Abstract
This work was the first investigation of the essential oil composition of Goniothalamus tortilipetalus M.R.Hend. The aim of this study is to investigate the essential oil composition extracted from different parts of Goniothalamus tortilipetalus M.R.Hend., including flowers, leaves, and twigs, and to evaluate their antioxidant and antibacterial activities. The Clevenger apparatus was used for hydrodistillation to prepare the essential oils. The essential oils were investigated using gas chromatography–mass spectrometry (GC-MS). The three major compounds of the flowers were bicyclogermacrene (15.81%), selin-11-en-4-α-ol (14.68%), and E-caryophyllene (7.02%), whereas the leaves were p-cymene (39.57%), ascaridole (9.39%), and α-copaene (9.12%). In the case of the twigs, α-copaene (10.34%), selin-11-en-4-α-ol (8.85%), and p-cymene (7.76%) were the major compounds. The flower essential oil showed antioxidant activities with IC50 values of 725.21 µg/mL and 123.06 µg/mL for DPPH and ABTS assays, respectively. The flower essential oil also displayed antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Micrococcus luteus, Salmonella typhimurium, and Shigella flexneri, with the same MIC value of 640 µg/mL.
1. Introduction
Plants have been known for their use in traditional medicines for centuries [1], and many of them have been reported for biological activities with potential therapeutic applications. The genus of Goniothalamus is one of the largest genera of the family of Annonaceae, and over 150 species were discovered worldwide [2]. Different parts of Goniothalamus species have been used for the treatment of fever, scabies, edema, typhoid fever [3], asthma, malaria, stomachache [4], and also as a mosquito repellent [5]. Goniothalamus species produced diverse types of chemical constituents, including styryl lactones [6], alkaloids [3], flavonoids [7,8], steroids, terpenoids [7], and acetogenins [9]. Many of these compounds showed various biological activities such as antimicrobial [10], antioxidant [7], anti-inflammatory [11,12], and cytotoxicity [5,7,9]. In the case of essential oils, many Goniothalamus species have been widely reported for their chemical compositions, including G. malayanus [13,14], G. uvariodes [14,15], G. macrophyllus [14,16], G. andersonii [14], G. cardiopetalus [17], G. clemensii [4], G. tapis [18], G. takhtajanii [19], and G. multiovulatus [19]. Nevertheless, the essential oil compositions and their biological activities have not been reported from Goniothalamus tortilipetalus M.R. Hend, according to the SciFinder Scholar database (Chemical Abstracts Service, Columbus, OH, USA). This information led us to investigate the essential oil compositions and their antioxidant and antibacterial activities from the flower, leaf, and twig essential oils of G. tortilipetalus. This work was the first investigation of essential oils compositions and their antioxidant and antibacterial activities in regard to G. tortilipetalus.
2. Materials and Methods
2.1. Plant Material
The flowers, leaves, and twigs of G. tortilipetalus were collected in April 2021 from Narathiwat Province, Thailand. The plant was identified by Mr. Abdulromae Baka, Independent Research Group on Plant Diversity in Thailand, Sichon, Nakhon Si Thammarat, Thailand. Specimens of this plant (MFU-NPR0214) have been deposited in the Natural Products Research Laboratory at the Mae Fah Luang University under specimen number MFU-NPR0214.
2.2. Extraction of the Essential Oils
The flowers, leaves, and twigs of G. tortilipetalus were washed and crushed with a grinder before being hydrodistilled. The materials were immersed in 500 mL distilled water and subjected to hydrodistillation at 100 °C for 4 h using Clevenger apparatus to obtain the volatile oils, which were dried over anhydrous sodium sulfate (Na2SO4). The % yields of the extracts were calculated using the material weights.
2.3. Analysis by GC/MS
The essential oils were characterized through GC/MS using Agilent Technologies, HP 6890 gas chromatography with the HP 5973 mass selective detector (Agilent Technologies, Santa Clara, CA, USA). An HP-5 ms (5% phenylpolymethylsiloxane) capillary column (30 m length × 0.25 mm id × 0.25 µm film thickness, Agilent Technologies, CA, USA) was used. Helium (99.9% purity) was used as carrier gas with a flow rate of 1 mL/min and injection in split mode 1:70. The oven temperature was set at 60 °C and was increased at 3 °C/min to 220 °C. The injector and detector temperatures were set at 250 °C and 280 °C, respectively. The mass spectrometer operated in Electron Impact mode at 70 eV, and the electron multiplier voltage was 1150 V. MS data were acquired in scan mode in the range of m/z 29–300. The ion source and quadrupole temperatures were set at 230 °C and 150 °C, respectively [20]. Furthermore, for the identification of the isolated compounds, the retention indices (RIs) were compared relative to a series of C8–C20 n-alkanes standard and the mass spectra of individual components with the reference mass spectra via the National Institute of Standards and Technology (NIST) mass spectral library. Also, their mass spectra were compared with their Kovats index (KI) [21] with that determined by the host lab. The chemical compositions of essential oils were summarized as a percent relative peak area, as shown in Table 1.

Table 1.
Chemical composition (relative area percentage) of the essential oils from flowers, leaves, and twigs of G. tortilipetalus by GC–MS.
2.4. Antioxidant Activity by DPPH Free Radical Scavenging Assay
The antioxidant activity of the flower, leaf, and twig essential oils of G. tortilipetalus was assessed based on the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method, as described previously [20]. DPPH (Sigma Aldrich, St. Louis, MI, USA) radical scavenging methanolic solution (6 × 10−5 M, 100 µL) was prepared. The essential oils were prepared in methanol at serially diluted concentrations of 100, 250, 500, 1000, 2000, and 3000 µg/mL. A mixture of diluted essential oils (100 µL) and DPPH methanolic solution (100 µL) was prepared in a 96-well microplate. The solution was incubated at room temperature in darkness for 30 min. The absorbance of the reaction solution was measured at 517 nm using the microplate reader (Biochrom Asys UVM 340 Microplate Reader, Biochrom, Cambridge, UK). Ascorbic acid was used as the positive control at serially diluted concentrations (1, 2, 5, 10, and 15 µg/mL in methanol). The DPPH radical scavenging activity was expressed as the inhibitory concentration at 50% (IC50).
2.5. Antioxidant Activity Using ABTS Radical Cation Scavenging Assay
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) radical cation scavenging assay was performed as previously described [22]. In brief, the working solution of ABTS radical cation (ABTS•+) was prepared from the reaction of equal volumes of 7 mM of ABTS with 2.45 mM of potassium persulfate in the dark at room temperature for 16 h before use. The working solution of ABTS•+ was adjusted to the absorbance of 0.70 ± 0.02 at 734 nm with ethanol. The essential oils were prepared at serially diluted concentrations of 25, 50, 100, 250, and 500 µg/mL. An aliquot of 20 µL of diluted essential oils was mixed with 180 µL of ABTS•+ solution and allowed to stand in the dark at room temperature for 5 min, then the absorbance of the reaction solution was measured at 734 nm using the microplate reader (Biochrom Asys UVM 340 Microplate Reader, Biochrom, Cambridge, UK). Serially diluted ascorbic acid concentrations (1.5, 3, 6, 12, and 25 µg/mL) were used as the positive controls. The ABTS radical cation scavenging activity of essential oils was expressed as the inhibitory concentration at 50% (IC50).
2.6. Antibacterial Activity
The antibacterial activities of the essential oils from G. tortilipetalus were demonstrated against four Gram-positive bacteria (Bacillus subtilis TISTR 1248, Listeria monocytogenes F2369, Staphylococcus aureus ATCC 25923, and Micrococcus luteus DMST 15503), five Gram-negative bacteria (Escherichia coli TISTR 780, Salmonella typhimurium DMST 562, Pseudomonas aeruginosa ATCC 10145, Shigella flexneri DMST 4423, and Salmonella typhi DMST 22842). The bacterial strains were obtained from the Microbiological Resources Centre of the Thailand Institute of Scientific and Technological Research. Microdilution in the Mueller–Hinton broth was performed in the 96-well plates to determine the minimum inhibitory concentration (MIC) [20]. The essential oils were diluted with DMSO and then loaded in the Muller–Hinton broth microdilution with serial dilution (twofold). One hundred microliters of microbial culture, approximately 1.0 × 106 CFU/mL, was added to the 96-well plates. The negative control contained only the extraction buffer without microorganisms. The broth cultures of each strain were incubated aerobically at 37 °C for 24 h. After incubation, the MIC value was measured as the lowest concentration of the essential oils that completely inhibit the growth of microorganisms. Ampicillin, vancomycin, and gentamicin were used as positive controls.
3. Results and Discussion
3.1. Essential Oil Yield and Chemical Composition
The essential oils of the flowers, leaves, and twigs were obtained using hydrodistillation with a yield of 122.9 mg (0.06%, w/w), 167.1 mg (0.08%, w/w), and 47.0 mg (0.02%, w/w), respectively. The analyses of those essential oils by GC/MS identified 32, 21, and 51 compounds, comprising 91.18%, 96.97%, and 95.10% of the total peak areas (Figure 1) from the flowers, leaves, and twigs, respectively. The chemical compositions of those essential oils are shown in (Table 1). The percentage of monoterpenes, oxygenated monoterpenes, sesquiterpenes, and oxygenated sesquiterpenes found in the flower essential oil was 3.61%, 0.54%, 49.12%, and 37.91%, the percentage found in the leaf essential oil was 71.36%, 4.24%, 13.49%, and 7.22% and in the twig essential oil showed percentage of 25.81%, 2.75%, 34.67%, and 30.73%, respectively. The chemical constituents of leaf oil were discernibly different from those of flower and twig oils in that the former was composed almost entirely of monoterpene hydrocarbons (71.36%). In contrast, the twig oil showed similar major components but considerable variation in the percentage of individual constituents. In comparing the flower and twig oils, the percentage of sesquiterpene hydrocarbons and oxygenated sesquiterpenes were slightly similar, but the major constituents were discernibly different. The major components of the flowers were identified as bicyclogermacrene (15.81 ± 0.47%), selin-11-en-4-α-ol (14.68 ± 0.16%), E-caryophyllene (7.02 ± 0.05%), and β-selinene (6.89 ± 0.04%), whereas p-cymene (39.57 ± 0.65%), ascaridole (9.39 ± 0.09%), α-copaene (9.12 ± 0.15%), and spathulenol (7.22 ± 0.19%) were the main components of the leaves. In the case of the twigs, the main components were similar to those of flowers and leaves, including α-copaene (10.34 ± 0.09%), selin-11-en-4-α-ol (8.85 ± 0.07%), p-cymene (7.76 ± 0.11%), and α-terpinene (7.08 ± 0.03%).
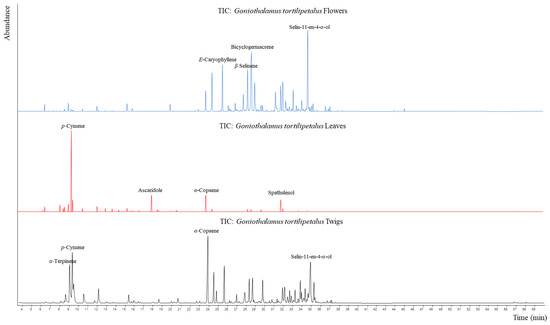
Figure 1.
GC–MS chromatogram of flower, leaf, and twig essential oils from G. tortilipetalus.
Previous chemical composition investigations of the leaf essential oils from Goniothalamus species found that the different species will produce different major compositions. For example, the G. uvariodes revealed 51 components, mainly comprising β-cubebene (15.2%), elemol (9.7%), epi-α-cadinol (6.2%), and α-muurolene (4.8%) [15]. The leaf essential oils of G. malayanus and G. andersonii consisted of 43 and 25 components, respectively. The major compounds of G. malayanus were β-selinene (33.6%), varidifloral (13.1%), epi-globulol (7.7%), and E-nerolidol (4.4%). In comparison, the major components present in G. andersonii were guaiol (28.6%), elemol (19.6%), β-caryophyllene (7.7%), and Z-nerolidol (3.7%) [13]. In addition, the leaf essential oils from G. tapis and G. tapisoides have been reported and the presence of 29 and 28 compounds, respectively, was revealed. The major components of G. tapis were α-copaene (23.8%), linalool (18.5%), β-caryophyllene (14.4%), and 1,8-cineole (7.6%), whereas G. tapisoides showed 1,8-cineole (79.0%), α-pinene (9.6%), α-terpineol (4.4%), and terpinene-4-ol (2.3%) as major compounds [18]. In another study, the chemical constituents of essential oils obtained from the leaf of G. takhtajanii, G. multiovulatus, and G. wightii were reported [19]. The major constituents of G. takhtajanii were linalool (17.6%), α-phellandrene (16.7%), bicycloelemene (8.3%), and bicyclogermacrene (8.0%) [19]. The G. multiovulatus mainly comprised β-caryophyllene (32.0%), α-humulene (21.2%), caryophyllene oxide (5.6%), and spathulenol (5.2%) [19]. The major constituents of G. wightii mainly comprised linalool (18.9%), δ-cadinene (15.5%), bicyclogermacrene (15.3%), and bicycloelemene (12.7%) [19]. Previous studies revealed that leaf oils from different Goniothalamus species showed variations in their major components, with variable quantities of percentage constituents. However, linalool and β-caryophyllene were identified as common major compounds in the leaves across this genus. A comparison of the leaf oil from G. tortilipetalus revealed a similarity to other species regarding α-copaene being one of the main components.
Regarding flower and twig essential oils, 116 and 21 components were identified from the flower essential oils of G. marcanii [20] and the twig essential oils of G. macrophyllus [16], respectively. Of these, caryophyllene oxide (19.2%), E-caryophyllene (14.5%), β-copaene (4.1%), and α-humulene (3.6%) were found as major components in the flower essential oils of G. marcanii [20]. Still, geranyl acetate (45.5%), geraniol (17.0%), linalool (12.7%), and camphene (7.5%) were identified as major components of twig essential oils [16]. This study revealed that the major compounds of essential oil differed from our results, which could be attributed to the different species. To the best of our knowledge, this study was the first report on the chemical compositions of the essential oils of G. tortilipetalus. It was observed that each of the Goniothalamus essential oils has its chemical compositions, which differ from others. Our data exhibited partial similarity to those previously reported in the essential oils of some Goniothalamus species, with the presence of some common major and minor compounds.
3.2. Antioxidant Activity
Antioxidant activity is one of the most valuable biological activities for the cosmetics, food, and beverage industries. This work was the first investigation of the antioxidant activity of essential oil compositions from G. tortilipetalus. The antioxidant potential of the essential oils from G. tortilipetalus was assessed through the DPPH radical scavenging and ABTS radical cation assay. The concentration that inhibits 50% of the DPPH and ABTS free radical (IC50) are presented in Table 2. The DPPH radical scavenging activity of the essential oils of the flowers, leaves, and twigs of G. tortilipetalus showed weak antioxidant activity with IC50 values of 725.21 µg/mL, 2017.39 µg/mL, and 2435.50 µg/mL, respectively. In the case of the ABTS radical scavenging activity, the percentage inhibitions of the ABTS radicals were similar to those of DPPH. The essential oils of flowers, leaves, and twigs showed IC50 values of 123.06 µg/mL, 290.63 µg/mL, and 382.17 µg/mL, respectively.

Table 2.
The DPPH and ABTS radical scavenging activities of essential oils from flowers, leaves, and twigs of G. tortilipetalus.
3.3. Antibacterial Activity
The essential oils extracted from flowers, leaves, and twigs of G. tortilipetalus were evaluated for their antibacterial activities against four Gram-positive bacteria, including B. subtilis, L. monocytogenes, S. aureus, and M. luteus, and five Gram-negative bacteria, E. coli, S. typhimurium, P. aeruginosa, S. flexneri, and S. typhi. The minimum inhibitory concentration (MIC) values obtained from the bacterial strains tested are shown in Table 3. The flower essential oil showed antibacterial activity against the Gram-positive bacteria, B. subtilis, S. aureus, and M. luteus, and the Gram-negative bacteria, S. typhimurium and S. flexneri, with the same MIC value of 640 µg/mL. Whereas the leaf oils showed weak antibacterial activity against S. aureus, M. luteus, S. typhimurium, and S. typhi with an MIC value of 1280 µg/mL. In the case of the twig essential oils, only M. luteus was inhibited with the MIC value of 1280 µg/mL. The antimicrobial activities of the essential oils obtained from Goniothalamus species have been reported. The flower essential oil from G. marcanii presented caryophyllene oxide (19.3%), E-caryophyllene (14.6%), β-copaene (4.2%), and α-humulene (3.6%) as main compounds [20]. It exhibited moderate activities against S. agalactiae, S. aureus, S. epidermidis, P. mirabilis, S. typhimurium, and E. coli with MIC values in the range of 15.62–1000 µg/mL using the paper disk diffusion method [20]. The stem bark essential oils of G. cardiopetalus contained linalool (11.7%), α-pinene (7.0%), trans-pinocarveol (5.2%), and caryophyllene oxide (5.0%) as major constituents and displayed antimicrobial activity with MIC values in the range of 1.0–1.5 mg/mL for the Gram-positive bacteria (S. aureus, S. albus, S. epidermidis, S. mitis, M. luteus, B. subtilis, and B. cereu) and 1.5–6.5 mg/mL for the Gram-negative bacteria (E. coli, E. aerogenes, K. pneumoniae, S. typhi, P. vulgaris, P. aeruginosa) [17]. In this study, the antibacterial activities of essential oils from G. tortilipetalus are in agreement with the previous report. Still, they are less active than essential oils from G. marcanii and G. cardiopetalus, probably due to the difference in the main chemical composition.

Table 3.
Antibacterial activity of essential oils from flowers, leaves, and twigs of G. tortilipetalus.
4. Conclusions
In conclusion, the findings of the present study indicated that essential oils obtained from the flowers, leaves, and twigs of G. tortilipetalus were rich in monoterpene and sesquiterpene hydrocarbons. Bicyclogermacrene, p-cymene, and α-copaene were the main constituents of the total oil compositions from the flower, leaf, and twig essential oils, respectively. The DPPH and ABTS radical scavenger test of essential oils showed weak antioxidant activities. The flower essential oils showed antibacterial activity against all tested microorganisms. Meanwhile, the leaf oil showed a moderate inhibitory power towards the Gram-positive (S. aureus and M. luteus) and the Gram-negative (S. typhimurium and S. typhi) strains.
Author Contributions
Conceptualization, A.A. and S.L.; methodology, A.A.; validation, P.P., S.D. and T.M.; formal analysis, A.A., R.C. and T.D.; investigation, A.A.; resources, S.L. and R.C.; data curation, A.A.; writing—original draft preparation, A.A. and S.L.; writing—review and editing, A.A., P.P. and S.L.; visualization, A.A.; supervision, S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Research Council of Thailand and Mae Fah Luang University, grant number N42A650373. The Postdoctoral Fellowship from Mae Fah Luang University to Dr. Aknarin Anatachodwanit was also acknowledged.
Data Availability Statement
Any request for further data should be made via contacting the author.
Acknowledgments
The authors gratefully acknowledge the Postdoctoral Fellowship from Mae Fah Luang University to Aknarin Anatachodwanit, and we would like to thank Mae Fah Luang University for its laboratory facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aslam, M.S.; Ahmad, M.S.; Mamat, A.S.; Ahmad, M.Z.; Salam, F. Goniothalamus: Phytochemical and ethnobotanical review. Recent Adv. Biol. Med. 2016, 2, 34–47. [Google Scholar] [CrossRef]
- Wiart, C. Goniothalamus species: A source of drugs for the treatment of cancers and bacterial infections? Evid. Based Complement. Altern. Med. 2007, 4, 635095. [Google Scholar] [CrossRef] [PubMed]
- Jaidee, W.; Andersen, R.J.; Patrick, B.O.; Pyne, S.G.; Muanprasat, C.; Borwornpinyo, S.; Laphookhieo, S. Alkaloids and styryllactones from Goniothalamus cheliensis. Phytochemistry 2019, 157, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Moharam, B.A.; Jantan, I.; Ahmad, F.; Jalil, J. Antiplatelet aggregation and platelet activating factor (PAF) receptor antagonistic activities of the essential oils of five Goniothalamus species. Molecules 2010, 15, 5124–5138. [Google Scholar] [CrossRef] [PubMed]
- Surivet, J.P.; Vatèle, J.M. Total synthesis of antitumor Goniothalamus styryllactones. Tetrahedron 1999, 55, 13011–13028. [Google Scholar] [CrossRef]
- Meesakul, P.; Jaidee, W.; Richardson, C.; Andersen, R.J.; Patrick, B.O.; Willis, A.C.; Muanprasat, C.; Wang, J.; Lei, X.; Hadsadee, S.; et al. Styryllactones from Goniothalamus tamirensis. Phytochemistry 2020, 171, 112248. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, E.; Salim, K.A.; Lim, L.B. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud. Univ. Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef]
- Trieu, Q.H.; Pham, V.C.; Retailleau, P.; Litaudon, M.; Doan, T.M.H. Rare flavonoids and sesquiterpenoids isolated from the leaves of Goniothalamus gracilipes. Fitoterapia 2021, 155, 105034. [Google Scholar] [CrossRef]
- Tantithanaporn, S.; Wattanapiromsakul, C.; Itharat, A.; Keawpradub, N. Cytotoxic activity of acetogenins and styryl lactones isolated from Goniothalamus undulatus Ridl. root extracts against a lung cancer cell line (COR-L23). Phytomedicine 2011, 18, 486–490. [Google Scholar] [CrossRef]
- Funnimid, N.; Pompimon, W.; Nuntasaen, N. In vitro evaluation of crude extracts and isolated compounds from Goniothalamus rongklanus and Goniothalamus latestigma for bioactive properties. J. Nat. Remedies 2019, 19, 146–152. [Google Scholar] [CrossRef]
- Zohdi, R.M.; Kaharudin, F.A.; Mukhtar, S.M.; Sidek, H.M.; Ismail, N.H. Dichloromethane stem bark extract of Goniothalamus lanceolatus Miq. modulates inflammatory cytokines and ameliorates tissue damage in Plasmodium berghei-infected mice. J. Appl. Pharm. Sci. 2022, 12, 149–155. [Google Scholar] [CrossRef]
- Suthiphasilp, V.; Maneerat, W.; Rujanapun, N.; Duangyod, T.; Charoensup, R.; Deachathai, S.; Andersen, R.J.; Patrick, B.O.; Pyne, S.G.; Laphookhieo, S. α-Glucosidase inhibitory and nitric oxide production inhibitory activities of alkaloids isolated from a twig extract of Polyalthia cinnamomea. Bioorg. Med. Chem. 2020, 28, 115462. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, F.; Ahmad, A.S. A comparative study of the essential oils of four Goniothalamus species. Acta Hortic. 2003, 677, 27–36. [Google Scholar] [CrossRef]
- Jantan, I.B.; Ahmad, F.B.; Din, L.B. Chemical constituents of the bark oil of Goniothalamus macrophyllus Hook. f. from Malaysia. J. Essent. Oil Res. 2005, 17, 181–183. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Jantan, I.B. Chemical constituents of the essential oils of Goniothalamus uvariodes King. Flavour. Fragr. J. 2003, 18, 128–130. [Google Scholar] [CrossRef]
- Humeirah, A.S.; Azah, M.N.; Mastura, M.; Mailina, J.; Saiful, J.A.; Muhajir, H.; Puad, A.M. Chemical constituents and anti-microbial activity of Goniothalamus macrophyllus (Annonaceae) from Pasoh Forest Reserve, Malaysia. Afr. J. Biotechnol. 2010, 9, 5511–5515. [Google Scholar]
- Hisham, A.; Pathare, N.; Al-Saidi, S.; Jayakumar, G.; Ajitha, M.D.; Harikumar, B. The composition and antimicrobial activity of stem bark essential oil of Goniothalamus cardiopetalus (Bl.) Hook. f. et Thoms. J. Essent. Oil Res. 2006, 18, 451–454. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Moharm, B.A.; Jantan, I. A comparative study of the constituents of the essential oils of Goniothalamus tapis Miq. and G. tapisoides Mat Salleh from Borneo. J. Essent. Oil Res. 2010, 22, 499–502. [Google Scholar] [CrossRef]
- Thang, T.D.; Dai, D.N.; Ogunwande, I.A. Identification of the volatile compounds in the leaf and stem bark of three Goni-othalamus species from Vietnam. J. Essent. Oil-Bear. Plants. 2016, 19, 743–749. [Google Scholar] [CrossRef]
- Monggoot, S.; Pripdeevech, P. Chemical composition and antibacterial activities of Goniothalamus marcanii flower essential oil. J. Appl. Pharm. Sci. 2017, 7, 144–148. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).