Abstract
Colorectal cancer is currently a public health concern due to its high incidence, morbidity, and mortality rates. Researchers have identified the intestinal microbiome as a crucial factor in the development of this disease. Currently, specialized literature data support the role of the microbiota in both the development of colorectal cancer and resistance to oncological therapies. Therefore, studying the composition of the gut microbiome can aid in creating risk assessment tools to identify specific populations that would benefit from tailored screening approaches. Also, manipulation of the intestinal microbiome can be useful in improving the response to chemotherapy or immunotherapy. Identifying the pathogenic mechanisms responsible for this causal link can aid in the discovery of novel treatment targets. This article will provide the latest information regarding the influence of the intestinal microbiota on the development and progression of colorectal cancer.
1. Introduction
Colorectal cancer (CRC) is associated with a significant burden on the global healthcare system. Among all gastrointestinal cancers, CRC is associated with the highest risk of occurrence during life (38.5%) and, secondly, the highest risk of death from cancer (28.2%) [1]. These results emphasize the need for research to improve the diagnostic and therapeutic management of this disease [1].
Regarding its prevalence, CRC ranks as the third most common malignancy worldwide, accounting for approximately 1,926,425 newly diagnosed cases each year [1,2]. In terms of mortality, it is the second leading cause of cancer deaths, after lung cancer, with an annual death toll of roughly 904,019 [2]. Geographic variation in CRC risk factors partially accounts for the geographic variation in the incidence and mortality rates of this malignant disease [2]. According to recent data, Australia and Europe had the highest rates of CRC, with 40.6 cases per 100,000 men, while Africa and South Asia had the lowest rates, with 4.4 occurrences per 100,000 women [1,2]. The mortality rate exhibited comparable trends, with the highest rate recorded in Eastern Europe (20.2 deaths per 100,000 men) and the smallest in South Asia (2.5 deaths per 100,000 women) [1,2,3]. Furthermore, recent epidemiological research indicates that the number of new cases of CRC is estimated to rise to 3.2 million by the year 2040, with a corresponding increase in mortality to 1.6 million deaths [2]. The rise in the prevalence of CRC appears to be directly related to the expansion of the economy [3]. This phenomenon is considered to be a result of lifestyle changes, including a higher intake of meat, processed foods, and alcohol, decreased levels of physical activity, and an increased prevalence of obesity [2,3]. Another potential explanation for this relationship could be the demographic phenomenon of population aging [3].
Advancements in oncological treatment and the adoption of screening strategies that enable the early detection of CRC contributed to a rise in the 5-year life expectancy from around 50% in 1970 to around 70% at present [4,5,6]. However, currently, there is also a documented rise in the occurrence of CRC among individuals under the age of 50 [7,8,9,10]. Furthermore, by 2030, according to concerning statistics, one in ten patients diagnosed with colon cancer and one in four patients diagnosed with rectal cancer will be under the age of 50 [11]. The increased incidence of CRC among younger patients is also accompanied by a corresponding rise in mortality rates, primarily because of delayed diagnosis and a more advanced stage of the disease [12]. All of these observations led to a decrease in the age of implementing CRC screening strategies to 45 years in the United States of America [13].
Another essential issue to consider when reflecting on the worldwide burden of CRC is the identification of particular risk factors for this malignancy [14]. These data have the potential to be used in the development of risk scores that might help identify populations that could benefit from individualized screening programs. In addition, they can be used to formulate precise recommendations on how to eliminate or minimize exposure to particular risk factors. Furthermore, for patients who have already received a diagnosis of CRC, the detection of pathogenic pathways implicated in colorectal oncogenesis can aid in the development of novel, targeted therapies. This review will focus on the pathogenic correlation between CRC and the intestinal microbiota, according to the most recent data available.
2. Risk Factors for CRC
The pathophysiological mechanisms that contribute to colorectal carcinogenesis include abnormal cell proliferation and differentiation, resistance to apoptosis, tumor cell invasion of neighboring structures, and distant organs [14]. Dietary and lifestyle choices may potentially initiate inflammation in the intestines and alter the composition of the intestinal microorganisms, thereby facilitating an immune reaction that supports the development and advancement of polyps into cancer [6]. In addition, mutations in oncogenes and tumor-suppressor genes, whether inherited or occurring spontaneously, might provide some mucosal cells a competitive advantage, stimulating excessive cell growth and ultimately resulting in the development of cancer [6].
The risk factors for CRC are categorized into two main groups: non-modifiable and modifiable factors (Figure 1) [6,14].
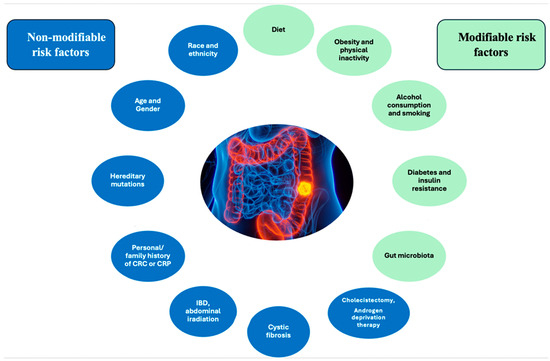
Figure 1.
Risk factors for CRC.
Factors such as access to healthcare, screening programs, diet, income, and education appear to influence the slightly higher prevalence of CRC among black individuals compared to white individuals more than genetics [6,15,16]. Regarding gender, males have approximately a 1.5-fold greater probability of developing CRC in comparison to females [6]. Additionally, females have a higher susceptibility to right-sided colon cancer, which is linked to a poorer prognosis when compared to CRC found in the left colon [6].
The likelihood of developing CRC rises in direct correlation with advancing age. For instance, in the United States, the probability of a diagnosis of CRC is approximately three times higher for individuals aged 65 and above compared to those aged 50–64 and approximately thirty times higher for those aged 25–49 [17]. As a result of the implementation of CRC screening strategies, morbidity and mortality rates associated with this condition have decreased. A significant issue in the past few decades has been the rise in the incidence of CRC cases identified in individuals under the age of 50. Research indicates that early-onset CRC has distinct clinical characteristics, more aggressive behavior, and different molecular profiles compared to CRC that occurs at an older age [18]. Early-onset CRC is linked to more aggressive histological cancer types and is diagnosed in more advanced stages [18]. The high prevalence of germline mutations in these patients underscores the need for a more thorough assessment of the family cancer risk [18].
The main mutations observed in CRC patients involve the following genes: adenomatous polyposis coli (APC—up to 80%), tumor protein P53 (TP53—35–55%), Kirsten rat sarcoma virus (KRAS—35–45%), transforming growth factor beta receptor 2 (TGFBR2—25–30%), MutL protein homolog 1 (MLH1), MutS protein homolog 2 (MSH2), MutS protein homolog 6 (MSH6), postmeiotic segregation increased 2 (PMS2, MLH1, MSH2, and MSH6—15–25%), mothers against decapentaplegic homolog 4 (SMAD4—10–35%), phosphatase and tensin homolog (PTEN—10–15%), and v-raf murine sarcoma viral oncogene homolog B1 (BRAF—8–12%) [19]. CRC can also be associated with several hereditary syndromes, including familial adenomatous polyposis (FAP), Li-Fraumeni syndrome, cardiofasciocutaneous syndrome, hereditary non-polyposis CRC (Lynch syndrome), familial juvenile polyposis, Cowden syndrome, Turcot syndrome, Peutz–Jeghers syndrome, or Gardner syndrome [14,19].
A recent case–control study conducted in Sweden found that individuals with a first-degree relative who had a colorectal polyp faced a 40% higher risk of developing polyps themselves [20]. Notably, the study’s authors observed that the risk of colorectal polyps rises with each additional first-degree relative affected by polyps, along with a decrease in the age at which polyps are diagnosed in family members [20]. According to other studies, people who have a first-degree relative with a history of CRC are two to four times more likely to develop the disease than the general population [21].
Regarding the personal history of colorectal polyps and the risk of developing CRC, this is enhanced by both the number and size of adenomatous polyps discovered on examination [22]. A pooled prospective analysis conducted by Martinez et al. found that patients who had more than five adenomas or at least one adenoma measuring 19 mm or larger had an approximate 20–25% increase in the absolute risk of developing metachronous advanced adenoma [22].
Individuals diagnosed with inflammatory bowel diseases (IBD) have a twofold increased risk of CRC in comparison to the general population [14]. The abnormal release of cytokines and metabolic products, as well as an increase in local blood flow, are all consequences of the chronic intestinal inflammation that defines these diseases. These factors collectively contribute to the development of cancer [14].
Patients with a history of abdominal irradiation are more likely to develop gastrointestinal neoplasms, particularly CRC, which is the most prevalent. For instance, individuals who have undergone radiotherapy for prostate cancer have a similar risk of CRC to patients with a family history of colonic adenomas [14]. Additionally, individuals with prostate cancer who are receiving androgen deprivation therapy involving the use of gonadotropin-releasing hormone (GnRH) agonists or orchiectomy are at a higher risk of CRC [23].
Obesity and a lack of physical activity are the main behavioral factors that greatly contribute to the development of CRC. These factors are also likely responsible for most of the differences in the occurrence of the disease across different regions [6]. Research suggests that people who participate in consistent physical activity have a 25% reduced likelihood of CRC, whereas those who have a sedentary lifestyle have a heightened risk of up to 50% [6,14]. Obesity can promote CRC carcinogenesis through abnormal lipid metabolism, gut microbiota dysbiosis, chronic inflammation, or disrupted bile acid homeostasis [24]. Tumor microenvironment adipocytes serve as a source of energy for the progression of CRC [24]. Furthermore, adipose tissue growth is associated with increased levels of proinflammatory cytokines such as interleukin 6, tumor necrosis factor-alpha (TNF-alpha), plasminogen activator inhibitor-1 (PAI-1), and chemokine ligand 2 (CCL2), as well as elevated levels of adipokines, insulin, and insulin-like growth factor (IGF), all of which contribute to the development of CRC [24].
The consumption of processed and red meats has been linked to an increased risk of developing gastric and intestinal cancer. This effect is especially pronounced when these meats are combined with high-temperature preparation methods and smoking [25]. Conversely, calcium, fiber, vitamin D, and a diet rich in fruits and vegetables have all demonstrated protective effects against CRC [26]. Typically found in fruits, vegetables, and whole grains, fiber accelerates intestinal peristalsis, reducing the contact time with potential cancer-causing substances [26]. Fiber can also interfere with bile acid metabolism, which has been shown to be involved in digestive carcinogenesis [27]. In addition, vegetables rich in fiber also contain significant quantities of antioxidant compounds, such as resveratrol, polyphenols, and phytoestrogens [27]. Moreover, fiber can modulate the intestinal microbiome; the commensal flora can digest it to produce compounds like butyrate, which have an antiproliferative effect [27].
Research has indicated that individuals who consume two to three alcoholic beverages daily have a 20% increased risk of CRC [28]. Furthermore, those who consume more than three drinks per day face a 40% greater risk of developing this disease [28].
In relation to smoking, the literature provides evidence of a distinct correlation between this detrimental behavior and both the onset of CRC and an unfavorable prognosis [29,30]. In a recent study conducted on mice, Bai et al. observed a correlation between tobacco exposure and intestinal microbiome modification [31]. Specifically, these authors noted an increase in the abundance of Eggerthella lenta and a decrease in the abundance of Parabacteroides distasonis and Lactobacillus spp. [31]. Furthermore, mice exposed to cigarette smoke showed an increase in the concentration of specific biliary metabolites (particularly taurodeoxycholic acid) in the colon and the activation of signaling pathways such as mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MAPK/ERK) [31].
Patients with diabetes mellitus have an increased risk of CRC, regardless of body mass index, level of physical activity, or smoking status [32]. The underlying pathophysiological mechanism involves an elevation in serum levels of insulin and IGF-1, both of which exhibit mitogenic properties, thereby promoting the proliferation of colorectal epithelial cells [32].
3. Gut Microbiota and CRC
The gastrointestinal tract (GIT) contains more than 100 trillion microorganisms, collectively known as the microbiome, which serves as the primary site for communication between host cells, the immune system, and the microbiota [33,34,35]. The gut microbiota has evolved alongside the host and actively participates in regulating metabolism and immunity, as well as maintaining homeostasis and facilitating nutritional absorption [36,37].
The intestinal microbiome plays a role in colorectal oncogenesis through direct interaction with enterocytes, as well as involvement in cellular metabolism and immune response processes [37]. Sequencing-based investigations have revealed alterations in the intestinal microbiota composition and a reduction in its diversity within patients diagnosed with CRC [37]. It has been reported that several species promote colorectal carcinogenesis, including Streptococcus gallolyticus, Enterococcus faecalis, Helicobacter pylori, Bacteroides fragilis, Clostridium septicum, Escherichia coli, and Fusobacterium nucleatum (Figure 2) [38].
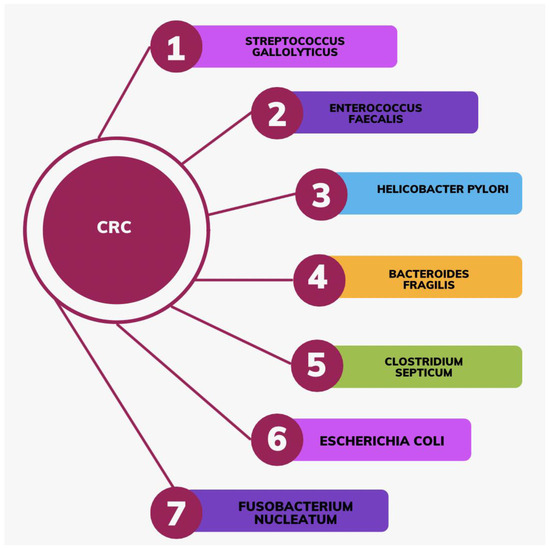
Figure 2.
Bacterial species that promote the development of CRC.
Enterococcus faecalis produces extracellular superoxide, which causes deoxyribonucleic acid (DNA) damage and genomic instability in intestinal epithelial cells [39]. Additionally, it has been demonstrated that this microorganism activates macrophages to generate 4-hydroxy-2non-enal, thereby promoting CRC [39]. Another mechanism by which Enterococcus faecalis promotes carcinogenesis is by inhibiting the activation of the protective transforming growth factor beta (TGF-beta)/Smad signaling pathway [40]. This germ can also enhance colonic tumorigenesis by secreting biliverdin, its key metabolite [41]. By modulating the PI3K/AKT/mTOR signaling pathway, biliverdin can enhance the levels of interleukin-8 and vascular endothelial growth factor A, thereby inducing an angiogenic phenotype that facilitates the progression of CRC [41].
Besides its clear involvement in gastric carcinogenesis, recent studies have reported an association between Helicobacter pylori and CRC [42,43]. A study involving 812,736 individuals found that Helicobacter pylori infection is associated with an 18% higher risk of developing CRC and a 12% higher risk of death from CRC [44]. An explanation for this pathogenic relationship can be found in the disruption of cell proliferation caused by the vacA toxin, which is produced by Helicobacter pylori [43]. Ralser et al. identified a specific immune pattern associated with Helicobacter pylori infection, characterized by a reduction in regulatory T cells and proinflammatory T cells [45]. This bacterium also induced the activation of the pro-oncogenic STAT3 signaling pathway and the loss of goblet cells, all of which are involved in colorectal oncogenesis [45].
Streptococcus gallolyticus subspecies gallolyticus (Sgg) has been found to significantly elevate the risk of CRC through its capacity to induce tumor cell proliferation [46]. Compared to the general population, Sgg bacteremia was associated with a 60% increased risk of colon adenomas and adenocarcinomas [46,47,48,49,50]. Furthermore, Corredoira et al. provided evidence that 45% of patients diagnosed with endocarditis caused by Sgg acquire colonic neoplastic tumors within 5 years of the initial infectious disease diagnosis [51]. Data from the literature has demonstrated a phenotypic variation across Sgg strains in terms of their capacity to induce cell proliferation [46]. For instance, the strain Sgg TX20005 has the ability to induce cell proliferation, whereas the strain ATCC_43143 does not have this ability [46]. Furthermore, research on in vitro cell cultures revealed that the TX20005 strain required beta-catenin to perform its function of increasing cell proliferation [46]. Taylor et al. demonstrated in a recent study that the deletion of the Sgg-pathogenicity-associated region (SPAR) locus leads to a reduction in colon colonization with Sgg, a reduction in Sgg’s ability to adhere to intestinal cells, and the loss of these microorganisms’ ability to stimulate cell proliferation and CRC development [46]. Infection with Sgg also activates certain pro-oncogenic signaling pathways, including Wnt/beta-catenin, c-Myc, and proliferating cell nuclear antigen (PCNA) [52].
Recent studies have revealed a significant correlation between the epidemiology of colon cancer and the presence of enterotoxigenic Bacteroides fragilis (ETBF), suggesting that this bacterium may play a role in the pathogenesis of the disease [53]. ETBF promotes the development of colon cancer by enhancing the activation of T helper type 17 cells (TH17-type) [54]. The activation of STAT3 signaling within the tumor microenvironment potentially contributes to a TH17-type immune response, which has procarcinogenic potential [54]. An additional physiological mechanism that can explain the connection between the colonization of Bacteroides fragilis and CRC is the secretion of a toxin called zinc-dependent metalloprotease. This toxin specifically targets and breaks down E-cadherin, leading to the movement of β-catenin into the nucleus, the increased expression of c-Myc, and heightened cell growth [55].
Escherichia coli synthesizes colibactin, a genotoxin that causes DNA damage [56,57,58]. It appears to have more of a ‘hit-and-run’ mechanism because short-term infection was sufficient to generate Wnt-independent organoids [59]. The Wnt-independent phenotype was the result of a disturbance of the p53/miR-34 axis, as well as extra synergistic mutations. These mutant cells with increased pro-survival signals can outperform normal cells, resulting in the formation of premalignant lesions such as polyps in affected individuals [59]. In a recent study, Nouri et al. found that 23% of CRC patients were colonized with enteropathogenic Escherichia coli, compared to only 7.1% of healthy individuals [57]. Furthermore, these authors found that phylogroups D and B2 were more prevalent in patients with CRC, while phylogroup A was more prevalent in healthy individuals [57]. Out of the Escherichia coli strains found in CRC samples, over 36.9% had the ability to create biofilms [57]. In comparison, only 16.6% of the Escherichia coli strains from the control group were capable of forming biofilms [57]. In addition, CRC patients had a greater frequency of genes encoding cyclomodulin, which was associated with the likelihood of generating a biofilm [57].
Fusobacterium nucleatum produces Fusobacterium nucleatum adhesin A, which promotes the development of CRC by altering the β-catenin/Wnt signaling pathway [60,61]. Zepeda-Rivera et al. have shown that elevated levels of Fusobacterium nucleatum within the tumor are linked to increased rates of tumor recurrence, distant metastasis, and a poorer prognosis [61]. Furthermore, the authors state that the Fusobacterium nucleatum animalis (Fna) subspecies represents the majority of the strains found at the tumor level [61]. In addition, genomic analysis revealed that the Fna subspecies consists of two clades: Fna C1 and Fna C2 [61]. Only Fna C2 dominates the colorectal tumor niche among these [61]. Fusobacterium nucleatum has the ability to promote a pro-oncogenic inflammatory environment [62]. It was thus demonstrated that this microorganism activates the NF-kB pathway, leading to the overexpression of some proinflammatory cytokines (cyclooxygenase 2, tumor necrosis factor, interleukin-6, interleukin-8, and interleukin-1 beta) and the selective recruitment of some myeloid-derived immune cells [62].
Currently, there are numerous studies that highlight the pathogenic link between Clostridium septicum and the development of CRC [63,64,65,66,67]. Clostridium septicum proliferates in the intestines as a result of its anaerobic nature and ability to generate spores. It has the ability to cross the mucosa and enter the bloodstream. However, the mechanisms by which this bacterium contributes to colorectal oncogenesis are only partially understood [63]. Although Clostridium septicum does not appear to cause CRC, the alpha-toxin it produces enhances the spread and circulation of tumor cells [68].
In addition to the microbes themselves, the development of CRC is influenced by microbial metabolites such as short-chain fatty acids (SCFAs), secondary bile acids, and glucuronidase [69]. SCFAs are major bacterial metabolites, with a role in both the maintenance of intestinal homeostasis and oncogenesis [69]. Okumura et al. demonstrated in a recent article, for example, that butyrate promotes carcinogenesis by promoting cellular senescence [70]. These authors also documented the cause-and-effect relationship between the abnormal proliferation of Porphyromonas species and the development of CRC, which may be attributed to the induction of cellular senescence by butyrate [70]. Another study showed that organic solute transporter β (OST β), an important subunit of the bile acid transporter OSTα-OSTβ, is significantly reduced in CRC patients, suggesting the important role of bile acids in the development of this neoplasia [71]. Kim et al. reported an elevation in glucuronidase activity in the feces of patients with CRC [72]. Researchers also discovered a link between glucuronidase activity inhibition and a decrease in colorectal tumors [71].
Recent studies have shown notable disparities in the composition of the intestinal microbiome between the tumor site and the rest of the gut [69]. As a result, there is currently evidence that signaling pathways in the tumor microenvironment (TME) are involved in the progression and metastasis of CRC [69]. The main components of the TME are intestinal cells, immune cells, endothelial cells, fibroblasts, and the extracellular matrix [37]. Besides these common components, the cells are in close contact with a large population of microorganisms [37]. Direct physical cellular interactions in the TME are essential for signal transduction [69]. In addition, molecules such as TGF-beta, Wnt, and other metabolites or exosomes secreted by tumor cells mediate cellular communication and are involved in cancer progression and distant metastasis [69]. MicroRNA, one of the essential components of exosomes, can play both pro- and anti-tumor roles. For example, miR-21 promotes tumor cell proliferation, invasion, and resistance to therapy, while miR-379 suppresses tumor cell proliferation and migration [73,74,75]. The TME microbiome may be involved in the response to chemotherapy [76,77,78,79,80]. For instance, the gammaproteobacteria present in the TME metabolize the medication gemcitabine, converting it into its inert state [81]. Furthermore, a study found that the antibiotic-induced reduction in this bacterium increased responsiveness to CRC chemotherapy [81].
Consuming meat that contains carcinogenic substances can also increase the incidence of CRC [82]. Factors such as gut microbiota metabolism, the presence of toxins, the activation of carcinogenic enzymes (including β-glucuronidase, β-glucosidase, azoreductase, nitroreductase, and alcohol dehydrogenase), the production of hydrogen sulfide (H2S) and reactive oxygen species (ROS), the transformation of secondary bile salts, and the by-products of protein fermentation can all influence the development of CRC [82].
Some microorganisms can suppress the progression and metastasis of CRC [83,84,85,86]. For instance, several probiotics have the potential to decrease DNA damage and inflammation. As a result, they may also hinder the progression of CRC [87]. Yue et al. reported that the administration of the Lactobacillus plantarum YYC-3 strain reduces intestinal inflammation and prevents CRC [88]. From a molecular point of view, this strain suppresses the NF-kB and Wnt signaling pathways in tumor cells to inhibit the production of proinflammatory cytokines [88]. Another study found that the administration of the probiotic strain Clostridium butyricum reduces tumor size by decreasing tumor infiltration with CD4+ and CD8+ T lymphocytes [89]. Oberreuther-Moschner et al. demonstrated that the use of the probiotic strains Lactobacillus acidophilus 145 and Bifidobacterium longum 913 prevents DNA damage at the cellular level [83]. The roles of probiotics or prebiotics in preventing the onset of CRC can be summarized as follows:
- (1)
- The inactivation of enzymes implicated in the development of cancer;
- (2)
- The enhancement of the population of beneficial gut bacteria with immune-modulating effects;
- (3)
- The establishment of a protective barrier against pathogen infection;
- (4)
- The ability to bind to carcinogens [82].
The strengths of our study are represented by the presentation of the most recent data, which indicate the role of the intestinal microbiota in the development of CRC. Gaining insight into the mechanisms that explain this pathogenic relationship can pave the way for the development of prognostic scores for CRC, as well as the development of novel targeted therapies. Despite substantial investigation and encouraging findings, the application of intestinal microbiota in the diagnostic and therapeutic management of CRC patients remains limited at present. This can be considered one of our research’s limitations.
4. Conclusions
Screening strategies have resulted in a decrease in CRC-associated mortality. Nevertheless, CRC continues to be a significant public health issue, currently ranking as the third most common cause of cancer-related mortality globally. The rise in CRC cases among the younger population, not included in screening programs, can explain this phenomenon. These findings emphasize the immediate necessity for further investigation focused on enhancing the diagnostic and therapeutic management of CRC. One of the pillars of the pathogenesis of this type of cancer is the intestinal microbiota. The manipulation of the microbiota can reduce the risk of evolution toward CRC, but at the same time, it can improve the prognosis of these patients by reducing their resistance to chemotherapy. Furthermore, researchers have identified the microbial composition and molecular functions of the intestinal microbiome as crucial biomarkers in predicting response to immunotherapy [78]. Thus, the evaluation of the intestinal microbiome can serve not only as a biomarker for predicting the risk of CRC but also as a potential target for increasing the efficiency of immunotherapy. Approaches to modulating the gut microbiome include dietary interventions, probiotic/prebiotic supplementation, fecal microbiota transplantation, and antibiotic therapy [78].
Author Contributions
Conceptualization, V.A.I. and G.G.; methodology, T.F.G. and V.B.; software, M.-S.C. and B.B.; validation, C.D.; formal analysis, B.B., M.B., N.B. and I.-A.C.; investigation, V.B., M.-S.C., M.B., B.B. and I.-A.C.; resources, N.B. and T.F.G.; data curation, V.A.I.; writing—original draft preparation, V.A.I. and G.G.; writing—review and editing, C.D.; visualization, C.D.; supervision, C.D.; project administration, C.D.; funding acquisition, T.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, S.; Zheng, R.; Li, J.; Zeng, H.; Li, L.; Chen, R.; Sun, K.; Han, B.; Bray, F.; Wei, W.; et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: A population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 2024, 9, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Gross, C.P.; Andersen, M.S.; Krumholz, H.M.; McAvay, G.J.; Proctor, D.; Tinetti, M.E. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA 2006, 296, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.C.; Harlan, L.C.; Lund, J.L.; Lynch, C.F.; Geiger, A.M. Patterns of colorectal cancer care in the United States: 1990-2010. J. Natl. Cancer Inst. 2015, 107, djv198. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.C.; Dixon, S.W.; White, P.; Williams, A.C.; Thomas, M.G.; Messenger, D.E. Demographic trends in the incidence of young-onsetcolorectal cancer: A population-based study. Br. J. Surg. 2020, 107, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Lui, R.N.; Tsoi, K.K.F.; Ho, J.M.W.; Lo, C.M.; Chan, F.C.H.; Kyaw, M.H.; Sung, J.J.Y. Global increasing incidence ofyoung-onset colorectal cancer across 5 continents: A joinpointregression analysis of 1,922,167 cases. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Done, J.Z.; Fang, S.H. Young-onset colorectal cancer: A review. World J. Gastrointest. Oncol. 2021, 13, 856–866. [Google Scholar] [CrossRef]
- Austin, H.; Henley, S.J.; King, J.; Richardson, L.C.; Eheman, C. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control. 2014, 25, 191–201. [Google Scholar] [CrossRef]
- Bailey, C.E.; Hu, C.Y.; You, Y.N.; Bednarski, B.K.; Rodruguez-Bigas, M.A.; Skibber, J.M.; Cantor, S.B.; Chang, G.J. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States,1975–2010. JAMA Surg. 2015, 150, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Yang, J.; Tan, Y.; Jiang, M.; Wen, F.; Huang, Y.; Chen, H.; Yi, C.; Zheng, S.; Yuan, Y. Young patients (≤35 years old) with colorectal cancer have worse outcomes due to more advanced disease: A 30-year retrospective review. Medicine 2014, 93, e135. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for Colorectal CancerUS Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer: From Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef] [PubMed]
- Irby, K.; Anderson, W.F.; Henson, D.E.; Devesa, S.S. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002). Cancer Epidemiol. Biomark. Prev. 2006, 15, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp-Vogelaar, I.; Kuntz, K.M.; Knudsen, A.B.; van Ballegooijen, M.; Zauber, A.G.; Jemal, A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol. Biomark. Prev. 2012, 21, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.K.; Ward, E.; Kohler, B.A.; Eheman, C.; Zauber, A.G.; Anderson, R.N.; Jemal, A.; Schymura, M.J.; Lansdorp-Vogelaar, I.; Seeff, L.C.; et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010, 116, 544–573. [Google Scholar] [CrossRef]
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-onset colorectal cancer: A review of current knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef]
- Song, M.; Emilsson, L.; Roelstraete, B.; Ludvigsson, J.F. Risk of colorectal cancer in first degree relatives of patients with colorectal polyps: Nationwide case-control study in Sweden. BMJ 2021, 373, n877. [Google Scholar] [CrossRef]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M.; et al. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Baron, J.A.; Lieberman, D.A.; Schatzkin, A.; Lanza, E.; Winawer, S.J.; Zauber, A.G.; Jiang, R.; Ahnen, D.J.; Bond, J.H.; et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009, 136, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Templeton, A.; Marra, G.; Kuo, Y.F.; Valtorta, E.; Shahinian, V.B. Risk of colorectal cancer in men on long-term androgen deprivation therapy for prostate cancer. J. Natl. Cancer Inst. 2010, 102, 1760–1770. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Coelho, D.; Blachier, F. Review of the association between meat consumption and risk of colorectal cancer. Nutr. Res. 2013, 33, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260e1216. [Google Scholar] [CrossRef] [PubMed]
- Celiberto, F.; Aloisio, A.; Girardi, B.; Pricci, M.; Iannone, A.; Russo, F.; Riezzo, G.; D’Attoma, B.; Ierardi, E.; Losurdo, G.; et al. Fibres and Colorectal cancer: Clinical and Molecular Evidence. Int. J. Mol. Sci. 2023, 24, 13501. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C.; et al. Alcohol drinking and colorectal cancer risk: An overall and dose-response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Wei, P.L.; Ho, C.H.; Yeh, C.C. Cigarette Smoking Associated with Colorectal Cancer Survival: A Nationwide, Population-Based Cohort Study. J. Clin. Med. 2022, 11, 913. [Google Scholar] [CrossRef]
- Limsui, D.; Vierkant, R.A.; Tillmans, L.S.; Wang, A.H.; Weisenberger, D.J.; Laird, P.W.; Lynch, C.F.; Anderson, K.E.; French, A.J.; Haile, R.W.; et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J. Natl. Cancer Inst. 2010, 102, 1012–1022. [Google Scholar] [CrossRef]
- Bai, X.; Wei, H.; Liu, W.; Coker, O.O.; Gou, H.; Liu, C.; Zhao, L.; Li, C.; Zhou, Y.; Wang, G.; et al. Cigarette smoke promotes colorectal cancer through modulation of gut microbiota and related metabolites. Gut 2022, 71, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Song, M.; Papadimitriou, N.; Carreras-Torres, R.; Langenberg, C.; Martin, R.M.; Tsilidis, K.K.; Barroso, I.; Chen, J.; Frayling, T.M.; et al. Association between Glycemic Traits and Colorectal cancer: A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2022, 114, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Varlas, V.; Stanescu, A.M.A.; Diaconu, C. Hepatobiliary impairments in patients with inflammatory bowel diseases: The current approach. Gastroenterology Insights 2022, 14, 13–26. [Google Scholar] [CrossRef]
- Nakatsu, G.; Li, X.; Zhou, H.; Sheng, J.; Wong, S.H.; Wu, W.K.K.; Ng, S.C.; Tsoi, H.; Dong, Y.; Zhang, N.; et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 2015, 6, 8727. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Garrett, W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016, 70, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Gagniere, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Huycke, M.M.; Abrams, V.; Moore, D.R. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. Carcinogenesis 2002, 23, 529–536. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Shkoda, A.; Kim, S.C.; Sartor, R.B.; Haller, D. Il-10 gene-deficient mice lack Tgf-Beta/Smad signaling and fail to inhibit proinflammatory gene expression in intestinal epithelial cells after the colonization with colitogenic Enterococcus Faecalis. J. Immunol. 2005, 174, 2990–2999. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Deng, M.; Chen, X.; Jiang, L.; Zhang, J.; Tao, L.; Yu, W.; Qiu, Y. Enterococcus faecalis promotes the progression of colorectal cancer via its metabolite: Biliverdin. J. Transl. Med. 2023, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mao, Y.; Du, J.; Xu, Y.; Zhu, Z.; Cao, H. Helicobacter pylori infection associated with an increased risk of colorectal adenomatous polyps in the Chinese population. BMC Gastroenterol. 2019, 19, 14. [Google Scholar]
- Coelho, L.G.V.; Coelho, M.C.F. Helicobacter pylori and colorectal neoplasms: A concise review. Arq. Gastroenterol. 2021, 58, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Shad, S.C.; Camargo, M.C.; Lamm, M.; Bustamante, R.; Roumie, C.L.; Wilson, O.; Halvorson, A.E.; Greevy, R.; Liu, L.; Gupta, S.; et al. Impact of Helicobacter pylori Infection and Treatment on Colorectal Cancer in a Large, Nationwide Cohort. J. Clin. Oncol. 2024, 42, 1881–1889. [Google Scholar]
- Ralser, A.; Dietl, A.; Jarosch, S.; Engelsberger, V.; Warnisch, A.; Janssen, K.P.; Middelhoff, M.; Vieth, M.; Quante, M.; Haller, D.; et al. Helicobacter pylori promotes colorectal carcinogenesis by deregulating intestinal immunity and inducing a mucus-degrading microbiota signature. Gut 2023, 72, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Kumar, R.; Xu, J.; Xu, Y. A pathogenicity locus of Streptococcus gallolyticus subspecies gallolyticus. Sci. Rep. 2023, 13, 6291. [Google Scholar] [CrossRef] [PubMed]
- Pasquereau-Kotula, E.; Martins, M.; Aymeric, L.; Dramsi, S. Significance of Streptococcus gallolyticus subsp. gallolyticusAssociation With Colorectal Cancer. Front. Microbiol. 2018, 9, 614. [Google Scholar] [CrossRef] [PubMed]
- Taddese, R.; Roelofs, R.; Draper, D.; Wu, X.; Swinkels, D.W.; Tjalsma, H.; Boleij, A. Streptococcus gallolyticus Increases Expression and Activity of Aryl Hydrocarbon Receptor-Dependent CYP1 Biotransformation Capacity in Colorectal Epithelial Cells. Front. Cell. Infect. Microbiol. 2021, 11, 740704. [Google Scholar] [CrossRef]
- Gheorghe, G.; Ceobanu, G.; Gheorghe, F.; Bratu, O.G.; Bacalbasa, N.; Bungau, S.; Diaconu, C.C. Fever of unknown origin. Rom. J. Mil. Med. 2020, 122, 213–218. [Google Scholar] [CrossRef]
- Abdulamir, A.S.; Hafidh, R.R.; Bakar, F.A. The association of Streptococcus bovis/gallolyticus with colorectal tumors: The nature and the underlying mechanisms of its etiological role. J. Exp. Clin. Cancer Res. 2011, 30, 11. [Google Scholar] [CrossRef]
- Corredoira, J.; Garcia-Pais, M.J.; Coira, A.; Rabunal, R.; Garcia-Garrote, F.; Pita, J.; Rodriguez-Marcias, A.; Blanco, M.; Lopez-Roses, L.; Lopez-Alvarez, J.; et al. Differences between endocarditis caused by Streptococcus bovis and Enterococcus spp. and their association with colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Herold, J.L.; Schady, D.; Davis, J.; Kopetz, S.; Martinez-Moczygemba, M.; Murray, B.E.; Han, F.; Li, Y.; Callaway, E.; et al. Streptococcus gallolyticus Subsp. gallolyticus promotes colorectal tumor development. PLOS Pathog. 2017, 13, e1006440. [Google Scholar] [CrossRef] [PubMed]
- Toprak, N.U.; Yagci, A.; Gulluoglu, B.M.; Akin, M.L.; Demirkalem, P.; Celenk, T.; Soyletir, G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 2006, 12, 782–786. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Wu, S.; Morin, P.J.; Maouyo, D.; Sears, C.L. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 2003, 124, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Nouri, R.; Hasani, A.; Shirazi, K.M.; Alivand, M.R.; Sepehri, B.; Sotoodeh, S.; Hemmati, F.; Rezaee, M.A. Escherichia coli and Colorectal Cancer: Unfolding the Enigmatic Relationship. Curr. Pharm. Biotechnol. 2022, 23, 1257–1268. [Google Scholar] [PubMed]
- Nouri, R.; Hasani, A.; Shirazi, K.M.; Alivand, M.R.; Sepehri, B.; Sotoudeh, S.; Hemmati, F.; Fattahzadeh, A.; Abdinia, B.; Rezaee, M.A. Mucosa-Associated Escherichia coli in Colorectal Cancer Patients and Control Subjects: Variations in the Prevalence and Attributing Features. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 2131787. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstern, C.R.; Lamichhane-Khadka, R. A tale of two bacteria—Bacteroides fragilis, Escherichia coli, and colorectal cancer. Front. Bacteriol. 2023, 2, 1229077. [Google Scholar] [CrossRef]
- Iftekhar, A.; Berger, H.; Bouznad, N.; Heuberger, J.; Boccellato, F.; Dobrindt, U.; Hermeking, H.; Sigal, M.; Meyer, T.F. Genomic aberrations after short-term exposure to colibactin-producing E. coli transform primary colon epithelial cells. Nat. Commun. 2021, 12, 1003. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Miguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef]
- Wang, N.; Fang, J.Y. Fusobacterium nucleatum, a key pathogenic factor and microbial biomarker for colorectal cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.T.; Padam, S. Clostridium septicum Bacteremia as the Presenting Sign of Colon Cancer. Cureus 2023, 15, e45343. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Diaconu, C.; Costache, R.S.; Florentina, G.; Andronesi, A.G.; Gheorghe, G. Predictive Factors for Death among Patients with Clostridium difficile Infection—A Single Center Experience Study. Rom. J. Mil. Med. 2023, 4, 492–501. [Google Scholar] [CrossRef]
- Nanjappa, S.; Dhah, S.; Pabbathi, S. Clostridium septicum Gas Gangrene in Colon Cancer: Importance of Early Diagnosis. Case Rep. Infect. Dis. 2015, 2015, 694247. [Google Scholar] [PubMed]
- Sidhu, J.S.; Mandal, A.; Virk, J.; Gayam, V. Early Detection of Colon Cancer Following Incidental Finding of Clostridium septicum Bacteremia. J. Investig. Med. High Impact Case Rep. 2019, 7, 2324709619832050. [Google Scholar]
- Chirikian, D.; Awsare, S.; Fitzgibbon, J.; Lee, L. Concurrent Clostridium septicum bacteremia and colorectal adenocarcinoma with metastasis to the brain—A Case Report. IDCases 2021, 25, e01189. [Google Scholar] [CrossRef]
- Dahmus, J.D.; Kotler, D.L.; Kastenberg, D.M.; Kistler, C.A. The gut microbiome and colorectal cancer: A review of bacterial pathogenesis. J. Gastrointest. Oncol. 2018, 9, 769–777. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Shen, M. Tumor Microenvironment Shapes Colorectal Cancer Progression, Metastasis, and Treatment Responses. Front. Med. 2022, 9, 869010. [Google Scholar] [CrossRef]
- Okumura, S.; Konishi, Y.; Narukawa, M.; Sugiura, Y.; Yoshimoto, S.; Arai, Y.; Sato, S.; Yoshida, Y.; Tsuji, S.; Uemura, K.; et al. Gut bacteria identified in colorectal cancer patients promote Tumourigenesis via butyrate secretion. Nat. Commun. 2021, 12, 5674. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, C.; Lou, Y.; Liu, J.; Ye, S.; Chen, L.; Lei, J.; Guo, S.; Zeng, S.; Yu, L. Epigenetic mechanisms underlying organic solute transporter beta repression in colorectal cancer. Mol. Pharmacol. 2020, 97, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jin, Y.H. Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Arch. Pharmacal Res. 2001, 24, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Kawee-Ai, A.; Kim, S.M. Application of microalgal fucoxanthin for the reduction of colon cancer risk: Inhibitory activity of fucoxanthin against beta-glucuronidase and Dld-1 cancer cells. Nat. Prod. Commun. 2014, 9, 921–924. [Google Scholar] [PubMed]
- Sun, L.H.; Tian, D.; Yang, Z.C.; Li, J.L. Exosomal Mir-21 promotes proliferation, invasion and therapy resistance of colon adenocarcinoma cells through its target Pdcd4. Sci. Rep. 2020, 10, 8271. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.; Khan, S.; Glynn, C.L.; Holian, E.; Dockery, P.; Lalor, P.; Brown, J.A.L.; Joyce, M.R.; Kerin, M.J.; Dwyer, R.M. Screening of exosomal micrornas from colorectal cancer cells. Cancer Biomark. 2016, 17, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Mei, J.X.; Tu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kolat, D.; Yang, K.; Hu, J.K. Role of the gut microbiota in anticancer therapy: From molecular mechanisms to clinical applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.K.; Xie, R.L.; You, R.; Liu, Y.P.; Chen, X.Y.; Chen, M.Y.; Huang, P.Y. The role of the bacterial microbiome in the treatment of cancer. BMC Cancer 2021, 21, 934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Xia, Q. Role of gut microbiome in cancer immunotherapy: From predictive biomarker to therapeutic target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Diaconu, C.; Bungau, S.G.; Jinga, V.; Gheorghe, G. Current Approaches in the Allocation of Liver Transplantation. J. Pers. Med. 2022, 12, 1661. [Google Scholar] [CrossRef]
- Guo, X.W.; Lei, R.; Zhou, Q.N.; Zhang, G.; HU, B.I.; Liang, Y.X. Tumor microenvironment characterization in colorectal cancer to identify prognostic and immunotherapy genes signature. BMC Cancer 2023, 23, 773. [Google Scholar] [CrossRef]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gaver, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, D.Y.; Kang, J.H.; Kim, J.H.; Jeong, J.W.; Kim, H.W.; Oh, D.H.; Yoon, S.H.; Hur, S.J. Retationship betwwn gut microbiota and colorectal cancer: Probiotics as a potential strategy for prevention. Food Res. Int. 2022, 156, 111327. [Google Scholar] [CrossRef] [PubMed]
- Oberreuther-Moschner, D.L.; Jahreis, G.; Rechkemmer, G.; Pool-Zobel, B.L. Dietary intervention with the probiotics Lactobacillus acidophilus 145 and Bifidobacterium longum 913 modulates the potential of human faecal water to induce damage in Ht29clone19a Cells. Br. J. Nutr. 2004, 91, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The role of Probiotics in Colorectal Cancer Management. Evid. Based Complement. Altern. Med. 2020, 2020, 3535982. [Google Scholar] [CrossRef] [PubMed]
- Drago, L. Probiotics and Colon Cancer. Microorganisms 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Dikeocha, I.J.; Al-Kabsi, A.M.; Hussin, S.; Alshawsh, M.A. Role of probiotics in patients with colorectal cancer: A systematic review protocol of randomised controlled trial studies. BMJ Open 2020, 10, e038128. [Google Scholar] [CrossRef] [PubMed]
- Ambalam, P.; Raman, M.; Purama, R.K.; Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Ye, K.; Lu, J.; Wang, X.; Zhang, S.; Liu, L.; Yang, B.; Nassar, K.; Xu, X.; Pang, X.; et al. Probiotic strain Lactobacillus plantarum Yyc-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed. Pharmacother. 2020, 127, 110159. [Google Scholar] [CrossRef]
- Chen, Z.F.; Ai, L.Y.; Wang, J.L.; Ren, L.L.; Yu, Y.N.; Xu, J.; Chen, H.Y.; Yu, J.; Li, M.; Qin, W.X.; et al. Probiotics Clostridium butyricum and Bacillus subtilis ameliorate intestinal tumorigenesis. Future Microbiol. 2015, 10, 1433–1445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).