Abstract
Background: Looking for anomalies and vascular control gains a central role in colon cancer surgery. Complete mesocolic excision (CME) presents technical challenges, primarily due to the considerable variability in the arterial configuration of the right colon. The importance of understanding colonic vascular anatomy has become more prominent with the adoption of this surgical technique. The aim of this study is to systematically review the vascular anatomical variations in the superior mesenteric artery (SMA) in the setting of extended lymphadenectomy for CME in right colon cancer and to show its impact in clinical practice. Methods: A systematic review of the literature on Medline (PubMed), Web of Science (WOS), and Scopus was performed according to PRISMA guidelines. The following criteria were set for inclusion: (1) studies reporting minimally invasive (robotic, laparoscopic, and hybrid techniques) or open CME/D3 lymphadenectomy; (2) studies reporting patients with right-sided colon cancer; (3) studies reporting the description or illustration of SMA variations. The methodological quality of all included studies was evaluated using the Newcastle–Ottawa Scale (NOS). Results: After the literature search, 800 studies were recorded, 31 studies underwent full-text reviews, and 9 studies met the inclusion criteria. All studies reported vascular variations in SMA, and the total number of patients was 813. No intraoperative complications were reported. In 6.4% of patients, post-operative bleeding occurred. Conclusions: Vascular anatomical variations are not a rare entity. In experienced centers, vascular anomalies are not associated with an increase in complications, both in traditional open and minimally invasive surgery (MIS). However, in MIS, full access to central vessels and intraoperative vascular control, moderate retraction, safety maneuvers, and accurate vascular dissection are mandatory.
1. Introduction
Colon cancer is among the most commonly diagnosed malignancies worldwide, representing a major health burden. According to recent global estimates, it ranks third in incidence and second in cancer-related mortality. Understanding the disease’s anatomical and oncological behavior is essential to improve surgical strategies and oncological outcomes.
In 2009, Hohenberger et al. [] first described the concept of complete mesocolic excision (CME) and the extended lymph node dissection with central vascular ligation (CVL) for curative resection of colon cancer. Through consistent application of this approach, a significant decrease in 5-year local recurrence rates has been reported, dropping from 6.5% to 3.6% when compared with conventional resections. Concurrently, 5-year cancer-specific survival rates improved from 82.1% to 89.1% in patients undergoing curative resection [].
Surgical management of right-sided colon cancer has sparked extensive debate, particularly regarding postoperative and oncological outcomes of CME compared to conventional right hemicolectomy (CRH). Over the last decade, the literature has gradually shown the oncological impact to be greater than conventional surgery [,,,].
In 2022, the Portsmouth Consensus Statements on CME for right-sided colon cancer agreed on essential components of a procedure to qualify for CME: (1) CVL, (2) exposure of the superior mesenteric vein (SMV), and (3) excision of an intact mesocolon [].
According to the Japanese Society for Cancer of the Colon and Rectum, D3 lymphadenectomy is grounded in principles similar to those of CME with CVL. When comparing D3 and CME specimens, both approaches demonstrated high rates of mesocolic plane surgery and extended distances from the central vascular tie to the bowel wall [,]. Dissection of D3 represents an extended lymphadenectomy that includes lymph nodes along the root vessel. A D3 dissection for a right-sided tumor includes lymph nodes along the anterior aspect of the SMV and superior mesenteric artery (SMA) (central lymph nodes).
Looking for anomalies and vascular control gains a central role in colon cancer surgery with the description of the “no-touch technique” [,], utilizing the principles of a medial-to-lateral approach to lymphovascular isolation before colon mobilization.
The high degree of variation in right colonic arterial anatomy makes this procedure particularly technically demanding, and the surgical significance of the colon vascular anatomy was amplified with the introduction of CME and D3 lymphadenectomy [].
Vascular anatomical variations are a common matter, involved in oncological right-sided colonic surgery approaching at superior mesenteric root. Pitfalls are compromised visceral vascular supply, unexpected bleeding, and oncological clearance.
This paper aims to review and summarize the vascular anatomical variations in SMA in the setting of extended lymphadenectomy for CME in right colon cancer and to show the impact on clinical practice.
2. Materials and Methods
2.1. Search Strategy
Medline (PubMed), Web of Science (WOS), and Scopus were systematically searched for the vascular anatomical variations in SMA in the setting of D3 lymphadenectomy and CME for right colon cancer surgery, following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) standards (Figure 1) [,]. Eligible studies were searched using distinct databases up to May 2025. The same following combinations of keywords were used for researching the 3 different databases: (1) (superior mesenteric artery), combined with (2) (vascular variations); (3) (aberrant anatomy); (4) (right hemicolectomy OR right colectomy); (5) (complete mesocolic excision OR CME); and (6) (right colon cancer). The search was limited to studies reported in the English language. Full-text papers were independently screened by one author (G.M.) for eligibility. Reports were assessed for eligibility after records were removed before screening (duplicate records, records marked as ineligible by automation tools, and records removed for other reasons) and records excluded from the title and abstract screening (Figure 1). The reference lists of all included studies were manually reviewed to ensure comprehensive coverage and inclusion of all pertinent publications. This systematic review was registered in the PROSPERO database (CRD42024623459).
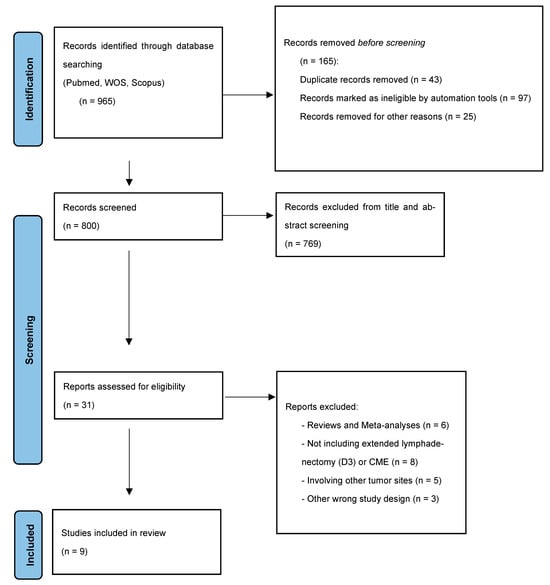
Figure 1.
PRISMA flow diagram.
2.2. Inclusion and Exclusion Criteria
In the systematic review, we restricted the search using the following exclusion criteria: (1) animal studies, (2) studies not including extended lymphadenectomy (D3) or CME for colon cancer; (3) studies involving other tumor sites except the right colon; (4) reviews and meta-analyses. The following criteria were set for inclusion: (1) studies reporting minimally invasive (robotic, laparoscopic, hybrid techniques) or open CME/D3 lymphadenectomy; (2) studies reporting patients with right-sided colon cancer; (3) studies reporting the description or illustration of SMA variations. No restrictions were set for the date of publication.
2.3. Data Extraction
After completing the full text revision of eligible studies, 1 author (G.M.) performed the data extraction and cross-checked all results. A database was created with all patients’ characteristics. Extracted variables included (1) general study characteristics, (2) patient demographics, (3) surgical characteristics, and (4) outcomes.
General study characteristics included (1) author, journal, year of publication, country, study design (intraoperative, radiologic, cadaveric) and the number of patients with SMA variations; patient demographics included (2) age, gender and body mass index (BMI); surgical characteristics included (3) type of resection and technique, SMA variations; outcomes included (4) intraoperative complications, conversion rate, and postoperative complications. When coding the data, any disagreements were adjudicated by a second reviewer (D.C.). Specifically, the outcomes domains that were considered “critical” for interpreting the review’s conclusions are the “intraoperative” and “radiologic” study design, techniques of surgery (robotic, laparoscopic, hybrid, or open), and SMA variations in relation to “intraoperative” and “postoperative” complications.
2.4. Statistical Analysis
Data were organized using Microsoft Excel (Microsoft 365), and cumulative analyses were performed where applicable. Categorical data were extracted as absolute counts and expressed as proportions; the postoperative complication was defined in the period between treatment and 30 days after surgery.
2.5. Quality Assessment
The methodological quality of the selected studies was evaluated using the Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies (Table 1).

Table 1.
Quality assessment of included studies.
3. Results
After the literature search, 800 studies were recorded, 31 studies underwent full-text reviews, and 9 studies met the inclusion criteria (Figure 1) [,,,,,,,,]. The study design and characteristics are reported in Table 2. Of the nine included studies, four were intraoperative studies, three were cadaveric studies, and two were radiology studies, published from 2015 to 2021. Only intraoperative and radiological findings were considered to define the postoperative outcomes. All studies included only significant data on patients with SMA vascular variations.

Table 2.
Baseline characteristics of the included studies.
3.1. Baseline Characteristics
Overall, a total of 813 patients were included in this review (sample size 1–507). All the studies (100%) included right-sided resections with CME/D3 lymphadenectomy for cancer. The mean age was 71.1 (58.5–84), and 55.9% were male. Four studies analyzed patients with a mean age under 70 years old [,,,], and two studies did not specify the sample mean age [,]; the mean BMI was 23, but six studies did not specify the BMI of the patients [,,,,,]. The studies reported every pathological T and N stage, according to the Union for International Cancer Control (UICC).
3.2. Surgical Characteristics and Vascular Variations
Among the 813 patients who underwent CME for right colon cancer, most of the procedures were performed through minimally invasive surgery (MIS) (92.7%) and a minor sample by the open technique (7.3%). However, five studies did not specify the surgical approach [,,,,]. Surgical characteristics and variations in SMA are reported in Table 3. In this review, all studies included report vascular variations in the SMA.

Table 3.
Surgical characteristics of the included studies.
A cadaveric study describes one replaced proper hepatic artery (PHA) from SMA []; the common hepatic artery (CHA) was absent, and the PHA was replaced directly to the SMA.
The celiac trunk was the source of both the gastroduodenal and right inferior phrenic arteries, while no distinct right gastric artery was observed. It gave rise to three primary branches: the splenic, left gastric, and gastroduodenal arteries. Consequently, the hepatic arterial supply originated entirely from the SMA.
A radiologic finding reports one replaced middle colic artery (MCA) from inferior mesenteric artery (IMA) and 10 accessory middle colic artery (aMCA) originating from the left-hand side of SMA []; MCA was replaced from an aberrant MCA, originating from IMA; the 10 aMCA are directed towards the splenic flexure. The bifurcation of MCA was positioned left to SMV in 4 patients (12.1%), anterior to SMV in 17 patients, including the aberrant MCA (53.1%), and right to SMV, over the duodenum or the pancreatic head, in 11 patients (34.4%).
An intraoperative finding during CME reports a case of a common trunk of the ileocolic artery (ICA) and the right colic artery (RCA) [].
Another cadaveric study describes nine RCA replaced from the right branch of MCA, eight RCA replaced from SMA, three RCA replaced from ICA, and three RCA replaced from the root of MCA []. One study (patients n = 116) reported an ICA that passed the SMV anteriorly or posteriorly in 58 patients (50.0%) each; the RCA originated from the SMA in 38 patients (32.7%); the RCA crossed the SMV anteriorly in 33 patients (86.8%) and posteriorly in 5 (13.2%) [].
CHA originated as the first branch of the SMA behind the SMV and pancreas in one case report [], and the CHA was 4 mm long until it divided into the two branches, distributing to the left and right lobes of the liver, respectively. The left hepatic artery (LHA) ran on the portal vein’s left side and divided the gastroduodenal artery at the cranial side of the duodenum and the accessory gastric artery beneath the hepatic portal. The right hepatic artery (RHA) coursed to the right of the portal vein and posterior to the bile duct, entering the right hepatic lobe after giving off the cystic artery, which passed between the common hepatic duct and the portal vein. Four retromesenteric MCAs (0.79%) were found around 507 patients in a radiology study [].
In case 1, the MCA embraces the SMV posteriorly and emerges at its right border just below the gastrocolic trunk of Henle (GTH) []. In case 2, the MCA courses to the right, behind the SMV, but in front and to the left of the common trunk for the inferior pancreaticoduodenal vein (IPDV), jejunal vein (JV), and GTH []. In case 3, the MCA takes an oblique course, crossing the SMV posteriorly, approaching the GTH from below, and bifurcating level with the point of the GTH junction []. In case 4, the MCA crosses the SMV posteriorly and emerges at its right border just below the GTH termination. After bifurcation, its branches follow the concomitant affluents of the GTH []. Finally, a study (patients n = 60) reports SMA left of the SMV in 57 cases and right of the SMV in 3 cases [].
In this systematic review, ICA was found in 99.5%, RCA in 72.1%, and MCA in 100% of patients.
About MCA, eight had a common trunk for the MCA and RCA, while one had a double MCA. Among 51 cases with one MCA (excluding those with common MCA/RCA trunk or double MCA), the reach of the MCA bifurcation from the pancreatic neck was ≤1 cm in 8, 1–2 cm in 34, and >2 cm in 9 [].
3.3. Outcomes
No intraoperative complications were reported in the studies included in the review (0%). Any vessel was ligated without defining its course, and always avoiding mass ligation. Dissection of the D3 area was performed in all the patients.
Complete dissection of the D3 region allowed for clear visualization of the origin and drainage patterns of all vessels supplying the right colon. The SMV, as well as the anterior and right surfaces of the SMA, were routinely exposed.
In 6.4% of patients, there was a post-operative bleeding [,]. 2 studies reported post-operative ileus in 14.7% and 54.2% of patients [,]. Intra- and post-operative outcomes are listed in Table 4.

Table 4.
Outcomes of the included studies.
4. Discussion
4.1. Anatomical Variability of the Mesenteric Arteries
CME for right-sided colon cancer is becoming more popular worldwide. This technique should be the standard of care resection for locally advanced colon cancer and is advisable for younger patients (under 50 years old) with a locally advanced colon cancer irrespective of the site [].
The components of a procedure to qualify for CME are (1) CVL, (2) exposure of the SMV, and (3) excision of an intact mesocolon. There was no agreement regarding the exposure of SMA as a routine part of the CME procedure []. Nevertheless, this technique is oncologically demanding due to the highly variable arterial pattern of the right colon.
In 1966, Michels was the first to distinguish between “accessory” and “replaced” hepatic arteries []. Among the ten variants of the hepatic artery in Michels classification, accessory or replaced RHA variants from SMA of interest in radical colon cancer surgery are described (Michels type 3, replaced RHA from the SMA, 11%; Michels type 4, replaced RHA and LHA, 1%; Michels type 6, accessory RHA, 7%; Michels type 7, accessory RHA and LHA, 1%) and an hepatic trunk as a branch of the SMA (Michels type 9, 4.5%).
Anatomical variants of the SMA were categorized into types according to Yada’s classification []. Recently, Anania et al. reported the results of a large study during intraoperative and radiological evaluations performed on patients who underwent laparoscopic right colectomy (CoDIG2) []. The RCA arises independently from the SMA (Yada type 1), was the most frequent finding during intraoperative dissection (55.2%), although the rate was lower during radiological preoperative evaluation (33.9%) []. Common trunk of the RCA and MCA (Yada type 2) was the most common finding during radiological preoperative evaluation (contrast-enhanced CT) (47.6%), but the rate was lower for intraoperative dissection (35.6%) []. Common trunk of the RCA and ICA (Yada type 3) was the least frequent, and the rates of radiological preoperative evaluation (12.5%) and intraoperative dissection (9.2%) were the same [].
An exceptional but important finding reported in the literature is prepancreatic CHA arising from SMA. Preoperative arterial 3D reconstruction was performed in this case, and no other arteries are directed to the liver []. Moreover, an SMA origin of the PHA was reported, with the absence of CHA and PHA replaced directly by the SMA; also in this case, the entire arterial blood supply to the liver was derived from the SMA [].
Therefore, it is essential to have a good knowledge of the vascular anatomy to avoid vascular insufficiency of other organs. Sometimes the arterial blood supply of the liver can arise exclusively from the SMA.
Overall, in this systematic review, particular variations are evident and could have both an intraoperative and postoperative impact on surgery: the PHA replaced from SMA, the replaced MCA from IMA or accessory MCA which could not be found, the variant position of MCA bifurcation that could be found over the duodenum or the pancreatic head, the RCA absent but replaced from other branches of ICA and MCA, the variant position of ICA or RCA that pass the SMV and SMA anteriorly or posteriorly, and finally the variant and tortuous position of SMV and SMA forming sharp and elongated curves, are the most complex anomalies to manage and control. A schematic summary of key findings is reported in Table 5.

Table 5.
Schematic summary of key findings of the systematic review.
Maybe, the most important finding is the wide range of possible MCA bifurcation positions. The fact that the MCA bifurcation can be found left to SMV, anterior to SMV, or more often right of the SMV is of crucial importance during operative planning. While lengths from the MCA origin to the bifurcation have been previously reported, the position of its bifurcation has to date not been described in the literature [].
4.2. Surgical Implications for CME and CVL
This review analyzes SMA vascular variations in right-sided colon resections with CME/D3 lymphadenectomy for cancer. Despite SMA anatomical variations not being rare, most of the procedures were performed through MIS, without the need for conversion. Especially in MIS, moderate retraction is mandatory, with accurate dissection of arterial-like tissues (lymphatics and nodes) and complete anatomical and vascular control. Vascular injury and unexpected bleeding may affect the visceral anastomoses. In this review, no studies report intraoperative complications or iatrogenic vascular injuries.
The most reported postoperative complication is prolonged ileus, and the most insidious is bleeding. Postoperative ileus is among the most frequently encountered complications after colorectal surgery, with an estimated incidence ranging from 10% to 20% following elective colectomies [,,]. This functional, non-mechanical inhibition of coordinated gastrointestinal activity may lead to a prolonged hospital stay.
A relatively rare condition is the chylous leak (CL) or chyloperitoneum (CP), with an estimated incidence of 1–6.5% after colorectal surgery []. It has been reported during surgical procedures where the dissection is performed close to the lymphatic, and nowadays, the rapid advancement in minimally invasive, more aggressive lymphadenectomy to achieve R0 resection, increased the risk of this complication. Risk factors identified for iatrogenic CP include right colonic surgery, tumor location, extent of lymphadenectomy, and number of lymph nodes harvested []. This condition was extremely rare in the era of open colorectal surgery, probably just due to less aggressive lymphadenectomy. The lymphatic system is a one-way drainage system that allows for the return of excess interstitial fluids and proteins to the vascular system. The thoracic duct, the main duct for the return of lymph to blood, begins as a dilation called the cisterna chyli anterior to the second lumbar vertebra. The cisterna chyli receives lymph from the right and left lumbar trunks and from the intestinal trunk. The cisterna chyli is in close contact with the SMA (Figure 2). Chylous effusions develop when these are injured or obstructed []. In this scenario, real-time ICG lymphangiography can guide the location and repair of CL after colonic resection, injecting the fluorescence dye under the serous layer at the proximal end of the anastomosis, at the end of the operation [].
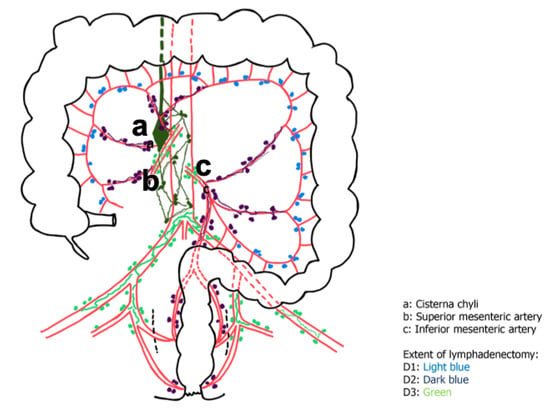
Figure 2.
Schematic representation of the lymphatic system of the colon and the extent of lymphadenectomy.
4.3. Preoperative Planning and Imaging
Preoperative review of CT imaging and reconstruction of the vascular anatomy may be useful prior to performing CME, particularly in the context of minimally invasive surgery []. Three-dimensional (3D) printing has recently gained attention as a promising innovation across multiple fields of medical practice. Three-dimensional printed models of the central mesenteric vascular anatomy provide sufficient anatomical accuracy to allow practical 3D anatomical orientation before and during radical D3 right hemicolectomy for cancer [].
4.4. Study Limitations and Future Perspectives
A limitation of this study is the sample size. A larger sample of patients may offer more pertinent data about the frequency of anatomical vascular variations, but the number of studies that meet the extensive inclusion criteria did not allow for the harvesting of a larger number of cases. Moreover, BMI was not well reported in all included studies. It should be underlined that patients with normal or low BMI have the most favorable anatomical situation for identifying anatomical landmarks during surgery. On the other hand, the careful restriction of the inclusion criteria, which concern only arterial variations in these types of patients, represents a strength of this study, underlining aspects that have not yet been discussed.
Regarding future perspectives, the application of manual editing and 3D reconstruction protocols allows navigation and visualization of vascular anatomy relative to regions of interest (ROIs), following trajectories of SMA branches []. All this could guarantee an improvement in preoperative vascular anatomy reconstruction and consequently gain oncological clearance.
5. Conclusions
Vascular anatomical variations are not a rare entity, playing a central role in right colon cancer surgery, especially in the era of CME and the extended lymphadenectomies.
The key observation of this study is the considerable variability in the MCA bifurcation positions, highlighting the importance of precise anatomical knowledge during surgical planning and which to date has not been described in the literature.
For preoperative radiological studies, the reconstruction of the vascular anatomy is unavoidable, although there is often no concordance between the intraoperative and radiological assessments. Radiologists and surgeons have different but complementary roles, and surgeons must critically review preoperative CT imaging, keeping in mind every single step of surgery. In MIS, full access to central vessels and intraoperative vascular control, moderate retraction, safety maneuvers, and accurate vascular dissection are mandatory.
This kind of oncological surgery needs vascular skills or available vascular surgeons, but in experienced centers, vascular anomalies are not associated with poor outcomes, both in traditional open and MIS.
Author Contributions
G.M.: study design, conceptualization, methodology, research, material preparation and data collection, data analysis, writing—original draft, review and editing. D.C.: methodology, data analysis, writing—review and editing. E.M.M.: methodology, writing—review and editing. B.P.: review and editing. S.R.: review and editing. A.S.: review and editing. S.M.: review and editing. B.C.: review and editing. G.B.: review and editing. M.A.: writing—review and editing, supervision. A.M.: conceptualization, methodology, writing—review and editing, supervision, funding Acquisition. I.A.M.: study design, conceptualization, methodology, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hohenberger, W.; Weber, K.; Matzel, K.; Papadopoulos, T.; Merkel, S. Standardized surgery for colonic cancer: Complete meso- colic excision and central ligation–technical notes and outcome. Colorectal Dis. 2009, 11, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Muttillo, E.M.; Picardi, B.; Rossi, S.; Muttillo, I.A. Complete mesocolic excision and D3 lymphadenectomy with central vascular ligation in right-sided colon cancer: A systematic review of postoperative outcomes, tumor recurrence and overall survival. Surg. Endosc. 2021, 35, 4945–4955. [Google Scholar] [CrossRef]
- Sica, G.S.; Vinci, D.; Siragusa, L.; Sensi, B.; Guida, A.M.; Bellato, V.; García-Granero, Á.; Pellino, G. Definition and reporting of lymphadenectomy and complete mesocolic excision for radical right colectomy: A systematic review. Surg. Endosc. 2023, 37, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Mazzarella, G.; Muttillo, E.M. Need to define right mesocolectomy and complete mesocolic excision concept: How, when and why? Colorectal Dis. 2021, 23, 3026. [Google Scholar] [CrossRef] [PubMed]
- Anania, G.; Davies, R.J.; Bagolini, F.; Vettoretto, N.; Randolph, J.; Cirocchi, R.; Donini, A. Right hemicolectomy with complete mesocolic excision is safe, leads to an increased lymph node yield and to increased survival: Results of a systematic review and meta-analysis. Tech. Coloproctol. 2021, 25, 1099–1113. [Google Scholar] [CrossRef]
- Tejedor, P.; Francis, N.; Jayne, D.; Hohenberger, W.; Khan, J.; on behalf the CME Project Working Group. Consensus statements on complete mesocolic excision for right-sided colon cancer-technical steps and training implications. Surg. Endosc. 2022, 36, 5595–5601. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese society for cancer of the colon and rectum. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- West, N.P.; Kobayashi, H.; Takahashi, K.; Perrakis, A.; Weber, K.; Hohenberger, W.; Sugihara, K.; Quirke, P. Understanding optimal colonic cancer surgery: Comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J. Clin. Oncol. 2012, 30, 1763–1769. [Google Scholar] [CrossRef]
- Turnbull, R.B., Jr. Current concepts in cancer. Cancer of the GI tract: Colon, rectum, anus. The no-touch isolation technique of resection. JAMA 1975, 231, 1181–1182. [Google Scholar] [CrossRef]
- Turnbull, R.B., Jr.; Kyle, K.; Watson, F.R.; Spratt, J. Cancer of the colon: The influence of the no-touch isolation technic on survival rates. Ann. Surg. 1967, 166, 420–427. [Google Scholar] [CrossRef]
- Cirocchi, R.; Randolph, J.; Davies, R.J.; Cheruiyot, I.; Gioia, S.; Henry, B.M.; Carlini, L.; Donini, A.; Anania, G. A systematic review and meta-analysis of variants of the branches of the superior mesenteric artery: The Achilles heel of right hemicolectomy with complete mesocolic excision? Colorectal Dis. 2021, 23, 2834–2845. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Prefferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. intern Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Büchler, M.W.; Diener, M.K. Optimal literature search for systematic reviews in surgery. Langenbecks Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Alakkam, A.; Hill, R.V.; Saggio, G. Superior mesenteric origin of the proper hepatic artery: Embryological and clinical implications. Surg. Radiol. Anat. 2016, 38, 747–750. [Google Scholar] [CrossRef]
- Alsabilah, J.F.; Razvi, S.A.; Albandar, M.H.; Kim, N.K. Intraoperative Archive of Right Colonic Vascular Variability Aids Central Vascular Ligation and Redefines Gastrocolic Trunk of Henle Variants. Dis. Colon. Rectum. 2017, 60, 22–29. [Google Scholar] [CrossRef]
- Andersen, B.T.; Stimec, B.V.; Edwin, B.; Kazaryan, A.M.; Maziarz, P.J.; Ignjatovic, D. Re-interpreting mesenteric vascular anatomy on 3D virtual and/or physical models: Positioning the middle colic artery bifurcation and its relevance to surgeons operating colon cancer. Surg. Endosc. 2022, 36, 100–108. [Google Scholar] [CrossRef]
- Pérez-Corbal, L.; Trujillo-Diaz, J.C.; Alarcón, I.; Licardie, E.; Senent, A.; Morales-Conde, S. Interactive 3D vascular reconstruction: A navigation tool to improve safety in laparoscopic D3 right colectomy—A video vignette. Colorectal Dis. 2021, 23, 3030–3032. [Google Scholar] [CrossRef]
- Haywood, M.; Molyneux, C.; Mahadevan, V.; Lloyd, J.; Srinivasaiah, N. The right colic artery: An anatomical demonstration and its relevance in the laparoscopic era. Ann. R. Coll. Surg. Engl. 2016, 98, 560–563. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, S.C.; Kim, M.J.; Sohn, D.K.; Oh, J.H. Vascular Anatomy in Laparoscopic Colectomy for Right Colon Cancer. Dis. Colon. Rectum. 2016, 59, 718–724. [Google Scholar] [CrossRef]
- Omotehara, T.; Naito, M.; Hayashi, S.; Kawata, S.; Shimada, K.; Itoh, M. Common hepatic artery originating from superior mesenteric artery with replaced right hepatic artery. Anat. Sci. Int. 2021, 96, 568–571. [Google Scholar] [CrossRef]
- Stimec, B.V.; Andersen, B.T.; Benz, S.R.; Fasel, J.H.D.; Augestad, K.M.; Ignjatovic, D. Retromesenteric course of the middle colic artery-challenges and pitfalls in D3 right colectomy for cancer. Int. J. Colorectal Dis. 2018, 33, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ye, K.; Wu, Y.; Chen, Q.; Xu, J.; Lin, J.; Kang, W. Variations in right colic vascular anatomy observed during laparoscopic right colectomy. World J. Surg. Oncol. 2019, 17, 16. [Google Scholar] [CrossRef]
- Michels, N.A. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am. J. Surg. 1966, 112, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Yada, H.; Sawai, K.; Taniguchi, H.; Hoshima, M.; Katoh, M.; Takahashi, T. Analysis of vascular anatomy and lymph node metastases warrants radical segmental bowel resection for colon cancer. World J. Surg. 1997, 21, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Anania, G.; Campagnaro, A.; Chiozza, M.; Randolph, J.; Resta, G.; Marino, S.; Pedon, S.; Agrusa, A.; Cuccurullo, D.; Cirocchi, R.; et al. A SICE (Società Italiana di Chirurgia Endoscopica e Nuove Tecnologie) observational prospective multicenter study on anatomical variants of the superior mesenteric artery: Intraoperative analysis during laparoscopic right hemicolectomy-CoDIG 2 database (ColonDx Italian Group). Updates Surg. 2024, 76, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, L.; Felli, E.; Muttillo, E.M.; Fiorentini, G.; Diana, M.; Pessaux, P.; Felli, E. Prepancreatic common hepatic artery arising from superior mesenteric artery: An exceptional but important finding during pancreaticoduodenectomy. Surg. Radiol. Anat. 2021, 43, 1413–1420. [Google Scholar] [CrossRef]
- Scarborough, J.E.; Schumacher, J.; Kent, K.C.; Heise, C.P.; Greenberg, C.C. Associations of specific postoperative complications with outcomes after elective colon resection: A procedure-targeted approach toward surgical quality improvement. JAMA Surg. 2017, 152, e164681. [Google Scholar] [CrossRef]
- Chapman, S.J. EuroSurg Collaborative Ileus management international (IMAGINE): Protocol for a multicentre, obser- vational study of ileus after colorectal surgery. Colorectal Dis. 2018, 20, O17–O25. [Google Scholar] [PubMed]
- Ng, Z.Q.; Han, M.; Beh, H.N.; Keelan, S. Chylous ascites in colorectal surgery: A systematic review. World J. Gastrointest. Surg. 2021, 13, 585–596. [Google Scholar] [CrossRef]
- Agustsdottir, E.E.S.; Stimec, B.V.; Stroemmen, T.T.; Sheikh, A.E.; Elaiyarajah, I.; Lindstroem, J.C.; Ignjatovic, D. Preventing chylous ascites after right hemicolectomy with D3 extended mesenterectomy. Langenbecks Arch. Surg. 2020, 405, 1017–1024. [Google Scholar] [CrossRef]
- Al-Busafi, S.A.; Ghali, P.; Deschênes, M.; Wong, P. Chylous Ascites: Evaluation and Management. ISRN Hepatol. 2014, 2014, 240473. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, Y.; Han, B.; Sun, P.; Wang, J.; Lin, Q.; Xu, M. Real-time indocyanine green lymphangiography in radical resection of right colon cancer allows the identification of chyle leakage. Contemp Oncol. 2021, 25, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Luzon, J.A.; Andersen, B.T.; Stimec, B.V.; Fasel, J.H.D.; Bakka, A.O.; Kazaryan, A.M.; Ignjatovic, D. Implementation of 3D printed superior mesenteric vascular models for surgical planning and/or navigation in right colectomy with extended D3 mesenterectomy: Comparison of virtual and physical models to the anatomy found at surgery. Surg. Endosc. 2019, 33, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Nesgaard, J.M.; Stimec, B.V.; Bakka, A.O.; Edwin, B.; Ignjatovic, D. Navigating the mesentery: A comparative pre- and perioperative visualization of the vascular anatomy. Colorectal Dis. 2015, 17, 810–819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).