Abstract
Recently, the bioactive potential of several functional ingredients and biomolecules has been evaluated regarding human and animal nutrition. The digestive process from food intake to absorption and metabolism are important events that induce changes in ingredients, which affect their bioactivity. Consequently, there is a need to assess the bioavailability and bioaccessibility of these compounds. The methodology for the simulation of the human gastrointestinal tract has been standardized (INFOGEST protocol), while a gastrointestinal protocol for other animals (e.g., ruminants or broilers) has yet to be established. However, INFOGEST allows us only to predict bioaccessibility, leaving a gap regarding a methodology able to assess bioavailability by mimicking intestinal permeability and absorption. Several approaches—including in vitro, ex vivo, in situ and in vivo methods—can be found in the literature, aiming to tackle transepithelial routes, but leading to different results concerning the bioefficiency of the compounds studied. Therefore, this review aims to assess the current state-of-the-art regarding monogastric intestinal dynamics, absorption, and permeability events. Moreover, it compiled methodologies for simulating intestinal absorption in several biological systems, while reasoning their advantages, disadvantages, applications in ingredient development and the existing gaps.
1. Introduction
In recent decades, the role of nutrition has been widely recognized as a way to improve health and prevent a vast array of diseases in both humans and animals [1,2]. Therefore, in recent years, the bioactive potential of ingredients along with the development of so-called functional foods have been extensively explored by the scientific community. Bioefficiency is broadly defined as the potential of foods to fulfil the nutritional and metabolic needs of the consumer, so that the organism may perform its basic functions, but also contribute to health maintenance, through its bioactive potential [3]. However, the bioefficiency of many compounds with promising bioactivity may be affected by digestion, food intake, enzymatic digestion and absorption to metabolization. Hence, their bioefficiency should be clarified through the assessment of bioaccessibility (BC) and bioavailability (BA). Bioaccessibility usually refers to the fraction of compounds that are released as a result of gastrointestinal digestion and are therefore available to be absorbed [3,4]. As for BA, it stands for the fraction of compounds or metabolites that are absorbed unchanged, reach systemic circulation and then exert a bioactive effect on a target site, triggering a metabolic and/or physiologic response [4,5]. Thus, a compound, to fulfil its bioefficiency purpose, must first become bioaccessible, through digestion, so it can be absorbed into the systemic circulation and become bioavailable and finally exert its bioactive potential (Figure 1). In another way, the BC concept is focused on the impact of the digestive processes, including the stability amidst acidic pH values or upon enzymatic action, while BA comprises the process of the intestinal absorption of the bioacessible fraction, at the prompting of a physiological effect.
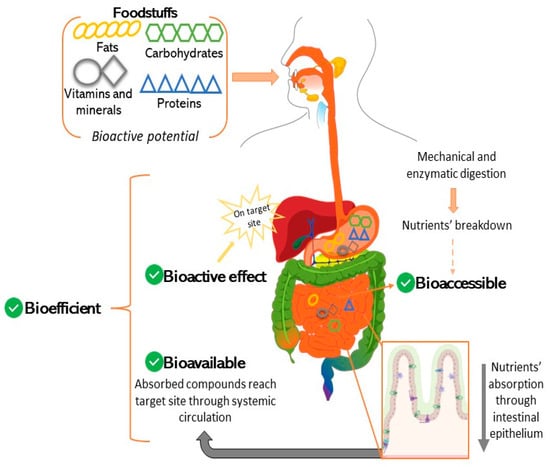
Figure 1.
From food intake to bioefficiency—relationship between bioacessibility, intestinal absorption, bioavailability and bioactivity.
Recently, several models of human and animal digestion simulation have been developed to predict the BC of different compounds. For example, INFOGEST is a widely acknowledged standardized static in vitro model of the human upper gastrointestinal tract (GIT), representing a valuable tool used to predict the BC of several ingredients [6,7]. Digestion simulation protocols have also been developed for chickens, since they have a distinct GIT among monogastric animals [8,9], despite not reuniting the same broad consensus as INFOGEST. From a different standpoint, BA represents a challenging concept to be accurately evaluated, since in vivo methodologies are the ‘gold standard’ and rely on measuring a compound of interest in blood plasma levels. However, there are currently several ethical constraints related, for example, with protecting animal’s welfare and human volunteers’ safety, in addition to being time and resource consuming [10,11,12]. As such, several models have been developed as attempts at predicting BA through the determination of the bioacessible fraction absorbed. These systems are essentially tissue based, namely ex vivo (e.g., InTESTineTM Ussing chambers, Franz diffusion cells, everted-sac technique) or cell-culture based (e.g., Transwell® inserts, 3D organoids) [4,12,13]. Additionally, other alternatives are still being developed, such as continuous flow dialysis, organ chip technologies [14] and a medium throughput micro-physiological system, the intestinal Explant Barrier Chip (IEBC) set-up [15], among others.
From a different perspective, studies regarding the human GIT dynamics in health and disease demand more appropriate models, namely non-rodent ones, due to the intrinsic differences between the early stage GIT development in this species versus that of a human. As such, pig-based models have established themselves as the most reliable alternative supported by the extensive research on this animal and given the anatomical and physiological similarities, nutritional requirements and microbiota diversity [16,17]. Therefore, many studies on human health use pig tissues to assess the BA of compounds and drugs [18,19], surpassing the constraints of obtaining human intestinal segments. From a different perspective, models with other animal tissues can also be found in the literature, within the scope of animal science and nutrition. For instance, Ringø et al., 2014 [20] used salmon intestines in a Ussing chamber assay to assess the damaging effect of a pathogen, and Kent-Dennis et al., 2021 [21] used cow’s ruminal tissue for ex vivo studies regarding the effects of lipopolysaccharide (LPS), among many other studies. Additionally, the establishment of nutritional claims regarding ingredients’ bioactive properties through studies involving animal tissues are also being banished by several players that commercialize functional ingredients, which opens the need for developing reliable permeation models based on artificial membranes.
The main purpose of this review was to address the lack of consensual and standardized methodologies and to assess the intestinal permeation of digested compounds by revising the existent models, as well as their adequacy, advantages and disadvantages. Moreover, it also intended to provide a comprehensive state-of-the-art review on the intestinal absorptive epithelium and its permeability. In addition to human and porcine absorption, given the acknowledged similarities, this review also compiled the most recent insights on chicken’s intestinal absorption, in line with the body of work developed by our research group in recent years concerning the development of GIT in vitro models for humans and animals [22].
2. Materials and Methods
The research was carried out to assess the extent of the application of distinct techniques in simulating intestinal absorption. Google Scholar and PubMed were used with a time interval between 2015 and 2022. The search strings used combinations between animal model (pig, human, chicken), techniques (Ussing chamber, Franz diffusion, etc.) and other keywords regarding food matrices and intestinal permeability. For example: “pig” AND “Ussing chamber” AND “ingredient” AND “absorption”.
Exclusion and Inclusion Criteria
The research papers were screened and selected based on whether their goal was to assess the effects of a nutrient/ingredient/supplement or molecule on health. Therefore, studies using different molecules (e.g., caffeine, among others) to compare distinct techniques and studies focused on the development/validation of new permeation models were excluded. In opposition, research papers not comprising GIT digestion before intestinal absorption simulation were included.
3. Overview of the Absorptive Epithelium—Structure and Physiology
The intestinal epithelium is a structure conserved among all vertebrates, serving the same purpose in distinct organism’s physiology [23]. It consists of a sheet of tightly linked cells that separate the lumen from the internal environments. Moreover, it represents a primary site of absorption and establishes a constant interplay between the metabolism, immune system and microbiota [24]. Some key elements are common to several species, namely the presence of similar absorptive enterocytes, goblet cells, enteroendocrine cells or even the process of cellular turnover from the crypt to the top of the villus [25]. Additionally, these common threads and the availability of animal tissues turn animal models into valuable tools for exploring intestinal dynamics [25]. Therefore, this section intends to provide a basic understanding of the intestinal epitheliums of the models considered in this review.
3.1. Human Intestinal Epithelium
The human intestinal epithelium has been thoroughly reviewed and studied, and its involvement in health and disease has become clear. The reader can refer to complete reviews focused on this topic, such as [24,26,27]. While the complex mechanisms of cell structure and function and cell-to-cell communication are beyond the scope of this review, it is still relevant to keep in mind the basic notions of normal intestinal and epithelial organization (Figure 2A). The concepts of intestinal permeability and intestinal barrier have been explored as two distinct features of the same structure—the intestinal wall. On one hand, the term intestinal barrier refers to the protective aspect of the gut, acting as a physical barrier against microorganisms’ invasion and preventing the passage of toxins. On the other hand, intestinal permeability relates to the transepithelial passage of fluids and nutrients [28]. When considering these two opposing characteristics, namely the provided protection, while allowing the absorption of fluids, it is important to understand how key structural and physiological elements of the epithelium contribute to these characteristics (Figure 2B). From a different perspective, at a cellular level, the epithelial monolayer is sealed through three types of intercellular junctions: tight junctions (TJs), adherens junctions (AJs) and desmosomes. Each one of these junctions is in a specific membrane region contributing to, at a cellular level, the barrier function by safeguarding the paracellular pathway, but also contributing to the overall structural integrity and cell-to-cell communication. TJs represent the main controlling factor in the paracellular pathway, and their number increases from crypt to villus, along with the size selectivity. From another perspective, changes in the TJs’ expression, often induced by proinflammatory cytokines, lipopolysaccharides (LPS) or pathogenic bacteria, have been linked to excessive intestinal permeability, which has been frequently associated with intestinal inflammation and diseases [29,30].
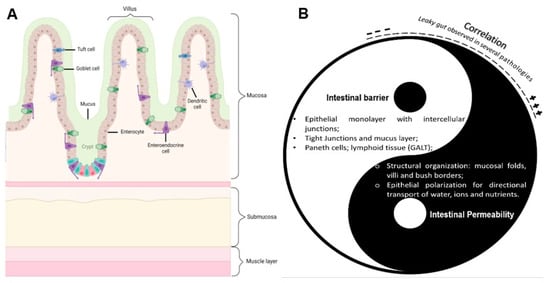
Figure 2.
Intestinal epithelium structure and homeostasis. (A) Schematic representation of the key elements found in the small intestine epithelium and its main layers. Image created with Biorender.com. (B) The intestinal epithelium Yin–Yang: Intestinal permeability and intestinal barrier as two fundamental but opposite “forces” of the same structure. The symbols “+” and “–“ express increases or decreases in gut permeability.
3.2. Pig and Chicken Intestinal Epitheliums
As mentioned above, a pig’s intestinal epithelium is very similar to that of a human, and therefore, it has also been extensively studied. Detailed reviews on the structural features of the intestinal epithelium can be found in Modina et al., 2019 and Modina et al., 2021 [31,32]. The epithelium is organized in four essential layers, as in most vertebrates. One macroscopic difference between human and pig intestines is regarding the intestinal length. The small intestine of a pig is ca. 20 m, that of a human averages around 6 m, and the pig’s large intestine has an average length of 5 m, while the human averages 1.5 m [33].
The chicken’s GIT has been described in the literature, in complete reviews such as those of Denbow, 2015 or Alshamy et al., 2018 [34,35]. Interestingly, the literature specifically targeting the intestinal epithelium and the absorption of nutrients is lacking. Briefly, the avian GIT is composed of an esophagus, crop, proventriculus, ventriculus/gizzard, small intestine, and large intestine. The crop is an out-pocket in the esophagus, where swallowed feed is stored until it passes on through the GIT. The proventriculus is the glandular stomach responsible for the secretion of acid and enzymes, as in chemical digestion. The gizzard or ventriculus, an organ unique in birds, functions as a mechanical stomach since it grinds, mixes and mashes the digesta and small stones. The small intestine is made up of the duodenum and the lower intestine, which is composed of the jejunum and ileum. The duodenum receives enzymes and bicarbonate from the pancreas and liver, which are particularly important for the digestion of proteins and lipids. Regarding nutrient absorption, it occurs in the small intestine, through a specialized intestinal mucosa, with similar features to the ones described previously: villi and cellular polarization, with brush borders in the apical region to increase the absorption area [35]. The intestinal epithelium has a dynamic nature, in part influenced by nutrition since the villus height varies both with age and with nutritional intake [36,37].
3.3. Nutrients’ Transportation Routes across Epithelium
Among monogastric organisms, the small intestine is usually the main site of nutrient absorption. The absorptive epithelial cells constitute ca. 90% of the epithelium, and their main function is to selectively uptake nutrients from the luminal environment, across the brush border. This is achieved through transepithelial transport, which is a dynamic and complex process, involving the modulation of the expressions of transmembrane transporting proteins, related to distinct transport mechanisms, but also varying according to the physicochemical attributes of the substances to be absorbed. Transport strategies can be essentially divided into paracellular transport—between two neighboring cells—and transcellular routes—across cells. The latter can be further subdivided into passive diffusion, carrier-mediated transport and endocytosis. The paracellular route is linked to the passive transport route, and it is mostly determined by the presence of TJs. The TJs permeability are mostly determined by the particle size. Therefore, the passage of substances through this route is minimal and essentially limited to solutes, water and ions, which cross the epithelium unaltered as they are not exposed to the intracellular environment [38]. Yu et al., 2016 [39] stated that the paracellular space, under normal physiological conditions, ranges from 0.3 to 1.0 nm. From a different perspective, the deregulation of the TJ complex has been linked to the serious impairment of the intestinal barrier (leaky gut) acting as a trigger for an inflammatory status and even systemic pathologies [40,41]. Transcellular transport is usually linked to the passage of proteins, macromolecules and larger particles.
As Kiela et al., 2016 [42] described, dietary sugars and amino acids are absorbed via facilitated diffusion, by transporters located in the brush border of the enterocytes. Glucose and galactose are absorbed via SGLT1 transporters, in a Na+-dependent manner (each sugar molecule is accompanied by two Na+ ions), driven by an electrochemical gradient. Fructose is transported via GLUT5 and GLUT2, in the brush border and the basolateral membrane, respectively [43]. Di and tripeptides are absorbed through the H+/peptide transporter, but longer peptides (>tetrapeptides) are poorly absorbed. Some peptides are degraded into amino acids, through proteases. The amino acid transport is complex, given that countless systems exist that are responsible for amino acid uptake, which is generally ion dependent. Peptide transport is not dependent on the hydrophobicity of peptides, but essentially on peptide length, and their sequences seem to be a critical factor [44]. Lipids are essentially digested in the duodenum, by pancreatic enzymes and bile acids. Then, they are absorbed by the enterocytes via simple diffusion and move to the intracellular environment through the actions of CD36 and FABP (Fatty Acid-Binding Proteins) transporters [45]. Generally, only medium-length fatty acids can be absorbed. As for vitamins, for some of them, the transport mechanisms have not been fully disclosed (for example, in vitamins A and K) [46], and while some are absorbed through a transporter, others require more complex transport pathways since they often reach the intestine and require metabolic conversions.
Regarding swine’s absorption mechanisms, they are similar to those of humans. The mechanisms for sugars, peptides and amino acid and lipid transport are the same [47,48]. For instance, fructose transporters are the same as in humans, belonging to the GLUT family. The only difference relates to the amino acid absorption in the large intestine, as previously observed in pigs, but not yet demonstrated in humans since it is generally accepted that amino acids that reach the large intestine are usually metabolized by the gut microbiota instead [49].
In chickens, the same basic mechanisms remain valid: sugars cross the epithelium through glucose transporters such as SGLT-1; the similar transporter applies to small peptide absorption (PepT-1), while the amino acid mechanism remains specific and complex beyond the scope of the present review. Lipids are hydrolyzed into free fatty acids or monoglycerides and absorbed through simple diffusion, although other chaperones (FABP) are responsible for their intracellular movements [50]. Interestingly, Karasov, 2017 pointed out that birds have a higher paracellular absorption among flying and non-flying vertebrates [51].
Aside from the transport mechanisms described, it is also worth mentioning the close relationship between intestinal transport and the physicochemical properties of the compounds, namely their solubility (lipophilicity and/or hydrophilicity). Transport through passive diffusion is usually related to lipophilic compounds, meaning that compounds with a high affinity to the enterocyte’s membrane, while hydrophilic compounds (water-soluble) cross the epithelium in a paracellular manner [42,52]. However, as Zhu et al., 2017 [53] stated, the paracellular route represents a minor pathway, as the intestinal surface area available for this route is reduced.
In summary, epithelial structure and physiology have been thoroughly described and studied in humans. However, despite humans’ transepithelial nutrient transport mechanisms being well studied in terms of membrane transporters and drug absorption mechanisms, from a nutritional standpoint there are some knowledge gaps that remain to be addressed. For instance, as previously noted, the transport mechanisms of some vitamins are not completely disclosed. The void is even more evident in monogastric animals. The transepithelial absorptive routes in chickens are still poorly explored as information is lacking on this topic. The acknowledged link between animal health, its performance and its impact on human consumption accentuates the relevance of further investigating this topic [22].
4. In Vitro Models for Predicting Bioaccessibility
Although the focus of the present review is on models that simulate intestinal absorption, this process is included in the broader concept of digestion, and therefore, it is important to understand what models exist upstream of absorption. During digestion, nutrients present in the food/feed matrices are broken down through enzymatic and mechanical action and made bioaccessible for absorption. This process is essential for well-being as digestion can be considered an interface between foods and their impact on health [54]. In vivo methods consist of feeding animals or humans under controlled conditions. However, these models are expensive, complex and time consuming and increasingly raise ethical concerns. As such, developing better in vitro digestion models remains highly relevant when studying the impacts of bioactive compounds on health. The current models can be essentially divided into static models, meaning that only replicate biochemical processes, such as enzymatic action and pH value oscillations, and the products are immobilized, not simulating mechanical actions like mixing, shearing and others. Dynamic models contemplate pH changes, mechanical action, enzymatic secretions and the release of fluids through time, among others. Finally, semi-dynamic models combine features from both dynamic and static models. The complex features of the dynamic models, in opposition to the static ones, might translate into different bioaccessibilities, affecting the release of the food matrix, its solubility and stability. Dynamic models are more suited for kinetic studies, such as for determining nutrients’ digestibility and for assessing nutrients’ bioaccessibility, while static models are more appropriate for mechanist studies, since there are fewer variables to control and are more reproducible. Overall, in comparison to in vivo options, in vitro models are considered cheap and versatile, and their simplicity (especially of the static models) allows for standardization and comparisons with the literature. Additionally, they are particularly useful for screening the bioactive potential of new formulations/ingredients. However, gastrointestinal in vitro models are unable to mimic hormonal features, their interactions with the immune system or with the gut microbiota, for instance.
Currently, several models mimic human digestion, or parts of it. Overall, the INFOGEST models are widely used, and this consortium was able to significantly contribute to the standardization of digestion protocols [6,55]. The INFOGEST protocol simulates almost the complete span of digestion, from the mouth to the small intestine. In each stage, conditions are standardized, concerning temperature, incubation time, pH value and fluid composition, and the enzymatic solutions are well defined. This protocol is very useful for either the kinetics of nutrient release or for monitoring the concentration or stability of a particular substance or nutrient. However, this model does not ensure complete fatty acid ionization in the small intestine step after lipid digestion and lacks dynamic feedback mechanisms. Nevertheless, studies have shown that despite these limitations, INFOGEST displays a good correlation with in vivo animal feeding studies. Other frequently cited in vitro models are the TNO Gastro-Intestinal Model (TIM), considering both the static and dynamic versions (TIM1 and TIM2), which simulate both stomach and intestinal digestion [56]; the Dynamic Gastrointestinal Simulator (GI Simulator—SIGMI) which is an automated in vitro gastrointestinal model specifically engineered to dynamically mimic the physiological events occurring during digestion, both in the stomach and small intestine. Additionally, it replicates the colonic microbiota, simulating its action within the large intestine [12]. SHIME is another unavoidable model since it mimics not only the stomach and the small intestine, but also the distinct regions of the colon [57].
As for animal in vitro models, there is a lack of standardization compared to that attained by INFOGEST. Regarding pig digestion, pigs have also been used as an animal model to perform in vivo–in vitro correlation studies for the INFOGEST protocol [58]. Additionally, INFOGEST has also been used in studies to predict amino acid digestion, given the similarities with the human gastrointestinal system [59]. The three-step method developed by Boisen et al., 1997 [60] represents a conventional approach to in vitro digestion simulation, with a general validity for the most used feedstuffs. This protocol contemplates proper particle size through grinding, incubation times, temperature and agitation, as well as pH variations, proper buffers and enzymatic solutions. These parameters are optimized for achieving the potential maximal digestibility. Most models mentioned in the literature aim to assess the digestibility of a particular nutrient, to better assess the energetic value of pig feeds. A more recent review on in vitro techniques for digestibility evaluation can be found in Święch et al. (2017) [61], although the author also acknowledges the model proposed by Boisen as the most generally applicable one. Concerning chicken in vitro gastrointestinal models, the resources in the literature are even more scarce. Nash et al., 2022 [62] pointed out the over-reliance on in vivo studies in the face of the absence of proper in vitro models. The authors essentially reviewed cell culture approaches to drug development and toxicity studies, although acknowledging that the right models depend on the scientific question behind them. When considering the development of new functional feed formulations, there is a lack of complete and standardized models comprising the oral phase to the cecum simulation. Carvalho et al., (2023) proposed a model as a combination of several other models [63,64,65]. It includes mechanical action, incubation times/temperatures and enzymatic solutions for each digestive step and includes cecal fermentations, using inocula previously collected, covering to some extent the potential interactions of the ingredients in the study with the microbiota. This approach has the potential to be easily reproducible across different labs.
Overall, in what concerns human health, there are plenty of models, adequate for answering distinct questions. In what concerns the study of the bioactive potential of new ingredients, INFOGEST represents a crucial landmark in this field, reproducible and attainable to less resourceful research centers. In what concerns animal studies, particularly in the development of functional feedstuffs, there are models focused on assessing the digestibility of particular nutrients of energic value, but there are no general or uniformized models for evaluating the potential impact of functional ingredients or additives on feeds. Additionally, in both humans and animals, there is a lack of studies that investigate how these upstream models of digestion could be combined with the downstream models focused in this review.
5. Intestinal Permeability Models and Applications
As previously stated, the BA depends on BC and absorption. While in vivo studies are the “gold standard” for assessing BA, they are laborious, bear high costs and pose ethical constraints that would be inadequate for the early screenings of ingredients or foodstuff development studies. The methodologies described are often grouped according to the underlying principle. Tissue-based methods usually refer to ex vivo procedures that rely on using an intestinal segment, for example, Franz diffusion cells, a Ussing chamber, or an everted gut sac. Other authors categorize according to biological or non-biological models, grouping cell-culture methods and ex vivo approaches, while differentiating them from nonbiological methods, such as artificial membranes. In the present review, the techniques described are classified into tissue-based methods, cell-culture-based methods, or other nonbiological methodologies that do not fit into those two major categories. Overall, ex vivo models can be considered a middle term between cell-based methods and non-biological models, concerning their physiological relevance. Since they maintain the tissue’s structure, including membrane transporters, nutrient absorption might take place through the active transport of passive diffusion mechanisms. Additionally, the integrity and structure of the epithelium are preserved. However, ex vivo models encompass a high tissue variability and lack a blood supply. Cell-based models display a wide range of complexity, from monolayer models to gut-on-chip models, which represent a promising technology. These models allow nutrient absorption through active transport or facilitated diffusion mechanisms. Furthermore, they do not have any ethical constraints and have a higher reproducibility. Non-biological methods usually have a higher throughput and are more cost-effective than ex vivo or cell-based methods, therefore being more suitable for initial screenings. Yet, they cannot mimic the complexity of the intestine. For instance, only the passive transport of nutrients has been considered [12,66]. The advantages and disadvantages of each model are also considered, as first systematized in Table 1, and further described in this section. Additionally, examples of their use in the ingredients of food formulation testing are also reviewed (Table 2).

Table 1.
Main techniques and models for the assessment of intestinal absorption.
5.1. Tissue-Based Methods
Ex vivo methods are commonly used to predict intestinal absorption, utilizing viable and functional tissues isolated from organisms properly incubated under controlled conditions. These methods include the Ussing chamber, everted sac, and Franz diffusion cells, among others (Figure 3) Nevertheless, these ex vivo techniques imply the loss of blood flow and nervous systems, which may impair the reliability of some results. Despite this, they constitute more attainable and practical alternatives to in vivo studies [12,67].
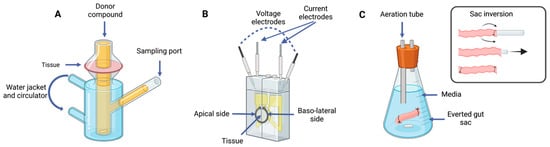
Figure 3.
Tissue-based methods: (A) Franz cells; (B) Ussing chamber; (C) everted gut sac technique coupled with sac inversion diagram. Image created with BioRender.com.
5.1.1. Ussing Chamber
A Ussing chamber assay represents the most common technique among the ex vivo models used to assess intestinal absorption. Since it was created in the 1950s to investigate sodium uptake, this system continues to be relevant nowadays for intestinal permeability studies [68]. This technique is frequently used to measure the transport of ions, nutrients, or drugs across epithelial tissues [69]. It consists of an opened intestinal segment supported, creating two isolated compartments: one corresponding to the luminal side, where the molecule of interest is placed, and the serosal side, corresponding to the basolateral region. This system also controls and maintains temperature and continuously gasses the chamber. Moreover, two voltage-sensing electrodes and two current-passing electrodes are placed on each side, which monitor and provide current, enabling the measurement of transepithelial electrical resistance (TEER) and the short-circuit current (Isc), as a structural integrity criterion [69,70]. On one hand, the Ussing chamber technique is well validated and can be used to study permeability across distinct intestinal regions, as it allows the assessment of carrier-mediated transport and works with drugs that are poorly absorbed. On the other hand, it requires the removal of the muscular layer since contractions might interfere with electrical monitoring [71]. Moreover, not all animal models are suitable for this technique, for instance, rabbit tissues, since they are considered too thick to allow diffusion processes. Other disadvantages are the low to medium throughput of this technique, the high cost of the system and the technical skills needed. More recently, modular Ussing chamber systems have been developed and commercialized to increase the throughput, such as NaviCyte [72]. Furthermore, Westerhout et al., 2014 [73] optimized the use of porcine intestinal segments in a newly developed InTESTineTM system to predict intestinal absorption, for drug development or digested foodstuffs.
5.1.2. Franz Diffusion Cells
Franz diffusion cells constitute an established system for transdermal diffusion experiments in skin tissues, as recently reviewed by Supe et al., 2021 [74]. In the literature, few studies regarding intestinal absorption rely on this apparatus. While the principle behind this system is very similar to that of the Ussing chamber, some differences are worth mentioning. For instance, Franz cells follow a vertical orientation instead of a horizontal one (as in Ussing chambers). The compartments have different volumes: the donor chamber, which receives the compound of study, has a smaller volume than the receiving chamber [13]. Moreover, there are no electrodes or gasification, but only controlled temperatures and the stirring of the receiving chamber, which may be the reason for the higher permeability when comparing with the Ussing chamber, as proposed by Dezani et al., 2013 [75].
5.1.3. Intestinal Rings and Segments
These techniques consist of exposing intestinal segments or rings, to the compound of interest, dissolved in a buffer, to assess its uptake by enterocytes and subsequent metabolism [12]. An intestinal segment is isolated and, after washing, is cut either into small rings or segments and submerged in a proper oxygenated medium. The intestinal rings might also be incubated in an everted position, as in the evert sac technique. While in the intestinal segments, the muscle layer is removed; in the intestinal rings, no layer is removed, which influences their viable time (1 h for intestinal rings and 2 h for segments). The main advantages of these methods are their practicality and simplicity, the ability to choose distinct intestinal regions and their high throughput. Additionally, one single animal can provide various rings from each intestinal region, which might be advantageous in case of a shortage of tissues [12]. The main constraints consist of the impossibility of discriminating the direction of the transepithelial transport since both sides—the serosal and mucosal surfaces—are exposed to the compound of interest and the tissue has reduced viability over time [13,67]. Additionally, the presence of all tissue layers impairs proper oxygenation. Given these limitations, intestinal rings or segments are becoming less relevant, with few recent research papers mentioning them.
5.1.4. Everted Gut Sac
The everted sac technique was created in the 1950s by Wilson et al., 1954 [76]. In this technique, an intestinal section is reverted, and both ends are tied, after filling it with an appropriate buffer solution. Then, this intestinal section is incubated with the chosen buffer and the compound of interest. The incubation usually involves a controlled temperature and aeration or stirring. The main advantages of this method are the possibility to test distinct intestinal regions and the small volume inside the sac leading to a sample concentration, which is better in terms of analytical analysis. Additionally, it is low-cost and practical, since several samples can be tested. Furthermore, it provides an increased absorption area with a mucosal surface. However, the major drawback relies on the tissue integrity, namely the damage in the eversion process and the viability during the assay. Luo et al., 2013 [67] reported changes after only 5 min of incubation, although Verhoeckx et al., 2015 [12] described the tissue as viable for up to 2 h. Moreover, the absorption time might be slower due to the presence of all layers of the intestinal wall, which may result in underestimating the absorption. From a different standpoint, several variations to this method are worth mentioning, namely not everting the intestine segment, or instead of tying both ends, inserting a cannula in the serosal side. The everted sac method has been used to tackle the mechanisms of carried mediated absorption and kinetics of drug absorption and even drug metabolism. In the literature, the use of different animal tissues, namely chickens and pigs, has been reported [77].
5.2. Cell-Based Methods
Cell-based methods can be divided according to the number of cell lines involved (monocultures or co-cultures) or the growth dimensions (2D or 3D), as represented in Figure 4. Additionally, the cell lines and their origin deeply influence the quality of the models generated. The use of Caco-2 monolayers (a cell line originally derived from a colon adenocarcinoma) is frequently used for permeability and mechanistic studies regarding the intestinal epithelium [78]. This line has been widely acknowledged for its morphological and physiological resemblance with small intestine enterocytes. They can differentiate into polar columnar absorptive cells with brush borders, tight junction expressions, similar enzymes and carrier-mediated transport systems [79]. As such, in vitro protocols have been developed, namely the use of plate inserts. Briefly, these protocols consist of growing cells in a permeable support in a plate and allowing them to properly differentiate for about 21 days. Then, the apical and basal sides are equilibrated using a proper buffer (e.g., Hank’s Balanced Salt Solution) and the formulation of interest is added to the apical side. Samples are collected from the basal side over time, and the volumes collected are balanced with a buffer. Additionally, the epithelial barrier function is monitored by measuring TEER values, before and during the assay [79,80]. While these assays with Caco-2 monolayers might be simple and practical, they fail to mimic the complexity of the intestinal environment, as they lack the diversity of cell types found in the epithelium and their interplay. For instance, goblet cells are found dispersed among enterocytes and produce the mucus that covers the whole epithelium, contributing to the barrier function and therefore affecting cellular uptake. Given these disadvantages of monocultures, nowadays, it is common to find in the literature protocols comprising two cell lines: Caco-2 and HT29-MTX [81,82]. This last cell line is also isolated from a human colon adenocarcinoma and upon treatment with methotrexate, expresses mucins, a component of mucus and differentiated into mature goblet cells. However, these cells do not express much TJs as Caco-2 does. Therefore, Caco-2 cells are more often used alone compared with HT29-MTX, which, in turn, are commonly used in co-cultures with Caco-2. These combined cell lines are used in protocols as described earlier but require an initial step of mixing both cells in proportion, before seeding them [83]. Thus, a more complex and realistic model is obtained, with fully differentiated enterocytes and a mucus layer.
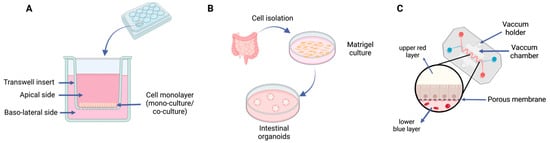
Figure 4.
Schematic representation of cell-based methods for assessing intestinal absorption: (A) 2D cell culture system, either monoculture or co-culture, using an insert; (B) development of an intestinal organoid; (C) gut-on-chip technology. Image created with BioRender.com.
While the models described earlier represent the most conventional approach based on 2D models, new strategies like 3D organoids or gut-on-chip technologies, are currently being developed and optimized. The 2D models, based on growing cells on flat surfaces, fail to represent the physiological and structural in vivo complexity, namely regarding cell-to-cell and cell-to-extracellular environment interactions. In turn, these interactions are responsible for cell differentiation, vitality and proliferation. Additionally, because of culturing, there is an accumulation of genetic changes through time, which induces a loss of a diverse phenotype [84]. In opposition, the development of 3D organoids leads to more accurate physiological models compared with the naturally occurring structure. These models are usually created by culturing intestinal stem cells, isolated from crypt cells [12,85,86], which then communicate and differentiate efficiently through the matrix into so-called “mini-guts”. Several cell types, usually found in the epithelium, such as goblet cells, enterocytes, Paneth Cells and enteroendocrine cells can be found. Some disadvantages of this approach involve the demanding technical skills required to carry out this laborious technique. Moreover, it still does not reproduce the immune, vascular, lymphatic and enteric nervous systems, as can be done in vivo [12,87]. While this kind of approach has begun to be considered for drug development and regenerative medicine purposes, it has not yet been established for permeability and interaction studies with food ingredients, despite its relevance. Additionally, gut-on-chip systems are microfluidic culture devices composed of a transparent silicone polymer that is made of two channels, separated by a porous membrane. On one side, human intestinal epithelial cells are cultured, and on the opposite side, there are human microvascular endothelial cells. Therefore, this high-resolution technique allows the control of parameters such as concentration gradients, tissue–organ interactions and cell patterning, among others [88]. This system has been improved to also include stable communities of microorganisms of the gut microbiota, which allows a better understanding of host–microbiome interactions, opening a path for the development of new therapeutics or nutraceuticals [89]. This system ensures a physiologically appropriate gut environment and allows the continuous collection of fluids that flow through the lumen of the intestinal chip. The samples collected can be used for the quantification of nutrient digestion, mucus secretion or even the assessment of the status of the intestinal barrier. Gut-on-chip systems represent a valuable tool not only for pharmacological and host–microbiome studies, but also for personalized medicine, and studies on nutrition and metabolism. However, as Xiang et al., 2020 [90] pointed out, while gut-on-chip systems might be very useful for nutraceutical science, as the effects of foods on health and nutrition-related diseases might be properly evaluated, the complexity of food represents a challenge, given the barriers in sample processing and analysis. Moreover, in general, organ-on chips have a low throughput, which can be useful for the late stages of the development/validation pipeline, but not in the early stages (screenings). Therefore, the development of high-throughput organ chips, such as on a single device with several chambers, would be highly valuable [91].
5.3. Nonbiological Methods
Another common strategy is to simulate intestinal absorption using semi-permeable membranes for dialysis purposes. The dialysis process, in general, depends on a concentration gradient as a driving force for molecule movement across the membrane and can be classified into positive or negative dialysis. In positive dialysis, the solution with the compound of interest is placed inside the dialysis bag, which, in turn, is surrounded by artificial intestinal fluid. In the reverse dialysis method, the dialysis bag is filled with intestinal fluid, and the bag is placed in a container with the compound of interest dissolved [92]. One critical factor in this technique is the pore size of the membrane, which is usually under 20 kDa [93,94,95]. From a different perspective, two approaches can be found in the literature regarding this process. Some authors consider dialysis a technique for simulating absorption in the small intestine, regarding it as step in BA determination. Others consider it a final step of the BC assessment, as a way to separate soluble and insoluble fractions, since only the soluble fraction can be eventually absorbed. For instance, Gayoso et al., 2016 [96] compared dialysis to centrifugation to determine the BC of food and extracts given their solubility; Moreno-Montoro et al., 2018 [93] used a combined strategy of centrifugation followed by dialysis to select which portion of the soluble fraction (centrifugation) supernatant would be absorbed (dialysate). On the other hand, it is also possible to find some standardized and complex systems for BC prediction for both drugs and foods, such as the TNO Gastrointestinal Model (TIM) or Engineered Stomach and Small Intestine (ESIN), which include dialysis membranes as a last digestion step for simulating intestinal absorption, namely passive absorption [12,97]. For example, González et al., 2020 [95] reported an improved dialysis system based on a continuous flow of fluid at a controlled temperature for a more realistic dynamic small intestine simulation. In 2007, another dialysis system arose: the AMI system. This system consists of a regenerated cellulose membrane with a molecular cutoff of 2 kDa, which is assembled between two rings. After proper validation through correlation tests with other techniques, this method established itself as a high-throughput and cost-effective tool for assessing the passive permeability of poor water-soluble compounds [52]. As such, dialysis itself represents a simple technique, accessible, but open to different approaches and improvements, given the heterogenicity of methodologies found in the literature.
Another approach for investigating intestinal absorption consists of artificial membranes, which mimic the compound’s interactions with the phospholipidic cell membrane, using nanomaterials or 3D printed templates with biomolecules [98]. These new technologies might represent an interesting tissue and cell-free alternative for mimicking intestinal absorption. For instance, Permeapad™ represents an innovative membrane that can be used in the replacement of ex vivo tissues in Franz diffusion cells or as an alternative to cell culture methods, as plate inserts [99]. Additionally, PAMPA systems have also been acknowledged for having a good correlation with Caco-2 permeation assays, although failing to mimic paracellular transport. This system consists of a supported filter and a membrane soaked in phospholipids dissolved in an organic solvent and is usually applied into a 96-well plate, separated into a donor and acceptor cassette. Through time, this technique has evolved to distinct support filters, membrane compositions, solvents and pH values in the acceptor and donor chambers. Another technique worth mentioning is PVPA, in which a liposome solution is deposited in a filter support (Transwell inserts, for example). These liposomes are considered building blocks resembling the phospholipidic bilayer. This technique presents a good correlation with absorbed fraction values found in the literature for Caco-2 models and PAMPA models [52].
Considering the limitations of the techniques reviewed above, it is worth mentioning the importance of carrying out these methodologies in combination with others. For instance, it is common to combine a Ussing chamber, Franz diffusion cells or everted gut sac techniques with further validation through cell culture-based methods, generally using Caco-2 cell lines for Transwell® assays. For instance, Westerhout et al., 2014 [73] developed a new approach to assess intestinal permeation and further validated the results of several drug permeations through a comparison with a co-culture of Caco-2 and HT29-MTX on a Transwell® system. B Sánchez et al., 2019 [100] also tested different drugs using Franz cell diffusion assays with porcine tissues, and the results were compared to the results found in the literature regarding permeation values obtained with Caco-2. Chen et al., 2019 [101] tested the permeation of chitobiose using an everted rat gut sac and compared it with the results of a Transwell® assay with Caco-2. While these examples refer to technique validation and drug permeability purposes, this approach could constitute a good practice to implement in biomolecule/ingredient development studies.
Table 2 summarizes the literature review regarding the application of the methodologies previously described, for ingredient/formulation development. It is possible to point out a clear gap in approaches that favor the in vitro pre-assessments of ingredients’ potential, especially concerning chicken models. From the literature review performed, it was possible to notice that most studies report only digestibility results, by simulating enzymatic GIT, while failing to consider intestinal absorption; other studies tackled the effects of some ingredients on intestinal permeability, either directly, with no prior digestion, or after their in vivo administration. Overall, studies on promising ingredients, reporting both BC and BA to some extent (GIT simulation and intestinal absorption), are scarce or inexistent. The outlook on in vitro studies looking toward human health is much more positive as studies including in vitro digestion simulation followed by intestinal absorption prediction could be found. Moreover, porcine models provide an opportunity for ex vivo studies on intestinal absorption. Cell culture models rely on well-established protocols, using human-derived cell lines. While for chicken feed, no cell culture protocol has been used for ingredient development, and only a few exploratory studies with primary cell lines or organoid development could be found [102].

Table 2.
Review of approaches found in the literature used to assess the intestinal permeation of functional compounds or ingredients.
Table 2.
Review of approaches found in the literature used to assess the intestinal permeation of functional compounds or ingredients.
| Technique | Food/Ingredient Tested | Model | Description | Reference |
|---|---|---|---|---|
| Ussing chamber | Cinnamon bark oil and coconut oil emulsions | Laying hen | No previous simulation of the gastrointestinal tract | [103] |
| Apple polyphenols | Pig | Direct application of polyphenols to a Ussing chamber; no previous digestion of the polyphenols was performed | [104] | |
| Organic acid supplementation in feed | Chicken | In vivo administration of the supplement was performed; a Ussing chamber was used to assess intestinal permeability changes | [105] | |
| Oligopeptides from whey protein hydrolysate | Pig | In vitro digestion and absorption simulation through Ussing chamber; | [106] | |
| Franz cells | Tetrahydrocurcumin-hyaluronic acid conjugate (metabolite of curcumin) | Pig | TNO dynamic gastrointestinal model-1 (TIM-1) followed by Franz cell assay | [107] |
| Monosaccharides, amino acids and a corn oil-in-water emulsion | Semi-permeable cellulose membrane | No gastrointestinal simulation was performed; no biological tissue was used | [108] | |
| Everted intestinal sac | Fructose uptake | Chicken | No previous digestive process was simulated | [109] |
| Garra fish meal | Chicken | In vivo studies carried out in chickens, followed by an evaluation of diet effects on intestinal permeability | [110] | |
| Encapsulation of β-carotene in zein protein | Chicken | Study focused on human health; human GIT digestion simulation was simulated, followed by absorption experiments with chicken intestines | [111] | |
| Phenolic compounds from non-extruded and extruded Mango Bagasse-added confections | Pig | Human in vitro GIT digestion, followed by permeability assessment | [112] | |
| Encapsulated curcumin and resveratrol | Pig | Human in vitro GIT digestion was carried out, followed by an everted gut sac for BA assessment | [113] | |
| Mono-cultures | Angiotensin I-converting enzyme inhibitory peptides, from cooked chicken breast/thighs | Caco-2 | Peptides identified after in vitro digestion, followed by PET inserts with a Caco-2 monoculture | [114] |
| Curcumin alone or with polyvinylpyrrolidone | Caco-2 | This study focused on chickens’ health; despite an in vitro simulation of the chicken GIT, a Transwell permeability assay was performed with the initial samples, not the digested ones | [115] | |
| Co-cultures | Sardine protein hydrolysate | Caco-2 + HT29-MTX | After human in vitro digestion, permeability was assessed using PET inserts (like the TranswellTM system) | [116] |
| Encapsulated rosemary extract | Caco-2 + HT29-MTX | In vitro human digestion followed by co-culture in a TranswellTM system | [117] | |
| Salmosan (derived from Mannan oligosaccharide) | Caco-2 + THP-1 (macrophages) | Salmosan and Salmosan with L. plantarum were tested for the effects on the intestinal permeability and barrier, as potential feed additives; nevertheless, no GIT digestion was simulated | [118] | |
| Dialysis membrane | Gelatinized starch dispersions | Hollow fiber membrane (synthetic) | Study on starch digestion and the consequent absorption of hydrolytic products generated in the human small intestine, using an in vitro intestinal digestion system (i-IDS) | [95] |
| Phenolics, flavonoids, rutin, β-carotene and lutein in six edible greens | Cellulose membrane (12,000 Da) | Bioaccessibility and BA was evaluated; GIT simulation was carried out followed by dialysis, as a simplified model of intestinal permeation | [119] | |
| PAMPA | Crude plant extracts (Angelica archangelica, Waltheria indica, Pueraria montana var. lobata) | Polycarbonate filter plate (5–20% porosity with a 0.45 µm pore size and 9–10 µm thickness) impregnated with a hexadecane/hexane (5/95 % (v/v)) solution | Prediction of the passive intestinal absorption of a representative set of frequently occurring natural products from Angelica archangelica, Waltheria indica and Pueraria montana var. lobata; no GIT digestion simulation was performed before PAMPA assay | [120] |
| Saponins and sapogenins from seed extracts from red quinoa and seeds of fenugreek. | Lipid mixture containing L-α-phosphatidylcholine and cholesterol in 1,7-octadiene solution added to the PVDF filter of each well; after membrane coating, the donor solutions were added | Evaluation and comparison of the permeability of saponins and sapogenins from fenugreek and quinoa extracts with and without previous in vitro digestion simulation, through the previous development of a GIT digestion protocol attached to PAMPA | [121] |
The literature revision, according to the defined criteria, underlines the lack of relevance of intestinal segments or of the ring technique since no research papers were found on these topics in the period considered. Moreover, the reduced number of Franz diffusion cell studies is indicative of the reduced applicability of intestinal permeability assays. Few papers are designed in a way to fully assess the influence of the gastrointestinal tract. For instance, most do not simulate the process from mouth to colon, neglecting this relevant topic. As for chickens, there is a relevant lack of cell culture-based methods and non-biological methods for feed/nutritional studies.
6. Conclusions and Opportunities
Overall, studies regarding physiology aspects and nutrient transport mechanisms for porcine and/or human models are more abundant than for chicken models. The same trend is observed for studies focused on the development of functional ingredients or bioactive compounds that include the GIT and intestinal absorption simulation.
The study of the impact of new ingredients and molecules on human and animal health demands reliable and complete evaluation methods, from mouth to colon, preferably including potential interactions with the microbiota. Concerning in vitro models for predicting bioaccessibility, there are several models, either static or dynamic, but also simulating one digestion compartment or several in sequence. Some models are more suited for mechanistic studies, while others are more adequate for kinetic studies. Overall, INFOGEST represents an adequate model for either the kinetics of nutrient release or for monitoring the concentration or stability of a particular substance or nutrient. Moreover, it is easy to implement and reproduce and allows comparisons between distinct laboratories and studies pointing to a good correlation with in vivo studies. However, the same broad consensus was not attained for animal digestion simulation models or concerning models that assess the potential interactions with the microbiota. Additionally, for both humans and animals, the absorption simulation step lacks standardization and consensus among the scientific community. The studies using absorption models for chickens’ nutrition and feedstuff development were overall scarce. Currently, there is no “one size fits all” technique for simulating intestinal permeation. Different techniques require distinct implementation costs and technical skills and present distinct pros and cons. Therefore, the strategies employed in some drug-related studies combining the use of two techniques—for instance, an ex vivo method along with a cell-based method—provide more reliable results and should constitute a general recommendation in the nutritional studies field. Moreover, in drug development studies regarding solubility and absorption, these follow highly standardized classification systems and testing protocols (for example, the Biopharmaceutical Classification System (BCS)). In fact, even dissolution in nutritional liquids has been explored [122]. The guidelines used in toxicology might be useful for reflecting on and in the application of the study of functional ingredients’ biopotential. Additionally, the need for complete GIT simulation models constitutes an important step before in vivo studies, in alignment with widespread ethical standards.
In summary, the purpose of this review was to bring awareness to the lack of complete standardized models—from mouth to colon—needed to achieve meaningful conclusions when studying the potential of bioactive ingredients, either for human or animal applications. Our recommendation for reliable in vitro screenings when developing new ingredients/formulations for food or feedstuffs would be to adopt an in vitro gastrointestinal digestion strategy: a model that is as standardized as possible with colonic/cecal fermentation followed by a cell culture-based method (a Transwell assay, for instance) with an ex vivo tissue-based model before laborious and expensive in vivo testing. Nevertheless, this general approach would benefit from a proper in vivo study to establish a correlation between them.
Author Contributions
Conceptualization, C.M.C. and D.L.d.O.; investigation, C.M.C. and N.M.d.C.; writing—original draft preparation, C.M.C.; writing—review and editing, C.M.C., N.M.d.C., D.L.d.O. and A.R.M.; supervision, A.R.M.; project administration, A.R.M.; funding acquisition, A.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundo Europeu de Desenvolvimento Regional (FEDER), through the Programa Operacional Competitividade e Internacionalização (POCI) under the Alchemy project: Capturing high value from industrial fermentation bioproducts (POCI-01-0247-FEDER-027578).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ozen, A.E.; Pons, A.; Tur, J.A. Worldwide Consumption of Functional Foods: A Systematic Review. Nutr. Rev. 2012, 70, 472–481. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Saxena, M.J. Feed Additives in Animal Health. In Nutraceuticals in Veterinary Medicine; Springer: Berlin/Heidelberg, Germany, 2019; pp. 345–362. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and Bioaccessibility of Food Bioactive Compounds; Overview and Assessment by in vitro Methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Santos, D.I.; Saraiva, J.M.A.; Vicente, A.A.; Moldão-Martins, M. Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Woodhead Publishing: Sawston, UK, 2019; Chapter 2; pp. 23–54. [Google Scholar]
- Sensoy, I. A Review on the Relationship Between Food Structure, Processing, and Bioavailability. Crit. Rev. Food Sci. Nutr. 2014, 54, 902–909. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Chitchumroonchokchai, C.; Mariutti, L.R.B.; Mercadante, A.Z.; Failla, M.L. Comparison of Two Static in vitro Digestion Methods for Screening the Bioaccessibility of Carotenoids in Fruits, Vegetables, and Animal Products. J. Agric. Food Chem. 2017, 65, 11220–11228. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Development of an in vitro Protein Digestibility Assay Mimicking the Chicken Digestive Tract. Anim. Nutr. 2018, 4, 401–409. [Google Scholar] [CrossRef]
- Bean, T.G.; Arnold, K.E.; Lane, J.; Pietravalle, S.; Boxall, A.B.A. An in vitro Method for Determining the Bioaccessibility of Pharmaceuticals in Wildlife. Environ. Toxicol. Chem. 2016, 35, 2349–2357. [Google Scholar] [CrossRef]
- Ng, J.C.; Juhasz, A.; Smith, E.; Naidu, R. Assessing the Bioavailability and Bioaccessibility of Metals and Metalloids. Environ. Sci. Pollut. Res. 2015, 22, 8802–8825. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Zhao, Q.; Xia, C.; Huang, Q. Using in vitro and in vivo Models To Evaluate the Oral Bioavailability of Nutraceuticals. J. Agric. Food Chem. 2015, 63, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: London, UK, 2015. [Google Scholar]
- Nunes, R.; Silva, C.; Chaves, L. Tissue-Based in vitro and Ex vivo Models for Intestinal Permeability Studies. In Concepts and Models for Drug Permeability Studies; Elsevier: Amsterdam, The Netherlands, 2016; pp. 203–236. [Google Scholar]
- Gleeson, J.P.; McCartney, F. Striving towards the Perfect In vitro Oral Drug Absorption Model. Trends Pharmacol. Sci. 2019, 40, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Amirabadi, H.E.; Donkers, J.M.; Wierenga, E.; Ingenhut, B.; Pieters, L.; Stevens, L.; Donkers, T.; Westerhout, J.; Masereeuw, R.; Bobeldijk-Pastorova, I.; et al. Intestinal Explant Barrier Chip: Long-Term Intestinal Absorption Screening in a Novel Microphysiological System Using Tissue Explants. Lab Chip 2022, 22, 326–342. [Google Scholar] [CrossRef]
- Yin, L.; Yang, H.; Li, J.; Li, Y.; Ding, X.; Wu, G.; Yin, Y. Pig Models on Intestinal Development and Therapeutics. Amino Acids 2017, 49, 2099–2106. [Google Scholar] [CrossRef]
- Zhang, Q.; Widmer, G.; Tzipori, S. A Pig Model of the Human Gastrointestinal Tract. Gut Microbes 2013, 4, 193–200. [Google Scholar] [CrossRef]
- Franz-Montan, M.; Serpe, L.; Martinelli, C.C.M.; da Silva, C.B.; dos Santos, C.P.; Novaes, P.D.; Volpato, M.C.; de Paula, E.; Lopez, R.F.V.; Groppo, F.C. Evaluation of Different Pig Oral Mucosa Sites as Permeability Barrier Models for Drug Permeation Studies. Eur. J. Pharm. Sci. 2016, 81, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Faustini, M.; Attard, E. In vitro Fermentation of Feed Ingredients by Fresh or Frozen Pig Fecal Inocula. Anim. Sci. J. 2014, 85, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Jutfelt, F.; Kanapathippillai, P.; Bakken, Y.; Sundell, K.; Glette, J.; Mayhew, T.M.; Myklebust, R.; Olsen, R.E. Damaging Effect of the Fish Pathogen Aeromonas salmonicida ssp. salmonicida on Intestinal Enterocytes of Atlantic Salmon (Salmo salar L.). Cell Tissue Res. 2004, 318, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kent-Dennis, C.; Penner, G.B. Effects of Lipopolysaccharide Exposure on the Inflammatory Response, Butyrate Flux, and Metabolic Function of the Ruminal Epithelium Using an Ex vivo Model. J. Dairy Sci. 2021, 104, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Mota de Carvalho, N.; Oliveira, D.L.; Saleh, M.A.D.; Pintado, M.E.; Madureira, A.R. Importance of Gastrointestinal in vitro Models for the Poultry Industry and Feed Formulations. Anim. Feed Sci. Technol. 2021, 271, 114730. [Google Scholar] [CrossRef]
- Lickwar, C.R.; Camp, J.G.; Weiser, M.; Cocchiaro, J.L.; Kingsley, D.M.; Furey, T.S.; Sheikh, S.Z.; Rawls, J.F. Genomic Dissection of Conserved Transcriptional Regulation in Intestinal Epithelial Cells. PLoS Biol. 2017, 15, e2002054. [Google Scholar] [CrossRef] [PubMed]
- Zachos, N.C. Viral Gastroenteritis; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 1–21. [Google Scholar]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell–Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The Intestinal Epithelial Barrier: A Therapeutic Target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Edelblum, K.L.; Turner, J.R. Epithelial Cells: Structure, Transport, and Barrier Function. Structure, Transport, and Barrier Function. In Mucosal Immunology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1–2, pp. 187–210. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight Junctions in Inflammatory Bowel Diseases and Inflammatory Bowel Disease Associated Colorectal Cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef]
- Modina, S.C.; Polito, U.; Rossi, R.; Corino, C.; di Giancamillo, A. Nutritional Regulation of Gut Barrier Integrity in Weaning Piglets. Animals 2019, 9, 1045. [Google Scholar] [CrossRef]
- Modina, S.C.; Aidos, L.; Rossi, R.; Pocar, P.; Corino, C.; di Giancamillo, A. Stages of Gut Development as a Useful Tool to Prevent Gut Alterations in Piglets. Animals 2021, 11, 1412. [Google Scholar] [CrossRef]
- Tako, E.; Bar, H.; Glahn, R.P. The Combined Application of the Caco-2 Cell Bioassay Coupled with In vivo (Gallus gallus) Feeding Trial Represents an Effective Approach to Predicting Fe Bioavailability in Humans. Nutrients 2016, 8, 732. [Google Scholar] [CrossRef]
- Denbow, D.M. Gastrointestinal Anatomy and Physiology. In Sturkie’s Avian Physiology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 337–366. [Google Scholar] [CrossRef]
- Alshamy, Z.; Richardson, K.C.; Hünigen, H.; Hafez, H.M.; Plendl, J.; al Masri, S. Comparison of the Gastrointestinal Tract of a Dual-Purpose to a Broiler Chicken Line: A Qualitative and Quantitative Macroscopic and Microscopic Study. PLoS ONE 2018, 13, e0204921. [Google Scholar] [CrossRef] [PubMed]
- Wijtten, P.J.A.; Langhout, D.J.; Verstegen, M.W.A. Small Intestine Development in Chicks after Hatch and in Pigs around the Time of Weaning and Its Relation with Nutrition: A Review. Acta Agric. Scand. Sect. A—Anim. Sci. 2012, 62, 1–12. [Google Scholar] [CrossRef]
- Hollemans, M.S.; van Baal, J.; de Vries Reilingh, G.; Kemp, B.; Lammers, A.; de Vries, S. Intestinal Epithelium Integrity after Delayed Onset of Nutrition in Broiler Chickens. Poult. Sci. 2020, 99, 6818–6827. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D. Claudins: Vital Partners in Transcellular and Paracellular Transport Coupling. Pflugers Arch. 2017, 469, 35–44. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Zhu, C.; Guo, S.; Gan, Y. Advances in the Transepithelial Transport of Nanoparticles. Drug Discov. Today 2016, 21, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Michielan, A.; D’Incà, R. Intestinal Permeability in Inflammatory Bowel Disease: Pathogenesis, Clinical Evaluation, and Therapy of Leaky Gut. Mediators Inflamm. 2015, 2015, 628157. [Google Scholar] [CrossRef]
- Ma, T.Y.; Nighot, P.; Al-Sadi, R. Tight Junctions and the Intestinal Barrier. In Physiology of the Gastrointestinal Tract, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1–2, pp. 587–639. [Google Scholar] [CrossRef]
- Kiela, P.R.; Ghishan, F.K. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of Sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Matsui, T. Current Knowledge of Intestinal Absorption of Bioactive Peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef]
- Ko, C.W.; Qu, J.; Black, D.D.; Tso, P. Regulation of Intestinal Lipid Metabolism: Current Concepts and Relevance to Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fat-Soluble Vitamin Intestinal Absorption: Absorption Sites in the Intestine and Interactions for Absorption. Food Chem. 2015, 172, 155–160. [Google Scholar] [CrossRef]
- Mace, O.J.; Marshall, F. Digestive Physiology of the Pig Symposium: Gut Chemosensing and the Regulation of Nutrient Absorption and Energy Supply. J. Anim. Sci. 2013, 91, 1932–1945. [Google Scholar] [CrossRef]
- Wijtten, P.J.A.; van der Meulen, J.; Verstegen, M.W.A. Intestinal Barrier Function and Absorption in Pigs after Weaning: A Review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- van der Wielen, N.; Moughan, P.J.; Mensink, M. Amino Acid Absorption in the Large Intestine of Humans and Porcine Models. J. Nutr. 2017, 147, 1493–1498. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Sheikhahmadi, A.; Wang, Y.; Jiao, H.; Lin, H.; Song, Z. Effects of Heat Stress on the Gene Expression of Nutrient Transporters in the Jejunum of Broiler Chickens (Gallus gallus domesticus). Int. J. Biometeorol. 2015, 59, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Karasov, W.H. Integrative Physiology of Transcellular and Paracellular Intestinal Absorption. J. Exp. Biol. 2017, 220, 2495–2501. [Google Scholar] [CrossRef]
- Berben, P.; Bauer-Brandl, A.; Brandl, M.; Faller, B.; Flaten, G.E.; Jacobsen, A.C.; Brouwers, J.; Augustijns, P. Drug Permeability Profiling Using Cell-Free Permeation Tools: Overview and Applications. Eur. J. Pharm. Sci. 2018, 119, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lu, L.; Wang, S.; Wu, J.; Shi, J.; Yan, T.; Xie, C.; Li, Q.; Hu, M.; Liu, Z. Oral Absorption Basics: Pathways and Physicochemical and Biological Factors Affecting Absorption. In Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 297–329. [Google Scholar] [CrossRef]
- Capuano, E.; Janssen, A.E.M. Food Matrix and Macronutrient Digestion. Annu. Rev. Food Sci. Technol. 2021, 12, 193–212. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST In vitro Digestion Model to Foods: A Review. Annu. Rev. Food Sci. Technol. 2023, 14, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Marteau, P.; Havenaar, R.; Veld, J.H.J.H. A Multicompartmental Dynamic Computer-Controlled Model Simulating the Stomach and Small Intestine. Altern. Lab. Anim. 1995, 23, 197–209. [Google Scholar] [CrossRef]
- Molly, K.; Woestyne, M.V.; Smet, I.D.; Verstraete, W. Validation of the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) Reactor Using Microorganism-Associated Activities. Microb. Ecol. Health Dis. 1994, 7, 191–200. [Google Scholar] [CrossRef]
- Egger, L.; Schlegel, P.; Baumann, C.; Stoffers, H.; Guggisberg, D.; Brügger, C.; Dürr, D.; Stoll, P.; Vergères, G.; Portmann, R. Physiological Comparability of the Harmonized INFOGEST in vitro Digestion Method to in vivo Pig Digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Tang, J.; van Milgen, J.; Renaudeau, D. Cell Wall of Feeds and Their Impact on Protein Digestibility: An in vitro Method Applied for Pig Nutrition. Anim. Feed Sci. Technol. 2022, 293, 115467. [Google Scholar] [CrossRef]
- Boisen, S.; Fernández, J.A. Prediction of the Total Tract Digestibility of Energy in Feedstuffs and Pig Diets by in vitro Analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Święch, E. Alternative Prediction Methods of Protein and Energy Evaluation of Pig Feeds. J. Anim. Sci. Biotechnol. 2017, 8, 39. [Google Scholar] [CrossRef]
- Nash, T.; Vervelde, L. Advances, Challenges and Future Applications of Avian Intestinal in vitro Models. Avian Pathol. 2022, 51, 317–329. [Google Scholar] [CrossRef]
- Martinez-Haro, M.; Taggart, M.A.; Green, A.J.; Mateo, R. Avian Digestive Tract Simulation To Study the Effect of Grit Geochemistry and Food on Pb Shot Bioaccessibility. Environ. Sci. Technol. 2009, 43, 9480–9486. [Google Scholar] [CrossRef]
- Meimandipour, A.; Shuhaimi, M.; Hair-Bejo, M.; Azhar, K.; Kabeir, B.M.; Rasti, B.; Yazid, A.M. In vitro Fermentation of Broiler Cecal Content: The Role of Lactobacilli and PH Value on the Composition of Microbiota and End Products Fermentation. Lett. Appl. Microbiol. 2009, 49, 415–420. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Costa, C.M.; Castro, C.; Saleh, M.A.D.; Pintado, M.E.; Oliveira, D.L.; Madureira, A.R. Development of a Chicken Gastrointestinal Tract (GIT) Simulation Model: Impact of Cecal Inoculum Storage Preservation Conditions. Appl. Microbiol. 2023, 3, 968–992. [Google Scholar] [CrossRef]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. An Overview of in vitro, Ex vivo and in vivo Models for Studying the Transport of Drugs across Intestinal Barriers. Adv. Drug Deliv. Rev. 2021, 175, 113795. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, Y.; Zhao, B.; Tang, M.; Dong, H.; Zhang, L.; Lv, B.; Wei, L. Ex vivo and in Situ Approaches Used to Study Intestinal Absorption. J. Pharmacol. Toxicol. Methods 2013, 68, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Ussing, H.H.; Zerahn, K. Active Transport of Sodium as the Source of Electric Current in the Short-Circuited Isolated Frog Skin. Acta Physiol. Scand. 1951, 23, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Smart, K.; Somerville, M.S.; Lauder, S.N.; Appanna, G.; Horwood, J.; Sunder Raj, L.; Srivastava, B.; Durai, D.; Scurr, M.J.; et al. The Ussing Chamber System for Measuring Intestinal Permeability in Health and Disease. BMC Gastroenterol. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.L. A Guide to Ussing Chamber Studies of Mouse Intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, 1151–1166. [Google Scholar] [CrossRef]
- Sjögren, E.; Eriksson, J.; Vedin, C.; Breitholtz, K.; Hilgendorf, C. Excised Segments of Rat Small Intestine in Ussing Chamber Studies: A Comparison of Native and Stripped Tissue Viability and Permeability to Drugs. Int. J. Pharm. 2016, 505, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wuyts, B.; Riethorst, D.; Brouwers, J.; Tack, J.; Annaert, P.; Augustijns, P. Evaluation of Fasted and Fed State Simulated and Human Intestinal Fluids as Solvent System in the Ussing Chambers Model to Explore Food Effects on Intestinal Permeability. Int. J. Pharm. 2015, 478, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Westerhout, J.; van de Steeg, E.; Grossouw, D.; Zeijdner, E.E.; Krul, C.A.M.; Verwei, M.; Wortelboer, H.M. A New Approach to Predict Human Intestinal Absorption Using Porcine Intestinal Tissue and Biorelevant Matrices. Eur. J. Pharm. Sci. 2014, 63, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Supe, S.; Takudage, P. Methods for Evaluating Penetration of Drug into the Skin: A Review. Ski. Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Dezani, A.B.; Pereira, T.M.; Caffaro, A.M.; Reis, J.M.; Serra, C.H. dos R. Determination of Lamivudine and Zidovudine Permeability Using a Different Ex vivo Method in Franz Cells. J. Pharmacol. Toxicol. Methods 2013, 67, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.H.; Wiseman, G. The Use of Sacs of Everted Small Intestine for the Study of the Transference of Substances from the Mucosal to the Serosal Surface. J. Physiol. 1954, 123, 116. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Everted Gut Sac Model as a Tool in Pharmaceutical Research: Limitations and Applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef]
- Noben, M.; Vanhove, W.; Arnauts, K.; Ramalho, A.S.; van Assche, G.; Vermeire, S.; Verfaillie, C.; Ferrante, M. Human Intestinal Epithelium in a Dish: Current Models for Research into Gastrointestinal Pathophysiology. United Eur. Gastroenterol. J. 2017, 5, 1073–1081. [Google Scholar] [CrossRef]
- Lechanteur, A.; Almeida, A.; Sarmento, B. Elucidation of the Impact of Cell Culture Conditions of Caco-2 Cell Monolayer on Barrier Integrity and Intestinal Permeability. Eur. J. Pharm. Biopharm. 2017, 119, 137–141. [Google Scholar] [CrossRef]
- Pereira, C.; Costa, J.; Sarmento, B.; Araújo, F. Cell-Based in vitro Models for Intestinal Permeability Studies. In Concepts and Models for Drug Permeability Studies: Cell and Tissue Based In Vitro Culture Models; Elsevier: Amsterdam, The Netherlands, 2016; pp. 57–81. [Google Scholar] [CrossRef]
- Volpe, D.A. Advances in Cell-Based Permeability Assays to Screen Drugs for Intestinal Absorption. Expert Opin. Drug Discov. 2020, 15, 539–549. [Google Scholar] [CrossRef]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 Co-Culture in vitro Cell Models for Permeability Studies. Int. J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B Coculture Models to Predict Intestinal and Colonic Permeability Compared to Caco-2 Monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Silva-Almeida, C.; Ewart, M.A.; Wilde, C. 3D Gastrointestinal Models and Organoids to Study Metabolism in Human Colon Cancer. Semin. Cell Dev. Biol. 2020, 98, 98–104. [Google Scholar] [CrossRef]
- Meneses, A.M.C.; Schneeberger, K.; Kruitwagen, H.S.; Penning, L.C.; van Steenbeek, F.G.; Burgener, I.A.; Spee, B. Intestinal Organoids—Current and Future Applications. Vet. Sci. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Dotti, I.; Mayorgas, A.; Salas, A. Generation of Human Colon Organoids from Healthy and Inflammatory Bowel Disease Mucosa. PLoS ONE 2022, 17, e0276195. [Google Scholar] [CrossRef]
- Fois, C.A.M.; Le, T.Y.L.; Schindeler, A.; Naficy, S.; McClure, D.D.; Read, M.N.; Valtchev, P.; Khademhosseini, A.; Dehghani, F. Models of the Gut for Analyzing the Impact of Food and Drugs. Adv. Healthc. Mater. 2019, 8, 1900968. [Google Scholar] [CrossRef]
- Signore, M.A.; de Pascali, C.; Giampetruzzi, L.; Siciliano, P.A.; Francioso, L. Gut-on-Chip Microphysiological Systems: Latest Advances in the Integration of Sensing Strategies and Adoption of Mature Detection Mechanisms. Sens. Biosensing Res. 2021, 33, 100443. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A Complex Human Gut Microbiome Cultured in an Anaerobic Intestine-on-a-Chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Xiang, Y.; Wen, H.; Yu, Y.; Li, M.; Fu, X.; Huang, S. Gut-on-Chip: Recreating Human Intestine in vitro. J. Tissue Eng. 2020, 11, 2041731420965318. [Google Scholar] [CrossRef]
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in Methods for Measuring the Intestinal Absorption of Nanoparticle-Bound Drugs. Int. J. Mol. Sci. 2016, 17, 1171. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Jauregi, P.; Navarro-Alarcón, M.; Olalla-Herrera, M.; Giménez-Martínez, R.; Amigo, L.; Miralles, B. Bioaccessible Peptides Released by in vitro Gastrointestinal Digestion of Fermented Goat Milks. Anal. Bioanal. Chem. 2018, 410, 3597–3606. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Benitez, F.J.; Pérez-Jiménez, J.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. In vitro Evaluation of the Kinetics of the Release of Phenolic Compounds from Guava (Psidium guajava L.) Fruit. J. Funct. Foods 2018, 43, 139–145. [Google Scholar] [CrossRef]
- González, C.; González, D.; Zúñiga, R.N.; Estay, H.; Troncoso, E. Simulation of Human Small Intestinal Digestion of Starch Using an In vitro System Based on a Dialysis Membrane Process. Foods 2020, 9, 913. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of Rutin, Caffeic Acid and Rosmarinic Acid: Influence of the in vitro Gastrointestinal Digestion Models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Guerra, A.; Denis, S.; le Goff, O.; Sicardi, V.; François, O.; Yao, A.F.; Garrait, G.; Manzi, A.P.; Beyssac, E.; Alric, M.; et al. Development and Validation of a New Dynamic Computer-Controlled Model of the Human Stomach and Small Intestine. Biotechnol. Bioeng. 2016, 113, 1325–1335. [Google Scholar] [CrossRef]
- Carrasco-Correa, E.J.; Ruiz-Allica, J.; Rodríguez-Fernández, J.F.; Miró, M. Human Artificial Membranes in (Bio)Analytical Science: Potential for in vitro Prediction of Intestinal Absorption-A Review. TrAC Trends Anal. Chem. 2021, 145, 116446. [Google Scholar] [CrossRef]
- di Cagno, M.; Bibi, H.A.; Bauer-Brandl, A. New Biomimetic Barrier PermeapadTM for Efficient Investigation of Passive Permeability of Drugs. Eur. J. Pharm. Sci. 2015, 73, 29–34. [Google Scholar] [CrossRef]
- B Sánchez, A.; C Calpena, A.; Mallandrich, M.; Clares, B. Validation of an Ex vivo Permeation Method for the Intestinal Permeability of Different BCS Drugs and Its Correlation with Caco-2 in vitro Experiments. Pharmaceutics 2019, 11, 638. [Google Scholar] [CrossRef]
- Chen, P.; Zhao, M.; Chen, Q.; Fan, L.; Gao, F.; Zhao, L. Absorption Characteristics of Chitobiose and Chitopentaose in the Human Intestinal Cell Line Caco-2 and Everted Gut Sacs. J. Agric. Food Chem. 2019, 67, 4513–4523. [Google Scholar] [CrossRef]
- Pierzchalska, M.; Panek, M.; Czyrnek, M.; Grabacka, M. The Three-Dimensional Culture of Epithelial Organoids Derived from Embryonic Chicken Intestine. Methods Mol. Biol. 2016, 1576, 135–144. [Google Scholar] [CrossRef]
- Baskara, A.P.; Sharma, S.; Sener-Aydemir, A.; Koger, S.; Ariyadi, B.; Dono, N.D.; Zuprizal, Z.; Metzler-Zebeli, B.U. Cinnamon Bark Oil and Coconut Oil Emulsions Modified Small Intestinal Motility and Barrier Function in Laying Hens in an Ex vivo Experiment. Br. Poult. Sci. 2021, 62, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Deußer, H.; Rogoll, D.; Scheppach, W.; Volk, A.; Melcher, R.; Richling, E. Gastrointestinal Absorption and Metabolism of Apple Polyphenols Ex vivo by the Pig Intestinal Mucosa in the Ussing Chamber. Biotechnol. J. 2013, 8, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, I.; Röhe, I.; Goodarzi Boroojeni, F.; Knorr, F.; Mader, A.; Hafeez, A.; Zentek, J. Feed Supplemented with Organic Acids Does Not Affect Starch Digestibility, nor Intestinal Absorptive or Secretory Function in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2015, 99, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ozorio, L.; Mellinger-Silva, C.; Cabral, L.M.C.; Jardin, J.; Boudry, G.; Dupont, D. The Influence of Peptidases in Intestinal Brush Border Membranes on the Absorption of Oligopeptides from Whey Protein Hydrolysate: An Ex vivo Study Using an Ussing Chamber. Foods 2020, 9, 1415. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, M.; Zhang, H.; Pan, Y.; Dong, Q.; Xin, Y.; Ho, C.T.; Huang, Q. Evaluation of the Bioaccessibility of Tetrahydrocurcumin-Hyaluronic Acid Conjugate Using in vitro and Ex vivo Models. Int. J. Biol. Macromol. 2021, 182, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E. Effect of Pectins on the Mass Transfer Kinetics of Monosaccharides, Amino Acids, and a Corn Oil-in-Water Emulsion in a Franz Diffusion Cell. Food Chem. 2016, 209, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Komaki, Y.; Fukano, N.; Bungo, T. Transporter Gene Expression and Transference of Fructose in Broiler Chick Intestine. J. Poult. Sci. 2018, 55, 0170095. [Google Scholar] [CrossRef]
- Mebratu, A.T.; Asfaw, Y.T.; Paul, G.; Janssens, J. Exploring the Functional and Metabolic Effects of Feeding Garra Fish Meal to Broiler Chickens. Prepint 2021. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Micro- and Nano-Encapsulation of β-Carotene in Zein Protein: Size-Dependent Release and Absorption Behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Herrera-Cazares, L.A.; Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Gutiérrez-Uribe, J.A.; Campos-Vega, R.; Gaytán-Martínez, M. Influence of Extrusion Process on the Release of Phenolic Compounds from Mango (Mangifera indica L.) Bagasse-Added Confections and Evaluation of Their Bioaccessibility, Intestinal Permeability, and Antioxidant Capacity. Food Res. Int. 2021, 148, 110591. [Google Scholar] [CrossRef]
- Leena, M.M.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Co-Delivery of Curcumin and Resveratrol through Electrosprayed Core-Shell Nanoparticles in 3D Printed Hydrogel. Food Hydrocoll. 2022, 124, 107200. [Google Scholar] [CrossRef]
- Sangsawad, P.; Roytrakul, S.; Choowongkomon, K.; Kitts, D.D.; Chen, X.M.; Meng, G.; Li-Chan, E.C.Y.; Yongsawatdigul, J. Transepithelial Transport across Caco-2 Cell Monolayers of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides Derived from Simulated in vitro Gastrointestinal Digestion of Cooked Chicken Muscles. Food Chem. 2018, 251, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Patlan, D.; Solis-Cruz, B.; Pontin, K.P.; Latorre, J.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Merino-Guzman, R.; Méndez-Albores, A.; Hargis, B.M.; Lopez-Arellano, R.; et al. Evaluation of a Solid Dispersion of Curcumin with Polyvinylpyrrolidone and Boric Acid against Salmonella Enteritidis Infection and Intestinal Permeability in Broiler Chickens: A Pilot Study. Front. Microbiol. 2018, 9, 1289. [Google Scholar] [CrossRef]
- Vieira, E.F.; das Neves, J.; Ferreira, I.M.P.L.V.O. Bioactive Protein Hydrolysate Obtained from Canned Sardine and Brewing By-Products: Impact of Gastrointestinal Digestion and Transepithelial Absorption. Waste Biomass Valorization 2021, 12, 1281–1292. [Google Scholar] [CrossRef]
- Arranz, E.; Guri, A.; Fornari, T.; Mendiola, J.A.; Reglero, G.; Corredig, M. In vitro Uptake and Immune Functionality of Digested Rosemary Extract Delivered through Food Grade Vehicles. Food Res. Int. 2017, 97, 71–77. [Google Scholar] [CrossRef]
- Brufau, M.T.; Campo-Sabariz, J.; Carné, S.; Ferrer, R.; Martín-Venegas, R. Salmosan, a β-Galactomannan-Rich Product, in Combination with Lactobacillus Plantarum Contributes to Restore Intestinal Epithelial Barrier Function by Modulation of Cytokine Production. J. Nutr. Biochem. 2017, 41, 20–24. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of Phenolics, Carotenoids, and Antioxidant Capacity Following Simulated Gastrointestinal Digestion and Dialysis of Selected Edible Green Leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Bujard, A.; Skalicka-Woźniak, K.; Cretton, S.; Houriet, J.; Christen, P.; Carrupt, P.A.; Wolfender, J.L. Prediction of the Passive Intestinal Absorption of Medicinal Plant Extract Constituents with the Parallel Artificial Membrane Permeability Assay (PAMPA). Planta Med. 2016, 82, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Navarro Del Hierro, J.; Piazzini, V.; Reglero, G.; Martin, D.; Bergonzi, M.C. In vitro Permeability of Saponins and Sapogenins from Seed Extracts by the Parallel Artificial Membrane Permeability Assay: Effect of in vitro Gastrointestinal Digestion. J. Agric. Food Chem. 2020, 68, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Charalabidis, A.; Sfouni, M.; Bergström, C.; Macheras, P. The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond Guidelines. Int. J. Pharm. 2019, 566, 264–281. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).