Leung, A.W.; Gao, L.; Jiang, S.; Xu, C. Discovery and Development of Natural Products and their Derivatives as Photosensitizers for Photodynamic Therapy. Curr. Med. Chem. 2018, 25, 839–860. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xia, X.; Leung, A.W.; Xiang, J.; Xu, C. Apoptosis of breast cancer cells induced by hypocrellin B under light-emitting diode irradiation. Photodiagnosis Photodyn. Ther. 2012, 9, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, P.W.G.; Chandran, R.; Abrahamse, H. Hypocrellin: A Natural Photosensitizer and Nano-Formulation for Enhanced Molecular Targeting of PDT of Melanoma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, J.E.; Cho, H.J.; Yi, E.; Kim, D.D.; Jheon, S. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid-ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J. Photochem. Photobiol. B Biol. 2016, 158, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.S.; Chandran, R.; Abrahamse, H. Advancing Photodynamic Therapy with Nano-Conjugated Hypocrellin: Mechanisms and Clinical Applications. Int. J. Nanomed. 2024, 19, 11023–11038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chin, W.; Lau, W.; Cheng, C.; Olivo, M. Evaluation of Hypocrellin B in a human bladder tumor model in experimental photodynamic therapy: Biodistribution, light dose and drug-light interval effects. Int. J. Oncol. 2004, 25, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Ma, L.P.; Wang, S.W.; Zhang, Z.Y.; Cao, G.D. Antisense bcl-2 retrovirus vector increases the sensitivity of a human gastric adenocarcinoma cell line to photodynamic therapy. Photochem Photobiol. 1999, 69, 582–586. [Google Scholar] [PubMed]
- Deininger, M.H.; Weinschenk, T.; Morgalla, M.H.; Meyermann, R.; Schluesener, H.J. Release of regulators of angiogenesis following Hypocrellin-A and -B photodynamic therapy of human brain tumor cells. Biochem. Biophys. Res. Commun. 2002, 298, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Ebermann, R.; Alth, G.; Kreitner, M.; Kubin, A. Natural products derived from plants as potential drugs for the photodynamic destruction of tumor cells. J. Photochem. Photobiol. B Biol. 1996, 36, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Okubo, A.; Yamazaki, S.; Fuwa, K. Biosynthesis of Cercosporin. Agric. Biol. Chem. 1975, 39, 1173–1175. [Google Scholar]
- Yamazaki, S.; Okubo, A.; Akiyama, Y.; Fuwa, K. Cercosporin, a novel photodynamic pigment isolated from Cercospora kikuchii. Agric. Biol. Chem. 1975, 39, 287–288. [Google Scholar] [CrossRef]
- Macrì, F.; Vianello, A. Photodynamic activity of compounds structurally related to cercosporin. Agric. Biol. Chem. 1980, 44, 2967–2970. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Ogawa, T. The chemistry and stereochemistry of cercosporin. Agric. Biol. Chem. 1972, 36, 1707–1718. [Google Scholar] [CrossRef][Green Version]
- Marchiando, N.C.; Zón, M.A.; Fernández, H. Determination of cercosporin (CER) phytotoxin isolated from infected peanut leaves by using adsorptive stripping square wave voltammetry. Anal. Chim. Acta 2005, 550, 199–203. [Google Scholar] [CrossRef]
- Gunasinghe, N.; You, M.P.; Cawthray, G.R.; Barbetti, M.J. Cercosporin From Pseudocercosporella capsellae and its Critical Role in White Leaf Spot Development. Plant Dis. 2016, 100, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Chaturvedi, A.K.; Javitt, G.; Brandis, A.; Fluhr, R. Multiple paths of plant host toxicity are associated with the fungal toxin cercosporin. Plant Cell Environ. 2023, 46, 2542–2557. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Yuan, Z.; Lu, L.; Liu, X.; Zhu, X.; Wang, L.; Liu, C.; Rao, Y. Cercosporin-bioinspired photoinactivation of harmful cyanobacteria under natural sunlight via bifunctional mechanisms. Water Res. 2022, 215, 118242. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liu, M.; Su, Z.; Xu, H.; Liu, C.; Lu, L.; Wang, L.; Zhu, X.; Zhang, Y.; Rao, Y. Designing a cercosporin-bioinspired bifunctional algicide with flocculation and photocatalysis for efficiently controlling harmful cyanobacterial blooms. J. Hazard. Mater. 2023, 459, 132110. [Google Scholar] [CrossRef] [PubMed]
- Cadelis, M.M.; Li, S.A.; van de Pas, S.J.; Grey, A.; Mulholland, D.; Weir, B.S.; Copp, B.R.; Wiles, S. Antimicrobial Natural Products from Plant Pathogenic Fungi. Molecules 2023, 28, 1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mastrangelopoulou, M.; Grigalavicius, M.; Berg, K.; Ménard, M.; Theodossiou, T.A. Cytotoxic and Photocytotoxic Effects of Cercosporin on Human Tumor Cell Lines. Photochem. Photobiol. 2019, 95, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Grigalavicius, M.; Mastrangelopoulou, M.; Arous, D.; Juzeniene, A.; Ménard, M.; Skarpen, E.; Berg, K.; Theodossiou, T.A. Photodynamic Efficacy of Cercosporin in 3D Tumor Cell Cultures. Photochem. Photobiol. 2020, 96, 699–707. [Google Scholar] [CrossRef] [PubMed]
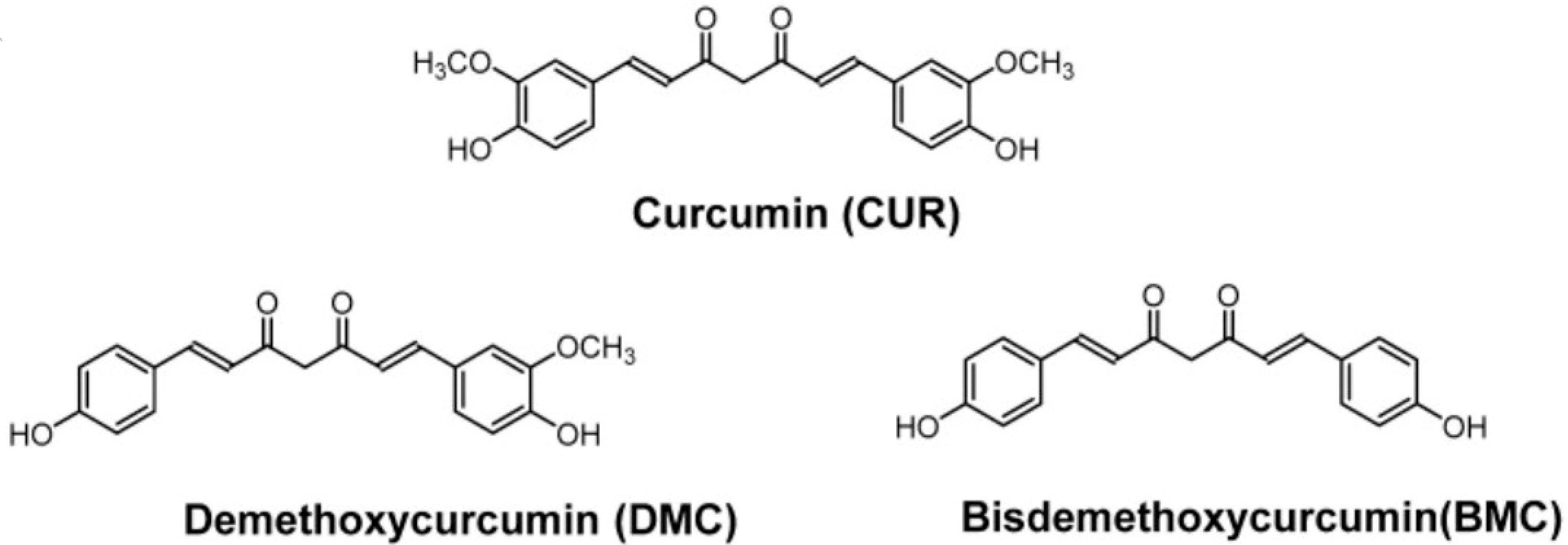
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic natural products as photosensitizers for antimicrobial photodynamic therapy: Recent advances and future prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reda, F.M.; El-Saadony, M.T.; Elnesr, S.S.; Alagawany, M.; Tufarelli, V. Effect of Dietary Supplementation of Biological Curcumin Nanoparticles on Growth and Carcass Traits, Antioxidant Status, Immunity and Caecal Microbiota of Japanese Quails. Animals 2020, 10, 754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashemi, B.; Madadlou, A.; Salami, M. Functional and in vitro gastric digestibility of the whey protein hydrogel loaded with nanostructured lipid carriers and gelled via citric acid-mediated crosslinking. Food Chem. 2017, 237, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nikoo, A.M.; Kadkhodaee, R.; Ghorani, B.; Razzaq, H.; Tucker, N. Electrospray-assisted encapsulation of caffeine in alginate microhydrogels. Int. J. Biol. Macromol. 2018, 116, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Hosseinzadeh, R.; Shahidi, F.K. Photodynamic treatment with anionic nanoclays containing curcumin on human triple-negative breast cancer cells: Cellular and biochemical studies. J. Cell. Biochem. 2019, 120, 4998–5009. [Google Scholar] [CrossRef] [PubMed]
- de Matos, R.P.A.; Calmon, M.F.; Amantino, C.F.; Villa, L.L.; Primo, F.L.; Tedesco, A.C.; Rahal, P. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Cervical Carcinoma Cell Lines. BioMed Res. Int. 2018, 2018, 4057959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fadeel, D.A.A.; Kamel, R.; Fadel, M. PEGylated lipid nanocarrier for enhancing photodynamic therapy of skin carcinoma using curcumin: In-vitro/in-vivo studies and histopathological examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and silver nanoparticles carried out from polysaccharide-based hydrogels improved the photodynamic properties of curcumin through metal-enhanced singlet oxygen effect. Mater. Sci. Eng. C 2020, 112, 110853. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, S.; Liu, C.; He, J.; Li, T.; Fu, C.; Meng, X.; Shao, H. Enhanced Photothermal-Photodynamic Therapy by Indocyanine Green and Curcumin-Loaded Layered MoS2 Hollow Spheres via Inhibition of P-Glycoprotein. Int. J. Nanomed. 2021, 16, 433–442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baghdan, E.; Duse, L.; Schüer, J.J.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Development of inhalable curcumin loaded Nano-in-Microparticles for bronchoscopic photodynamic therapy. Eur. J. Pharm. Sci. 2019, 132, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kouhpeikar, H.; Butler, A.E.; Bamian, F.; Barreto, G.E.; Majeed, M.; Sahebkar, A. Curcumin as a therapeutic agent in leukemia. J. Cell. Physiol. 2019, 234, 12404–12414. [Google Scholar] [CrossRef] [PubMed]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Latest Innovations and Nanotechnologies with Curcumin as a Nature-Inspired Photosensitizer Applied in the Photodynamic Therapy of Cancer. Pharmaceutics 2021, 13, 1562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagnosis Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, A.; Wawryka, P.; Przystupski, D.; Rossowska, J.; Szewczyk, A.; Saczko, J.; Kulbacka, J.; Chwiłkowska, A. Effects of Photosensitization of Curcumin in Human Glioblastoma Multiforme Cells. In Vivo 2019, 33, 1857–1864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sadeghi, M.; Dehnavi, S.; Asadirad, A.; Xu, S.; Majeed, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Curcumin and chemokines: Mechanism of action and therapeutic potential in inflammatory diseases. Inflammopharmacology 2023, 31, 1069–1093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marton, L.T.; Pescinini-E-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.d.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Bueno, P.C.d.S. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Zhu, Q.; Chen, Y.; Ji, K.; Li, S.; Wu, Q.; Pan, Q.; Li, J. Review of the Protective Mechanism of Curcumin on Cardiovascular Disease. Drug Des. Dev. Ther. 2024, 18, 165–192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kao, Y.W.; Hsu, S.K.; Chen, J.Y.F.; Lin, I.L.; Chen, K.J.; Lee, P.Y.; Ng, H.S.; Chiu, C.C.; Cheng, K.C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2020, 22, 212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bideshki, M.V.; Jourabchi-Ghadim, N.; Radkhah, N.; Behzadi, M.; Asemani, S.; Jamilian, P.; Zarezadeh, M. The efficacy of curcumin in relieving osteoarthritis: A meta-analysis of meta-analyses. Phytother. Res. 2024, 38, 2875–2891. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, S.; Morasso, C.; Stivaktakis, P.; Pandini, C.; Tinelli, V.; Tsatsakis, A.; Prosperi, D.; Hickey, M.; Corsi, F.; Cereda, C. Curcumin Formulations and Trials: What’s New in Neurological Diseases. Molecules 2020, 25, 5389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
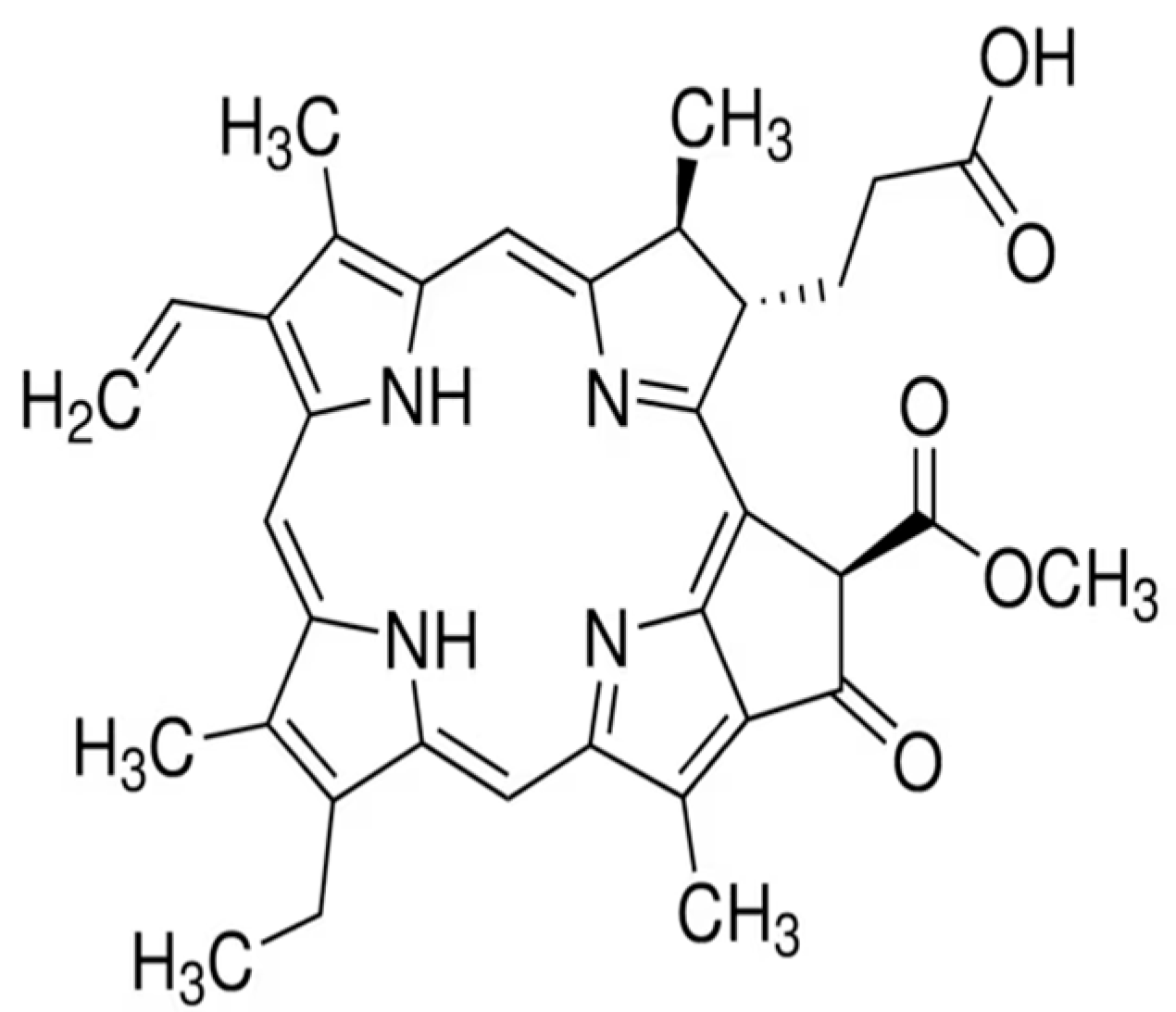
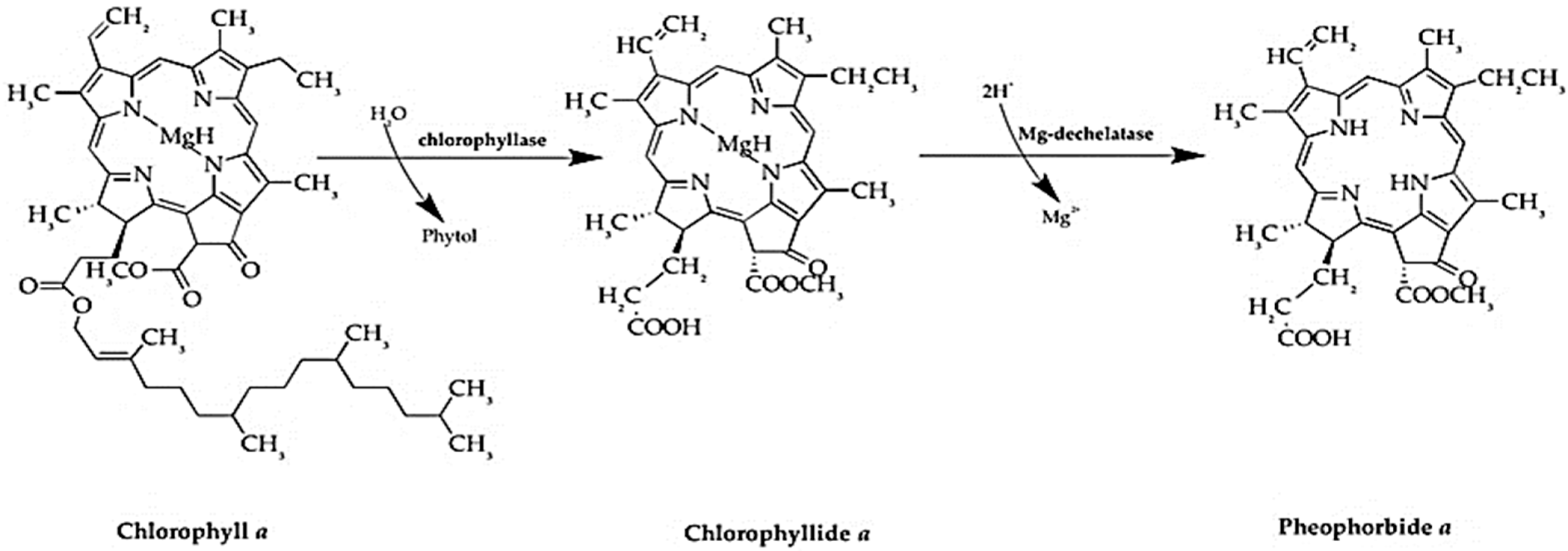
- Xodo, L.E.; Rapozzi, V.; Zacchigna, M.; Drioli, S.; Zorzet, S. The chlorophyll catabolite pheophorbide a as a photosensitizer for the photodynamic therapy. Curr. Med. Chem. 2012, 19, 799–807. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Yoon, H.E.; Yoon, J.H.; Ko, H.; Kim, Y.C. Synthesis of pheophorbide-a conjugates with anticancer drugs as potential cancer diagnostic and therapeutic agents. Bioorg. Med. Chem. 2011, 19, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.B.; Mitchell, M.O.; Weinberg, J.S.; D’Amico, A.V.; Bubley, G.J. Towards enzyme activated antiprostatic agents. Bioorg. Med. Chem. Lett. 2000, 10, 1987–1989. [Google Scholar] [CrossRef]
- Pickaert, G.; Cesario, M.; Ziessel, R. A convenient protocol for the synthesis of ligands from a 4-methyl-3, 5-diacylaminophenyl platform. J. Org. Chem. 2004, 69, 5335. [Google Scholar] [CrossRef]
- Ohta, S.; Ono, F.; Shiomi, Y.; Nakao, T.; Aozasa, O.; Nagate, T.; Kitamura, K.; Yamaguchi, S.; Nishi, M.; Miyata, H. Anti-herpes simplex virus substances produced by the marine green alga, Dunaliella primolecta. J. Appl. Phycol. 1998, 10, 349–356. [Google Scholar] [CrossRef]
- Ratnoglik, S.L.; Aoki, C.; Sudarmono, P.; Komoto, M.; Deng, L.; Shoji, I.; Fuchino, H.; Kawahara, N.; Hotta, H. Antiviral activity of extracts from Morinda citrifolia leaves and chlorophyll catabolites, pheophorbide a and pyropheophorbide a, against hepatitis C virus. Microbiol. Immunol. 2014, 58, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E.H. Lysophosphatidylcholines and Chlorophyll-Derived Molecules from the Diatom Cylindrotheca closterium with Anti-Inflammatory Activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lanfer-Marquez, U.M.; Barros, R.M.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Bui-Xuan, N.H.; Tang, P.M.K.; Wong, C.K.; Chan, J.Y.W.; Cheung, K.K.Y.; Jiang, J.L.; Fung, K.P. Pheophorbide a: A photosensitizer with immunostimulating activities on mouse macrophage RAW 264.7 cells in the absence of irradiation. Cell. Immunol. 2011, 269, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Miranda, N.; Volpato, H.; da Silva Rodrigues, J.H.; Caetano, W.; Ueda-Nakamura, T.; de Oliveira Silva, S.; Nakamura, C.V. The photodynamic action of pheophorbide a induces cell death through oxidative stress in Leishmania amazonensis. J. Photochem. Photobiol. B Biol. 2017, 174, 342–354. [Google Scholar] [CrossRef]
- Kim, J.; Gousopoulos, E.; Faleschini, T.M.; Hamburger, M.; Potterat, O.; Detmar, M. Pheophorbide a identified in an Eupatorium perfoliatum extract is a novel lymphatic vascular activator. Biomed. Pharmacother. 2022, 147, 112664. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.K.; Chan, J.Y.W.; Au, S.W.N.; Kong, S.K.; Tsui, S.K.W.; Waye, M.M.Y.; Mak, T.C.W.; Fong, W.P.; Fung, K.P. Pheophorbide a, an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol. Ther. 2006, 5, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.Y.; Yoon, H.; Kwon, S.; Lee, J.; Min, S.; Kim, Y.; Ahn, S.; Yoon, J. Synthesized Pheophorbide a-mediated photodynamic therapy induced apoptosis and autophagy in human oral squamous carcinoma cells. J. Oral Pathol. Med. 2013, 42, 17–25. [Google Scholar] [CrossRef]
- Tang, P.M.K.; Liu, X.Z.; Zhang, D.M.; Fong, W.P.; Fung, K.P. Pheophorbide a based photodynamic therapy induces apoptosis via mitochondrial-mediated pathway in human uterine carcinosarcoma. Cancer Biol. Ther. 2009, 8, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photodynamic therapy using pheophorbide and 670nm LEDs exhibits anti-cancer effects in-vitro in androgen dependent prostate cancer. Photodiagnosis Photodyn. Ther. 2018, 21, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bui-Xuan, N.H.; Tang, P.M.K.; Wong, C.K.; Fung, K.-P. Photo-activated pheophorbide-a, an active component of Scutellaria barbata, enhances apoptosis via the suppression of ERK-mediated autophagy in the estrogen receptor-negative human breast adenocarcinoma cells MDA-MB-231. J. Ethnopharmacol. 2010, 131, 95–103. [Google Scholar] [CrossRef]
- Hoi, S.W.; Wong, H.M.; Chan, J.Y.; Yue, G.G.L.; Tse, G.M.; Law, B.K.; Fong, W.P.; Fung, K.P. Photodynamic Therapy of Pheophorbide a Inhibits the Proliferation of Human Breast Tumour via Both Caspase-dependent and-independent Apoptotic Pathways in In Vitro and In Vivo Models. Phytother. Res. 2012, 26, 734–742. [Google Scholar] [CrossRef]
- Cho, M.; Park, G.M.; Kim, S.N.; Amna, T.; Lee, S.; Shin, W.S. Glioblastoma-specific anticancer activity of pheophorbide a from the edible red seaweed Grateloupia elliptica. Microbiol. Biotechnol. 2014, 24, 346–353. [Google Scholar] [CrossRef] [PubMed]
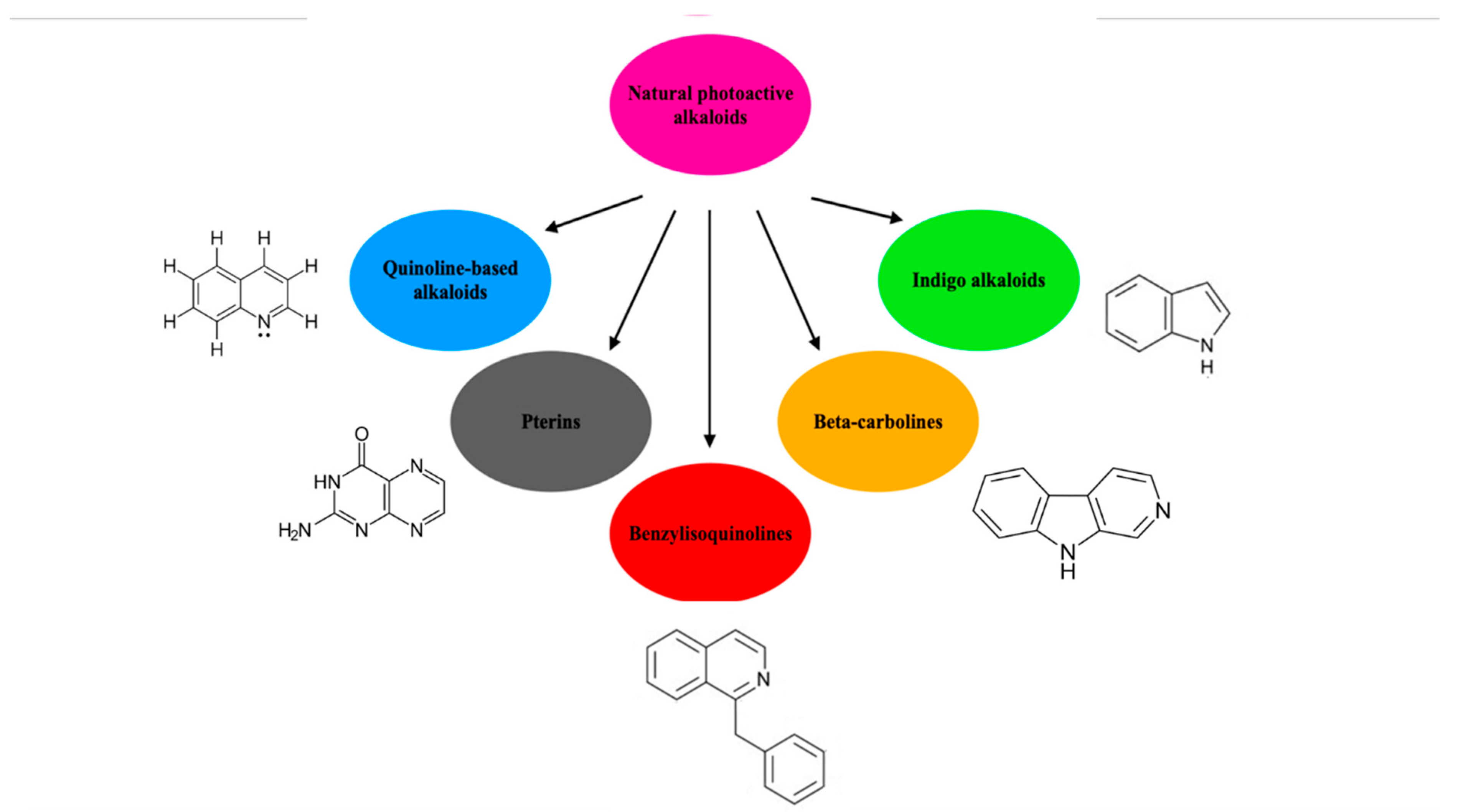
- Olofinsan, K.; Abrahamse, H.; George, B.P. Therapeutic Role of Alkaloids and Alkaloid Derivatives in Cancer Management. Molecules 2023, 28, 5578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine-A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Peng, L.Y.; Wei, G.H.; Ge, H. Therapeutic Effects of Berberine Capsule on Patients with Mild Hyperlipidemia. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi Chin. J. Integr. Tradit. West. Med. 2016, 36, 681–684. [Google Scholar] [PubMed]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef] [PubMed]
- Rui, R.; Yang, H.; Liu, Y.; Zhou, Y.; Xu, X.; Li, C.; Liu, S. Effects of Berberine on Atherosclerosis. Front. Pharmacol. 2021, 12, 764175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shan, M.Y.; Dai, Y.; Ren, X.D.; Zheng, J.; Zhang, K.B.; Chen, B.; Yan, J.; Xu, Z.H. Berberine mitigates nonalcoholic hepatic steatosis by downregulating SIRT1-FoxO1-SREBP2 pathway for cholesterol synthesis. J. Integr. Med. 2021, 19, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, D.; Yu, B.; Sun, S.; Chen, B.; Harkare, H.V.; Wang, L.; Pan, J.; Huang, B.; Song, Y.; Ma, T.; et al. Effects of adjuvant berberine therapy on acute ischemic stroke: A meta-analysis. Phytother. Res. 2023, 37, 3820–3838. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; O’neill, E.J.; Gagacev, F.; Tsiani, E. Effects of Berberine against Pancreatitis and Pancreatic Cancer. Molecules 2022, 27, 8630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, C.; Li, K.; Peng, X.X.; Yao, T.J.; Wang, Z.Y.; Hu, P.; Cai, D.; Liu, H.Y. Berberine a traditional Chinese drug repurposing: Its actions in inflammation-associated ulcerative colitis and cancer therapy. Front. Immunol. 2022, 13, 1083788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, M.; Ren, L.; Fan, J.; Huang, L.; Zhou, L.; Li, X.; Ye, X. Berberine inhibits gastric cancer development and progression by regulating the JAK2/STAT3 pathway and downregulating IL-6. Life Sci. 2022, 290, 120266. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Pallathadka, H.; Gupta, J.; Ma, H.; Al-Shukri, H.H.K.; Kareem, A.K.; Zwamel, A.H.; Mustafa, Y.F. Berberine and berberine nanoformulations in cancer therapy: Focusing on lung cancer. Phytotherapy Res. 2024, 38, 4336–4350. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, J.; Ai, G.; Liu, J.; Luo, N.; Li, C.; Cheng, Z. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol. Res. 2019, 52, 37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carriero, F.; Martinelli, C.; Gabriele, F.; Barbieri, G.; Zanoletti, L.; Milanesi, G.; Casali, C.; Azzalin, A.; Manai, F.; Paolillo, M.; et al. Berberine Photo-Activation Potentiates Cytotoxicity in Human Astrocytoma Cells through Apoptosis Induction. J. Pers. Med. 2021, 11, 942. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
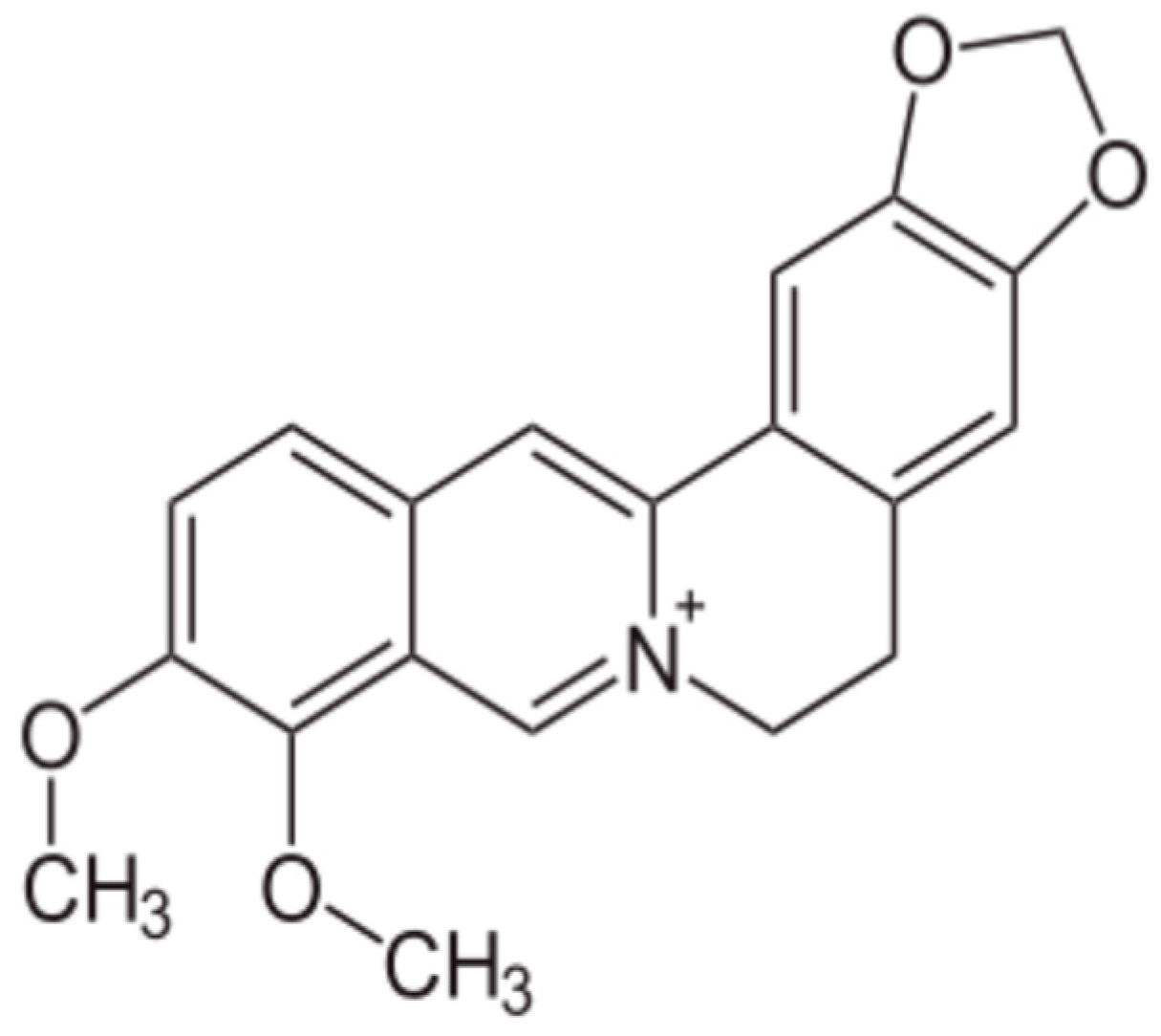
- Luiza Andreazza, N.; Vevert-Bizet, C.; Bourg-Heckly, G.; Sureau, F.; José Salvador, M.; Bonneau, S. Berberine as a photosensitizing agent for antitumoral photodynamic therapy: Insights into its association to low density lipoproteins. Int. J. Pharm. 2016, 510, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Yeh, C.C.; Lee, J.H.; Hung, C.F.; Chung, J.G. Berberine inhibited arylamine N-acetyltransferase activity and gene expression and DNA adduct formation in human malignant astrocytoma (G9T/VGH) and brain glioblastoma multiforms (GBM 8401) cells. Neurochem. Res. 2002, 27, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Obregón-Mendoza, M.A.; Meza-Morales, W.; Alvarez-Ricardo, Y.; Estévez-Carmona, M.M.; Enríquez, R.G. High Yield Synthesis of Curcumin and Symmetric Curcuminoids: A “Click” and “Unclick” Chemistry Approach. Molecules 2023, 28, 289. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zheng, J.; Li, W.D.Z. Concise total syntheses of berberine and its analogues enabled by trifluoroacetic anhydride-promoted decarbonylative-elimination reaction. Tetrahedron Lett. 2023, 132, 154826. [Google Scholar] [CrossRef]

| Limited Tissue Penetration: |
| Many photosensitizers absorb light in the visible spectrum, which has limited tissue penetration, hindering the treatment of deeper-seated tumors. |
| Lack of Tumor Specificity: |
| Photosensitizers can accumulate in both tumor and healthy tissues, leading to off-target effects and potential toxicity. |
| Inadequate Efficacy: |
| Some photosensitizers exhibit poor efficacy due to factors like low quantum yield, aggregation, or rapid clearance from the body. |
| Side Effects: |
| Photosensitizers can cause skin and eye photosensitivity, requiring careful patient management during and after treatment. |
| Variability in Clinical Protocols: |
| Early PDT studies faced challenges due to inconsistent light sources, varying photosensitizer formulations, and a lack of standardization in clinical protocols. |
| Type of Cell Death: |
| The type of cell death induced by PDT (apoptosis vs. necrosis) can be influenced by the photosensitizer, light intensity, and oxygen levels. Necrosis can trigger inflammation, while apoptosis is generally considered more desirable. |
| Dosage and Delivery: |
| Optimal dosage and delivery methods are critical for maximizing therapeutic efficacy and minimizing toxicity. This includes finding the right balance between light fluence, photosensitizer concentration, and targeting strategies. |
| Development of Next-Generation Photosensitizers: |
| Ongoing research focuses on developing photosensitizers with improved properties, such as enhanced NIR absorption, better targeting capabilities, and increased biocompatibility. |
| Areas for Improvement: |
| NIR-Absorbing Photosensitizers: |
| Developing photosensitizers that absorb in the near-infrared (NIR) region of the spectrum is crucial for deeper tissue penetration. |
| Targeted PDT: |
| Conjugating photosensitizers to antibodies, peptides, or other targeting agents can improve their selectivity for tumor cells. |
| Nanotechnology-Based Delivery: |
| Nanoparticles can be used to encapsulate and deliver photosensitizers, enhancing their solubility, stability, and tumor targeting. |
| Multifunctional Photosensitizers: |
| Developing photosensitizers with additional functionalities, such as imaging capabilities or drug delivery, can improve PDT’s therapeutic potential. |
| Standardization of Clinical Protocols: |
| Further research is needed to optimize light sources, photosensitizer dosages, and treatment protocols for various cancer type |
| 1. Generation | 2. Generation | 3. Generation | |
|---|---|---|---|
| examples of substances |
|
| modified existing PS with biological conjugates such as peptides, antibodies or antisense molecules, chemical coupling or encapsulation of PS in carriers |
| feature |
|
|
|
| Red to Near-Infrared (650–800 nm): |
| This range offers better tissue penetration compared to shorter wavelengths, allowing for deeper treatment in PDT. Examples: Some natural photosensitizers that absorb in this range include the following: Chlorophyll derivatives: Found in plants and algae, these molecules can be used in PDT applications, as shown in the study focusing on antibacterial nanoparticles. Phycobilins: Found in red algae and cyanobacteria, these pigments absorb light in the red and far-red regions. |
| Blue Light (405–435 nm): |
| While not as deeply penetrating as red light, blue light can be effective for treating superficial conditions. Examples: Curcumin: A compound found in turmeric, curcumin absorbs strongly in the blue region and is being investigated for its potential as a photosensitizer. |
| Other Wavelengths: |
| 470 nm: Some natural photosensitizers, like hypocrellin and cercosporin, have a maximum absorption peak around 470 nm. 500–600 nm: Anthocyanins, found in flowers like Hibiscus rosa-sinensis, have absorption peaks in this range. 635 nm: Blue light around this wavelength is also used in PDT. |
| Important Considerations: |
| Light Source: |
| The specific wavelength used depends on the photosensitizer and the target tissue. |
| Tissue Penetration: |
| Shorter wavelengths are less effective at penetrating tissue than longer wavelengths. |
| ROS Production: |
| Photosensitizers must be able to generate reactive oxygen species (ROS) upon light activation to be effective in PDT |
1. Emodin/Emodin Anthrone as a Starting Material [32] |
| Emodin and its reduced form, emodin anthrone, are common starting materials for hypericin synthesis. |
| Emodin can be obtained from various plant sources like the Hypericum species. |
| Emodin anthrone can be synthesized from emodin through reduction, for example, using tin(II) chloride. |
2. Dimerization to Protohypericin [33]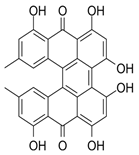 |
| A crucial step is the dimerization of emodin anthrone to form protohypericin. |
| This dimerization can be achieved through various methods, including oxidation with iron(III) chloride or other oxidizing agents. |
| For instance, in some methods, emodin anthrone is dimerized in the presence of pyridine and pyridine N-oxide, followed by oxidation to form protohypericin. |
3. Conversion to Hypericin [34]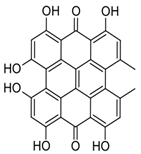 |
| Protohypericin, the immediate precursor, is then converted to hypericin through irradiation with visible light. |
| This light-induced conversion is a key step in the final stage of hypericin synthesis. |
| 4. Biosynthesis [35] |
| Hypericin biosynthesis in plants is believed to involve polyketide synthase (PKS) enzymes. |
| The PKS pathway leads to the formation of an octaketide chain, which undergoes cyclization and decarboxylation to form emodin anthrone, a precursor to hypericin. |
| The hyp-1 gene, identified in Hypericum species, is also involved in hypericin biosynthesis, potentially catalyzing the dimerization of emodin or emodin anthrone and subsequent steps. |
| 5. Recent Advances [36]: |
| Recent research has focused on improving the efficiency and yield of hypericin synthesis, including exploring green synthesis methods and optimizing reaction conditions. |
| For example, some studies have investigated the use of ultrasonic-assisted extraction for hypericin from Hypericum perforatum. |
| Other studies have focused on developing more efficient chemical synthesis routes, such as those involving Diels–Alder reactions. |
| Hypericin’s Positive Aspects in PDT: |
| Effective Photosensitizer: |
| Hypericin is a strong photosensitizer, meaning it becomes activated by light and can then destroy targeted cells. |
| Tumor-Tropic: |
| It tends to accumulate in tumor tissue, enhancing its effectiveness in cancer treatment. |
| Antiviral Activity: |
| Light-activated hypericin has shown effectiveness against various viruses, including HIV. |
| Low Cytotoxicity: |
| In the absence of light, hypericin exhibits low toxicity, making it a safer option compared to some other photosensitizers. |
| Induces Cell Death: |
| Hypericin-PDT can induce both apoptosis (programmed cell death) and necrosis (uncontrolled cell death) in cancer cells. |
| Immunomodulatory Effects: |
| Sublethal doses of hypericin-PDT can have immunomodulatory effects, potentially helping in the treatment of persistent skin inflammation. |
| Promising in Onychomycosis: |
| Studies suggest hypericin-PDT is a promising treatment for onychomycosis (nail fungal infection). |
| Potential “Bed” Issues with Hypericin-PDT: |
| Phototoxic Skin Reactions: |
| High doses of hypericin can cause phototoxic skin reactions, even without detectable antiviral activity. |
| Timing of Light Application: |
| The efficacy of hypericin-PDT can be highly dependent on the timing between drug administration and light exposure. Studies suggest maximal effect is achieved when light is applied shortly after drug administration (e.g., 0.5 h), with efficacy decreasing as the interval increases. |
| Skin Distribution: |
| Hypericin distribution in the skin may not be homogenous, with accumulation in the stratum corneum and less in deeper layers. |
| Limited Topical Efficacy: |
| When applied topically, hypericin-PDT can be less efficient than other photosensitizers like methyl aminolevulinate (Me-ALA). |
| Vascular Damage: |
| Studies suggest vascular damage is a primary effect of hypericin-PDT, and its distribution within the tumor vasculature is crucial for PDT response. |
| Potential for Reduced Efficacy: |
| In some cases, hypericin’s efficacy in PDT may decrease when used in combination with other treatments, such as with 17-DMAG. |
| In conclusion, while hypericin offers several advantages as a photosensitizer in PDT, clinicians and researchers need to be mindful of its potential limitations, particularly regarding skin reactions and the timing of light application. Further research is needed to optimize hypericin-PDT protocols and minimize potential adverse effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inglot, J.; Strzelczyk, J.; Inglot, J.; Bartusik-Aebisher, D.; Aebisher, D. Isolation, Synthesis, and Use of Natural Photosensitizers in the Treatment of Central Nervous System Tumors. Chemistry 2025, 7, 148. https://doi.org/10.3390/chemistry7050148
Inglot J, Strzelczyk J, Inglot J, Bartusik-Aebisher D, Aebisher D. Isolation, Synthesis, and Use of Natural Photosensitizers in the Treatment of Central Nervous System Tumors. Chemistry. 2025; 7(5):148. https://doi.org/10.3390/chemistry7050148
Chicago/Turabian StyleInglot, Julia, Joanna Strzelczyk, Jadwiga Inglot, Dorota Bartusik-Aebisher, and David Aebisher. 2025. "Isolation, Synthesis, and Use of Natural Photosensitizers in the Treatment of Central Nervous System Tumors" Chemistry 7, no. 5: 148. https://doi.org/10.3390/chemistry7050148
APA StyleInglot, J., Strzelczyk, J., Inglot, J., Bartusik-Aebisher, D., & Aebisher, D. (2025). Isolation, Synthesis, and Use of Natural Photosensitizers in the Treatment of Central Nervous System Tumors. Chemistry, 7(5), 148. https://doi.org/10.3390/chemistry7050148








