Abstract
The use of Liquid Organic Hydrogen Carriers (LOHC) is one of the potential options to store hydrogen. Today, the vast majority of compounds used as LOHC come from the oil industry. Using biosourced LOHC would be a step forward in the development of this CO2-free solution. This article looks at LOHC candidates that can be obtained from biomass. The special case of formic acid and methanol, which do not fall within the definition of LOHC, is also considered. The synthesis of alcohols, polyols, amines, aminoalcohols and N-heterocyclic compounds from biosourced compounds is reviewed.
1. Introduction
Liquid Organic Hydrogen Carriers (LOHC) are now well recognized as a potential solution to store hydrogen, either to be used onboard or on stationary equipment [1]. The storage/release system is based on the hydrogenation of hydrogen-lean molecules and dehydrogenation of the corresponding hydrogen-rich molecules. The LOHC systems can be classified into three categories: (a) the cycloalkane/aromatic couples, (b) the N-heterocyclic compounds and (c) other molecules. The two first categories represent more than 95% of the publications on LOHC and present the highest level of maturity. In a recent article, Modisha and Bessarabov share their opinion on the challenges concerning some of the most mature systems: benzyltoluene (BT) and dibenzyltoluene (DBT). The high dehydrogenation reaction temperature and the need for efficient, selective and stable catalysts are the major concerns [2]. The energy efficiency of the system is also seen as the major challenge by Li et al. [3] who investigated the benefits of waste-heat recovery in the global chain from the electrolyzer to the fuel cell. They demonstrate that it leads to better efficiency than traditional hydrogen storage options in the case of the use of LOHC and specifically DBT. Due to the reversibility of the hydrogenation/dehydrogenation reactions involved in the storing/releasing process, the source of the LOHC compounds may appear as a minor issue. Most of them come from the petroleum industry. However, due to the fact that cycling is never 100%, finding LOHC that can be bio-sourced appears as a necessary survey. Moreover, this can pave the way to the use of less nocive molecules.
Very few articles deal with the use of biosourced LOHC. Valentini et al. used biosourced formic acid (FA), methanol and isopropanol as hydrogen donors to hydrogenate platform molecules [4]. The molecules they obtain could also be seen as LOHC, but that is not the purpose of the paper. Theoretically, the term LOHC excludes FA and methanol because of the indirect reversibility of the reactions (CO2 is not captured from the dehydrogenation reaction). Nevertheless, the authors are committed to using bio-based products and it is one of the rare examples.
There are two approaches when it comes to finding the right LOHC candidate produced from biomass. The first one, a conventional approach, attempts to obtain molecules that have been proven to be efficient in this application, this time as green derivatives instead of petrol derivatives. The second one, a bolder approach, suggests new molecules due to their availability or ease of obtention and evaluates them with performance parameters in this field. Examples of both these approaches will be discussed in the following sections, presenting their particularities and giving insights on what direction research should follow in the future years to develop this new concept.
To begin with, it is necessary to understand the physical and chemical parameters that define an attractive LOHC pair. After identifying such criteria, the search in the biobased library of compounds becomes less demanding and more logical. The parameters to be considered, as recommended by Paragian et al. [5], Niermann et al. [1] and Rao and Yoon [6] are:
- Storage capacity: The hydrogen-rich molecule in the pair is expected to have volumetric and gravimetric densities of about 56 kg/m3 and 6 wt.% or higher, respectively.
- Dehydrogenation enthalpy: it must be less than or in the range of 40–60 kJ/mol H2 to enable the reaction to be carried out below 200 °C and at near-atmospheric pressures. This is key to introducing the LOHC system into a heat integration plan where waste heat supplies the dehydrogenation requirement.
- Availability or ease of synthesis: LOHC pairs must be either widely available or easy to be synthesized, while also being inexpensive in order to satisfy economic constraints.
- Synergy with catalysts: The hydrogenation/dehydrogenation cycle must be able to be undertaken at low temperatures by means of a low-production-cost catalyst. Either heterogeneous or homogeneous (but especially for the latter), the activity and stability, separation and recycling must be favored by the LOHC pair.
- Material handling: it comprises a set of properties that facilitate the manipulation of the LOHC along the supply chain. The boiling point should be high enough (>300 °C) so the LOHC does not volatilize during dehydrogenation, implying an additional separation step prior to hydrogen utilization. The melting point should be low (<0 °C), avoiding possible solidification during storage and/or the use of solvents that ultimately reduce their storage capacity. Viscosity and eco-toxicity are considered to ease pumping during transport and storage and to avoid environmental and health hazards along the supply chain; these values are expected to be the same or lower than those of conventional fuels.
- LOHC and catalyst stability relative to the formation of undesired byproducts, either during the storage or hydrogenation cycles. The longer a LOHC pair works without losses or degradation, the more H2 delivery cycles it can undergo. Lower temperatures at dehydrogenation and an active and selective catalyst play a crucial role in extending the carrier’s lifespan.
- Gas uptake: This represents the rate at which hydrogen gas is released from the hydrogen-rich LOHC.
- Technology Readiness Level relative to its compatibility with the existing fuel and chemistry infrastructure of storage and transport and the maturity of its obtention process.
None of the well-known LOHC pairs fulfill these characteristics completely. Therefore, the ideal candidate is still undiscovered. This is roughly why this technology has not been commercialized on a wider scale [7]. Promissory prospects from biomass valorization have emerged in the previous years and are expected to provide the right balance between performance and sustainability.
2. Circular Hydrogen Carriers
Some molecules with high gravimetric H2 storage capacity after releasing their hydrogen content produce a stable gaseous species that is normally released to the environment, leaving an open door in the inherent cycle of the carrier. Examples of such compounds are Formic acid, Methanol and Dimethyl ether, which release CO2, and Ammonia, releasing N2. This poses an additional constraint to the process, which is the capture and further conversion of such hydrogen-lean gas species through hydrogenation or other chemical routes [8]. In addition to ammonia not being organic, the other compounds are not considered to be LOHC either, since the hydrogen-lean molecules are not always recovered and transported back to the hydrogenation facilities. Formic acid synthesis is net zero in carbon if the CO2 used as raw material comes directly from flue gases or from the atmosphere. However, the carbon dioxide released in the high-energy demand site is never the same as the one used in FA synthesis given the time and distance offset in the energy supply scheme. An indirect way to return the released carbon into the cycle is also proposed by Preuster and Albert [9]: biomass is the feedstock for FA synthesis, but also the sink of carbon dioxide through the photosynthetic mechanism. Several chemical routes have been proposed for obtaining formic acid from biomass sources, most of which are presented in Table 1.

Table 1.
Reported methods for obtaining formic acid from biomass. LCB: Lignocellulosic biomass, GLU: Glucose, XYL: Xylose, FA: Formic acid, AA: Acetic acid, LA: Lactic acid, HPA: Heteropolyacids, T: Temperature, P: Pressure.
The presented technologies shed light on two main facts: First, the formic acid yield from lignocellulose through most existing technologies is low due to the complexity of the lignocellulosic biomass (LCB) structure and second, technologies still need development or optimization to reach industrial application. The use of formic acid as a hydrogen carrier is justified because of its high density (1.22 g/cm3) and, thus, its volumetric storage capacity that attains 53 g H2/L, regardless of its low hydrogen content (4.4 wt.%) [14]. Many developments have been proposed in terms of suitable catalysts [14,15,16] and regarding the way this technology may reach the industrial scale [8]. The inclusion of biomass sources in such implementation strategies will be of central importance to guarantee the real circularity of the process.
The final separation and purification of FA from aqueous mixtures obtained in the methods presented in Table 1 requires additional processing steps. Typically, FA recovery is performed via extractive distillation on a laboratory scale, although using solvents entails safety and health concerns [10]. Other methods observed use a reactive liquid extraction via complexation using either a mixture of an aliphatic amine and a phosphorus-bonded extractant [17] or a mixture of alcohols and aliphatic amines [18,19]. Such methods require the subsequent FA stripping from the organic phase. Additionally, organic acids can also be recovered using polysulfone membranes and ethers/acetates as solvent [20].
In the case of methanol, hydrogen storage capacity is greater, as 3 moles of hydrogen gas (2 from methanol, 1 from water) can be released per mole of methanol when the dehydrogenation reaction is performed in aqueous medium [21]. The hydrogen release is achieved either by high-temperature steam reforming of methanol or by low-temperature dehydrogenation. The first one corresponds to the reaction with water vapor at 420 °C on Ir, Pt-supported catalysts, showing a high gas flow. The second one, on the other hand, operates at less than 100 °C but requires water as a solvent to be added, needing an additional H2/H2O separation step at the end [1]. The main routes for obtaining methanol from biomass are presented in Table 2. Methanol-containing streams need to be purified before their application as LOHC. From the presented routes, diluted methanol in liquefaction methods is not easily recovered and its separation results are cumbersome [22]. For the case of the promising biosynthesis routes, similar methods as for ethanol recovery from fermentation broth (distillation, pervaporation, solvent extraction, vacuum fermentation, etc.) may be used to purify the methanol obtained [23].

Table 2.
Overview of the methods for the obtention of methanol from biomass, containing main stages and suggestions for operation conditions.
In this table, an overview of known biomass-based methanol synthesis routes is presented with key information reported in the literature. Nevertheless, it only represents a first glance at such methods, so the references cited must be considered as a starting point to delve into the corresponding topic. Table 2 reveals that bio-methanol production routes depend on both mature (anaerobic digestion, gasification, steam reforming) and immature technologies (CO2 hydrogenation, biosynthesis). Hence, additional research endeavors are needed to completely replace the conventional fossil chemical route for the provision of methanol that might be used as a hydrogen carrier.
It is important to know that methanol can also be applied in chemical synthesis as a hydrogen donor [21,27]. These two applications, along with the vast conventional ones for these commodity molecules, will need to coexist to guarantee their effective insertion into the energy production matrix. Since circular storage outside LOHC escapes the rest of the scope of this work, no additional discussion will be performed in the following sections. For the other mentioned “circular storage” compounds, ammonia and dimethyl ether, further details are to be found in the works of Sun et al. [28], Kojima and Yamaguchi [29], Babson et al. [30], Papadias et al. [31], Kojima [32], Pawelczyk et al. [33] and Catizzone et al. [34], shedding light on the details of hydrogen carrying capacity and potential, but also in the obtention of these molecules from biomass sources.
3. Alcohols
3.1. Primary and Secondary Alcohols
It is already well known that alcohols can be obtained as products from biomass, with ethanol and butanediol production from fermentation being classic examples. These, among other products, have well-established synthesis routes, that make them inexpensive, widely available and easy to produce. For the present review, alcohols and diols also attain interest since they can undergo Acceptorless Alcohol Dehydrogenation (AAD) to produce H2 and a hydrogen-lean molecule (aldehydes, esters or carboxylic acids) that can be reversibly hydrogenated afterward to produce the initial alcohol in the presence of a noble metal catalyst [35]. Ethanol, the most studied biobased alcohol, can be readily obtained from different biomass sources, as reviewed by Bušić et al. [36], Gamage et al. [37], Rajeswari et al. [38] and Aditiya et al. [39]. In such reviews, all reported biomass pretreatment methods, which depend upon the composition and source of the raw material, are listed and compared. The pretreatment technique prepares the biomass for the most common transformation process, which is microbial fermentation. Moreover, ethanol separation and purification methods are also discussed. Very detailed literature on these processes is widely available and constantly updated. There are several mechanisms that allow the hydrogen content in ethanol to be released, but the most efficient ones in the literature are:
- Ethanol steam reforming (ESR): Conversion of a steam atmosphere into carbon dioxide and 6 moles of H2 per mol of ethanol [40]. However, this route is not of interest to the current work, as discussed previously.
- AAD: Alcohol dehydrogenation can yield ethyl acetate or acetaldehyde depending on the catalyst and reaction conditions (Figure 1). It can be extended for general primary and secondary alcohols and their corresponding hydrogen-lean ketones or esters, respectively.
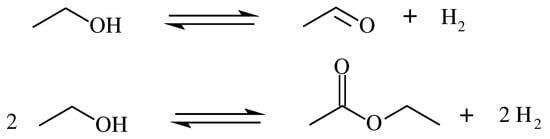 Figure 1. Ethanol dehydrogenation routes into ethyl acetate and acetaldehyde.
Figure 1. Ethanol dehydrogenation routes into ethyl acetate and acetaldehyde.
Typical AAD catalysts based on Ru and Fe coordinated molecular catalysts fulfill the main requirements for this application [41], which are:
- Mild reaction conditions are required for temperature (80–100 °C) and pressure (H2 from electrolyzers at low pressure).
- Turn over number (TON) is relatively constant after the high number of hydrogenation/dehy drogenation cycles.
- Stability, selectivity and reactivity, to avoid the over-formation of reaction products and allow catalyst reuse.
Heterogeneous catalysts have also been used to dehydrogenate alcohols like ethanol in the gas phase, but they require higher temperatures (200–250 °C) and undergo C-C coupling reactions, forming long chain hydrocarbons and reducing the final H2 yield. Examples of supported metal catalysts are Cu over Alumina, Zirconia or Copper chromite [42,43]. These previous facts point research attention towards molecular organometallic catalysts as the most suitable catalysts for this purpose. Tran et al. [41] presented the example of Ru-MACHO, a promising Ru complex containing bis(2-diphenylphosphinoethyl)amine, because of its activity and selectivity to ethyl acetate in AAD, its commercial availability and the past industrial experiences that have validated its performance. Nevertheless, this system still suffers from deactivation affecting its recyclability. Further research is expected to include diverse ligands that improve the overall robustness of the molecular catalyst. Moreover, research to achieve an efficient replacement of noble metals for earth-abundant transition metals in homogeneous catalysis would make this approach more economically and environmentally attractive [35,44]. For the case of other bio alcohols, like 2-propanol and n-butanol coming from ABE fermentation by Clostridium strains (which produces H2 as a gaseous byproduct) or by the Guerbet reaction of bioethanol, similar conclusions as in the case of ethanol can be drawn [45]. However, their hydrogen storage capacity might be low if they undergo the acetate pathway in dehydrogenation (2.7 wt.%) making them unsuitable as LOHC candidates [44,46]. An example of biobased alcohols as LOHC candidates was presented by Verevkin et al. [47], where bioderived furfuryl alcohol serves as the hydrogen-rich molecule, undergoing dehydrogenation to form tetrahydrofurfuryl alcohol. Despite being only a theoretical study, it shed light on the hydrogen storage capacity of this LOHC system, while also revealing that its dehydrogenation enthalpy shows a higher value than most systems, which will probably hinder its application due to energetic inefficiency. New bio-sourced LOHC were proposed in a recent patent [48]. The couples are 1-Cyclohexylethanol/Acetophenone, Cyclohexylmethanol/Benzaldehyde, 4-Methylcyclohexanemethanol/(4-Methylphenyl)methanol, Dicyclohexylcarbinol/Benzophenone and Cyclohexanepropanol/Cinnamaldehyde. Cinnamaldehyde is present in essential oils [49] and can react to form benzaldehyde [50] and acetophenone [51]. These molecules can also be obtained from lignin [52].
3.2. Polyols
Another type of alcohol fits in the purpose of the present review: Diols and polyols are obtained from lignocellulosic biomass and other natural sources but can also undergo hydrogenation/dehydrogenation cycles storing and releasing hydrogen gas [35]. These kinds of compounds are widely available but traditionally produced from fossil resources for the manufacturing of polymers, cosmetics, and pharmaceuticals, among others. The production technologies for polyol production from biomass sources, on the other hand, are presented in Table 3. The strategies arising to produce biobased diols are diverse and promising in terms of yield and operating conditions. Particularly, the biosynthesis represents an important improvement in the conventional production routes since metabolic engineering strategies allow the bacterial strains to diversify their substrates (C5 sugars from hemicellulose, raw glycerol or even syngas), optimize the expression or inhibition of enzyme-production genes according to their function and reduce their vulnerability and dependence on certain nutrients (like growth cofactors) [53]. The separation of diols, in particular, those coming from fermentation pathways recurs to methods such as membrane separation, chromatography, solvent extraction and reactive extraction, among others, as thoroughly described by Xiu and Zeng [54].

Table 3.
An overview of the methods for obtaining diols and polyols from biomass, including main stages and operation conditions reported in the literature.
In the context of LOHC, two different schemes for diol/polyol hydrogenation cycles exist. The scheme of Figure 2 represents the formation of a lactone starting from a C4 or higher diol, for which the hydrogen storage capacity will depend on the length of the carbon chain. The one in Figure 3 represents the dehydrogenative coupling of diols to form multiple ester bonds, whose storage capacity will depend on the number of diol molecules that undergo this reaction.
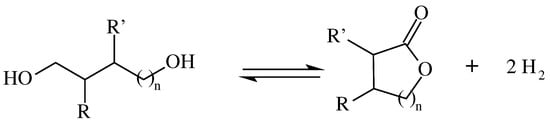
Figure 2.
Dehydrogenative lactonization of a diol [35].

Figure 3.
Acceptorless dehydrogenative coupling of ethylene glycol [35].
A LOHC pair that works using the first dehydrogenation mechanism is the one formed by 1,4-butanediol, a biosourced diol (Table 3), and its conjugated hydrogen-lean pair -butyrolactone [59]. Onoda et al. [60] proved that the Ir-catalyzed (homogeneous) hydrogenation/dehydrogenation of this system worked appropriately in terms of yield (up to 99% for the two reactions) and selectivity. Then, Mevawala et al. proved in 2022 that this reaction can occur both in the liquid and gas phases in an equally efficient manner over a Copper Chromite heterogeneous catalyst [61]. Many other copper-based heterogeneous catalysts have been used for this dehydrocyclization reaction [62,63,64]. The final decision will depend solely on catalytic considerations, but also having in mind the reactor design specificities and the overall economics of the process. The use of propylene glycol or 2,3-butanediol as LOHC is also proposed in a recent patent [65]. The idea is based on the principle that for each system, the dehydrogenation product is much more volatile than the reactant. Thus, shifting the equilibrium is possible by simple separation at temperatures between both boiling points. The dehydrogenation of 2,3-butanediol was also studied by Al-Auda et al., using a Cu-Al2O3 catalyst [66] with the objective of a selective synthesis of acetoin (partial dehydrogenation).
3.3. Coupling with Alcohols
Alcohols and diols can lead to dehydrogenative coupling as presented in Figure 4. A first validation of this system was carried out by Zou et al. [67], where they obtained an 80% hydrogen yield with a Ru-based pincer complex as a catalyst and 50 bar H2 pressure under neat conditions to hydrogenate the diverse ester mixture back into alcohols with 72% yield. Similarly, Zhou et al. [68] reduced the hydrogenation pressure to 5 bar, also carrying out the dehydrogenation under base and solvent-free conditions. The produced esters have a storage capacity of over 5%.

Figure 4.
Dehydrogenative coupling of ethylene glycol and ethanol over a Ru-complex molecular catalyst [35].
4. Amines
Biomass-derived amines are possible thanks to the catalytic amination of diols and carbonyl-derivative compounds obtained in biorefineries. A wide range of products can be obtained: amines, diamines, amino alcohols and cyclic amines, but also the starting biomass derivative molecules. The amination reaction requires an amine source and a catalyst. Gupta et al. reported amine sources that range from ammonia to aniline and other alkyl-/allylamines [69]. On the other hand, the catalysts can either be organometallic, predominantly Ru-based, but also Ir-based, or heterogeneous with supported metals like Ru, Ni, Pt, Cu, Cr, Mo and even Fe. The yield of the reaction strictly depends on the substrate and the amino source, with temperatures around 150–200 °C. The system may require the use of a special reaction atmosphere (often H2 but also Ar or N2) and the use of an organic solvent. This kind of conversion provides very useful precursor amines from readily available biomass derivatives, significantly reducing the previous dependency on fossil resources. However, the purification of the amine formed, solvent and catalyst recovery depends on the reaction system configuration and the achieved yield, but little to no mention of such a scheme out of the laboratory scale is found in related literature [69,70].
4.1. Amines and Diamines
Amines and diamines undergo a LOHC cycle that yields hydrogen-lean nitriles and hydrogen gas (Figure 5). Although their hydrogen storage capacity is relatively high (around 6.8 wt.%) and their dehydrogenation enthalpy has an appropriate value (around 60 kJ/mol H2), there are some thermodynamic and process limitations for amines in this application. This type of amine requires high temperatures (300 °C) for dehydrogenation or the presence of an inert gas to lower the partial pressure of the product hydrogen and thus favor its release at lower temperatures [71]. Some examples were described using a solvent and an inert diluting gas [72].

Figure 5.
Acceptorless dehydrogenation of amines to nitriles. Example of hexan-1,6-diamine [71].
4.2. Aminoalcohols
Next, obtaining amino alcohols from biomass-derived diols is of great interest for the LOHC market. Aminoalcohols are another family of compounds with high potential capacity to store and release hydrogen, using the same catalysts and at mild reaction conditions. Verevkin et al. studied the thermodynamic modelling of the system composed by H2-rich 2-amino-ethanol and H2-lean piperazine-2,5-dione (Figure 6), which has a storage capacity of 6.56 wt.% [73,74].

Figure 6.
Acceptorless dehydrogenative coupling of 2-ethanolamine and the production of glycine anhydride (piperazine-2,5-dione) with a byproduct linear oligopeptide.
The formation of the oligopeptide may change the overall energy efficiency of the process since its enthalpy of dehydrogenation is hard to determine. Nevertheless, it does not affect the hydrogenation part of the cycle, obtaining a total yield of the amino alcohol at the end [75]. PNN Ruthenium pincer molecular catalysts have proven to be the most suitable for this reaction. The most crucial finding of Verevkin et al. was related to the enthalpy of dehydrogenation of 2-ethanolamine into piperazine-2,5-dione, with a value of only 24.2 kJ/mol H2 [76]. This value contrasts with the values of conventional LOHC systems like N-ethyl-carbazole (50.5 kJ/mol H2) and benzyltoluenes (63.5 kJ/mol H2). This opens the door to further optimization and application on a larger scale. The study of other LOHC pairs coming from amino alcohols is to be found in the work by Verevkin et al., but none of them reach the attractiveness of the one previously presented [73].
4.3. N-Heterocyclic Compounds
Subsequently, the third family of amines obtained from biomass-derived platform molecules is N-heterocyclic amines. This is a very special kind of compound for LOHC systems, especially considering that many traditional pairs contained one N-heterocycle, so biomass can now provide molecules that even or surpass their performance. N-containing heterocycles are preferable because they present lower dehydrogenation enthalpy and higher kinetic rate as compared with alicyclic compounds, especially in 5 and 6-member rings [6,76]. Care must be taken, however, because this may also lead to undesired degradation pathways and thermal vulnerability [77]. An example of a biosourced N-heterocyclic LOHC system is the pair Pyrrole/Pyrrolidine. Pyrrolidine can be obtained by amination of (bio) 1,4-butanediol with ammonia. This system can store up to 5.63 wt.% hydrogen, which can be released by using an Ir-pincer complex at temperatures around 150 °C [78]. In the cited study, it was determined that dehydrogenation might be limited (high temperature needed) by sterics around the catalyst metal center and not only by the C-H bond strength. Another example presents the production of pyrrolidones and quinolines, a couple of N-heterocyclic compounds suitable as LOHC, starting from levulinic acid (LA), a widely available byproduct of biomass processing [79]. It was performed as an acid-assisted condensation reaction of LA and 2-alkynylanilines. The yield towards pyrrolidone reaches 93% and towards quinoline derivatives, it reaches 48%. This shows an interesting application of levulinic acid obtaining significant yields of compounds that are adequate not only for LOHC schemes but also in the pharmaceutical and cosmetic industry, just to mention some. Two last emerging examples of N-heterocyclic LOHC systems have been studied by the teams of Tang [80] and Forberg [81]. The first one is related to the use of natural Amaryllidaceae alkaloids as LOHC because of their low molecular weight and high hydrogen storage capacity. The second one relates to the obtention of phenazine from lignin hydrogenolysis via cyclohexane 1,2-diol, taking advantage of its high aromatic content. The results obtained by Tang et al. revealed that the two main alkaloids of Amaryllidaceae flowers (trisphaeridine and norbelladine) undergo two different dehydrogenation pathways, that were modeled and followed along the hydrogenation reaction [80]. Only trisphaeridine (Figure 7) seemed suitable for LOHC systems, since thermodynamics are favorable for its hydrogen release (54 kJ/mol H2) and its storage capacity of up to 5.9 wt.%. Norbelladine, on the contrary, dehydrogenates through an irreversible chemical route, making it unfeasible to apply a LOHC scheme with it. Alternatively, Forberg et al. studied lignin hydrogenolysis to produce an N-heterocycle, octahydrophenazine, and its reversible hydrogenation by means of a bimetallic catalyst [81]. As Pd and Ru-based catalysts were the most efficient in hydrogenation reactions, a bimetallic synergy was intended, over a novel silicon carbonitride support. The catalyst was tested for the hydrogenation cycle of N-ethyl carbazole showing the best results in hydrogen uptake and low temperature requirements when compared with commercial catalyst options. Then, a suitable hydrogen storage molecule was proposed having lignin as a starting material to valorize one of the main byproducts of biomass processing all over the world. The lignin macrostructure is hydrogenated and hydrolyzed to yield cyclohexane-1,2-diol. This compound is aminated with NH3 to form octa-hydrophenazine with a yield of 74%. The conditions for the reactions in the cycle H14-Phenazine/Phenazine (Figure 7) were 115 °C and 50 bar H2 with the addition of dioxane for the hydrogenation (24 h), compared to 190 °C and the addition of diglyme (24 h). Since the molecule obtained can reach 7.2 wt.% hydrogen storage capacity, releasing 7 moles of hydrogen gas per mole of H14-phenazine, and added to the Pd2Ru catalyst proposed by Forberg et al., it seems to be a very attractive way to valorize lignin and supply the hydrogen market at the same time [76,81].

Figure 7.
Representation of Trisphaeridine (left) and Phenazine (right).
4.4. Coupling with Alcohols
Diamines and alcohols can undergo acceptorless dehydrogenative coupling, forming a bis-cyclic imide species as hydrogen-lean LOHC (Figure 8). Ruthenium catalysts once again prove superior for this application and the hydrogen storage capacity of the mixture is high compared to all other LOHC systems presented in this review (6.7 wt.%). The dehydrogenation temperature is low, corresponding to 135 °C at 40 bar H2, which makes it a very promising reaction system for this application. The reader is advised to consider the work of Yadav et al. [35] and Kumar et al. [82] to fully understand the rest of the coupling reactions that can happen between diamines and alcohol species, together with its potential in the LOHC technology.

Figure 8.
Production of hydrogen-lean bis-cyclic imide from 1,4-butanediol and ethylenediamine [35].
5. Discussion
Table 4 is presented as a summary of the critical LOHC parameters of the LOHC pairs in this review. Such parameters were gathered to allow the reader to carry out a quick revision of the best alternatives considering his/her own priorities or the particularities of the process he/she is invested in. The temperatures at which the compounds are liquid are also reported to help decide whether they can easily be used and in which process type (reactive distillation, gas-phase dehydrogenation, etc.). In the introduction section, it was mentioned that the ideal range at which LOHC are liquid should be (0–300 °C). However, as can be seen in Table 4, no system satisfies this recommendation. Some adapted processes have to be designed to cope with the actual physical properties of the compounds. If one of the compounds is solid at RT, an option may be not to work at full conversion. If the boiling point is low, the process may be used for stationary storage using gas-phase reactors. Some of the LOHC can be directly used in fuel cells like isopropanol, but also methanol and formic acid if we agree to consider these compounds as pseudo-LOHC [4]. The latter ones have reached a certain level of maturity, namely the methanol fuel cell which is commercialized. In the case of the formic acid fuel cell, the formic acid oxidation at the anode still needs research activities [83,84]. Concerning the direct isopropanol fuel cell (DIFC), it presents the advantage of producing acetone instead of CO2, and acetone can be further reduced to isopropanol. Furthermore, the DIFC presents a much lower theoretical cell voltage (1.07 V) than a hydrogen fuel cell (1.23 V). Some research works are in progress to find an appropriate catalytic system for the electrooxidation of isopropanol avoiding poisoning of the surface by acetone [85,86].

Table 4.
Summary of the properties of the biosourced H2 carrier pairs presented throughout the document.
Having the alternative to produce LOHC compounds completely from renewable sources is a great option that fits in the contemporary context of a circular bioeconomy and complying with climate protection regulations. Various families of such compounds were presented and understood from the operational point of view. Nevertheless, the information presented in this review is certainly evolving with time, so the reader is advised to stay updated on new innovative technologies from the researchers in this field. From the options presented in Table 4, some represent a striking opportunity for valorization of previously non-valorized biomass sources, like lignocellulosic residues in the case of phenazine synthesis from lignin. This application attains the most attention because of its high hydrogen storage capacity, the comparable enthalpy of dehydrogenation as compared to the conventional LOHC and the robustness of the catalysts. However, other options on the table may require less energy in the pretreatment sections or may be more mature at the time, so their lower performance would turn out to be preferred. The understanding of the mechanistic and thermodynamic particularities of each system, including its catalyst, is a crucial task that will help in the development of more robust, efficient and cheap systems that allow for the deployment of the hydrogen bioeconomy that the LOHC are trying to accelerate. There is a large amount of literature on the liquid organic hydrogen carrier concept, but the biosourced molecules are only starting to grow and represent a significant part of the new research. Nevertheless, most of the information is up-to-date and represents the real state of the art of the technology, so researchers will not have a tough time understanding what the needs of this market are.
6. Conclusions
The LOHC technology currently has many constraints that will require technological enablers to be overcome. To achieve this, research and investment need to be conducted in a multidimensional manner, covering the H2-producing technology, the biomass conversion technologies, the catalyst development and even the carbon capture strategies. Finally:
- Hydrogen production technology needs to be optimized to achieve higher yields and production capacities. Moreover, the development of LOHC fuel cells that allow direct utilization of a LOHC system would allow performing the dehydrogenation reactions at room temperature by reducing the O2 species inside the cell.
- Catalysts play a central role in this application, as they guarantee the selectivity (and thus the purity of the hydrogen), but also the time-efficacy of the process. Understanding the mechanisms to increase the kinetics of the process will bring it closer to its industrial-scale application.
- “Circular” organic hydrogen carriers require efficient carbon dioxide capture technologies in order to provide the hydrogen-lean molecule for the pair, but also to guarantee its circularity and sustainability.
- Thorough research on natural molecules may lead science to isolate potential candidates with a higher performance than any known LOHC at the time. A very efficient example of this was obtained from the Amaryllidaceae alkaloids, from which only two were analyzed in the presented work, but more than 500 exist only in this plant species. This fact shows the research potential in this regard.
- Technological tools are becoming involved in human personal and professional decisions since their calculation capacity is higher than ours. This is why it is recommended to use modeling software to determine potential candidates from biomass sources or biomass transformation that fulfill all LOHC criteria, reducing significantly the innovation time this (or any) topic is currently having. An example of this approach is presented in the work published by Paragian et al. [5], where they evaluated over 1 million molecules in a database and obtained 37 LOHC candidates at the end by conditioning their characteristics like minimum hydrogen storage capacity and dehydrogenation enthalpies, among others.
Author Contributions
Writing—original draft preparation, N.A.B.A. and V.M.; writing—review and editing, N.A.B.A. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work has been conducted in the context of the “BioRef” Erasmus Mundus master’s degree at the University of Lille. The authors gratefully extend their sincere appreciation to the academic staff among the consortium members for their guidance and support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)–Assessment based on chemical and economic properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Modisha, P.; Bessarabov, D. Aromatic liquid organic hydrogen carriers for hydrogen storage and release. Curr. Opin. Green Sustain. Chem. 2023, 42, 100820. [Google Scholar] [CrossRef]
- Li, L.; Vellayani Aravind, P.; Woudstra, T.; van den Broek, M. Assessing the waste heat recovery potential of liquid organic hydrogen carrier chains. Energy Convers. Manag. 2023, 276, 116555. [Google Scholar] [CrossRef]
- Valentini, F.; Marrocchi, A.; Vaccaro, L. Liquid Organic Hydrogen Carriers (LOHCs) as H-Source for Bio-Derived Fuels and Additives Production. Adv. Energy Mater. 2022, 12, 2103362. [Google Scholar] [CrossRef]
- Paragian, K.; Li, B.; Massino, M.; Rangarajan, S. A computational workflow to discover novel liquid organic hydrogen carriers and their dehydrogenation routes. Mol. Syst. Des. Eng. 2020, 5, 1658–1670. [Google Scholar] [CrossRef]
- Rao, P.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, Y.; Kim, K.; Lee, U. Implementation of Formic Acid as a Liquid Organic Hydrogen Carrier (LOHC): Techno-Economic Analysis and Life Cycle Assessment of Formic Acid Produced via CO2 Utilization. Catalysts 2022, 12, 1113. [Google Scholar] [CrossRef]
- Preuster, P.; Albert, J. Biogenic Formic Acid as a Green Hydrogen Carrier. Energy Technol. 2018, 6, 501–509. [Google Scholar] [CrossRef]
- Shen, F.; Smith Jr., R.; Li, J.; Guo, H.; Zhang, X.; Qi, X. Critical assessment of reaction pathways for conversion of agricultural waste biomass into formic acid. Green Chem. 2021, 23, 1536–1561. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Wu, J. Sustainable production of formic acid from biomass and carbon dioxide. Mol. Catal. 2020, 483, 110716. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Qi, M.; Wu, J.; Gözaydın, G.; Yan, N.; Zhong, H.; Jin, F. Room temperature, near-quantitative conversion of glucose into formic acid. Green Chem. 2019, 21, 6089–6096. [Google Scholar] [CrossRef]
- Sahoo, P.; Zhang, T.; Das, S. Oxidative Transformation of Biomass into Formic Acid. Eur. J. Org. Chem. 2021, 2021, 1331–1343. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2016, 2, 188–195. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, J.; Ma, X.; Huang, Y.; Li, Q.; Qiu, H. An effective low Pd-loading catalyst for hydrogen generation from formic acid. Int. J. Hydrogen Energy 2017, 42, 18375–18382. [Google Scholar] [CrossRef]
- Zhong, H.; Iguchi, M.; Chatterjee, M.; Himeda, Y.; Xu, Q.; Kawanami, H. Formic Acid-Based Liquid Organic Hydrogen Carrier System with Heterogeneous Catalysts. Adv. Sustain. Syst. 2018, 2, 1700161. [Google Scholar] [CrossRef]
- Sahin, S.; Bayazit, S.S.; Bilgin, M.; Inci, I. Investigation of Formic Acid Separation from Aqueous Solution by Reactive Extraction: Effects of Extractant and Diluent. J. Chem. Eng. Data 2010, 55, 1519–1522. [Google Scholar] [CrossRef]
- Mungma, N.; Kienberger, M.; Siebenhofer, M. Reactive Extraction of Lactic Acid, Formic Acid and Acetic Acid from Aqueous Solutions with Tri-n-octylamine/1-Octanol/n-Undecane. ChemEngineering 2019, 3, 43. [Google Scholar] [CrossRef]
- Zeidan, H.; Marti, M.E. Selective and efficient separation of levulinic, acetic and formic acids from multi-acid solutions by adjusting process parameters. J. Water Process Eng. 2023, 56, 104299. [Google Scholar] [CrossRef]
- Karunanithi, S.; e, P.; Kapoor, A.; Delfino, P. Separation of Carboxylic Acids from Aqueous Solutions using Hollow Fiber Membrane Contactors. J. Membr. Sci. Res. 2019, 5, 233–239. [Google Scholar] [CrossRef]
- Garg, N.; Sarkar, A.; Sundararaju, B. Recent developments on methanol as liquid organic hydrogen carrier in transfer hydrogenation reactions. Coord. Chem. Rev. 2021, 433, 213728. [Google Scholar] [CrossRef]
- Gautam, P.; Neha; Upadhyay, S.; Dubey, S. Bio-methanol as a renewable fuel from waste biomass: Current trends and future perspective. Fuel 2020, 273, 117783. [Google Scholar] [CrossRef]
- Zentou, H.; Abidin, Z.Z.; Yunus, R.; Awang Biak, D.R.; Korelskiy, D. Overview of Alternative Ethanol Removal Techniques for Enhancing Bioethanol Recovery from Fermentation Broth. Processes 2019, 7, 458. [Google Scholar] [CrossRef]
- Feng, W.; Ji, P.; Chen, B.; Zheng, D. Analysis of Methanol Production from Biomass Gasification. Chem. Eng. Technol. 2011, 34, 307–317. [Google Scholar] [CrossRef]
- Shamsul, N.; Kamarudin, S.; Rahman, N.; Kofli, N. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33, 578–588. [Google Scholar] [CrossRef]
- Moioli, E.; Schildhauer, T. Eco-Techno-Economic Analysis of Methanol Production from Biogas and Power-to-X. Ind. Eng. Chem. Res. 2022, 61, 7335–7348. [Google Scholar] [CrossRef]
- Ferlin, F.; Valentini, F.; Marrocchi, A.; Vaccaro, L. Catalytic Biomass Upgrading Exploiting Liquid Organic Hydrogen Carriers (LOHCs). ACS Sustain. Chem. Eng. 2021, 9, 9604–9624. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, Q.; Zhao, D.; Cao, T.; Sha, H.; Zhang, C.; Song, H.; Da, Z. Ammonia as hydrogen carrier: Advances in ammonia decomposition catalysts for promising hydrogen production. Renew. Sustain. Energy Rev. 2022, 169, 112918. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Babson, D.; Bellman, K.; Prakash, S.; Fennell, D. Anaerobic digestion for methane generation and ammonia reforming for hydrogen production: A thermodynamic energy balance of a model system to demonstrate net energy feasibility. Biomass Bioenergy 2013, 56, 493–505. [Google Scholar] [CrossRef]
- Papadias, D.; Peng, J.K.; Ahluwalia, R. Hydrogen carriers: Production, transmission, decomposition, and storage. Int. J. Hydrogen Energy 2021, 46, 24169–24189. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrogen Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Łukasik, N.; Wysocka, I.; Rogala, A.; Gębicki, J. Recent Progress on Hydrogen Storage and Production Using Chemical Hydrogen Carriers. Energies 2022, 15, 4964. [Google Scholar] [CrossRef]
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 2021, 58, 55–77. [Google Scholar] [CrossRef]
- Yadav, V.; Sivakumar, G.; Gupta, V.; Balaraman, E. Recent Advances in Liquid Organic Hydrogen Carriers: An Alcohol-Based Hydrogen Economy. ACS Catal. 2021, 11, 14712–14726. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56. [Google Scholar] [CrossRef]
- Gamage, J.; Lam, H.; Zhang, Z. Bioethanol Production from Lignocellulosic Biomass, A Review. J. Biobased Mater. Bioenergy 2010, 4, 3–11. [Google Scholar] [CrossRef]
- Rajeswari, S.; Baskaran, D.; Saravanan, P.; Rajasimman, M.; Rajamohan, N.; Vasseghian, Y. Production of ethanol from biomass–Recent research, scientometric review and future perspectives. Fuel 2022, 317, 123448. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Sharma, Y.; Kumar, A.; Prasad, R.; Upadhyay, S. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Tran, B.; Johnson, S.; Brooks, K.; Autrey, S. Ethanol as a Liquid Organic Hydrogen Carrier for Seasonal Microgrid Application: Catalysis, Theory, and Engineering Feasibility. ACS Sustain. Chem. Eng. 2021, 9, 7130–7138. [Google Scholar] [CrossRef]
- Gao, D.; Feng, Y.; Yin, H.; Wang, A.; Jiang, T. Coupling reaction between ethanol dehydrogenation and maleic anhydride hydrogenation catalyzed by Cu/Al2O3, Cu/ZrO2, and Cu/ZnO catalysts. Chem. Eng. J. 2013, 233, 349–359. [Google Scholar] [CrossRef]
- Santacesaria, E.; Carotenuto, G.; Tesser, R.; Di Serio, M. Ethanol dehydrogenation to ethyl acetate by using copper and copper chromite catalysts. Chem. Eng. J. 2012, 179, 209–220. [Google Scholar] [CrossRef]
- Rana, J.; Sahoo, S.; Daw, P. Homogeneous first-row transition metal catalyst for sustainable hydrogen production and organic transformation from methanol, formic acid, and bio-alcohols. Tetrahedron 2021, 99, 132473. [Google Scholar] [CrossRef]
- Schubert, T. Production routes of advanced renewable C1 to C4 alcohols as biofuel components—A review. Biofuels Bioprod. Biorefining 2020, 14, 845–878. [Google Scholar] [CrossRef]
- Abo, B.; Gao, M.; Wang, Y.; Wu, C.; Wang, Q.; Ma, H. Production of butanol from biomass: Recent advances and future prospects. Environ. Sci. Pollut. Res. 2019, 26, 20164–20182. [Google Scholar] [CrossRef]
- Verevkin, S.P.; Siewert, R.; Pimerzin, A.A. Furfuryl alcohol as a potential liquid organic hydrogen carrier (LOHC): Thermochemical and computational study. Fuel 2020, 266, 117067. [Google Scholar] [CrossRef]
- D’ambra, F.; Faucheux, V.; Nicolas, E.; Silva Costa, R. Nouveaux Liquides Organiques Porteurs D’hydrogene, Leurs Utilisations Pour Le Transport Et Le Stockage D’hydrogene, Et Les Procedes De Generation D’hydrogene Les Utilisant. Patent WO2023006956, 2 February 2023. [Google Scholar]
- Jardim, I.N.; Oliveira, D.F.; Silva, G.H.; Campos, V.P.; de Souza, P.E. (E)-cinnamaldehyde from the essential oil of Cinnamomum cassia controls Meloidogyne incognita in soybean plants. J. Pest Sci. 2018, 91, 479–487. [Google Scholar] [CrossRef]
- Buck, K.T.; Boeing, A.J.; Dolfini, J.E. Method of Producing Benzaldehyde. Patent US4673766A, 16 June 1987. [Google Scholar]
- Ma, L.; Liu, X.; Liang, J.; Zhang, Z. Biotransformations of cinnamaldehyde, cinnamic acid and acetophenone with Mucor. World J. Microbiol. Biotechnol. 2011, 27, 2133–2137. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Chen, Z. Production of C2–C4 diols from renewable bioresources: New metabolic pathways and metabolic engineering strategies. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.L.; Zeng, A.P. Present state and perspective of downstream processing of biologically produced 1,3-propanediol and 2,3-butanediol. Appl. Microbiol. Biotechnol. 2008, 78, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Issariyakul, T.; Dalai, A. Biodiesel from vegetable oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Tomishige, K.; Nakagawa, Y.; Tamura, M. Production of Diols from Biomass. In Production of Platform Chemicals from Sustainable Resources; Springer: Singapore, 2017; pp. 343–373. [Google Scholar] [CrossRef]
- Pang, J.; Zheng, M.; Sun, R.; Song, L.; Wang, A.; Wang, X.; Zhang, T. Catalytic conversion of cellulosic biomass to ethylene glycol: Effects of inorganic impurities in biomass. Bioresour. Technol. 2015, 175, 424–429. [Google Scholar] [CrossRef] [PubMed]
- te Molder, T.; Kersten, S.; Lange, J.P.; Ruiz, M.P. Ethylene Glycol from Lignocellulosic Biomass: Impact of Lignin on Catalytic Hydrogenolysis. Ind. Eng. Chem. Res. 2021, 60, 7043–7049. [Google Scholar] [CrossRef]
- Chappaz, A.; Bengaouer, A. Use of Hydrogenated Organic Liquids, in Particular in Devices for Converting Energy. Patent EP3725738A1, 21 October 2020. [Google Scholar]
- Onoda, M.; Nagano, Y.; Fujita, K.i. Iridium-catalyzed dehydrogenative lactonization of 1,4-butanediol and reversal hydrogenation: New hydrogen storage system using cheap organic resources. Int. J. Hydrogen Energy 2019, 44, 28514–28520. [Google Scholar] [CrossRef]
- Mevawala, C.; Autrey, T.; Brooks, K.; Bowden, M.; Tran, B.; Müller, K. 1,4-Butanediol as a Hydrogen Carrier: Liquid- versus Gas-Phase Dehydrogenation. Energy Fuels 2022, 37, 560–566. [Google Scholar] [CrossRef]
- Hwang, D.W.; Kashinathan, P.; Lee, J.M.; Lee, J.H.; Lee, U.h.; Hwang, J.S.; Hwang, Y.K.; Chang, J.S. Production of γ-butyrolactone from biomass-derived 1,4-butanediol over novel copper-silica nanocomposite. Green Chem. 2011, 13, 1672–1675. [Google Scholar] [CrossRef]
- Nagaiah, P.; Venkat Rao, M.; Thirupathaiah, K.; Venkateshwarlu, V.; David Raju, B.; Rama Rao, K.S. Selective vapour phase dehydrogenation of biomass-derived 1,4-butanediol to gamma butyrolactone over Cu/ZrO2 catalysts: Influence of La2O3 promotor. Res. Chem. Intermed. 2018, 44, 5817–5831. [Google Scholar] [CrossRef]
- Patil, K.N.; Prasad, D.; Manoorkar, V.K.; Bhanushali, J.T.; Jadhav, A.H.; Nagaraja, B.M. Selective vapour-phase dehydrocyclization of biomass-derived 1,4-butanediol to γ-butyrolactone over Cu/ZnAl2O4-CeO2 catalyst. J. Ind. Eng. Chem. 2022, 106, 142–151. [Google Scholar] [CrossRef]
- Alagy, J.; Trambouze, P.J.L. Procede et Systeme de Recuperation d’Energie Pour Dispositif Consommateur de Dihydrogene. Patent FR3097218A1, 18 December 2020. [Google Scholar]
- Al-Auda, Z.; Li, X.; Hohn, K.L. Dehydrogenation of 2,3-Butanediol to Acetoin Using Copper Catalysts. Ind. Eng. Chem. Res. 2022, 61, 3530–3538. [Google Scholar] [CrossRef]
- Zou, Y.Q.; Von Wolff, N.; Anaby, A.; Xie, Y.; Milstein, D. Ethylene glycol as an efficient and reversible liquid-organic hydrogen carrier. Nat. Catal. 2019, 2, 415–422. [Google Scholar] [CrossRef]
- Zhou, Q.Q.; Zou, Y.Q.; Ben-David, Y.; Milstein, D. A Reversible Liquid-to-Liquid Organic Hydrogen Carrier System Based on Ethylene Glycol and Ethanol. Chem. Eur. J. 2020, 26, 15487–15490. [Google Scholar] [CrossRef]
- Gupta, N.; Reif, P.; Palenicek, P.; Rose, M. Toward Renewable Amines: Recent Advances in the Catalytic Amination of Biomass-Derived Oxygenates. ACS Catal. 2022, 12, 10400–10440. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, H.; Shi, F. Sustainable Catalytic Amination of Diols: From Cycloamination to Monoamination. ACS Sustain. Chem. Eng. 2018, 6, 1061–1067. [Google Scholar] [CrossRef]
- Müller, K. Acceptorless Dehydrogenation of Amines to Nitriles for Hydrogen Storage: Reality or Wishful Thinking? Energy Technol. 2022, 10, 2200468. [Google Scholar] [CrossRef]
- Grellier, M.; Sabo-Etienne, S. New Perspectives in Hydrogen Storage Based on RCH2NH2/RCN Couples. Dalton Trans. 2014, 43, 6283–6286. [Google Scholar] [CrossRef] [PubMed]
- Verevkin, S.; Konnova, M.; Zherikova, K.; Pimerzin, A. Sustainable hydrogen storage: Thermochemistry of amino-alcohols as seminal liquid organic hydrogen carriers. J. Chem. Thermodyn. 2021, 163, 106591. [Google Scholar] [CrossRef]
- Verevkin, S.; Andreeva, I.; Konnova, M.; Portnova, S.; Zherikova, K.; Pimerzin, A. Paving the way to the sustainable hydrogen storage: Thermochemistry of amino-alcohols as precursors for liquid organic hydrogen carriers. J. Chem. Thermodyn. 2021, 163, 106610. [Google Scholar] [CrossRef]
- Hu, P.; Fogler, E.; Diskin-Posner, Y.; Iron, M.; Milstein, D. A novel liquid organic hydrogen carrier system based on catalytic peptide formation and hydrogenation. Nat. Commun. 2015, 6, 6859. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R. Nitrogen-Containing Liquid Organic Hydrogen Carriers: Progress and Prospects. ACS Sustain. Chem. Eng. 2017, 5, 4491–4498. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Accounts Chem. Res. 2016, 50, 74–85. [Google Scholar] [CrossRef]
- Brayton, D.F.; Jensen, C.M. Dehydrogenation of pyrrolidine based liquid organic hydrogen carriers by an iridium pincer catalyst, an isothermal kinetic study. Int. J. Hydrogen Energy 2015, 40, 16266–16270. [Google Scholar] [CrossRef]
- Ortiz-Cervantes, C.; Flores-Alamo, M.; García, J. Synthesis of pyrrolidones and quinolines from the known biomass feedstock levulinic acid and amines. Tetrahedron Lett. 2016, 57, 766–771. [Google Scholar] [CrossRef]
- Tang, C.; Fei, S.; Lin, G.D.; Liu, Y. Natural liquid organic hydrogen carrier with low dehydrogenation energy: A first principles study. Int. J. Hydrogen Energy 2020, 45, 32089–32097. [Google Scholar] [CrossRef]
- Forberg, D.; Schwob, T.; Zaheer, M.; Friedrich, M.; Miyajima, N.; Kempe, R. Single-catalyst high-weight% hydrogen storage in an N-heterocycle synthesized from lignin hydrogenolysis products and ammonia. Nat. Commun. 2016, 7, 13201. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Janes, T.; Espinosa-Jalapa, N.; Milstein, D. Selective Hydrogenation of Cyclic Imides to Diols and Amines and Its Application in the Development of a Liquid Organic Hydrogen Carrier. J. Am. Chem. Soc. 2018, 140, 7453–7457. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.S.; Ahmad Alwi, M.M.; Saleem, J.; Al-Odail, F.; Basu, A.; Mozahar Hossain, M. Recent Advances in Anode Electrocatalysts for Direct Formic Acid Fuel Cell-II-Platinum-Based Catalysts. Chem. Rec. 2022, 22, e202200156. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.S.; Saleem, J.; Mudassir Ahmad Alwi, M.; Al-Odail, F.A.; Mozahar Hossain, M. Recent Advances in Anode Electrocatalysts for Direct Formic Acid Fuel Cells–Part I–Fundamentals and Pd Based Catalysts. Chem. Rec. 2022, 22, e202200045. [Google Scholar] [CrossRef] [PubMed]
- Brodt, M.; Müller, K.; Kerres, J.; Katsounaros, I.; Mayrhofer, K.; Preuster, P.; Wasserscheid, P.; Thiele, S. The 2-Propanol Fuel Cell: A Review from the Perspective of a Hydrogen Energy Economy. Energy Technol. 2021, 9, 2100164. [Google Scholar] [CrossRef]
- Hauenstein, P.; Mangoufis-Giasin, I.; Seeberger, D.; Wasserscheid, P.; Mayrhofer, K.J.J.; Katsounaros, I.; Thiele, S. Impact of Catalyst Loading, Ionomer Content, and Carbon Support on the Performance of Direct Isopropanol Fuel Cells. J. Power Sources Adv. 2021, 10, 100064. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy–Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Dong, Z.; Mukhtar, A.; Lin, H. Heterogeneous Catalysis on Liquid Organic Hydrogen Carriers. Top. Catal. 2021, 64, 481–508. [Google Scholar] [CrossRef]
- Mevawala, C.; Brooks, K.; Bowden, M.; Breunig, H.; Tran, B.; Gutiérrez, O.; Autrey, T.; Müller, K. The Ethanol–Ethyl Acetate System as a Biogenic Hydrogen Carrier. Energy Technol. 2022, 11, 2200892. [Google Scholar] [CrossRef]
- Ventura-Espinosa, D.; Marzá-Beltrán, A.; Mata, J. Catalytic Hydrogen Production by Ruthenium Complexes from the Conversion of Primary Amines to Nitriles: Potential Application as a Liquid Organic Hydrogen Carrier. Chem. Eur. J. 2016, 22, 17758–17766. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).