Abstract
The reactivity of the complex [(dpp-bian)GaNa(DME)2] (1) (dpp-bian = 1,2-bis[(2,6-di-isopropylphenyl)imino]acenaphthene) towards isocyanates, benzophenone, diphenylketene, and 1,2-dibenzylidenehydrazine has been studied. Treatment of 1 with isocyanates led to derivatives of imidoformamide [(dpp-bian)Ga{C(=NPh)2}2–NPh][Na(DME)3] (2), biuret [(dpp-bian)Ga(NCy)2(CO)2NCy][Na(DME)] (3), or carbamic acids [(dpp-bian)GaN(Cy)C(O)O]2[Na(THF)(Et2O)] (4), [(dpp-bian)GaC(=NCy)N(Cy)C(O)O][Na(Py)3] (5). Treatment of 1 with 2 equiv. of Ph2CO resulted in gallium pinacolate [(dpp-bian)GaO(CPh2)2O][Na(Py)2] (9), while the reaction of 1 with 2 equiv. Ph2CCO gave divinyl ether derivative [(dpp-bian)Ga{C(=CPh2)O}2][Na(DME)3] (10). Complex 1 treated with 2 equiv. 1,2-dibenzylidenehydrazine underwent [1+2+2] cycloaddition to give C–C coupling product [(dpp-bian)Ga{N(NCHPh)}2(CHPh)2][Na(DME)3] (11). When complex 1 was sequentially treated with 1 equiv. of 1,2-dibenzylidenehydrazine and 1 equiv. of pyridine or pyridine-d5; it gave [1+2+2] cycloaddition product [(dpp-bian)GaN(NCHPh)C(Ph)CN][Na(DME)3] (12). Compounds 2–12 were characterized by NMR and IR spectroscopy, and their molecular structures were established by single-crystal X-ray diffraction analysis.
Keywords:
redox-active ligand; low-valent gallium; isocyanate; benzophenone; ketene; azine; cycloaddition 1. Introduction
The discovery of stable carbenes at the end of the 20th century marked a new era in chemistry. These compounds underwent rapid examination and garnered significant favor as ligands for transition metal atoms. Notably, palladium and ruthenium complexes based on N-heterocyclic carbenes have proven effective as catalysts for cross-coupling and olefin metathesis reactions [1,2]. Carbenes themselves are highly reactive and showcase unique properties [3,4,5], including cycloadditions [6].
Similar carbene-like reactivity is observed in certain compounds of group 13 elements. The entities denoted as RM:, in which M represents gallium or aluminum, have been identified as stable complexes [Cp*Al]4 (Cp* = C5Me5) [7] and [(TMS)3CGa]4 (TMS = SiMe3) [8]. Other spacious ligands, such as amido–imino ligands (e.g., NacNac = (HC(CMeNAr)2) and aromatic ligands like 2,6-terphenyls, proved to be useful in stabilizing [RM:]n (where n = 1 or 2, M = group 13 metal) [9,10,11,12]. Another class of reactive group 13 species emerges when [R2M•] (M = group 13 metal) undergo reduction, forming anionic carbenoid [R2M:]− species. These anionic species find stabilization through various bisamido ligands, including diaminoxanthene ligands [13], NON-ligands (NON = [O(SiMe2NAr)2]2−) [14], SiNDipp (SiNDipp = CH2SiMe2N(2,6-iPr2C6H3))2, deprotonated β-diketiminate [15], 1,1,4,4-tetrakis(trimethylsilyl)butane-1,4-diyl [16], and 1,4-diazadiene ligands [17,18,19,20,21,22]. Additionally, they can be part of cyclic(alkyl)(amino)aluminyl anions [23,24].
Molecules of R2−M–MR2−-type exhibit carbene-like behavior if the ligand R is redox-active. Due to the non-innocence of the ligand, these species may exhibit an equilibria R2-M–MR2−↔R1−M=MR1−⇄R1−M (Scheme 1). While controlling electron transfer between the non-innocent ligand and the metal atom remains a challenge [25,26], the chemical properties of R2-M–MR2- suggest reactivity resembling heterocyclopentadiene (II) [27,28], dimetallene (III) [29,30], and metallylene (IV) [31,32] types (Scheme 1).
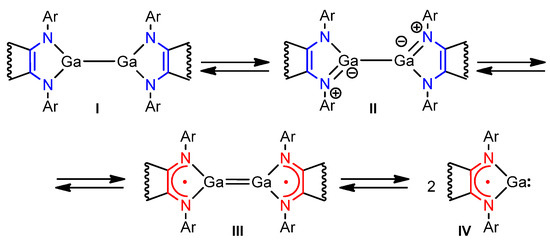
Scheme 1.
Structural versatility of R2−M–MR2− species (R = non-innocent ligand) reflecting various reactivity types.
While the synthesis and structural features of low-valent 13 carbenoid compounds are captivating, their reactivity remains an equally fascinating aspect [33,34,35,36,37,38]. First, they are good σ-donor/π-acceptor ligands for transition metal centers [39,40], have high reactivity against organic molecules, can cleave C–C aromatic bonds [41], and insert into robust C–H [16,42,43,44,45], C–F, C–O [46], C=N [47], C=S, P=S bonds [48]. Low-valent 13 group compounds were reported to undergo transition metal-like oxidative addition [31,49,50,51] and reductive elimination [52,53]. This made them environmentally safe candidates to replace expensive transition metal complexes in catalysis [54].
Despite structural and electronic differences, all the aforementioned metallylenes can undergo cycloadditions [55]. Dialumenes, reminiscent of olefins (Scheme 2, A), unveil their intriguing propensity for [2+2] cycloaddition reactions with an array of substrates—ranging from CO2 [56], olefins, and alkynes [57,58] to unactivated benzene rings [59,60]. Gallium terphenyl dimetallene ArGaGaAr (Ar = 2,6-terphenyl) was reported to react under ambient conditions with ethylene, propene, 1-hexene, and styrene to give 1,4-digallacycloalkanes [61].
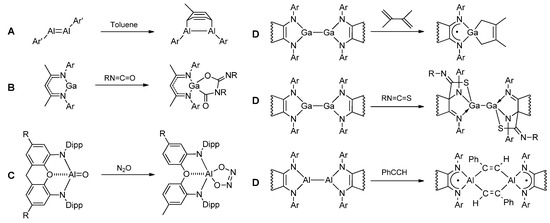
Scheme 2.
Cycloaddition on 13 group low-valent species.
Meanwhile, RM: species proceed with [2+1] or [4+1] cycloadditions. [NacNacAl:] reacts with alkynes to form alumacyclopropenes [62], alkenes to form alumacyclopropanes [63,64], and cyclic, acyclic and aromatic dienes [64,65], carbonyl compounds [66], and 1,4-diphosphinine [67]. Anionic aluminum(I) complexes react with 1,3,5,7-cyclooctatetrene [14] or unsaturated hydrocarbons [68]. [NacNacGa:] undergoes a cycloaddition reaction with two equivalents of RNCO (R = Ph; 3,5-Me2C6H3) or two equivalents (p-Tol)N=C=N(p-Tol) (Scheme 2, B) [69]. Cycloaddition may occur at polar groups M=E (E = O, S, N(2,6-iPr2C6H3)) [70,71,72,73,74] (Scheme 2, C). Noteworthy examples include cycloadditions of iso(thio)cyanates to monomeric aluminum sulfide [NacNacAl=S] [48]. At the same time, alumylene oxide {[(NON)Al=O]K}2 was reported to react with an equivalent of CO2, PhNCO, or N2O via a [2+2]-cycloaddition mechanism to produce aluminum carbonate, carbamate or hyponitrite [75]. The structural flexibility of low-valent species containing redox-active ligands leads to a larger scope of cycloaddition reactions, including[4+1]-, [4+2]-, and [2+2+1+1]- cycloaddition [76,77,78] (Scheme 2, D).
Attempts have been made to apply these concepts, but as of now, the achievements in this area are quite limited, in contrast to applied low-valent surfaces and solid-state chemistry [79,80]. Metallylenes were employed for selective coupling of carbonyl compounds, isocyanates, nitriles, imines, and azides with pyridine [81] or benzophenone [66]. They promoted a facile synthesis of phosphine PH3 from white phosphorus and ammonia [82]. Alumene’s adducts of olefins can undergo a reversible insertion of CO [83]. Benzene was converted into acyclic 1,3,5-triene products at a low-valent aluminum center [84]. Recently, we reported a direct transformation of RN=C=NR to guanidinates and iminoguanidinates [85,86], activation and modification of CO2 into carboxylic acid derivatives [87] mediated by gallylene complex [(dpp-bian)GaNa(DME)2] (1).
Here, we report on novel transformations of RN=C=O, Ph2C=O, Ph2C=C=O, N2O, COS and PhHC=N-N=CHPh at the gallium center of complex 1 and draw certain generalizations regarding the patterns that govern them.
2. Materials and Methods
General procedure for synthesis of 2–12. All the manipulations with air- and moisture-sensitive compounds were carried out in a vacuum or under argon using the standard Schlenk technique or under an argon atmosphere in a drybox. A solution of 1 equiv. of [(dpp-bian)GaNa(DME)2] [88] was prepared in situ by stirring 0.5 equiv. [(dpp-bian)Ga]2 with 1 equiv. of sodium metal in a relevant ether solvent until the metal completely dissolved. The solution became yellow-green. Then, 1 (or 2) equiv. of heteroalkene was added to the solution. Within a few minutes, the solution’s color became green/green-blue. Further crystallization afforded green/green-blue diamagnetic crystals of compounds 2–12.
Details of synthesis, characterization, X-ray crystal structure determination details, and crystal data are given in the electronic supplementary materials. CCDC 2314238 (2), 2314239 (3), 2314240 (4), 2314241 (5), 2314242 (6), 2314245 (7), 2314246 (8), 2314247 (9), 2314248 (10), 2314249 (11) and 2314250 (12) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via ccdc.cam.ac.uk/structures (accessed on 13 December 2023).
3. Results and Discussion
Metallylene’s [(dpp-bian)GaNa(DME)2] (1) reactivity against isocyanates was tested in three conditions: (1) 1 with 1 equiv. of PhNCO in DME solution, (2) 1 with 1 equiv. of CyNCO in DME solution, and (3) 1 with 2 equiv. of CyNCO in THF solution at 25 °C. A rapid solution color change from yellow-green to green-blue was observed. However, despite similar reaction conditions, the isolated products were strikingly different in each case (Scheme 3). The imidoformamide 2 was isolated from the first reaction, biuret (3) and carbamic acid (4) derivatives from the second reaction, and carbamic acid (5) from the third reaction, respectively. The products appeared to be especially moisture- and air-sensitive crystals of various green-blue tones indicative of dpp-bian2− [31] that were isolated in moderate 11–18% yields. Compounds 3 and 4 were isolated from the same reaction mixture simultaneously.
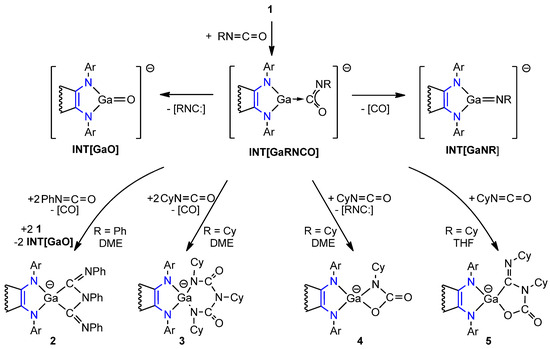
Scheme 3.
Reaction of gallylene 1 with CyNCO and PhNCO to yield complexes 2–5. Counter ions and acenaphthene parts are omitted for clarity.
Formation of 2–5 can be rationalized through the mechanism suggested earlier for the reaction of 1 with O=C=O [87]. It anticipates the formation of intermediate adduct INT[GaRNCO] (Scheme 3). The reaction is followed by the [3+2] or [3+2+2] cycloaddition of a second and a third molecule of isocyanate with concomitant extrusion of isonitrile or CO. Moreover, the intermediate INT[GaRNCO] is supposed to have two competitive decomposition pathways to the oxide INT[GaO] and the imide INT[GaNR], which are unstable towards dimerization [87]. According to the proposed mechanism, the generation of species 2 should have led to a noticeable amount of INT[GaO], which was well supported by an experiment. A deeper inspection of the reaction mixture of 1 with PhNCO allowed the isolation of green crystals of an INT[GaO] dimer, the gallium oxide [(dpp-bian)GaO]2[Na(DME)2]2 (6), on top of crystals of complex 2. The relatively low isolated yields of 2–5 may be attributed to the existence of few energetically close intermediates INT[GaO], INT[GaNR], INT[GaRNCO] along with the presence of competitive reaction pathways that, in fact, resulted in a mixture of similar products. Complex 6 was also synthesized by the direct reaction of gallylene 1 with 1 equiv. N2O (Scheme 4) as green crystals in 66% yield. Spectral characteristics of 6 from both experiments coincided. For the sake of 6 synthesis, compound 1 was also treated with 1 equiv. carbonyl sulfide. That, however, resulted in sulfide 7 as green crystals in 35% yield.
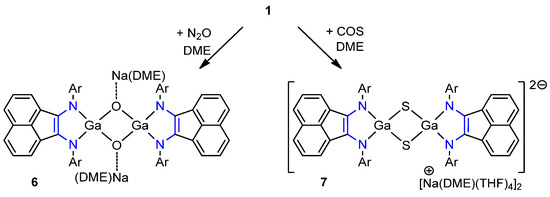
Scheme 4.
Reaction of gallylene 1 with 1 equiv. of N2O and 1 equiv. of COS.
Compounds 2–6 are diamagnetic and produce resolved NMR spectra. Complexes of dpp-bian that have symmetry elements produce a characteristic pattern of the ligand NMR [87]. Likewise, the NMR spectra of a solution of 2 contain a simplified set of signals due to two symmetry planes. Eight methyl groups and four methine groups of iPr are presented by two doublets (δ 0.75, 0.89 ppm, 12H each) and one septet (3.74 ppm, 4H), respectively. Protons of the phenyl group produce signals at 7.56 (2H), 7.25 (2H), 7.01 (4H), 6.78 (4H), 7.06 (1H), and 6.57 (2H) ppm. The GaC(NPh)N carbon atom produces a signal at 152.8 ppm that is similar to 158.1 ppm of azacyclobutane C(=NPh)C(Me2)N(Ph)C(=NPh) [89]. Compounds 3 and 4 were characterized only by XRD analysis because of very close solubilities. The NMR spectrum of complex 5 demonstrates a simplified signal set due to a symmetry plane. Protons of the methyl and methine groups of iPr give four doublets (6H each) and two septets (2H each). Protons of the naphthalene part appear as two doublets (2H each) at 6.29 and 7.12 ppm and one doublet of doublets (2H) at 6.86 ppm. Protons of methylene groups cyclohexyl substitutes give six multiplets at 2.79 (2H), 1.89 (2H), 1.70 (4H), 1.57 (4H), 1.37 (4H), and 1.18 (4H) ppm. Protons of the methine groups produce two broad singlets (1H each) at 4.82 and 3.54 ppm. Compound 6 is poorly soluble in ether solvents, aggravating its NMR identification. The 1H NMR spectra of 6 exhibited two doublets (24H each) at 0.96 and 0.86 ppm and one septet (8H) at 3.77 ppm for the protons isopropyl group indicative of three symmetry planes. The reactions from Scheme 3 seem to be unselective; NMR-scale experiments resulted in untrackable mixtures of products.
The IR spectra of the compounds 2–6 support the proposed structures. Compound 2 features a strong C=N absorption band (1640 cm−1). The mixture of 3 and 4 exhibits absorption at 1628 cm−1 and 1602 cm−1, corresponding to C=O and C=N vibrations. The IR spectrum of compound 5 consists of the absorption of 1714 cm−1 and 1661 cm−1, which are characteristics of double C=O and C=N bonds, respectively. No -N=C=O absorption bands were registered in the 2000–2300 cm−1 region.
In a separate experiment, adventitious contact of a reaction mixture of 1 with 1 equiv. CyNCO in THF with air led to the isolation of green crystals of compound [(dpp-bian)GaOC(NHCy)O(O)][Na(DME)3] (8) in 9% yield (Scheme 5).
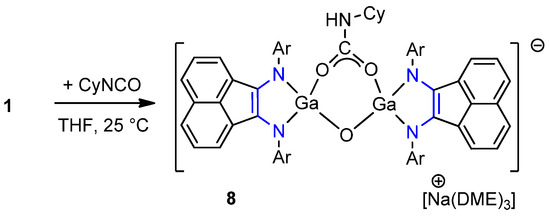
Scheme 5.
Reaction of gallylene 1 with 1 equiv. of CyNCO to yield 8.
Complex 8 is diamagnetic but shows no resolved NMR spectra at room temperature, probably due to molecular motion in the cyclohexyl substituent. The spectrum resolution improved at −20 °C (Figure S19). Protons from the isopropyl group produced signals at 1.20, 1.03, 0.74, and 0.71 ppm (12H each) and 3.84 and 3.48 ppm (4H each). Protons of cyclohexyl substitutions appeared at 3.07 (1H), 2.20 (4H), 1.99 (4H), and 1.12 (2H) ppm. Atom H(5) of the imino group presented as a doublet at 5.79 ppm. The IR spectrum of product 8 contains absorption bands corresponding to stretching vibrations of N–H (3364 cm−1) bonds and a carboxyl group (1591 and 1438 cm−1).
Even though the isocyanate oligomerization phenomenon under basic or reductive conditions is known [90,91], and we reported a few isocyanate transformations on the gallium center [27], most of the structures and transformations reported herein are novel. For example, complex 2 is the first metallocycloiminoacylamidine of the main group metal. Three metallocycloiminoacylamidines of the transition metals were characterized, though. They were isolated by the insertion of 2,6-dimethylphenyl isocyanide to the M–N bond of platinumazacyclopropane [(RCN)2PtC(=NR)NR)] (R = 2,6-dimethylphenyl) [92] and transformation of isonitrile in the coordination sphere of iron in the complex [Fe(dppe)(CNR)4](ClO4)2] (R = p-Tol; dppe = 1,2-bis(diphenylphosphino)ethane)) by its treatment with excess KOH [93]. Similarly, complex 3 is the second main group metal biuret to be deposited in the Cambridge Structural Database (CSD) [27]. Isocyanate oligomerizations were reported to give six-member metallacycles on nickel [94], palladium [95], and chromium [96] centers. To the best of our knowledge, the generation of compounds with five-membered metallacycle MC(=NR)C(=O)O, like in complex 5, is also unprecedented.
To better understand the reactivity of 1 against C=O-containing compounds, it was treated with 2 equiv. of benzophenone or 2 equiv. of diphenylketene. Green crystalline products [(dpp-bian)GaO(CPh2)2O][Na(Py)2] (9) (53% yield) and [(dpp-bian)Ga{C(=CPh2)O}2][Na(DME)3] (10) (22% yield) were isolated from the reaction mixtures, respectively (Scheme 6).
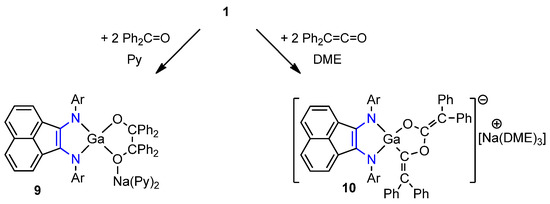
Scheme 6.
Reaction of 1 with 2 equiv. of Ph2CO and with 2 equiv. of Ph2CCO.
NMR spectra of the compounds 9 and 10 supported their structure. Protons of isopropyl substituents have shown a set of two doublets (12H each) and one septet (4H) associated with two symmetry planes. At the same time, those of 10 produced four doubles and two septets indicative of only one symmetry plane. The chemical shift of the quaternary carbon atom of the pinacolate fragment of δ 88.6 ppm was significantly shifted to a stronger field (cf. δ (13C) = 196.5 ppm in Ph2CO). 13C NMR chemical shifts of sp2-hybridized carbon atoms of the galladioxolane fragment (161.1 (OCO), 158.1 (OCGa), 127.6 (Ph2C=COGa), 88.6 (Ph2C=CO2) ppm) are close to those in the phosphorus derivative [PhPOC(=CPh2)OC(=CPh2)] (156.4 (OCO), 150.7 (PCO), 125.9 (Ph2C=COP), 95.5 (Ph2C=CO2 ppm) [97].
Pinacolate coupling was reported in the reaction of [NacNacAl] and benzophenone [98]. Magnesium complex [(dpp-bian)Mg(THF)3] also reacts with benzophenone to create binuclear magnesium pinacolate [{(dpp-bian)Mg(THF)}2{µ-O2C2Ph4}], which can dissociate in toluene solution into two biradical species [99]. Analogously to these cases, generation of pinacolate 9 can be viewed through ketyl radical formation. Meanwhile, C–O coupling instead of C–C in the reaction of 1 with diphenylketene may account for the [1+2+2] cycloaddition mechanism. Besides transition metals [100] and phosphorus [97], ketene cyclization product 10 is the first to be reported across the main group metals.
A similar mechanism ambiguity between reduction and [1+2+2] cycloaddition appeared in the reaction of complex 1 with 2 equiv. of 1,2-dibenzylidenehydrazine. It resulted in complex [(dpp-bian)Ga{N(NCHPh)}2(CHPh)2][Na(DME)3] (11) in the form of turquoise crystals at 39% yield. During the reaction, two benzaldazine molecules combined in the gallium atom coordination sphere. One C–C bond and two Ga–N bonds formed to create a five-membered galladiazametallacycle (Scheme 7).
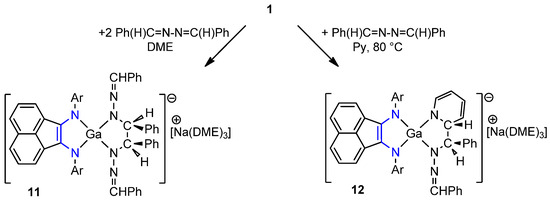
Scheme 7.
Reaction of 1 with 1,2-dibenzylidenehydrazine.
Compound 11 is diamagnetic and exhibits resolved NMR spectra. In the THF-d8 solution, isopropyl substituents of the ligand appeared as a set of four doublets 6H and two septets 2H, indicative of a symmetry plane. The methine protons of benzaldazine are represented by two singlets at 7.37 and 5.08 ppm (2H each). The latter signal corresponds to the hydrogen atoms at the carbon atoms that formed a new bond. It shifted into the higher field compared to the starting 1,2-dibenzylidenehydrazine (cf. δ (NCHPh2)2 = 8.65 ppm). The naphthalene protons were not equivalent, which allowed us to conclude that the symmetry plane coincides with the naphthalene plane. The addition reaction was also nicely illustrated by 13C NMR signals of 131.2 ppm (H–C=N) and 68.8 ppm (H–C–N) shifted in comparison to the starting material (cf. δ (–N=CHPh)2 = 161.8 ppm). The presence of the symmetry plane confirmed the formation of one optical isomer—the meso-form.
Curiously, when the DME solution of 1 was treated with 1 equiv. of 1,2-dibenzylidenehydrazine and then the products were dissolved in pyridine, a pyridine–benzaldazine coupling product [(dpp-bian)GaN(NCHPh)C(Ph)CN][Na(DME)3] (12) was isolated in 40% yield as green crystals (Scheme 7). Using pyridine-d5 as a solvent, an isostructural complex 12-d5 was isolated.
In the THF-d8 solution, the molecule of 12 was asymmetric, which was seen from eight doublets and four septets arising from iPr-substituents of dpp-bian (Figure 1). The methine protons of the azine fragment produced two distinct signals (1H each) at 7.73 and 4.58 ppm. The last corresponded to the hydrogen atom at the sp3-hybrydized carbon atom, forming a new bond. Five signals of protons in the pyridine fragment (6.62, 5.44, 4.29, 4.23, 4.00 ppm) were significantly shifted in comparison to free pyridine (8.61, 7.66, 7.28 ppm), which confirmed its dearomatization. The assignment of these signals became clear when 1H NMR spectra of 12 and 12-d5 were compared (Figure 1). The 13C NMR spectrum also confirmed the structure of the resulting compound. The signal of the sp3-hybridized carbon atom H–C–N of the azine fragment shifted upfield (73.3 ppm), and the sp2-hybridized carbon atom H–C=N produced a signal at 131.5 ppm (cf. δ (–N=CHPh)2 161.8 ppm). The carbon atoms of the pyridine fragment of 12 produced 13C NMR signals at 143.7, 127.5, 104.6, 92.5, and 62.5 ppm. (cf. C5H5N 149.9, 135.9, and 123.75 ppm in pyridine). Since only one diastereomer was identified in both reactions of 1 with benzaldazine, it may be believed to proceed through the cycloaddition mechanism.
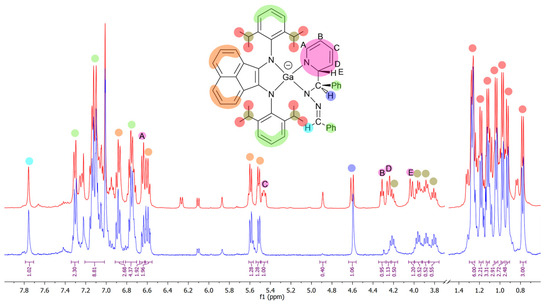
Figure 1.
Selected area of 1H NMR spectrum of 12 (red) and 12-d5 (blue) (THF-d8, 400 MHz, 25 °C).
Similar reductions of benzaldazine were reported by [(C5Me5)2Sm(THF)2] [101] and [Cp2Ti(η2-Me3SiC2SiMe3)] [102]. The authors suggested a one-electron reduction of the imino group, which led to C–C coupling binuclei complexes. Processes of chemoselective coupling of carbonyl compounds with pyridine were previously reported for [NacNacAl:] (NacNac = [ArNC-(Me)CHC(Me)NAr]−, Ar = 2,6-iPr2C6H3) [81]. However, the coupling of two azine molecules and one azine molecule with a pyridine molecule in the coordination sphere of one metal atom was observed for the first time.
Molecular Structures of Compounds 2–12
According to XRD analysis of 2–12, interatomic bond distances within five-membered metallacycles correspond to single C–N and double C=C bonds and agree well with the geometry of complex 1 [103]. This anticipates that dpp-bian preserved the dianion state during the reactions, while electron transfer occurred exclusively from the metal atom.
The XRD analysis of 2 (Figure 2) indicated that radial interatomic distances N(3)–C(37) (1.277(2) Å) and N(5)–C(50) (1.284(2) Å) are close to the double C=N bonds (1.29 Å) distances, while N(4)–C(37) (1.414(2) Å), N(4)–C(50) (1.412(2) Å) are close to single C–N (1.47 Å). These values correlate well with distances in platinum complexes [(RCN)2PtC(=NR)NRC(=NR))] (av. C=N 1.42, C–N 1.28 Å) [92] and the organic molecule imidoformamide (av. C=N 1.268, C–N 1.375 Å) [104].
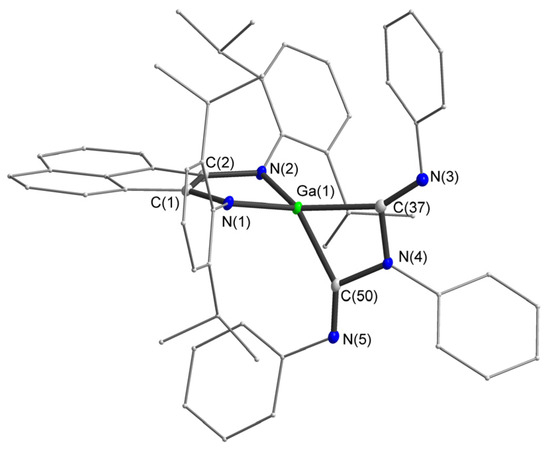
Figure 2.
Molecular structure of anion 2. Thermal ellipsoids are drawn at 30% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (°): N(1)–C(1) 1.380(2), N(2)–C(2) 1.401(2), C(1)–C(2) 1.379(2), N(3)–C(37) 1.277(2), N(4)–C(37) 1.414(2), N(4)–C(50) 1.412(2), N(5)–C(50) 1.284(2), Ga(1)-C(37) 2.0621(16), Ga(1)-C(50) 2.0635(16), N(3)–C(37)–N(4) 119.12(14), C(50)–N(4)–C(37) 109.86(13), N(5)–C(50)–N(4) 119.23(14) C(37)–Ga(1)–C(50) 68.20(6).
In the crystal, molecules of 3 presented by species dimerized through sodium cations and placed on the crystallographic center of symmetry (Figure 3). Distances O(1)–C(43) (1.2397(18) Å), O(2)–C(50) (1.2448(18) Å) are close to each other and correspond to a double C=O bond. The C–N bonds (N(3)–C(43) (1.3352(19) Å), N(5)–C(50) (1.3436(19) Å) are averaged between C=N (1.27 Å) and C–N (1.46 Å), probably due to conjugation with C=O bonds. Interatomic distances N(4)–C(50) (1.4174(19) Å) and N(4)–C(43) (1.4300(19) Å) in turn fall into the range typical to single C–N bonds. Interatomic distances corroborate those of N,N’,N”-triphenylbiuret [105]. Complex 4, like 3, is a dimer in a crystal state (Figure 4). Interatomic distance O(2)–C(37) (1.239(6) Å) corresponds to a double C=O bond, while the distances O(1)–C(37) (1.345(6) Å) and N(3)–C(37) (1.366(6) Å) correspond to single C–O and C–N bonds. Similar four-member metallacycles MNCO were previously reported on aluminum [75]. Admittedly, structures 3 and 4 are reminiscent of reaction products of 1 with 1 equiv. of CO2 and 1 equiv. of PhNCO or CyNCO [(dpp-bian)GaN(Cy)C(O)N(Cy)C(O)O]2[Na(DME)2]2 and [(dpp-bian)GaN(Ph)C(O)O]2[Na(DME)2]2, respectively [87].
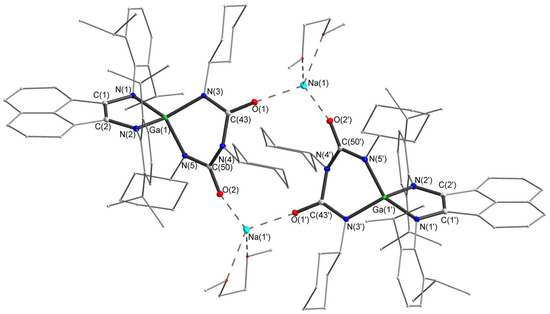
Figure 3.
Molecular structure of compound 3. Thermal ellipsoids are drawn at 30% probability level. Hydrogen atoms are omitted. Selected bond lengths (Å) and angles (°): Ga(1)-N(3) 1.9029(12), Ga(1)-N(5) 1.9301(13), O(1)-C(43) 1.2397(18), O(2)-C(50) 1.2448(18), N(4)-C(50) 1.4174(19), N(4)-C(43) 1.4300(19), N(5)-C(50) 1.3436(19), N(3)-C(43) 1.3352(19), N(3)-Ga(1)-N(5) 95.76(5), C(43)-N(3)-Ga(1) 117.45(10), N(3)-C(43)-N(4) 116.44(13), C(50)-N(4)-C(43) 124.69(12), C(50)-N(5)-Ga(1) 114.47(10), N(5)-C(50)-N(4) 117.95(13).
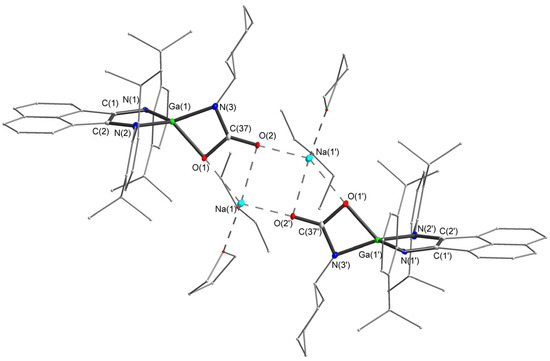
Figure 4.
Molecular structure of compound 4. Thermal ellipsoids are drawn at 30% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (°): Ga(1)-N(2) 1.882(5), Ga(1)-N(1) 1.889(5), Ga(1)-N(3) 1.902(4), Ga(1)-O(1) 1.984(3), N(1)-C(1) 1.389(9), C(1)-C(2) 1.394(7), N(2)-C(2) 1.403(9), O(1)-C(37) 1.345(6), O(2)-C(37) 1.239(6), N(3)-C(37) 1.366(6), C(37)-N(3)-Ga(1) 92.6(3), N(3)-Ga(1)-O(1) 68.96(17), C(37)-O(1)-Ga(1) 89.8(3), O(1)-C(37)-N(3) 108.6(5), O(2)-C(37)-O(1) 122.4(5), O(2)-C(37)-N(3) 129.0(5).
Metallacycle GaOCNC is not planar; the gallium atom is positioned out of a plane at 0.127 Å (Figure 5). Interatomic distances O(1)–C(37) (1.305(2) Å), N(3)–C(37) (1.383(3) Å), N(3)–C(44) (1.415(3) Å) are single C–O and C–N bonds, but O(2)–C(37) (1.228(2) Å) and N(4)–C(44) (1.265(3) Å) are shorter and correspond to double bonds. The structure of the organic fragment in 5 is parallel to the product of the insertion of carbodiimide into a carbamate, propane-2-yl (diphenylcarbamimidoyl)(phenyl)carbamate PhNHC(=NPh)NPhC(=O)OiPr, that was isolated from the reaction of titanium isopropoxide with phenylisocyanate [106].
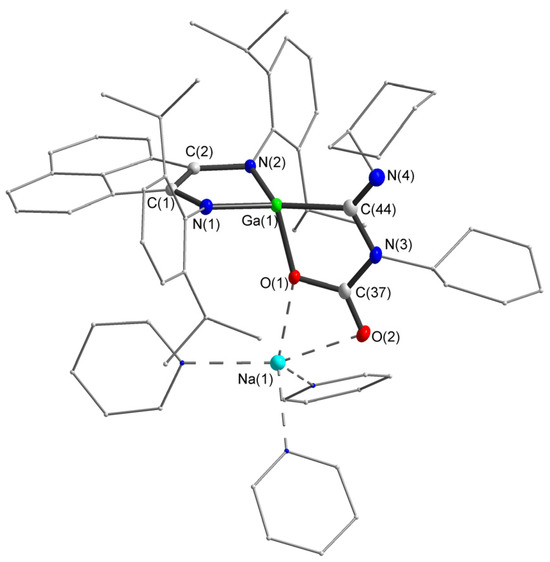
Figure 5.
Molecular structure of compound 5. Thermal ellipsoids are drawn at 30% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (°): N(1)-C(1) 1.394(2), N(2)-C(2) 1.391(2), C(1)-C(2) 1.368(3), N(3)-C(37) 1.383(3), O(1)-C(37) 1.305(2), O(2)-C(37) 1.228(2), N(3)-C(44) 1.415(3), N(4)-C(44) 1.265(3), Ga(1)-N(1) 1.8874(15), Ga(1)-N(2) 1.8908(15), Ga(1)-O(1) 1.9520(13), Ga(1)-C(44) 1.979(2), N(1)-Ga(1)-N(2) 90.29(7), N(1)-Ga(1)-O(1) 105.07(6), N(2)-Ga(1)-O(1) 109.23(6), N(1)-Ga(1)-C(44) 129.61(7), N(2)-Ga(1)-C(44) 134.14(8), O(1)-Ga(1)-C(44) 84.00(7), O(2)-C(37)-O(1) 121.95(19), O(2)-C(37)-N(3) 121.76(17), O(1)-C(37)-N(3) 116.29(17), N(4)-C(44)-N(3) 118.76(18), N(4)-C(44)-Ga(1) 133.90(16), N(3)-C(44)-Ga(1) 107.34(13).
According to XRD analysis, molecular structure of 6 (Figure S1) contains almost square fragment Ga2O2 with inner angles about 90° and close interatomic distances Ga–O (Ga(1)–O(1) 1.8322(16) Å, Ga(1)–O(1a) 1.8830(17) Å). Plane Ga2O2 is orthogonal to the plane of dpp-bian moiety (89.9°). This feature is reminiscent of other gallium oxo-complexes [107]. The Ga–Ga interatomic distance (2.6336(5) Å) is significantly less than the doubled value van der Waals radius (3.74 Å) [108]. However, given the significant ionic nature of gallium atoms, the Ga–Ga interactions are likely small.
Crystals of 8 suitable for XRD analysis were obtained by recrystallization from C6D6 (Figure 6). Bonds lengths in carbamate fragment O(2)–C(73) (1.2744(19) Å), O(3)–C(73) (1.2759(19) Å) are approximately equal and in good agreement with phenanthroline–carboxylate gallium complex (C(30)–O(5) (1.269(12) Å) and C(30)–O(6) (1.272(11) Å)) [109]. The gallium oxide bridge geometry is similar that of 6. The sum of the angles at the C(73) is approximately 360°, which indicates its sp2-hybridization.
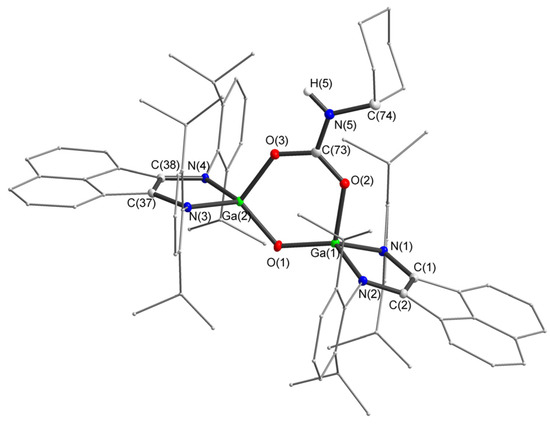
Figure 6.
Molecular structure of anion 8. Thermal ellipsoids are drawn at 30% probability level. Hydrogen atoms (except H(5)) are omitted. Selected bond lengths (Å) and angles (°): Ga(1)–N(1) 1.9047(12), Ga(1)–N(2) 1.9046(12), O(2)–C(73) 1.2743(19), O(3)–C(73) 1.2759(18), N(1)-C(1) 1.3929(19), N(2)–C(2) 1.3920(19), N(5)–C(73) 1.342(2), O(1)–Ga(1)–N(1) 124.00(5), O(1)–Ga(1)–O(2) 106.72(5), Ga(1)–O(1)–Ga(2) 123.34(7), O(2)–C(73)–O(3) 125.90(14), O(2)–C(73)–N(5) 118.38(14), O(3)–C(73)–N(5) 115.73(14).
The molecular structure of products 9 and 10 is shown in Figure 7. They consist of galladioxolene metallacycles. Bonds within the metallacycles (O(1)-C(37) 1.4184(15), O(2)-C(50) 1.4377(15), C(37)-C(50) 1.6408(18), Ga(1)-O(1) 1.8310(9), Ga(1)-O(2) 1.8654(9) Å in 9 and O(43)–C(44) 1.313(6), O(45)–C(44) 1.370(6), O(45)–C(46) 1.408(6), Ga(1)–O(43) 1.921(4), Ga(1)–C(46) 1.978(5) Å in 10) are in perfect agreement with expected values (C–C 1.52, C–O 1.42, C(sp2)–O 1.39, Ga–O 1.88, Ga–C(sp2) 1.95 Å) derived from corresponding covalent radii [110]. Compared to 9, the metallacycle in 10 is almost flat; the deviation of atoms from this plane is less than 0.033 Å. The sum of the angles at the carbon atoms C(44) and C(46) in 10 is approximately 360°, indicating their sp2-hybridization. Bond lengths C(44)–C(66) (1.372(8) Å) and C(46)–C(47) (1.333(8) Å) correspond to double C=C bonds (1.34 Å). Pinacolate fragments can be compared with similar complexes, for example, aluminum pinacolate [LAl(OCPh2)2] (L = HC[(CMe)(NAr)]2, Ar = 2,6-i-Pr2C6H3) [98]. Comparison of metallacycle in 10 is limited to germanium cycloadduct [LGeC(Ph)OC(Ph)O] (O(1)–C(21) 1.416(2) Å, O(2)–C(21) 1.433(2) Å, O(2)–C(28) 1.435(2) Å) [111], and the titanium complex [(Cp)2TiC(=CPh2)OC(=CPh2)O)] (O(1)–C(11) 1.36(2) Å, O(2)–C(11) 1.33(2) Å, O(2)–C(13) 1.42(2) Å) [100].
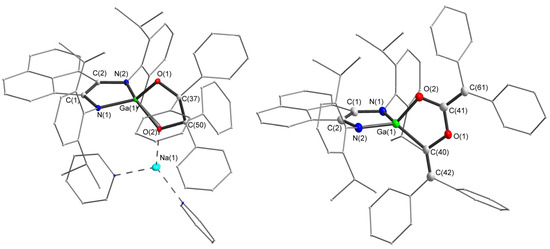
Figure 7.
Molecular structures of compound 9 and anion of 10. Thermal ellipsoids are drawn at 30% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (°) in 9: N(1)-C(1) 1.3988(16), C(1)-C(2) 1.3799(18), N(2)-C(2) 1.3901(16), Ga(1)-O(1) 1.8310(9), Ga(1)-O(2) 1.8654(9), O(1)-C(37) 1.4184(15), O(2)-C(50) 1.4377(15), C(37)-C(50) 1.6408(18), O(1)-Ga(1)-O(2) 89.30(4), O(1)-C(37)-C(50) 107.94(10), C(37)-O(1)-Ga(1) 114.14(8), C(50)-O(2)-Ga(1) 112.25(7), O(2)-C(50)-C(37) 105.97(10), C(57)-C(50)-C(51) 113.06(11), C(38)-C(37)-C(44) 107.86(10). Selected bond lengths (Å) and angles (°) in 10: N(2)–C(15) 1.392(7), C(16)–C(15) 1.385(7), N(17)–C(16) 1.375(7), Ga(1)–O(43) 1.921(4), Ga(1)–C(46) 1.978(5), O(43)–C(44) 1.313(6), O(45)–C(44) 1.370(6), O(45)–C(46) 1.408(6), C(44)–C(66) 1.372(8), C(46)–C(47) 1.333(8), O(43)–Ga(1)–C(46) 84.67(19), O(43)–C(44)–O(45) 117.1(5), O(43)–C(44)–C(66) 123.4(5), O(45)–C(44)–C(66) 119.5(5).
The compounds 11 and 12 were characterized by XRD (Figure 8). During the reaction, the double C=N bond is reduced to a single one, as can be clearly seen from a comparison of C(38)–N(5) (1.4578(16) Å) and N(6)-C(58) (1.2903(18) Å) interatomic distances in 11. Bonds N(5)–N(6) (1.3584(15) Å) and N(3)–N(4) (1.3547(16) Å) are also elongated by 0.6 Å in comparison to free benzaldazine (1.418 Å) [112] due to loss of conjugation. These changes are consistent with the geometry of the reduced benzaldazine fragment in [Cp2Ti(η2-Me3SiC2SiMe3)] [102]. The same considerations are true for the benzaldazine fragment of 12. On top of that, the pyridine molecule in 12 loses its aromaticity, which is clear from a comparison of the interatomic distances of a pyridine fragment, where N(5)–C(41) (1.428(8) Å), N(5)–C(37) (1.363(8) Å) are single C–N bonds, C(38)–C(39) (1.443(10) Å) are C–C bonds, and C(37)–C(38) (1.347(7) Å), C(40)–C(39) (1.383(10) Å) are double C=C bonds. Compound 12 is chiral due to the two asymmetric centers, C(41) and C(42), with an S-configuration. Compound 12 crystallizes in the centrosymmetric group P21/C, so the enantiomer is also present in the crystal.
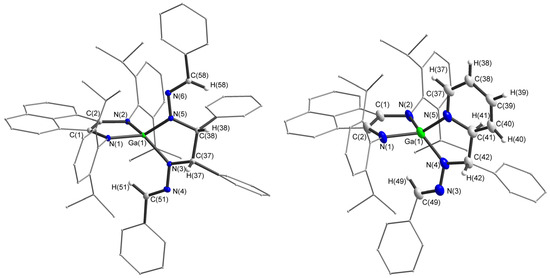
Figure 8.
Molecular structures of anions 11 and 12. Thermal ellipsoids are drawn at 30% probability level. Hydrogen atoms except for H(58), H(38), H(37), H(51) in 11 and H(37), H(38), H(39), H(40), H(41), H(42), H(49) in 12 are omitted. Selected bond lengths (Å) and angles (°) in 11: N(1)-C(1) 1.3871(16), C(1)-C(2) 1.3805(18), N(2)-C(2) 1.3884(16), N(5)-N(6) 1.3584(15), N(5)-C(38) 1.4578(16), N(6)-C(58) 1.2903(18), C(37)-C(38) 1.5629(19), N(3)-C(37) 1.4637(17), N(3)-N(4) 1.3547(16), N(4)-C(51) 1.2721(19), N(4)-N(3)-C(37) 111.43(10), C(51)-N(4)-N(3) 119.86(12), N(6)-N(5)-C(38) 120.00(11), C(58)-N(6)-N(5) 120.79(11), N(3)-C(37)-C(38) 108.19(10), N(5)-C(38)-C(37) 106.35(10), N(4)-C(51)-C(52) 120.14(13), N(6)-C(58)-C(59) 121.14(13). Selected bond lengths (Å) and angles (°) in 12: Ga(1)–N(5) 1.897(4), Ga(1)–N(4) 1.890(6), Ga(1)–N(1) 1.926(4), Ga(1)–N(2) 1.886(4), N(5)–C(41) 1.428(8), C(42)–C(41) 1.550(9), N(4)–C(42) 1.476(6), N(3)–C(49) 1.290(7), N(4)–N(3) 1.375(7), N(1)–C(1) 1.375(7), C(1)–C(2) 1.369(7), N(2)–C(2) 1.409(7), N(4)–Ga(1)–N(5) 87.9(2), N(2)–Ga(1)–N(1) 89.79(18), N(4)–C(42)–C(41) 106.5(5), N(5)–C(41)–C(42) 113.3(5), N(5)–C(41)–C(40) 116.7(6), C(41)–N(5)–Ga(1) 111.2(4), C(42)–N(4)–Ga(1) 113.9(4), C(49)–N(3)–N(4) 116.3(5).
4. Conclusions
In conclusion, transformations of isocyanates, nitrous oxide, carbonyl sulfide, benzophenone, diphenylketene, benzaldazine, and pyridine at the GaI center combined with redox-active ligand dpp-bian were reported. They primarily resulted in cycloaddition products that reflected the gallium center’s transition metal-like properties. The reactions were always aggravated by strong reductive behavior of low-valent centers, and a complete oxygen atom or other group transfer was sometimes observed. Simultaneously, the gallium center exhibited an exciting balance between the strong oxo- and carbophilicity, giving rise to products like imidoformamide 2. This manuscript, together with papers concerning transformations of RN=C=NR [86] and O=C=O [87] substrates, illustrates that the Ga(I)-center supported with bisamide ligand can promote coupling of several C=O− and C=N− substrates to give plentiful organic products. One of the most notable features was coupling benzaldazine molecules with a robust substrate such as pyridine. That may open up more sustainable and selective alternatives for syntheses of imidoformamide, biuret, carbamic acid compounds, as well as opportunities to use gallylene reagents in McMurry- and Wittig-type reactions, selective C–C reductive couplings, and cyclizations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/reactions5010009/s1, Details of synthesis, characterization, X-ray crystal structure determination details, and crystal data are given in the electronic supporting information. Figure S1: Molecular structure of compound 6; Figure S2: Molecular structure of compound 7; Figures S2–S46: NMR spectra of compounds 2, 5, 6, 8–12. Table S1. Crystallographic data and refinement details for compounds 2–12. References [88,113,114,115,116,117,118,119,120,121,122,123] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, I.L.F.; Methodology, O.A.K.; Validation, O.A.K. and E.V.B.; Formal analysis, O.A.K. and E.V.B.; Investigation, V.A.D.; Writing—original draft, V.A.D. and O.A.K.; Writing—review & editing, V.A.D., O.A.K., E.V.B. and I.L.F.; Supervision, I.L.F.; Project administration, V.A.D.; Funding acquisition, V.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 21-73-20153.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors thank the Analytical Center of the G.A. Razuvaev Institute of Organometallic Chemistry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Scholl, M.; Trnka, T.M.; Morgan, J.P.; Grubbs, R.H. Increased ring closing metathesis activity of ruthenium-based olefin metathesis catalysts coordinated with imidazolin-2-ylidene ligands. Tetrahedron Lett. 1999, 40, 2247–2250. [Google Scholar] [CrossRef]
- Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)(Amino)Carbenes (CAACs): Stable Carbenes on the Rise. Acc. Chem. Res. 2015, 48, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Bourissou, D.; Guerret, O.; Gabbaï, F.P.; Bertrand, G. Stable Carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef] [PubMed]
- Nesterov, V.; Reiter, D.; Bag, P.; Frisch, P.; Holzner, R.; Porzelt, A.; Inoue, S. NHCs in Main Group Chemistry. Chem. Rev. 2018, 118, 9678–9842. [Google Scholar] [CrossRef]
- Moerdyk, J.P.; Bielawski, C.W. Reductive generation of stable, five-membered N,N′-diamidocarbenes. Chem. Commun. 2014, 50, 4551–4553. [Google Scholar] [CrossRef]
- Dohmeier, C.; Robl, C.; Tacke, M.; Schnöckel, H. The Tetrameric Aluminum(I) Compound [{Al(η5-C5Me5)}4]. Angew. Chem. Int. Ed. 1991, 30, 564–565. [Google Scholar] [CrossRef]
- Uhl, W.; Hiller, W.; Layh, M.; Schwarz, W. [Ga4{C(SiMe3)3}4] with a Tetrahedral Ga4 Skeleton. Angew. Chem. Int. Ed. 1992, 31, 1364–1366. [Google Scholar] [CrossRef]
- Su, J.; Li, X.-W.; Crittendon, R.C.; Robinson, G.H. How Short is a -Ga⋮Ga- Triple Bond? Synthesis and Molecular Structure of Na2[Mes*2C6H3-Ga⋮Ga-C6H3Mes*2] (Mes* = 2,4,6-i-Pr3C6H2): The First Gallyne. J. Am. Chem. Soc. 1997, 119, 5471–5472. [Google Scholar] [CrossRef]
- Wright, R.J.; Brynda, M.; Power, P.P. Synthesis and Structure of the “Dialuminyne” Na2[Ar′AlAlAr′] and Na2[(Ar′′Al)3]: Al-Al Bonding in Al2Na2 and Al3Na2 Clusters. Angew. Chem. Int. Ed. 2006, 45, 5953–5956. [Google Scholar] [CrossRef]
- Bag, P.; Porzelt, A.; Altmann, P.J.; Inoue, S. A Stable Neutral Compound with an Aluminum–Aluminum Double Bond. J. Am. Chem. Soc. 2017, 139, 14384–14387. [Google Scholar] [CrossRef]
- Queen, J.D.; Lehmann, A.; Fettinger, J.C.; Tuononen, H.M.; Power, P.P. The Monomeric Alanediyl: AlAriPr8 (AriPr8 = C6H-2,6-(C6H2-2,4,6-Pri3)2-3,5-Pri2): An Organoaluminum(I) Compound with a One-Coordinate Aluminum Atom. J. Am. Chem. Soc. 2020, 142, 20554–20559. [Google Scholar] [CrossRef]
- Hicks, J.; Vasko, P.; Goicoechea, J.M.; Aldridge, S. Synthesis, structure and reaction chemistry of a nucleophilic aluminyl anion. Nature 2018, 557, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Schwamm, R.J.; Anker, M.D.; Lein, M.; Coles, M.P. Reduction vs. Addition: The Reaction of an Aluminyl Anion with 1,3,5,7-Cyclooctatetraene. Angew. Chem. Int. Ed. 2019, 58, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Grams, S.; Eyselein, J.; Langer, J.; Färber, C.; Harder, S. Boosting Low-Valent Aluminum(I) Reactivity with a Potassium Reagent. Angew. Chem. Int. Ed. 2020, 59, 15982–15986. [Google Scholar] [CrossRef] [PubMed]
- Kurumada, S.; Takamori, S.; Yamashita, M. An alkyl-substituted aluminium anion with strong basicity and nucleophilicity. Nat. Chem. 2020, 12, 36–39. [Google Scholar] [CrossRef]
- Schmidt, E.S.; Jockisch, A.; Schmidbaur, H. A Carbene Analogue with Low-Valent Gallium as a Heteroatom in a quasi-Aromatic Imidazolate Anion. J. Am. Chem. Soc. 1999, 121, 9758–9759. [Google Scholar] [CrossRef]
- Asay, M.; Jones, C.; Driess, M. N-Heterocyclic Carbene Analogues with Low-Valent Group 13 and Group 14 Elements: Syntheses, Structures, and Reactivities of a New Generation of Multitalented Ligands. Chem. Rev. 2011, 111, 354–396. [Google Scholar] [CrossRef]
- Abdalla, J.A.B.; Aldridge, S. Group 13 Metal–Metal Bonds. In Molecular Metal-Metal Bonds; Wiley: Hoboken, NJ, USA, 2015; pp. 455–484. [Google Scholar]
- Liu, Y.; Li, S.; Yang, X.-J.; Li, Q.-S.; Xie, Y.; Schaefer, H.F.; Wu, B. Alkali metal compounds of a gallium(I) carbene analogue {:Ga[N(Ar)C(Me)]2} (Ar = 2,6-iPr2C6H3). J. Organomet. Chem. 2011, 696, 1450–1455. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Li, Q.-S.; Su, J.-H. Synthesis and structures of mononuclear and dinuclear gallium complexes with α-diimine ligands: Reduction of the metal or ligand? Dalton Trans. 2016, 45, 246–252. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, Y.; Xu, W.; Su, J.-H.; Shen, L.; Liu, L.; Wu, B.; Yang, X.-J. Reductive linear- and cyclo-trimerization of isocyanides using an Al–Al-bonded compound. Chem. Commun. 2019, 55, 9452–9455. [Google Scholar] [CrossRef]
- Koshino, K.; Kinjo, R. Construction of σ-Aromatic AlB2 Ring via Borane Coupling with a Dicoordinate Cyclic (Alkyl)(Amino)Aluminyl Anion. J. Am. Chem. Soc. 2020, 142, 9057–9062. [Google Scholar] [CrossRef]
- Yan, C.; Kinjo, R. A Three-Membered Diazo-Aluminum Heterocycle to Access an Al=C π Bonding Species. Angew. Chem. Int. Ed. 2022, 61, e202211800. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Skatova, A.A.; Dodonov, V.A.; Chudakova, V.A.; Bazyakina, N.L.; Piskunov, A.V.; Demeshko, S.V.; Fukin, G.K. Digallane with Redox-Active Diimine Ligand: Dualism of Electron-Transfer Reactions. Inorg. Chem. 2014, 53, 5159–5170. [Google Scholar] [CrossRef] [PubMed]
- Dodonov, V.A.; Makarov, V.M.; Zemnyukova, M.N.; Razborov, D.A.; Baranov, E.V.; Bogomyakov, A.S.; Ovcharenko, V.I.; Fedushkin, I.L. Stability and Solution Behavior of [(dpp-Bian)Ln] and [(dpp-Bian)LnX] (Ln = Yb, Tm, or Dy; X = I, F, or N3). Organometallics 2023, 42, 2558–2567. [Google Scholar] [CrossRef]
- Dodonov, V.A.; Chen, W.; Zhao, Y.; Skatova, A.A.; Roesky, P.W.; Wu, B.; Yang, X.J.; Fedushkin, I.L. Gallium “Shears” for C=N and C=O Bonds of Isocyanates. Chem. Eur. J. 2019, 25, 8259–8267. [Google Scholar] [CrossRef] [PubMed]
- Dodonov, V.A.; Chen, W.; Liu, L.; Sokolov, V.G.; Baranov, E.V.; Skatova, A.A.; Zhao, Y.; Wu, B.; Yang, X.-J.; Fedushkin, I.L. Reactions of Iso(thio)cyanates with Dialanes: Cycloaddition, Reductive Coupling, or Cleavage of the C=S or C=O Bond. Inorg. Chem. 2021, 60, 14602–14612. [Google Scholar] [CrossRef] [PubMed]
- Fedushkin, I.L.; Skatova, A.A.; Dodonov, V.A.; Yang, X.-J.; Chudakova, V.A.; Piskunov, A.V.; Demeshko, S.; Baranov, E.V. Ligand “Brackets” for Ga–Ga Bond. Inorg. Chem. 2016, 55, 9047–9056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dodonov, V.A.; Chen, W.; Zhang, S.; Roesky, P.W.; Zhao, Y.; Fedushkin, I.L.; Yang, X.-J. Reactions of Low-Valent Gallium Species with Organic Azides: Formation of Imido-, Azoimido-, and Tetrazene Complexes. Inorg. Chem. 2023, 62, 6288–6296. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Dodonov, V.A.; Skatova, A.A.; Sokolov, V.G.; Piskunov, A.V.; Fukin, G.K. Redox-Active Ligand-Assisted Two-Electron Oxidative Addition to Gallium(II). Chem. Eur. J. 2018, 24, 1877–1889. [Google Scholar] [CrossRef]
- Dodonov, V.A.; Morozov, A.G.; Rumyantsev, R.V.; Fukin, G.K.; Skatova, A.A.; Roesky, P.W.; Fedushkin, I.L. Synthesis and ε-Caprolactone Polymerization Activity of Electron-Deficient Gallium and Aluminum Species Containing a Charged Redox-Active dpp-Bian Ligand. Inorg. Chem. 2019, 58, 16559–16573. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Sinhababu, S.; Roesky, H.W. The unique β-diketiminate ligand in aluminum(I) and gallium(I) chemistry. Dalton Trans. 2020, 49, 1351–1364. [Google Scholar] [CrossRef]
- Nagendran, S.; Roesky, H.W. The Chemistry of Aluminum(I), Silicon(II), and Germanium(II). Organometallics 2008, 27, 457–492. [Google Scholar] [CrossRef]
- Lopez, C.A. Aluminium, gallium, indium and thallium. Annu. Rep. Sect. A 2009, 105, 98–116. [Google Scholar] [CrossRef]
- Schoeller, W.W. Neutral Carbene Analogues of Group 13 Elements: The Dimerization Reaction to a Biradicaloid. Inorg. Chem. 2011, 50, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, W.; Li, J.; Cui, C. Chemistry of s-, p- and f-block metal complexes with ene-diamido ligands. Coord. Chem. Rev. 2019, 383, 132–154. [Google Scholar] [CrossRef]
- Helling, C.; Schulz, S. Thallium. In Comprehensive Organometallic Chemistry IV; Parkin, G., Meyer, K., O’hare, D., Eds.; Elsevier: Oxford, UK, 2022; pp. 370–406. [Google Scholar]
- Linti, G.; Schnöckel, H. Low valent aluminum and gallium compounds—Structural variety and coordination modes to transition metal fragments. Coord. Chem. Rev. 2000, 206–207, 285–319. [Google Scholar] [CrossRef]
- González-Gallardo, S.; Bollermann, T.; Fischer, R.A.; Murugavel, R. Cyclopentadiene Based Low-Valent Group 13 Metal Compounds: Ligands in Coordination Chemistry and Link between Metal Rich Molecules and Intermetallic Materials. Chem. Rev. 2012, 112, 3136–3170. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.L. Modulating the Frontier Orbitals of an Aluminylene for Facile Dearomatization of Inert Arenes. Angew. Chem. Int. Ed. 2022, 61, e202116658. [Google Scholar] [CrossRef]
- Dhara, D.; Jayaraman, A.; Härterich, M.; Dewhurst, R.D.; Braunschweig, H. Generation of a transient base-stabilised arylalumylene for the facile deconstruction of aromatic molecules. Chem. Sci. 2022, 13, 5631–5638. [Google Scholar] [CrossRef]
- Dmitrienko, A.; Pilkington, M.; Britten, J.F.; Gabidullin, B.M.; van der Est, A.; Nikonov, G.I. Shedding Light on the Diverse Reactivity of NacNacAl with N-Heterocycles. Angew. Chem. Int. Ed. 2020, 59, 16147–16153. [Google Scholar] [CrossRef] [PubMed]
- Kassymbek, A.; Vyboishchikov, S.F.; Gabidullin, B.M.; Spasyuk, D.; Pilkington, M.; Nikonov, G.I. Sequential Oxidation and C−H Bond Activation at a Gallium(I) Center. Angew. Chem. Int. Ed. 2019, 58, 18102–18107. [Google Scholar] [CrossRef] [PubMed]
- Kassymbek, A.; Spasyuk, D.; Dmitrienko, A.; Pilkington, M.; Nikonov, G.I. Facile C–H bond activation on a transient gallium imide. Chem. Commun. 2022, 58, 6946–6949. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Boyko, Y.; Korobkov, I.; Nikonov, G.I. Transition Metal-like Oxidative Addition of C–F and C–O Bonds to an Aluminum(I) Center. Organometallics 2015, 34, 5363–5365. [Google Scholar] [CrossRef]
- Chu, T.; Vyboishchikov, S.F.; Gabidullin, B.M.; Nikonov, G.I. Oxidative Cleavage of the C=N Bond on Al(I). J. Am. Chem. Soc. 2017, 139, 8804–8807. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Vyboishchikov, S.F.; Gabidullin, B.; Nikonov, G.I. Oxidative Cleavage of C=S and P=S Bonds at an AlI Center: Preparation of Terminally Bound Aluminum Sulfides. Angew. Chem. Int. Ed. 2016, 55, 13306–13311. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Scheid, D.; Linti, G.; Zessin, T. Oxidative Addition Reactions of Element–Hydrogen Bonds with Different Polarities to a Gallium(I) Compound. Chem. Eur. J. 2009, 15, 12114–12120. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Korobkov, I.; Nikonov, G.I. Oxidative Addition of σ Bonds to an Al(I) Center. J. Am. Chem. Soc. 2014, 136, 9195–9202. [Google Scholar] [CrossRef]
- Chu, T.; Boyko, Y.; Korobkov, I.; Kuzmina, L.G.; Howard, J.A.K.; Nikonov, G.I. Oxidative Addition of Disulfides, Alkyl Sulfides, and Diphosphides to an Aluminum(I) Center. Inorg. Chem. 2016, 55, 9099–9104. [Google Scholar] [CrossRef]
- Falconer, R.L.; Nichol, G.S.; Smolyar, I.V.; Cockroft, S.L.; Cowley, M.J. Reversible Reductive Elimination in Aluminum(II) Dihydrides. Angew. Chem. Int. Ed. 2021, 60, 2047–2052. [Google Scholar] [CrossRef]
- Chu, T.; Nikonov, G.I. Oxidative Addition and Reductive Elimination at Main-Group Element Centers. Chem. Rev. 2018, 118, 3608–3680. [Google Scholar] [CrossRef]
- Weetman, C.; Inoue, S. The Road Travelled: After Main-Group Elements as Transition Metals. ChemCatChem 2018, 10, 4213–4228. [Google Scholar] [CrossRef]
- Ota, K.; Kinjo, R. Heavier element-containing aromatics of [4n + 2]-electron systems. Chem. Soc. Rev. 2021, 50, 10594–10673. [Google Scholar] [CrossRef] [PubMed]
- Weetman, C.; Bag, P.; Szilvási, T.; Jandl, C.; Inoue, S. CO2 Fixation and Catalytic Reduction by a Neutral Aluminum Double Bond. Angew. Chem. Int. Ed. 2019, 58, 10961–10965. [Google Scholar] [CrossRef] [PubMed]
- Weetman, C.; Porzelt, A.; Bag, P.; Hanusch, F.; Inoue, S. Dialumenes—Aryl vs. silyl stabilisation for small molecule activation and catalysis. Chem. Sci. 2020, 11, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Li, X.; Wang, C.; Zhang, J.; Cheng, J.; Zhu, X. Isolation of a 1,2-Dialuminacyclobutene. Angew. Chem. Int. Ed. 2006, 45, 2245–2247. [Google Scholar] [CrossRef] [PubMed]
- Agou, T.; Nagata, K.; Tokitoh, N. Synthesis of a Dialumene-Benzene Adduct and Its Reactivity as a Synthetic Equivalent of a Dialumene. Angew. Chem. Int. Ed. 2013, 52, 10818–10821. [Google Scholar] [CrossRef] [PubMed]
- Queen, J.D.; Power, P.P. Comproportionation of a dialuminyne with alane or dialane dihalides as a clean route to dialuminenes. Chem. Commun. 2023, 59, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Caputo, C.A.; Zhu, Z.; Brown, Z.D.; Fettinger, J.C.; Power, P.P. Activation of olefins with low-valent gallium compounds under ambient conditions. Chem. Commun. 2011, 47, 7506–7508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chai, J.; Fan, H.; Roesky, H.W.; He, C.; Jancik, V.; Schmidt, H.-G.; Noltemeyer, M.; Merrill, W.A.; Power, P.P. A Stable Aluminacyclopropene LAl(η2-C2H2) and Its End-On Azide Insertion to an Aluminaazacyclobutene. Angew. Chem. Int. Ed. 2005, 44, 5090–5093. [Google Scholar] [CrossRef]
- Bakewell, C.; White, A.J.P.; Crimmin, M.R. Reversible alkene binding and allylic C–H activation with an aluminium(I) complex. Chem. Sci. 2019, 10, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Bakewell, C.; Garçon, M.; Kong, R.Y.; O’Hare, L.; White, A.J.P.; Crimmin, M.R. Reactions of an Aluminum(I) Reagent with 1,2-, 1,3-, and 1,5-Dienes: Dearomatization, Reversibility, and a Pericyclic Mechanism. Inorg. Chem. 2020, 59, 4608–4616. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienko, A.; Pilkington, M.; Nikonov, G.I. Reactions of an aluminium(I) diketiminate compound with arenes. Mendeleev Commun. 2022, 32, 68–70. [Google Scholar] [CrossRef]
- Dmitrienko, A.; Britten, J.F.; Spasyuk, D.; Nikonov, G.I. Adduct of NacNacAl with Benzophenone and Its Coupling Chemistry. Chem. Eur. J. 2020, 26, 206–211. [Google Scholar] [CrossRef]
- Koner, A.; Gabidullin, B.M.; Kelemen, Z.; Nyulászi, L.; Nikonov, G.I.; Streubel, R. 7-Metalla-1,4-diphosphanorbornadienes: Cycloaddition of monovalent group 13 NacNac complexes to a stable 1,4-diphosphinine. Dalton Trans. 2019, 48, 8248–8253. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Nakano, R.; Yamashita, M. Cycloaddition of Dialkylalumanyl Anion toward Unsaturated Hydrocarbons in (1 + 2) and (1 + 4) Modes. Chem. Eur. J. 2020, 26, 2174–2177. [Google Scholar] [CrossRef]
- Kassymbek, A.; Britten, J.F.; Spasyuk, D.; Gabidullin, B.; Nikonov, G.I. Interaction of Multiple Bonds with NacNacGa: Oxidative Cleavage vs. Coupling and Cyclization. Inorg. Chem. 2019, 58, 8665–8672. [Google Scholar] [CrossRef]
- Loh, Y.K.; Aldridge, S. Acid–Base Free Main Group Carbonyl Analogues. Angew. Chem. Int. Ed. 2021, 60, 8626–8648. [Google Scholar] [CrossRef]
- Fooken, U.; Saak, W.; Weidenbruch, M. Diarylstannylene reactions with some aryl azides: Formation of different ring systems. J. Organomet. Chem. 1999, 579, 280–284. [Google Scholar] [CrossRef]
- Anker, M.D.; Coles, M.P. Aluminium-Mediated Carbon Dioxide Reduction by an Isolated Monoalumoxane Anion. Angew. Chem. Int. Ed. 2019, 58, 18261–18265. [Google Scholar] [CrossRef]
- Cui, C.; Roesky, H.W.; Schmidt, H.-G.; Noltemeyer, M. [HC{(CMe)(NAr)}2]Al[(NSiMe3)2N2] (Ar = 2,6-iPr2C6H3): The First Five-Membered AlN4 Ring System. Angew. Chem. Int. Ed. 2000, 39, 4531–4533. [Google Scholar] [CrossRef]
- Hicks, J.; Vasko, P.; Goicoechea, J.M.; Aldridge, S. The Aluminyl Anion: A New Generation of Aluminium Nucleophile. Angew. Chem. Int. Ed. 2020, 60, 1702–1713. [Google Scholar] [CrossRef]
- Hicks, J.; Heilmann, A.; Vasko, P.; Goicoechea, J.M.; Aldridge, S. Trapping and Reactivity of a Molecular Aluminium Oxide Ion. Angew. Chem. Int. Ed. 2019, 58, 17265–17268. [Google Scholar] [CrossRef] [PubMed]
- Dodonov, V.A.; Kushnerova, O.A.; Rumyantsev, R.V.; Novikov, A.S.; Osmanov, V.K.; Fedushkin, I.L. Cycloaddition of isoselenocyanates to sodium and magnesium metallacycles. Dalton Trans. 2022, 51, 4113–4121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dodonov, V.A.; Chen, W.; Zhao, Y.; Skatova, A.A.; Fedushkin, I.L.; Roesky, P.W.; Wu, B.; Yang, X.-J. Cycloaddition versus Cleavage of C=S Bond of Isothiocyanates Promoted by Digallane Compounds with Non-Innocent α-Diimine Ligands. Chem. Eur. J. 2018, 24, 14994–15002. [Google Scholar] [CrossRef] [PubMed]
- Fedushkin, I.L.; Moskalev, M.V.; Lukoyanov, A.N.; Tishkina, A.N.; Baranov, E.V.; Abakumov, G.A. Dialane with a Redox-Active Bis-amido Ligand: Unique Reactivity towards Alkynes. Chem. Eur. J. 2012, 18, 11264–11276. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, X.; Wang, Y.; Meng, C. Recent Advances on Gallium-Modified ZSM-5 for Conversion of Light Hydrocarbons. Molecules 2021, 26, 2234. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, X.; Meng, C. Speciation and interconversion of atomically dispersed extra-framework Ga in ZSM-5 zeolite. Appl. Surf. Sci. 2023, 636, 157811. [Google Scholar] [CrossRef]
- Dmitrienko, A.; Pilkington, M.; Nikonov, G.I. Selective Cross-Coupling of Unsaturated Substrates on AlI. Chem. Eur. J. 2021, 27, 5730–5736. [Google Scholar] [CrossRef]
- Roy, M.M.D.; Heilmann, A.; Ellwanger, M.A.; Aldridge, S. Generation of a π-Bonded Isomer of [P4]4− by Aluminyl Reduction of White Phosphorus and its Ammonolysis to PH3. Angew. Chem. Int. Ed. 2021, 60, 26550–26554. [Google Scholar] [CrossRef]
- Kong, R.Y.; Crimmin, M.R. Reversible insertion of CO into an aluminium–carbon bond. Chem. Commun. 2019, 55, 6181–6184. [Google Scholar] [CrossRef]
- Hicks, J.; Vasko, P.; Goicoechea, J.M.; Aldridge, S. Reversible, Room-Temperature C-C Bond Activation of Benzene by an Isolable Metal Complex. J. Am. Chem. Soc. 2019, 141, 11000–11003. [Google Scholar] [CrossRef] [PubMed]
- Boronski, J.T.; Thomas-Hargreaves, L.R.; Ellwanger, M.A.; Crumpton, A.E.; Hicks, J.; Bekiş, D.F.; Aldridge, S.; Buchner, M.R. Inducing Nucleophilic Reactivity at Beryllium with an Aluminyl Ligand. J. Am. Chem. Soc. 2023, 145, 4408–4413. [Google Scholar] [CrossRef] [PubMed]
- Dodonov, V.A.; Xiao, L.; Kushnerova, O.A.; Baranov, E.V.; Zhao, Y.; Yang, X.-J.; Fedushkin, I.L. Transformation of carbodiimides to guanidine derivatives facilitated by gallylenes. Chem. Commun. 2020, 56, 7475–7478. [Google Scholar] [CrossRef] [PubMed]
- Dodonov, V.A.; Kushnerova, O.A.; Baranov, E.V.; Novikov, A.S.; Fedushkin, I.L. Activation and modification of carbon dioxide by redox-active low-valent gallium species. Dalton Trans. 2021, 50, 8899–8906. [Google Scholar] [CrossRef] [PubMed]
- Fedushkin, I.L.; Lukoyanov, A.N.; Tishkina, A.N.; Fukin, G.K.; Lyssenko, K.A.; Hummert, M. Reduction of digallane [(dpp-bian)Ga-Ga(dpp-bian)] with Group 1 and 2 metals. Chem. Eur. J. 2010, 16, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-T.; Huang, C.-A.; Chen, C.-T. Palladacyclic Complexes Containing C,N-Type Ligands as Catalysts in Cross-Coupling Reactions. Eur. J. Inorg. Chem. 2008, 2008, 3142–3150. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.H.; Slocombe, R.J. The Chemistry of the Organic Isocyanates. Chem. Rev. 1948, 43, 203–218. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamazaki, H. Low-valent isocyanide complexes and clusters of palladium and platinum. Crystal structure of [Pt{C(=NR)N(R)C(=NR}(RNC)2](R = 2,6-Me2C6H3). J. Chem. Soc. Dalton Trans. 1989, 1989, 2161–2166. [Google Scholar] [CrossRef]
- Riera, V.; Ruiz, J.; Tiripicchio, A.; Camellini, M.T. Synthesis of coordinated carbon monoxide from isocyanide iron(II) compounds. Crystal structure of [Fe(dppe)(CO)(CN-p-tol)(p-tolN=C-N-p-tol-C=N-p-tol)]. J. Organomet. Chem. 1987, 327, C5–C8. [Google Scholar] [CrossRef]
- Hoberg, H.; Oster, B.W.; Krüger, C.; Tsay, Y.H. Nickela-heteroringe aus nickel(0) und phenylisocyanat. J. Organomet. Chem. 1983, 252, 365–373. [Google Scholar] [CrossRef]
- Paul, F.; Fischer, J.; Ochsenbein, P.; Osborn, J.A. Syntheses, interconversions and reactivity of heteropalladacycles made from aryl isocyanates and various phenanthroline Pd(II) precursors with small molecules. Comptes Rendus Chim. 2002, 5, 267–287. [Google Scholar] [CrossRef]
- Lam, H.-W.; Wilkinson, G.; Hussain-Bates, B.; Hursthouse, M.B. Reactions of tert-butyl isocyanate and trimethylsilyl azide with imidoamido compounds of chromium, molybdenum and tungsten. J. Chem. Soc. Dalton Trans. 1993, 1993, 781–788. [Google Scholar] [CrossRef]
- Weber, L.; Lassahn, U.; Stammler, H.-G.; Neumann, B. Inversely Polarized Phosphaalkenes as Phosphinidene- and Carbene-Transfer Reagents. Eur. J. Inorg. Chem. 2005, 2005, 4590–4597. [Google Scholar] [CrossRef]
- Cui, C.; Köpke, S.; Herbst-Irmer, R.; Roesky, H.W.; Noltemeyer, M.; Schmidt, H.-G.; Wrackmeyer, B. Facile Synthesis of Cyclopropene Analogues of Aluminum and an Aluminum Pinacolate, and the Reactivity of LAl[η2-C2(SiMe3)2] toward Unsaturated Molecules (L = HC[(CMe)(NAr)]2, Ar = 2,6-i-Pr2C6H3). J. Am. Chem. Soc. 2001, 123, 9091–9098. [Google Scholar] [CrossRef] [PubMed]
- Fedushkin, I.L.; Skatova, A.A.; Cherkasov, V.K.; Chudakova, V.A.; Dechert, S.; Hummert, M.; Schumann, H. Reduction of Benzophenone and 9(10H)-Anthracenone with the Magnesium Complex [(2,6-iPr2C6H3-bian)Mg(thf)3]. Chem. Eur. J. 2003, 9, 5778–5783. [Google Scholar] [CrossRef] [PubMed]
- Fachinetti, G.; Biran, C.; Floriani, C.; Chiesi Villa, A.; Guastini, C. C:O and C:C bond activation in diphenylketene promoted by dicarbonylbis(.eta.-cyclopentadienyl)titanium(II). Inorg. Chem. 1978, 17, 2995–3002. [Google Scholar] [CrossRef]
- Evans, W.J.; Drummond, D.K. Reductive coupling of pyridazine and benzaldehyde azine and reduction of bipyridine by samarium complex (C5Me5)2Sm(THF)2. J. Am. Chem. Soc. 1989, 111, 3329–3335. [Google Scholar] [CrossRef]
- Ohff, A.; Zippel, T.; Arndt, P.; Spannenberg, A.; Kempe, R.; Rosenthal, U. Reactions of Azines with Titanocene: C-H Activation, C-C Coupling, and N-N Cleavage to Heterobimetallic Complexes. Organometallics 1998, 17, 1649–1651. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Lukoyanov, A.N.; Fukin, G.K.; Ketkov, S.Y.; Hummert, M.; Schumann, H. Synthesis, Molecular Structure and DFT Study of (dpp-bian)Ga-M(Et2O)3 (M = Li, Na; dpp-bian=1,2-bis(2,6-diisopropylphenyl)imino acenaphthene)). Chem. Eur. J. 2008, 14, 8465–8468. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, T.H.; Shin, Y.W.; Jeon, Y.; Kim, J. Amitraz. Acta Crystallogr. Sect. E 2013, 69, o1300. [Google Scholar] [CrossRef] [PubMed]
- Carugo, O.; Poli, G.; Manzoni, L. Structure of N,N′,N″-triphenylbiuret. Acta Crystallogr. Sect. C 1992, 48, 2013–2016. [Google Scholar] [CrossRef]
- Ghosh, R.; Samuelson, A.G. Catalytic metathesis of carbon dioxide with heterocumulenes mediated by titanium isopropoxide. Chem. Commun. 2005, 2005, 2017–2019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hardman, N.J.; Power, P.P. Dimeric Gallium Oxide and Sulfide Species Stabilized by a Sterically Encumbered β-Diketiminate Ligand. Inorg. Chem. 2001, 40, 2474–2475. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 90th ed.; CD-ROM Version 2010; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Wang, X.M.; Fan, R.Q.; Qiang, L.S.; Li, W.Q.; Wang, P.; Zhang, H.J.; Yang, Y.L. Tunable luminescence from rare 2D Ga(III)/In(III) coordination polymers coexisting with three different conjugated system aromatic ligands. Chem. Commun. 2014, 50, 5023–5026. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.; Gomez, V.; Platero-Prats, A.E.; Reves, M.; Echeverria, J.; Cremades, E.; Barragan, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 2008, 2832–2838. [Google Scholar] [CrossRef]
- Arii, H.; Amari, T.; Kobayashi, J.; Mochida, K.; Kawashima, T. Low-Coordinate Germanium(II) Centers Within Distorted Axially Chiral Seven-Membered Chelates: Stereo- and Enantioselective Cycloadditions. Angew. Chem. Int. Ed. 2012, 51, 6738–6741. [Google Scholar] [CrossRef]
- Mom, V.; de With, G. A reinvestigation on benzalazine, influence of TDS and comparison with different experiments. Acta Crystallogr. Sect. B 1978, 34, 2785–2789. [Google Scholar] [CrossRef]
- Bruker APEX3. Bruker Molecular Analysis Research Tool; v. 2018.7-2; Bruker AXS: Madison, WI, USA, 2018. [Google Scholar]
- Data Collection, Reduction and Correction Program; CrysAlisPro 1.171.40.67a—Software Package; Rigaku OD: Tokyo, Japan, 2019.
- Bruker. SAINT Data Reduction and Correction Program v. 8.40B; Bruker AXS: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. SADABS v.2016/2, Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS: Madison, WI, USA, 2016. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- SCALE3 ABSPACK: Empirical Absorption Correction; CrysAlisPro 1.171.40.67a—Software Package; Rigaku OD: Tokyo, Japan, 2019.
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXTL. Structure Determination Software Suite; Version 6.14; Bruker AXS: Madison, WI, USA, 2003. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).