Abstract
This study aimed to investigate the relationship between chronic kidney disease (CKD) and different gastric diseases by conducting a population-based retrospective analysis using National Inpatient Sample (NIS) data. A total of 7,159,694 patients diagnosed with gastric diseases with or without CKD were included, and the diagnoses of gastritis, gastric polyps, peptic ulcer disease (PUD), and Helicobacter pylori infection were based on ICD-10-CM codes. The study found a higher prevalence of gastritis and gastric polyps in patients with CKD compared to patients without CKD, especially in the late stages of CKD. After adjustment, patients with CKD also had a higher risk of developing these gastric diseases than patients without CKD. However, there was no significant association between all stages of CKD and PUD or Helicobacter pylori infection. These results underscored the importance of monitoring gastric health in patients with CKD.
1. Introduction
Chronic kidney disease (CKD) has had an increased prevalence over the past decade and, in a recent study in 2015–2018, was found to affect around 14.9% of US adults [1,2]. Etiologies of CKD can vary and can have lasting effects on the rate of progression, pathology, and severity of CKD [3]. Individuals with a past medical history of uncontrolled hypertension (HTN) or diabetes mellitus are at the highest risk of developing kidney disease with other common causes, including older age, heart disease, family history of kidney disease, and inherited kidney disorders [4]. Long-term patients are known to be more prone to multiple complications involving the cardiovascular system, hematology, endocrine, neurologic, skin, and even psychiatry [5,6,7]. Gastric symptoms such as nausea, vomiting, early satiety, and epigastric pain are commonly reported in patients with CKD, with an estimated incidence of 70% in those with renal insufficiency [8,9]. Despite this, the underlying physiology causing gastric symptoms in patients with CKD is not yet fully understood. Some studies suggest that factors such as delayed gastric emptying, acute/chronic gastritis, peptic ulcer disease (PUD), and asymptomatic gastric polyps might play a role; however, no clear association between CKD and gastric diseases has been established [10,11].
Gastritis is a type of inflammation affecting the gastric mucosal lining and causes abdominal pain, regurgitation, nausea, and vomiting symptoms [12,13]. Diagnosis is usually dependent on the underlying cause and can include a urea breath test, endoscopy with biopsy, and, in some cases, blood tests. Complications of gastritis are usually related to the formation of gastric ulcers and bleeding, but, in rare cases, can lead to gastric cancer. The etiology of gastritis is often related to autoimmune disorders or H. pylori infection [14,15], with other causes, including reactive gastritis that can result from radiation, alcohol, or medications. The underlying physiologic mechanism for this disease is still being determined, however, so exact associations cannot be made. Interestingly, a previous study reported that over 40% of patients with renal failure experienced epigastric pain of unknown origin [16]. Thomas et al. [17] showed that 13 out of 38 patients with CKD had gastritis confirmed by biopsy after endoscopy. Another prospective study by Margolis et al. [16] in 1987 found a high incidence of mucosal inflammation in chronic renal failure patients undergoing long-term hemodialysis.
Polyps are abnormal growths of cells that can frequently present in the lining of the stomach and, though mostly benign, can occasionally be malignant. Clinically, the presentation of these polyps is generally silent and is found incidentally during routine or diagnostic exams for other reasons. Additionally, the increased use of endoscopy in recent times led to a higher detection rate of gastric polyps, with them presenting in 6% of patient examinations in a recent study in 2020 [18]. The most common etiologies related to gastric polyps are H. pylori infection and proton pump inhibitors (PPIs) in thought due to alterations to stomach pH by gastric acid. However, there are some genetic conditions, such as familial adenomatous polyposis (FAP), in which they can develop in vast quantities, leading to further diagnostic studies being recommended, often completed upon finding a polyp [19]. Although there is no recent research linking CKD to the development of gastric polyps, a high prevalence was found in patients with CKD undergoing endoscopy due to anemia [20]. So frequent, in fact, that a study completed in 2018 reported that individuals diagnosed with CKD stages 3–5 could benefit from screening for polyps to search for adenomatous polyps and even colorectal cancer [20].
Peptic ulcer disease (PUD), in relation to other gastric diseases, has a decreased prevalence and still is a source of significant morbidity in general populations [21]. A study in 2022 found that the lifetime incidence of PUD was between 5%–10% and had decreased over recent years due to improved treatment standards [22]. Clinically, PUD can vary with mild cases presenting with imprecise epigastric pain and nausea, whereas severe complicated cases can lead to severe abdominal pain with guarding. Complications include developing severe gastric ulcers that can bleed and even perforate, leading to emergent situations [23]. In diagnosing PUD, the gold standard usually involves upper GI endoscopy or EGD to visualize the ulcer and can sometimes include biopsy to rule out [23]. Etiologic factors for PUD are similar to other gastric conditions, including H. pylori infections and the use of NSAIDs [21,24]. Moreover, in patients with impaired renal function, there has been noted to be a higher risk of developing PUD than those with normal function [25]. Liang et al. [26] found that the risk of PUD was 10–12 times higher in a patient with CKD in Taiwan from 1998–2008. Additionally, the recurrence of PUD after H. pylori eradication was significantly higher in patients undergoing hemodialysis [27,28].
Patients with CKD commonly present with symptoms involving the gastrointestinal tract, which often can lead to a difficult and lesser quality of life for those affected. Persistent symptoms can become debilitating and lead to frequent provider visits, inevitably leading to hospitalization with serious complications in those not treated appropriately. With a large percentage of patients with CKD having generalized upper gastrointestinal symptoms, unveiling the underlying association between the two becomes an ever-greater priority. While, currently, the relationship between CKD and gastric diseases such as gastritis, gastric polyps, PUD, and H. pylori infection is still widely unknown, some small studies suggest a relationship between them. This study aimed to analyze and investigate whether CKD was associated with a greater prevalence of these gastric diseases by using a large national inpatient database.
2. Results
A total of 7,159,694 hospitalized patients during 2017 were included in this study, with 103,015 having gastritis, 7594 with gastric polyps, 18,110 with PUD, and 5679 with H. pylori infection, with or without CKD, respectively (Figure 1). Patients with CKD were generally older than those without CKD. A higher proportion of males were diagnosed with gastritis, gastric polyps, and H. pylori infection, and this trend was more pronounced in the CKD group compared to the non-CKD group. The prevalence of type 2 diabetes mellitus (T2DM) was lower in those with gastric diseases and CKD versus the groups without CKD (p < 0.01). A higher proportion of patients with these gastric diseases and CKD also had hyperlipidemia than those without CKD (Table 1 and Table 2).
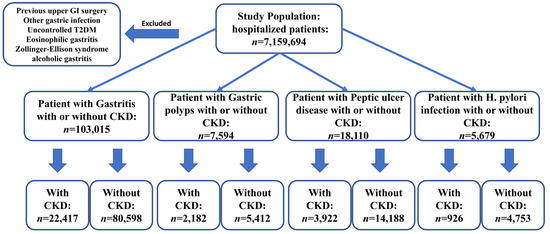
Figure 1.
Sample selection and study design flowchart. A total of 7,159,694 hospitalized patients were analyzed and selected based on ICD-10 and exclusion criteria. The sample included patients diagnosed with gastritis, gastric polyps, peptic ulcer disease, and H. pylori infection. The following numbers represent the sample size of patients with each condition, categorized by the presence or absence of CKD.

Table 1.
Demographics, risk factors, comorbidities, prevalence, and odds ratios for patients with CKD with gastritis, gastric polyps. CKD, chronic kidney disease; HTN, hypertension; HLD, hyperlipidemia, T2DM, type 2 diabetes; and PUD, peptic ulcer disease.

Table 2.
Demographics, risk factors, comorbidities, prevalence, and odds ratios for patients with CKD with PUD, and H. pylori infection. CKD, chronic kidney disease; HTN, hypertension; HLD, hyperlipidemia, T2DM, type 2 diabetes; and PUD, peptic ulcer disease.
The risk of gastritis was higher in subjects with CKD (OR: 1.257, 95%CI: 1.238–1.227, p < 0.001). The incidence of gastritis in CKD was 2.4%, and the incidence in those without CKD was 1.4% (p < 0.01). The risk and incidence of gastritis increased as the CKD stage progressed, with the highest being seen in those with ESRD (OR: 1.676 95%CI: 1.631–1.723 p < 0.01; incidence: 2.9%) (Figure 2).
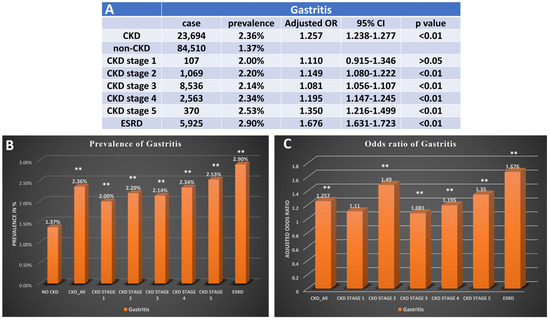
Figure 2.
Prevalence and odds ratio for patients with CKD with gastritis. (A) The case number, prevalence, and adjusted odds ratio for gastritis in patients with diagnosis of different stages of CKD. (B) Bar graph for prevalence of gastritis in patients without CKD, patients with CKD, and different stages of CKD. (C) Bar graph presents adjusted odds ratio of gastritis in patients with different stages of CKD. CKD, chronic kidney disease; OR, odds ratio; ESRD, end-stage renal disease, ** p < 0.01. Adjusted for age, gender, race, obesity, smoking history, T2DM, hypertension, and hyperlipidemia.
Patients with CKD were more likely to develop gastric polyps compared to those without CKD (OR: 1.437 95%CI: 1.364–1.513, p < 0.001), with a prevalence of 22.9 per 10,000 cases among those with CKD and 9.4 per 10,000 cases among patients without CKD (p < 0.01). The odds of gastric polyps also had a positive association with the stage of CKD, similar to gastritis (CKD stage 2: 1.344, 95%CI: 1.106–1.634 VS ESRD: 2.030, 95%CI: 1.850–2.228, p < 0.01) (Figure 3).
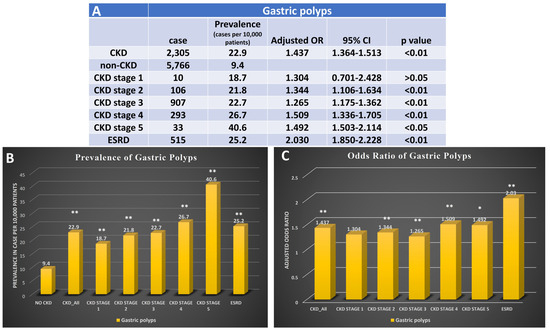
Figure 3.
Prevalence and odds ratio for patients with CKD with gastric polyps. (A) The case number, prevalence, and adjusted odds ratio for gastric polyps in patients with diagnosis of different stages of CKD. (B) Bar graph for prevalence of gastric polyps in patients without CKD, patients with CKD, and different stages of CKD. (C) Bar graph presents adjusted odds ratio of gastric polyps in patients with different stages of CKD. CKD, chronic kidney disease; OR, odds ratio; ESRD, end-stage renal disease, * p < 0.05, and ** p < 0.01. Adjusted for age, gender, race, obesity, smoking history, T2DM, hypertension, and hyperlipidemia.
Patients with CKD had a higher incidence of peptic ulcer disease (PUD) compared to those without CKD (40.7 vs. 24.0 per 10,000 cases, p < 0.001). However, there were no clinically significant differences in the risk of having PUD between patients with early stages of CKD. However, patients with ESRD had a higher risk of developing PUD compared to those without CKD (OR: 1.311 95%CI: 1.220–1.409, p < 0.001), with a prevalence of 40.2 per 10,000 cases among those with ESRD (p < 0.01) (Figure 4).
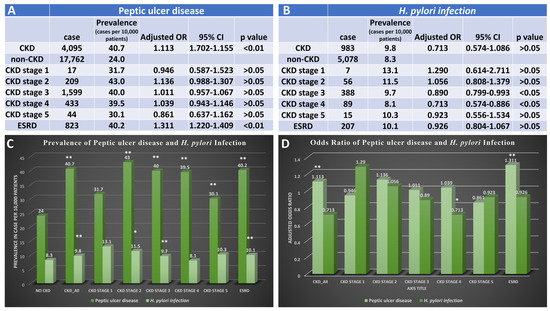
Figure 4.
Prevalence and odds ratio for patients with CKD with peptic ulcer disease and H. pylori infection. (A) The case number, prevalence, and adjusted odds ratio for peptic ulcer disease in patients with diagnosis of different stages of CKD. (B) The case number, prevalence, and adjusted odds ratio for H. pylori infection in patients with diagnosis of different stages of CKD. (C) Bar graph for prevalence of peptic ulcer disease or H. pylori infection in patients without CKD, patients with CKD, and different stages of CKD. (D) Bar graph presents an adjusted odds ratio of peptic ulcer disease or H. pylori infection in patients with different stages of CKD. CKD, chronic kidney disease; OR, odds ratio; ESRD, end-stage renal disease, * p < 0.05, and ** p < 0.01. Adjusted for age, gender, race, obesity, smoking history, T2DM, hypertension, and hyperlipidemia.
Furthermore, there were no significant clinical differences in the prevalence or odds of risk of H. pylori infection between patients with CKD and those without (Figure 4).
3. Discussion
The results from this study had some important findings, revealing an increased risk and incidence of gastritis and gastric polyps in those with CKD compared to those without CKD. As mentioned earlier, the quality-of-life measures and future complication rates are greater in those diagnosed with these certain gastric conditions and, as such, can have larger outcomes than originally expected. Preventative measurements and proper management of these conditions become more vital in clinical practice in patients with these conditions due to this. Additionally, it was found that the highest risk and incidence of gastric diseases were found in the patients with the most severe stage of CKD ESRD. The significance of these findings is due to this being the first extensive inpatient data analysis to specifically investigate gastric, and gastric polyps in patients with CKD whose nonspecific gastric symptoms might often otherwise be misinterpreted. Data from prior research studies that showed a significant correlation between increased rates of PUD in those with CKD also aligned with our data. If clinically correlated, investigation and prevention in those with CKD can potentially help alleviate complications and thus health disparity. The findings are supported by previous prospective studies conducted on patients with CKD and further emphasize the importance of establishing the relationship between the two.
Previously, there have been a few studies that showed a greater prevalence of gastritis in CKD patients, especially ESRD [16,17]. However, our study is the first to demonstrate that CKD is an independent risk factor for the development of gastritis, even in the early stage of CKD. The hypothesized mechanisms include electrolyte abnormalities, uremia, and hormone imbalances that affect gastric mucosa stability and thus the ability to protect itself from acidic harm. For example, Quintero et al. [29] found that basal gastric mucus thickness was significantly reduced in a renal failure rat model, making the gastric mucosal barrier more susceptible to acid injury. Another animal model of CKD showed a correlation between uremia status and disrupted stomach epithelial tight junctions, resulting in signs of gastritis [30]. Additionally, it has been suggested that elevated urea can lead to an increased rate of synthesis and decreased clearance of gastrin, leading to a more acidic pH that can injure the mucosa. Hyperkalemia and acidosis in patients with CKD can also disrupt the protective mechanism of gastric mucosal by weakening the pH gradient across the epithelial cell leading to potential erosion and ulceration [31]. Some studies have even suggested developing a method to target potassium as a way of gastric acid suppression due to its role in H/K ATPase in the stomach, though future trials are still underway. Even further, the development of gastritis in patients with CKD may also be caused by a reduction in blood flow to the gastric mucosa and the amplification of reactive oxygen species due to the inhibition of prostaglandin production [5,31]. Similar to the mechanism that chronic NSAIDs use, this can lead to the development of gastric ulcers and gastritis.
The occurrence of gastrointestinal lesions in uremia has long been recognized through methods of edema, inflammation, erosion, and potential ulceration. Clinical symptoms of uremia are believed to result as a consequence of delayed gastric motility, delayed gastric emptying, and changes in the myoelectric activity of the gastric mucosa [32]. Jaffe et al. [33] first reported in 1934 that mild mucosa edema and hemorrhages were found in 136 cases of patients with uremia. Paimela et al. [34] reported a significantly higher prevalence of duodenal polyps in patients with CKD compared to the control group, which was associated with high mean values of gastric resting pH. Another study of approximately 1000 patients found a high prevalence of adenomatous polyps in late-stage patients with CKD over 50 years old [20]. However, to truly evaluate the association, further research is needed to comprehensively study the relationship between gastric polyps and CKD in a larger patient database. From this study, we have been the first to show that there is an evident association between the development of gastric polyps and CKD, presenting that all stages of CKD can be independent risk factors for gastric polyps. The potential mechanism has been thought to involve the dysregulation of reactive oxygen species (ROS) in patients with CKD through physiologic oxidative injury to cellular DNA leading to increased rates of mutative changes and unregulated replication. Beno et al. [35] found increased activity of antioxidant enzymes secondary to a higher ROS concentration in the cellular membranes of gastric polyps. Elevated oxidative stress markers have also been found in gastric cancer patients in part thought due to products of lipid peroxidation, superoxide dismutase (SOD), catalase activity, and malondialdehyde (MDA) concentrations [15,20,36].
Formerly, there have been studies that have indicated a strong connection between CKD and peptic ulcer disease (PUD), particularly in countries and regions of Asian descent. A study in Taiwan using data from 1998–2008 found that patients with CKD had a significantly higher incidence of PUD compared to those without CKD, with a risk nine times higher than patients without CKD [26]. Similar results were reported in another study in Taiwan [25]. In Korea, a study conducted between 2004–2016 by Kim et al. [37] found a high prevalence of PUD in dialysis patients, linked to low serum albumin and high blood urea nitrogen levels. From our findings based solely in the United States, there was a significant increase in PUD incidence found among patients with CKD compared to those without CKD, consistent with previous studies. Another study showed increased rates of PUD in those who were receiving long-term hemodialysis compared to those without. However, with our data, we did not observe a significant association between PUD and CKD. We also found no difference in incidence or risk of H. pylori infection between patients with CKD and without CKD, which may have been due to the relatively low prevalence of H. pylori infection and PUD in the US population or due to another confounding variable. Additionally, future studies with a longer duration, greater prevalence, and larger population size may provide diverse results. With some pathologies correlated to certain regions, specifically H. pylori, a larger study with geographical patient populations can be studied specifically better analyze the relationship between the two.
In this study, several limitations have been identified. Firstly, a nationwide inpatient database from which all the data were collected was used as the primary source. However, outpatient information, which is another large percentage of clinical encounters, was not included. From this data collection, the diagnosis of the gastric diseases analyzed in this study was based on the use of ICD-10-CM codes, which were entered among various hospital system electronic medical records. The diagnosis of each gastric disease was assumed to be based on images, endoscopy, gastric emptying test, and pathology. However, the methods used were not explicitly stated. The gold standard for diagnosing many gastric diseases is pathologic confirmation and, in the absence of that, clinical correlation is used, which can oftentimes be skewed. The risk factors of gastric diseases, such as smoking, diabetes, and alcohol abuse, were also determined using ICD-10-CM codes, but no timeline was identified for these risk factors. Individuals who met had a history of these risk factors cannot be evaluated until a greater analysis of the length of the factor is established to rule out any confounding variables. Furthermore, stages of CKD were also determined using ICD-10-CM codes, which were assumed to be diagnosed based on glomerular filtration rate (GFR). The basis of CKD can vary depending on the institution or hospital. The study was based on the current retrospective database, and other factors should also be noted as limitations as well. Pharmacologic treatment was not able to be evaluated and used in this study, which could contribute to some progression of gastric disease. New developments in glucagon-like peptide 1 (GLP-1) agonists have been used for diabetics and weight loss and could invariably affect the development of gastric diseases. In recent studies, there has been an association between chronic proton pump inhibitor (PPI) use and the development of gastric polyps. Moreover, multiple additional medications can lead to other gastric diseases such as gastritis and PUD, such as NSAIDs; therefore, more analysis of their potential alternative mechanism for symptoms needs to be evaluated. Additionally, other factors, such as the timeline and initiation of the disease, which were not included in this study, could be further investigated to determine the disease progression involvement. Allowing for the identification of precipitating causes can help determine the onset of disease and possibly help unravel the underlying cause. Future studies can confirm and address these concerns to alleviate some of the corresponding limitations and limit any confounding variables. A prospective study could prove invaluable in establishing a greater correlation between the diseases.
4. Materials and Methods
4.1. Database
We conducted a retrospective analysis utilizing the 2017 NIS database. The NIS is the most extensive publicly available all-payer inpatient healthcare database, specifically designed to estimate inpatient utilization, access, cost, quality, and outcomes. It comprises un-weighted data from approximately 7 million hospital stays annually. The NIS represents a 20% stratified sample of all discharges from US community hospitals, excluding rehabilitation and long-term acute-care hospitals.
4.2. Data Collection and Outcomes
A total of 7,159,694 adult patients admitted to the hospital in 2017 were included in this study. Patients diagnosed with different gastric diseases with or without CKD (ICD-10-CM N18.1-9) were compared to patients without each gastric disease. We included diagnoses of PUD (ICD-10-CM K27.0-9), gastritis (ICD-10-CM K29.0-K29.7), gastric polyps (ICD-10-CM K31.7), and H. pylori infection (ICD-10-CM B9681). We excluded subjects with a history of upper GI surgeries or uncontrolled T2DM. More specifically, eosinophilic gastritis or gastroenteritis, Zollinger-Ellison syndrome, and alcoholic gastritis were excluded from the gastritis groups, whereas alcohol abuse was excluded from the PUD group. Risk factors of CKD, including controlled T2DM, essential HTN, hyperlipidemia, and demographic data were collected, including age, race, and gender. To assess the odds ratio of different gastric diseases in different stages of CKD, we included those with a specific disease (cases) compared with those without each disease (controls). All diagnoses included or excluded from this study were selected by the ICD-10-CM code.
4.3. Statistical Analysis
All demographic and risk factor data in this study collected from NIS were categorical and thus were presented as several cases and percentages. Chi-squared analysis was used to analyze the association between different gastric diseases and CKD and compared to those without CKD. Multivariate logistic regression analysis was used to assess the risk of, in the form of odds ratios, gastric diseases with and without the different stages of CKD. We adjusted for age, gender, race, obesity, smoking history, T2DM, hypertension, and hyperlipidemia as covariates to mitigate the effect of confounding factors. A two-sample test for equal proportions was used, and a p-value < 0.05 was considered significant. IBM SPSS 28.0.1.1 (IBM Corp., Chicago, IL, USA, Version 28.0.1.1.) was used for the statistical analysis in this study.
5. Conclusions
Chronic kidney disease patients present with multitudes of symptoms other than the expected decrease in kidney function and are generally broad in nature. Individuals with higher risk factors are generally monitored through kidney function tests, and the presentations of symptoms are often difficult to correlate with severity. Symptoms can vary in severity themselves and often can lead to hospitalization if misdiagnosed or left untreated. Severe outcomes associated with CKD and its symptoms can lead to lifetime complications, quality of life, and even death. Due to the wide variety of presentations and the different organ systems that are involved, it is easy to correlate the symptoms to separate conditions and not associate secondary to CKD. With the increasing prevalence of gastric disease and CKD, it is important to identify and monitor for these conditions, in part, due to decrease the chance of complications but also to ensure a better quality of life. Gastric symptoms can present similarly, and mistreatment can often worsen or mask the underlying cause. By evaluating the associations, our study highlights the importance of thoroughly examining patients with CKD who present with epigastric symptoms for the presence of gastritis, gastric polyps, or PUD. Early detection and treatment of these gastric conditions should be considered, especially for patients in the early stages of CKD in order to prevent more serious and unreversible injury. Symptoms of patients with late stages of CKD should be treated as well as possible due to the difficulty in kidney function repair. The socioeconomic burden of CKD can also be improved by managing the disease and its complications before they occur. The data from our study have clinical implications to suggest that individuals who are diagnosed with CKD disease and who present with gastric symptoms should be investigated further.
Author Contributions
X.W.: Conceptualization, investigation, data curation, data analysis, visualization, drafting the manuscript. Z.W.: Editing manuscript. J.W.: Editing manuscript. W.M.F.: Editing manuscript. G.S.: Conceptualization, project supervision, editing manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Marshall University School of Medicine Institutional Review Board has deemed studies using the NIS database as exempt from requiring IRB approval due to the de-identified and aggregated nature of the data in the database. The Case Western Reserve University/Metrohealth Medical Center Institutional Review Board has deemed studies using the NIS database as exempt from requiring IRB approval due to the de-identified and aggregated nature of the data in the database at the standard defined in Section 164.514(a) of the HIPAA Privacy Rule.
Informed Consent Statement
Patient consent was waived due to no patient’s identity was collected in this database.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patient and hospital information privacy and the requirement of H.CUP.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Vart, P.; Powe, N.R.; McCulloch, C.E.; Saran, R.; Gillespie, B.W.; Saydah, S.; Crews, D.C. National Trends in the Prevalence of Chronic Kidney Disease Among Racial/Ethnic and Socioeconomic Status Groups, 1988-2016. JAMA Netw. Open 2020, 3, e207932. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Remuzzi, G.; Glassock, R.; Levin, A.; Jager, K.J.; Tonelli, M.; Massy, Z.; Wanner, C.; Anders, H.-J. Chronic kidney disease. Nat. Rev. Dis. Primers 2017, 3, 17088. [Google Scholar] [CrossRef]
- Wang, X.; Shapiro, J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019, 15, 159–175. [Google Scholar] [CrossRef]
- Bello, A.K.; Alrukhaimi, M.; Ashuntantang, G.E.; Basnet, S.; Rotter, R.C.; Douthat, W.G.; Kazancioglu, R.; Köttgen, A.; Nangaku, M.; Powe, N.R.; et al. Complications of chronic kidney disease: Current state, knowledge gaps, and strategy for action. Kidney Int. Suppl. 2017, 7, 122–129. [Google Scholar] [CrossRef]
- Zoccali, C.; Vanholder, R.; Massy, Z.A.; Ortiz, A.; Sarafidis, P.; Dekker, F.W.; Fliser, D.; Fouque, D.; Heine, G.H.; Jager, K.J.; et al. The systemic nature of CKD. Nat. Rev. Nephrol. 2017, 13, 344–358. [Google Scholar] [CrossRef]
- Karahan, D.; Şahin, İ. Comparison of gastrointestinal symptoms and findings in renal replacement therapy modalities. BMC Nephrol. 2022, 23, 261. [Google Scholar] [CrossRef]
- Cano, A.E.; Neil, A.K.; Kang, J.Y.; Barnabas, A.; Eastwood, J.B.; Nelson, S.R.; Hartley, I.; Maxwell, D. Gastrointestinal symptoms in patients with end-stage renal disease undergoing treatment by hemodialysis or peritoneal dialysis. Am. J. Gastroenterol. 2007, 102, 1990–1997. [Google Scholar] [CrossRef]
- Lew, S.Q.; Radhakrishnan, J. Chapter 33—Chronic Kidney Disease and Gastrointestinal Disorders. In Chronic Renal Disease, 2nd ed.; Kimmel, P.L., Rosenberg, M.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 521–539. [Google Scholar]
- Grant, C.J.; Harrison, L.E.; Hoad, C.L.; Marciani, L.; Gowland, P.A.; McIntyre, C.W. Patients with chronic kidney disease have abnormal upper gastro-intestinal tract digestive function: A study of uremic enteropathy. J. Gastroenterol. Hepatol. 2017, 32, 372–377. [Google Scholar] [CrossRef]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Neumann, W.L.; Coss, E.; Rugge, M.; Genta, R.M. Autoimmune atrophic gastritis—Pathogenesis, pathology and management. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 529–541. [Google Scholar] [CrossRef]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e1327. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.; Fossmark, R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 6548. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.M.; Saylor, J.L.; Geisse, G.; DeSchryver-Kecskemeti, K.; Harter, H.R.; Zuckerman, G.R. Upper gastrointestinal disease in chronic renal failure. A prospective evaluation. Arch. Intern. Med. 1978, 138, 1214–1217. [Google Scholar] [CrossRef]
- Thomas, R.; Panackal, C.; John, M.; Joshi, H.; Mathai, S.; Kattickaran, J.; Iqbal, M. Gastrointestinal Complications in Patients with Chronic Kidney Disease—A 5-Year Retrospective Study from a Tertiary Referral Center. Ren. Fail. 2013, 35, 49–55. [Google Scholar] [CrossRef]
- Kővári, B.; Kim, B.H.; Lauwers, G.Y. The pathology of gastric and duodenal polyps: Current concepts. Histopathology 2021, 78, 106–124. [Google Scholar] [CrossRef]
- Markowski, A.R.; Markowska, A.; Guzinska-Ustymowicz, K. Pathophysiological and clinical aspects of gastric hyperplastic polyps. World J. Gastroenterol. 2016, 22, 8883–8891. [Google Scholar] [CrossRef]
- García Agudo, R.; Aoufi Rabih, S.; González Carro, P.; Pérez Roldán, F.; Proy Vega, B.; Arias Arias, Á.; Cazalla Cadenas, F.; Tenías Burillo, J.M.; Fernández Rodríguez, A. Gastrointestinal lesions in chronic kidney disease patients with anemia. Nefrol. (Engl. Ed.) 2019, 39, 50–57. [Google Scholar] [CrossRef]
- Kavitt, R.T.; Lipowska, A.M.; Anyane-Yeboa, A.; Gralnek, I.M. Diagnosis and Treatment of Peptic Ulcer Disease. Am. J. Med. 2019, 132, 447–456. [Google Scholar] [CrossRef]
- Abbasi-Kangevari, M.; Ahmadi, N.; Fattahi, N.; Rezaei, N.; Malekpour, M.R.; Ghamari, S.H.; Moghaddam, S.S.; Azadnajafabad, S.; Esfahani, Z.; Kolahi, A.A.; et al. Quality of care of peptic ulcer disease worldwide: A systematic analysis for the global burden of disease study 1990–2019. PLoS ONE 2022, 17, e0271284. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Reddy, K.M.; Marsicano, E. Peptic Ulcer Disease and Helicobacter pylori infection. Mo. Med. 2018, 115, 219–224. [Google Scholar]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-M.; Hsu, C.-N.; Tai, W.-C.; Yang, S.-C.; Wu, C.-K.; Shih, C.-W.; Ku, M.-K.; Yuan, L.-T.; Wang, J.-W.; Tseng, K.-L.; et al. Risk factors influencing the outcome of peptic ulcer bleeding in chronic kidney disease after initial endoscopic hemostasis: A nationwide cohort study. Medicine 2016, 95, e4795. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Muo, C.H.; Wang, I.K.; Chang, C.T.; Chou, C.Y.; Liu, J.H.; Yen, T.H.; Huang, C.C.; Chung, C.J. Peptic ulcer disease risk in chronic kidney disease: Ten-year incidence, ulcer location, and ulcerogenic effect of medications. PLoS ONE 2014, 9, e87952. [Google Scholar] [CrossRef] [PubMed]
- Tseng, G.Y.; Lin, H.J.; Fang, C.T.; Yang, H.B.; Tseng, G.C.; Wang, P.C.; Hung, T.L.; Deng, Y.C.; Cheng, Y.T.; Huang, C.H. Recurrence of peptic ulcer in uraemic and non-uraemic patients after Helicobacter pylori eradication: A 2-year study. Aliment. Pharmacol. Ther. 2007, 26, 925–933. [Google Scholar] [CrossRef]
- Suzuki, H.; Mori, H. Helicobacter pylori gastritis—A novel distinct disease entity. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 556–557. [Google Scholar] [CrossRef]
- Quintero, E.; Kaunitz, J.; Nishizaki, Y.; De Giorgio, R.; Sternini, C.; Guth, P.H. Uremia increases gastric mucosal permeability and acid back-diffusion injury in the rat. Gastroenterology 1992, 103, 1762–1768. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Nazertehrani, S.; Ni, Z.; Liu, S. Chronic kidney disease causes disruption of gastric and small intestinal epithelial tight junction. Am. J. Nephrol. 2013, 38, 99–103. [Google Scholar] [CrossRef]
- Forssell, H. Gastric mucosal defence mechanisms: A brief review. Scand. J. Gastroenterol. 1988, 155, 23–28. [Google Scholar] [CrossRef]
- Falconi, C.A.; Junho, C.V.d.C.; Fogaça-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.H.; Laing, D.R. Changes of the digestive tract in uremia: A pathologic anatomic study. Arch. Intern. Med. 1934, 53, 851–864. [Google Scholar] [CrossRef]
- Paimela, H.; Tallgren, L.G.; Stenman, S.; von Numers, H.; Scheinin, T.M. Multiple duodenal polyps in uraemia: A little known clinical entity. Gut 1984, 25, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Beno, I.; Volkovová, K.; Bátovsky, M.; Staruchová, M. Increased mucosal antioxidant enzyme activities in chronic gastritis and benign gastric polyps. Eur. J. Cancer Prev. 1993, 2, 461–465. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, F.; Yuan, X.; Yang, T.; Liang, X.; Wang, Y.; Tu, H.; Chang, J.; Nan, K.; Wei, Y. Reactive Oxygen Species Are Involved in the Development of Gastric Cancer and Gastric Cancer-Related Depression through ABL1-Mediated Inflammation Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 5813985. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Kim, S.W. Risk factors for peptic ulcer disease in patients with end-stage renal disease receiving dialysis. Kidney Res. Clin. Pract. 2019, 38, 81–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).