Abstract
Therapeutic nanomaterials serve as an important platform for drug delivery under image guidance. Despite significant growth and broad applications, their design specifics remain a subject of continued interest primarily due to multifunctional factors involved, ranging from nanomaterial properties, imaging modalities, and therapeutic agents to activation strategies. This review article summarizes key findings on their design characteristics with a particular interest in strategies developed for therapeutic activation (release). First, their activation can be controlled using either an endogenous factor including low pH and glutathione or an external stimulation by light, ultrasound, or electromagnetic field. The former is passively controlled from a spatiotemporal aspect compared to the latter, which is otherwise actively controlled through drug linker photolysis, nanomaterial disassembly, or gate opening. Second, light stimulation serves a most notable strategy due to its essential role in controlled drug release, photothermal activation (hyperthermia), and photodynamic production of reactive oxygen species (ROS). Third, some of those activation strategies that rely on ultrasound, photothermal, photoacoustic, magnetic field, or X-ray radiation are dually functional due to their role in imaging modalities. In summary, this review article presents recent advances and new insights that pertain to nanotherapeutic delivery systems. It also addresses their technical limitations associated with tissue penetration (light), spatial resolution (ultrasound, hyperthermia), and occurrence of cellular resistance (ROS).
1. Introduction
Over recent decades, we have seen a rapid growth in and significant contribution by nanoscale therapeutic systems in image-guided delivery [1]. These systems utilize an integrative approach that combines both imaging and delivery functions in a single nanoscale entity. Their design is composed of three complementary modules that include an imaging probe, a therapeutic component, and a nanomaterial platform. Their imaging function plays a fundamental role in detecting and tracking the nanotherapeutic system following its administration (Figure 1). This helps to identify whether it is well distributed at its targeted site from a pharmacokinetic perspective and thus to determine an optimal timing for its therapeutic activation. Its therapeutic module is achieved with many strategies collectively by drug release [2,3,4,5], photothermal activation [6], photodynamic activation [7,8], or the combination of these. Nanomaterial platforms are more variable in the range of their structure and function as illustrated in dendrimer polymers [9,10,11], upconversion nanocrystals (UCN) [12,13,14], metal-organic frameworks [15], magnetic nanoparticles [16], and hollow nanostructures [17]. This modular approach employed in nanotherapeutic design offers a combinatorial convenience and broad applicability in various therapeutic areas and personalized medicine [1].
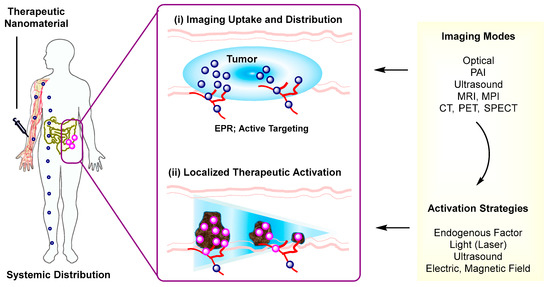
Figure 1.
A schematic for image-guided nanotherapeutic delivery in tumors. A therapeutic nanomaterial is taken up in a diseased tissue via passive infiltration through leaky blood vessels or active biomarker targeting, imaged and therapeutically activated by endogenous factors or external stimulation.
In this article, therapeutic strategies are presented with regard to their activation mechanisms. Activation strategies which are covered here involve stimulations by either endogenous factors (pH, glutathione) [18,19] or external stimuli including light [2,3,4,5], ultrasound (US) [20,21,22], alternating magnetic field [4,16], or electric field [23]. These stimuli provide various mechanisms applicable for therapeutic activation including drug release that occurs through linker cleavage, nanomaterial disassembly, or gate opening. These strategies are described along with design factors involved in the selection and integration of imaging probes, payloads, and linkers to nanomaterials. Of particular focus is their individual role in therapeutic activation that occurs via stimulus-controlled linker cleavage, nanomaterial degradation, or induction of porosity.
Another important aspect described here relates to how therapeutic strategies are integrated with imaging modalities. For this purpose, delivery systems are to be introduced briefly for their imaging capability, which is achieved with ultrasound (US), magnetic resonance imaging (MRI), magnetic particle imaging (MPI), thermal imaging, photoacoustic imaging (PAI), X-ray computed tomography (CT), γ-ray positron emission tomography (PET), or single photon-emission computerized tomography (SPECT). It should be noted that these imaging methods have already been reviewed individually and comprehensively in several excellent articles [24,25,26,27,28,29,30,31,32,33], including those focused on optical [34], magnetic resonance [35], magnetic particle [32,33], photothermal [36], photoacoustic [37], and ultrasound [38].
Briefly, nanoscale systems are engineered for image-guided delivery through modular assembly. They are variable in modular principles, design characteristics, and activation strategies. Identifying a strategy for most optimal therapeutic efficacy remains an objective of significant interest [39]. This article offers new insights learned from recent advances in image-guided systems.
2. Therapeutic Activation by Endogenous Factors
2.1. Low pH
Cells provide several stimulating conditions applicable for controlled drug release. Some of these relate to low pH conditions in subcellular endosomes (pH 5.0–6.0) [40] and lysosomes [41,42] where nanomaterials initially reside after intracellular uptake through receptor-mediated endocytosis [43,44]. Similarly, acid-mediated release is reported to occur at extracellular matrices in tumors because they are more acidic than normal tissues [18]. This release strategy controlled by lowered pH has been used frequently in delivery systems. Some of its mechanistic basis is attributed to acid-catalyzed linker hydrolysis [3] or degradation of acid-susceptible nanomaterials that include metal-organic frameworks [45], nanogels [35], and mesoporous silica oxide (mSiO2) [46].
Figure 2 shows representative delivery systems based on pH-mediated therapeutic release. These include single-walled carbon nanotubes (SWNT) [43] designed for endosomal release of its cisplatin (IV) prodrug [3] (Figure 2A) and bortezomib-loaded polydopamine nanoparticulates [47] in which bortezomib linked through a boronic acid–catechol bonding is demonstrated for faster release at pH 5.4 than 7.4 (Figure 2B). The schematic in Figure 2D illustrates polymer micelles designed for extracellular activation catalyzed by acidic pH or proteinases [18]. This endogenous control is likewise applied in the release of doxorubicin loaded in acid-labile nanogels [35] or on the surface of iron oxide nanoparticles (IONP; Fe3O4) [48]. Delivery systems based on such magnetic nanomaterials as IONP allow MRI-guided drug delivery [35,48]. Another system controlled by low pH is porous mSiO2 applied in the delivery of camptothecin, an anticancer drug that inhibits topoisomerase I in the nucleus [46]. Here, the drug molecule loaded in the porous shell layer of mSiO2 is released when the silica layer is degraded in acidic subcellular compartments.
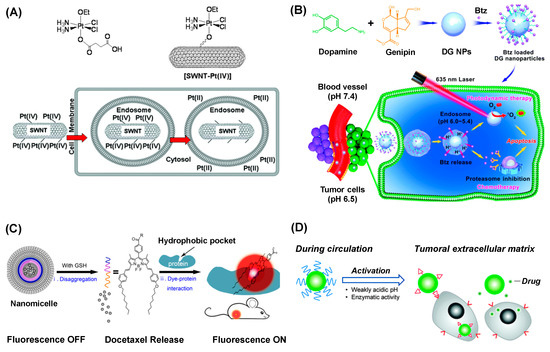
Figure 2.
Representative strategies of therapeutic release by endogenous factors. (A) Single-walled carbon nanotube (SWNT) carrying a cisplatin (IV) prodrug, and its activation at endosomes where lowered pH facilitates reductive release of platinum (II) complex [43]. Reproduced with permission, Copyright 2007, American Chemical Society. (B) Bortezomib (Btz)-loaded polydopamine nanoparticle designed for pH-mediated drug release in combination with photodynamic therapy [47]. Reproduced with permission, Copyright 2019, Royal Society of Chemistry. (C) Docetaxel-loaded nanomicelle for fluorescence-guided tumor imaging and glutathione (GSH)-mediated drug release [49]. Reproduced with permission, Copyright 2016, American Chemical Society. (D) pH-responsive polymer micelles designed for extracellular activation triggered by acidic pH or proteinases in a tumor microenvironment [18]. Reproduced with permission, Copyright 2009, American Chemical Society.
2.2. Glutathione (GSH)
Cellular cytosols contain glutathione (GSH) at higher levels (2–10 mM) than the extracellular side [19]. This differential GSH expression serves as a trigger for facilitated intracellular release which occurs through a thiol (GSH)-disulfide exchange [50]. Release systems controlled by GSH are composed of disulfide drug linkers [50], reducible prodrugs or nanomaterials [49,51] made with disulfide building blocks. Figure 2A and Figure 2C each illustrate such release that includes a cisplatin (IV) prodrug loaded on SWNT which undergoes reductive activation to a platinum (II) species [43] and docetaxel release from degradable polymer micelles made of GSH-cleavable poly(ethylene glycol) (PEG) [49]. The latter micelle system is of relevance to image-guided drug delivery due to its ability for induction of fluorescence by a boron dipyrromethene (BODIPY) derivative that serves as a fluorescent probe when it is co-released with docetaxel.
Other pathophysiological conditions play a significant role in therapeutic activation as well. These are related with upregulated redox enzymes [52,53] and tumor hypoxia (low oxygen) [54]. Each of these is exploitable for prodrug activation or release as reported in prodrugs based on SN38 [53], naloxone [52], and Pt [43,55]. Another condition involves matrix metalloproteinase [56,57,58], a hydrolytic enzyme upregulated at tumors which is applicable for catalytic linker cleavage. It plays a role in the tumor-targeted delivery of methotrexate carried by dendrimer [56,57] or hyaluronic acid polymer [58]. In short, though passively controlled, drug release that occurs under endogenous conditions constitutes one of principal strategies explored in delivery systems.
3. Photocontrolled Therapeutic Release
Light stimulation serves as one of the important strategies that allow an active control in drug release [5,39]. It occurs via linker photolysis, nanomaterial disassembly [59,60,61,62,63,64,65,66,67], and pore gating [68,69,70,71,72,73,74]. It shows a higher degree of spatiotemporal resolution [3,75,76,77] than other external strategies stimulated by ultrasound or magnetic field [13,78]. Collectively, this accounts for its relatively higher precision in imaging and therapeutic activation [9,79]. However, it is notable that despite its benefits in spatial resolution and greater photochemical selectivity, light irradiation suffers from relatively lower tissue penetration, light scattering, and absorption by non-targeted biological molecules and tissues, each contributing to reducing its efficiency for drug activation [80].
3.1. Linker Photolysis
Release controlled by linker photolysis involves drug conjugation through a photocleavable linker made of o-nitrobenzyl (ONB) [11,81,82], thioacetal ONB [9,79], coumarin [51,83,84], quinolone [85,86], pyrene [87], acridin-9-methanol [88], or N-methyl-4-picolinium [89]. Figure 3 illustrates two linker systems made of either gold nanoparticle (AuNP) (Figure 3A) [81] or poly(amidoamine) (PAMAM) dendrimer (Figure 3B) [10], each carrying ONB linked 5-fluorouracil (5-FU) or ciprofloxacin, respectively. Upon exposure to long wavelength UV (365 nm), linker photolysis occurs, leading to rapid drug release. This also occurs consistently in cell studies in which 5-FU release results in induction of cytotoxicity in MCF-7 tumor cells (Figure 3A) as does ciprofloxacin in induction of antibacterial activity against Escherichia coli (Figure 3B). This release strategy is broadly applicable to various payloads from anticancer drugs (doxorubicin [9,11,14,90], methotrexate [91,92], 5-fluorouracil [81,83,89], paclitaxel [93], camptothecin [94], chlorambucil [84,87,95], platinum prodrug [96,97,98]), antibacterial ciprofloxacin [10,99,100], and biomolecules (DNA [101], oligonucleotide [102,103], Cas9-sgRNA [104]) to gas molecules (CO [105], NO [106,107,108,109], SO2 [110], H2S [111,112]).
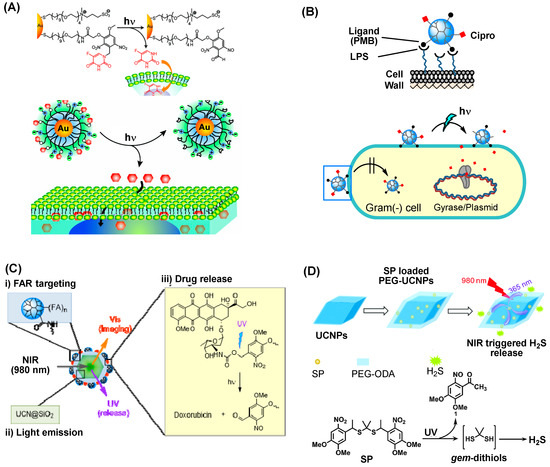
Figure 3.
Representative strategies of therapeutic release via linker photolysis. (A) UV controlled release of 5-fluorouracil (5-FU)@AuNP [81]. Reproduced with permission, Copyright 2009, American Chemical Society. (B) Light-controlled delivery of ciprofloxacin at Gram (−) cells using lipopolysaccharide (LPS)-targeting dendrimer [10]. Reproduced with permission, Copyright 2016, Royal Society of Chemistry. (C) Near IR (NIR)-controlled doxorubicin release from folate receptor (FAR)-targeting upconversion nanocrystal (UCN) via linker photolysis by luminescence emission from UCN [14]. Reproduced with permission, Copyright 2015, John Wiley & Sons. (D) NIR-controlled H2S release from its precursor and UCN-loaded nanogel [111]. Reproduced with permission, Copyright 2015, Royal Society of Chemistry.
Selecting light wavelengths that are optimal for linker photolysis is dependent on specific photophysical properties defined by linkers. This is illustrated with UV-cleavable ONB [11,81,82], visible light-cleavable coumarin [51,83,84], and near IR (NIR)-responsive cyanine [113,114]. However, linker integration with UCN opens an indirect route for NIR-triggered linker photolysis due to UCN luminescent emission at the UV and visible region upon irradiation at 980 nm [9,14,90]. This concept is illustrated in Figure 3 with UCN-based delivery systems designed for doxorubicin (Figure 3C) [14] or hydrogen sulfide (Figure 3D) [111], each linked through ONB to the UCN shell layer. Thus, NIR irradiation at 980 nm results in doxorubicin-induced cytotoxicity in folate receptor (FAR)-positive KB cancer cells as potent as UV irradiation (Figure 3C), whereas it accounts for more effective H2S release in a pork skin model due to its deep tissue irradiation than UV (Figure 3D). This NIR control is equally demonstrated in a range of payloads from doxorubicin [9,14,90] to macromolecular CRISPR-Cas9 [104]. Further benefits conferred by UCN relate to irradiation at NIR, which shows deeper tissue penetration compared to UV or visible light. Additionally, its luminescent bands contribute to UCN imaging and tracking as well [14,60,104,115].
In brief, release strategies via linker photolysis have yielded a remarkable growth in delivery systems. They have shown broad utilities in a variety of nanoscale systems including polymer [83,87,106], polymer micelle [87,94,97,116], PAMAM dendrimer [9,10,11,91,92,100], UCN [14,90,98,103,104,105,110] (LiNbO3 [84]), AuNP [81,101], silver nanoparticle (AgNP) [102], quantum dot (QD) [89,107], TiO2 [109], mSiO2 [95], graphene [108], RNA nanocage [93], and hydrogel [99].
3.2. Disassembly
Release systems via disassembly are designed in diverse ways though their mechanisms occur mainly through linker photolysis [51,59,117,118,119,120,121,122,123,124,125] or photoisomerization. Systems that are applicable for photolytic disassembly rely on photodegradable soft nanomaterials made of liposome [117,118], polymer [125], polymer micelle [119,120,121,123,124,126,127], polymersome [51], tubisome [122], and functionalized UCN [59,60,61]. These are applicable for loading various therapeutic agents that include doxorubicin [51,59,121,122,123,126,127], camptothecin [125], siRNA [61], and aptamer Sgc8 [124]. Co-loading a fluorescent dye (6-carboxyfluorescein [117], Nile red [119,120]) or an MRI contrast agent [118] serves as a route for monitoring drug release under image guidance. This is highlighted in Figure 4 with photocleavable liposomes loaded with Gd chelates demonstrated for MRI-guided delivery (Figure 4A) [118]. Upon irradiation at 400 nm, this liposome shows a time-dependent decrease in relaxivity (mM−1 s−1) in a consistent manner with a decrease in its ONB absorbance at 365 nm.
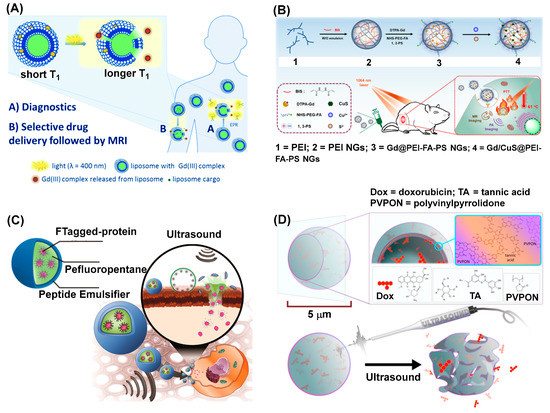
Figure 4.
Representative strategies of therapeutic release via controlled disassembly. (A) Light-activated liposome disassembly for magnetic resonance imaging (MRI) contrast imaging and therapeutic release [118]. Reproduced with permission, Copyright 2019, Royal Society of Chemistry. (B) MRI and photoacoustic image-guided photothermal ablation therapy using a polyethylenimine (PEI)-based nanogel carrying Gd/CuS@PEI-FA-PS [139]. Reproduced with permission, Copyright 2020, American Chemical Society. (C) Ultrasound (US)-triggered cytosolic delivery of green fluorescent protein (GFP) loaded in fluorous nanoemulsions [140]. Reproduced with permission, Copyright 2020, American Chemical Society. (D) US triggered release of encapsulated doxorubicin (Dox) from polymer microcapsules via capsule perforation and destruction [141]. Reproduced with permission, Copyright 2017, American Chemical Society.
Disassembly via photoisomerization occurs in systems incorporated with light-responsive units that undergo bond isomerization including azobenzene [128] or donor–acceptor Stenhouse adduct [129]. These units are readily incorporated in a range of nanomaterials from liposomes [128], polymer micelles [129,130,131,132,133,134], hydrogel [135,136], and metal-organic frameworks [137], to UCN [138]. These disassembly systems are successfully applied in the delivery of various payloads including erlotinib [130], 5-fluorouracil [137], doxorubicin [134], ciprofloxacin [136], propranolol [131], siRNA [132], and green fluorescent protein [135].
3.3. Gating
Gating refers to a process in which a payload is retained in a carrier until it is released upon gate opening. One approach involves a mechanism that induces photolytic alteration at a gate entrance by which its gate is opened (Figure 5). As shown in Figure 5A, this often occurs in porous materials like mesoporous silica nanoparticle (MSN), which is temporarily blocked at its gate entrance with a photocleavable valve [69,142,143] or polymer brush [68,115,144]. This system is effective for NIR (974 nm)-controlled doxorubicin delivery in HeLa cells as evidenced with the induction of potent cytotoxicity [69]. Another approach involves valve isomerization at the entrance as illustrated in Figure 5B [143]. Its delivery application is consistent with UV-controlled release of doxorubicin which results in the induction of cytotoxicity in macrophages. Molecular valves commonly utilized in this isomerization approach comprise azobenzne [71,143,145,146], coumarate [147], fumaramide [148], spiropyran [149], and donor–acceptor Stenhouse adduct [150]. Their role in gated release is well established by applications in various payloads including doxorubicin [71,143,145,146,150], camptothecin [150], naproxen [147], curcumin [151], DNA [152], and dye molecules including rhodamine B [148] and 4′,6-diamidino-2-phenylindole [149].
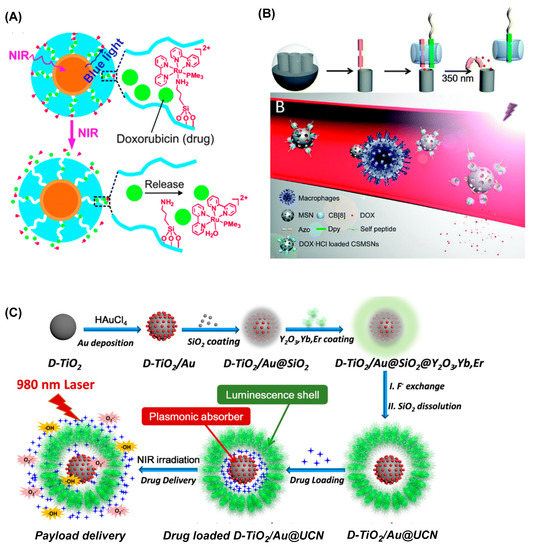
Figure 5.
Representative strategies of therapeutic release via controlled gating. (A) Doxorubicin release via NIR-triggered cleavage of a Ru complex valve in mSiO2-coated UCN [69]. Reproduced with permission, Copyright 2015, Royal Society of Chemistry. (B) Doxorubicin release via photoisomerization at a gate entrance in mesoporous silica nanoparticle (MSN) [143]. Reproduced with permission, Copyright 2015, Royal Society of Chemistry. (C) NIR light-controlled release of ampicillin from TiO2/Au@UCN nanocomposite [153]. Reproduced with permission, Copyright 2020, American Chemical Society.
Whereas most gating systems are designed for activation by UV or visible light, they can be functionalized for opening by NIR. This is achieved by integration of valve-attached mSiO2 with nano gold [68] or UCN [153], each serving as an NIR-responsive nanomaterial. Of note is a TiO2/Au@UCN nanocomposite (Figure 5C), a dual action system designed for NIR-controlled ampicillin release and ROS production by irradiation at 980 nm. This shows potent antibacterial activities against E. coli and methicillin-resistant Staphylococcus aureus (MRSA) in vitro [153]. Their gate opening occurs through valve alteration or isomerization in response to photothermal heating by nano Au or UV–VIS luminescence emitted from NIR-excited UCN. This NIR control has been successfully applied to several payloads including doxorubicin [68,69,143], fluorescein [115,144], and ruthenium-bipyridyl [142].
Briefly, light control constitutes one of principal strategies employed in the activation of nanotherapeutic systems. Its mechanisms for therapeutic release occur via linker photolysis, disassembly, or pore gating.
4. Photodynamic Activation
4.1. Reactive Oxygen Species (ROS)
Photodynamic activation involves the production of reactive oxygen species (ROS) which comprise singlet oxygen (1O2) and oxygen-based radical species [154,155]. This occurs through photosensitizer (PS) excitation as applicable to zinc phthalocyanine (ZnPc) [156,157], chlorin e6 (Ce6) [158], rose bengal [12,13], and NIR-responsive indocyanine dye [159]. Alternatively, ROS are produced by other types of PS based on photoactive polymers including polydopamine (Figure 2B) [47] and photoactive nanomaterials [76,160] including nanoTiO2 [161]. ROS production by most PS systems occurs by light absorption at visible wavelengths. However, UCN-integrated PS systems [12,13,153,157,162,163] are able to produce ROS by NIR irradiation at 980 nm due to UCN luminescence at UV–VIS bands. This NIR-based PS activation is more beneficial in therapeutic applications in vivo than in vitro due to its deeper penetration [12,163].
4.2. Photodynamic Delivery Systems
A typical system designed for photodynamic delivery consists of PS molecules encapsulated or conjugated in nanomaterials like mesoporous nanoparticles (NPs) [156], liposomes [164,165,166], polymers [47,167,168], polymer micelles [65,67,159,169,170], and lipid NPs [171]. Due to high chemical reactivity, ROS produced from these systems are able to make a broad range of non-selective cellular damages from lipid oxidation responsible for membrane ruptures to oxidative degradation in proteins and nucleic acids [76,160]. This accounts for its potent cytotoxicity induced in mammalian cells and bacterial pathogens [154,172], and it serves as a therapeutic basis for photodynamic therapy (PDT) [155,173,174,175].
Moreover, ROS contributes to controlled drug release through its participation in oxidative linker cleavage, disassembly or gating [66]. This is illustrated with Ce6-bound IgG nanocomplexes, an anticancer system demonstrated for its ability to deliver an immune checkpoint inhibitor under fluorescent image guidance [176]. Photodynamic release is equivalently applied to a broad range of therapeutic agents from anticancer drugs including doxorubicin [65,66,167], paclitaxel [166], bortezomib [47], SN38 [67], camptothecin [159], mitoxantrone [169], and 7-dehydrocholesterol [165] to antibacterial agents vancomycin [168] and ampicillin [153]. UCN integration allows NIR-controlled photodynamic drug release as illustrated in Figure 5C where ampicillin is released from a UCN-coated TiO2/Au nanocomposite [153].
In short, photodynamic activation serves as an important route for a dual therapy due to ROS-mediated cytotoxicity and the ability to participate in controlled drug release. PS systems also offer an optical imaging capability due to their fluorescence emission from PS or photoactive nanomaterials themselves, as frequently applied in image-guided delivery [170,174,177]. Their fluorescence detection is coupled with other imaging modes. This is illustrated with a Ce6-based system co-loaded with an Mn2+ chelate, which is designed for photodynamic therapy under dual image guidance by fluorescence and MRI [170,174,177].
4.3. Challenges in Photodynamic Systems
PS systems are faced with certain challenges that are attributable to unfavorable physicochemical properties and cellular occurrence of ROS resistance. Most PS molecules show poor aqueous solubility and high aggregation tendency because of their hydrophobic aromatic rings that engage in intermolecular π–π stacking interaction. As a consequence, these are responsible for lowered efficiency in ROS production, fluorescence quenching, or suboptimal imaging resolution. This issue can be overcome in part by ROS production using either photoactive nanomaterials or polymer particulates that display aggregation induced emission (AIE) [178,179], including integrin-targeting polymer nanodots [179].
Another challenge relates to occurrence of cellular ROS resistance, a known mechanism responsible for reduction in PDT efficacy. This occurs through reductive inactivation of ROS by intracellular antioxidant thiols, primarily, GSH. A few approaches are reported to address this problem including co-delivery of an Mn2+ contrast agent [177] or MnO2, which releases thiol-oxidizing manganese ions [170]. Their oxidative role contributes to a decrease in GSH level via thiol oxidation. An alternative approach involves facilitating the rate of ROS production by increasing the intracellular concentration of O2, a precursor molecule for ROS. This involves co-delivery of catalase, an oxidative enzyme that engages in catalyzing endogenous hydrogen peroxide to O2 [170,180]. The latter approach is of most relevance to applications in tumors where their oxygen level remains relatively lower (hypoxia) than normal tissues. Potent antitumor efficacy is achieved by catalase co-delivery systems made of Ce6-modified chitosan micelles [170] or indocyanine green-loaded albumin complexes [180].
In short, photodynamic activation produces ROS for application in photodynamic therapy [155,158] as well as dual therapy by engagement in controlled drug release [176]. Photodynamic systems are applied for image-guided delivery through their fluorescence alone or in combination with MRI [170,177], photoacoustic imaging (PAI) [180], and/or thermal imaging [170].
5. Photothermal Activation
Certain classes of nanomaterials are able to produce localized heat through plasmonic photoactivation [181]. These comprise nano Au (AuNP, gold nanorod (AuNR) [182], hollow Au nanosphere (AuNS) [183]), black phosphorus [36], CuS [184], and MoS2 nanosheet [185]. Their heat production results in the induction of cytotoxic hyperthermia, and this constitutes a therapeutic basis in photothermal ablation therapy (PTT) [186,187]. The heat is further exploited in release mechanisms for dual therapy. Additionally, it serves as an imaging modality applicable in photothermal imaging, which is widely applied in image-guided systems.
5.1. Photothermal Drug Release
Despite its cytotoxicity, it is possible that hyperthermia alone is not potent enough for complete tumor ablation, leaving a marginal area of tumor for potential recurrence. This insufficiency is improved by combination with drug release. Delivery systems ideal for photothermal release are designed through nanomaterial integration that consists of a drug-loadable porous nanomaterial and, separately, a photothermally-active nanomaterial. Their integration is illustrated with various combinations of nano Au and mSiO2 including smaller AuNR-loaded mSiO2 [188], Au-coated mSiO2 [74], mSiO2-coated AuNR [72], and UCN coated with carbon dot-loaded mSiO2 [73].
Photothermal drug release occurs through one of two main mechanisms, pore opening and disassembly. First, pore opening occurs in a system when a local temperature is elevated by photothermal activation. This photothermal release is applied to doxorubicin [72,73], naproxen [188], and ibuprofen [74] loaded in porous silica oxide. Second, disassembly occurs in thermally unstable nanomaterials including liposomes co-loaded with nano Au [189] or black phosphorous QD [190]. Polymer micelles are equivalently applied for photothermal disassembly as illustrated with those encapsulated with nano Au (AuNR [63,191,192], Au nanoflower [193]), polydopamine [194], or photothermal dyes including cypate [195], indocyanine green [196,197], and BODIPY [64]. Other systems designed for the photothermal disassembly include drug-loaded Au nanoshell [198] and nano Au-coated polymer particulates [199]. Overall, nanomaterials integrated for photothermal activation are successfully applied to a variety of payloads from doxorubicin [62,190,192,196], camptothecin [64], berberine [189], cisplatin [200], Pt (IV) prodrug [195,197], plasmid DNA [191,199], and siRNA [199] to precursor DNAzyme [198].
5.2. Photothermal Imaging Delivery
Heat production itself serves as an imaging modality in photothermal systems. This is illustrated with AuNR-coated mSiO2 developed for photothermal image-guided dual therapy via hyperthermia and 5-fluorouracil release [182]. A few other systems have been developed similarly that include UCN (Gd2O3:Yb,Er)-coated nano Au [201], black phosphorus-loaded hydrogel [36], polydopamine-coated IONP [202], or extracellular vesicles loaded with Au–IONP [203]. Each of these is effectively applied in the photothermal delivery of doxorubicin [36] or anti-miR-21 aptamer [203] under imaging guidance. Yet, unlike purely imaging modalities, such as MRI scans, photothermal imaging has a risk of inducing premature cytotoxicity or drug activation by photothermal heat produced during its scanning process. Therefore, its application is limited on photothermal verification or optimization purposes at target tissues instead of pre-activation imaging guidance, which is achieved by supplementary modalities such as MRI [201], fluorescence [36,182], and PAI [182,202].
5.3. Photoacoustic Imaging (PAI) Delivery
The technique of PAI is based on detecting a sound wave (photoacoustic signal) which is generated upon photoexcitation at certain functional systems. It occurs via photothermal expansion (thermoelastic), vaporization (perfluoro molecule), and nanomaterial breakdown [37]. Photoacoustic systems are designed with chromophore molecules or photoactive nanomaterials including polydopamine [202], AuNR [204], CuS [139], and MoS2 [205]. These are integrated in various ways by nanomaterial coating [202], encapsulation in nanobubbles [204], or loading in a nanogel [139]. These include a paclitaxel-loaded multifunctional nanobubble composed of AuNR and perfluorohexane [204]. Its photoacoustic activation involves perfluorohexane vaporization that occurs in response to temperature elevation induced by photoactivated AuNR. This leads to shockwave production that triggers bubble implosion, serving as a mechanism for drug release and PAI [204]. Systems loaded with CuS [139] and MoS2 [205] are reported as well for PAI-guided photothermal therapy as illustrated with a FAR-targeted polyethylenimine (PEI)-based nanogel loaded with CuS and Gd chelates (Figure 4B) [139]. This nanogel system allows dual image (PAI, MRI)-guided PTT in FAR-overexpressing tumors by laser irradiation at 1064 nm. In a similar way, MoS2 nanosheet modified with IONP and 64Cu on its shell surface shows a multifunctional capability that allows PAI-guided PTT in combination with two complementary imaging modes, MRI and PET [205].
5.4. Computed Tomography (CT) Imaging Delivery
CT-guided photothermal systems rely on nanomaterials with ability for X-ray absorption. These comprise nano Au [206], CuS [184], CuS-Pt [207], Bi2Se3 nanosponge [208], and MoS2 nanosheet [185], and many of these exhibit strong absorbance at NIR wavelengths. Therefore, CT systems allow additional imaging capabilities including thermal imaging and PAI [208]. Examples of CT-guided delivery include doxorubicin-loaded MoS2 nanosheet [185] and CuS-encapsulated hyaluronic acid polymer [184]. Liposomes loaded with IONP@Au are effectively applicable for dual CT and magnetic particle imaging as demonstrated in the delivery of tenofovir disoproxil, an antiviral drug, in HIV-infected microglia cells [206]. Using Bimetallic CuS-Pt is dually beneficial for CT guidance as well as Pt chemotherapy in which cytotoxic Pt (II) is released inside the cell following its photothermal activation at 808 nm [207].
5.5. Positron Emission Tomography (PET) Imaging Delivery
The basis of PET imaging relies on detecting γ-rays generated in a tissue when tissue electrons react with positrons emitted from radionucleotide tracers carried in a delivery system [209]. PET tracers used in clinical agents include 11C, 13N, 15O, and 18F, each incorporated as an isotope in a ligand or drug molecule. Post-transition metal elements constitute another class of PET tracers that includes 89Zr [45], 64Cu [205,210,211], and Au [212]. PET systems developed for drug delivery are designed in a number of ways. First, PET tracers are incorporated as a part of the carrier as illustrated with 89Zr incorporated in a metal-organic framework [45], a system developed for doxorubicin delivery under PET guidance. Second, some of these tracers are available as chelated species as seen in 64Cu chelated in polymer micelles [211] and 64Cu-NOTA conjugated to mSiO2 [213]. Third, PET tracers allow direct integration through chemoadsorption or coating as evident in 64Cu-adsorbed MoS2 nanosheets [205], Au-coated PdCu nanotripods [212], and 64Cu-coated AuNR [210]. Overall, CT imaging systems designed in these ways are applied for therapeutic delivery. This includes doxorubicin-loaded mSiO2@64Cu-NOTA demonstrated for tumor vasculature-targeted delivery under PET imaging [213]. Nanodots composed of 64CuS display a similar capability for PET-guided photothermal ablation as demonstrated in tumor-bearing mice [214].
5.6. Single Photon-Emission Computerized Tomography (SPECT) Imaging Delivery
SPECT is a dual imaging modality composed of CT and radiation imaging [209]. Like PET, radionuclide elements are required that include 111In, 123I, 131I, 99mTc, and 188Re, and they are often incorporated in aromatic compounds through 123I/131I labeling [139] and in pharmaceuticals through 99mTc-chelation [209]. Radionucleotide tracers used in SPECT have longer half-lives (6–68 h) on average than PET tracers. This property is highly beneficial during their radiolabeling, nanomaterial incorporation, and uses because it allows more time until full decay.
Several systems have been reported for SPECT-guided therapy based on nano Au [183], dendrimer [139], and liposomes [215]. These include 111In-labeled hollow AuNS designed for epidermal growth factor receptor (EGFR)-targeted photothermal ablation in head and neck cancer [183]. 131I-labeled dendrimer is effectively used as a tumor-targeted radionuclide therapy after ligand functionalization with a tumor targeting the LyP-1 peptide [139]. Micelle serves as another platform for SPECT-guided delivery as illustrated with 188Re-labeled micelles loaded with IR-780 dye applied for PTT in tumors [215].
6. Ultrasound Activation
6.1. Mechanism of Ultrasound Activation
Low-intensity ultrasound (US) is commonly used in diagnostic ultrasonography [38], however high-intensity US can serve as an external stimulus strong enough for inducing therapeutic release. This US activation occurs in nanomaterial systems composed of gas-filled cavities or US-responsive molecules including perfluorocarbon [140,216], fluorous tag [20], and polymer [141]. Like photoacoustic activation described above, the physical basis of US activation involves cavitation which occurs upon vaporization of molecules loaded in the cavity. This leads to induction of bubble ruptures or open pores, both contributing to drug release as illustrated in Figure 4C and Figure 4D [20]. This is evident in A549 cells treated with GFP-loaded fluorous nanoemulsions that show a significant increase in intracellular fluorescence following US application compared to free GFP as a control (Figure 4C). Similarly, stimulating microcapsule-treated MCF-7 cells by therapeutic US leads to induction of cytotoxicity, which does not occur without microcapsule treatment or by diagnostic weaker US (Figure 4D).
High-intensity focused ultrasound (HIFU) is also able to induce hyperthermia, which serves as another mechanism applicable for controlled drug release [22]. Similar to photothermal activation, this occurs in thermo-sensitive systems [22] including doxorubicin-loaded liposomes, which show controlled drug release in response to high-intensity US [217,218]. In spite of its potential and recent advances in transducer designs, the efficacy of HIFU activation is limited by its focal length that can reduce spatial resolution, its slow onset of activation which takes hours, and its challenges in certain tissue areas that require crossing a bone or intact skull [219].
6.2. Ultrasound Delivery Systems
Delivery systems that are responsive to US activation comprise microbubbles [20,21,220], nanobubbles [216], probubbles [221], nanoemulsions [140], nanodroplets [222], and microcapsules [141]. Microbubbles are popularly used in US applications due to their large shell or cavity space available for loading supplementary imaging probes and therapeutic agents. These include IONP (Fe3O4)-coated microbubbles developed for dual MRI and US-guided delivery of tPA [21]. Multifunctional nanobubbles that are loaded with 5-fluorouracil, a Gd chelate and IR-780 dye, are comparably used in US-controlled drug release under guidance by multiple imaging modalities [216]. US activation is also applied in the controlled release of gas molecules as illustrated with liposomal probubbles loaded with a H2S precursor for application in tumor ablation therapy [221]. Non-bubble US systems are made of nanoemulsions (Figure 4C) [140], microcapsules (Figure 4D) [141], and nanodroplets [222], each applied in the delivery of doxorubicin [141], green fluorescent protein and antibodies [140], and paclitaxel [222], respectively. Porous nanomaterials are useful as well for US activation. This is illustrated with mSiO2 loaded with an NO precursor and superparamagnetic iron oxide nanoparticle (SPION) for MRI-guided, US-controlled NO release in tumor [223].
One of the limitations in US systems relates to bubble sizes. Most microbubble systems are excellent in contrast imaging capability, although they are considerably too large for efficient tissue penetration and cellular uptake. On the other hand, nanometer-sized systems are reversed in the trend with greater tissue infiltration at lower imaging contrast. This conflicting unbalance has been recently addressed using size-variable polymersome (~200 nm), which is able to grow in size under acidic conditions [224]. Thus, once taken up in tumor tissues and subcellular compartments, its signal intensity in US imaging is enhanced as a result of its enlarged size.
In summary, US has been actively explored for therapeutic activation in image-guided delivery systems. This strategy is applied to a variety of payloads from drugs (doxorubicin [141,224], paclitaxel [20,222]) and proteins (tissue plasminogen activators [21], green fluorescent protein [140], antibodies [140]) to gas molecules of therapeutic significance (H2S [221], NO [223]).
7. Electric and Magnetic Field Activation
Stimulation under an electrical field is potentially applicable in controlled delivery systems. A notable approach involves electroporation, a technique that applies an electrical pulse to induce temporary pores across cell membranes. This technique has played an important role in enhancing cell permeability as often applied to small drug molecules, biomolecules, and genes [225,226]. However, it is rarely used in nanomaterial delivery though it can be beneficial as illustrated with doxorubicin-loaded SPION demonstrated for tumor uptake through electroporation [227]. Unlike normal electroporation, this system offers an imaging guidance through magnetic particle imaging that helps precise positioning of electrodes at its uptake area.
Applying an alternating magnetic field (AMF) on magnetic nanomaterials is more often used in delivery systems. It is able to produce a localized heat applicable for controlled drug release [3]. This magnetic hyperthermia is observed in IONP systems loaded with DNA [228] or doxorubicin linked through a thermally unstable azo bond [229]. SPION is equally applicable for magnetically induced hyperthermia demonstrated in photothermal therapy [230]. Its use also plays a critical role in MPI-guided spatial and temporal control of magnetic hyperthermia, drug delivery, or the combination of these [230,231,232]. This approach helps to attain precise localization and positioning of magnetic nanocarriers within a target region prior to drug release through AMF-triggered hyperthermia. This is demonstrated with SPION-encapsulated poly(lactic-co-glycolic acid) (PLGA) [232] and doxorubicin-loaded liposomes [231]. Furthermore, applying a magnetic field serves as a physical force that enables the control of particle movement. Therefore, drug-loaded magnetic nanomaterials are guided and transported by a magnet for accumulation in a targeted tissue, which has been effectively applied in a few systems. These include a magnetic nanospear designed for gene transfection in glioblastoma cells [233], SPION-loaded microbubbles for doxorubicin delivery in brain tumors [217], and IONP-coated microbubbles for accumulation at specific blood vessels [21]. Overall, delivery systems developed for magnetic control offer several benefits including magnetically inducible hyperthermia, magnetic particle imaging, and magnetic particle guidance.
8. Conclusions and Perspectives
This article described recent advances in nanotherapeutic systems developed for image-guided delivery with a focus on their design concepts and mechanisms in therapeutic activation. Some of their mechanisms are attributed to cellular and pathophysiological factors, primarily low pH conditions [35,45,46] or elevated GSH levels [49]. Other mechanisms involve external stimulations which are more actively controlled via light-triggered linker cleavage [11,81,91,92,101], disassembly [59,60,61,62,63,64,65,66,67], pore gating [68,69,70,71,72,73,74], photothermal activation [36,182,204], photodynamic activation [156,164,165,166], US-mediated disassembly [140,141,216], hyperthermia [217], electroporation [227], and magnetic thermal activation [230]. Developing these activation strategies is making a significant impact on advancing knowledge and creating a new capability in nanotherapeutic delivery systems.
It is however worth noting current limitations associated with activation strategies. The degree of spatial resolution conferred by each activation strategy is variable as it is defined by the perimeter of its stimulus. Some strategies characterized by US, electromagnetic field, or thermal stimulation show relatively lower precision in spatiotemporal control compared to light activation. On the other hand, light shows a lower level of tissue penetration compared to US or electromagnetic stimulation [80]. This light limitation is currently worked out using an optical technique that allows tissue bypassing in which laser irradiation is delivered through a catheter inserted in a needle injected in tissue [201].
Another critical aspect which is of broad interest involves how to achieve specific nanomaterial uptake and localization in targeted cells only. Many systems discussed here are designed for tumor uptake via enhanced permeation and retention (EPR), a passive targeting strategy that facilitates particle infiltration through leaky vessels in tumors (Figure 1) [234]. However, this passive targeting is not applicable for distinguishing specific tumor biomarkers or targeted binding and uptake at specific biomarker-overexpressing tumor cells. This lack of specificity is achieved otherwise by an active targeting strategy [3,235,236] in which a drug-loaded nanomaterial is functionalized through multivalent conjugation with a target-specific ligand or antibody. This active targeting has been applied to a few tumor biomarkers that include FAR [46,204,216], αvβ3 integrin [179,210,237,238], IGF1 receptor [48], EGFR [183], CD105 [213], CCR5 [212], and nucloelin [45]. It would be equally applicable to other promising but less explored biomarkers that include prostate-specific membrane antigen (PSMA) receptors [239], Her2 [240], riboflavin receptors [241,242], and transferrin receptors [243] for brain delivery. In brief, multivalent ligand conjugation serves as an important strategy in the design of actively targeted systems.
Finally, clinical translation of nanotherapeutic agents is associated with significant challenges due to their multifunctional design, difficulty in synthetic scalability, and paucity of efficient clinical devices needed for their optimal activation. Nevertheless, they show a growing potential as evident with numerous types of nanotherapeutic agents either approved or advanced to clinical studies [244]. Of those, release control by endogenous factors is most actively engaged as shown in PLGA NPs encapsulated with leuprolide (Eligard®) [245], albumin NPs bound with paclitaxel (Abraxane®) [246], and liposomes encapsulated with doxorubicin (Doxil®) [247], mifamurtide (Mepact®) [248], vincristine (Marqibo®) [249], or irinotecan (Onivyde®) [250]. Thermal control of drug release is also successfully applied in heat-sensitive liposomes loaded with doxorubicin (ThermoDox®) [251]. Clinical development of photoactivated nanotherapeutics has been relatively slower but was already demonstrated by PDT-based verteporfin liposome (Visudyne®), which is approved for age-related macular degeneration and is currently being investigated for locally advanced pancreatic cancer [252,253]. This strategy is also applicable in topical and superficial treatments as illustrated with PDT nanoemulsion (BF-200) [254], a topical agent investigated for treating actinic keratosis [255]. In summary, nanotherapeutic activation strategies are currently being evaluated for their clinical translation [39,244].
Funding
This research received no external funding.
Acknowledgments
The author acknowledges support for cited works in part by the Michigan Nanotechnology Institute for Medicine and Biological Sciences.
Conflicts of Interest
The author declares no competing financial interests.
References
- Ojha, T.; Rizzo, L.; Storm, G.; Kiessling, F.; Lammers, T. Image-guided drug delivery: Preclinical applications and clinical translation. Expert Opin. Drug Deliv. 2015, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K. (Ed.) Photocleavable linkers: Design and applications in nanotechnology. In Photonanotechnology for Therapeutics and Imaging; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–275. [Google Scholar]
- Wong, P.T.; Choi, S.K. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chem. Rev. 2015, 115, 3388–3432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Yang, X.; Pu, K. Photoactivatable Protherapeutic Nanomedicine for Cancer. Adv. Mater. 2020, 32, 2002661. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Yin, R.; Agrawal, T.; Khan, U.; Gupta, G.K.; Rai, V.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial photodynamic inactivation in nanomedicine: Small light strides against bad bugs. Nanomedicine 2015, 10, 2379–2404. [Google Scholar] [CrossRef]
- Zhao, J.; Duan, L.; Wang, A.; Fei, J.; Li, J. Insight into the efficiency of oxygen introduced photodynamic therapy (PDT) and deep PDT against cancers with various assembled nanocarriers. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1583. [Google Scholar] [CrossRef]
- Wong, P.T.; Tang, S.; Cannon, J.; Chen, D.; Sun, R.; Lee, J.; Phan, J.; Tao, K.; Sun, K.; Chen, B.; et al. Photocontrolled Release of Doxorubicin Conjugated through a Thioacetal Photocage in Folate-Targeted Nanodelivery Systems. Bioconjug. Chem. 2017, 28, 3016–3028. [Google Scholar] [CrossRef]
- Wong, P.; Tang, S.; Mukherjee, J.; Tang, K.; Gam, K.; Isham, D.; Murat, C.; Sun, R.; Baker, J.R.; Choi, S.K. Light-Controlled Active Release of Photocaged Ciprofloxacin for Lipopolysaccharide-Targeted Drug Delivery using Dendrimer Conjugates. Chem. Commun. 2016, 52, 10357–10360. [Google Scholar] [CrossRef]
- Choi, S.K.; Thomas, T.; Li, M.; Kotlyar, A.; Desai, A.; Baker, J.R., Jr. Light-Controlled Release of Caged Doxorubicin from Folate Receptor-Targeting PAMAM Dendrimer Nanoconjugate. Chem. Commun. 2010, 46, 2632–2634. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, Y.; Hu, M.; Zhang, P.; Kong, N.; Liu, R.; Liu, C.; Choi, S.K. Lanthanide-doped core-shell nanoparticles as a multimodality platform for imaging and photodynamic therapy. Chem. Commun. 2018, 54, 9525–9528. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Hu, M.; Liu, C.; Choi, S.K. Yolk-structured multifunctional up-conversion nanoparticles for synergistic photodynamic-sonodynamic antibacterial resistance therapy. Biomater. Sci. 2017, 5, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Chen, D.; Tang, S.; Yanik, S.; Payne, M.; Mukherjee, J.; Coulter, A.; Tang, K.; Tao, K.; Sun, K.; et al. Modular Integration of Upconversion Nanocrystal-Dendrimer Composites for Folate Receptor-Specific Near Infrared Imaging and Light Triggered Drug Release. Small 2015, 11, 6078–6090. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal–Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Orza, A.; Lu, Q.; Guo, P.; Wang, L.; Yang, L.; Mao, H. Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Adv. Funct. Mater. 2016, 26, 3818–3836. [Google Scholar] [CrossRef]
- Wang, J.; Li, N. Functional hollow nanostructures for imaging and phototherapy of tumors. J. Mater. Chem. B 2017, 5, 8430–8445. [Google Scholar] [CrossRef]
- Gullotti, E.; Yeo, Y. Extracellularly Activated Nanocarriers: A New Paradigm of Tumor Targeted Drug Delivery. Mol. Pharmaceutics 2009, 6, 1041–1051. [Google Scholar] [CrossRef]
- Chen, H.H.W.; Song, I.-S.; Hossain, A.; Choi, M.-K.; Yamane, Y.; Liang, Z.D.; Lu, J.; Wu, L.Y.-H.; Siddik, Z.H.; Klomp, L.W.J.; et al. Elevated Glutathione Levels Confer Cellular Sensitization to Cisplatin Toxicity by Up-Regulation of Copper Transporter hCtr1. Mol. Pharmacol. 2008, 74, 697–704. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, J.; He, C.; Geng, H.; Yu, G.; Li, J.; Zheng, H.; Ji, X.; Yan, F. Ultrasound triggered image-guided drug delivery to inhibit vascular reconstruction via paclitaxel-loaded microbubbles. Sci. Rep. 2016, 6, 21683. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Xiu, W.; Liu, Y.; Ren, L.; Xiao, H.; Yang, F.; Gao, Y.; Xu, C.; Wang, L. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Sci. Adv. 2020, 6, eaaz8204. [Google Scholar] [CrossRef]
- Grüll, H.; Langereis, S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Control. Release 2012, 161, 317–327. [Google Scholar] [CrossRef]
- Li, F.; Qin, Y.; Lee, J.; Liao, H.; Wang, N.; Davis, T.P.; Qiao, R.; Ling, D. Stimuli-responsive nano-assemblies for remotely controlled drug delivery. J. Control. Release 2020, 322, 566–592. [Google Scholar] [CrossRef]
- Hatefi, A.; Minko, T. Advances in image-guided drug delivery. Drug Deliv. Transl. Res. 2012, 2, 1–2. [Google Scholar] [CrossRef]
- Solorio, L.; Patel, R.B.; Wu, H.; Krupka, T.; Exner, A.A. Advances in image-guided intratumoral drug delivery techniques. Ther. Deliv. 2010, 1, 307–322. [Google Scholar] [CrossRef]
- Chakravarty, R.; Hong, H.; Cai, W. Positron Emission Tomography Image-Guided Drug Delivery: Current Status and Future Perspectives. Mol. Pharm. 2014, 11, 3777–3797. [Google Scholar] [CrossRef] [PubMed]
- Tomitaka, A.; Arami, H.; Ahmadivand, A.; Pala, N.; McGoron, A.J.; Takemura, Y.; Febo, M.; Nair, M. Magneto-plasmonic nanostars for image-guided and NIR-triggered drug delivery. Sci. Rep. 2020, 10, 10115. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Brock, K. Image-guided radiation therapy: Looking beyond what we currently see. Future Oncol. 2017, 13, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Guo, X.; Zhang, X.; Chen, S.; Wang, Y.; Chen, T.; Huang, G.; Gao, Y.; Tian, Z.; Yang, Z. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater. Today Bio 2020, 5, 100035. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Wojtynek, N.E.; Mohs, A.M. Image-guided tumor surgery: The emerging role of nanotechnology. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1624. [Google Scholar] [CrossRef]
- Pablico-Lansigan, M.H.; Situ, S.F.; Samia, A.C.S. Magnetic particle imaging: Advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale 2013, 5, 4040–4055. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Saha, R.; Liu, J.; Chugh, V.K.; Wang, J.-P. Magnetic Particle Spectroscopy: A Short Review of Applications Using Magnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 4972–4989. [Google Scholar] [CrossRef]
- Jiang, S.; Gnanasammandhan, M.K.; Zhang, Y. Optical imaging-guided cancer therapy with fluorescent nanoparticles. J. R. Soc. Interface 2010, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, D.; Wang, Y.; Ouyang, Z.; Peng, Y.; Tomás, H.; Xia, J.; Rodrigues, J.; Shen, M.; Shi, X. Polyethylenimine Nanogels Incorporated with Ultrasmall Iron Oxide Nanoparticles and Doxorubicin for MR Imaging-Guided Chemotherapy of Tumors. Bioconjug. Chem. 2020, 31, 907–915. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Wilson, K.; Homan, K.; Emelianov, S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nat. Commun. 2012, 3, 618. [Google Scholar] [CrossRef]
- Fisher, D.G.; Price, R.J. Recent Advances in the Use of Focused Ultrasound for Magnetic Resonance Image-Guided Therapeutic Nanoparticle Delivery to the Central Nervous System. Front. Pharmacol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Choi, S.K. Photoactivation Strategies for Therapeutic Release in Nanodelivery Systems. Adv. Ther. 2020, 3, 2000117. [Google Scholar] [CrossRef]
- Geisow, M.J.; Evans, W.H. pH in the endosome: Measurements during pinocytosis and receptor-mediated endocytosis. Exp. Cell Res. 1984, 150, 36–46. [Google Scholar] [CrossRef]
- Chan, P.; Lovrić, J.; Warwicker, J. Subcellular pH and predicted pH-dependent features of proteins. Proteomics 2006, 6, 3494–3501. [Google Scholar] [CrossRef]
- Geisow, M.J. Fluorescein conjugates as indicators of subcellular pH: A critical evaluation. Exp. Cell Res. 1984, 150, 29–35. [Google Scholar] [CrossRef]
- Feazell, R.P.; Nakayama-Ratchford, N.; Dai, H.; Lippard, S.J. Soluble Single-Walled Carbon Nanotubes as Longboat Delivery Systems for Platinum(IV) Anticancer Drug Design. J. Am. Chem. Soc. 2007, 129, 8438–8439. [Google Scholar] [CrossRef] [PubMed]
- Dubowchik, G.M.; Walker, M.A. Receptor-mediated and enzyme-dependent targeting of cytotoxic anticancer drugs. Pharmacol. Therap. 1999, 83, 67–123. [Google Scholar] [CrossRef]
- Chen, D.; Yang, D.; Dougherty, C.A.; Lu, W.; Wu, H.; He, X.; Cai, T.; Van Dort, M.E.; Ross, B.D.; Hong, H. In Vivo Targeting and Positron Emission Tomography Imaging of Tumor with Intrinsically Radioactive Metal–Organic Frameworks Nanomaterials. ACS Nano 2017, 11, 4315–4327. [Google Scholar] [CrossRef]
- Li, Z.; Dong, K.; Huang, S.; Ju, E.; Liu, Z.; Yin, M.; Ren, J.; Qu, X. A Smart Nanoassembly for Multistage Targeted Drug Delivery and Magnetic Resonance Imaging. Adv. Funct. Mater. 2014, 24, 3612–3620. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Jia, Y.; Qu, C.; Li, J. Covalently assembled dopamine nanoparticle as an intrinsic photosensitizer and pH-responsive nanocarrier for potential application in anticancer therapy. Chem. Commun. 2019, 55, 15057–15060. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, W.; Uckun, F.M.; Wang, L.; Wang, Y.A.; Chen, H.; Kooby, D.; Yu, Q.; Lipowska, M.; Staley, C.A.; et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano 2015, 9, 7976–7991. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.; Hu, Q.; Bai, H.; Zhang, S.; Shen, Y.; Tang, G.; Ping, Y. Redox-Activated Light-Up Nanomicelle for Precise Imaging-Guided Cancer Therapy and Real-Time Pharmacokinetic Monitoring. ACS Nano 2016, 10, 11385–11396. [Google Scholar] [CrossRef]
- Ojima, I. Guided Molecular Missiles for Tumor-Targeting Chemotherapy;Case Studies Using the Second-Generation Taxoids as Warheads. Acc. Chem. Res. 2008, 41, 108–119. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, G.; Hu, J.; Liu, S. Photo- and Reduction-Responsive Polymersomes for Programmed Release of Small and Macromolecular Payloads. Biomacromolecules 2018, 19, 2071–2081. [Google Scholar] [CrossRef]
- Huang, B.; Tang, S.; Desai, A.; Cheng, X.-m.; Kotlyar, A.; Spek, A.V.D.; Thomas, T.P.; Baker, J.R., Jr. Human plasma-mediated hypoxic activation of indolequinone-based naloxone pro-drugs. Bioorg. Med. Chem. Lett. 2009, 19, 5016–5020. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Desai, A.; Tang, S.; Thomas, T.P.; Baker, J.R. The Synthesis of a c(RGDyK) Targeted SN38 Prodrug with an Indolequinone Structure for Bioreductive Drug Release. Org. Lett. 2010, 12, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Naughton, D.P. Drug targeting to hypoxic tissue using self-inactivating bioreductive delivery systems. Adv. Drug Deliv. Rev. 2001, 53, 229–233. [Google Scholar] [CrossRef]
- Dhar, S.; Liu, Z.; Thomale, J.; Dai, H.; Lippard, S.J. Targeted Single-Wall Carbon Nanotube-Mediated Pt(IV) Prodrug Delivery Using Folate as a Homing Device. J. Am. Chem. Soc. 2008, 130, 11467–11476. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; Kelly, B.D.; McLeod, V.M.; Sberna, G.; Boyd, B.J.; Owen, D.J.; Porter, C.J.H. Capping Methotrexate α-Carboxyl Groups Enhances Systemic Exposure and Retains the Cytotoxicity of Drug Conjugated PEGylated Polylysine Dendrimers. Mol. Pharm. 2011, 8, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, L.M.; Kelly, B.D.; McLeod, V.M.; Boyd, B.J.; Krippner, G.Y.; Williams, E.D.; Porter, C.J.H. Pharmacokinetics and Tumor Disposition of PEGylated, Methotrexate Conjugated Poly-l-lysine Dendrimers. Mol. Pharm. 2009, 6, 1190–1204. [Google Scholar] [CrossRef]
- Homma, A.; Sato, H.; Okamachi, A.; Emura, T.; Ishizawa, T.; Kato, T.; Matsuura, T.; Sato, S.; Tamura, T.; Higuchi, Y.; et al. Novel hyaluronic acid-methotrexate conjugates for osteoarthritis treatment. Bioorg. Med. Chem. 2009, 17, 4647–4656. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.-C.; Branda, N.R.; Zhao, Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J. Am. Chem. Soc. 2011, 133, 19714–19717. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, G.; Yu, Y.; Zhang, H.; Gao, J.; Sun, Z.; Lu, Y.; Zou, H. NIR-responsive copolymer upconversion nanocomposites for triggered drug release in vitro and in vivo. ACS Appl. Bio Mater. 2019, 2, 495–503. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, W.; Ma, H.; Jiang, R.; Tang, Y.; Ji, Y.; Lu, X.; Hou, B.; Deng, W.; Huang, W.; et al. Photo-Induced Charge-Variable Conjugated Polyelectrolyte Brushes Encapsulating Upconversion Nanoparticles for Promoted siRNA Release and Collaborative Photodynamic Therapy under NIR Light Irradiation. Adv. Funct. Mater. 2017, 27, 1702592. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, C.; Cheng, L.; Meng, F.; Zhong, Z.; Liu, Z. Gold Nanorod-Cored Biodegradable Micelles as a Robust and Remotely Controllable Doxorubicin Release System for Potent Inhibition of Drug-Sensitive and -Resistant Cancer Cells. Biomacromolecules 2013, 14, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.; Wu, X.; Zhao, Y.; Chen, C. Near Infrared Laser-Induced Targeted Cancer Therapy Using Thermoresponsive Polymer Encapsulated Gold Nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Ren, N.; Gu, L.; Xu, G.; Wang, R.; Zhu, T.; Zhu, Y.; Fan, C.; Zhao, C.; Tian, H. Theranostic Nanoplatform with Hydrogen Sulfide Activatable NIR Responsiveness for Imaging-Guided On-Demand Drug Release. Angew. Chem. Int. Ed. 2019, 58, 16826–16830. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, G.; Lee, J.; Kim, J.; Kim, W.J. Visible light-induced singlet oxygen-mediated intracellular disassembly of polymeric micelles co-loaded with a photosensitizer and an anticancer drug for enhanced photodynamic therapy. Chem. Commun. 2015, 51, 9995–9998. [Google Scholar] [CrossRef]
- Brega, V.; Scaletti, F.; Zhang, X.; Wang, L.-S.; Li, P.; Xu, Q.; Rotello, V.M.; Thomas, S.W. Polymer Amphiphiles for Photoregulated Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 2814–2820. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Huang, Y.; Chen, Y.; Wu, W.; Liu, Y.; Zhang, J.; Feng, Y.; Jiang, X.; Gou, M. Light-activated drug release from prodrug nanoassemblies by structure destruction. Chem. Commun. 2019, 55, 13128–13131. [Google Scholar] [CrossRef]
- Hernández-Montoto, A.; Gorbe, M.; Llopis-Lorente, A.; Terrés, J.M.; Montes, R.; Cao-Milán, R.; Díaz de Greñu, B.; Alfonso, M.; Orzaez, M.; Marcos, M.D.; et al. A NIR light-triggered drug delivery system using core–shell gold nanostars–mesoporous silica nanoparticles based on multiphoton absorption photo-dissociation of 2-nitrobenzyl PEG. Chem. Commun. 2019, 55, 9039–9042. [Google Scholar] [CrossRef]
- He, S.; Krippes, K.; Ritz, S.; Chen, Z.; Best, A.; Butt, H.-J.; Mailänder, V.; Wu, S. Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chem. Commun. 2015, 51, 431–434. [Google Scholar] [CrossRef]
- Wang, D.; Wu, S. Red-Light-Responsive Supramolecular Valves for Photocontrolled Drug Release from Mesoporous Nanoparticles. Langmuir 2016, 32, 632–636. [Google Scholar] [CrossRef]
- Liu, J.; Bu, W.; Pan, L.; Shi, J. NIR-Triggered Anticancer Drug Delivery by Upconverting Nanoparticles with Integrated Azobenzene-Modified Mesoporous Silica. Angew. Chem. Int. Ed. 2013, 52, 4375–4379. [Google Scholar] [CrossRef]
- Li, M.; Yan, H.; Teh, C.; Korzh, V.; Zhao, Y. NIR-triggered drug release from switchable rotaxane-functionalized silica-covered Au nanorods. Chem. Commun. 2014, 50, 9745–9748. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, P.; He, F.; Gai, S.; Li, C.; Dai, Y.; Yang, G.; Lin, J. A Yolk-like Multifunctional Platform for Multimodal Imaging and Synergistic Therapy Triggered by a Single Near-Infrared Light. ACS Nano 2015, 9, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Yagüe, C.; Arruebo, M.; Santamaria, J. NIR-enhanced drug release from porous Au/SiO2 nanoparticles. Chem. Commun. 2010, 46, 7513–7515. [Google Scholar] [CrossRef]
- Chan, J.M.; Zhang, L.; Tong, R.; Ghosh, D.; Gao, W.; Liao, G.; Yuet, K.P.; Gray, D.; Rhee, J.-W.; Cheng, J.; et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc. Natl. Acad. Sci. USA 2010, 107, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K. Mechanistic Basis of Light Induced Cytotoxicity of Photoactive Nanomaterials. NanoImpact 2016, 3-4, 81–89. [Google Scholar] [CrossRef]
- Xue, X.; Zhao, Y.; Dai, L.; Zhang, X.; Hao, X.; Zhang, C.; Huo, S.; Liu, J.; Liu, C.; Kumar, A.; et al. Spatiotemporal Drug Release Visualized through a Drug Delivery System with Tunable Aggregation-Induced Emission. Adv. Mater. 2014, 26, 712–717. [Google Scholar] [CrossRef]
- Deepagan, V.G.; You, D.G.; Um, W.; Ko, H.; Kwon, S.; Choi, K.Y.; Yi, G.-R.; Lee, J.Y.; Lee, D.S.; Kim, K.; et al. Long-Circulating Au-TiO2 Nanocomposite as a Sonosensitizer for ROS-Mediated Eradication of Cancer. Nano Lett. 2016, 16, 6257–6264. [Google Scholar] [CrossRef]
- Wong, P.T.; Tang, S.; Cannon, J.; Mukherjee, J.; Isham, D.; Gam, K.; Payne, M.; Yanik, S.A.; Baker, J.R.; Choi, S.K. A Thioacetal Photocage Designed for Dual Release: Application in the Quantitation of Therapeutic Release by Synchronous Reporter Decaging. ChemBioChem 2017, 18, 126–135. [Google Scholar] [CrossRef]
- Choi, S.K. (Ed.) Chapter 1—Light sources for photonanotechnology. In Photonanotechnology for Therapeutics and Imaging; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–21. [Google Scholar]
- Agasti, S.S.; Chompoosor, A.; You, C.-C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef]
- Mahmoodi, M.M.; Abate-Pella, D.; Pundsack, T.J.; Palsuledesai, C.C.; Goff, P.C.; Blank, D.A.; Distefano, M.D. Nitrodibenzofuran: A One- and Two-Photon Sensitive Protecting Group That Is Superior to Brominated Hydroxycoumarin for Thiol Caging in Peptides. J. Am. Chem. Soc. 2016, 138, 5848–5859. [Google Scholar] [CrossRef]
- Jin, Q.; Mitschang, F.; Agarwal, S. Biocompatible Drug Delivery System for Photo-Triggered Controlled Release of 5-Fluorouracil. Biomacromolecules 2011, 12, 3684–3691. [Google Scholar] [CrossRef] [PubMed]
- Vuilleumier, J.; Gaulier, G.; De Matos, R.; Mugnier, Y.; Campargue, G.; Wolf, J.-P.; Bonacina, L.; Gerber-Lemaire, S. Photocontrolled Release of the Anticancer Drug Chlorambucil with Caged Harmonic Nanoparticles. Helv. Chim. Acta 2020, 103, e1900251. [Google Scholar] [CrossRef]
- Gore, S.; Ukhanov, K.; Herbivo, C.; Asad, N.; Bobkov, Y.V.; Martens, J.R.; Dore, T.M. Photoactivatable Odorants for Chemosensory Research. ACS Chem. Biol. 2020, 15, 2516–2528. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pavlos, C.M.; Toscano, J.P.; Dore, T.M. 8-Bromo-7-hydroxyquinoline as a Photoremovable Protecting Group for Physiological Use: Mechanism and Scope. J. Am. Chem. Soc. 2006, 128, 4267–4276. [Google Scholar] [CrossRef]
- Yu, G.; Yu, W.; Mao, Z.; Gao, C.; Huang, F. A Pillararene-Based Ternary Drug-Delivery System with Photocontrolled Anticancer Drug Release. Small 2015, 11, 919–925. [Google Scholar] [CrossRef]
- Janett, E.; Bernardinelli, Y.; Müller, D.; Bochet, C.G. Synthesis of FMRFaNV, a Photoreleasable Caged Transmitter Designed to Study Neuron–Glia Interactions in the Central Nervous System. Bioconjug. Chem. 2015, 26, 2408–2418. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, Q.; Huang, Q.; Liu, H.; Bao, C.; Zhang, W.; Zhong, X.; Zhu, L. Semiconductor quantum dots photosensitizing release of anticancer drug. Chem. Commun. 2011, 47, 1482–1484. [Google Scholar] [CrossRef]
- Dcona, M.M.; Yu, Q.; Capobianco, J.A.; Hartman, M.C.T. Near infrared light mediated release of doxorubicin using upconversion nanoparticles. Chem. Commun. 2015, 51, 8477–8479. [Google Scholar] [CrossRef]
- Choi, S.K.; Verma, M.; Silpe, J.; Moody, R.E.; Tang, K.; Hanson, J.J.; Baker, J.R., Jr. A photochemical approach for controlled drug release in targeted drug delivery. Bioorg. Med. Chem. 2012, 20, 1281–1290. [Google Scholar] [CrossRef]
- Choi, S.K.; Thomas, T.P.; Li, M.-H.; Desai, A.; Kotlyar, A.; Baker, J.R. Photochemical release of methotrexate from folate receptor-targeting PAMAM dendrimer nanoconjugate. Photochem. Photobiol. Sci. 2012, 11, 653–660. [Google Scholar] [CrossRef]
- Xu, C.; Li, H.; Zhang, K.; Binzel, D.W.; Yin, H.; Chiu, W.; Guo, P. Photo-controlled release of paclitaxel and model drugs from RNA pyramids. Nano Res. 2019, 12, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, J.; Liu, T.; Zhang, G.; Liu, S. Photo-Triggered Release of Caged Camptothecin Prodrugs from Dually Responsive Shell Cross-Linked Micelles. Macromolecules 2013, 46, 6243–6256. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Y.; Zhang, H.; Huang, K.; Yang, J.; Han, G. Expanding Anti-Stokes Shifting in Triplet–Triplet Annihilation Upconversion for In Vivo Anticancer Prodrug Activation. Angew. Chem. Int. Ed. 2017, 56, 14400–14404. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, J.; Liu, F.; Tan, E.W.P.; Khezri, B.; Webster, R.D.; Yeow, E.K.L.; Xing, B. Human Transport Protein Carrier for Controlled Photoactivation of Antitumor Prodrug and Real-Time Intracellular Tumor Imaging. Bioconjug. Chem. 2015, 26, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, W.; Qi, R.; Yan, L.; Jing, X.; Zheng, M.; Xiao, H. Delivering a photosensitive transplatin prodrug to overcome cisplatin drug resistance. Chem. Commun. 2015, 51, 11493–11495. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xiao, H.; Liu, J.; Yuan, Q.; Ma, P.A.; Yang, D.; Li, C.; Cheng, Z.; Hou, Z.; Yang, P.; et al. In Vivo Multimodality Imaging and Cancer Therapy by Near-Infrared Light-Triggered trans-Platinum Pro-Drug-Conjugated Upconverison Nanoparticles. J. Am. Chem. Soc. 2013, 135, 18920–18929. [Google Scholar] [CrossRef]
- Shi, Y.; Truong, V.X.; Kulkarni, K.; Qu, Y.; Simon, G.P.; Boyd, R.L.; Perlmutter, P.; Lithgow, T.; Forsythe, J.S. Light-triggered release of ciprofloxacin from an in situ forming click hydrogel for antibacterial wound dressings. J. Mater. Chem. B 2015, 3, 8771–8774. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Tang, S.; Tang, K.; Coulter, A.; Mukherjee, J.; Gam, K.; Baker, J.R.; Choi, S.K. A lipopolysaccharide binding heteromultivalent dendrimer nanoplatform for Gram negative cell targeting. J. Mater. Chem. B 2015, 3, 1149–1156. [Google Scholar] [CrossRef]
- Han, G.; You, C.-C.; Kim, B.-j.; Turingan, R.S.; Forbes, N.S.; Martin, C.T.; Rotello, V.M. Light-Regulated Release of DNA and Its Delivery to Nuclei by Means of Photolabile Gold Nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 3165–3169. [Google Scholar] [CrossRef]
- Brown, P.K.; Qureshi, A.T.; Moll, A.N.; Hayes, D.J.; Monroe, W.T. Silver Nanoscale Antisense Drug Delivery System for Photoactivated Gene Silencing. ACS Nano 2013, 7, 2948–2959. [Google Scholar] [CrossRef]
- Jayakumar, M.K.G.; Bansal, A.; Huang, K.; Yao, R.; Li, B.N.; Zhang, Y. Near-Infrared-Light-Based Nano-Platform Boosts Endosomal Escape and Controls Gene Knockdown in Vivo. ACS Nano 2014, 8, 4848–4858. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yang, J.; Luan, X.; Liu, X.; Li, X.; Yang, J.; Huang, T.; Sun, L.; Wang, Y.; Lin, Y.; et al. Near-infrared upconversion–activated CRISPR-Cas9 system: A remote-controlled gene editing platform. Sci. Adv. 2019, 5, eaav7199. [Google Scholar] [CrossRef]
- Pierri, A.E.; Huang, P.-J.; Garcia, J.V.; Stanfill, J.G.; Chui, M.; Wu, G.; Zheng, N.; Ford, P.C. A photoCORM nanocarrier for CO release using NIR light. Chem. Commun. 2015, 51, 2072–2075. [Google Scholar] [CrossRef] [PubMed]
- Fraix, A.; Kandoth, N.; Manet, I.; Cardile, V.; Graziano, A.C.E.; Gref, R.; Sortino, S. An engineered nanoplatform for bimodal anticancer phototherapy with dual-color fluorescence detection of sensitizers. Chem. Commun. 2013, 49, 4459–4461. [Google Scholar] [CrossRef]
- Fowley, C.; McHale, A.P.; McCaughan, B.; Fraix, A.; Sortino, S.; Callan, J.F. Carbon quantum dot–NO photoreleaser nanohybrids for two-photon phototherapy of hypoxic tumors. Chem. Commun. 2015, 51, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xiang, H.-J.; Wang, Y.; Zhang, Q.-L.; An, L.; Yang, S.-P.; Ma, Y.; Wang, Y.; Liu, J.-G. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem. Commun. 2017, 53, 3253–3256. [Google Scholar] [CrossRef]
- Xiang, H.-J.; An, L.; Tang, W.-W.; Yang, S.-P.; Liu, J.-G. Photo-controlled targeted intracellular delivery of both nitric oxide and singlet oxygen using a fluorescence-trackable ruthenium nitrosyl functional nanoplatform. Chem. Commun. 2015, 51, 2555–2558. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Jiang, X.; Qiu, Y.; Song, X.; Huang, G.; Fu, N.; Lin, L.; Song, J.; Chen, X.; et al. Near-Infrared Light-Triggered Sulfur Dioxide Gas Therapy of Cancer. ACS Nano 2019, 13, 2103–2113. [Google Scholar] [CrossRef]
- Chen, W.; Chen, M.; Zang, Q.; Wang, L.; Tang, F.; Han, Y.; Yang, C.; Deng, L.; Liu, Y.-N. NIR light controlled release of caged hydrogen sulfide based on upconversion nanoparticles. Chem. Commun. 2015, 51, 9193–9196. [Google Scholar] [CrossRef]
- Chen, W.; Ni, D.; Rosenkrans, Z.T.; Cao, T.; Cai, W. Smart H2S-Triggered/Therapeutic System (SHTS)-Based Nanomedicine. Adv. Sci. 2019, 6, 1901724. [Google Scholar] [CrossRef]
- Nani, R.R.; Gorka, A.P.; Nagaya, T.; Kobayashi, H.; Schnermann, M.J. Near-IR Light-Mediated Cleavage of Antibody–Drug Conjugates Using Cyanine Photocages. Angew. Chem. Int. Ed. 2015, 54, 13635–13638. [Google Scholar] [CrossRef] [PubMed]
- Gorka, A.P.; Nani, R.R.; Zhu, J.; Mackem, S.; Schnermann, M.J. A Near-IR Uncaging Strategy Based on Cyanine Photochemistry. J. Am. Chem. Soc. 2014, 136, 14153–14159. [Google Scholar] [CrossRef]
- Xiang, J.; Ge, F.; Yu, B.; Yan, Q.; Shi, F.; Zhao, Y. Nanocomplexes of Photolabile Polyelectrolyte and Upconversion Nanoparticles for Near-Infrared Light-Triggered Payload Release. ACS Appl. Mater. Interfaces 2018, 10, 20790–20800. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Kang, X.; Sun, J.; Jing, X.; Wang, Z.; Yan, L.; Qi, R.; Zheng, M. Nanoparticle delivery of sterically hindered platinum(iv) prodrugs shows 100 times higher potency than that of cisplatin upon light activation. Chem. Commun. 2016, 52, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Chandra, B.; Mallik, S.; Srivastava, D.K. Design of photocleavable lipids and their application in liposomal “uncorking”. Chem. Commun. 2005, 3021–3023. [Google Scholar] [CrossRef] [PubMed]
- Reeßing, F.; Stuart, M.C.A.; Samplonius, D.F.; Dierckx, R.A.J.O.; Feringa, B.L.; Helfrich, W.; Szymanski, W. A light-responsive liposomal agent for MRI contrast enhancement and monitoring of cargo delivery. Chem. Commun. 2019, 55, 10784–10787. [Google Scholar] [CrossRef]
- Wu, H.; Dong, J.; Li, C.; Liu, Y.; Feng, N.; Xu, L.; Zhan, X.; Yang, H.; Wang, G. Multi-responsive nitrobenzene-based amphiphilic random copolymer assemblies. Chem. Commun. 2013, 49, 3516–3518. [Google Scholar] [CrossRef]
- Patil, N.G.; Basutkar, N.B.; Ambade, A.V. Visible light-triggered disruption of micelles of an amphiphilic block copolymer with BODIPY at the junction. Chem. Commun. 2015, 51, 17708–17711. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Y.; Liu, T.; Zhang, G.; Liu, S. Light-Triggered Concomitant Enhancement of Magnetic Resonance Imaging Contrast Performance and Drug Release Rate of Functionalized Amphiphilic Diblock Copolymer Micelles. Biomacromolecules 2012, 13, 3877–3886. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.-I.; Song, Q.; Rho, J.Y.; Mansfield, E.D.H.; Hall, S.C.L.; Sambrook, M.; Huang, F.; Perrier, S. Hierarchical Self-Assembled Photo-Responsive Tubisomes from a Cyclic Peptide-Bridged Amphiphilic Block Copolymer. Angew. Chem. Int. Ed. 2020, 59, 8860–8863. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, P.; Liu, Y.; Lu, C.; Qiu, Y.; Mu, H.; Duan, J. A photo-controlled hyaluronan-based drug delivery nanosystem for cancer therapy. Carbohydr. Polym. 2019, 206, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sun, H.; Liu, Y.; Hou, W.; Yang, Y.; Cai, R.; Cui, C.; Zhang, P.; Pan, X.; Li, X.; et al. Self-Assembled Aptamer-Grafted Hyperbranched Polymer Nanocarrier for Targeted and Photoresponsive Drug Delivery. Angew. Chem. Int. Ed. 2018, 57, 17048–17052. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Manouras, T.; Vamvakaki, M.; Argitis, P. Harnessing photochemical internalization with dual degradable nanoparticles for combinatorial photo–chemotherapy. Nat. Commun. 2014, 5, 3623. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, M.; Liang, S.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. Synthesis of Photo- and pH Dual-Sensitive Amphiphilic Copolymer PEG43-b-P(AA76-co-NBA35-co-tBA9) and Its Micellization as Leakage-Free Drug Delivery System for UV-Triggered Intracellular Delivery of Doxorubicin. ACS Appl. Mater. Interfaces 2016, 8, 22127–22134. [Google Scholar] [CrossRef] [PubMed]
- Abebe Alemayehu, Y.; Tewabe Gebeyehu, B.; Cheng, C.-C. Photosensitive Supramolecular Micelles with Complementary Hydrogen Bonding Motifs to Improve the Efficacy of Cancer Chemotherapy. Biomacromolecules 2019, 20, 4535–4545. [Google Scholar] [CrossRef]
- Liang, X.; Yue, X.; Dai, Z.; Kikuchi, J.-i. Photoresponsive liposomal nanohybrid cerasomes. Chem. Commun. 2011, 47, 4751–4753. [Google Scholar] [CrossRef]
- Poelma, S.O.; Oh, S.S.; Helmy, S.; Knight, A.S.; Burnett, G.L.; Soh, H.T.; Hawker, C.J.; Read de Alaniz, J. Controlled drug release to cancer cells from modular one-photon visible light-responsive micellar system. Chem. Commun. 2016, 52, 10525–10528. [Google Scholar] [CrossRef]
- Namazi, H.; Jafarirad, S. Invitro Photo-Controlled Drug Release System Based on Amphiphilic Linear-Dendritic Diblock Copolymers; Self-Assembly Behavior and Application as Nanocarrier. J. Pharm. Pharm. Sci. 2011, 14, 162–180. [Google Scholar] [CrossRef]
- Yan, Q.; Xin, Y.; Zhou, R.; Yin, Y.; Yuan, J. Light-controlled smart nanotubes based on the orthogonal assembly of two homopolymers. Chem. Commun. 2011, 47, 9594–9596. [Google Scholar] [CrossRef]
- Li, F.-Q.; Yu, Q.-L.; Liu, Y.-H.; Yu, H.-J.; Chen, Y.; Liu, Y. Highly efficient photocontrolled targeted delivery of siRNA by a cyclodextrin-based supramolecular nanoassembly. Chem. Commun. 2020, 56, 3907–3910. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, L.; Guo, D.; Yasen, W.; Wu, Y.; Su, Y.; Chen, D.; Qiu, F.; Yan, D.; Zhu, X. A NIR-triggered gatekeeper of supramolecular conjugated unimicelles with two-photon absorption for controlled drug release. Chem. Commun. 2019, 55, 6735–6738. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, P.; Li, Q.; Al-Khalaf, A.A.; Hozzein, W.N.; Zhang, F.; Li, X.; Zhao, D. Near-Infrared Triggered Decomposition of Nanocapsules with High Tumor Accumulation and Stimuli Responsive Fast Elimination. Angew. Chem. Int. Ed. 2018, 57, 2611–2615. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Tomatsu, I.; Kros, A. Light controlled protein release from a supramolecular hydrogel. Chem. Commun. 2010, 46, 4094–4096. [Google Scholar] [CrossRef]
- Karcher, J.; Pianowski, Z.L. Photocontrol of Drug Release from Supramolecular Hydrogels with Green Light. Chem. Eur. J. 2018, 24, 11605–11610. [Google Scholar] [CrossRef] [PubMed]
- Roth Stefaniak, K.; Epley, C.C.; Novak, J.J.; McAndrew, M.L.; Cornell, H.D.; Zhu, J.; McDaniel, D.K.; Davis, J.L.; Allen, I.C.; Morris, A.J.; et al. Photo-triggered release of 5-fluorouracil from a MOF drug delivery vehicle. Chem. Commun. 2018, 54, 7617–7620. [Google Scholar] [CrossRef] [PubMed]
- Möller, N.; Hellwig, T.; Stricker, L.; Engel, S.; Fallnich, C.; Ravoo, B.J. Near-infrared photoswitching of cyclodextrin–guest complexes using lanthanide-doped LiYF4 upconversion nanoparticles. Chem. Commun. 2017, 53, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, W.; Wang, Y.; Xu, F.; Qu, J.; Xia, J.; Shen, M.; Shi, X. Gd-/CuS-Loaded Functional Nanogels for MR/PA Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 9107–9117. [Google Scholar] [CrossRef]
- Sloand, J.N.; Nguyen, T.T.; Zinck, S.A.; Cook, E.C.; Zimudzi, T.J.; Showalter, S.A.; Glick, A.B.; Simon, J.C.; Medina, S.H. Ultrasound-Guided Cytosolic Protein Delivery via Transient Fluorous Masks. ACS Nano 2020, 14, 4061–4073. [Google Scholar] [CrossRef]
- Chen, J.; Ratnayaka, S.; Alford, A.; Kozlovskaya, V.; Liu, F.; Xue, B.; Hoyt, K.; Kharlampieva, E. Theranostic Multilayer Capsules for Ultrasound Imaging and Guided Drug Delivery. ACS Nano 2017, 11, 3135–3146. [Google Scholar] [CrossRef]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.Y. Functionalized mesoporous silica nanoparticle-based visible light responsive controlled release delivery system. Chem. Commun. 2011, 47, 2817–2819. [Google Scholar] [CrossRef]
- Ma, N.; Wang, W.-J.; Chen, S.; Wang, X.-S.; Wang, X.-Q.; Wang, S.-B.; Zhu, J.-Y.; Cheng, S.-X.; Zhang, X.-Z. Cucurbit[8]uril-mediated supramolecular photoswitching for self-preservation of mesoporous silica nanoparticle delivery system. Chem. Commun. 2015, 51, 12970–12973. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Mu, X.; Xu, Y.; Wu, X.; Wu, C.; Li, C.; Chen, J.; Zhao, Y. Light-responsive nanogated ensemble based on polymer grafted mesoporous silica hybrid nanoparticles. Chem. Commun. 2010, 46, 7370–7372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Yan, Y.; Huang, J. Smart Nanocarrier: Self-Assembly of Bacteria-like Vesicles with Photoswitchable Cilia. ACS Nano 2014, 8, 11341–11349. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, P.; Li, X.; Hu, X.; Hou, J.; Wang, L.; Zhang, F. Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance. Adv. Mater. 2016, 28, 9341–9348. [Google Scholar] [CrossRef] [PubMed]
- Beňová, E.; Zeleňák, V.; Halamová, D.; Almáši, M.; Petrul’ová, V.; Psotka, M.; Zeleňáková, A.; Bačkor, M.; Hornebecq, V. A drug delivery system based on switchable photo-controlled p-coumaric acid derivatives anchored on mesoporous silica. J. Mater. Chem. B 2017, 5, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cuezva, A.; Valero-Moya, S.; Alajarin, M.; Berna, J. Light-responsive peptide [2]rotaxanes as gatekeepers of mechanised nanocontainers. Chem. Commun. 2015, 51, 14501–14504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, J.; Liu, G.; Tian, J.; Wang, H.; Gong, M.; Liu, S. Reversibly Switching Bilayer Permeability and Release Modules of Photochromic Polymersomes Stabilized by Cooperative Noncovalent Interactions. J. Am. Chem. Soc. 2015, 137, 15262–15275. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, T.; Zhou, L.; Gu, Q.; Liu, L.; Lv, F.; Wang, S. Conjugated Polymer Nanoparticles with Appended Photo-Responsive Units for Controlled Drug Delivery, Release, and Imaging. Angew. Chem. Int. Ed. 2018, 57, 13114–13119. [Google Scholar] [CrossRef]
- Yan, H.; Teh, C.; Sreejith, S.; Zhu, L.; Kwok, A.; Fang, W.; Ma, X.; Nguyen, K.T.; Korzh, V.; Zhao, Y. Functional Mesoporous Silica Nanoparticles for Photothermal-Controlled Drug Delivery In Vivo. Angew. Chem. Int. Ed. 2012, 51, 8373–8377. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Zhang, Y.; Chen, T.; Lu, D.; Zhao, Z.; Zhang, X.; Li, Z.; Yan, C.-H.; Tan, W. Photon-Manipulated Drug Release from a Mesoporous Nanocontainer Controlled by Azobenzene-Modified Nucleic Acid. ACS Nano 2012, 6, 6337–6344. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, N.; Wu, D.; Gao, Z.; Song, Y.-Y.; Schmuki, P. Upconversion Nanoparticle-Assisted Payload Delivery from TiO2 under Near-Infrared Light Irradiation for Bacterial Inactivation. ACS Nano 2020, 14, 337–346. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.; Singha, K.; Kim, W.J. Mesoporous silica nanoparticle facilitated drug release through cascade photosensitizer activation and cleavage of singlet oxygen sensitive linker. Chem. Commun. 2013, 49, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, P.; Wang, D.; Chen, J.; Liu, W.; Hu, P.; Huang, M.; Chen, X.; Chen, Z. Near-infrared-triggered antibacterial and antifungal photodynamic therapy based on lanthanide-doped upconversion nanoparticles. Nanoscale 2018, 10, 15485–15495. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shao, X.; Zhao, J.; Wu, M. Controllable Photodynamic Therapy Implemented by Regulating Singlet Oxygen Efficiency. Adv. Sci. 2017, 4, 1700113. [Google Scholar] [CrossRef]
- Ji, C.; Gao, Q.; Dong, X.; Yin, W.; Gu, Z.; Gan, Z.; Zhao, Y.; Yin, M. A Size-Reducible Nanodrug with an Aggregation-Enhanced Photodynamic Effect for Deep Chemo-Photodynamic Therapy. Angew. Chem. Int. Ed. 2018, 57, 11384–11388. [Google Scholar] [CrossRef]
- Mu, Q.; Jiang, G.; Chen, L.; Zhou, H.; Fourches, D.; Tropsha, A.; Yan, B. Chemical Basis of Interactions Between Engineered Nanoparticles and Biological Systems. Chem. Rev. 2014, 114, 7740–7781. [Google Scholar] [CrossRef]
- Luo, L.; Guo, Y.; Yang, J.; Liu, Y.; Chu, S.; Kong, F.; Wang, Y.; Zou, Z. An efficient visible light controlled protein delivery system. Chem. Commun. 2011, 47, 11243–11245. [Google Scholar] [CrossRef]
- Ye, Y.; Li, Y.; Fang, F. Upconversion nanoparticles conjugated with curcumin as a photosensitizer to inhibit methicillin-resistant Staphylococcus aureus in lung under near infrared light. Int. J. Nanomed. 2014, 9, 5157–5165. [Google Scholar] [CrossRef]
- Li, S.; Cui, S.; Yin, D.; Zhu, Q.; Ma, Y.; Qian, Z.; Gu, Y. Dual antibacterial activities of a chitosan-modified upconversion photodynamic therapy system against drug-resistant bacteria in deep tissue. Nanoscale 2017, 9, 3912–3924. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, S.; Xue, Y.; Cao, H.; Zhang, W. Light-controllable toxicity recovery from selenium-based amphiphiles. Chem. Commun. 2016, 52, 14208–14211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, N.; Li, C.; Hou, Y.; Wang, X.; Zhou, Q. Incorporation of 7-dehydrocholesterol into liposomes as a simple, universal and efficient way to enhance anticancer activity by combining PDT and photoactivated chemotherapy. Chem. Commun. 2019, 55, 14081–14084. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Hu, X.; Zheng, X.; Liu, S.; Li, Y.; Jing, X.; Xie, Z. Light-Activatable Red Blood Cell Membrane-Camouflaged Dimeric Prodrug Nanoparticles for Synergistic Photodynamic/Chemotherapy. ACS Nano 2018, 12, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, C.-S.; Na, K. Light-controlled reactive oxygen species (ROS)-producible polymeric micelles with simultaneous drug-release triggering and endo/lysosomal escape. Chem. Commun. 2016, 52, 2839–2842. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zheng, L.; Sun, Y.; Zhang, D. Multifunctional semiconducting polymer dots for imaging, detection, and photo-killing of bacteria. J. Mater. Chem. B 2014, 2, 4818–4825. [Google Scholar] [CrossRef]
- Guo, S.; Liu, X.; Yao, C.; Lu, C.; Chen, Q.; Hu, X.-Y.; Wang, L. Photolysis of a bola-type supra-amphiphile promoted by water-soluble pillar[5]arene-induced assembly. Chem. Commun. 2016, 52, 10751–10754. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, T.; Zhang, J.; Che, H.; Shi, Y.; Shi, X.; van Hest, J.C.M. Surface-Charge-Switchable Nanoclusters for Magnetic Resonance Imaging-Guided and Glutathione Depletion-Enhanced Photodynamic Therapy. ACS Nano 2020, 14, 11225–11237. [Google Scholar] [CrossRef]
- Rout, B.; Liu, C.-H.; Wu, W.-C. Photosensitizer in lipid nanoparticle: A nano-scaled approach to antibacterial function. Sci. Rep. 2017, 7, 7892. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial Photodynamic Therapy and Photodynamic Inactivation, or Killing Bugs with Dyes and Light—A Symposium-in-Print. Photochem. Photobiol. 2012, 88, 496–498. [Google Scholar] [CrossRef]
- Nishiyama, N.; Morimoto, Y.; Jang, W.-D.; Kataoka, K. Design and development of dendrimer photosensitizer-incorporated polymeric micelles for enhanced photodynamic therapy. Adv. Drug Deliv. Rev. 2009, 61, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Moussaron, A.; Youssef, Z.; Ben-Mihoub, A.; Vanderesse, R.; Frochot, C.; Acherar, S. Chapter 5—Dual imaging and photodynamic therapy anticancer theranostic nanoparticles. In Photonanotechnology for Therapeutics and Imaging; Choi, S.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 105–146. [Google Scholar]
- Xu, J.; Yu, S.; Wang, X.; Qian, Y.; Wu, W.; Zhang, S.; Zheng, B.; Wei, G.; Gao, S.; Cao, Z.; et al. High Affinity of Chlorin e6 to Immunoglobulin G for Intraoperative Fluorescence Image-Guided Cancer Photodynamic and Checkpoint Blockade Therapy. ACS Nano 2019, 13, 10242–10260. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Li, S.; Xin, X.; Yuan, S.; Ma, G.; Yan, X. Self-Assembled Minimalist Multifunctional Theranostic Nanoplatform for Magnetic Resonance Imaging-Guided Tumor Photodynamic Therapy. ACS Nano 2018, 12, 8266–8276. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Feng, G.; Qin, W.; Tang, B.Z.; Liu, B. Targeted and image-guided photodynamic cancer therapy based on organic nanoparticles with aggregation-induced emission characteristics. Chem. Commun. 2014, 50, 8757–8760. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Y.; Yuan, Y.; Wu, Y.; Song, Z.; Tang, B.Z.; Liu, B.; Zheng, Q.C. One-Step Formulation of Targeted Aggregation-Induced Emission Dots for Image-Guided Photodynamic Therapy of Cholangiocarcinoma. ACS Nano 2017, 11, 3922–3932. [Google Scholar] [CrossRef]
- Yang, Z.; Du, Y.; Sun, Q.; Peng, Y.; Wang, R.; Zhou, Y.; Wang, Y.; Zhang, C.; Qi, X. Albumin-Based Nanotheranostic Probe with Hypoxia Alleviating Potentiates Synchronous Multimodal Imaging and Phototherapy for Glioma. ACS Nano 2020, 14, 6191–6212. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Fang, S.; Lin, J.; Li, C.; Huang, P.; Hou, W.; Zhang, C.; Liu, J.; Huang, S.; Luo, Y.; Fan, W.; et al. Dual-Stimuli Responsive Nanotheranostics for Multimodal Imaging Guided Trimodal Synergistic Therapy. Small 2017, 13, 1602580. [Google Scholar] [CrossRef]
- Melancon, M.P.; Zhou, M.; Zhang, R.; Xiong, C.; Allen, P.; Wen, X.; Huang, Q.; Wallace, M.; Myers, J.N.; Stafford, R.J.; et al. Selective Uptake and Imaging of Aptamer- and Antibody-Conjugated Hollow Nanospheres Targeted to Epidermal Growth Factor Receptors Overexpressed in Head and Neck Cancer. ACS Nano 2014, 8, 4530–4538. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, S.; Zhang, F.; Yang, K.; Ma, Q.; Zhu, L. Activatable Hyaluronic Acid Nanoparticle as a Theranostic Agent for Optical/Photoacoustic Image-Guided Photothermal Therapy. ACS Nano 2014, 8, 12250–12258. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yan, L.; Yu, J.; Tian, G.; Zhou, L.; Zheng, X.; Zhang, X.; Yong, Y.; Li, J.; Gu, Z.; et al. High-Throughput Synthesis of Single-Layer MoS2 Nanosheets as a Near-Infrared Photothermal-Triggered Drug Delivery for Effective Cancer Therapy. ACS Nano 2014, 8, 6922–6933. [Google Scholar] [CrossRef]
- Vigderman, L.; Zubarev, E.R. Therapeutic platforms based on gold nanoparticles and their covalent conjugates with drug molecules. Adv. Drug Delivery Rev. 2013, 65, 663–676. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-dimensional graphene analogues for biomedical applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef] [PubMed]
- Marquez, D.T.; Scaiano, J.C. Visible and Near-Infrared Plasmon-Mediated Molecular Release from Cucurbit[6]uril Mesoporous Gated Systems. Langmuir 2016, 32, 13764–13770. [Google Scholar] [CrossRef]
- An, X.; Zhang, F.; Zhu, Y.; Shen, W. Photoinduced drug release from thermosensitive AuNPs-liposome using a AuNPs-switch. Chem. Commun. 2010, 46, 7202–7204. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Wu, L.; Cui, H.; Tan, W.; Chen, T.; Chu, P.K.; Yu, X.-F. Synthesis of lipid–black phosphorus quantum dot bilayer vesicles for near-infrared-controlled drug release. Chem. Commun. 2018, 54, 6060–6063. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Niidome, Y.; Yamada, S. Controlled release of plasmid DNA from gold nanorods induced by pulsed near-infrared light. Chem. Commun. 2005, 2247–2249. [Google Scholar] [CrossRef]
- Song, J.; Yang, X.; Jacobson, O.; Lin, L.; Huang, P.; Niu, G.; Ma, Q.; Chen, X. Sequential Drug Release and Enhanced Photothermal and Photoacoustic Effect of Hybrid Reduced Graphene Oxide-Loaded Ultrasmall Gold Nanorod Vesicles for Cancer Therapy. ACS Nano 2015, 9, 9199–9209. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Babu, T.; Liu, Y.; Gong, J.; Nie, Z. Near-infrared light-responsive vesicles of Au nanoflowers. Chem. Commun. 2013, 49, 576–578. [Google Scholar] [CrossRef]
- Ding, Y.; Du, C.; Qian, J.; Dong, C.-M. NIR-Responsive Polypeptide Nanocomposite Generates NO Gas, Mild Photothermia, and Chemotherapy to Reverse Multidrug-Resistant Cancer. Nano Lett. 2019, 19, 4362–4370. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Tian, X.; Ke, H.; Guo, M.; Zhu, A.; Yang, T.; Guo, Z.; Ge, Z.; Yang, X.; et al. Multipronged Design of Light-Triggered Nanoparticles To Overcome Cisplatin Resistance for Efficient Ablation of Resistant Tumor. ACS Nano 2015, 9, 9626–9637. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-Step Assembly of DOX/ICG Loaded Lipid–Polymer Nanoparticles for Highly Effective Chemo-photothermal Combination Therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, G.; Zhang, W.; Xing, D.; Hu, X. Cascade-Promoted Photo-Chemotherapy against Resistant Cancers by Enzyme-Responsive Polyprodrug Nanoplatforms. Chem. Mater. 2018, 30, 3486–3498. [Google Scholar] [CrossRef]
- Wang, W.; Satyavolu, N.S.R.; Wu, Z.; Zhang, J.-R.; Zhu, J.-J.; Lu, Y. Near-Infrared Photothermally Activated DNAzyme–Gold Nanoshells for Imaging Metal Ions in Living Cells. Angew. Chem. Int. Ed. 2017, 56, 6798–6802. [Google Scholar] [CrossRef]
- Jeong, E.H.; Ryu, J.H.; Jeong, H.; Jang, B.; Lee, H.Y.; Hong, S.; Lee, H.; Lee, H. Efficient delivery of siRNAs by a photothermal approach using plant flavonoid-inspired gold nanoshells. Chem. Commun. 2014, 50, 13388–13390. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.; Cai, J.; Cheng, D.; Ma, Y.; Zhang, J.; Zhou, C.; Liu, S.; Shi, H.; Zhang, Y.; et al. A Multifunctional Platform for Tumor Angiogenesis-Targeted Chemo-Thermal Therapy Using Polydopamine-Coated Gold Nanorods. ACS Nano 2016, 10, 10404–10417. [Google Scholar] [CrossRef]
- Parchur, A.K.; Sharma, G.; Jagtap, J.M.; Gogineni, V.R.; LaViolette, P.S.; Flister, M.J.; White, S.B.; Joshi, A. Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano 2018, 12, 6597–6611. [Google Scholar] [CrossRef]
- Lin, L.-S.; Cong, Z.-X.; Cao, J.-B.; Ke, K.-M.; Peng, Q.-L.; Gao, J.; Yang, H.-H.; Liu, G.; Chen, X. Multifunctional Fe3O4@Polydopamine Core–Shell Nanocomposites for Intracellular mRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef]
- Jc Bose, R.; Uday Kumar, S.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R.; et al. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS Nano 2018, 12, 10817–10832. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, S.; Wen, L.; Xing, D. Imaging-guided photoacoustic drug release and synergistic chemo-photoacoustic therapy with paclitaxel-containing nanoparticles. J. Control. Release 2016, 226, 77–87. [Google Scholar] [CrossRef]
- Liu, T.; Shi, S.; Liang, C.; Shen, S.; Cheng, L.; Wang, C.; Song, X.; Goel, S.; Barnhart, T.E.; Cai, W.; et al. Iron Oxide Decorated MoS2 Nanosheets with Double PEGylation for Chelator-Free Radiolabeling and Multimodal Imaging Guided Photothermal Therapy. ACS Nano 2015, 9, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Tomitaka, A.; Arami, H.; Huang, Z.; Raymond, A.; Rodriguez, E.; Cai, Y.; Febo, M.; Takemura, Y.; Nair, M. Hybrid magneto-plasmonic liposomes for multimodal image-guided and brain-targeted HIV treatment. Nanoscale 2018, 10, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, P.; Xu, X.; Lei, P.; Du, K.; Zhang, M.; Wang, D.; Feng, J.; Li, W.; Zhang, H. Simple construction of Cu2−xS:Pt nanoparticles as nanotheranostic agent for imaging-guided chemo-photothermal synergistic therapy of cancer. Nanoscale 2018, 10, 10945–10951. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, J.; Hu, Y.; Howard, K.A.; Li, Z.; Fan, X.; Chang, M.; Sun, Y.; Besenbacher, F.; Chen, C.; et al. Multimodal Imaging-Guided Antitumor Photothermal Therapy and Drug Delivery Using Bismuth Selenide Spherical Sponge. ACS Nano 2016, 10, 9646–9658. [Google Scholar] [CrossRef]
- Pimlott, S.L.; Sutherland, A. Molecular tracers for the PET and SPECT imaging of disease. Chem. Soc. Rev. 2011, 40, 149–162. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Yan, X.; Wang, Y.; Guo, J.; Jacobson, O.; Liu, D.; Szajek, L.P.; Zhu, W.; Niu, G.; et al. Chelator-Free 64Cu-Integrated Gold Nanomaterials for Positron Emission Tomography Imaging Guided Photothermal Cancer Therapy. ACS Nano 2014, 8, 8438–8446. [Google Scholar] [CrossRef]
- Cheng, L.; Kamkaew, A.; Sun, H.; Jiang, D.; Valdovinos, H.F.; Gong, H.; England, C.G.; Goel, S.; Barnhart, T.E.; Cai, W. Dual-Modality Positron Emission Tomography/Optical Image-Guided Photodynamic Cancer Therapy with Chlorin e6-Containing Nanomicelles. ACS Nano 2016, 10, 7721–7730. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, Y.; Luehmann, H.; Yang, X.; Detering, L.; You, M.; Zhang, C.; Zhang, L.; Li, Z.-Y.; Ren, Q.; et al. 64Cu-Doped PdCu@Au Tripods: A Multifunctional Nanomaterial for Positron Emission Tomography and Image-Guided Photothermal Cancer Treatment. ACS Nano 2016, 10, 3121–3131. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radiolabeled Mesoporous Silica Nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Liang, S.; Sood, A.K.; Liang, D.; Li, C. CuS Nanodots with Ultrahigh Efficient Renal Clearance for Positron Emission Tomography Imaging and Image-Guided Photothermal Therapy. ACS Nano 2015, 9, 7085–7096. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-L.; Shih, Y.-H.; Lee, P.-C.; Hsieh, T.M.-H.; Luo, T.-Y.; Shieh, M.-J. Multimodal Image-Guided Photothermal Therapy Mediated by 188Re-Labeled Micelles Containing a Cyanine-Type Photosensitizer. ACS Nano 2011, 5, 5594–5607. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhou, J.; Zhang, C.; Zhi, X.; Niu, J.; Fu, H.; Song, J.; Cui, D. Surface-engineered nanobubbles with pH-/light-responsive drug release and charge-switchable behaviors for active NIR/MR/US imaging-guided tumor therapy. NPG Asia Mater. 2018, 10, 1046–1060. [Google Scholar] [CrossRef]
- de Smet, M.; Heijman, E.; Langereis, S.; Hijnen, N.M.; Grüll, H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. J. Control. Release 2011, 150, 102–110. [Google Scholar] [CrossRef]
- Hijnen, N.; Kneepkens, E.; de Smet, M.; Langereis, S.; Heijman, E.; Grüll, H. Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proc. Natl. Acad. Sci. USA 2017, 114, E4802–E4811. [Google Scholar] [CrossRef]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Pang, X.; Jung, S.; Zhang, G.; Kong, M.; Liu, G.; Chen, X. Smart gold nanoparticle-stabilized ultrasound microbubbles as cancer theranostics. J. Mater. Chem. B 2018, 6, 3235–3239. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yuan, C.; Li, M.; Wang, T.; Chen, B.; Jin, J.; Zhao, P.; Tong, J.; Luo, S.; et al. Magnetic Nanoliposomes as in Situ Microbubble Bombers for Multimodality Image-Guided Cancer Theranostics. ACS Nano 2017, 11, 1509–1519. [Google Scholar] [CrossRef]
- Lee, J.Y.; Carugo, D.; Crake, C.; Owen, J.; de Saint Victor, M.; Seth, A.; Coussios, C.; Stride, E. Nanoparticle-Loaded Protein–Polymer Nanodroplets for Improved Stability and Conversion Efficiency in Ultrasound Imaging and Drug Delivery. Adv. Mater. 2015, 27, 5484–5492. [Google Scholar] [CrossRef]
- Jin, Z.; Wen, Y.; Hu, Y.; Chen, W.; Zheng, X.; Guo, W.; Wang, T.; Qian, Z.; Su, B.-L.; He, Q. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale 2017, 9, 3637–3645. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, T.; Li, B.; Zheng, R.; Qiu, C.; Lam, K.S.; Zhang, Q.; Shuai, X. Size-Modulable Nanoprobe for High-Performance Ultrasound Imaging and Drug Delivery against Cancer. ACS Nano 2018, 12, 3449–3460. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, L.; He, L.; Hinkle, K.; Wu, Y.; Ma, J.; Chang, L.; Zhao, X.; Perez, D.G.; Eckardt, S.; et al. Design of a Microchannel-Nanochannel-Microchannel Array Based Nanoelectroporation System for Precise Gene Transfection. Small 2014, 10, 1015–1023. [Google Scholar] [CrossRef]
- Mouli, S.K.; Tyler, P.; McDevitt, J.L.; Eifler, A.C.; Guo, Y.; Nicolai, J.; Lewandowski, R.J.; Li, W.; Procissi, D.; Ryu, R.K.; et al. Image-Guided Local Delivery Strategies Enhance Therapeutic Nanoparticle Uptake in Solid Tumors. ACS Nano 2013, 7, 7724–7733. [Google Scholar] [CrossRef]
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart Drug Delivery through DNA/Magnetic Nanoparticle Gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef]
- Dunn, A.E.; Dunn, D.J.; Macmillan, A.; Whan, R.; Stait-Gardner, T.; Price, W.S.; Lim, M.; Boyer, C. Spatial and temporal control of drug release through pH and alternating magnetic field induced breakage of Schiff base bonds. Polym. Chem. 2014, 5, 3311–3315. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]
- Liu, J.F.; Neel, N.; Dang, P.; Lamb, M.; McKenna, J.; Rodgers, L.; Litt, B.; Cheng, Z.; Tsourkas, A.; Issadore, D. Radiofrequency-Triggered Drug Release from Nanoliposomes with Millimeter-Scale Resolution Using a Superimposed Static Gating Field. Small 2018, 14, 1802563. [Google Scholar] [CrossRef]
- Fuller, E.G.; Sun, H.; Dhavalikar, R.D.; Unni, M.; Scheutz, G.M.; Sumerlin, B.S.; Rinaldi, C. Externally Triggered Heat and Drug Release from Magnetically Controlled Nanocarriers. ACS Appl. Mater. Interfaces 2019, 1, 211–220. [Google Scholar] [CrossRef]
- Xu, X.; Hou, S.; Wattanatorn, N.; Wang, F.; Yang, Q.; Zhao, C.; Yu, X.; Tseng, H.-R.; Jonas, S.J.; Weiss, P.S. Precision-Guided Nanospears for Targeted and High-Throughput Intracellular Gene Delivery. ACS Nano 2018, 12, 4503–4511. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef] [PubMed]
- Gianella, A.; Jarzyna, P.A.; Mani, V.; Ramachandran, S.; Calcagno, C.; Tang, J.; Kann, B.; Dijk, W.J.R.; Thijssen, V.L.; Griffioen, A.W.; et al. Multifunctional Nanoemulsion Platform for Imaging Guided Therapy Evaluated in Experimental Cancer. ACS Nano 2011, 5, 4422–4433. [Google Scholar] [CrossRef] [PubMed]
- Kunjachan, S.; Pola, R.; Gremse, F.; Theek, B.; Ehling, J.; Moeckel, D.; Hermanns-Sachweh, B.; Pechar, M.; Ulbrich, K.; Hennink, W.E.; et al. Passive versus Active Tumor Targeting Using RGD- and NGR-Modified Polymeric Nanomedicines. Nano Lett. 2014, 14, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Foss, C.A.; Byun, Y.; Nimmagadda, S.; Pullambhatla, M.; Fox, J.J.; Castanares, M.; Lupold, S.E.; Babich, J.W.; Mease, R.C.; et al. Radiohalogenated Prostate-Specific Membrane Antigen (PSMA)-Based Ureas as Imaging Agents for Prostate Cancer. J. Med. Chem. 2008, 51, 7933–7943. [Google Scholar] [CrossRef]
- Shukla, R.; Thomas, T.P.; Desai, A.M.; Kotlyar, A.; Park, S.J.; Baker, J.R., Jr. HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology 2008, 19, 295102. [Google Scholar] [CrossRef]
- Thomas, T.P.; Choi, S.K.; Li, M.-H.; Kotlyar, A.; Baker, J.R., Jr. Design of Riboflavin-Presenting PAMAM Dendrimers as a New Nanoplatform for Cancer-Targeted Delivery. Bioorg. Med. Chem. Lett. 2010, 20, 5191–5194. [Google Scholar] [CrossRef]
- Plantinga, A.; Witte, A.; Li, M.-H.; Harmon, A.; Choi, S.K.; Banaszak Holl, M.M.; Orr, B.G.; Baker, J.R., Jr.; Sinniah, K. Bioanalytical Screening of Riboflavin Antagonists for Targeted Drug Delivery—A Thermodynamic and Kinetic Study. ACS Med. Chem. Lett. 2011, 2, 363–367. [Google Scholar] [CrossRef]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted Drug Delivery via the Transferrin Receptor-Mediated Endocytosis Pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines—Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61, 25–31. [Google Scholar] [CrossRef]
- Sparreboom, A.; Scripture, C.D.; Trieu, V.; Williams, P.J.; De, T.; Yang, A.; Beals, B.; Figg, W.D.; Hawkins, M.; Desai, N. Comparative Preclinical and Clinical Pharmacokinetics of a Cremophor-Free, Nanoparticle Albumin-Bound Paclitaxel (ABI-007) and Paclitaxel Formulated in Cremophor (Taxol). Clin. Cancer Res. 2005, 11, 4136–4143. [Google Scholar] [CrossRef] [PubMed]
- Lyass, O.; Uziely, B.; Ben-Yosef, R.; Tzemach, D.; Heshing, N.I.; Lotem, M.; Brufman, G.; Gabizon, A. Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer 2000, 89, 1037–1047. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Liu, Y.; Noe, D.; Mertz, J.; Bargfrede, M.; Marbury, T.; Farbakhsh, K.; Oliva, C.; Milton, A. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. Br. J. Clin. Pharmacol. 2014, 77, 998–1010. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef]
- Zhang, H. Onivyde for the therapy of multiple solid tumors. OncoTargets Ther. 2016, 9, 3001–3007. [Google Scholar] [CrossRef]
- Dunne, M.; Epp-Ducharme, B.; Sofias, A.M.; Regenold, M.; Dubins, D.N.; Allen, C. Heat-activated drug delivery increases tumor accumulation of synergistic chemotherapies. J. Control. Release 2019, 308, 197–208. [Google Scholar] [CrossRef]
- Huggett, M.T.; Jermyn, M.; Gillams, A.; Illing, R.; Mosse, S.; Novelli, M.; Kent, E.; Bown, S.G.; Hasan, T.; Pogue, B.W.; et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. Br. J. Cancer 2014, 110, 1698–1704. [Google Scholar] [CrossRef]
- Obaid, G.; Broekgaarden, M.; Bulin, A.-L.; Huang, H.-C.; Kuriakose, J.; Liu, J.; Hasan, T. Photonanomedicine: A convergence of photodynamic therapy and nanotechnology. Nanoscale 2016, 8, 12471–12503. [Google Scholar] [CrossRef]
- Maisch, T.; Santarelli, F.; Schreml, S.; Babilas, P.; Szeimies, R.-M. Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Exp. Dermatol. 2010, 19, e302–e305. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, H.S.; Brooks, S.; van der Ploeg-van den Heuvel, A.; ten Hagen, T.L.M.; de Haas, E.R.M.; Robinson, D.J. Light Fractionation Significantly Increases the Efficacy of Photodynamic Therapy Using BF-200 ALA in Normal Mouse Skin. PLoS ONE 2016, 11, e0148850. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).