Abstract
Swallowing is a complex neuromuscular activity regulated by the autonomic central nervous system, and impairment can lead to dysphagia, which is difficulty in swallowing. This research presents a novel approach that utilizes wireless, wearable technology for the continuous mechano-acoustic tracking of respiratory activities and swallowing. To address the challenge of accurately tracking swallowing amidst potential confounding activities or significant body movements, we employ two accelerometers. These accelerometers help distinguish between genuine swallowing events and other activities. By monitoring movements and vibrations through the skin surface, the developed device enables non-intrusive monitoring of swallowing dynamics and respiratory patterns. Our focus is on the development of both the wireless skin-interfaced device and an advanced algorithm capable of detecting swallowing dynamics in conjunction with respiratory phases. The device and algorithm demonstrate robustness in detecting respiratory patterns and swallowing instances, even in scenarios where users exhibit periodic movements due to disease or daily activities. Furthermore, peak detection using an adaptive threshold automatically adjusts to an individual’s signal strength, facilitating the detection of swallowing signals without the need for individual adjustments. This innovation has significant potential for enhancing patient training and rehabilitation programs aimed at addressing dysphagia and related respiratory issues.
1. Introduction
Swallowing, deemed a paramount function essential for human survival, represents a remarkably intricate synergy of rapid and interdependent movements, meticulously orchestrated by sensory end organs situated within the oral cavity, pharynx, and larynx [1,2]. These orchestrated movements, far from being mere mechanical actions, serve the dual purpose of safeguarding the delicate airway from the perils of aspiration while simultaneously propelling ingested material, commonly referred to as bolus, throughout the convoluted maze of the upper aerodigestive tract [3,4]. The profound significance of swallowing function cannot be overstated, particularly in light of the staggering number of adults afflicted by various forms of dysphagia, stemming from a plethora of neurologic conditions, head and neck cancers, as well as gastrointestinal and respiratory diseases [5,6,7]. Moreover, the spectrum of diseases or conditions that impair the central nervous system’s intricate mechanisms governing swallowing or directly affront peripheral structures of swallowing movements can result in a spectrum of swallowing difficulties, ranging from minor inconveniences to life-threatening impediments [8,9]. Dysphagia, in its multifaceted manifestations, may encompass alterations in sensory perception that precipitate delays in the initiation of swallowing movements, diminishing muscle strength, as well as compromising the range and coordination of the temporal aspects of these movements [10,11]. Furthermore, the already intricate control and execution of swallowing are further compounded by the intricacies of coordinating breathing patterns with swallowing initiation. This synchronization is indispensable as the pharyngeal cavity, serving dual roles in both respiration and swallowing, seamlessly transitions from a state of patency during breathing to facilitate the smooth passage of air, to a state of tight compression during swallowing, thereby generating substantial positive pressures requisite for the efficient clearance of the bolus through the upper aerodigestive tract. The intricate interplay between these physiological processes underscores the complexity of the human body’s mechanisms, necessitating comprehensive approaches to address the multifaceted challenges posed by dysphagia and to optimize swallowing function and quality of life for affected individuals [12].
The coordination between breathing and swallowing is vital for the safe passage of food and liquids through the throat. In healthy adults, swallowing typically aligns with a pause in breathing, ensuring airway protection and efficient swallowing. This synchronized coordination involves various physiological mechanisms that aid in bolus transit and clearance [13,14]. However, individuals with dysphagia experience disruptions in this coordination, leading to swallowing difficulties and reduced quality of life. Traditional dysphagia treatments focus on strengthening throat muscles, but often yield incomplete results [15]. Recent innovations explore training individuals to swallow during specific phases of breathing, showing promising outcomes in some cases [16]. However, challenges remain in accurately detecting swallowing events, ensuring effective intervention, and maintaining long-term improvements, highlighting the complexity of swallowing rehabilitation.
Against the backdrop of burgeoning research efforts aimed at enhancing dysphagia management, there arises a growing imperative for the development of user-centric monitoring solutions, particularly those harnessing the capabilities of wireless technologies. The advent of such technologies holds immense promise in liberating dysphagia patients from the constraints of traditional monitoring setups, affording them the freedom to engage in daily activities without tethering them to specific locations. The biometric vibration signal collected through devices attached to the body can be used to track breathing and swallowing. These devices can be utilized by clinical therapists to aid in the swallowing training of patients with dysphagia [17]. This has the potential to alleviate the psychological burden and stress associated with the management of dysphagia, thereby enhancing overall quality of life [18,19]. Furthermore, as dysphagia patients acclimate to utilizing home-based monitoring devices, an intriguing phenomenon emerges wherein initial attempts at swallowing may be characterized by rigid postures. However, with gradual adaptation and increased familiarity with the monitoring apparatus, these rigidities often give way to more naturalistic swallowing movements, necessitating the development of sophisticated algorithms capable of discerning and accurately capturing these nuanced variations in swallowing patterns amidst varying activities. Hence, against this multifaceted backdrop, this study embarks on an exploration of not only the theoretical underpinnings but also the practical implementation of a cutting-edge monitoring device adept at capturing swallowing patterns in dynamic, real-world scenarios. Through meticulous experimentation conducted across diverse subject profiles, this research endeavors to shed light on the efficacy and reliability of the proposed monitoring solution, offering valuable insights poised to inform future advancements in the realm of dysphagia management and rehabilitation.
The fundamental concepts underlying mechano-acoustic measurements of bodily processes, employing soft, skin-mounted devices positioned near the neck, have been extensively discussed [20,21,22,23]. In summary, a broad-spectrum inertial measurement unit (IMU) captures surface motions, encompassing vibrational patterns linked to vocalizations and cardiac activity, as well as overall body movements associated with activities like walking and jumping. The resultant data streams encompass a rich array of information, amenable to analysis through digital filtering and machine learning methodologies [24,25,26,27,28]. Recent advancements have highlighted the efficacy of mechano-acoustic (MA) devices incorporating multiple IMUs for differential detection, amplifying their capacity to monitor physiological parameters, particularly those stemming from cardiopulmonary dynamics, even in settings susceptible to substantial motion-induced artifacts [23,28,29,30,31].
This paper introduces the MA sensor meticulously engineered for the real-time monitoring of both swallowing and breathing processes, underscoring the increasingly pivotal role of education and training initiatives tailored to individuals grappling with dysphagia. The innovative system capitalizes on the integration of dual IMUs strategically arranged in a differential configuration and affixed to specific anatomical locations such as the neck and upper chest. This configuration serves to not only amplify the robustness of the captured signals but also effectively counteract any potential distortions introduced by motion artifacts, thereby ensuring the fidelity of the acquired data.
2. Materials and Methods
To diagnose and train dysphagia accurately, it is crucial to monitor the breathing cycle and swallowing process simultaneously. In this section, we explore the methodology for capturing signals associated with breathing and swallowing within the body while mitigating the effects of overall body movement, achieved through using two accelerometers. Subsequently, we analyze the experimental outcomes. Following the assessment of signals in a less motion-affected location, the results are validated through data from experiments conducted based on predefined scenarios for the test subjects. The device employed in these experiments is identical to the one referenced in [29]. In contrast to previous studies, which utilized a single IMU, this study employs two IMUs to elucidate the process of eliminating routine motion using differential signals.
2.1. Physical Analysis of Differential Signals
The wireless wearable device incorporates two accelerometers, one located near the laryngeal prominence (LP) and the other on the sternal manubrium (SM), to capture biometric vibration signals. During body movement, both accelerometers measure acceleration. While walking or experiencing overall body shaking, the acceleration value may be affected. However, since both accelerometers are impacted, the utilization of a differential signal, derived from the variance between the signals detected by the two accelerometers, enables the removal of acceleration attributed to overall movement.
Figure 1 shows a schematic diagram of body changes and the location of the accelerometer during the exhalation phase of breathing. The acceleration utilized in Figure 1 is the acceleration perpendicular to the attachment surface of the device. In Figure 1a, the change in sternum position corresponding to lung volume alteration during exhalation is depicted. During normal exhalation, lung volume decreases, causing the angle between the SM and near the LP to diminish. The gray dotted line represents the SM’s normal position, while the solid black line depicts its position during exhalation. Figure 1b presents a schematic diagram replicating the SM’s position from Figure 1a. Here, let us denote the angle between LP and SM as and estimate the actual acceleration measured by the accelerometer. Both accelerometers gauge acceleration resulting from overall body movement and the angle θ between them. If the acceleration in LP is denoted as and the acceleration in SM as , the differential signal, i.e., the difference between the two signals, can be expressed as Equation (1).
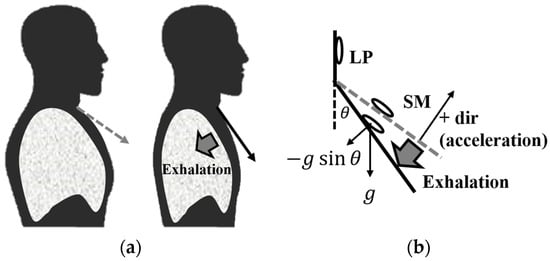
Figure 1.
Physical changes during the exhalation phase of respiration. (a) Schematic diagram of sternal manubrium (SM) changes; (b) schematic diagram with vectors.
Therefore, upon obtaining the differential signal, the accelerometer measures , influenced by external forces (), gravitational acceleration, and the angle . During exhalation, as the angle decreases, the value also decreases, resulting in a decrease in the measured acceleration value. Conversely, during inhalation, as increases, the value also increases.
2.2. Location of Device Attachment to Acquire Biometric Vibration Signal
The objective of this study was to effectively monitor swallowing and breathing activities amidst movement. Therefore, an experiment was conducted involving swallowing food while walking. It was observed that depending on the location to which the device was attached, there was a significant variance in the obtained biometric signal levels related to swallowing activity. To capture signals that best represented both swallowing and respiratory cycles, an experiment was designed, focusing on a location near the neck that was closely associated with both actions.
The experimental scenario entailed the subject taking 10 deep breaths, swallowing saliva three times, holding a glass of water, and sequentially swallowing water three to four times. Dots were marked at 1 cm intervals on the subject’s neck to denote positions, and data were recorded at each position. The 4th position corresponded to the placement of both accelerometers on the sternum, with subsequent positions indicating upward movement at 1 cm intervals.
Figure 2 shows the data collected at each location. The blue solid line represents the measurements from the upper accelerometer, while the red solid line represents those from the lower accelerometer, positioned approximately 2 cm below the upper accelerometer. The solid yellow line illustrates the differential signal computed from the signals obtained by the two accelerometers. Black arrows indicate instances of saliva or simulated swallowing, while red arrows denote actual swallowing actions, such as drinking water.
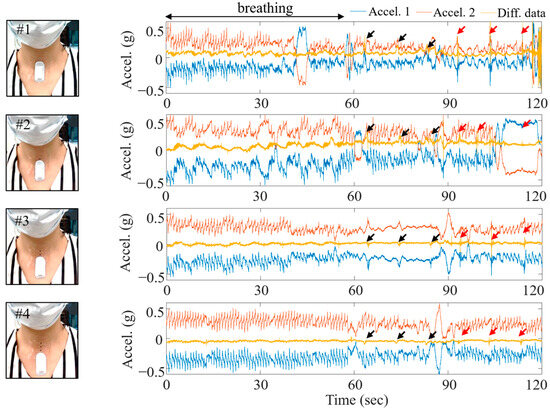
Figure 2.
Locations of the attached wireless measurement device and the signals acquired at each location.
The experiment was conducted at a brisk walking pace, occurring one step per second. All transient vibrations between the ground and the foot were recorded from accelerometers 1 and 2. However, the transient signal due to the walking was eliminated in the differential signal, leaving only the breathing and swallowing signals in the yellow line. Although the breathing signal was most accurately captured at the #2 position out of the four, the swallowing signal was weak, and external activity (walking) was not completely eliminated. In the case of position #3, it demonstrated clear removal of external disturbance signals due to walking compared to position #2, while also providing clear swallowing signals. In position #4, where both accelerometers are located on the SM, the sternum movement during breathing resulted in no angle difference between the two accelerations, as described in Equation (1).
In experiments involving multiple individuals, the device was attached between positions #2 and #3, depending on the subject, for conducting experiments. Consequently, the upper accelerometer is positioned near the LP, while the lower accelerometer is placed near the SM.
2.3. Data Collection
To verify the effectiveness of tracking swallowing and breathing despite movement, an experiment was conducted involving healthy subjects. Table 1 presents details regarding the participants and the experiment. The ages of the eight participants ranged from 20 to 60 s, with an equal distribution between males and females. The experiment had a duration of approximately 25 min. Following the presentation of a scenario involving swallowing along with various types of actions, data acquisition commenced upon receiving a cue signal.

Table 1.
Subject information.
2.3.1. Protocol for Swallowing Test Scenario
To detect swallowing in daily life, a behavioral protocol involving various activities was assigned to the participants in the experiment (Table 2). First, a saliva swallowing activity that is similar to actual swallowing occurs. Afterward, the subjects were asked to perform seven types of swallowing: swallowing saliva, sitting still with the least external movement, swallowing while rocking the body back and forth, swallowing while rocking the body left and right, swallowing while walking, swallowing while riding a stationary bicycle, and swallowing while lightly running. Afterward, for various chaotic activities, the subjects were asked to talk with the device attached, tap or rub the device to imitate a situation where a blanket or clothing touches the device, and move the body forward, backward, and left and right. If coughing occurred during the experimental protocol, the time of the specific action was recorded, and the protocol continued as planned. Additionally, participants chewed gum that produced vibrations in the jaw and neck, distinct from swallowing. There might be unplanned swallowing due to saliva accumulation, prompted by inadvertent swallowing during confounding activities or chewing gum. Each participant executed the protocol, marking the time of each swallow using a mobile phone app. For accuracy, a test supervisor also independently recorded the time of each observed swallow. Thus, the accuracy of the final detection results was assessed by comparing the event marker times recorded by the subjects and time stamps by the supervisor.

Table 2.
Scenario of swallowing detection test.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Northwestern University Institutional Review Board in Chicago, IL, USA (STU00212981 for UMA device validation studies). In each study, a medical-grade double-sided silicone acrylate adhesive (3 M, 2477 p) was used to affix the sensor to the neck region. The silicone layer of the adhesive ensured a soft contact with the skin, enabling wear periods of up to 12 h without any risk of detachment.
2.3.2. Detection Algorithm
An adaptive threshold-based peak detection algorithm was utilized to identify swallowing events within the swallowing signals. This algorithm adjusted the threshold value dynamically, with signal averaging performed over a temporal window of 7 s. Swallowing was recognized when the signal’s amplitude surpassed this threshold. However, signals exceeding a magnitude of 0.08 g were categorized as non-swallowing events, such as coughs or physical impacts, as detailed in the confounding actions outlined in Table 2. This adaptive threshold approach effectively accommodated variations in individual subject behavior and behavioral changes.
3. Results and Discussion
An experimental scenario was conducted to determine whether swallowing signals can be detected in swallowing activities accompanied by various external movements that can occur in daily life and pathological symptoms. In this section, the acquired signal from the experimental scenario was analyzed, and the accuracy of swallowing detection was assessed in each situation. Additionally, for comparison with previous studies, the results obtained with one accelerometer were examined with those obtained using two accelerometers.
3.1. Differential Signal with Motion Removed
The method for capturing signals associated with swallowing was developed using two accelerometers. External movement was eliminated and vibration transmitted from within was confirmed. Figure 3a is a signal acquired four times during swallowing when the body was swaying side to side. The light blue and red solid lines are signals acquired from each accelerometer (IMU1, IMU2). The wave moving once every two seconds is observed due to the body movement of swaying. The black solid line indicates the differential signal which is the difference between the two accelerometer signals. The waveform in which the body sways side to side is removed from the original accelerometer signal. Additionally, the differential signal has a more distinct internal biometric signal caused by swallowing and heartbeat.
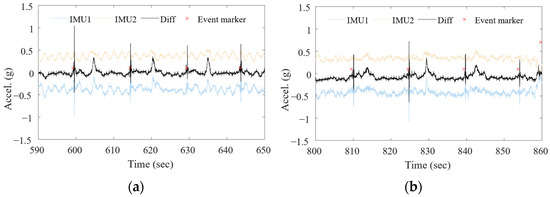
Figure 3.
Differential sensing for measurement of swallowing during physical activities. (a) Swallowing while body swaying side to side; (b) swallowing while walking.
Figure 3b is the waveform acquired when swallowing while walking. It was confirmed that changes due to body movement while walking were observed in the signals acquired from each accelerometer, but the walking-related signals were eliminated in the differential signal represented by the black solid line. Additionally, swallowing signals were also observed more clearly.
3.2. Experimental Results
Eight healthy normal subjects were recruited, generating a total of 65 swallows each for seven types of swallowing. The experiment was conducted according to the protocol sequence described in Table 2. In Figure 4a, the gray line is the differential signal from two accelerometers, and the black line is the bandpass-filtered signal with 90–120 Hz. The red dot is an event detected as swallowing using the adaptive threshold described in the previous section, and the purple cross is the time of the event marker checked by the subject and the experimental supervisor. True positives were determined and the accuracy of the algorithm was calculated by comparing the swallowing detected by the algorithm with the time recorded in the event marker. Notably, accounting for human reaction speed, any swallowing signal detected by the algorithm within a time frame of ±1 s from the subject-recorded event marker was adjudged as a true positive.
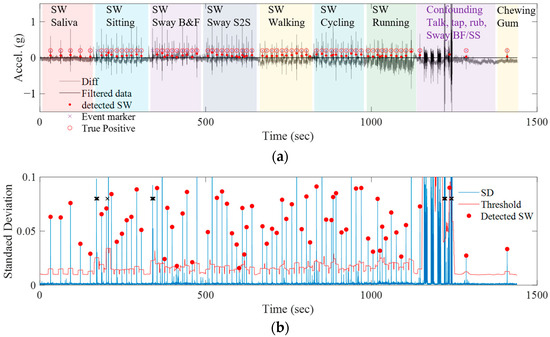
Figure 4.
(a) Detection result of swallowing events during 7 types of activities and confounding motions; (b) standard deviation (SD) of the filtered signal and the adaptive thresholds for detecting the swallowing events.
Figure 4b shows the standard deviation calculated in a 0.05 s window using the filtered signal. The blue line is the standard deviation (SD), the red line is the adaptive threshold, and the red dot is the detected swallowing (SW) by the algorithm. Swallowing was mostly detected in each activity during the scenario that lasted until about 1100 s. In the confounding phase that takes place after 1100 s, the SD increases significantly, and the adaptive threshold also increases accordingly. Additionally, although there was no swallowing scenario in the protocol after 1200 s, there were cases where the subject swallowed a large amount of saliva accumulated in the mouth while speaking or chewing gum. The swallowing event was recorded by the subject in the event marker system, and also detected as swallowing in the algorithm.
Two accelerometers were used to develop a device and algorithm that can demonstrate robust performance even when accompanied by movements of the entire body other than swallowing. To compare the results with previous studies, the results of detecting swallowing using one accelerometer were compared with the results calculated using two accelerometers. Table 3 shows the results of the analysis using signals acquired from one of the two accelerometers for each swallowing type, and Table 4 shows the results of the analysis using both accelerometers. ‘Saliva’ means swallowing saliva, ‘Sitting’ indicates swallowing while sitting, ‘B and F’ indicates swallowing while the body is swaying back and forth, ‘S2S’ indicates swallowing while the body is swaying side to side, ‘Walking’ indicates swallowing while walking, ‘Cycling’ indicates swallowing while cycling, and ‘Running’ indicates swallowing while running. In Table 3, for swallowing saliva, the precision is 48.9 and the sensitivity is 55, which is very low. The swallowed saliva is difficult to classify as swallowing when the amount of liquid is small. However, in the results in Table 4 using two accelerometers, saliva swallowing is more accurately classified as swallowing than when only one accelerometer is used. Except for swallowing in body movement from side to side, all values show an improvement of about 5% points. In the case of food intake without any special events, the dominant situation is that swallowing is performed while sitting still in a chair. Even in this case, detecting swallowing was improved when two accelerometers were used rather than a single accelerometer.

Table 3.
Experimental results using one accelerometer.

Table 4.
Experimental results using two accelerometers.
4. Conclusions and Discussion
The accurate diagnosis and effective training of dysphagia necessitate the simultaneous monitoring of both the breathing cycle and swallowing process. Through the methodology explored in this study involving the capture of signals associated with swallowing within the body while mitigating the impact of overall body movement, significant advancements have been achieved. By utilizing two accelerometers in the wireless wearable device, differential signals were effectively employed to eliminate routine motion, enabling the isolation of signals pertinent to swallowing activities. Experimental outcomes validated the efficacy of this approach, particularly in scenarios involving swallowing activities amidst movement, such as walking and swaying the body. The location of the device attachment played a crucial role in acquiring biometric vibration signals accurately, with optimal positions identified near the neck, closely associated with both the swallowing and respiratory cycles. Furthermore, the detection algorithm, employing an adaptive threshold-based peak detection approach, demonstrated robust performance across various activities, ensuring accurate identification of swallowing events. The comparison between results obtained using one accelerometer versus two accelerometers highlighted the superiority of using two accelerometers, showcasing improved precision and sensitivity in classifying swallowing events. However, there were limitations in the experimental scenarios utilized within this study. The focus was primarily on detecting swallowing and the respiratory phase during physical activities, consequently restricting food intake to the use of a cup. Notably, the study excluded the participation of elderly subjects and did not assess their swallowing ability through non-instrumental methods, which may restrict the generalizability of the findings across all potential clinical scenarios. This selection bias highlights an area for further research to extend the applicability of the technological solutions developed in this study to a broader range of dysphagic patients and conditions. Overall, this study presents a comprehensive framework for reliable monitoring and detection of swallowing activities, even in dynamic environments, laying the groundwork for enhanced dysphagia diagnosis and therapeutic interventions.
Funding
This research was supported by the 2023 scientific promotion program funded by Jeju National University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Northwestern University Institutional Review Board, Chicago, IL, USA (STU00212981 for UMA device validation studies).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to concerns for the privacy of the subjects.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Matsuo, K.; Palmer, J.B. Anatomy and physiology of feeding and swallowing: Normal and abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef]
- Logemann, J.A. Swallowing disorders. Best Pract. Res. Clin. Gastroenterol. 2007, 21, 563–573. [Google Scholar] [CrossRef]
- Bhattacharyya, N. The prevalence of dysphagia among adults in the United States. Otolaryngol. Head Neck Surg. 2014, 151, 765–769. [Google Scholar] [CrossRef]
- Panebianco, M.; Marchese-Ragona, R.; Masiero, S.; Restivo, D.A. Dysphagia in neurological diseases: A literature review. Neurol. Sci. 2020, 41, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; Abbass, M.A.; Soliman, R.K.; Zaki, A.F.; Gamea, A. Post-stroke dysphagia: Frequency, risk factors, and topographic representation: Hospital-based study. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57, 1–8. [Google Scholar] [CrossRef]
- Krebbers, I.; Simon, S.R.; Pilz, W.; Kremer, B.; Winkens, B.; Baijens, L.W. Patients with head-and-neck cancer: Dysphagia and affective symptoms. Folia Phoniatr. Et Logop. 2021, 73, 308–315. [Google Scholar] [CrossRef]
- Hsiang, C.-C.; Chen, A.W.-G.; Chen, C.-H.; Chen, M.-K. Early postoperative oral exercise improves swallowing function among patients with oral cavity cancer: A randomized controlled trial. Ear Nose Throat J. 2019, 98, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J. Neurophysiological basis of swallowing. Dysphagia 1986, 1, 91–100. [Google Scholar] [CrossRef]
- Gallois, Y.; Neveu, F.; Gabas, M.; Cormary, X.; Gaillard, P.; Verin, E.; Speyer, R.; Woisard, V. Can swallowing cerebral neurophysiology be evaluated during ecological food intake conditions? A Systematic Literature Review. J. Clin. Med. 2022, 11, 5480. [Google Scholar] [CrossRef]
- Hao, N.; Sasa, A.; Kulvanich, S.; Nakajima, Y.; Nagoya, K.; Magara, J.; Tsujimura, T.; Inoue, M. Coordination of respiration, swallowing, and chewing in healthy young adults. Front. Physiol. 2021, 12, 696071. [Google Scholar] [CrossRef]
- Curtis, J.A.; Troche, M.S. Effects of verbal cueing on respiratory-swallow patterning, lung volume initiation, and swallow apnea duration in Parkinson’s disease. Dysphagia 2020, 35, 460–470. [Google Scholar] [CrossRef]
- Hopkins-Rossabi, T.; Curtis, P.; Temenak, M.; Miller, C.; Martin-Harris, B. Respiratory phase and lung volume patterns during swallowing in healthy adults: A systematic review and meta-analysis. J. Speech Lang. Hear. Res. 2019, 62, 868–882. [Google Scholar] [CrossRef]
- Hopkins-Rossabi, T.; Rowe, M.; McGrattan, K.; Rossabi, S.; Martin-Harris, B. Respiratory–swallow training methods: Accuracy of automated detection of swallow onset, respiratory phase, lung volume at swallow onset, and real-time performance feedback tested in healthy adults. Am. J. Speech Lang. Pathol. 2020, 29, 1012–1021. [Google Scholar] [CrossRef]
- Krishnan, G.; Goswami, S.P.; Rangarathnam, B. A systematic review of the influence of bolus characteristics on respiratory measures in healthy swallowing. Dysphagia 2020, 35, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Pauloski, B.R.; Colangelo, L.; Lazarus, C.; Fujiu, M.; Kahrilas, P.J. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J. Speech Lang. Hear. Res. 1995, 38, 556–563. [Google Scholar] [CrossRef]
- Ghannouchi, I.; Speyer, R.; Doma, K.; Cordier, R.; Verin, E. Swallowing function and chronic respiratory diseases: Systematic review. Respir. Med. 2016, 117, 54–64. [Google Scholar] [CrossRef]
- Kang, Y.J.; Arafa, H.M.; Yoo, J.-Y.; Kantarcigil, C.; Kim, J.-T.; Jeong, H.; Yoo, S.; Oh, S.; Kim, J.; Wu, C.; et al. Soft skin-interfaced mechano-acoustic sensors for real-time monitoring and patient feedback on respiratory and swallowing biomechanics. NPJ Digit. Med. 2022, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Shune, S.E.; Namasivayam-MacDonald, A. Dysphagia-related caregiver burden: Moving beyond the physiological impairment. Perspect. ASHA Spec. Interest Groups 2020, 5, 1282–1289. [Google Scholar] [CrossRef]
- Pizzorni, N. Social and psychologic impact of dysphagia. In Dysphagia: Diagnosis and Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 873–886. [Google Scholar]
- Chun, K.S.; Kang, Y.J.; Lee, J.Y.; Nguyen, M.; Lee, B.; Lee, R.; Jo, H.H.; Allen, E.; Chen, H.; Kim, J.; et al. A skin-conformable wireless sensor to objectively quantify symptoms of pruritus. Sci. Adv. 2021, 7, eabf9405. [Google Scholar] [CrossRef]
- Liu, Y.; Norton, J.J.S.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.-I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2016, 2, e1601185. [Google Scholar] [CrossRef]
- Lee, K.; Ni, X.; Lee, J.Y.; Arafa, H.; Pe, D.J.; Xu, S.; Avila, R.; Irie, M.; Lee, J.H.; Easterlin, R.L.; et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 2020, 4, 148–158. [Google Scholar] [CrossRef]
- Jeong, H.; Kwak, S.S.; Sohn, S.; Lee, J.Y.; Lee, Y.J.; O’brien, M.K.; Park, Y.; Avila, R.; Kim, J.-T.; Yoo, J.-Y.; et al. Miniaturized wireless, skin-integrated sensor networks for quantifying full-body movement behaviors and vital signs in infants. Proc. Natl. Acad. Sci. USA 2021, 118, e2104925118. [Google Scholar] [CrossRef]
- Atallah, L.; Lo, B.; King, R.; Yang, G.-Z. Sensor positioning for activity recognition using wearable accelerometers. IEEE Trans. Biomed. Circuits Syst. 2011, 5, 320–329. [Google Scholar] [CrossRef]
- Patel, S.; Park, H.; Bonato, P.; Chan, L.; Rodgers, M. A review of wearable sensors and systems with application in rehabilitation. J. Neuroeng. Rehabil. 2012, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, Y.; Coyle, J.L.; Sejdić, E. Non-invasive identification of swallows via deep learning in high resolution cervical auscultation recordings. Sci. Rep. 2020, 10, 8704. [Google Scholar] [CrossRef]
- Chung, H.U.; Rwei, A.Y.; Hourlier-Fargette, A.; Xu, S.; Lee, K.; Dunne, E.C.; Xie, Z.; Liu, C.; Carlini, A.; Rogers, J.A.; et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 2020, 26, 418–429. [Google Scholar] [CrossRef]
- Ni, X.; Ouyang, W.; Jeong, H.; Kim, J.-T.; Tzavelis, A.; Mirzazadeh, A.; Wu, C.; Lee, J.Y.; Keller, M.; Mummidisetty, C.K.; et al. Automated, multiparametric monitoring of respiratory biomarkers and vital signs in clinical and home settings for COVID-19 patients. Proc. Natl. Acad. Sci. USA 2021, 118, e2026610118. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.Y.; Lee, K.; Kang, Y.J.; Kim, J.-T.; Avila, R.; Tzavelis, A.; Kim, J.; Ryu, H.; Kwak, S.S.; et al. Differential cardiopulmonary monitoring system for artifact-canceled physiological tracking of athletes, workers, and COVID-19 patients. Sci. Adv. 2021, 7, eabg3092. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, A.; Sikeridis, D.; Antonakopoulos, T. Wearable smart health advisors: An IMU-enabled posture monitor. IEEE Cons. Elec. Mag. 2020, 9, 20–27. [Google Scholar] [CrossRef]
- Monaco, V.; Giustinoni, C.; Ciapetti, T.; Maselli, A.; Stefanini, C. Assessing Respiratory Activity by Using IMUs: Modeling and Validation. Sensors 2022, 22, 2185. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).