Abstract
The gut microbiota greatly influences host physiology, including immune regulation, metabolic balance, and brain health. Aging is associated with alterations in the gut microbiome, including reduced microbial diversity and increased pro-inflammatory bacteria, which are linked to age-related decline and chronic diseases. This review examines the impact of the gut microbiota on key indicators of aging, including cellular senescence, mitochondrial dysfunction, alterations in gene expression, and immune system modifications. It also examines microbiome-related diseases associated with aging, including neurodegeneration, cardiovascular issues, metabolic syndrome, and frailty. Additionally, it highlights evidence-based methods to restore a youthful microbial profile. New findings suggest that certain microbial substances, including short-chain fatty acids, urolithins, and bile acids, play a role in regulating inflammation, maintaining barrier integrity, and influencing metabolism. Age-related diseases are often associated with molecular pathways driven by an imbalance in the gut microbiome. Various intervention strategies, from dietary changes and probiotics to personalized nutrition and fecal microbiota transplantation, have shown promise in reversing signs of microbial aging and improving health outcomes in both lab and human studies. Overall, the gut microbiome serves as both a marker and a regulator of healthy aging. Treatments that restore microbial balance offer hopeful ways to extend healthy living. Future studies should focus on developing long-term, multifaceted, and personalized methods to identify causal pathways and enhance microbiota-based strategies for various aging populations.
1. Introduction
The global population is aging at an unprecedented rate, with those aged 60 and above expected to exceed 2 billion by 2050 [1]. While this reflects remarkable advances in medicine and public health, it also presents complex challenges. Aging often leads to a gradual decline in physical and cognitive function, increased susceptibility to chronic illnesses, and a heightened risk of frailty and multimorbidity [2,3]. Yet, not all aging follows the same trajectory. The concept of “healthy aging” has shifted focus from merely extending lifespan to enhancing health span—the period of life spent in good health and functional independence [4].
In recent years, gut microbiota has emerged as a potent modulator of aging processes [5]. This vast ecosystem, comprising bacteria, archaea, fungi, viruses, and protozoa, plays a central role in human physiology [6]. It influences nutrient metabolism, immune regulation, epithelial barrier integrity, and even brain function [7,8]. Aging is typically accompanied by notable shifts in microbial composition and function, often driven by factors such as diet, medication use, changes in the immune system, and comorbid conditions [9]. A typical pattern includes reduced microbial diversity, loss of beneficial taxa such as Bifidobacterium and Akkermansia, and enrichment of pro-inflammatory microbes [10]. These microbial changes are increasingly linked to a phenomenon known as “inflammaging”, a state of persistent low-grade inflammation that underlies many age-related conditions [11]. Meta-analyses involving over 21,000 individuals found that overall diversity does not always predict healthy aging; instead, the emergence of unique, individualized microbial profiles—reflected in metrics like Kendall uniqueness—may be more informative [12].
Notably, centenarians often display microbial patterns enriched in butyrate-producing bacteria (Ruthenibacterium lactatiformans, Intestinimonas butyriciproducens, Butyricimonas virosa, Eubacterium ramulus, and Bacteroides fragilis) [13]. This suggests that specific microbial configurations may confer resilience and delay the onset of aging-related decline.
The gut microbiota exerts its effects through several biological mechanisms. It produces short-chain fatty acids (SCFAs), such as butyrate and acetate, which enhance gut barrier function, reduce inflammation [14]. On the other hand, dysbiosis may lead to the production of harmful compounds, such as trimethylamine N-oxide (TMAO) and phenylacetic acid, which lead to the development of cardiovascular and neurological diseases [15]. Microbial metabolites also influence the gut–brain axis, modulating blood–brain barrier integrity and neuroinflammation, and potentially playing roles in conditions such as Alzheimer’s and Parkinson’s [16].
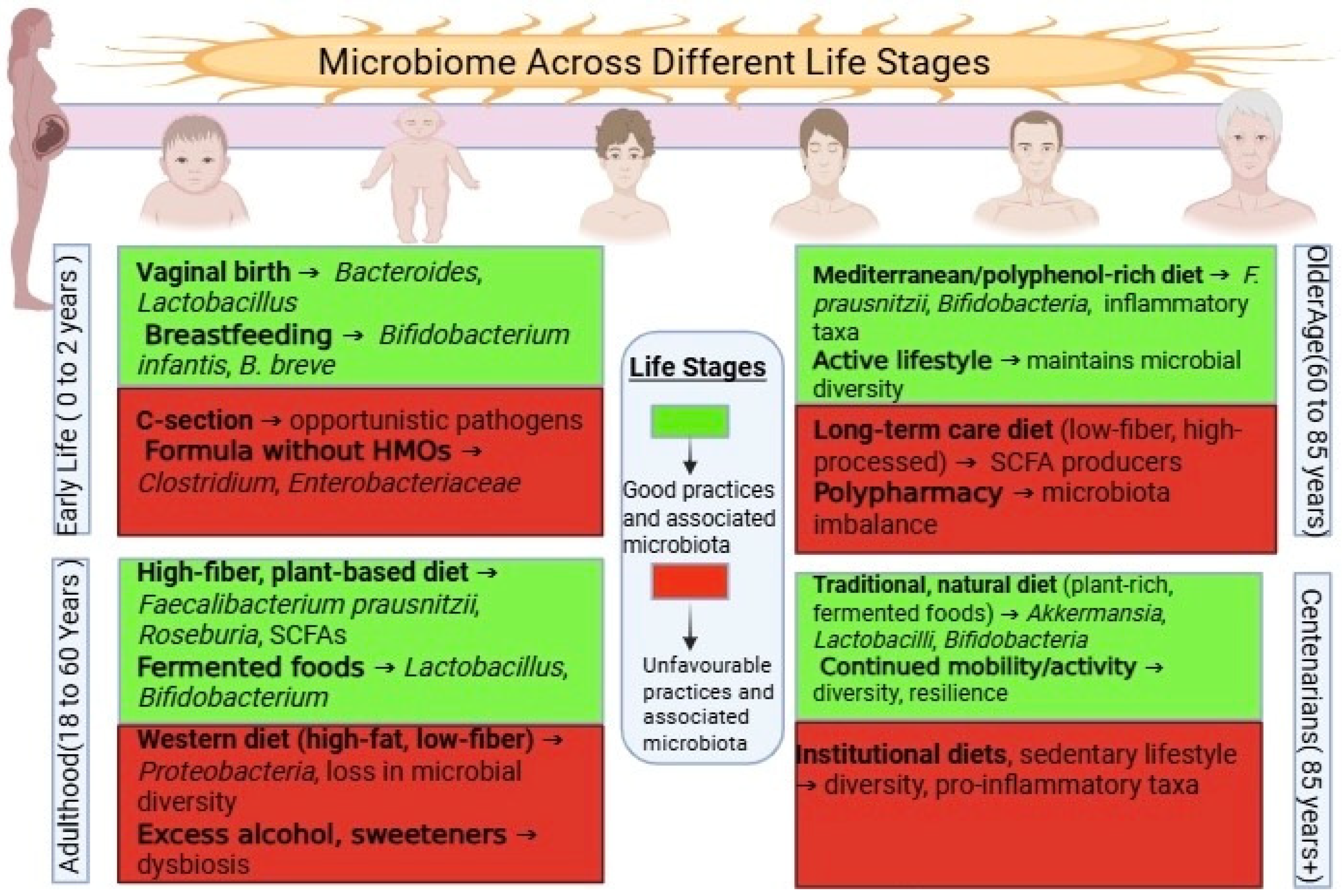
Immune regulation is particularly relevant in later life. As immune function declines—a process known as immunosenescence—the microbiota helps modulate T-regulatory cells and cytokine production, potentially delaying immune aging [17]. Additionally, early-life microbial acquisition, particularly through vaginal birth and breastfeeding, lays the foundation for lifelong health, as illustrated in Figure 1 [18].
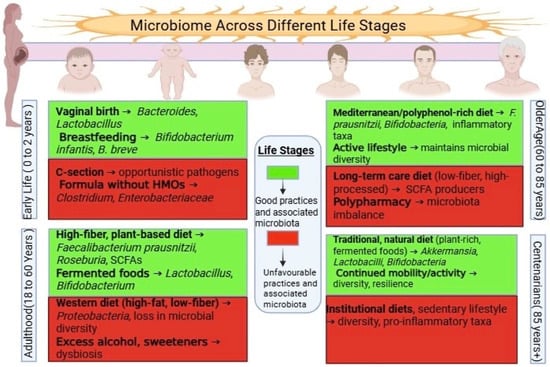
Figure 1.
Lifespan Dynamics of the Gut Microbiota. This image illustrates the changes in composition and diversity of the gut microbiota from infancy to old age. Early life is marked by rapid microbial assembly and increasing diversity, adulthood by relative stability, and older age by reduced diversity and a shift toward pro-inflammatory taxa, all of which influence health outcomes.
An emerging body of research supports the use of microbiome-based interventions, including fiber-rich diets, probiotics, postbiotics, and fecal microbiota transplantation (FMT) [19]. Lifestyle factors, such as physical activity and reduced stress, also display beneficial effects [20]. However, in older adults—especially those in institutional care—unmet needs such as poor nutrition or social isolation may intensify microbial decline and health deterioration [21], as illustrated in Figure 1.
2. Microbiome Dynamics Across the Lifespan
The human gut microbiome is not static—it is a highly dynamic and responsive ecosystem that evolves across the lifespan. From birth to the later decades of life, the gut microbiota continuously adapts to shifts in host biology, environment, diet, and lifestyle, as illustrated in Figure 1. Understanding how the gut microbiome develops, stabilizes, and transforms over time is crucial for revealing its role in both maintaining health and age-related disease risk.
2.1. Early Life and Microbiome Assembly
Our microbial life begins at birth, and early events during this stage have long-lasting effects on health. The mode of delivery is one of the first key factors shaping the infant’s gut. Babies born via vaginal delivery are exposed to beneficial maternal microbes, particularly species such as Lactobacillus and Bacteroides [22]. On the other hand, infants delivered by cesarean section often acquire skin-associated bacteria, including Staphylococcus, resulting in reduced microbial diversity [22,23]. Feeding practices further shape microbial communities—breast milk not only provides essential nutrients but also delivers beneficial microbes, notably Bifidobacterium [18].
This early colonization is not just about microbial presence—it plays an essential role in immune training and metabolic programming [24,25]. Factors such as antibiotic exposure, infection, or malnutrition during infancy can disrupt microbiota development, setting the stage for increased susceptibility to chronic diseases, including allergies, metabolic disorders, and autoimmune conditions, later in life [26].
By the age of 2 to 3 years, the gut microbiome typically matures into a more stable, adult-like structure. At this point, diversity increases and is known to support gut barrier integrity and regulate inflammation [27].
2.2. Microbiome Stability in Adulthood
Once mature, the adult microbiome tends to be relatively stable under normal conditions, acting as a metabolic and immunological ally. The dominant bacterial phyla—Firmicutes and Bacteroidetes—maintain a balance that reflects long-term dietary and lifestyle patterns [28]. A fiber-rich, plant-based diet helps sustain SCFA-producing bacteria, while high-fat, low-fiber diets may disrupt this equilibrium [29].
In adulthood, the gut microbiota supports a wide range of physiological functions, including nutrient absorption, antimicrobial protection, and immune modulation [7]. However, stability does not mean rigidity. The microbiota remains sensitive to external disruptions—stress, illness, antibiotic use, and abrupt dietary changes can lead to dysbiosis. Fortunately, a well-resilient gut ecosystem, backed by microbial redundancy and immune competence, can often recover [30].
2.3. Microbial Shifts in Older Age
Aging leads to physiological changes that alter the intestinal environment, including reduced gut motility, increased intestinal permeability, altered bile acid profiles, and a weakened immune system. These changes, in turn, affect the composition and function of the gut microbiota [31].
Older adults commonly exhibit a decline in microbial diversity, accompanied by a loss of beneficial genera, such as Bifidobacterium and Faecalibacterium, and an increase in potentially harmful taxa such as Enterobacteriaceae [32]. This shift might contribute to low-grade chronic inflammation—or “inflammaging”—which has been linked to frailty, metabolic disorders, and cognitive decline [32].
Another notable age-related shift is the reduction in SCFA production [33]. SCFAs, such as butyrate, are essential for maintaining gut barrier integrity and regulating immune responses [34]. A decrease in their availability can compromise gut function and lead to increased systemic exposure to microbial toxins [35,36].
It is essential to recognize that these changes are highly individual [37]. Factors such as diet, medication, underlying health conditions, and even living arrangements (e.g., institutional care) can either accelerate or moderate microbial decline [38]. This variability highlights the potential for modifiable lifestyle and clinical interventions to support microbial health in the aging population [39].
2.4. Centenarians and Microbial Resilience
Centenarians represent an exceptional model of successful aging. Despite their advanced age, many retain functional independence and demonstrate unique gut microbial profiles. Their microbiomes are often enriched in groups such as Akkermansiaceae, Lactobacillaceae, and Christensenellaceae [40], which are associated with maintaining metabolic balance, regulating the immune system, and exhibiting anti-inflammatory activity [41].
These findings suggest that microbial deterioration with age is not an inevitable process. Instead, specific microbial configurations may actively contribute to resilience and longevity, supporting the idea that promoting a healthy gut microbiota could enhance quality of life in later years [41].
2.5. Insights from Longitudinal Animal Models
Animal models, especially those observed in the wild, offer additional insights. For instance, a long-term study of wild Seychelles warblers revealed that microbial diversity and composition remained largely stable across the adult lifespan [42]. This contrasts with findings in humans and laboratory animals, suggesting that lifestyle factors—such as diet, physical activity, and exposure to environmental microbes—may play a larger role in shaping the gut microbiome than chronological age alone [39].
These observations suggest that age-related microbial decline may be, to a large extent, a consequence of modern living conditions—such as processed diets, reduced environmental exposure, sedentary behavior, and medical interventions—rather than age itself. This raises the possibility that microbiome health in older adults could be preserved or even improved through targeted lifestyle and environmental modifications [43].
3. Microbiome–Hallmarks of Aging Interface
The aging process is characterized by progressive physiological deterioration, driven by complex interactions among genetic, metabolic, immunological, and environmental factors. Among the key drivers of this process are the nine hallmarks of aging originally proposed by López-Otín et al., which have since been expanded to include additional mechanisms such as inflammaging, impaired mitophagy, and gut microbial dysbiosis. Recent research increasingly highlights the gut microbiome as a critical modulator of many of these hallmarks. These multifaceted interactions are illustrated in Figure 2, which maps key microbial metabolites—such as SCFAs and bile acids—to the major hallmarks of aging, including senescence, mitochondrial dysfunction, and inflammaging (38). As such, the microbiome is not only considered an additional hallmark of aging but also an upstream regulator influencing several canonical aging pathways [44].
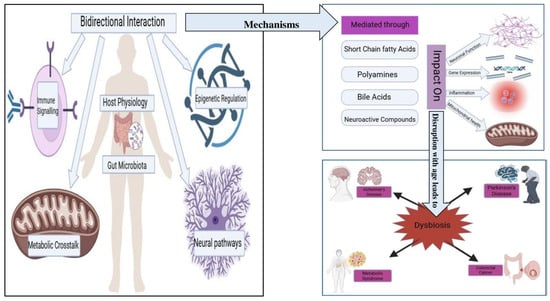
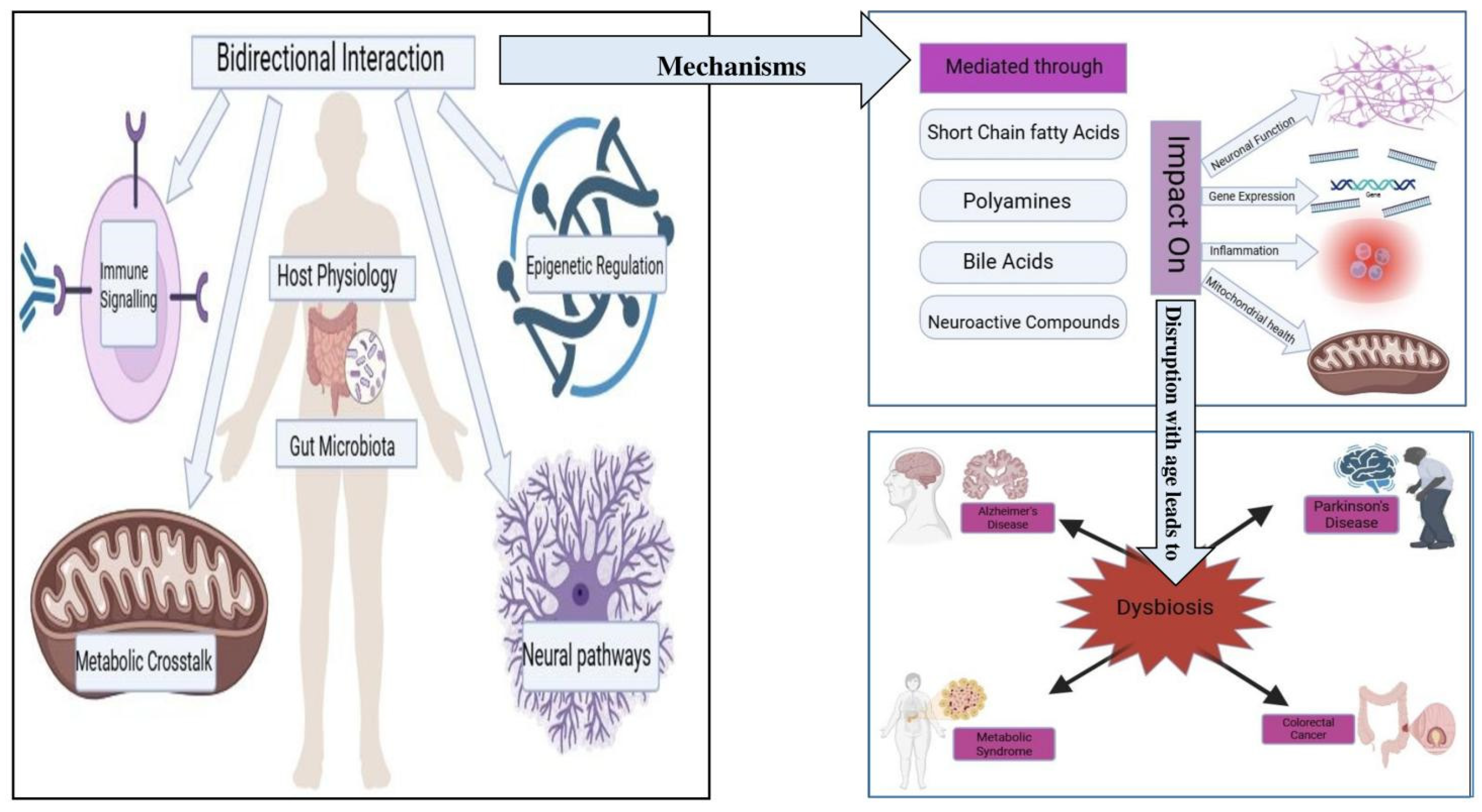
Figure 2.
Microbiome–Hallmarks of Aging Interface. A schematic overview showing how gut microbial metabolites (such as short-chain fatty acids and bile acids) interact with key hallmarks of aging—including cellular senescence, mitochondrial dysfunction, inflammaging, and epigenetic regulation—thereby modulating the aging process and susceptibility to age-related diseases.
As illustrated in Figure 2, the bidirectional interactions between host physiology and the gut microbiota involve several mechanisms. These interactions are mediated through microbial metabolites, including SCFAs, polyamines, bile acids, and neuroactive compounds, which can influence gene expression, inflammation, mitochondrial health, and neuronal function [45]. Disruptions in microbiota composition with age—characterized by reduced diversity and overrepresentation of pro-inflammatory taxa—are linked to systemic dysfunction across these biological axes [46].
These microbial influences are particularly relevant in the context of age-related diseases such as Alzheimer’s, Parkinson’s, colorectal cancer, and metabolic syndrome [47], where dysbiosis contributes to disease progression via mechanisms including epigenetic dysregulation, increased gut permeability, mitochondrial dysfunction, and neuroinflammation [47,48].
This section examines how gut microbiota interfaces with five critical mechanisms of aging—senescence, mitophagy, inflammaging, epigenetic regulation, and neurocognitive decline—highlighting the microbial drivers and modulators of these pathways.
3.1. Senescence and SASP Modulation
3.1.1. Cellular Senescence and Its Role in Aging
As we age, our cells accumulate stress from environmental insults, DNA damage, and internal wear and tear. One typical cellular response to such stress is senescence—a state where cells stop dividing but remain metabolically active. While this process is protective in the short term, persistent senescence can become harmful. Senescent cells begin to secrete a mix of pro-inflammatory signals, enzymes, and growth factors collectively known as the senescence-associated secretory phenotype (SASP). This inflammatory environment disrupts tissue structure, promotes chronic inflammation, and fuels the progression of aging-related diseases [49].
3.1.2. Gut Microbiome Changes in Aging and Their Impact on Senescence
Recent evidence highlights a surprising but critical player in this process: the gut microbiome. In aging individuals, gut microbial diversity typically declines, beneficial SCFA-producing bacteria decrease, and inflammatory taxa, such as Enterobacteriaceae, increase [50]. These changes do not just reflect the aging process—they actively influence senescence and SASP pathways through direct molecular signaling [51].
3.1.3. Molecular Pathways Regulating SASP
At the heart of SASP regulation are transcription factors like Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), CCAAT/Enhancer Binding Protein beta (C/EBPβ), and GATA binding protein 4 (GATA4), which are activated by persistent DNA damage responses [52]. Age-associated mitochondrial dysfunction, often linked to gut dysbiosis, amplifies these stress signals, leading to overactivation of the p38 mitogen-activated protein kinase (p38 MAPK) and mammalian target of rapamycin (mTOR) pathways that drive SASP expression [53,54].
3.1.4. Microbial Metabolites Modulating SASP
Crucially, microbiota-derived metabolites influence these pathways. Butyrate, a key SCFA produced during fiber fermentation, acts as a histone deacetylase inhibitor (HDACi) [55]. It suppresses NF-κB activation and reduces SASP-related gene expression, and thus, this might help maintain a balanced inflammatory tone in aging tissues [56]. On the other hand, harmful microbial components, such as lipopolysaccharide (LPS), can leak from the gut during dysbiosis, activating Toll-like receptor 4 (TLR4) receptors on host cells and triggering SASP-like inflammatory responses even in otherwise healthy cells [57,58].
3.1.5. Cyclic GMP-AMP Synthase (cGAS)–Stimulator of Interferon Genes (STING) Pathway and Microbiome-Linked Inflammation
Another link is the cGAS–STING pathway, a cellular defense mechanism that detects misplaced DNA in the cytoplasm. In senescent cells, this includes chromatin fragments and even leaked mitochondrial DNA [59,60]. When cGAS binds these fragments, it activates STING, initiating interferon production and thereby intensifying SASP signaling [60,61]. Gut-derived mitochondrial stress and inflammation further amplify this cascade [62].
3.1.6. Microbiome Influence on Cellular Metabolism and Nicotinamide Adenine Dinucleotide (NAD+) Pathway
The microbiome also plays a role in cellular metabolism, primarily through the NAD+-pathway [63]. NAD+ levels decline with age, impairing the activity of sirtuins [64], which represses typical senescence by stabilizing mitochondrial health and regulating p53/p21 signaling [65]. Microbial balance influences the availability of NAD+ precursors [63], and enzymes like extracellular Nicotinamide Phosphoribosyltransferase (eNAMPT)—a component of SASP—are affected by microbial metabolites [66].
3.1.7. Tissue-Specific Effects of Senescence and Microbiome Dysbiosis
The real-world effects of these changes are visible in tissue-specific outcomes. In aging bone, for example, senescent osteoblasts secrete Interleukin-6 (IL-6) and Transforming Growth Factor-beta (TGF-β), tipping the balance toward bone loss [67]. Dysbiotic microbiota exacerbate this by impairing calcium metabolism and promoting systemic inflammation [67,68].
3.1.8. Immune Clearance of Senescent Cells and Microbiome Impact
Complicating matters, the immune system, which is responsible for clearing senescent cells, becomes less effective with age [69]. Natural killer (NK) cells and macrophages, which usually eliminate damaged or senescent cells [70], lose potency in aged individuals—especially under the influence of gut dysbiosis, which alters immune tone and reduces cytotoxic activity [70,71].
3.1.9. Senescence, Microbiome, and Cancer Risk
Senescent cells, when not cleared, accumulate and can even contribute to tumor formation [72]. A striking example is obesity-related dysbiosis, where microbial metabolites, such as deoxycholic acid, drive the production of SASP cytokines in liver tissue, promoting hepatocarcinogenesis [73].
3.1.10. Epigenetic Regulation of SASP by Microbial Signals
Epigenetic regulation further complicates the picture. Proteins such as high mobility group (HMG) proteins, including HMGB2 and HMGA1, influence the accessibility of SASP gene regions on chromatin. Their activity is sensitive to microbial signals such as butyrate and polyamines, linking the gut microbiota to long-term transcriptional control of senescence pathways [74].
3.1.11. Therapeutic Potential of Microbiome Modulation
Encouragingly, interventions that restore a healthy microbiota show potential for mitigating senescence and SASP [51]. Probiotics, prebiotics, and even FMT have been shown to reduce pro-inflammatory cytokines like IL-6 and Tumor Necrosis Factor Alpha (TNF-α) and improve tissue regeneration by rebalancing microbial communities and increasing SCFA levels [75].
3.2. Mitochondrial Health and Mitophagy
3.2.1. Mitochondrial Decline and Its Impact on Aging
Mitochondria are central to cellular health, acting as the cell’s energy hubs and regulators of metabolism, apoptosis, and oxidative stress [76]. As we age, mitochondrial function naturally declines—an issue compounded by reduced turnover of damaged mitochondria. This inefficiency is closely linked to age-related dysfunctions in the muscle, brain, and immune systems [77]. A key quality-control process, mitophagy, which eliminates defective mitochondria, is essential for maintaining mitochondrial integrity and cellular homeostasis [78]. And now, compelling research is uncovering the gut microbiome’s role in regulating this process—linking gut health to mitochondrial resilience [79].
3.2.2. Mechanisms of Mitophagy: The PTEN-Induced Kinase 1 (PINK1)–Parkin Pathway and Beyond
Mitophagy is primarily orchestrated through the PINK1–Parkin pathway [80]. Under healthy conditions, PINK1 is imported and degraded within the mitochondria. But when a mitochondrion is damaged, its membrane potential drops, leading to PINK1 accumulation on its surface. This signals Parkin, an E3 ubiquitin ligase, to tag outer mitochondrial membrane proteins for degradation, thereby triggering the recruitment of autophagosomes and mitochondrial clearance [81,82]. Additionally, Parkin-independent mitophagy also occurs via receptors such as BNIP3, NIX, and FUNDC1, which interact directly with the autophagic machinery [83].
3.2.3. Microbial Metabolites Enhancing Mitophagy
Significantly, this entire quality control process is influenced by microbial metabolites. For instance, SCFAs, particularly butyrate, are known to stimulate mitophagy by upregulating genes involved in autophagy and mitochondrial biogenesis, mainly through the Peroxisome Proliferator-activated Receptor Gamma Coactivator 1 Alpha (PGC-1α)–Silent Information Regulator 1 (SIRT1) signaling axis [84,85]. SCFAs also help balance NAD+/NADH levels, which are essential for activating sirtuins, thereby regulating mitochondrial turnover and longevity [63].
3.2.4. Gut Dysbiosis, Inflammation, and Impaired Mitophagy
On the other hand, gut dysbiosis has the opposite effect. A disrupted microbial community often leads to an overproduction of LPS, an endotoxin that compromises mitophagy by activating the NOD-like receptor (NLR) [86]. This increases mitochondrial reactive oxygen species (ROS), damages mitochondrial DNA, and inhibits mitophagic efficiency [87]. When this cycle becomes chronic, it contributes to the development of inflammaging and systemic mitochondrial decline [86,87,88].
3.2.5. Mitophagy Decline in Age-Related Muscle Wasting and Exercise Benefits
In tissues such as skeletal muscle, aging reduces the expression of mitophagy regulators, including PINK1, Parkin, and LC3, which contributes to sarcopenia, a condition characterized by age-related muscle wasting [89]. Exercise, especially endurance training, reverses this by increasing mitochondrial turnover, improving metabolic health, and also shifting the gut microbiome toward species that produce SCFAs [90].
3.2.6. Dietary and Pharmacological Modulation of Mitophagy
Dietary components and their interaction with the microbiome further influence mitophagy [91]. Urolithin A, a gut-derived metabolite from ellagitannins, enhances mitophagy and mitochondrial function by activating the AMP-activated protein kinase (AMPK) and Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) pathway- (AMPK–PGC-1α) pathway [92]. However, only specific bacteria can convert ellagitannins into bioactive urolithins, meaning individual microbiota composition determines therapeutic benefit [93]. Likewise, drugs such as metformin have been found to enhance mitophagy in human immune cells by increasing Parkin and Lysosome-Associated Membrane Protein (LAMP2) expression [94]. Caloric restriction, another proven longevity strategy, also induces mitophagy [95].
3.2.7. Mitophagy and Neurodegenerative Diseases
In the nervous system, impaired mitophagy is strongly associated with neurodegenerative diseases like Alzheimer’s and Parkinson’s [96]. In aged brain tissues, accumulation of phosphorylated ubiquitin indicates stalled mitophagy [97]. AD patients often exhibit reduced expression of genes such as Autophagy-Related Gene (ATG5), Parkin, and Unc-51-like Autophagy-Activating Kinase (ULK1) [98], suggesting a breakdown in mitochondrial maintenance and function. Gut dysbiosis further exacerbates this by reducing SCFA production and increasing the output of neuroinflammatory mediators [99].
3.2.8. Cardiac Mitophagy and Microbiome Interventions
In the heart, mitophagy regulates mitochondrial turnover, a process crucial for cardioprotection [100]. Mice lacking PINK1 or Parkin are more prone to cardiac injury [101]. Notably, probiotic and dietary strategies that restore microbial balance have been shown to improve AMPK signaling [102], and enhance mitochondrial quality in cardiac tissues [103].
3.2.9. Emerging Non-Canonical Mitophagy Pathways
Emerging research is also shedding light on non-canonical mitophagy. For example, mitochondrial-derived vesicles (MDVs) help isolate and remove damaged mitochondrial components [104], and microbial-derived lipid signals, such as oxidized cardiolipin, modulate their formation [105]. Another lesser-known pathway, megamitochondria formation, involves stress-induced mitochondrial fusion followed by internal degradation [106], and may be influenced by gut-related hypoxic signals.
3.2.10. Mitochondrial Dynamics: Fission, Fusion, and Microbial Influence
Fission and fusion dynamics are critical to mitophagy [107]. Proteins like Dynamin-Related Protein (DRP1) fragment damaged mitochondria into smaller units for degradation [108], while Parkin suppresses fusion proteins like Mitofusin 1 and 2 (MFN1/2) to prevent the reintegration of faulty mitochondria [109]. These processes are regulated by AMPK, which is in turn influenced by microbial activity and metabolite levels [110].
3.2.11. Coordination of Mitophagy and Mitochondrial Biogenesis
Finally, effective mitophagy must be coupled with mitochondrial biogenesis to ensure the constant renewal of mitochondrial populations [111]. The AMPK coordinates this PGC-1α–NRF1 axis [112], which may suggest a link between microbial health and mitochondrial rejuvenation.
3.3. Inflammaging and Immune System Rewiring
Aging is often accompanied by a subtle but persistent rise in inflammation—a phenomenon known as inflammaging. Unlike the acute inflammation that helps fight infections or heal wounds, inflammaging is a chronic, low-grade, and systemic process [113]. It is characterized by elevated levels of cytokines, such as IL-6, TNF-α, and IL-1β [114]. It is a major contributor to the development of age-related diseases, including Alzheimer’s, cardiovascular disorders, diabetes, and cancer [114]. While many factors contribute to this smoldering inflammation, the gut microbiota has emerged as a central orchestrator of immune remodeling with age [9].
3.3.1. Gut Microbiota and Immune Homeostasis in Youth
In youth, a balanced and diverse gut microbiota helps maintain immune homeostasis by supporting anti-inflammatory pathways and promoting the development of regulatory T cells (Tregs) [115]. These effects are primarily mediated by SCFAs, such as butyrate and propionate, which are produced through microbial fermentation of dietary fibers [116]. These SCFAs enhance the intestinal barrier, suppress NF-κB activation, and promote mucosal tolerance [117].
3.3.2. Age-Related Microbiome Shifts and Gut Barrier Dysfunction
With age, the composition of the gut microbiome shifts. Beneficial bacteria like Bifidobacterium and Faecalibacterium decline, while pro-inflammatory taxa—particularly from the Proteobacteria and Enterobacteriaceae families—increase [118]. This dysbiosis compromises the gut barrier, resulting in increased permeability, commonly referred to as a “leaky gut” [119]. As a result, bacterial products such as LPS can enter the bloodstream, where they activate Toll-like receptors (TLRs) and NLRs, triggering immune activation and perpetuating systemic inflammation [120].
3.3.3. Mucosal Immune Changes and Local-Global Spillover
The intestinal mucosa, the body’s largest immune organ, is among the first to exhibit age-related immune changes [121]. In older individuals, dendritic cells and macrophages within the gut shift toward a more pro-inflammatory phenotype. They become less efficient at sampling antigens and at inducing T regulatory cells (Tregs), thereby tipping the balance toward inflammation. Over time, this local immune dysregulation spills over into systemic immune compartments [122,123].
3.3.4. Monocyte and Macrophage Remodeling
Changes in the monocyte and macrophage populations are especially prominent in inflammaging [124]. Aging is associated with an expansion of CD14+CD16+ monocytes [125], which produce substantial amounts of IL-6 and TNF-α upon stimulation [126]. These monocytes are hyper-responsive to bacterial products, such as LPS [127], linking microbial shifts directly to immune overactivation. Additionally, harmful metabolites such as indoxyl sulfate and TMAO are produced by dysbiotic microbiota, thereby contributing to vascular inflammation [128,129].
3.3.5. T Cell Aging, Polyamines, and Microbial Influence
Aging also skews T cell homeostasis, with a decline in naïve T cells and an increase in senescent CD8+CD28− T cells, which are pro-inflammatory and less effective at immune surveillance [130]. Gut dysbiosis exacerbates this shift by reducing SCFA levels, which are essential for thymic function and T cell diversity [131,132]. Moreover, polyamines such as spermidine, produced by gut microbes, help sustain T cell mitochondrial function and autophagy; however, their levels decline with age [133,134].
3.3.6. The cGAS–STING Pathway and Amplification of Inflammation
Another pivotal pathway connecting microbial signals to inflammaging is the cGAS–STING axis [135]. This pathway detects cytosolic DNA, including leaked mitochondrial or bacterial DNA, and activates type I interferons and inflammatory cytokines [136]. In aging tissues, impaired mitophagy leads to increased mitochondrial DNA entering the cytoplasm, which activates the cGAS–STING pathway and amplifies SASP-like responses [137].
3.3.7. Neuroinflammation and the Gut–Brain–Immune Axis
The effects of inflammaging are not confined to the gut. In the brain, aging-associated microbial shifts correlate with increased microglial activation [138] and higher levels of IL-1β and TNF-α—hallmarks of neuroinflammation [139]. Animal studies show that transferring microbiota from young to old mice reduces inflammation and improves cognitive function and synaptic plasticity by modulating gut permeability [140].
3.3.8. Microbial Extracellular Vesicles (MEVs) and Systemic Inflammation
Another emerging factor is microbial extracellular vesicles (MEVs)—nano-sized packages secreted by gut microbes. MEVs from healthy microbes promote anti-inflammatory signaling and tissue repair [141], while those from dysbiotic communities intensify NF-κB activation and systemic inflammation [142]. With aging, the balance of MEV types shifts unfavorably, contributing to immune dysfunction in multiple organs [143].
3.3.9. B Cell Dysfunction and Age-Associated B Cells
B-cell function also deteriorates with age, with reduced antibody diversity and the emergence of age-associated B cells (ABCs), which produce inflammatory cytokines [144,145]. LPS from the gut microbiota can drive the expansion of these ABCs, further fueling immune aging [146].
3.3.10. Microbiome-Targeted Interventions to Counter Inflammaging
Encouragingly, interventions that target the gut microbiota can help reverse aspects of inflammaging [147]. Probiotics and prebiotics restore microbial diversity, increase SCFA production, and reduce inflammation in both animal models and elderly humans [148]. For instance, supplementation with Lactobacillus plantarum improved gut barrier integrity and reduced plasma IL-6 levels in aged mice [149], while Bifidobacterium breve enhanced Treg cell numbers and mucosal immune tolerance [150]. Dietary strategies, such as caloric restriction and intermittent fasting, also modulate the microbiome in favor of Akkermansia and SCFA-producing bacteria, leading to reduced systemic inflammation [151]. Likewise, polyphenols such as resveratrol have anti-inflammatory effects by reshaping the microbiome and inhibiting TLR and NLRP3 inflammasome signaling [152].
3.4. Epigenetic and Transcriptomic Regulation
3.4.1. Epigenetics: Beyond the Genetic Code
Aging is not only a story written in our genes but also in how those genes are expressed—or silenced—over time. This delicate orchestration is managed by epigenetic mechanisms [153], which involve reversible changes like DNA methylation, histone modifications, and non-coding RNA regulation [154]. Together, these processes shape the transcriptomic landscape, guiding how cells respond to internal and external stimuli [153]. Recent evidence shows that the gut microbiome plays a surprising yet crucial role in influencing these epigenetic pathways, adding a new layer to our understanding of how environmental signals and microbial metabolites modulate gene expression in aging [155].
3.4.2. Microbial Metabolites and Histone Modification
Among the most influential microbial signals are SCFAs—especially butyrate, acetate, and propionate—produced by gut bacteria during the fermentation of dietary fibers [156]. Butyrate, in particular, is known to inhibit histone deacetylases (HDACs) [157], enzymes that normally compact chromatin and suppress gene expression [158]. By blocking HDACs, butyrate promotes a more relaxed chromatin structure [157], enhancing the transcription of genes involved in anti-inflammation, oxidative stress response, and cellular metabolism [159].
3.4.3. Microbiome Influence on DNA Methylation
These microbial metabolites also influence DNA methylation, another core epigenetic modification [160]. Bacteria such as F. prausnitzii and Bifidobacterium spp. synthesize B vitamins, especially B2, B9, and B12, which are essential cofactors in the one-carbon metabolism pathway [161]. This pathway supplies S-adenosylmethionine (SAM), the primary methyl donor for DNA methyltransferases (DNMTs) [162]. When microbial diversity is lost, as often seen in older adults, the availability of these cofactors drops, leading to global DNA hypomethylation, which contributes to genomic instability and aberrant gene expression [155,163,164].
3.4.4. Epigenetic Clocks and Microbial Correlates
One of the best-known biomarkers of aging is the epigenetic clock, which estimates biological age based on patterns of DNA methylation across the genome [165]. Interestingly, these patterns are shaped, in part, by gut microbial composition [166]. Studies have found that beneficial bacteria, such as Subdoligranulum and Erysipelotrichaceae, are linked to slower epigenetic aging [167], whereas pro-inflammatory microbes, including Christensenellaceae and Peptococcaceae, accelerate methylation-based aging phenotypes [168].
3.4.5. Pathological Epigenetic Modifications Induced by Dysbiosis
Beyond aging rates, dysbiosis can directly promote pathological epigenetic changes [48,169]. For instance, chronic Helicobacter pylori infection has been shown to increase methylation of genes in gastric mucosa [170], while uropathogenic Escherichia coli strains induce methylation alterations in urogenital tissues [171]. Some of these bacteria even produce nucleomodulins, proteins that mimic host histone modifiers, directly impacting chromatin architecture [172].
3.4.6. Microbiome and Non-Coding RNA Regulation
Gut microbes also modulate non-coding RNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), which regulate gene expression post-transcriptionally [173]. Specific microbial signals can alter the expression of host miRNAs linked to inflammation and aging [174]. Conversely, host-derived fecal miRNAs influence microbial community composition, creating a feedback loop between the microbiome and the transcriptome [175].
3.4.7. Polyamines and Epigenetic Flexibility
Polyamines, including spermidine, are another group of microbial metabolites with epigenetic roles [176]. Produced through microbial metabolism of amino acids, polyamines regulate histone acetylation, support chromatin remodeling, and enhance expression of stress response genes [177]. Their decline in aging corresponds to a reduction in transcriptional flexibility and impaired cellular repair mechanisms [178].
3.4.8. Microbial Associations with Brain Epigenetics
In aging brains, large-scale methylome analyses have revealed that critical genes are involved in critical roles during aging and neurodegenerative diseases [179]. These methylation patterns have been correlated with specific microbial taxa. For example, Muribaculaceae abundance has been linked to methylation near ApoE, a key gene in Alzheimer’s risk [180], while Lachnospiraceae correlates with neuroprotective gene networks [181].
3.4.9. Microbiota Transplantation and Epigenetic Rejuvenation
Animal studies reinforce this connection. In aged mice, microbiota transplantation from young donors not only improved cognitive function but also reversed several age-related methylation changes [182]. This rejuvenation was mediated through the restoration of SCFA levels [182].
3.4.10. Transcriptomic Shifts in Aging and Microbial Modulation
At the transcriptomic level, aging is associated with upregulation of inflammatory genes [183] and downregulation of DNA repair and autophagy pathways [184]. Dysbiosis can exacerbate these trends, tipping the balance toward frailty and disease [185]. However, beneficial microbes can mitigate these effects by promoting anti-inflammatory expression or by supporting stress resilience mechanisms [186].
3.4.11. Dietary Polyphenols, Microbiome, and Epigenetic Modulation
Environmental factors also shape both the epigenome and the microbiome. Diets rich in polyphenols—such as resveratrol and green tea catechins—have been shown to inhibit DNMTs and HDACs, leading to beneficial epigenetic changes [187]. These effects are often microbiome-dependent, as microbes convert polyphenols into bioactive forms that can modulate gene expression in the host [188].
3.4.12. Mitochondrial DNA Epigenetics and Microbial Influence
Interestingly, recent research suggests that epigenetic modifications in mitochondrial DNA (mtDNA), a lesser explored yet potentially vital aspect of aging, play a role [189]. These mtDNA methylation changes vary with age and neurodegenerative disease, and may also be modulated by SCFA levels and microbial signals, though this area remains under active investigation [190,191].
3.5. Neurocognitive Resilience via the Gut–Brain Axis
3.5.1. The Gut–Brain Axis: A Bidirectional Communication Network
The idea that the gut can influence the brain might once have sounded implausible, but today it is at the heart of one of the most exciting frontiers in aging science. The gut–brain axis—a bidirectional communication network linking the gut microbiota to the central nervous system—plays a significant role in shaping cognition, mood, and emotional regulation [192]. As we age, maintaining neurocognitive resilience, or the brain’s capacity to resist age-related decline, depends increasingly on the integrity of this gut–brain connection [193].
3.5.2. Pathways of Microbiome-to-Brain Communication
The gut microbiome communicates with the brain through multiple pathways: neural (via the vagus nerve), immune (via cytokine signaling), endocrine (via the hypothalamic–pituitary–adrenal axis, also known as the HPA axis), and metabolic (via microbial metabolites) [194]. Among the most important of these messengers are SCFAs, such as butyrate, propionate, and acetate, which are known to cross the blood–brain barrier (BBB) and modulate neuroinflammation, synaptic plasticity, and neurotransmitter systems [195].
3.5.3. Age-Related Microbial Shifts and Their Impact on Brain Health
In healthy individuals, a balanced gut microbiome helps maintain BBB integrity and regulates microglial activation, thereby protecting against neurodegenerative processes [196]. However, with aging, the gut ecosystem undergoes significant changes, declining in diversity and losing beneficial microbes such as Bifidobacterium and Lactobacillus [197], while increasing pro-inflammatory taxa, including Proteobacteria [198,199]. These microbial shifts are associated with reduced SCFA production and increased systemic inflammation, both of which contribute to cognitive decline [200].
3.5.4. Experimental Evidence from Animal Models
Animal studies have provided compelling evidence of this link. In one model, FMT from young mice into aged recipients significantly improved performance in cognitive tasks and reduced markers of hippocampal inflammation [140]. In contrast, FMT from individuals with major depressive disorder (MDD) induced anxiety-like behaviors and impaired reward processing in mice [201], suggesting that the microbiome can transfer neuropsychiatric traits via microbial signaling [202].
3.5.5. SCFAs and Epigenetic Neuroprotection
SCFAs exert their neuroprotective effects, in part, through epigenetic modulation [203]. For example, butyrate acts as a histone deacetylase inhibitor, enhancing the expression of brain-derived neurotrophic factor (BDNF)—a critical regulator of synaptic plasticity and memory [203,204]. In aging and Alzheimer’s disease (AD), lower SCFA levels are consistently observed, correlating with increased microglial activation and reduced BDNF signaling [205].
3.5.6. Microbial Metabolites Linked to Neurodegeneration
Beyond SCFAs, other microbial metabolites play darker roles. TMAO and secondary bile acids have been implicated in AD progression [206]. Elevated TMAO levels in cerebrospinal fluid are associated with β-amyloid deposition and tau phosphorylation, hallmarks of Alzheimer’s pathology [207]. Similarly, increased deoxycholic acid and other bile acid metabolites have been found in the brains and serum of AD patients, linking dysbiosis to neurotoxicity [208].
3.5.7. Neural Pathways: Vagus Nerve and HPA Axis
The vagus nerve is a key neural highway connecting the gut to the brain [209]. Certain probiotics, such as Lactobacillus rhamnosus, can modulate vagal activity and alter GABA receptor expression, affecting anxiety and mood regulation [210]. Vagal stimulation is known to enhance neuroplasticity and may contribute to the antidepressant effect observed with specific microbial strains [211].
Another key player is the HPA axis, which governs the body’s response to stress [212]. Aging, combined with gut dysbiosis, can lead to hyperactivation of the HPA axis, resulting in elevated corticosterone levels and impaired cognitive resilience [213]. In germ-free mice, this stress response is exaggerated, but can be normalized through colonization with beneficial microbial strains [213].
3.5.8. Microbiome Influence on Synaptic Structure and Function
In terms of structure and function, the gut microbiota also influences synaptic architecture [214]. Resilient animal models have demonstrated increased dendritic spine density and higher BDNF expression in key brain regions, such as the prefrontal cortex and hippocampus, effects that are modifiable by gut microbial composition [215]. For instance, mice receiving microbiota from behaviorally resilient donors exhibited improved cognition and emotional regulation, alongside healthier neurotransmitter profiles [201].
3.5.9. Electrophysiological and Imaging Evidence
Electrophysiological and imaging studies support this. Altered microbial states have been linked to shifts in default mode network (DMN) connectivity—an essential brain network involved in memory and introspection [216]. Probiotic supplementation has also been shown to affect EEG markers of stress and resilience [217], supporting the idea that gut microbes influence not only structural changes but also real-time brain function.
3.5.10. Human Clinical Evidence and Cognitive Outcomes
Human clinical data is still evolving but increasingly promising. In elderly adults, probiotic supplementation with Lactobacillus and Bifidobacterium has been shown to improve cognitive scores, mood, and metabolic markers [218]. One study showed that older adults receiving probiotics had better Mini-Mental State Examination (MMSE) scores and reduced insulin resistance [219]—suggesting improved brain–glucose metabolism, which is vital for cognitive health.
3.5.11. Microbial Epigenetics and Future Directions
There is also emerging interest in how microbial signals influence the epigenetic regulation of cognitive genes. SCFAs, such as butyrate, may modify DNA methylation in neurons, thereby altering the expression of genes related to learning, memory, and synaptic integrity [220,221]. Although still in its early stages, these insights suggest that microbial epigenetics may be a promising future target for interventions aimed at preserving brain health in the aging population.
4. Age-Related Diseases and Microbial Imbalance
Aging is not simply a matter of accumulating years—it is a complex, systemic transformation influenced by interconnected biological processes, including cellular senescence, immune dysfunction, oxidative stress, and, more recently, the human microbiome [222]. As we age, the rich and diverse microbial communities that inhabit our gut begin to shift. This change, known as microbial dysbiosis, is characterized by a loss of microbial diversity, a decline in beneficial bacteria, and an increase in harmful pathobionts [223]. These microbial changes are far from benign—they actively contribute to the development and worsening of many chronic diseases that disproportionately affect older adults [224].
4.1. Inflammaging and Immune Dysfunction
A dysbiotic microbiome is a key driver of inflammaging—a chronic, low-grade inflammatory state that underlies many age-related disorders. As microbial diversity decreases, the gut barrier becomes compromised, allowing microbial products, such as LPS, to leak into the bloodstream [225]. These LPS molecules activate TLRs and NF-κB pathways, triggering widespread systemic inflammation [226]. Compromised mucosal immunity and epithelial barrier integrity further exacerbate immunosenescence [227], reducing the body’s ability to defend against infections and increasing vulnerability to autoimmune disorders [228].
4.2. Neurodegeneration and the Gut–Brain Axis
The gut–brain axis is an essential link between gut health and brain function. In aging, reductions in microbial taxa such as Bifidobacterium are associated with lower brain-derived neurotrophic factor (BDNF) levels and increased brain inflammation [229]. Animal studies demonstrate that FMT from young donors can restore synaptic plasticity and reverse behavioral impairments in aged mice [202]. Moreover, distinct microbial signatures found in Alzheimer’s and Parkinson’s disease patients are being explored as early biomarkers of neurodegeneration [230].
4.3. Metabolic Syndrome and Obesity
Age-related microbial changes are strongly implicated in the development of metabolic disorders, including obesity, insulin resistance, and type 2 diabetes [231]. The decline of SCFA-producing microbes impairs glucose regulation [232]. At the same time, the overgrowth of Prevotella and Ruminococcus promotes the synthesis of branched-chain amino acids (BCAAs), which worsens insulin resistance [233]. Notably, FMT from lean donors has resulted in transient improvements in insulin sensitivity in individuals with metabolic syndrome, highlighting the causal role of the microbiota in maintaining metabolic health [234].
4.4. Cardiovascular Disease
The gut microbiota also influences the aging cardiovascular system. Certain bacteria metabolize choline and carnitine into trimethylamine, which is converted into TMAO in the liver—a compound now known to be pro-atherogenic. Elevated TMAO levels are associated with increased risk of heart attacks, stroke, and heart failure [235].
4.5. Sarcopenia and Frailty
The age-related decline in muscle mass and strength, known as sarcopenia, may also be linked to the microbiome. Aging alters gut flora and reduces SCFA levels, which compromises mitochondrial function in muscle cells [236]. Inflammatory shifts in the gut also interfere with amino acid absorption and increase the catabolism of these amino acids [237]. Notably, probiotics such as Lactobacillus acidophilus DDS-1 have been shown to improve muscle mass and reduce frailty markers in aging models [238].
4.6. Osteopenia and Bone Health
The gut–bone connection is another emerging field. Microbiota influence calcium absorption, SCFA levels, and immune signaling, all of which are vital for bone health [239]. Dysbiosis leads to increased gut permeability and systemic inflammation, activating osteoclasts (bone-resorbing cells) while inhibiting osteoblasts (bone-forming cells), resulting in osteopenia and osteoporosis [240]. SCFAs like butyrate and propionate play anabolic roles and are reduced in older adults with fragile bones [241].
4.7. Non-Alcoholic Fatty Liver Disease (NAFLD)
In older people, NAFLD is increasingly understood as a microbiome-mediated condition [242]. Microbial products crossing a leaky gut barrier reach the liver via the portal circulation, triggering hepatic inflammation [243,244]. Clinical studies have demonstrated that FMT can enhance liver function and restore intestinal barrier integrity in patients with NAFLD [245], highlighting the therapeutic potential of targeting the gut–liver axis.
4.8. Cognitive Impairment and Mood Disorders
The gut microbiome affects the central nervous system through modulation of tryptophan metabolism and GABAergic pathways [246]. Older adults with cognitive decline often have diminished levels of microbes that produce neuroactive metabolites [247]. In aged rodents, probiotics have been shown to reverse anxiety-like behaviors and restore synaptic plasticity [248], further supporting the link between microbial health and emotional well-being.
4.9. Colorectal and Gastric Cancer
Certain microbes can increase cancer risk by metabolizing bile acids into genotoxins and producing carcinogenic compounds such as hydrogen sulfide and N-nitrosamines [249]. Fusobacterium nucleatum is frequently found in colorectal tumors, where it enhances cancer progression by modulating the immune response and activating β-catenin signaling [250]. Coupled with the decline in aging-related DNA repair, these microbial factors significantly increase the mutational burden [251].
4.10. Autoimmunity and Immune Regulation
With age, the gut microbiota loses Treg-inducing species, such as Clostridium cluster XIVa, and accumulates pro-inflammatory taxa [252,253]. This imbalance contributes to autoimmune diseases such as rheumatoid arthritis, lupus, and multiple sclerosis [254]. SCFAs, such as butyrate, are critical for Treg stability and IL-10 production, both of which decline in older people, thereby compromising immune tolerance [255].
4.11. Gut Barrier Dysfunction
One of the most direct impacts of dysbiosis is the erosion of gut barrier integrity [256]. The loss of beneficial microbes, such as A. muciniphila spp. compromises the mucin layer, leading to microbial translocation and endotoxemia [257]. This breakdown often precedes or worsens conditions like inflammatory bowel disease (IBD) and metabolic syndrome [258].
4.12. Chronic Respiratory Disease
The gut–lung axis is another pathway affected by aging and microbial imbalance [259]. Dysbiosis can suppress alveolar macrophages and exacerbate neutrophilic inflammation, contributing to respiratory conditions such as Chronic Obstructive Pulmonary Disease (COPD) and increasing vulnerability to pneumonia [260]. SCFA supplementation has been shown to mitigate lung inflammation in animal models, suggesting potential therapeutic applications for the future [261].
4.13. Urogenital Infections
Postmenopausal shifts in the vaginal and gut microbiota often lead to recurrent urinary tract infections (UTIs) [262]. The elderly community fosters an environment for uropathogenic bacteria, such as E. coli [263]. In older adults with gut barrier dysfunction, these pathogens can more easily migrate and establish infections in the urinary tract [264].
4.14. Diet, Lifestyle, and Microbial Aging
Diet profoundly shapes microbial composition. Fiber- and polyphenol-rich diets support a diverse, anti-inflammatory microbiota [265], whereas high-fat, processed diets favor the growth of pathogenic Firmicutes and Proteobacteria, contributing to obesity and inflammation [266]. Unfortunately, many older adults consume low-fiber diets, which exacerbates microbial aging and increases the risk of chronic disease [267].
4.15. Longevity and Microbial Profiles
Centenarians offer a blueprint for microbial resilience. Their gut microbiota are often enriched with SCFA producers and anti-inflammatory taxa, such as Akkermansia and Bifidobacterium [268]. These microbial configurations are associated with lower inflammation, better immune profiles, and delayed onset of frailty [269]. Remarkably, FMT from centenarian donors into aged mice has extended both lifespan and health span [270].
5. Interventions to Promote a Youthful Microbiome
Aging is no longer viewed as an isolated biological process, but rather as a systemic progression influenced by intricate relationships between host physiology and the gut microbiome [222]. One of the most striking revelations in recent geroscience is that the microbial changes occurring in the gastrointestinal tract are not merely consequences of aging—they may actively drive it [271]. With age, the gut microbiota becomes less diverse, loses beneficial organisms like Bifidobacterium and Faecalibacterium, and accumulates potentially harmful taxa [222]. These shifts contribute to chronic inflammation, metabolic dysregulation, cognitive decline, and immune deterioration [48]. Fortunately, the gut ecosystem is remarkably plastic. Emerging research suggests that lifestyle, nutrition, and targeted interventions can reshape the microbiota and restore youthful microbial patterns—offering a promising route to promote healthy aging [43]. A comprehensive summary of these intervention strategies and their microbiome-level effects is provided in Figure 3.
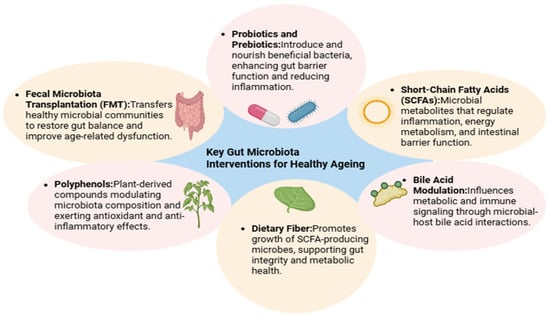
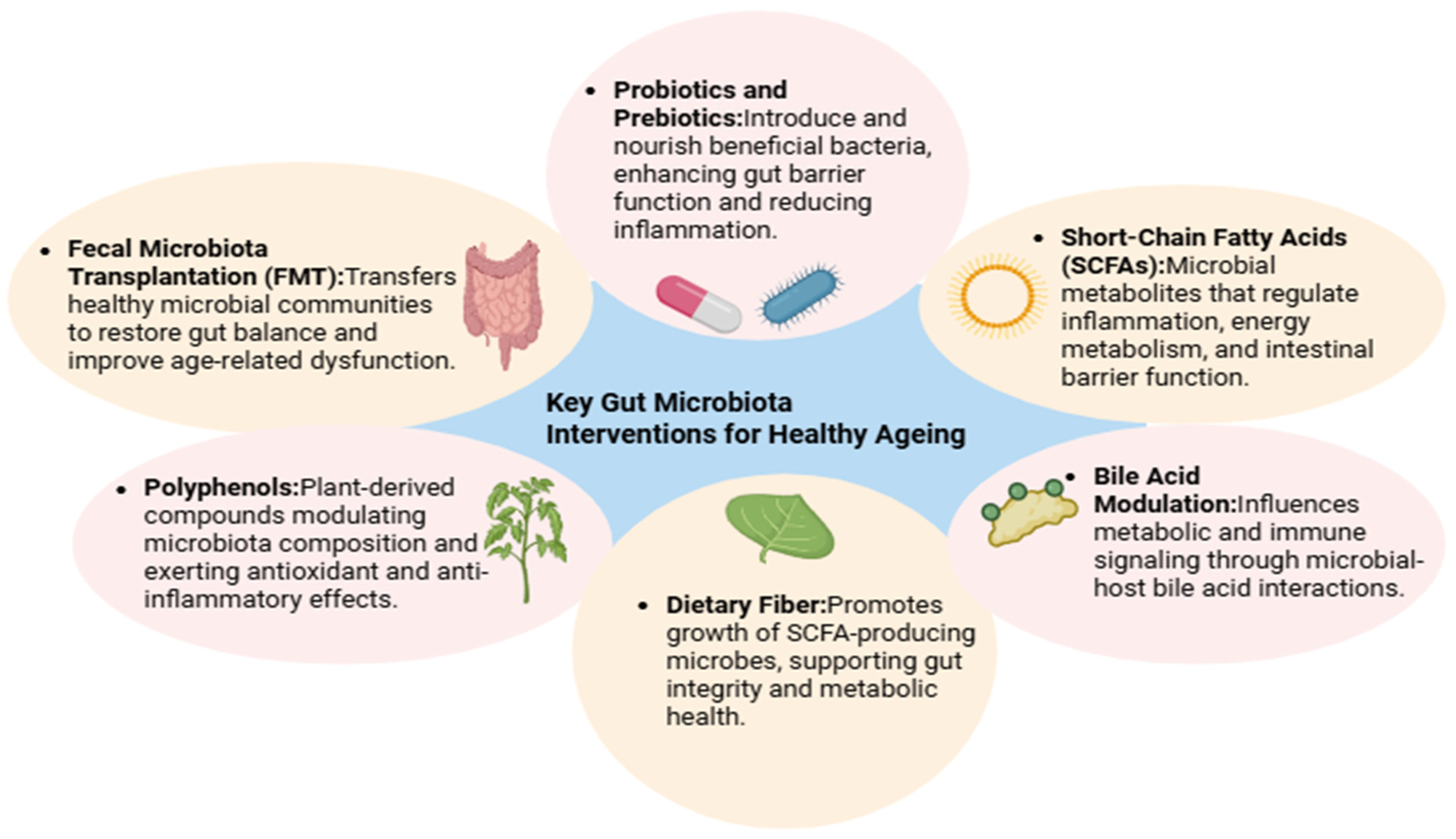
Figure 3.
Interventions Targeting the Aging Microbiome. This figure summarizes evidence-based interventions—such as dietary fiber, probiotics, and fecal microbiota transplantation—that can restore microbial diversity and function in older adults, highlighting their potential to promote healthy aging and extend health span.
5.1. Dietary Strategies to Rejuvenate the Microbiota
Among all interventions, diet is the most potent and modifiable factor influencing gut microbial composition, as depicted in Figure 3. The nutrients we consume shape microbial metabolism, dictate the abundance of SCFA-producing bacteria, and modulate immune responses [272]. Diets rich in fermentable fibers, such as whole grains, legumes, fruits, and vegetables, enhance the growth of beneficial microbes like A. muciniphila and F. prausnitzii—both of which are known for their anti-inflammatory effects and role in maintaining epithelial integrity [273].
The Mediterranean diet has garnered particular attention for its beneficial effects on the microbiota [274]. High in polyphenols and fiber, this dietary pattern has been associated with improved microbial diversity and reductions in frailty and systemic inflammation in older adults [275]. In the landmark Nutrition for Aging (NU-AGE) trial—a one-year intervention across five European countries—participants who adopted the Mediterranean diet showed significant improvements in cognitive function, which were linked to microbial shifts [275].
Furthermore, phytochemicals such as polyphenols and terpenoids act as prebiotics and microbial substrates [276]. These bioactive compounds promote the growth of beneficial bacteria while inhibiting the expansion of pathogens [277]. Their fermentation by gut microbes produces bioactive metabolites, such as urolithins, which have been shown to reduce inflammation and enhance both muscle and cognitive performance [278].
5.2. Probiotic-Based Interventions
Probiotics—live microorganisms that confer health benefits when administered in appropriate doses—are another cornerstone of microbiome-based strategies. Strains of Lactobacillus and Bifidobacterium have been shown to exhibit anti-aging properties in model organisms [279]. For instance, Lactobacillus gasseri SBT2055 has been shown to increase lifespan in Caenorhabditis elegans by enhancing oxidative stress resistance and boosting innate immunity [280].
In human studies, probiotics have shown promise in improving skin health, reducing oxidative stress, and mitigating signs of biological aging [148]. Supplementation with L. casei LC122 and B. longum BL986 has been found to enhance hippocampal neurogenesis and elevate neurotrophic factors [281].
Clinically, probiotics are being tested for conditions ranging from osteoporosis to inflammatory bowel disease [282]. In postmenopausal women, supplementation with L. reuteri ATCC 6475 improved bone mineral density [283], while fermented dairy products containing probiotics enhanced bone turnover markers in osteoporotic patients [284].
5.3. Personalized Nutrition and Microbiome Configuration
One-size-fits-all nutritional advice often falls short because microbiomes vary significantly between individuals. This interindividual variability influences how people respond to the same foods, particularly in aging populations with complex health profiles [285]. Personalized nutrition, guided by baseline microbial and metabolic profiles, is emerging as a more precise and effective strategy for promoting health span [286].
AI-driven models that integrate microbiome data with genetics, dietary patterns, and anthropometric data can predict post-meal glucose responses with remarkable accuracy. This was demonstrated in a study by Zeevi et al. [287], which laid the foundation for microbiome-guided nutritional interventions. More recently, microbial signatures were shown to predict improvements in cardiometabolic health during diet-based trials [288].
For aging adults, tailoring dietary and probiotic interventions to microbiome profiles holds the potential to delay or prevent age-related diseases [289]. Though still in development, this personalized approach is likely to reshape the future of geriatric medicine.
5.4. Exercise and Physical Activity
Physical activity is not just beneficial for muscles; it is also suitable for microbes. Moderate to vigorous exercise increases the abundance of SCFA-producing bacteria, such as Lachnospiraceae, Ruminococcaceae, and Bifidobacterium [290]. In older women, increased physical activity has been associated with improved gut microbial diversity and reduced risk of sarcopenic obesity [291].
Animal studies further reveal that exercise-induced shifts in the microbiome can influence muscle metabolism, energy expenditure, and immune modulation [292]. Notably, endurance athletes have a higher abundance of Veillonella atypica, a bacterium that converts lactate into propionate, potentially enhancing recovery and performance [293]. These findings support integrating exercise with microbiome-enhancing dietary strategies to maximize physiological benefits during aging.
5.5. Drug–Microbiota Interactions
Elderly individuals often take multiple medications (polypharmacy), which can significantly alter the gut microbiome. Common drugs, including metformin, proton-pump inhibitors, and statins, can reshape microbial communities, sometimes leading to adverse outcomes [294]. Metformin, however, has been shown to promote A. muciniphila, a bacterium linked to improved glucose regulation and metabolic health [295].
Understanding drug–microbiota interactions opens the door to co-administering probiotics or prebiotics alongside pharmaceuticals. Such combinations may enhance drug efficacy, reduce side effects, and restore microbial balance in aging populations [296].
5.6. Fecal Microbiota Transplantation (FMT)
FMT, which involves the transfer of stool from a healthy donor to a recipient with dysbiosis, is gaining attention beyond its conventional use in treating Clostridioides difficile infections [297]. In aging models, FMT from young or healthy donors has been shown to restore microbial diversity, reduce inflammatory markers, and improve overall health span. Mouse studies using FMT from young animals into progeroid (prematurely aging) models showed not only improvements in gut integrity and systemic inflammation but also positive effects on cognitive and muscular function [140,182]. Though human applications remain limited, FMT represents a promising frontier for rejuvenation therapies, as illustrated in Figure 3.
5.7. Integration of AI and Wearable Technologies
As microbiome science evolves, technology is accelerating its clinical application. Machine learning tools now allow researchers to predict biological age using gut microbial signatures with surprising accuracy [298]. Combined with wearable devices that monitor sleep, movement, and nutrition, these tools enable dynamic, real-time health assessments.
These integrated platforms could one day provide fully personalized microbiome care—adjusting diet, activity, and medication in response to microbial trends. However, they raise ethical concerns regarding data privacy, bias in algorithmic predictions, and access to digital health tools—all of which must be addressed as the field matures [299].
5.8. Limitations in Microbiome-Based Based Intervention
Growing evidence suggests that gut microbiome–based interventions can meaningfully influence health and aging, though the effects are often subtle and context-dependent [300]. Probiotic supplementation has shown measurable changes in both microbial composition and host physiology [301]. In a controlled study, individuals with attention-deficit/hyperactivity disorder (ADHD) who received Bifidobacterium supplements showed a 1.4–1.6-fold increase in Bifidobacterium levels, along with significant alterations in dopamine precursor pathways and reduced ventral striatal reactivity during reward anticipation (t(85) = 2.1, p = 0.038) [302]. Similarly, in a Chinese cohort of 51 untreated ADHD children, Faecalibacterium abundance was about 35% lower than in 32 healthy controls (p < 0.05), and its reduction correlated with more severe symptoms (β = −0.42, p = 0.048) [303]. Antibiotic-based modulation also produced behavioral and microbiota shifts [304]: minocycline treatment reduced depressive-like behavior and increased Lachnospiraceae abundance, while decreasing microglial density in the prefrontal cortex [305]. On a population level, findings from the Flemish Gut Flora Project suggest that diet-driven microbiota changes explain only 5–10% of total variance, indicating that host factors like age, BMI, and stool consistency play larger roles [306].
However, these interventions have several important limitations. For example, individual responses to probiotics or dietary changes vary widely—up to 40–60% of people show little to no measurable benefit [307]. Most clinical trials are also short-term (typically under 12 weeks) and lack follow-up to determine whether changes in the gut microbiota persist over time [308]. Moreover, probiotic benefits depend heavily on strain type and dosage, and many commercial strains fail to effectively colonize the gut or maintain long-term presence [309]. Antibiotic-based approaches, while mechanistically informative, risk disrupting beneficial microbial communities and metabolic networks [310]. Finally, most studies still rely on single-omics data; moving forward with personalized and longitudinal approaches, combined with multi-omics integration [311] and standardized methodologies, is crucial to transform these modest statistical effects into reliable, long-term therapeutic strategies [312].
6. Research Gaps and Future Directions
Despite the exciting strides made in unraveling the connection between the gut microbiome and healthy aging, several key gaps continue to limit the application of this knowledge in real-world clinical and public health settings. A significant limitation of most existing studies is their design: they are often cross-sectional and lack ethnically and geographically diverse cohorts, making it difficult to distinguish causation from correlation [313]. To move forward, we urgently need longitudinal, multi-omic investigations that integrate not only microbial sequencing but also metabolomics, immune profiling, and host transcriptomics to capture the dynamic interplay between the gut and aging biology over time [314]. An integrated schematic of microbiota shifts across lifespan, aging mechanisms, and potential interventions is depicted in Figure 4.
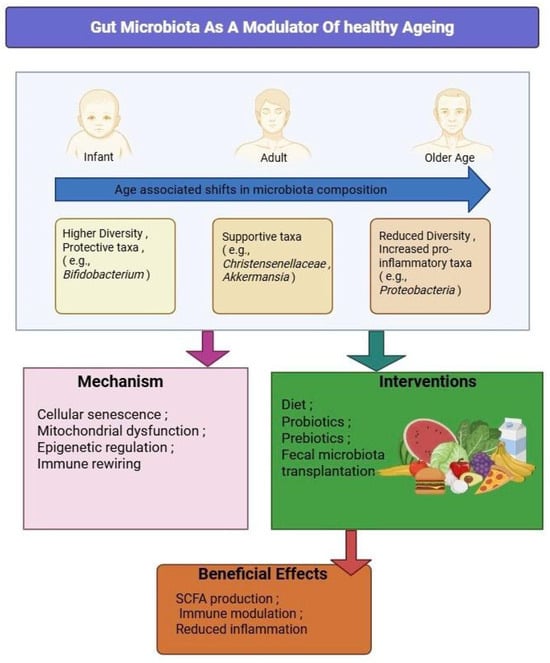
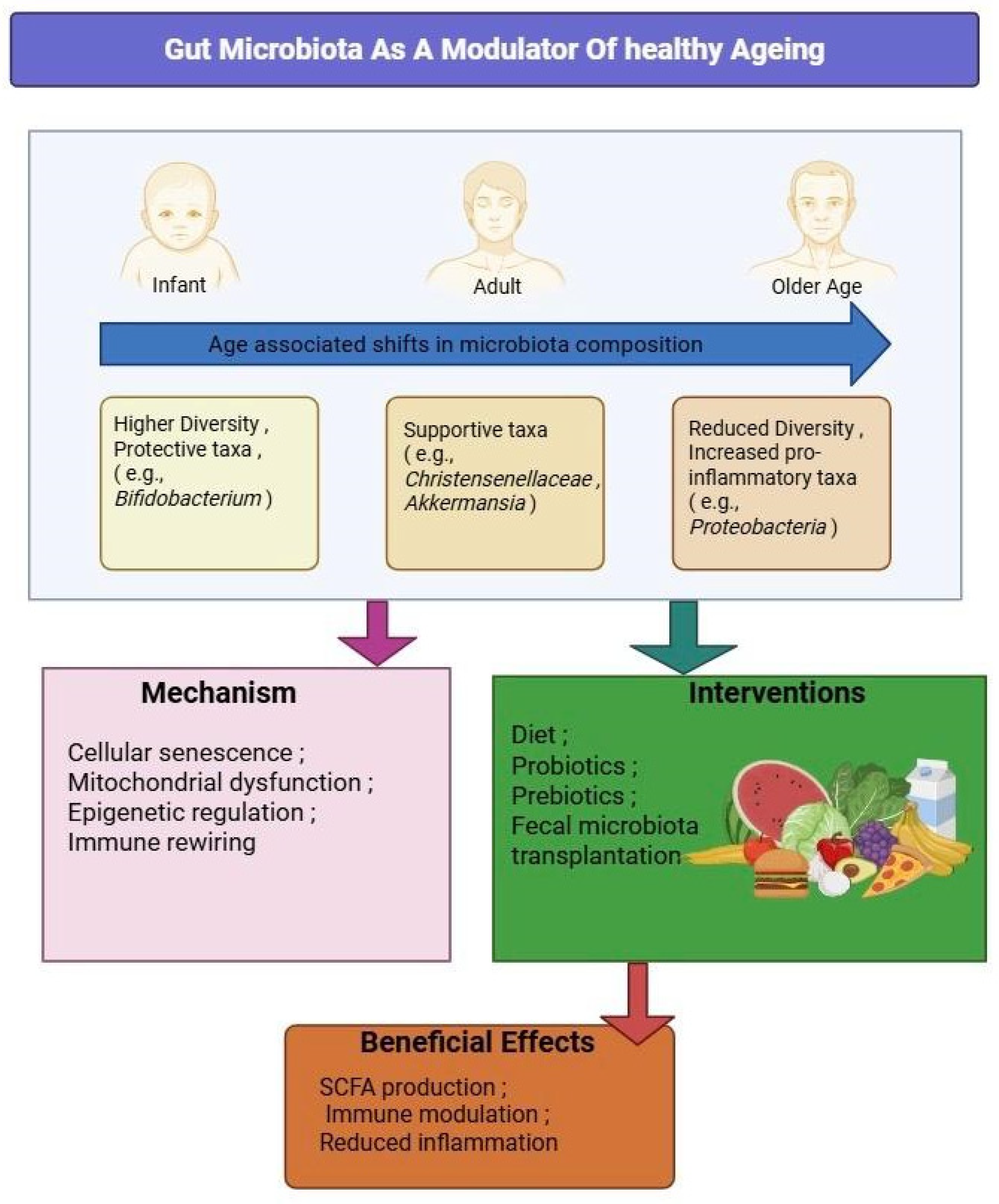
Figure 4.
Gut Microbiota as a Modulator of Healthy Aging. This figure illustrates how age-associated microbiota shifts—from infancy through older age—contribute to mechanisms of aging, including cellular senescence, mitochondrial dysfunction, epigenetic alterations, and immune rewiring. Intervention strategies, such as dietary modulation, probiotics, prebiotics, and fecal microbiota transplantation, may help restore microbial balance, leading to beneficial effects including the production of short-chain fatty acids (SCFAs), reduced inflammation, and enhanced immune regulation.
In addition, the current research landscape remains bacteriocentric, often overlooking the broader ecosystem of the gut microbiota. Non-bacterial components—such as fungi, viruses, and archaea—remain underexplored, despite evidence suggesting they can significantly influence host immune responses, intestinal barrier function, and systemic inflammation [315]. Understanding these microbial kingdoms and their crosstalk is crucial for developing a more holistic model of gut–host interactions.
Mechanistically, while many studies highlight the importance of microbial metabolites—including SCFAs, bile acids, and polyphenol-derived compounds—the precise ways these molecules influence host epigenetics, mitochondrial function, and chronic inflammation remain only partially understood [316]. Animal models have provided important insights, showing how fecal transplants or dietary modulation can extend lifespan or delay frailty [317]. However, translating these findings to humans is complicated by interindividual variability in diet, lifestyle, medication use, and environment.
Medication–microbiota interactions present another overlooked frontier. Older adults are often exposed to polypharmacy, yet our understanding of how drugs such as metformin, statins, or proton pump inhibitors affect microbial diversity and function over time remains limited [318]. These interactions could either enhance or undermine therapeutic effects, depending on how they shift microbial metabolism or immune tone.
Furthermore, while interventions shown in Figure 3, such as probiotics, prebiotics, and high-fiber diets, are widely recommended, the evidence base remains fragmented. Clinical trials vary widely in terms of strain selection, dosage, duration, and outcome measures, making it difficult to draw firm conclusions [319]. Moreover, an individual’s response to interventions is heavily influenced by their baseline microbiota, genetic background, and lifestyle—factors that current general guidelines often overlook [308].
The growing field of personalized microbiome medicine offers a compelling way forward. Studies using AI-based algorithms have shown that microbiota composition can predict glycemic responses to specific meals in younger populations [320]. However, such tools have yet to be validated in older adults, whose microbial profiles and metabolic responses differ markedly [321].
FMT, a more radical approach, has demonstrated rejuvenating effects in animal models, including the restoration of immune balance and improvement in cognition [140,182]. Yet, concerns around regulation, safety, ethics, and donor standardization currently limit its application in older populations.
Finally, lifestyle factors such as physical activity, sleep quality, and psychosocial well-being are well known to influence microbiota composition but are rarely integrated into microbiome intervention studies [322]. This is a critical oversight. To truly promote healthy aging, future research must adopt a systems biology perspective, blending multi-kingdom microbial analytics, wearable technology, AI-powered diagnostics, and personalized nutrition. Only through such interdisciplinary integration can we move from understanding to intervention—and from promise to practice.
Beyond the conceptual and biological challenges explored, researchers in microbiome and aging studies face a host of practical hurdles that continue to complicate what we can and cannot discover [271]. The reality is that most of our current understanding comes from laboratory experiments using cell cultures and animal models—valuable tools, certainly, but ones that fall short of capturing the intricate reality of the human gut [323].
For example, the cell lines that researchers rely on daily, such as Caco-2 and HT-29. While these have been workhorses in the field, they present a simplified view of gut biology [324]. They lack the diverse immune cells that patrol our intestines, the oxygen gradients that create distinct microbial neighborhoods, and the rich microbial communities that call our guts home [325,326]. Even when scientists turn to more sophisticated approaches like 3D organoids and organ-on-chip technologies, they encounter new challenges. While these cutting-edge models are promising, they often differ from lab to lab, making it difficult to compare results and build consistent knowledge [327]. This variability might suggest that what works in one laboratory setting may not translate seamlessly to human health and disease.
The computational side of microbiome research presents its own set of limitations. To understand what genes and proteins actually do in gut bacteria, researchers turn to databases like UniProt, KEGG, and EggNOG [328]. However, these repositories have significant blind spots. Many gut bacteria remain poorly characterized, leaving researchers with incomplete or inconsistent functional predictions [328]. It is like trying to solve a puzzle with half the pieces missing and no clear picture of what the final image should look like.
Adding to these challenges, the technical aspects of sequencing and data analysis introduce their own biases [329]. The process of sorting genetic material into distinct bacterial “bins” is not always accurate [330], and different research groups often use different analytical approaches, making it difficult to compare studies meaningfully [331]. Thus, these technical hurdles compound the interpretive challenges.
What becomes clear is that the field desperately needs a more integrated approach—one that brings together experimental microbiologists working with better human-relevant models, computational scientists developing more accurate databases and analytical tools, and clinical researchers conducting long-term studies of real people [332,333]. Future research efforts should prioritize developing culture systems that better mirror human gut conditions, improving the computational tools we use to interpret microbial functions, and expanding long-term human studies that account for the messy realities of human life—our varied diets, medications, and lifestyles [332,333].
Only by tackling these challenges head-on, through coordinated efforts across disciplines, can we move beyond correlation to truly understand how our gut microbes influence healthy aging. The path forward requires not just better tools and methods, but also the patience to build knowledge systematically, piece by piece, until we can see the complete picture of how our microbial partners shape our journey through life.
7. Conclusions
The gut microbiome plays a decisive role in shaping the trajectory of human aging, influencing inflammation, metabolism, cellular repair, and cognitive health. As we age, disruptions in this delicate microbial ecosystem can accelerate disease, frailty, and functional decline. However, targeted interventions—ranging from dietary strategies and probiotics to personalized nutrition and fecal transplants—hold immense promise in restoring microbial balance and promoting resilience. With continued investment in mechanistic research, customized tools, and ethical translational studies, microbiome-based therapies could soon become a cornerstone of healthy aging strategies for a rapidly graying world.
Author Contributions
Conceptualization, A.V.H. and K.S.; methodology, S.C., A.V.H. and K.S.; software, S.C., A.V.H. and K.S.; validation, S.C., A.V.H. and K.S.; writing—original draft preparation, S.C., A.V.H. and K.S.; writing—review and editing, S.C., A.V.H. and K.S.; visualization, S.C., A.V.H. and K.S.; supervision, A.V.H. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- UN. World Population Ageing 2017-Highlights (ST/ESA/SER.A/397). 2017. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2017_Highlights.pdf (accessed on 10 May 2025).
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef]
- Jugran, D. Too well to die; too ill to live: An update on the lifespan versus health span debate. J. Glob. Health 2025, 15, 03022. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Salazar, J.; Durán, P.; Díaz, M.P.; Chacín, M.; Santeliz, R.; Mengual, E.; Gutiérrez, E.; León, X.; Díaz, A.; Bernal, M.; et al. Exploring the Relationship between the Gut Microbiota and Ageing: A Possible Age Modulator. Int. J. Environ. Res. Public Health 2023, 20, 5845. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Kim, G.H.; Shim, J.O. Gut microbiota affects brain development and behavior. Clin. Exp. Pediatr. 2023, 66, 274–280. [Google Scholar] [CrossRef]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes. Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Z.; Chu, J.; Han, S. Aging Gut Microbiome in Healthy and Unhealthy Aging. Aging Dis. 2024, 16, 980–1002. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, M.; Rio, P.; Marrone, A.; Giambra, V.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Inflammaging: The Next Challenge-Exploring the Role of Gut Microbiota, Environmental Factors, and Sex Differences. Biomedicines 2024, 12, 1716. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. Toward an improved definition of a healthy microbiome for healthy aging. Nat. Aging 2022, 2, 1054–1069. [Google Scholar] [CrossRef]
- Wang, J.; Qie, J.; Zhu, D.; Zhang, X.; Zhang, Q.; Xu, Y.; Wang, Y.; Mi, K.; Pei, Y.; Liu, Y.; et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging-relevant neural and immune function. Gut Microbes 2022, 14, 2107288. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Dolkar, P.; Deyang, T.; Anand, N.; Rathipriya, A.G.; Hediyal, T.A.; Chandrasekaran, V.; Krishnamoorthy, N.K.; Gorantla, V.R.; Bishir, M.; Rashan, L.; et al. Trimethylamine-N-oxide and cerebral stroke risk: A review. Neurobiol. Dis. 2024, 192, 106423. [Google Scholar] [CrossRef]
- Chen, C.; Wang, G.Q.; Li, D.D.; Zhang, F. Microbiota-gut-brain axis in neurodegenerative diseases: Molecular mechanisms and therapeutic targets. Mol. Biomed. 2025, 6, 64. [Google Scholar] [CrossRef]
- Farhadi Rad, H.; Tahmasebi, H.; Javani, S.; Hemati, M.; Zakerhamidi, D.; Hosseini, M.; Alibabaei, F.; Banihashemian, S.Z.; Oksenych, V.; Eslami, M. Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines 2024, 12, 2776. [Google Scholar] [CrossRef]
- Brown, J.A.; Bashir, H.; Zeng, M.Y. Lifelong partners: Gut microbiota-immune cell interactions from infancy to old age. Mucosal Immunol. 2025, 18, 509–523. [Google Scholar] [CrossRef]
- Kadyan, S.; Park, G.; Singh, T.P.; Patoine, C.; Singar, S.; Heise, T.; Domeier, C.; Ray, C.; Kumar, M.; Behare, P.V.; et al. Microbiome-based therapeutics towards healthier aging and longevity. Genome Med. 2025, 17, 75. [Google Scholar] [CrossRef]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The Connection Between Physical Exercise and Gut Microbiota: Implications for Competitive Sports Athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, S.S.; Garcez, F.B.; Alencar, J.C.G.; Bastos, A.A.; Morley, J.E.; Cederholm, T.; Aprahamian, I.; de Souza, H.P.; Avelino-Silva, T.J.; Bindels, L.B.; et al. Gut microbiota disturbances in hospitalized older adults with malnutrition and clinical outcomes. Nutrition 2024, 122, 112369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jin, B.; Xu, X.; Zuo, X.; Li, Y.; Li, Z. The Effects of Delivery Mode on the Gut Microbiota and Health: State of Art. Front. Microbiol. 2021, 12, 724449. [Google Scholar] [CrossRef]
- Shin, H.; Pei, Z.; Martinez, K.A., 2nd; Rivera-Vinas, J.I.; Mendez, K.; Cavallin, H.; Dominguez-Bello, M.G. The first microbial environment of infants born by C-section: The operating room microbes. Microbiome 2015, 3, 59. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Monaco, M.H.; Donovan, S.M. Impact of early gut microbiota on immune and metabolic development and function. Semin. Fetal Neonatal Med. 2016, 21, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Dogra, S.K.; Kwong Chung, C.; Wang, D.; Sakwinska, O.; Colombo Mottaz, S.; Sprenger, N. Nurturing the Early Life Gut Microbiome and Immune Maturation for Long Term Health. Microorganisms 2021, 9, 2110. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451 Pt A, 97–102. [Google Scholar] [CrossRef]
- Fu, J.; Zheng, Y.; Gao, Y.; Xu, W. Dietary Fiber Intake and Gut Microbiota in Human Health. Microorganisms 2022, 10, 2507. [Google Scholar] [CrossRef]
- Safarchi, A.; Al-Qadami, G.; Tran, C.D.; Conlon, M. Understanding dysbiosis and resilience in the human gut microbiome: Biomarkers, interventions, and challenges. Front. Microbiol. 2025, 16, 1559521. [Google Scholar] [CrossRef]
- Gyriki, D.; Nikolaidis, C.G.; Bezirtzoglou, E.; Voidarou, C.; Stavropoulou, E.; Tsigalou, C. The gut microbiota and aging: Interactions, implications, and interventions. Front. Aging 2025, 6, 1452917. [Google Scholar] [CrossRef] [PubMed]
- Salazar, N.; Valdés-Varela, L.; González, S.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. Nutrition and the gut microbiome in the elderly. Gut Microbes 2017, 8, 82–97. [Google Scholar] [CrossRef]
- Liu, L.; Yi, Y.; Yan, R.; Hu, R.; Sun, W.; Zhou, W.; Zhou, H.; Si, X.; Ye, Y.; Li, W.; et al. Impact of age-related gut microbiota dysbiosis and reduced short-chain fatty acids on the autonomic nervous system and atrial fibrillation in rats. Front. Cardiovasc. Med. 2024, 11, 1394929. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Kirschner, S.K.; Engelen, M.P.; Haas, P.; Bischoff, S.C.; Deutz, N.E. Short-chain fatty acid kinetics and concentrations are higher after inulin supplementation in young and older adults: A randomized trial. Am. J. Clin. Nutr. 2025, 121, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, D.; De Commer, L.; Tito, R.Y.; Kathagen, G.; Sabino, J.; Vermeire, S.; Faust, K.; Raes, J. Temporal variability in quantitative human gut microbiome profiles and implications for clinical research. Nat. Commun. 2021, 12, 6740. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C. Dysbiotic drift: Mental health, environmental grey space, and microbiota. J. Physiol. Anthr. 2015, 34, 23. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- Santoro, A.; Ostan, R.; Candela, M.; Biagi, E.; Brigidi, P.; Capri, M.; Franceschi, C. Gut microbiota changes in the extreme decades of human life: A focus on centenarians. Cell. Mol. Life Sci. 2018, 75, 129–148. [Google Scholar] [CrossRef]
- Wu, L.; Xie, X.; Li, Y.; Liang, T.; Zhong, H.; Yang, L.; Xi, Y.; Zhang, J.; Ding, Y.; Wu, Q. Gut microbiota as an antioxidant system in centenarians associated with high antioxidant activities of gut-resident Lactobacillus. npj Biofilms Microbiomes 2022, 8, 102. [Google Scholar] [CrossRef]
- Worsley, S.F.; Davies, C.S.; Mannarelli, M.E.; Komdeur, J.; Dugdale, H.L.; Richardson, D.S. Assessing the causes and consequences of gut mycobiome variation in a wild population of the Seychelles warbler. Microbiome 2022, 10, 242. [Google Scholar] [CrossRef]
- Tamayo, M.; Olivares, M.; Ruas-Madiedo, P.; Margolles, A.; Espín, J.C.; Medina, I.; Moreno-Arribas, M.V.; Canals, S.; Mirasso, C.R.; Ortín, S.; et al. How Diet and Lifestyle Can Fine-Tune Gut Microbiomes for Healthy Aging. Annu. Rev. Food Sci. Technol. 2024, 15, 283–305. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, N.V.; Sukumar, M. Aging through the lens of the gut microbiome: Challenges and therapeutic opportunities. Arch. Gerontol. Geriatr. Plus 2025, 2, 100142. [Google Scholar] [CrossRef]
- Wu, S.; Bu, X.; Chen, D.; Wu, X.; Wu, H.; Caiyin, Q.; Qiao, J. Molecules-mediated bidirectional interactions between microbes and human cells. npj Biofilms Microbiomes 2025, 11, 38. [Google Scholar] [CrossRef]
- Holmes, A.; Finger, C.; Morales-Scheihing, D.; Lee, J.; McCullough, L.D. Gut dysbiosis and age-related neurological diseases; an innovative approach for therapeutic interventions. Transl. Res. 2020, 226, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut microbiota dysbiosis, oxidative stress, inflammation, and epigenetic alterations in metabolic diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Mylonas, A.; O’Loghlen, A. Cellular Senescence and Ageing: Mechanisms and Interventions. Front. Aging 2022, 3, 866718. [Google Scholar] [CrossRef]
- Jang, D.H.; Shin, J.W.; Shim, E.; Ohtani, N.; Jeon, O.H. The connection between aging, cellular senescence and gut microbiome alterations: A comprehensive review. Aging Cell 2024, 23, e14315. [Google Scholar] [CrossRef]
- Sharma, R. Emerging Interrelationship Between the Gut Microbiome and Cellular Senescence in the Context of Aging and Disease: Perspectives and Therapeutic Opportunities. Probiotics Antimicrob. Proteins 2022, 14, 648–663. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Ding, S.; Zhang, M. Cross-talk of inflammation and cellular senescence: A new insight into the occurrence and progression of osteoarthritis. Bone Res. 2024, 12, 69. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone deacetylase inhibition and dietary short-chain Fatty acids. Int. Sch. Res. Not. 2011, 2011, 869647. [Google Scholar] [CrossRef] [PubMed]
- Place, R.F.; Noonan, E.J.; Giardina, C. HDAC inhibition prevents NF-kappa B activation by suppressing proteasome activity: Down-regulation of proteasome subunit expression stabilizes I kappa B alpha. Biochem. Pharmacol. 2005, 70, 394–406. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Loo, T.M.; Miyata, K.; Tanaka, Y.; Takahashi, A. Cellular senescence and senescence-associated secretory phenotype via the cGAS-STING signaling pathway in cancer. Cancer Sci. 2020, 111, 304–311. [Google Scholar] [CrossRef]
- He, X.; Wedn, A.; Wang, J.; Gu, Y.; Liu, H.; Zhang, J.; Lin, Z.; Zhou, R.; Pang, X.; Cui, Y. IUPHAR ECR review: The cGAS-STING pathway: Novel functions beyond innate immune and emerging therapeutic opportunities. Pharmacol. Res. 2024, 201, 107063. [Google Scholar] [CrossRef]
- Wan, D.; Jiang, W.; Hao, J. Research Advances in How the cGAS-STING Pathway Controls the Cellular Inflammatory Response. Front. Immunol. 2020, 11, 615. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Yu, L.; Qiao, G.; Qin, D.; Yuen-Kwan Law, B.; Ren, F.; Wu, J.; Wu, A. Mitophagy and cGAS-STING crosstalk in neuroinflammation. Acta Pharm. Sin. B 2024, 14, 3327–3361. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, Y.; Li, T.; Sun, W.; Tang, Z.; Wang, Y.; Zhou, K.; Li, J.; Ding, Q.; Liang, K.; et al. NAD(+) and its possible role in gut microbiota: Insights on the mechanisms by which gut microbes influence host metabolism. Anim. Nutr. 2022, 10, 360–371. [Google Scholar] [CrossRef]
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Kuehnemann, C.; Hu, K.Q.; Butera, K.; Patel, S.K.; Bons, J.; Schilling, B.; Aguayo-Mazzucato, C.; Wiley, C.D. Extracellular Nicotinamide Phosphoribosyltransferase Is a Component of the Senescence-Associated Secretory Phenotype. Front. Endocrinol. 2022, 13, 935106. [Google Scholar] [CrossRef]
- Wang, T.; Huang, S.; He, C. Senescent cells: A therapeutic target for osteoporosis. Cell Prolif. 2022, 55, e13323. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, S.; Zhang, Y.; Yang, J.; Hu, Z. Gut microbiota and calcium balance. Front. Microbiol. 2022, 13, 1033933. [Google Scholar] [CrossRef]
- Prata, L.; Ovsyannikova, I.G.; Tchkonia, T.; Kirkland, J.L. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin. Immunol. 2018, 40, 101275. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef]
- Biragyn, A.; Ferrucci, L. Gut dysbiosis: A potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018, 19, e295–e304. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer-role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Seki, E. Microbiome-obesity-liver cancer interaction: Senescence of hepatic stellate cells and bile acids play new roles. Gastroenterology 2014, 146, 860–861. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Huang, Y.; Tang, Q. The role of high mobility group proteins in cellular senescence mechanisms. Front. Aging 2024, 5, 1486281. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef]
- Wu, N.N.; Zhang, Y.; Ren, J. Mitophagy, Mitochondrial Dynamics, and Homeostasis in Cardiovascular Aging. Oxid. Med. Cell. Longev. 2019, 2019, 9825061. [Google Scholar] [CrossRef]
- Imdad, S.; Lim, W.; Kim, J.H.; Kang, C. Intertwined Relationship of Mitochondrial Metabolism, Gut Microbiome and Exercise Potential. Int. J. Mol. Sci. 2022, 23, 2679. [Google Scholar] [CrossRef]
- Iorio, R.; Celenza, G.; Petricca, S. Mitophagy: Molecular Mechanisms, New Concepts on Parkin Activation and the Emerging Role of AMPK/ULK1 Axis. Cells 2021, 11, 30. [Google Scholar] [CrossRef]
- Rambold, A.S.; Lippincott-Schwartz, J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 2011, 10, 4032–4038. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, C.; Kazlauskaite, A.; Zhang, N.; Woodroof, H.I.; Campbell, D.G.; Gourlay, R.; Burchell, L.; Walden, H.; Macartney, T.J.; Deak, M.; et al. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012, 2, 120080. [Google Scholar] [CrossRef]
- Uoselis, L.; Nguyen, T.N.; Lazarou, M. Mitochondrial degradation: Mitophagy and beyond. Mol. Cell 2023, 83, 3404–3420. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; MacFabe, D.F.; et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, S.; Li, Y.; Yu, S.; Zhao, Y. SIRT1/PGC-1α Signaling Promotes Mitochondrial Functional Recovery and Reduces Apoptosis after Intracerebral Hemorrhage in Rats. Front. Mol. Neurosci. 2017, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Elias-Oliveira, J.; Leite, J.A.; Pereira, Í.S.; Guimarães, J.B.; Manso, G.; Silva, J.S.; Tostes, R.C.; Carlos, D. NLR and Intestinal Dysbiosis-Associated Inflammatory Illness: Drivers or Dampers? Front. Immunol. 2020, 11, 1810. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in oxidative stress, inflammation and aging: From mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef]
- Xu, X.; Wen, Z. The mediating role of inflammaging between mitochondrial dysfunction and sarcopenia in aging: A review. Am. J. Clin. Exp. Immunol. 2023, 12, 109–126. [Google Scholar]
- Lei, Y.; Gan, M.; Qiu, Y.; Chen, Q.; Wang, X.; Liao, T.; Zhao, M.; Chen, L.; Zhang, S.; Zhao, Y.; et al. The role of mitochondrial dynamics and mitophagy in skeletal muscle atrophy: From molecular mechanisms to therapeutic insights. Cell. Mol. Biol. Lett. 2024, 29, 59. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport. Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Varghese, N.; Werner, S.; Grimm, A.; Eckert, A. Dietary Mitophagy Enhancer: A Strategy for Healthy Brain Aging? Antioxidants 2020, 9, 932. [Google Scholar] [CrossRef]
- Jayatunga, D.P.W.; Hone, E.; Khaira, H.; Lunelli, T.; Singh, H.; Guillemin, G.J.; Fernando, B.; Garg, M.L.; Verdile, G.; Martins, R.N. Therapeutic Potential of Mitophagy-Inducing Microflora Metabolite, Urolithin A for Alzheimer’s Disease. Nutrients 2021, 13, 3744. [Google Scholar] [CrossRef]
- Leng, P.; Wang, Y.; Xie, M. Ellagic Acid and Gut Microbiota: Interactions, and Implications for Health. Food Sci. Nutr. 2025, 13, e70133. [Google Scholar] [CrossRef]
- Bhansali, S.; Bhansali, A.; Dutta, P.; Walia, R.; Dhawan, V. Metformin upregulates mitophagy in patients with T2DM: A randomized placebo-controlled study. J. Cell. Mol. Med. 2020, 24, 2832–2846. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, S.; Bagherniya, M.; Askari, G.; Read, M.I.; Sahebkar, A. The effect of fasting or calorie restriction on mitophagy induction: A literature review. J. Cachexia Sarcopenia Muscle 2020, 11, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xue, H.; Yue, Y.; Hao, S.; Huang, S.H.; Zhang, Z. Role of mitophagy in the neurodegenerative diseases and its pharmacological advances: A review. Front. Mol. Neurosci. 2022, 15, 1014251. [Google Scholar] [CrossRef]
- Lambourne, O.A.; Bell, S.; Wilhelm, L.P.; Yarbrough, E.B.; Holly, G.G.; Russell, O.M.; Alghamdi, A.M.; Fdel, A.M.; Varricchio, C.; Lane, E.L.; et al. PINK1-Dependent Mitophagy Inhibits Elevated Ubiquitin Phosphorylation Caused by Mitochondrial Damage. J. Med. Chem. 2023, 66, 7645–7656. [Google Scholar] [CrossRef]
- Hein, Z.M.; Vishnumukkala, T.; Karikalan, B.; Alkatiri, A.; Hussan, F.; Jagadeesan, S.; Kamaruzzaman, M.A.; Che Ramli, M.D.; Che Mohd Nassir, C.M.N.; Gopalakrishna, P.K. Autophagy and Alzheimer’s Disease: Mechanisms and Impact Beyond the Brain. Cells 2025, 14, 911. [Google Scholar] [CrossRef]
- Palanivelu, L.; Chang, C.W.; Li, S.J.; Liang, Y.W.; Lo, Y.C.; Chen, Y.Y. Interplay of Neuroinflammation and Gut Microbiota Dysbiosis in Alzheimer’s Disease Using Diffusion Kurtosis Imaging Biomarker in 3 × Tg-AD Mouse Models. ACS Chem. Neurosci. 2025, 16, 1511–1528. [Google Scholar] [CrossRef]
- Moyzis, A.G.; Sadoshima, J.; Gustafsson, Å.B. Mending a broken heart: The role of mitophagy in cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H183–H192. [Google Scholar] [CrossRef]
- Siddall, H.K.; Yellon, D.M.; Ong, S.B.; Mukherjee, U.A.; Burke, N.; Hall, A.R.; Angelova, P.R.; Ludtmann, M.H.; Deas, E.; Davidson, S.M.; et al. Loss of PINK1 increases the heart’s vulnerability to ischemia-reperfusion injury. PLoS ONE 2013, 8, e62400. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, C.; Zhao, H.; Zhao, C.; Chen, Y.; Wang, Y.; McClain, C.; Feng, W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J. Nutr. Biochem. 2015, 26, 337–344. [Google Scholar] [CrossRef]
- Wu, X.; Yang, J.; Liao, J.; Zalán, Z.; Kan, J.; Suo, H.; Zhang, Y.; Song, J. Identification of Critical Gut Metabolites Mediating the Protective Effects of Lacticaseibacillus paracasei on Myocardial Injury in Mice. J. Agric. Food Chem. 2025, 73, 18822–18840. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bucci, C.; Marzetti, E. Mitochondrial-Derived Vesicles: The Good, the Bad, and the Ugly. Int. J. Mol. Sci. 2023, 24, 13835. [Google Scholar] [CrossRef]
- Praharaj, P.P.; Naik, P.P.; Panigrahi, D.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Sethi, G.; Bhutia, S.K. Intricate role of mitochondrial lipid in mitophagy and mitochondrial apoptosis: Its implication in cancer therapeutics. Cell. Mol. Life Sci. 2019, 76, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, Z.; Cai, P.; Li, W.; Xu, Y.; Zhao, Y.; Xia, S.; Shao, Q.; Wang, H. Megamitochondria plasticity: Function transition from adaption to disease. Mitochondrion 2023, 71, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tan, J.; Miao, Y.; Zhang, Q. Mitochondrial Dynamics, Mitophagy, and Mitochondria-Endoplasmic Reticulum Contact Sites Crosstalk Under Hypoxia. Front. Cell Dev. Biol. 2022, 10, 848214. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Chen, Y.; Dorn, G.W., 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Zanfardino, P.; Amati, A.; Perrone, M.; Petruzzella, V. The Balance of MFN2 and OPA1 in Mitochondrial Dynamics, Cellular Homeostasis, and Disease. Biomolecules 2025, 15, 433. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Bremer, K.; Kocha, K.M.; Snider, T.; Moyes, C.D. Sensing and responding to energetic stress: The role of the AMPK-PGC1α-NRF1 axis in control of mitochondrial biogenesis in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 4–12. [Google Scholar] [CrossRef]
- Singh, A.; Schurman, S.H.; Bektas, A.; Kaileh, M.; Roy, R.; Wilson, D.M., 3rd; Sen, R.; Ferrucci, L. Aging and Inflammation. Cold Spring Harb. Perspect. Med. 2024, 14, a041197. [Google Scholar] [CrossRef]
- de Oliveira Neto, L.; Tavares, V.D.O.; Agrícola, P.M.D.; de Oliveira, L.P.; Sales, M.C.; de Sena-Evangelista, K.C.M.; Gomes, I.C.; Galvão-Coelho, N.L.; Pedrosa, L.F.C.; Lima, K.C. Factors associated with inflamm-aging in institutionalized older people. Sci. Rep. 2021, 11, 18333. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, G.; Im, S.H. Gut microbiota in regulatory T cell generation and function: Mechanisms and health implications. Gut Microbes 2025, 17, 2516702. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Liang, H.; Wang, W.; Li, B.; Liu, T.; Huang, Y.; Zhang, Z.; Qin, Y.; Zhou, X.; et al. The roles and applications of short-chain fatty acids derived from microbial fermentation of dietary fibers in human cancer. Front. Nutr. 2023, 10, 1243390. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Wen, S.; Long-kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D.V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [Google Scholar] [CrossRef]
- Chae, Y.R.; Lee, Y.R.; Kim, Y.S.; Park, H.Y. Diet-Induced Gut Dysbiosis and Leaky Gut Syndrome. J. Microbiol. Biotechnol. 2024, 34, 747–756. [Google Scholar] [CrossRef]
- Kumar, P.; Schroder, E.A.; Rajaram, M.V.S.; Harris, E.N.; Ganesan, L.P. The Battle of LPS Clearance in Host Defense vs. Inflammatory Signaling. Cells 2024, 13, 1590. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, C.; Wang, Q.; Feng, S.; Fang, Y.; Zhang, S. The impact of aging on intestinal mucosal immune function and clinical applications. Front. Immunol. 2022, 13, 1029948. [Google Scholar] [CrossRef]
- Babakhani, K.; Kucinskas, A.L.; Ye, X.; Giles, E.D.; Sun, Y. Aging immunity: Unraveling the complex nexus of diet, gut microbiome, and immune function. Immunometabolism 2025, 7, e00061. [Google Scholar] [CrossRef]
- Jagger, A.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T cells and the immune aging process: A mini-review. Gerontology 2014, 60, 130–137. [Google Scholar] [CrossRef]
- Austermann, J.; Roth, J.; Barczyk-Kahlert, K. The Good and the Bad: Monocytes’ and Macrophages’ Diverse Functions in Inflammation. Cells 2022, 11, 1979. [Google Scholar] [CrossRef]
- Seidler, S.; Zimmermann, H.W.; Bartneck, M.; Trautwein, C.; Tacke, F. Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults. BMC Immunol. 2010, 11, 30. [Google Scholar] [CrossRef]
- Mandala, W.; Harawa, V.; Munyenyembe, A.; Soko, M.; Longwe, H. Optimization of stimulation and staining conditions for intracellular cytokine staining (ICS) for determination of cytokine-producing T cells and monocytes. Curr. Res. Immunol. 2021, 2, 184–193. [Google Scholar] [CrossRef]
- Frauenlob, T.; Neuper, T.; Mehinagic, M.; Dang, H.H.; Boraschi, D.; Horejs-Hoeck, J. Helicobacter pylori Infection of Primary Human Monocytes Boosts Subsequent Immune Responses to LPS. Front. Immunol. 2022, 13, 847958. [Google Scholar] [CrossRef] [PubMed]
- Tacconi, E.; Palma, G.; De Biase, D.; Luciano, A.; Barbieri, M.; de Nigris, F.; Bruzzese, F. Microbiota Effect on Trimethylamine N-Oxide Production: From Cancer to Fitness-A Practical Preventing Recommendation and Therapies. Nutrients 2023, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Mubbashir, A.; Khan, M.S.; Shaikh, Z.; Memon, A.; Alvares, J.; Azhar, A.; Jain, H.; Ahmed, R.; Kanagala, S.G. Trimethylamine N-oxide in cardiovascular disease: Pathophysiology and the potential role of statins. Life Sci. 2025, 361, 123304. [Google Scholar] [CrossRef]
- Weng, N.P.; Akbar, A.N.; Goronzy, J. CD28− T cells: Their role in the age-associated decline of immune function. Trends Immunol. 2009, 30, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.A.; Ryu, J.H.; Jo, Y.; Hong, C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int. J. Biol. Sci. 2023, 19, 1178–1191. [Google Scholar] [CrossRef]
- Cheng, J.; Yuan, Y.; Zhao, F.; Chen, J.; Chen, P.; Li, Y.; Yan, X.; Luo, C.; Shu, D.; Qu, H.; et al. Thymic T-Cell Production Is Associated With Changes in the Gut Microbiota in Young Chicks. Front. Immunol. 2021, 12, 700603. [Google Scholar] [CrossRef]
- Wang, Z. Polyamines instruct T-cell differentiation. Nat. Cell Biol. 2021, 23, 811. [Google Scholar] [CrossRef]
- Nakamura, A.; Matsumoto, M. Role of polyamines in intestinal mucosal barrier function. Semin. Immunopathol. 2025, 47, 9. [Google Scholar] [CrossRef]
- Xu, Q.; Zhu, J. The cGAS-STING signaling pathway in the regulation of pulmonary infections: A systematic review. Front. Cell. Infect. Microbiol. 2025, 15, 1628481. [Google Scholar] [CrossRef]
- Kwon, J.; Bakhoum, S.F. The Cytosolic DNA-Sensing cGAS-STING Pathway in Cancer. Cancer Discov. 2020, 10, 26–39. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, R.; He, Q.; Cui, C.; Song, J.; Guo, X.; Zang, N.; Yang, M.; Zou, Y.; Yang, J.; et al. cGAS-STING mediates cytoplasmic mitochondrial-DNA-induced inflammatory signal transduction during accelerated senescence of pancreatic β-cells induced by metabolic stress. FASEB J. 2022, 36, e22266. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Wen, J.; Wang, S.; Yuan, M.; Sun, H.; Shu, L.; Yang, X.; Pu, Y.; Cai, Z. Microglia in brain aging: An overview of recent basic science and clinical research developments. J. Biomed. Res. 2024, 38, 122–136. [Google Scholar] [CrossRef]
- Rizzo, F.R.; Musella, A.; De Vito, F.; Fresegna, D.; Bullitta, S.; Vanni, V.; Guadalupi, L.; Stampanoni Bassi, M.; Buttari, F.; Mandolesi, G.; et al. Tumor Necrosis Factor and Interleukin-1β Modulate Synaptic Plasticity during Neuroinflammation. Neural Plast. 2018, 2018, 8430123. [Google Scholar] [CrossRef] [PubMed]
- Cerna, C.; Vidal-Herrera, N.; Silva-Olivares, F.; Álvarez, D.; González-Arancibia, C.; Hidalgo, M.; Aguirre, P.; González-Urra, J.; Astudillo-Guerrero, C.; Jara, M.; et al. Fecal Microbiota Transplantation from Young-Trained Donors Improves Cognitive Function in Old Mice Through Modulation of the Gut-Brain Axis. Aging Dis. 2025, 16, 6. [Google Scholar] [CrossRef]
- Sultan, S.; Mottawea, W.; Yeo, J.; Hammami, R. Gut Microbiota Extracellular Vesicles as Signaling Molecules Mediating Host-Microbiota Communications. Int. J. Mol. Sci. 2021, 22, 13166. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1387. [Google Scholar] [CrossRef]
- Manni, G.; Buratta, S.; Pallotta, M.T.; Chiasserini, D.; Di Michele, A.; Emiliani, C.; Giovagnoli, S.; Pascucci, L.; Romani, R.; Bellezza, I.; et al. Extracellular Vesicles in Aging: An Emerging Hallmark? Cells 2023, 12, 527. [Google Scholar] [CrossRef]
- de Mol, J.; Kuiper, J.; Tsiantoulas, D.; Foks, A.C. The Dynamics of B Cell Aging in Health and Disease. Front. Immunol. 2021, 12, 733566. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.I.; Catalan-Dibene, J.; Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015, 74, 318–326. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. The interaction between gut microbiota and age-related changes in immune function and inflammation. Immun. Ageing 2013, 10, 31. [Google Scholar] [CrossRef]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Forslund, S.K. Fasting intervention and its clinical effects on the human host and microbiome. J. Intern. Med. 2023, 293, 166–183. [Google Scholar] [CrossRef]
- Ding, M.H.; Xu, P.G.; Wang, Y.; Ren, B.D.; Zhang, J.L. Resveratrol Attenuates Ankylosing Spondylitis in Mice by Inhibiting the TLR4/NF-κB/NLRP3 Pathway and Regulating Gut Microbiota. Immunol. Investig. 2023, 52, 194–209. [Google Scholar] [CrossRef]
- Booth, L.N.; Brunet, A. The Aging Epigenome. Mol. Cell 2016, 62, 728–744. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Epigenetic Mechanisms in Aging: Extrinsic Factors and Gut Microbiome. Genes 2024, 15, 1599. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Davie, J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003, 133 (Suppl. S7), 2485s–2493s. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Y.; Zhang, C.; Wu, R.; Yang, A.Y.; Gaspar, J.; Kong, A.N. Dietary Phytochemicals and Cancer Chemoprevention: A Perspective on Oxidative Stress, Inflammation, and Epigenetics. Chem. Res. Toxicol. 2016, 29, 2071–2095. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, C. Impact of dietary gut microbial metabolites on the epigenome. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2018, 373, 20170359. [Google Scholar] [CrossRef]
- Hossain, K.S.; Amarasena, S.; Mayengbam, S. B Vitamins and Their Roles in Gut Health. Microorganisms 2022, 10, 1168. [Google Scholar] [CrossRef]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef]
- Sheaffer, K.L.; Elliott, E.N.; Kaestner, K.H. DNA Hypomethylation Contributes to Genomic Instability and Intestinal Cancer Initiation. Cancer Prev. Res. 2016, 9, 534–546. [Google Scholar] [CrossRef]
- Mutirangura, A. A Hypothesis to Explain How the DNA of Elderly People Is Prone to Damage: Genome-Wide Hypomethylation Drives Genomic Instability in the Elderly by Reducing Youth-Associated Gnome-Stabilizing DNA Gaps. In Epigenetics; Meccariello, R., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Kiselev, I.S.; Baulina, N.M.; Favorova, O.O. Epigenetic Clock: DNA Methylation as a Marker of Biological Age and Age-Associated Diseases. Biochemistry 2025, 90 (Suppl. S1), S356–S372. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Lund, R.; Laiho, A.; Lundelin, K.; Ley, R.E.; Isolauri, E.; Salminen, S. Gut microbiota as an epigenetic regulator: Pilot study based on whole-genome methylation analysis. mBio 2014, 5, e02113-14. [Google Scholar] [CrossRef]
- Singh, S.; Giron, L.B.; Shaikh, M.W.; Shankaran, S.; Engen, P.A.; Bogin, Z.R.; Bambi, S.A.; Goldman, A.R.; Azevedo, J.; Orgaz, L.; et al. Distinct intestinal microbial signatures linked to accelerated systemic and intestinal biological aging. Microbiome 2024, 12, 31. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Ye, J.; Yi, H.; Zheng, X. Causal effect of gut microbiota on DNA methylation phenotypic age acceleration: A two-sample Mendelian randomization study. Sci. Rep. 2023, 13, 18830. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, A.; Sandeep, K.; Yadav, D. Epigenetic Regulation of Gut Microbial Dysbiosis. Indian J. Microbiol. 2021, 61, 125–129. [Google Scholar] [CrossRef]
- Chan, A.O.; Lam, S.K.; Wong, B.C.; Wong, W.M.; Yuen, M.F.; Yeung, Y.H.; Hui, W.M.; Rashid, A.; Kwong, Y.L. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut 2003, 52, 502–506. [Google Scholar] [CrossRef]
- Tolg, C.; Sabha, N.; Cortese, R.; Panchal, T.; Ahsan, A.; Soliman, A.; Aitken, K.J.; Petronis, A.; Bägli, D.J. Uropathogenic E. coli infection provokes epigenetic downregulation of CDKN2A (p16INK4A) in uroepithelial cells. Lab. Investig. 2011, 91, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Bierne, H. Cross Talk Between Bacteria and the Host Epigenetic Machinery. In Epigenetics of Infectious Diseases; Doerfler, W., Casadesús, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 113–158. [Google Scholar]
- Fardi, F.; Khasraghi, L.B.; Shahbakhti, N.; Salami Naseriyan, A.; Najafi, S.; Sanaaee, S.; Alipourfard, I.; Zamany, M.; Karamipour, S.; Jahani, M.; et al. An interplay between non-coding RNAs and gut microbiota in human health. Diabetes Res. Clin. Prac. 2023, 201, 110739. [Google Scholar] [CrossRef] [PubMed]
- Teodori, L.; Petrignani, I.; Giuliani, A.; Prattichizzo, F.; Gurău, F.; Matacchione, G.; Olivieri, F.; Coppari, S.; Albertini, M.C. Inflamm-aging microRNAs may integrate signals from food and gut microbiota by modulating common signalling pathways. Mech. Ageing Dev. 2019, 182, 111127. [Google Scholar] [CrossRef]
- Sarshar, M.; Scribano, D.; Ambrosi, C.; Palamara, A.T.; Masotti, A. Fecal microRNAs as Innovative Biomarkers of Intestinal Diseases and Effective Players in Host-Microbiome Interactions. Cancers 2020, 12, 2174. [Google Scholar] [CrossRef]
- Gevrekci, A. The roles of polyamines in microorganisms. World J. Microbiol. Biotechnol. 2017, 33, 204. [Google Scholar] [CrossRef]
- Sakamoto, A.; Terui, Y.; Uemura, T.; Igarashi, K.; Kashiwagi, K. Polyamines regulate gene expression by stimulating translation of histone acetyltransferase mRNAs. J. Biol. Chem. 2020, 295, 8736–8745. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Jho, E.H. A concise review of human brain methylome during aging and neurodegenerative diseases. BMB Rep. 2019, 52, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Parikh, I.J.; Estus, J.L.; Zajac, D.J.; Malik, M.; Maldonado Weng, J.; Tai, L.M.; Chlipala, G.E.; LaDu, M.J.; Green, S.J.; Estus, S. Murine Gut Microbiome Association With APOE Alleles. Front. Immunol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, F.R.; Ren, L.; Bai, J.M.; Wang, S.C.; Li, X.Y.; Yang, H.J.; Xiao, H.H. Multi-omics reveal the neuroprotective mechanisms of Xinshubao tablet against scopolamine-induced cognitive dysfunction in mice. Front. Pharmacol. 2025, 16, 1596728. [Google Scholar] [CrossRef]
- D’Amato, A.; Di Cesare Mannelli, L.; Lucarini, E.; Man, A.L.; Le Gall, G.; Branca, J.J.V.; Ghelardini, C.; Amedei, A.; Bertelli, E.; Regoli, M.; et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome 2020, 8, 140. [Google Scholar] [CrossRef]
- Starr, M.E.; Hu, Y.; Stromberg, A.J.; Carmical, J.R.; Wood, T.G.; Evers, B.M.; Saito, H. Gene expression profile of mouse white adipose tissue during inflammatory stress: Age-dependent upregulation of major procoagulant factors. Aging Cell 2013, 12, 194–206. [Google Scholar] [CrossRef]
- Gomes, L.R.; Menck, C.F.M.; Leandro, G.S. Autophagy Roles in the Modulation of DNA Repair Pathways. Int. J. Mol. Sci. 2017, 18, 2351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Fan, L.; Meng, C.C.; Wang, Y.J.; Deng, L.E.; Yuan, Z.; Zhang, J.P.; Li, Y.Y.; Lv, S.C. Gut microbiota influence frailty syndrome in older adults: Mechanisms and therapeutic strategies. Biogerontology 2024, 25, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, T.; Liu, F.; Huang, Y.; Xiao, Z.; Qian, Y.; Zhou, W. Influence of gut microbiota on resilience and its possible mechanisms. Int. J. Biol. Sci. 2023, 19, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Nowrasteh, G.; Zand, A.; Raposa, L.B.; Szabó, L.; Tomesz, A.; Molnár, R.; Kiss, I.; Orsós, Z.; Gerencsér, G.; Gyöngyi, Z.; et al. Fruit Extract, Rich in Polyphenols and Flavonoids, Modifies the Expression of DNMT and HDAC Genes Involved in Epigenetic Processes. Nutrients 2023, 15, 1867. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Mongelli, A.; Mengozzi, A.; Geiger, M.; Gorica, E.; Mohammed, S.A.; Paneni, F.; Ruschitzka, F.; Costantino, S. Mitochondrial epigenetics in aging and cardiovascular diseases. Front. Cardiovasc. Med. 2023, 10, 1204483. [Google Scholar] [CrossRef]
- Coppedè, F.; Stoccoro, A. Mitoepigenetics and Neurodegenerative Diseases. Front. Endocrinol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Fonseca Cabral, G.; Schaan, A.P.; Cavalcante, G.C.; Sena-Dos-Santos, C.; de Souza, T.P.; Souza Port’s, N.M.; Dos Santos Pinheiro, J.A.; Ribeiro-Dos-Santos, Â.; Vidal, A.F. Nuclear and Mitochondrial Genome, Epigenome and Gut Microbiome: Emerging Molecular Biomarkers for Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 9839. [Google Scholar] [CrossRef]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef]
- Gu, Y.; Wong, N.M.L.; Chan, C.C.H.; Wu, J.; Lee, T.M.C. The negative relationship between brain-age gap and psychological resilience defines the age-related neurocognitive status in older people. Geroscience 2025, 47, 4023–4040. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Liu, R.; Sun, B. Lactic Acid Bacteria and Aging: Unraveling the Interplay for Healthy Longevity. Aging Dis. 2023, 15, 1487–1498. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Boehme, M.; Guzzetta, K.E.; Wasén, C.; Cox, L.M. The gut microbiota is an emerging target for improving brain health during ageing. Gut Microbiome 2023, 4, e2. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Warner, B.B. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr. Res. 2019, 85, 216–224. [Google Scholar] [CrossRef]
- Alpino, G.; Pereira-Sol, G.A.; Dias, M.M.E.; Aguiar, A.S.; Peluzio, M. Beneficial effects of butyrate on brain functions: A view of epigenetic. Crit. Rev. Food Sci. Nutr. 2024, 64, 3961–3970. [Google Scholar] [CrossRef]
- Karpova, N.N. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology 2014, 76 Pt CI, 709–718. [Google Scholar] [CrossRef]
- Qian, X.H.; Xie, R.Y.; Liu, X.L.; Chen, S.D.; Tang, H.D. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252–1266. [Google Scholar] [CrossRef]
- Ji, X.; Wang, J.; Lan, T.; Zhao, D.; Xu, P. Gut microbial metabolites and the brain-gut axis in Alzheimer’s disease: A review. Biomol. Biomed. 2025, 26, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jian, W. Signal Pathways and Intestinal Flora through Trimethylamine N-oxide in Alzheimer’s Disease. Curr. Protein Pept. Sci. 2023, 24, 721–736. [Google Scholar] [CrossRef] [PubMed]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Du, X.; Zhu, Y.; Wang, L.; Hu, J.; Sun, Y. Vagus Nerve and Gut-Brain Communication. Neuroscientist 2025, 31, 262–278. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Fiocco, A.J. Chronic Stress and Cognitive Aging. In PsychoNeuroImmunology: Volume 1: Integration of Psychology, Neurology, and Immunology; Rezaei, N., Yazdanpanah, N., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 381–406. [Google Scholar]
- Glinert, A.; Turjeman, S.; Elliott, E.; Koren, O. Microbes, metabolites and (synaptic) malleability, oh my! The effect of the microbiome on synaptic plasticity. Biol. Rev. Camb. Philos. Soc. 2022, 97, 582–599. [Google Scholar] [CrossRef]
- Han, H.; Yao, J.; Wu, J.; Mao, S.; Pan, H.; Qv, L.; Zhu, G.; Ren, J.; Yu, Y.; Xuan, F.; et al. Implications of neurogenesis in depression through BDNF: Rodent models, regulatory pathways, gut microbiota, and potential therapy. Mol. Psychiatry 2025, 30, 4409–4421. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zheng, L.J.; Liu, Y.; Ye, Y.B.; Luo, S.; Lu, G.M.; Gong, D.; Zhang, L.J. The gut microbiota-inflammation-brain axis in end-stage renal disease: Perspectives from default mode network. Theranostics 2019, 9, 8171–8181. [Google Scholar] [CrossRef]
- Adikari, A.; Appukutty, M.; Kuan, G. Effects of Daily Probiotics Supplementation on Anxiety Induced Physiological Parameters among Competitive Football Players. Nutrients 2020, 12, 1920. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Liu, L.; van Groen, T.; Kadish, I.; Tollefsbol, T.O. DNA methylation impacts on learning and memory in aging. Neurobiol. Aging 2009, 30, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Ensink, E.; Li, P.; Gordevičius, J.; Marshall, L.L.; George, S.; Pospisilik, J.A.; Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov. Disord. 2022, 37, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2022, 62, 3509–3534. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.; Weinkove, D. The Gut Microbiota and Ageing. Subcell. Biochem. 2018, 90, 351–371. [Google Scholar] [CrossRef]
- Haran, J.P.; McCormick, B.A. Aging, Frailty, and the Microbiome-How Dysbiosis Influences Human Aging and Disease. Gastroenterology 2021, 160, 507–523. [Google Scholar] [CrossRef]
- Kociszewska, D.; Vlajkovic, S. Age-Related Hearing Loss: The Link between Inflammaging, Immunosenescence, and Gut Dysbiosis. Int. J. Mol. Sci. 2022, 23, 7348. [Google Scholar] [CrossRef]
- Zhang, G.; Ghosh, S. Molecular mechanisms of NF-kappaB activation induced by bacterial lipopolysaccharide through Toll-like receptors. J. Endotoxin Res. 2000, 6, 453–457. [Google Scholar] [CrossRef]
- Hohman, L.S.; Osborne, L.C. A gut-centric view of aging: Do intestinal epithelial cells contribute to age-associated microbiota changes, inflammaging, and immunosenescence? Aging Cell 2022, 21, e13700. [Google Scholar] [CrossRef]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef]
- Dehghani, F.; Abdollahi, S.; Shidfar, F.; Clark, C.C.T.; Soltani, S. Probiotics supplementation and brain-derived neurotrophic factor (BDNF): A systematic review and meta-analysis of randomized controlled trials. Nutr. Neurosci. 2023, 26, 942–952. [Google Scholar] [CrossRef]
- Cheslow, L.; Snook, A.E.; Waldman, S.A. Biomarkers for Managing Neurodegenerative Diseases. Biomolecules 2024, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Stirling, K.; Yang, J.J.; Zhang, L. Gut microbiota and diabetes: From correlation to causality and mechanism. World J. Diabetes 2020, 11, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Mei, Y.; Li, W.; Wang, B.; Chen, Z.; Wu, X.; Lin, Y.; Wang, M. Gut microbiota: An emerging target connecting polycystic ovarian syndrome and insulin resistance. Front. Cell. Infect. Microbiol. 2025, 15, 1508893. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e616. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Fernandez, M.L. Trimethylamine N-Oxide (TMAO), Diet and Cardiovascular Disease. Curr. Atheroscler. Rep. 2021, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Park, S. Sarcopenia Is a Cause and Consequence of Metabolic Dysregulation in Aging Humans: Effects of Gut Dysbiosis, Glucose Dysregulation, Diet and Lifestyle. Cells 2022, 11, 338. [Google Scholar] [CrossRef]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. Biomed. Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef]
- Vemuri, R.; Gundamaraju, R.; Shinde, T.; Perera, A.P.; Basheer, W.; Southam, B.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Tristram, S.; et al. Lactobacillus acidophilus DDS-1 Modulates Intestinal-Specific Microbiota, Short-Chain Fatty Acid and Immunological Profiles in Aging Mice. Nutrients 2019, 11, 1297. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Sassi, F. Gut Microbiota, Immune System, and Bone. Calcif. Tissue Int. 2018, 102, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Seely, K.D.; Kotelko, C.A.; Douglas, H.; Bealer, B.; Brooks, A.E. The Human Gut Microbiota: A Key Mediator of Osteoporosis and Osteogenesis. Int. J. Mol. Sci. 2021, 22, 9452. [Google Scholar] [CrossRef]
- Feng, B.; Lu, J.; Han, Y.; Han, Y.; Qiu, X.; Zeng, Z. The role of short-chain fatty acids in the regulation of osteoporosis: New perspectives from gut microbiota to bone health: A review. Medicine 2024, 103, e39471. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, N.; Tanwar, S.S. The current findings on the gut-liver axis and the molecular basis of NAFLD/NASH associated with gut microbiome dysbiosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 11541–11579. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Solís-Urra, P.; Rodríguez-Rodríguez, F.; Olivares-Arancibia, J.; Navarro-Oliveros, M.; Abadía-Molina, F.; Álvarez-Mercado, A.I. The Gut Barrier, Intestinal Microbiota, and Liver Disease: Molecular Mechanisms and Strategies to Manage. Int. J. Mol. Sci. 2020, 21, 8351. [Google Scholar] [CrossRef]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef]
- Long, C.; Zhou, X.; Xia, F.; Zhou, B. Intestinal Barrier Dysfunction and Gut Microbiota in Non-Alcoholic Fatty Liver Disease: Assessment, Mechanisms, and Therapeutic Considerations. Biology 2024, 13, 243. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan Metabolism by Gut Microbiome and Gut-Brain-Axis: An in silico Analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Connell, E.; Le Gall, G.; Pontifex, M.G.; Sami, S.; Cryan, J.F.; Clarke, G.; Müller, M.; Vauzour, D. Microbial-derived metabolites as a risk factor of age-related cognitive decline and dementia. Mol. Neurodegener. 2022, 17, 43. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.F.; Ma, C.L.; Wei, H.; Li, B.M.; Luo, J. Alleviation of Anxiety/Depressive-Like Behaviors and Improvement of Cognitive Functions by Lactobacillus plantarum WLPL04 in Chronically Stressed Mice. Can. J. Infect. Dis. Med. Microbiol. = J. Can. Mal. Infect. Microbiol. Medicale 2021, 2021, 6613903. [Google Scholar] [CrossRef]
- Zhou, C.B.; Fang, J.Y. The regulation of host cellular and gut microbial metabolism in the development and prevention of colorectal cancer. Crit. Rev. Microbiol. 2018, 44, 436–454. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Yu, T.; Fang, X.; Lou, L.; Xin, S.; Ji, L.; Jiang, F.; Lou, Y. Fusobacterium nucleatum Promotes the Progression of Colorectal Cancer Through Cdk5-Activated Wnt/β-Catenin Signaling. Front. Microbiol. 2020, 11, 545251. [Google Scholar] [CrossRef]
- Kong, M.; Guo, L.; Xu, W.; He, C.; Jia, X.; Zhao, Z.; Gu, Z. Aging-associated accumulation of mitochondrial DNA mutations in tumor origin. Life Med. 2022, 1, 149–167. [Google Scholar] [CrossRef]
- Palatella, M.; Guillaume, S.M.; Linterman, M.A.; Huehn, J. The dark side of Tregs during aging. Front. Immunol. 2022, 13, 940705. [Google Scholar] [CrossRef] [PubMed]
- Sittipo, P.; Choi, J.; Lee, S.; Lee, Y.K. The function of gut microbiota in immune-related neurological disorders: A review. J. Neuroinflamm. 2022, 19, 154. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, P.; Kulkarni, A.; Bandre, A.; Subramanian, S.; Mahajan, S. Role of gut microbiota in autoimmune diseases: A review. J. Vaccines Immunol. 2021, 6, 163. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Sunder, S.; Verma, S.R. Disease-associated dysbiosis and potential therapeutic role of Akkermansia muciniphila, a mucus degrading bacteria of gut microbiome. Folia Microbiol. 2022, 67, 811–824. [Google Scholar] [CrossRef]
- Adolph, T.E.; Meyer, M.; Schwärzler, J.; Mayr, L.; Grabherr, F.; Tilg, H. The metabolic nature of inflammatory bowel diseases. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 753–767. [Google Scholar] [CrossRef]
- Eladham, M.W.; Selvakumar, B.; Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Ibrahim, S.M.; Halwani, R. Unraveling the gut-Lung axis: Exploring complex mechanisms in disease interplay. Heliyon 2024, 10, e24032. [Google Scholar] [CrossRef]
- Paudel, K.R.; Dharwal, V.; Patel, V.K.; Galvao, I.; Wadhwa, R.; Malyla, V.; Shen, S.S.; Budden, K.F.; Hansbro, N.G.; Vaughan, A.; et al. Role of Lung Microbiome in Innate Immune Response Associated With Chronic Lung Diseases. Front Med. 2020, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Bhagchandani, T.; Rai, A.; Sardarni, U.K.; Bhavesh, N.S.; Gulati, S.; Malik, R.; Tandon, R. Short-Chain Fatty Acid (SCFA) as a Connecting Link between Microbiota and Gut-Lung Axis-A Potential Therapeutic Intervention to Improve Lung Health. ACS Omega 2024, 9, 14648–14671. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, M.H.; Mao, J.; Karstens, L.A.; Ma, L.; Amundsen, C.L.; Schmader, K.E.; Siddiqui, N.Y. The Urinary Microbiome in Postmenopausal Women with Recurrent Urinary Tract Infections. J. Urol. 2021, 206, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Zagaglia, C.; Ammendolia, M.G.; Maurizi, L.; Nicoletti, M.; Longhi, C. Urinary Tract Infections Caused by Uropathogenic Escherichia coli Strains-New Strategies for an Old Pathogen. Microorganisms 2022, 10, 1425. [Google Scholar] [CrossRef]
- Rodriguez-Mañas, L. Urinary tract infections in the elderly: A review of disease characteristics and current treatment options. Drugs Context 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Meiners, F.; Ortega-Matienzo, A.; Fuellen, G.; Barrantes, I. Gut microbiome-mediated health effects of fiber and polyphenol-rich dietary interventions. Front. Nutr. 2025, 12, 1647740. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Jin, J.; Bian, Y.; Gu, Z.; Lin, M. Association Between Dietary Fiber Intake and Prevalence of Chronic Obstructive Pulmonary Disease in a Middle-Aged and Elderly Population: A Study Based on the National Health and Nutrition Examination Survey Database. Chronic Obs. Pulm. Dis. 2024, 11, 216–228. [Google Scholar] [CrossRef]
- Juárez-Fernández, M.; Porras, D.; García-Mediavilla, M.V.; Román-Sagüillo, S.; González-Gallego, J.; Nistal, E.; Sánchez-Campos, S. Aging, Gut Microbiota and Metabolic Diseases: Management through Physical Exercise and Nutritional Interventions. Nutrients 2020, 13, 16. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef]
- Theis, B.F.; Park, J.S.; Kim, J.S.A.; Zeydabadinejad, S.; Vijay-Kumar, M.; Yeoh, B.S.; Saha, P. Gut Feelings: How Microbes, Diet, and Host Immunity Shape Disease. Biomedicines 2025, 13, 1357. [Google Scholar] [CrossRef]
- Verhoog, S.; Taneri, P.E.; Roa Díaz, Z.M.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients 2019, 11, 1565. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean diet and health: Food effects on gut microbiota and disease control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Dingeo, G.; Brito, A.; Samouda, H.; Iddir, M.; La Frano, M.R.; Bohn, T. Phytochemicals as modifiers of gut microbial communities. Food Funct. 2020, 11, 8444–8471. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef]
- Ishaq, M.; Khan, A.; Bacha, A.S.; Shah, T.; Hanif, A.; Ahmad, A.A.; Ke, W.; Li, F.; Ud Din, A.; Ding, Z.; et al. Microbiota Targeted Interventions of Probiotic Lactobacillus as an Anti-Ageing Approach: A Review. Antioxidants 2021, 10, 1930. [Google Scholar] [CrossRef]
- Nakagawa, H.; Shiozaki, T.; Kobatake, E.; Hosoya, T.; Moriya, T.; Sakai, F.; Taru, H.; Miyazaki, T. Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 2016, 15, 227–236. [Google Scholar] [CrossRef]
- Salami, M. Interplay of Good Bacteria and Central Nervous System: Cognitive Aspects and Mechanistic Considerations. Front. Neurosci. 2021, 15, 613120. [Google Scholar] [CrossRef]
- Zhong, Y.; Zheng, C.; Zheng, J.H.; Xu, S.C. The relationship between intestinal flora changes and osteoporosis in rats with inflammatory bowel disease and the improvement effect of probiotics. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5697–5702. [Google Scholar] [CrossRef]
- Li, P.; Ji, B.; Luo, H.; Sundh, D.; Lorentzon, M.; Nielsen, J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. NPJ Biofilms Microbiomes 2022, 8, 84. [Google Scholar] [CrossRef]
- Bashir, H.H.; Hasnain, M.A.; Abbas, A.; Lee, J.H.; Moon, G.S. The Impact of Fermented Dairy Products and Probiotics on Bone Health Improvement. Food Sci. Anim. Resour. 2025, 45, 449–467. [Google Scholar] [CrossRef]
- Davidson, G.L.; Cooke, A.C.; Johnson, C.N.; Quinn, J.L. The gut microbiome as a driver of individual variation in cognition and functional behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170286. [Google Scholar] [CrossRef]
- Singh, V.K.; Hu, X.H.; Singh, A.K.; Solanki, M.K.; Vijayaraghavan, P.; Srivastav, R.; Joshi, N.K.; Kumari, M.; Singh, S.K.; Wang, Z.; et al. Precision nutrition-based strategy for management of human diseases and healthy aging: Current progress and challenges forward. Front. Nutr. 2024, 11, 1427608. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Pellanda, P.; Ghosh, T.S.; O’Toole, P.W. Understanding the impact of age-related changes in the gut microbiome on chronic diseases and the prospect of elderly-specific dietary interventions. Curr. Opin. Biotechnol. 2021, 70, 48–55. [Google Scholar] [CrossRef]
- Varghese, S.; Rao, S.; Khattak, A.; Zamir, F.; Chaari, A. Physical Exercise and the Gut Microbiome: A Bidirectional Relationship Influencing Health and Performance. Nutrients 2024, 16, 3663. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Xu, Y.; Lai, H.Y.; Yang, M.; Cheng, L.; Liu, X.; Sun, X.; Yang, Y.; Wang, J.; Lv, W.; et al. Effects of combined aerobic and resistance training on gut microbiota and cardiovascular risk factors in physically active elderly women: A randomized controlled trial. Front. Physiol. 2022, 13, 1004863. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, Y.H.; Chuang, H.L.; Chiu, C.C.; Huang, C.C. Investigation of the Effects of Microbiota on Exercise Physiological Adaption, Performance, and Energy Utilization Using a Gnotobiotic Animal Model. Front. Microbiol. 2019, 10, 1906. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Zawistowska-Rojek, A.; Tyski, S. Drugs Versus Microbiota: How Pharmacotherapy Affects Gut and Probiotic Bacteria. Pharmaceuticals 2025, 18, 1372. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated With Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid-Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef]
- Ji, J.; Jin, W.; Liu, S.J.; Jiao, Z.; Li, X. Probiotics, prebiotics, and postbiotics in health and disease. MedComm 2023, 4, e420. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Staley, C.; Sadowsky, M.J. Faecal microbiota transplantation for Clostridioides difficile: Mechanisms and pharmacology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Xu, C.; Kumar, A. Advanced computational tools, artificial intelligence and machine-learning approaches in gut microbiota and biomarker identification. Front. Med. Technol. 2024, 6, 1434799. [Google Scholar] [CrossRef] [PubMed]
- Probul, N.; Huang, Z.; Saak, C.C.; Baumbach, J.; List, M. AI in microbiome-related healthcare. Microb. Biotechnol. 2024, 17, e70027. [Google Scholar] [CrossRef]
- Rubas, N.C.; Torres, A.; Maunakea, A.K. The Gut Microbiome and Epigenomic Reprogramming: Mechanisms, Interactions, and Implications for Human Health and Disease. Int. J. Mol. Sci. 2025, 26, 8658. [Google Scholar] [CrossRef]
- Pyne, D.B.; West, N.P.; Cox, A.J.; Cripps, A.W. Probiotics supplementation for athletes-clinical and physiological effects. Eur. J. Sport Sci. 2015, 15, 63–72. [Google Scholar] [CrossRef]
- Aarts, E.; Ederveen, T.H.A.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Zhou, Y.Y.; Zhou, G.L.; Li, Y.C.; Yuan, J.; Li, X.H.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Schmidtner, A.K.; Slattery, D.A.; Gläsner, J.; Hiergeist, A.; Gryksa, K.; Malik, V.A.; Hellmann-Regen, J.; Heuser, I.; Baghai, T.C.; Gessner, A.; et al. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl. Psychiatry 2019, 9, 223. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Trush, E.A.; Poluektova, E.A.; Beniashvilli, A.G.; Shifrin, O.S.; Poluektov, Y.M.; Ivashkin, V.T. The Evolution of Human Probiotics: Challenges and Prospects. Probiotics Antimicrob. Proteins 2020, 12, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Sakagianni, A.; Koufopoulou, C.; Koufopoulos, P.; Feretzakis, G.; Anastasiou, A.; Theodorakis, N.; Myrianthefs, P. Influence of Microbiome Interactions on Antibiotic Resistance Development in the ICU Environment: Insights and Opportunities with Machine Learning. Acta Microbiol. Hell. 2025, 70, 14. [Google Scholar] [CrossRef]
- Muller, E.; Shiryan, I.; Borenstein, E. Multi-omic integration of microbiome data for identifying disease-associated modules. Nat. Commun. 2024, 15, 2621. [Google Scholar] [CrossRef]
- Schupack, D.A.; Mars, R.A.T.; Voelker, D.H.; Abeykoon, J.P.; Kashyap, P.C. The promise of the gut microbiome as part of individualized treatment strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 7–25. [Google Scholar] [CrossRef]
- Dwiyanto, J.; Ayub, Q.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Geographical separation and ethnic origin influence the human gut microbial composition: A meta-analysis from a Malaysian perspective. Microb. Genom. 2021, 7, 000619. [Google Scholar] [CrossRef]
- Yang, S.Y.; Han, S.M.; Lee, J.Y.; Kim, K.S.; Lee, J.E.; Lee, D.W. Advancing Gut Microbiome Research: The Shift from Metagenomics to Multi-Omics and Future Perspectives. J. Microbiol. Biotechnol. 2025, 35, e2412001. [Google Scholar] [CrossRef]
- Chin, V.K.; Yong, V.C.; Chong, P.P.; Amin Nordin, S.; Basir, R.; Abdullah, M. Mycobiome in the Gut: A Multiperspective Review. Mediat. Inflamm. 2020, 2020, 9560684. [Google Scholar] [CrossRef]
- Shaughnessy, D.T.; McAllister, K.; Worth, L.; Haugen, A.C.; Meyer, J.N.; Domann, F.E.; Van Houten, B.; Mostoslavsky, R.; Bultman, S.J.; Baccarelli, A.A.; et al. Mitochondria, energetics, epigenetics, and cellular responses to stress. Environ. Health Perspect. 2014, 122, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Xiang, H.; Wang, J.; Jiang, Y.; Pan, C.; Ji, B.; Zhang, A. Fecal microbiota transplantation: A novel strategy for treating Alzheimer’s disease. Front. Microbiol. 2023, 14, 1281233. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Kochar, B.D.; Kennelty, K.; Ernst, M.E.; Chan, A.T. Emerging approaches to polypharmacy among older adults. Nat. Aging 2021, 1, 347–356. [Google Scholar] [CrossRef]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef]
- Rouskas, K.; Guela, M.; Pantoura, M.; Pagkalos, I.; Hassapidou, M.; Lalama, E.; Pfeiffer, A.F.H.; Decorte, E.; Cornelissen, V.; Wilson-Barnes, S.; et al. The Influence of an AI-Driven Personalized Nutrition Program on the Human Gut Microbiome and Its Health Implications. Nutrients 2025, 17, 1260. [Google Scholar] [CrossRef]
- Asar, R.; Erenler, S.; Devecioglu, D.; Ispirli, H.; Karbancioglu-Guler, F.; Ozturk, H.I.; Dertli, E. Understanding the Functionality of Probiotics on the Edge of Artificial Intelligence (AI) Era. Fermentation 2025, 11, 259. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X. Current in Vitro and Animal Models for Understanding Foods: Human Gut-Microbiota Interactions. J. Agric. Food Chem. 2022, 70, 12733–12745. [Google Scholar] [CrossRef]
- Hoffmann, P.; Burmester, M.; Langeheine, M.; Brehm, R.; Empl, M.T.; Seeger, B.; Breves, G. Caco-2/HT29-MTX co-cultured cells as a model for studying physiological properties and toxin-induced effects on intestinal cells. PLoS ONE 2021, 16, e0257824. [Google Scholar] [CrossRef]
- Kim, R.; Attayek, P.J.; Wang, Y.; Furtado, K.L.; Tamayo, R.; Sims, C.E.; Allbritton, N.L. An in vitro intestinal platform with a self-sustaining oxygen gradient to study the human gut/microbiome interface. Biofabrication 2019, 12, 015006. [Google Scholar] [CrossRef]
- Mohebali, N.; Ekat, K.; Kreikemeyer, B.; Breitrück, A. Barrier Protection and Recovery Effects of Gut Commensal Bacteria on Differentiated Intestinal Epithelial Cells In Vitro. Nutrients 2020, 12, 2251. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Nadda, R.; Repaka, R. Advances and challenges in organ-on-chip technology: Toward mimicking human physiology and disease in vitro. Med. Biol. Eng. Comput. 2024, 62, 1925–1957. [Google Scholar] [CrossRef]
- Dias, C.K.; Starke, R.; Pylro, V.S.; Morais, D.K. Database limitations for studying the human gut microbiome. PeerJ Comput. Sci. 2020, 6, e289. [Google Scholar] [CrossRef]
- Fox, J.D.; Sims, A.; Ross, M.; Bettag, J.; Wilder, A.; Natrop, D.; Borsotti, A.; Kolli, S.; Mehta, S.; Verma, H.; et al. Bioinformatic Methodologies in Assessing Gut Microbiota. Microbiol. Res. 2024, 15, 2554–2574. [Google Scholar] [CrossRef]
- Hugerth, L.W.; Andersson, A.F. Analysing Microbial Community Composition through Amplicon Sequencing: From Sampling to Hypothesis Testing. Front. Microbiol. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Qian, X.B.; Chen, T.; Xu, Y.P.; Chen, L.; Sun, F.X.; Lu, M.P.; Liu, Y.X. A guide to human microbiome research: Study design, sample collection, and bioinformatics analysis. Chin. Med. J. 2020, 133, 1844–1855. [Google Scholar] [CrossRef]
- Dave, M.; Higgins, P.D.; Middha, S.; Rioux, K.P. The human gut microbiome: Current knowledge, challenges, and future directions. Transl. Res. 2012, 160, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Bertorello, S.; Cei, F.; Fink, D.; Niccolai, E.; Amedei, A. The Future Exploring of Gut Microbiome-Immunity Interactions: From In Vivo/Vitro Models to In Silico Innovations. Microorganisms 2024, 12, 1828. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).