Abstract
Uncaria rhynchophylla is a plant highly used in the traditional Chinese and Japanese medicines. It has numerous health benefits, which are often attributed to its alkaloid components. Recent studies in humans show that drugs containing Uncaria ameliorate sleep quality and increase sleep time, both in physiological and pathological conditions. Rhynchophylline (Rhy) is one of the principal alkaloids in Uncaria species. Although treatment with Rhy alone has not been tested in humans, observations in rodents show that Rhy increases sleep time. However, the mechanisms by which Rhy could modulate sleep have not been comprehensively described. In this review, we are highlighting cellular pathways that are shown to be targeted by Rhy and which are also known for their implications in the regulation of wakefulness and sleep. We conclude that Rhy can impact sleep through mechanisms involving ion channels, N-methyl-d-aspartate (NMDA) receptors, tyrosine kinase receptors, extracellular signal-regulated kinases (ERK)/mitogen-activated protein kinases (MAPK), phosphoinositide 3-kinase (PI3K)/RAC serine/threonine-protein kinase (AKT), and nuclear factor-kappa B (NF-κB) pathways. In modulating multiple cellular responses, Rhy impacts neuronal communication in a way that could have substantial effects on sleep phenotypes. Thus, understanding the mechanisms of action of Rhy will have implications for sleep pharmacology.
1. Introduction
Plant compounds have been substantially explored to treat human illnesses, especially in the traditional Chinese medicine. This includes their utilization to ameliorate sleep or induce sedation [1,2]. However, given that the use of such compounds began early in the human history, the knowledge of their beneficial effects on health is rarely accompanied by studies providing the details of the underlying mechanisms.
Uncaria rhynchophylla has been used in Asia as a component of numerous Chinese and Japanese treatments such as Gou-teng (or Chotoko; name given to Uncaria medicinal herbs), and Yi-gan-san (a blend of seven herbs also known as Yokukansan [YKS]). It has been reported to alleviate hypertension, arrhythmia, convulsions, dizziness, pain, sleep disturbances, and cognitive impairments [3,4,5,6,7,8]. Alkaloids account for 0.2% of the composition of U. rhynchophylla (in hook, stem, and leaves) and were proposed to underlie the majority of health benefits resulting from the use of Uncaria [4,9]. Rhynchophylline (Rhy) is one of the most abundant of these alkaloids and seems to associate with nearly the same benefits as those obtained with U. rhynchophylla in nonhuman mammals [3,4,10].
1.1. Rhynchophylline Pharmacology
Rhy is a tetracyclic oxindole alkaloid that represents about 10–30% of Uncaria alkaloids [9,11,12]. Rhy is interconvertible with its isomer isorhynchophylline (Isorhy), which accounts for another 30–50% of the alkaloid fraction [9,11,12] (Figure 1). Their rate of interconversion depends on pH and temperature [13,14]. Both forms are absorbed quickly by the intestine but, when provided intravenously or orally, Rhy seems more available than Isorhy in the plasma, likely because the latter is more unstable and metabolized faster by the liver and intestine [13]. Rhy easily crosses the blood–brain barrier, as it is highly detectable in the rat brain from 15 min to 6 h after oral administration [15]. Another study has shown that an in vitro blood–brain barrier model was more permeable to Isorhy than Rhy [16]. Therefore, even if Rhy could be more prevalent than Isorhy in the body, the administration of Rhy may trigger the presence of Isorhy, which effect should be considered.
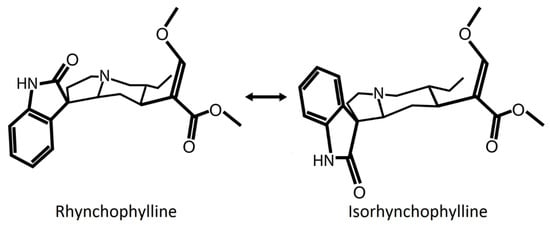
Figure 1.
Representation of the chemical structure of Rhynchophylline (Rhy) and Isorhynchophylline (Isorhy). The position of the oxindole structure (N-C=O in the second ring) of the alkaloids Rhy and Isorhy is different. Both molecules are diastereoisomers, interconvertible with each other depending on pH and temperature. Temperature is suggested to induce a break and reclosure of the third ring that results in a twisted conformation [14].
Rhy (like Isorhy) has been proposed to mainly act on the cardiovascular system and central nervous system (CNS) [3,10]. Although there is no clinical trial investigating the effects of Rhy alone, animal research suggests that Rhy has beneficial properties such as anti-inflammatory, antihypertensive, anti-arrhythmic, anticonvulsant and neuroprotective effects [3,10]. Moreover, it seems to reduce memory impairments, mood dysregulation, and addictive behaviors in rodents [17,18,19,20,21]. Interestingly, one study [22] and recent unpublished data from our group point to an effect of Rhy on sleep in rodents, which is in line with the beneficial effects of Chotoko and YKS on human sleep time and quality (see details in Section 1.3).
1.2. Sleep and Its Regulation
In mammals and other species, sleep is an essential behavior during which the organism isolates from environmental stimuli. Although the precise roles of sleep remain elusive, it could serve recovery from sustained activity (and associated oxidative stress) occurring during wakefulness in mammals and insects [23,24]. Moreover, sleep is beneficial for immune function, memory consolidation, and mood [25,26,27,28]. Mammalian sleep studies usually identify three main vigilance states: wakefulness, non-rapid eye movement (NREM) sleep (analogous to slow wave sleep in rodents), and rapid eye movement (REM) sleep (or paradoxical sleep) [29]. Wakefulness is characterized by a predominance of high frequency electroencephalographic (EEG) activity, NREM sleep by predominant low-frequency and high-amplitude EEG activity, and REM sleep by theta (4–9 Hz) EEG activity [29,30,31,32,33]. Delta activity (1–4 Hz) and slow oscillations (<1 Hz) during NREM sleep originate from synchronized up and down states of neuronal firing in cortical and thalamocortical networks [34,35]. Delta activity (or slow wave activity: 0.5–4.5 Hz) was proposed to reflect a sleep homeostatic/recovery process [31,32,36,37,38], which relationship was recently shown to differ between slower and faster delta [32].
The transitions between vigilance states are operated by the activation/inhibition of specific brain circuits [39,40]. During wakefulness, wake-promoting brain regions contribute to sustained neuronal activity and/or inhibit sleep promoting centers. Amongst the major wake-promoting centers are Hypocretin/Orexin neurons in the lateral hypothalamus, neurons in the basal forebrain (BF), and neurons in several nuclei of the reticular formation (laterodorsal tegmentum [LDT], pedunculopontine tegmentum [PPT], raphe nucleus [RN], locus cœruleus [LC]) [39,41,42,43,44,45,46]. Sleep promoting neurons are found in the hypothalamus, with the ventrolateral preoptic area having a particular relevance [47]. During REM sleep, neurons from several nuclei of the reticular formation, including the LDT and PPT, allow cortical activation while behavioral sleep is maintained [48,49]. The knowledge of sleep neurobiology is important to refine pharmacological approaches for sleep disturbances.
1.3. Rhynchophylline and Sleep
Drugs containing Uncaria appear to ameliorate sleep in different ways. For instance, YKS was shown to improve sleep disturbances (sleep time, quality, and sleep-related limb movements) in adults suffering from REM sleep behavior disorder or dementia [6,50,51,52]. It was also reported to improve sleep quality in patients with insomnia [7] and children with nocturnal enuresis [53]. Other drugs containing Uncaria (although in smaller proportion) were also shown to increase total sleep time in healthy subjects and sleep quality in patients with Parkinson’s disease or perimenopausal sleep disorder [54,55,56]. Fundamental research also suggests that Uncaria benefits sleep in rodents. Indeed, the administration of both YKS and a drug containing YKS was found to increase sleep time in socially isolated mice while having no impact in group-housed mice [57,58]. YKS was also shown to increase NREM sleep (and to decrease wake time) in a rat model of dementia [59], and Chotoko was reported to enhance the hypnotic-induced sleep time in mice [60]. Interestingly, Yoo and collaborators showed that Rhy increases sleep time in wild-type rats and mice [22]. This is in line with our recent observation of a longer time spent asleep after Rhy administration in mice, especially during the active (dark) period (Ballester Roig et al., in preparation). Moreover, Rhy, Isorhy or Uncaria were all shown to reduce spontaneous locomotor activity in mice [61,62,63].
Very few of these studies have investigated the cellular pathways underlying modifications of sleep. Three of them suggested that the increased sleep time in mice is linked to gamma-aminobutyric acid (GABA) neurotransmission because these effects were blocked by GABA receptor antagonists and since increased levels of GABAA receptor subunits were found in hypothalamic neurons following Rhy-containing drug administration (see also Section 2.8) [22,57,58]. Another study in rats with cerebral ischemia has linked the effects of YKS on sleep to a change in the mRNA level of prostaglandin receptors in the prefrontal cortex (PFC) and hypothalamus [59]. However, it appears that multiple cellular pathways impacted by Rhy may drive modifications in sleep. Therefore, this review is assembling findings on potential targets and cellular pathways affected by Rhy that are likely to impact the regulation of sleep. The literature demonstrates that Rhy could affect the activity of ion channels, N-methyl-d-aspartate (NMDA) receptors, receptor tyrosine kinases (RTK), extracellular signal-regulated kinases (ERK)/mitogen-activated protein kinases (MAPK), phosphoinositide 3-kinase (PI3K)/RAC serine/threonine-protein kinase (AKT), and nuclear factor-kappa B (NF-κB). A detailed overview of the effects of Rhy, including different types and durations of administration, is presented in Table 1. In addition, Table 2 lists the literature reporting effects of Rhy on specific sleep-relevant targets/pathways, and Figure 2 depicts a global scheme of the sleep-relevant pathways affected by Rhy and their interrelationships.

Table 1.
Compilation of datasets showing molecular and cellular (and some electrophysiological and behavioral) effects of rhynchophylline (Rhy) organized as a function of treatment type and duration, and by measurement timing.

Table 2.
List of literature showing effects of Rhynchophylline (Rhy) on sleep-related pathways under physiological (baseline) and/or pathological (disease-modeled) conditions.
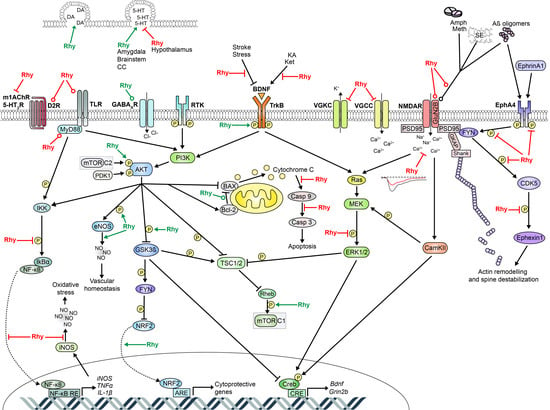
Figure 2.
Schematic representation of cellular pathways targeted by Rhy and relevant to sleep regulation. Red flat-head lines: Rhy inhibition; Green arrows: Rhy induction; Red round-head lines: Rhy-dependent decrease in expression level; Green round-head lines: Rhy-dependent increase in expression level. Additional interactions between these cellular pathways are not represented but could also be relevant to sleep molecular physiology. For instance, L-VGCC can activate ERK/MAPK [144] and are suggested to induce CaMKII, NR2B phosphorylation, and CREB activation [106,145]. NMDARs may also activate the PI3K/AKT pathway [143]. In addition, NF-κB and ERK/MAPK pathways were shown to interact with each other [82,142]. 5-HT: 5-hydroxytryptamine or serotonin; 5-HT2R: serotonin receptor 2; Aβ: amyloid β; Amph: amphetamine; AKT: RAC serine/threonine-protein kinase; ARE: antioxidant response element; BAX: Bcl-2 associated X protein; BDNF: brain-derived neurotrophic factor; CamKII: Ca2+/calmodulin-dependent protein kinase; Casp 3: caspase 3; Casp 9: caspase 9; CC: cerebral cortex; CDK5: cyclin dependent kinase 5; CRE: cAMP response element; CREB: cAMP response element-binding protein; D2R: dopamine D2 receptor; DA: dopamine; eNOS: endothelial nitric oxide synthase; EphA4: Eph receptor A4; ERK1/2: extracellular signal-regulated kinases 1 and 2; FYN: tyrosine-protein kinase Fyn; GABAAR: gamma-aminobutyric acid type A receptor; GKAP: guanylate kinase-associated protein; GluN2B: NMDAR subunit 2B; Grin2b: glutamate ionotropic receptor NMDA type subunit 2B gene; GSK3β: glycogen synthase kinase-3 β; IκBa: NF-kappa-B inhibitor alpha; IKK: IκB kinase; IL: interleukin; iNOS: inducible nitric oxide synthase; KA: kainic acid; Ket: ketamine; m1AchR: m1-type muscarinic acetylcholine receptor; MEF2D: myocyte enhancer factor 2D; MEK: mitogen-activated protein kinase kinase; Meth: methamphetamine; mTOR: mechanistic target of rapamycin; mTORC1: mTOR complex 1; mTORC2: mTOR complex 2; MyD88: myeloid differentiation primary response protein; NF-κB: nuclear factor-kappa B; NMDAR: N-methyl-D-aspartate receptor; NO: nitric oxide; NRF2: nuclear factor E2 related factor 2; PDK1: phosphoinositide-dependent protein kinase-1; PI3K: phosphoinositide 3-kinase; PSD95: postsynaptic density protein 95; RE: response element; Rheb: GTP-binding protein Rheb; Rhy: rhynchophylline; SE: status epilepticus; Shank: SH3 and multiple ankyrin repeat domains protein; TLR: toll-like receptors; TNFα: tumor necrosis factor α; TrkB: tropomyosin or tyrosine receptor kinase B; TSC1/2: tuberous sclerosis complex 1/2; VGCC: voltage-gated calcium channel; VGKC: voltage-gated potassium channel.
2. Rhy Targets and Links to Sleep Regulation
2.1. Ion Channels
2.1.1. Voltage-Gated Calcium Channels
Rhy was first described as a calcium channel blocker in arteries, heart and neuronal cultures from the rat, rabbit, guinea pig, and human [10]. Some studies suggest an inhibitory effect specifically on L-type voltage-gated calcium channels (L-VGCCs; Cav1 family of calcium channels), which are high-voltage activated channels present notably in neurons, retinal photoreceptors, vascular smooth muscle cells, and cardiomyocytes [102]. For example, acute in vitro incubation of rat cortical neurons, rat ventricular myocytes, and rat and human arteries with Rhy was shown to inhibit Ca2+ influx through L-VGCCs [66,67,69,103] (Table 1). In vessels, this Rhy-dependent inhibition of VGCCs and the inhibition of intracellular Ca2+ release were found to block the contractile response and induce vasodilation [69,103,104]. In cortical neurons, it was suggested that Rhy blocks L-VGCCs by decreasing the channel opening time and increasing its closing time under hypoxic conditions [66]. In neurons, L-VGCCs are mainly postsynaptic and contribute to Ca2+ influx, Ca2+ intracellular signaling, neuronal firing, and synaptic plasticity [105,106,107,108]. These roles affect neuronal responsiveness and synchronization, which is relevant to sleep regulation.
L-VGCCs were shown to modulate the synchronization of cortical and hippocampal neuronal oscillations, including in theta frequencies in vitro [109,110], and to affect the excitation/inhibition ratio in cortical slices [111]. In fact, Ca2+ signaling and ion channels including VGCCs are also proposed to be involved in the generation of the up and down states composing the slow oscillations characteristic of the NREM sleep EEG [112,113]. Cav1.2 channels represent more than 80% of L-VGCCs in the mouse brain [114]. Mice heterozygous for Cacna1c (gene encoding a Cav1.2 subunit) have less REM sleep during recovery after sleep deprivation (SD), as well as decreased beta and gamma activity (20–64 Hz) during wakefulness and REM sleep [115]. In addition, Cacna1c genetic variants, which have also been linked to psychiatric disorders, are associated with longer sleep latency in infants [116]. Therefore, although the effect of Rhy on neuronal L-VGCCs seems to have only been studied in vitro, Rhy may impact sleep stages and EEG activity through the blockage of L-VGCC-mediated currents. Moreover, Cav1.2 mRNA is expressed rhythmically in the mouse suprachiasmatic nucleus (SCN), and Cav1.2 KO mice have altered circadian adjustments to light [117]. This suggests that the effect of Rhy on VGCCs may also impact the circadian regulation of wakefulness and sleep.
2.1.2. Potassium Channels
Other ion channels targeted by Rhy which have important roles in CNS functions are voltage-gated potassium channels (VGKC). VGKC, by allowing K+ efflux, regulate neuronal repolarization and the timing of neuronal excitability [118]. Rhy was shown to speed up the inactivation of VGKC in N2A neuroblastoma cells [64] (Table 1). This study has also reported a specific effect on VGKC containing the Kv1.2 subunit expressed in HEK293 cells, in which Rhy accelerated Kv1.2 channels activation and inactivation times [64]. Noteworthy, the Kv1.2 subunit is highly expressed in the thalamocortical system [119,120], and potassium channels Kv1.2, Kv3.1 and Kv3.2 have been shown to regulate sleep [121,122,123,124]. In particular, Kv1.2 knockout (KO) mice spend less time in NREM sleep and more time in wakefulness [122], and Kv1.2 inhibition was reported to decrease NREM sleep and alter the NREM sleep EEG [124]. In Drosophila, mutation of VGKC subunits that are close to the mammalian Kv1.2 channels was also shown to induce a decrease in sleep time [24,125]. These findings suggest that the effect of Rhy on VGKCs may contribute to alterations in sleep features as well. Of note, Rhy also affects calcium-activated potassium channels in the vascular system [10]. This has not been investigated in the CNS but might be of relevance considering that these channels can impact sleep duration [126]. Interestingly, both VGKCs and calcium-activated potassium channels are also suggested to be involved in the generation of up and down states of NREM sleep oscillations [112,113].
2.2. NMDA Receptors
Among the most studied targets of Rhy are glutamate NMDA receptors (NMDARs), which are crucial for neurotransmission and brain plasticity [127]. Rhy was described as a non-competitive NMDAR antagonist due to its blocking effect on NMDAR current in xenopus oocytes [68]. In entorhinal cortex slices of epileptic rats, Rhy was found to cause an immediate attenuation of the potentiated NMDAR-mediated currents, which was associated to a decrease of seizures in vivo [19]. Moreover, Rhy was often shown to decrease the expression of the NMDAR subunit GluN2B, which is predominant in extrasynaptic NMDARs, responds to high spreads of glutamate such as in excitotoxic conditions, and activates apoptotic pathways [128,129]. In rodents, conditions such as pilocarpine-induced status epilepticus, injections of amyloid-beta (Aβ), and administration of amphetamine (amph) or methamphetamine (meth), are increasing GluN2B protein levels, effects that were diminished by Rhy in the medial PFC, entorhinal cortex, and hippocampal CA1 region [19,20,83,93] (Table 1 and Table 2). This modulation of GluN2B by Rhy could depend on an effect at the gene expression level because Rhy was shown to reduce Grin2b mRNA levels in rat hippocampal neurons and also after an amph-induced increase in PFC and CA1 [20,72]. Additionally, the effects of Rhy on NMDAR and GluN2B have been linked to a decrease in the frequency of discharge or population spike amplitude in brain regions including the entorhinal cortex and dentate gyrus (DG) [19,80,83]. Moreover, the Rhy-driven decreases in GluN2B are often observed in parallel with improvements in cognitive functions in rodents, such as spatial memory or drug-conditioned place preference (CPP) [20,83,93]. Similar findings were made in the zebrafish, in which Rhy was found to reduce the meth-induced increase in GluN2B protein level and CPP [88]. In contrast to the aforementioned studies, Rhy was shown to increase GluN2B protein in human mesenchymal cells [78]. Despite the fact that these last findings were from relatively long bath incubations of Rhy (72 h), they are difficult to reconcile with most of the effects reported in vivo in rodents. Also, it is important to keep in mind that only one study has reported an effect of Rhy on NMDARs in baseline conditions, and this was in vitro, which may raise the question whether Rhy can modulate NMDARs under baseline conditions in vivo. Nonetheless, the literature adds up in favor of an effect of Rhy on NMDAR function.
With regard to sleep, glutamatergic signaling and NMDARs have been implicated both in arousal- and sleep-promoting pathways, with very distinct implications depending on the brain region [39]. On the one hand, NMDA or glutamate injected in the rat BF or tuberomammillary nucleus was shown to increase time spent awake [130,131], and injection of glutamate in the PPT induces neocortical desynchronization, wakefulness and REM sleep in the rat and cat [132,133]. Similarly, intraperitoneal (i.p.) injection of the MK-801 NMDAR antagonist was found to cause a delayed increase in NREM sleep time in rats [134,135]. Also, Alzheimer’s disease patients treated with a non-competitive antagonist of NMDARs showed an increase in total sleep time (mainly NREM sleep), and reduced sleep fragmentation [136]. On the other hand, glutamate injection in the rat medial preoptic area (mPOA) or medial septum was shown to promote NREM sleep [137,138], and MK-801 was reported to decrease both NREM and REM sleep in mice [126]. Other data in rats have shown that peripheral administration of NMDAR antagonists induces cortical gamma activity (30–50 Hz) in all vigilance states, while a specific blockade of GluN2B increases it solely in REM sleep [139]. The discrepancies between some of these studies could be explained by differences in the time of administration, time of recording, and/or species. Nonetheless, all support a role for NMDAR-mediated neurotransmission in sleep regulation. Therefore, the ‘generally antagonistic’ effect of Rhy on NMDARs should modulate cortical activity and show vigilance state-specific effects on wake/sleep architecture and EEG activity. Moreover, downstream effectors of NMDARs, including components of the ERK/MAPK and PI3K/AKT pathways, also seem to be altered by Rhy and involved in sleep regulation [82,94,140,141,142,143] (Figure 2, and Section 2.5 and Section 2.6). These interrelationships may reinforce the association between Rhy and NMDARs but could also imply that Rhy affects these pathways in a NMDAR-independent manner.
2.3. EphA4 and Downstream Pathways
Ephrins and their Eph RTKs are cell adhesion molecules widely expressed in neurons, glia, lymphocytes, epithelial cells, fibroblasts, myocytes, and bone cells [146,147,148,149]. In the CNS, they are crucial for axon guidance and plasticity [150]. In particular, Eph receptor A4 (EphA4) has roles in the regulation of α-amino-3-hydroxy-5-methyl-4 -isoxazolepropionic acid (AMPA) receptors, glial glutamate transport, and spine morphology [150,151,152]. In 2014, Fu and collaborators proposed that Rhy inhibits EphA4 activation by direct high-affinity interaction with its extracellular domain [18]. In this study, it was shown that Rhy inhibited both the EphrinA1-induced and Aβ-induced phosphorylation of EphA4 in rat hippocampal neurons, and that oral administration of Rhy inhibited the elevated phosphorylation of EphA4 in the hippocampus of mice mutant for the amyloid precursor protein (APP) and presenilin 1 (PS1) [18]. These observations were associated with a restorative effect of Rhy on long-term potentiation and spine number. A subsequent study also showed that one Rhy i.p. injection reduces p-EphA4 in mice susceptible to stress, specifically in the PFC, hippocampal CA3, and DG, which correlated with an improvement of depressive-like behaviors and spine number [17]. In these same stress-susceptible mice, the phosphorylation of the tyrosine-protein kinase Fyn, cyclin dependent kinase 5 (Cdk5) and ephexin1 was increased, and Rhy attenuated these increments [17]. This could originate from an effect of Rhy directly on EphA4 because the Cdk5/ephexin1 pathway is downstream of EphA4 phosphorylation and linked to actin remodeling and spine destabilization [153] (Figure 2).
Research from our group supports a role for EphA4 in the regulation of sleep [154,155]. Indeed, we found that EphA4 KO mice spend less time in REM sleep and have longer bouts of wakefulness and NREM sleep during the light phase in comparison to wild-type littermates [154]. Also, EphA4 KO mice manifested a blunted 24-h rhythm of NREM sleep sigma (10–13 Hz) activity [154]. In addition, EphA4 KO mice showed a shorter duration of slow waves (0.5–4 Hz) during NREM sleep [155]. These observations suggest that Rhy might modulate sleep through EphA4-dependent pathways, which may alter sleep variables such as REM sleep amount or EEG properties in the sigma or delta frequency ranges. In parallel, EphA4 was shown to be expressed in the mouse and rat SCN, and EphA4 KO mice to have altered circadian responses to light [154,156]. This suggests an implication of EphA4 in the circadian timing system and, as a consequence, a potential effect of Rhy on circadian physiology.
2.4. BDNF/TrkB Signaling
Brain-derived neurotrophic factor (BDNF) is upregulated by neuronal activity and involved in cell survival and neuroplasticity [157,158,159,160,161]. It generally acts on p75 neurotrophin receptor (p75NTR) and tropomyosin or tyrosine receptor kinase B (TrkB) [162], and TrkB can activate other signaling pathways including PI3K and ERK/MAPK [160,163,164,165,166]. In a rat model of epilepsy, kainic acid was found to increase BDNF protein in the cerebral cortex and hippocampus, which was attenuated by Rhy or Uncaria [85]. Similarly, ketamine-addicted rats were shown to have an increased expression of BDNF in the hippocampus, which was diminished by Rhy [21,92]. Rhy was also observed to reduce the levels of extracellular and intracellular BDNF in human bone marrow mesenchymal cells [78]. In contrast, Rhy appears to restore BDNF level when it is decreased in pathological conditions instead of increased, such as in the cortex or hippocampus of a rat stroke model [94] or of chronic/social-defeat stressed mice [17,99]. TrkB phosphorylation was also found to be increased by Rhy in the PFC, hippocampal CA3 and DG regions of stressed mice, and in the striatum of a rat model of Tourette syndrome [17,96]. Therefore, Rhy may downregulate the BDNF pathway under some conditions of neuronal activation such as epilepsy or after ketamine administration, while it may upregulate it in specific pathological conditions such as stroke, stress or Tourette syndrome (Table 1). This could also suggest that Rhy effects on BDNF depend on distinct upstream pathways.
Both BDNF and TrkB signaling have been linked to sleep regulation [167,168,169]. Firstly, BDNF has long been considered a sleep-promoting substance. For example, intracerebroventricular injection of BDNF was found to induce NREM sleep in rats and NREM and REM sleep in rabbits [170]. Studies in humans also report that lower levels of BDNF associate with shorter sleep duration or with decreased amount of deep NREM and REM sleep [171,172]. Interestingly, TrkB KO mice have more REM sleep, reduced REM sleep latency, and shorter bouts of wake and NREM sleep [173]. Secondly, the BDNF/TrkB pathway was found to impact the sleep EEG. Indeed, intracerebroventricular injection of BDNF was shown to reduce NREM sleep slow wave activity (SWA) in rabbits [170], whereas BDNF injection in the rat cortex during wakefulness was shown to increase SWA in the following NREM sleep period, and cortical injection of a BDNF antibody or a TrkB inhibitor to reduce NREM sleep SWA [174]. Moreover, the Val66Met BDNF polymorphism in humans has been linked to decreased NREM sleep delta and theta activity, and REM sleep theta, sigma and alpha activity [175,176]. Carriers of this polymorphism also lost the positive correlation between sleep consolidation and declarative memory [177]. Thirdly, the phosphorylation of BDNF and TrkB responds to SD. Acute SD was shown to enhance BDNF levels and p-TrkB in the rat BF [178], and REM sleep deprivation (RSD) to increase BDNF in the PPT and subcœruleus nucleus, as well as in the ventromedial medulla of the spinal cord in a rat pain model [179,180,181]. SD was also found to increase BDNF levels in patients with major depressive disorder [182], and severe insomnia has been associated to lower BDNF [183]. Lastly, different inhibitors of TrkB were found to decrease REM sleep rebound after RSD [180]. Therefore, the literature suggests that the effects of Rhy on the BDNF/TrkB pathway could impact wakefulness and sleep phenotypes in numerous ways. However, the diverse roles of BDNF also suggest that the modulation by Rhy is likely context dependent.
2.5. ERK/MAPK Pathway
Rhy was shown to influence the phosphorylation (indicative of the activation) of ERK/MAPK. For instance, i.p. injection of Rhy diminished the elevated ERK phosphorylation (p-ERK) in trigeminal nucleus caudalis of rats stimulated with nitroglycerin (a rat migraine model) [82]. P-ERK level was also reported to be decreased by Rhy in rat and mouse microglia [71,76] and by U. rhynchophylla in murine macrophages [184]. In murine peripheral tissues, after several weeks of oral administration, Rhy was found to decrease the level of p-ERK in the lungs [100] and Isorhy to decrease it in the heart [101]. In contrast, others have reported that p-ERK levels were unaltered in the cortex or hippocampus after i.p. Rhy injections [86,87], which might be explained by a smaller dosage (i.e., 0.25 vs. 10–30 mg/kg). ERK and MAPK belong to a signaling cascade downstream of several membrane receptors, including NMDAR, TrkB, and toll-like receptors (TLRs), and can modulate multiple cellular responses via cAMP response element-binding protein (CREB) and activity-regulated genes such as Arc, Dbp, Homer1a, and Bdnf [163,164,165,166,185,186,187,188] (Figure 2). Therefore, the impact of Rhy on the ERK pathway may be linked to effects on both upstream and downstream elements.
CREB is a downstream effector of ERK/MAPK particularly relevant to understand the effects of Rhy. CREB is activated by neuronal activity and acts downstream of numerous other pathways including NMDAR and PI3K/AKT [164,166,187,188] (Figure 2). Rhy was shown to reduce p-CREB positive cells in the striatum and hippocampus in rats with meth and ketamine-dependent p-CREB increase [21,90,92]. Rhy was also found to rescue the meth-induced decrease in the number of c-fos positive cells in the striatum and CA1, which was suggested to depend on CREB [90].
With regard to the neurophysiology of sleep, the ERK pathway was shown to associate with both wake/sleep history and regulation. Indeed, ERK phosphorylation has been reported to increase after 15 min of wakefulness and to decrease after 15 min of NREM sleep in the mouse cerebral cortex [186]. Moreover, RSD was found to decrease p-ERK level in the rat hippocampus [189]. In parallel, the deletion of Erk1 or Erk2 genes, as well as the inhibition of ERK phosphorylation, was found to increase the time spent awake in mice, generally at the expense of NREM sleep [186]. The level of p-ERK was also reported to correlate with sleep time in Drosophila [190]. Interestingly, the inhibition of ERK phosphorylation was shown to increase NREM sleep delta power in mice [186]. In the cat visual cortex, ERK1 phosphorylation was observed to associate with REM sleep beta-gamma activity (20–40 Hz), and has been linked to REM sleep-dependent plasticity [191]. Several datasets are also supporting that sleep is regulated by CREB in both rodents and insects. For instance, mice mutant for CREB α and Δ isoforms show an increase in NREM sleep duration and a decrease in theta activity during wake and REM sleep [192]. Likewise, a specific mutation of CREB in forebrain excitatory neurons was found to reduce time spent awake and increase NREM sleep time and bout number in rats [193]. Moreover, SD was found to increase p-CREB in the rat cerebral cortex [194,195], but RSD decreases it in the rat hippocampus [189]. In flies, SD was found to enhance CREB transcriptional activity, while the inhibition of CREB activity was found to increase rest [196]. In sum, effects of Rhy on both ERK and CREB could impact wake/sleep duration and modulate EEG activity including NREM sleep delta power.
2.6. PI3K/AKT Signaling Network
The signaling by PI3K/AKT represents a major pathway regulating cell survival and growth [197]. Various receptors such as RTK and cytokine receptors directly stimulate PI3K upon ligand binding, which enables site-specific phosphorylation (and activation) of AKT by 3-Phosphoinositide-dependent protein kinase-1 (PDK1) and mechanistic target of rapamycin complex 2 (mTORC2) [198,199]. AKT controls numerous cellular processes such as apoptosis, anabolic metabolism, and angiogenesis notably via the phosphorylation of glycogen synthase kinase-3 (GSK3) and mTORC1 [200,201,202].
Both Rhy and Isorhy seem to activate the PI3K/AKT pathway [73,75,94,98,99] (Table 1). This pathway likely mediates neuroprotective effects of Rhy given that AKT induces anti-apoptotic and pro-survival effects [203,204,205,206]. In a Parkinson’s disease model in which cerebellar neurons are exposed to 1-Methyl-4-phenylpyridinium (MPP+, a potent neurotoxin), pre-treatment with Rhy was shown to decrease neuronal death [75]. This effect was abolished by the addition of a specific PI3K inhibitor, indicating that the effect of Rhy on cell survival is PI3K/AKT-dependent [75]. Also, Rhy and Isorhy were shown to prevent the shift towards apoptosis as measured with the Bax to Bcl-2 ratio [75,79,98]. In similar experimental conditions, U. Rhyncophylla has been shown to favor anti-apoptotic over pro-apoptotic proteins in vitro [207]. Moreover, Rhy, Isorhy and U. Rhyncophylla were all shown to prevent the increase of caspase-3 cleavage in various models of neurotoxicity [70,79,94,98,207,208]. The cleavage of caspase-3, known as an ‘executor of apoptosis’, is often considered the ultimate step in the apoptotic cascade [209].
GSK3, a major downstream effector of AKT [200], is a serine/threonine protein kinase particularly abundant in the CNS [210,211]. In mammals, GSK3 has two paralogs (i.e., homologous proteins derived from different genes), GSK3α and GSK3β [212]. Unlike most enzymes, GSK3 is constitutively active and pathways converging on it tend to decrease its activity by phosphorylation. GSK3 has repeatedly been linked to mood disorders [213,214]. The literature shows that Rhy inhibits GSK3β under pathological conditions, which mainly depends on the activation of PI3K/AKT. Indeed, Rhy was shown to reverse the decrease in GSK3β phosphorylation induced by MPP+ in cerebellar granule neurons, which was found to be PI3K-dependent [75]. Similarly, daily administration of Isorhy to chronically stressed mice or to Aβ-treated rats was reported to revert the decrease in GSK3β and AKT phosphorylation in the hippocampus and/or cerebral cortex [98,99]. Of interest is also that GSK3 is part of a pathway controlling NRF2 (nuclear factor E2 related factor 2) [215], which levels and translocation to the nucleus are enhanced by Rhy in hippocampal neurons of rats subjected to subarachnoid hemorrhage [208]. Isorhy had the same effect on NRF2 [74,101] and was also shown to induce transcription of ARE (antioxydant response element)-dependent genes [74]. The transcription of those genes is activated by NRF2 under oxidative stress conditions [216,217].
Few data are directly linking PI3K/AKT to sleep regulation. AKT was shown to respond to chronic sleep restriction, which decreases its phosphorylation in the hippocampus [218], thereby inhibiting the pathway. On the other hand, downstream targets of PI3K/AKT have been associated to sleep regulation, with in particular GSK3β activity that seems to impact sleep and the response to sleep loss. Firstly, mutant mice with constitutively active GSK3β were shown to have indications of an increased fragmentation of wakefulness and sleep states [219], and GSK3β knockdown in the cerebral cortex modifies the wakefulness and sleep EEG under baseline conditions and after SD in mice (Leduc et al. in preparation). Of note is that a genetic polymorphism decreasing GSK3β activity was found to ameliorate the clinical response to total SD in depressed patients [220,221]. Secondly, sleep-wake history appears to modify GSK3β activity. Chronic sleep restriction over a week was indeed shown to increase GSK3β phosphorylation in the hippocampus [218], and spontaneous wakefulness during the dark period to increase it in the hippocampus [222]. In a recent study, GSK3β activation was shown to occur at the transition to and during sleep and was proposed to act as major regulator of sleep-dependent plasticity [223]. In fact, GSK3β downregulation was found to abolish the SD-driven increase in mEPSCs (miniature excitatory post-synaptic currents) amplitude in the mouse PFC [224], supporting a role in wake/sleep-dependent plasticity. Thirdly, lithium, which is a direct inhibitor of GSK3 (α and β) [214], and the first-line treatment for bipolar disorders [225], was shown to affect sleep quality. For instance, lithium was reported to improve sleep efficiency in bipolar type I patients [226], to increase NREM sleep and decrease REM sleep in healthy volunteers [227], and to reduce REM sleep in mice [228]. The literature thus strongly supports a bidirectional relationship between GSK3 and sleep, which likely represents a key pathway by which Rhy could impact sleep architecture and EEG activity during sleep due to its inhibitory activity on GSK3β.
mTORC1, another serine/threonine kinase downstream of AKT [201,229], is an additional possible target of Rhy potentially underlying a role in wake/sleep regulation. Indeed, Rhy was shown to increase the phosphorylation of mTOR in a rat stroke model [94]. In parallel, sleep-wake history modifies mTORC1 activity, with sleep loss decreasing mTORC1 phosphorylation and thus attenuating mTORC1-dependent protein synthesis in the mouse hippocampus [230]. In addition, we have observed that mice heterozygous for mTOR are showing more SWA during wakefulness and REM sleep, and less theta activity during NREM sleep in comparison to wild-type mice (Areal et al., unpublished). Globally, considering that main downstream effectors of PI3K/AKT shown to be modulated by Rhy have been linked to sleep, these represent pathways by which Rhy could impact wake/sleep phenotypes.
2.7. NF-κB and Neuroinflammation
NF-κB is a transcription factor with implications in multiple cellular processes including neuroinflammation [231]. It can be activated by cytokine receptors and TLRs, which drive its nuclear translocation via the phosphorylation/degradation of NF-κB inhibitors (IkBs) [231]. The administration of Rhy has repeatedly been shown to diminish NF-κB activation in pathological contexts both in vitro [71,76,96,97] and in vivo [82,86,94,96,97] (Table 1). For example, in a rat nitroglycerin-induced migraine model, pre-treatment with Rhy almost completely prevented nuclear translocation of NF-κB in the trigeminal nucleus caudalis [82]. Moreover, it was shown that Rhy could decrease abnormal degradation of IkBα in pathological conditions such as treatments with lipopolysaccharide (LPS), nitroglycerin or 2,5-dimethoxy-4-iodoamphetamine [76,82,84,96,97]. In addition, there is growing literature supporting that Rhy reduces some effects associated with NF-κB activation: (i) the upregulation of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor α (TNFα) [71,76,84,85,96,97] and (ii) the increase in oxidative stress caused, in part, by nitric oxide (NO) [71,76,82,86]. Indeed, the incubation of rat microglial cells with LPS in the presence of Rhy for 24 h diminished the increase in NO, IL-1β and TNFα, and the increase in inducible NO synthase (iNOS) expression [71]. In contrast to its effect on iNOS-dependent NO synthesis, Rhy was shown to enhance endothelial NOS (eNOS)-dependent NO production in renal arteries of constitutively hypertensive rats via PI3K/AKT activation [73]. Thus, Rhy has different effects on NO synthesis depending on the context (here neuroinflammation/oxidative stress vs. vascular tone control). In pathological models such as ischemic brain injury and Tourette syndrome, Rhy was also shown to attenuate the upregulation of TLRs and MyD88 [94,97], the latter being an adaptor protein linking TLR activation to NF-κB nuclear translocation [232]. This led to the suggestion that the anti-inflammatory effects of Rhy in pathological contexts could result from an inhibition/downregulation of the TLR pathway [94,97]. However, a causative link remains to be defined.
The effect of Rhy on NF-κB and related pathways could impact sleep, at least in pathological contexts. Indeed, Rhy reduces the pathological upregulation of IL-1β, TNFα and NO, which are proposed to act as somnogenic substances [233,234]. More precisely, the administration of IL-1β, TNFα and NO (or of their precursors) was shown to increase NREM sleep duration in different mammalian species [235,236,237,238,239,240]. Moreover, the inhibition of these molecules and/or their transcription factor NF-κB was shown to decrease NREM sleep duration, again in multiple mammals [235,236,237,239,241,242,243,244,245,246,247,248,249,250,251]. In addition, SD was shown to upregulate IL-1β, TNFα, NO, and even NF-κB [252,253,254,255], and the inhibition of IL-1β, TNFα and NO can also reduce/block the NREM sleep rebound that is normally caused by sleep loss [242,243,246,248,256]. Finally, the administration of both IL-1β and TNFα was shown to increase slow wave amplitude during NREM sleep [238,239,257], and the inhibition of IL-1β, TNFα and NOS (non-selective NOS inhibition) was shown to reduce NREM sleep SWA [243,247,249]. The reduced NREM sleep SWA was also observed after SD for the inhibition of IL-1β and TNFα [246,248]. Accordingly, Rhy administration could, by inhibiting/downregulating NF-κB and IL-1β, TNFα and NO, reduce NREM sleep amount and SWA in pathological contexts. However, given that Rhy was shown not to impact IL-1β, TNFα, and p-IkBα levels in peripheral tissues (e.g., cardiomyocytes and macrophages) of healthy mice [84] (Table 2), support for a modulatory role of Rhy on sleep via this pathway under normal physiological conditions remains to be collected.
2.8. Neurotransmitters Signaling
Rhy has also been suggested to affect neurotransmitter signaling. For instance, a 3-min incubation with Rhy was shown to inhibit muscarinic acetylcholine receptor 1 (mAChR1) and serotonin receptor 2 (5-HT2)-mediated currents in xenopus oocytes [65]. Also, i.p. injection of Rhy in rats was found to decrease the release of 5-HT in the hypothalamus, and to increase it in the amygdala, cerebral cortex, and brainstem [61]. In this last study, dopamine (DA) release was increased in all these brain regions after Rhy administration [61]. Furthermore, Rhy was reported to rescue the amph-induced decrease of ACh, and the amph- and meth-induced increase in DA [88,91]. Rhy was also shown to attenuate the elevated DA and D2 receptor levels in the striatum of a rat Tourette syndrome model [96]. This provides support for a direct impact of Rhy on neurotransmitters in a manner that depends on the (patho)physiological condition and brain region (Table 1). Importantly, mAChRs and DA receptors are metabotropic receptors, which activity has respectively been linked to Kv1.2 channels and L-VGCCs [258,259] (Figure 2), emphasizing that Rhy could act at multiple levels of neurotransmitter function (see Section 2.1).
Interestingly, ACh, 5-HT and DA are important wake/sleep modulators and components of the ascending arousal system [39]. Cholinergic activation in pontine regions increases cortical activation and REM sleep, and suppresses NREM sleep and SWA [44,260]. In fact, mAChR1 and mAChR3 seem important for REM sleep regulation in both rodent and healthy subjects [261,262]. Furthermore, mAChR1 and other mAChRs modulate thalamocortical and hippocampal oscillations [263,264,265,266,267,268,269]. This suggests that the inhibitory effect of Rhy on mAChR1 (or its modulation of ACh release) may decrease REM sleep and cortical activation and modify EEG activity.
5-HT, mainly originating from the RN, is another contributor to arousal [270], but its effects on wake/sleep regulation and EEG activity are more controversial. Indeed, optogenetic activation of dorsal RN 5-HT neurons was found to induce cortical activation and wakefulness [45,271], whereas the administration of 5-HT or drugs enhancing 5-HT transmission was shown to enhance EEG synchronization and sleep [270]. These opposite roles likely originate from the variety of 5-HT projections, such as to the BF [272], tegmental regions [273], and hypothalamic sleep regulatory neurons [274,275]. Moreover, different 5-HT receptors may be differentially involved [276], given that the activation of 5-HT1A receptors can induce REM and theta activity [277,278,279,280], while that of 5-HT1B, 5-HT2A, 5-HT2A/2C or 5-HT7 is suggested to reduce REM sleep [281,282,283,284,285]. Dopaminergic signaling was also found to be involved in wake/sleep regulation. Briefly, DA cells in the ventral tegmental area (VTA) discharge with different firing patterns during NREM and REM sleep [286], and DA stimulation in the VTA induces behavioral arousal [287]. Overall, more research is required to determine the mechanisms by which Rhy impacts 5-HT and DA neurotransmissions in order to eventually predict the 5-HT- and DA-dependent effects on sleep of Rhy.
Finally, the only literature directly linking Rhy and sleep (see also introduction) suggests that Rhy and Rhy-containing drugs are inducing sleep in rodents via GABAA receptors. In fact, the sleep-promoting effects of the two Uncaria-containing drugs were found to be suppressed by the GABAA receptor inhibitor bicuculline [57,58]. The only study using Rhy has linked the increased sleep time to increased level of GABAA receptor subunits and increased glutamic acid decarboxylase (GAD)65/67 ratio (indicative of increased GABA synthesis at the synapse) in hypothalamic neurons [22]. Many GABAergic neurons regulate the activity of arousal and sleep circuits [39]. The majority of sedatives/hypnotics, such as benzodiazepines, are GABAA receptor agonists and promote ‘light’ (as opposed to ‘deep’) NREM sleep [288,289]. In addition, GABAergic signaling is implicated in cell synchronization during sleep in brain circuits such as the thalamocortical network [30,34]. Therefore, GABAergic signaling is likely a pathway by which Rhy could increase sleep time and should be further investigated in vivo.
3. Conclusions
This review describes how Rhy affects diverse cellular pathways showing a particular relevance to sleep regulation, including VGCC, VGKC, NMDAR, RTK, ERK/MAPK, PI3K/AKT, NF-κB, and neurotransmitter signaling. The literature reveals both acute and delayed/chronic effects of Rhy on these different pathways. This suggests that Rhy may exert rapid effects on wakefulness/sleep quantity and quality, as well as effects that could last for some weeks after exposure. It is worth noting that the effects of Rhy on ion channels have only been characterized under acute conditions. This underlines the need to investigate the delayed and long-term effects of Rhy on ion channels in particular.
Interestingly, almost all studies describing effects of Rhy in vivo have reported effects solely under pathological/disturbed conditions (e.g., stress, treatments with psychostimulants, inflammation, animal models of diseases including stroke, epilepsy, and Alzheimer’s disease), and not in control animals. In fact, apart from effects of Rhy under normal/undisturbed conditions reported in vitro for ion channels, neurotransmitter receptors, NMDAR and BDNF, only two in vivo studies demonstrate effects of Rhy under normal conditions. In the first, Rhy altered DA and 5-HT levels in the rat hippocampus [61], whereas the second showed that Rhy increases total sleep time and REM sleep in rats [22]. Therefore, the literature suggests that Rhy impacts molecular/cellular pathways predominantly under disturbed/diseased conditions. This indicates that Rhy could be particularly beneficial for some pathological conditions involving sleep disturbances. Nevertheless, the physiological effects (assessed under normal conditions) of Rhy on molecular/cellular targets such as ERK/MAPK, NF-κB (and TLR), or D2 receptors should be characterized in the CNS, given that effects have only been described in the context of neurotoxicity, inflammation or epilepsy.
Sex-dependent effects of Rhy also represent an area of need for future research. Indeed, among all studies reviewed in this article, only three have studied females. Two of these used both sexes to show effects of Rhy on EphA4 phosphorylation or neurotransmitter levels [18,61] and did not report sex-dependent effects. The last study used only females and reported that Rhy reduces inflammatory responses and impacts the MAPK/ERK pathway in an asthma model [100], effects that are comparable to those in males reported in other studies [82,101]. Therefore, there is a clear need to investigate whether Rhy has sex-dependent effects. This is particularly relevant with regard to Rhy targets that have been shown to be differentially involved in sleep in the two sexes. For example, genetic variants in CACNA1C were associated with increased sleep latency in male infants but not in females [116].
Another neglected sleep-related research area concerns the potential for effects of Rhy on circadian functions. Many of the pathways presented in this review have been linked to the circadian timing system [290]. For instance, NMDARs (including the GluN2B subunit), TrkB receptors, and D2Rs show circadian rhythms of mRNA or protein levels in specific brain regions [291,292,293,294,295,296]. This strongly suggests that the effects of Rhy on these specific targets will depend on time-of-day and/or internal circadian time. Thus, it appears crucial to consider the effects of Rhy separately, for instance, for the light and dark periods, at least for targets with known circadian regulation. Such investigation would notably help to determine the relevance of Rhy in chronotherapy.
This review has compiled the effects of Rhy with a particular focus on the CNS. However, Rhy impacts, among others, the cardiovascular and immune systems [3,10,84,297] (see also Section 2.1 and Section 2.7). Rhy was indeed shown to have antihypertensive roles via anti-sympathetic and vasodilatory effects that are mainly linked to ion channels [10]. Heart rate and heart rate variability differ between sleep stages [298,299], while systemic inflammation impacts sleep [28]. Thus, future research on Rhy should also consider the interplay between peripheral tissues and sleep.
As indicated in the introduction, Rhy is one of the most abundant alkaloids in Uncaria, which has been highly used in Chinese and Japanese traditional medicine [3,4,10]. The composition of Uncaria and, as a consequence, the components present in traditional treatments such as Chotoko could vary depending on the geographic region and plant growing conditions [300]. This may explain variations in the therapeutic effects of Uncaria, which might be overcome by the use of purified Rhy. Therefore, describing the specific mechanisms of action of Rhy will help defining the medical applications of this chemical. Nevertheless, multiple compounds in Uncaria may have synergistic actions in contributing to health benefits associated with the plant (e.g., chemicals helping the absorption of others [301]). Thus, studies comparing the benefits of Rhy to those of blends of Uncaria will help to identify the best treatment strategies for sleep disturbances and associated pathological conditions.
To conclude, Rhy may impact sleep architecture and oscillations by targeting a diversity of cellular pathways. These effects may specifically underlie the impacts of Chotoko, YKS, and other Uncaria treatments on sleep. Further studies are required to precisely determine the effects of Rhy on sleep as well as on other CNS functions (e.g., memory) under undisturbed/normal conditions. A better understanding of the cellular mechanisms of action of Rhy that are relevant to sleep physiology may eventually help to determine whether this alkaloid could be used in sleep medicine.
Author Contributions
Conceptualization M.N.B.R., T.L., C.C.A. and V.M.; literature search M.N.B.R., T.L. and C.C.A.; visualization C.C.A.; writing-original draft preparation M.N.B.R., T.L. and C.C.A.; writing-review and editing M.N.B.R., T.L., C.C.A. and V.M.; project administration M.N.B.R.; supervision V.M.; funding acquisition M.N.B.R., T.L. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Vanier Canada Graduate Scholarship (M.N.B.R.), a J.A. De Sève fellowship from the Recherche CIUSSS-NIM (T.L.), and the Canada Research Chair in Sleep Molecular Physiology (V.M.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- Singh, A.; Zhao, K. Treatment of insomnia with traditional chinese herbal medicine. Int. Rev. Neurobiol. 2017, 135, 97–115. [Google Scholar] [CrossRef]
- Yang, W.; Ip, S.P.; Liu, L.; Xian, Y.F.; Lin, Z.X. Uncaria rhynchophylla and its major constituents on central nervous system: A review on their pharmacological actions. Curr. Vasc. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Ndagijimana, A.; Wang, X.; Pan, G.; Zhang, F.; Feng, H.; Olaleye, O. A review on indole alkaloids isolated from Uncaria rhynchophylla and their pharmacological studies. Fitoterapia 2013, 86, 35–47. [Google Scholar] [CrossRef]
- Shi, J.S.; Yu, J.X.; Chen, X.P.; Xu, R.X. Pharmacological actions of Uncaria alkaloids, rhynchophylline and isorhynchophylline. Acta Pharmacol. Sin. 2003, 24, 97–101. [Google Scholar]
- Shinno, H.; Inami, Y.; Inagaki, T.; Nakamura, Y.; Horiguchi, J. Effect of Yi-Gan San on psychiatric symptoms and sleep structure at patients with behavioral and psychological symptoms of dementia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Ozone, M.; Yagi, T.; Chiba, S.; Aoki, K.; Kuroda, A.; Mitsui, K.; Itoh, H.; Sasaki, M. Effect of yokukansan on psychophysiological insomnia evaluated using cyclic alternating pattern as an objective marker of sleep instability. Sleep Biol. Rhythm 2012, 10, 157–160. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tajima, K.; Kawagoe, I.; Kanai, M.; Mitsuhata, H. Efficacy of traditional herbal medicine, Yokukansan on patients with neuropathic pain. Masui 2009, 58, 1248–1255. [Google Scholar]
- Yamanaka, E.; Kimizuka, Y.; Aimi, N.; Sakai, S.; Haginiwa, J. Studies of plants containing indole alkaloids. IX. Quantitative analysis of tertiary alkaloids in various parts of Uncaria rhynchophylla MIQ. Yakugaku Zasshi 1983, 103, 1028–1033. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, S. Antihypertensive and neuroprotective activities of rhynchophylline: The role of rhynchophylline in neurotransmission and ion channel activity. J. Ethnopharmacol. 2010, 132, 15–27. [Google Scholar] [CrossRef]
- Laus, G.; Teppner, H. The alkaloids of an Uncaria rhynchophylla (Rubiaceae-Coptosapelteae). Phyton (Horn Austria) 1996, 36, 185–196. [Google Scholar]
- Laus, G.; Brössner, D.; Keplinger, K. Alkaloids of peruvian Uncaria tomentosa. Phytochemistry 1997, 45, 855–860. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, M.; Liu, J.; Huang, Z.; Bai, Y.; Ren, Z.; Wang, Z.; Tian, Y.; Qiao, Z.; Liu, W.; et al. Differences of first-pass effect in the liver and intestine contribute to the stereoselective pharmacokinetics of rhynchophylline and isorhynchophylline epimers in rats. J. Ethnopharmacol. 2017, 209, 175–183. [Google Scholar] [CrossRef]
- Wu, Z.F.; Wang, Y.Q.; Wan, N.; Ke, G.; Yue, P.F.; Chen, H.; Zhan, J.J.; Yang, M. Structural stabilities and transformation mechanism of Rhynchophylline and Isorhynchophylline by ultra performance liquid chromatography/time-of-flight mass spectrometry (UPLC/Q-TOF-MS). Molecules 2015, 20, 14849–14859. [Google Scholar] [CrossRef]
- Lee, C.J.; Hsueh, T.Y.; Lin, L.C.; Tsai, T.H. Determination of protein-unbound rhynchophylline brain distribution by microdialysis and ultra-performance liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2014, 28, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Yang, Y.F.; Xu, W.; Yang, X.W. The blood-brain barrier permeability of six indole alkaloids from Uncariae Ramulus cum Uncis in the MDCK-pHaMDR cell monolayer model. Molecules 2017, 22, 1944. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Qu, Y.; Nakamura, M.; Dong, C.; Yang, C.; Ren, Q.; Ma, M.; Han, M.; Shirayama, Y.; et al. Increased EphA4-ephexin1 signaling in the medial prefrontal cortex plays a role in depression-like phenotype. Sci. Rep. 2017, 7, 7133. [Google Scholar] [CrossRef]
- Fu, A.K.; Hung, K.W.; Huang, H.; Gu, S.; Shen, Y.; Cheng, E.Y.; Ip, F.C.; Huang, X.; Fu, W.Y.; Ip, N.Y. Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer's disease. Proc. Natl. Acad. Sci. USA 2014, 111, 9959–9964. [Google Scholar] [CrossRef]
- Shao, H.; Yang, Y.; Mi, Z.; Zhu, G.X.; Qi, A.P.; Ji, W.G.; Zhu, Z.R. Anticonvulsant effect of Rhynchophylline involved in the inhibition of persistent sodium current and NMDA receptor current in the pilocarpine rat model of temporal lobe epilepsy. Neuroscience 2016, 337, 355–369. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Mo, Z.X.; Zhou, S.W. Rhynchophylline down-regulates NR2B expression in cortex and hippocampal CA1 area of amphetamine-induced conditioned place preference rat. Arch. Pharm. Res. 2010, 33, 557–565. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, C.; Tu, G.; Li, C.; Liu, Y.; Liu, W.; Lam Yung, K.K.; Mo, Z. Rhynchophylline downregulates phosphorylated cAMP response element binding protein, nuclear receptor-related-1, and brain-derived neurotrophic factor expression in the hippocampus of ketamine-induced conditioned place preference rats. Pharmacogn. Mag. 2018, 14, 81–86. [Google Scholar] [CrossRef]
- Yoo, J.H.; Ha, T.W.; Hong, J.T.; Oh, K.W. Rhynchophylline, one of major constituents of Uncariae Ramulus et Uncus enhances pentobarbital-induced sleep behaviors and Rapid Eye Movement Sleep in rodents. Nat. Prod. Sci. 2016, 22, 263–269. [Google Scholar] [CrossRef]
- Frank, M.G.; Heller, H.C. The function(s) of sleep. Handb. Exp. Pharmacol. 2019, 253, 3–34. [Google Scholar] [CrossRef] [PubMed]
- Kempf, A.; Song, S.M.; Talbot, C.B.; Miesenbock, G. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature 2019, 568, 230–234. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Walton, M.E.; Peirson, S.N.; Bannerman, D.M. Sleep homeostasis, habits and habituation. Curr. Opin. Neurobiol. 2017, 44, 202–211. [Google Scholar] [CrossRef]
- Boyce, R.; Williams, S.; Adamantidis, A. REM sleep and memory. Curr. Opin. Neurobiol. 2017, 44, 167–177. [Google Scholar] [CrossRef]
- Timofeev, I.; Chauvette, S. Sleep slow oscillation and plasticity. Curr. Opin. Neurobiol. 2017, 44, 116–126. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Abel, T.; Havekes, R.; Saletin, J.M.; Walker, M.P. Sleep, plasticity and memory from molecules to whole-brain networks. Curr. Biol. 2013, 23, R774–R788. [Google Scholar] [CrossRef]
- Headley, D.B.; Pare, D. Common oscillatory mechanisms across multiple memory systems. NPJ Sci. Learn. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.; Dijk, D.J.; Tobler, I.; Borbely, A.A. Sleep deprivation in rats: Effects on EEG power spectra, vigilance states, and cortical temperature. Am. J. Physiol. 1991, 261, R198–R208. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.; Gent, T.C.; Hoekstra, M.M.B.; Emmenegger, Y.; Mongrain, V.; Landolt, H.P.; Adamantidis, A.R.; Franken, P. Rapid fast-delta decay following prolonged wakefulness marks a phase of wake-inertia in NREM sleep. Nat. Commun. 2020, 11, 3130. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.M.; Sirota, A.; Buzsaki, G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J. Neurosci. 2008, 28, 6731–6741. [Google Scholar] [CrossRef]
- Steriade, M.; McCormick, D.A.; Sejnowski, T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science 1993, 262, 679–685. [Google Scholar] [CrossRef]
- Steriade, M.; Timofeev, I.; Grenier, F. Natural waking and sleep states: A view from inside neocortical neurons. J. Neurophysiol. 2001, 85, 1969–1985. [Google Scholar] [CrossRef]
- Borbely, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [PubMed]
- Daan, S.; Beersma, D.G.; Borbely, A.A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol. 1984, 246, R161–R183. [Google Scholar] [CrossRef] [PubMed]
- Dijk, D.J.; Czeisler, C.A. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J. Neurosci. 1995, 15, 3526–3538. [Google Scholar] [CrossRef]
- Jones, B.E. Arousal and sleep circuits. Neuropsychopharmacology 2020, 45, 6–20. [Google Scholar] [CrossRef]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef]
- Adamantidis, A.R.; Zhang, F.; Aravanis, A.M.; Deisseroth, K.; de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 2007, 450, 420–424. [Google Scholar] [CrossRef]
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; de Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef]
- Han, Y.; Shi, Y.F.; Xi, W.; Zhou, R.; Tan, Z.B.; Wang, H.; Li, X.M.; Chen, Z.; Feng, G.; Luo, M.; et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr. Biol. 2014, 24, 693–698. [Google Scholar] [CrossRef]
- Kroeger, D.; Ferrari, L.L.; Petit, G.; Mahoney, C.E.; Fuller, P.M.; Arrigoni, E.; Scammell, T.E. Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J. Neurosci. 2017, 37, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.R.; Leibold, N.K.; Rappoport, D.A.; Ginapp, C.M.; Purnell, B.S.; Bode, N.M.; Alberico, S.L.; Kim, Y.C.; Audero, E.; Gross, C.T.; et al. Dorsal raphe serotonin neurons mediate CO2-induced arousal from sleep. J. Neurosci. 2018, 38, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Boucetta, S.; Cisse, Y.; Mainville, L.; Morales, M.; Jones, B.E. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. 2014, 34, 4708–4727. [Google Scholar] [CrossRef]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.T.; Hormann, N.; Chang, W.C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef]
- Van Dort, C.J.; Zachs, D.P.; Kenny, J.D.; Zheng, S.; Goldblum, R.R.; Gelwan, N.A.; Ramos, D.M.; Nolan, M.A.; Wang, K.; Weng, F.J.; et al. Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc. Natl. Acad. Sci. USA 2015, 112, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P.H.; Billwiller, F.; Fort, P. Selective activation of a few limbic structures during paradoxical (REM) sleep by the claustrum and the supramammillary nucleus: Evidence and function. Curr. Opin. Neurobiol. 2017, 44, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Shinno, H.; Kamei, M.; Nakamura, Y.; Inami, Y.; Horiguchi, J. Successful treatment with Yi-Gan San for rapid eye movement sleep behavior disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sasai-Sakuma, T.; Ishigooka, J.; Nishimura, K.; Inoue, Y. Effect of Yokukansan for the treatment of idiopathic rapid eye movement sleep behavior disorder: A retrospective analysis of consecutive patients. J. Clin. Sleep Med. 2019, 15, 1173–1178. [Google Scholar] [CrossRef]
- Ozone, M.; Shimazaki, H.; Ichikawa, H.; Shigeta, M. Efficacy of yokukansan compared with clonazepam for rapid eye movement sleep behaviour disorder: A preliminary retrospective study. Psychogeriatrics 2020. [Google Scholar] [CrossRef] [PubMed]
- Ohtomo, Y.; Umino, D.; Nijama, S.; Fujinaga, S.; Shimizu, T. Yokukansan: A treatment option for nocturnal enuresis in children by improving sleep quality. Juntendo Med. J. 2014, 60, 536–542. [Google Scholar] [CrossRef]
- Aizawa, R.; Kanbayashi, T.; Saito, Y.; Ogawa, Y.; Sugiyama, T.; Kitajima, T.; Kaneko, Y.; Abe, M.; Shimizu, T. Effects of Yoku-kan-san-ka-chimpi-hange on the sleep of normal healthy adult subjects. Psychiatry Clin. Neurosci. 2002, 56, 303–304. [Google Scholar] [CrossRef]
- Pan, W.; Kwak, S.; Li, G.; Chen, Y.; Cai, D. Therapeutic effect of Yang-Xue-Qing-Nao granules on sleep dysfunction in Parkinson’s disease. Chin. Med. 2013, 8, 14. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Liu, R. Therapeutic evaluation on needling method of regulating the conception vessel and calming the mind for perimenopausal sleep disorder. J. Acupunct. Tuina Sci. 2013, 11, 142–146. [Google Scholar] [CrossRef]
- Egashira, N.; Nogami, A.; Iwasaki, K.; Ishibashi, A.; Uchida, N.; Takasaki, K.; Mishima, K.; Nishimura, R.; Oishi, R.; Fujiwara, M. Yokukansan enhances pentobarbital-induced sleep in socially isolated mice: Possible involvement of GABA(A)-benzodiazepine receptor complex. J. Pharmacol. Sci. 2011, 116, 316–320. [Google Scholar] [CrossRef]
- Murata, K.; Li, F.; Shinguchi, K.; Ogata, M.; Fujita, N.; Takahashi, R. Yokukansankachimpihange improves the social isolation-induced sleep disruption and allopregnanolone reduction in mice. Front. Nutr. 2020, 7, 8. [Google Scholar] [CrossRef]
- Nagao, M.; Takasaki, K.; Nogami, A.; Hirai, Y.; Moriyama, H.; Uchida, N.; Kubota, K.; Katsurabayashi, S.; Mishima, K.; Nishimura, R.; et al. Effect of Yokukansan on sleep disturbance in a rat model of cerebrovascular dementia. Tradit. Kampo Med. 2014, 1, 19–26. [Google Scholar] [CrossRef]
- Jeenapongsa, R.; Tohda, M. Effects of Choto-san and Chotoko on thiopental-induced sleeping time. J. Tradit. Med. 2003, 20, 165–167. [Google Scholar]
- Shi, J.S.; Huang, B.; Wu, Q.; Ren, R.X.; Xie, X.L. Effects of rhynchophylline on motor activity of mice and serotonin and dopamine in rat brain. Zhongguo Yao Li Xue Bao 1993, 14, 114–117. [Google Scholar]
- Sakakibara, I.; Terabayashi, S.; Kubo, M.; Higuchi, M.; Komatsu, Y.; Okada, M.; Taki, K.; Kamei, J. Effect on locomotion of indole alkaloids from the hooks of uncaria plants. Phytomedicine 1999, 6, 163–168. [Google Scholar] [CrossRef]
- Quílez, A.; Saenz, M.T.; García, M.D. Uncaria tomentosa (Willd. ex. Roem. & Schult.) DC. and Eucalyptus globulus Labill. interactions when administered with diazepam. Phytother. Res. 2012, 26, 458–461. [Google Scholar]
- Chou, C.H.; Gong, C.L.; Chao, C.C.; Lin, C.H.; Kwan, C.Y.; Hsieh, C.L.; Leung, Y.M. Rhynchophylline from Uncaria rhynchophylla functionally turns delayed rectifiers into A-Type K+ channels. J. Nat. Prod. 2009, 72, 830–834. [Google Scholar] [CrossRef]
- Kang, T.H.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H.; Matsumoto, K. Protective effect of rhynchophylline and isorhynchophylline on in vitro ischemia-induced neuronal damage in the hippocampus: Putative neurotransmitter receptors involved in their action. Life Sci. 2004, 76, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Wang, Z.F.; Xue, C.H. Effects of Rhynchophylline on L-type calcium channels in isolated rat cortical neurons during acute hypoxia. J. Chin. Pharm. Sci. 1998, 7, 205–208. [Google Scholar]
- Wang, X.L.; Zhang, L.M.; Hua, Z. Blocking effect of rhynchophylline on calcium channels in isolated rat ventricular myocytes. Zhongguo Yao Li Xue Bao 1994, 15, 115–118. [Google Scholar] [PubMed]
- Kang, T.H.; Murakami, Y.; Matsumoto, K.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Rhynchophylline and isorhynchophylline inhibit NMDA receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 2002, 455, 27–34. [Google Scholar] [CrossRef]
- Li, P.Y.; Zeng, X.R.; Cheng, J.; Wen, J.; Inoue, I.; Yang, Y. Rhynchophylline-induced vasodilation in human mesenteric artery is mainly due to blockage of L-type calcium channels in vascular smooth muscle cells. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.J.; Cui, L.Q.; Li, P.; Wang, Y.B.; Zhang, X.Z.; Guo, M.L. Rhynchophylline ameliorates myocardial ischemia/reperfusion injury through the modulation of mitochondrial mechanisms to mediate myocardial apoptosis. Mol. Med. Rep. 2019, 19, 2581–2590. [Google Scholar] [CrossRef]
- Song, Y.; Qu, R.; Zhu, S.; Zhang, R.; Ma, S. Rhynchophylline attenuates LPS-induced pro-inflammatory responses through down-regulation of MAPK/NF-kappaB signaling pathways in primary microglia. Phytother. Res. 2012, 26, 1528–1533. [Google Scholar] [CrossRef]
- He, Y.; Zeng, S.Y.; Zhou, S.W.; Qian, G.S.; Peng, K.; Mo, Z.X.; Zhou, J.Y. Effects of rhynchophylline on GluN1 and GluN2B expressions in primary cultured hippocampal neurons. Fitoterapia 2014, 98, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.F.; Liu, L.M.; Pan, C.S.; Wang, C.S.; Gao, Y.S.; Fan, J.Y.; Han, J.Y. Rhynchophylline ameliorates endothelial dysfunction via Src-PI3K/Akt-eNOS cascade in the cultured intrarenal arteries of spontaneous hypertensive rats. Front. Physiol. 2017, 8, 928. [Google Scholar] [CrossRef]
- Li, Q.; Niu, C.; Zhang, X.; Dong, M. Gastrodin and Isorhynchophylline synergistically inhibit MPP(+)-induced oxidative stress in SH-SY5Y cells by targeting ERK1/2 and GSK-3beta pathways: Involvement of Nrf2 nuclear translocation. ACS Chem. Neurosci. 2018, 9, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Mak, S.; Zuo, X.; Li, H.; Wang, Y.; Han, Y. Neuroprotection against MPP(+)-induced cytotoxicity through the activation of PI3-K/Akt/GSK3beta/MEF2D signaling pathway by Rhynchophylline, the major tetracyclic oxindole alkaloid isolated from Uncaria rhynchophylla. Front. Pharmacol. 2018, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Ma, B.; Yang, J.Y.; Xie, Y.Y.; Wang, L.; Zhang, L.J.; Kano, Y.; Wu, C.F. Anti-inflammatory effects of rhynchophylline and isorhynchophylline in mouse N9 microglial cells and the molecular mechanism. Int. Immunopharmacol. 2009, 9, 1549–1554. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Chen, J.; Zhou, S.W.; Mo, Z.X. Individual and combined effects of rhynchophylline and ketamine on proliferation, NMDAR1 and GluA2/3 protein expression in PC12 cells. Fitoterapia 2013, 85, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Coats, A.B.; Tuazon, J.P.; Jo, M.; Borlongan, C.V. Rhynchophylline promotes stem cell autonomous metabolic homeostasis. Cytotherapy 2020, 22, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Chen, M.; Wang, W.; Zhou, M.; Liu, C.; Fan, Y.; Shi, D. Protection by rhynchophylline against MPTP/MPP(+)-induced neurotoxicity via regulating PI3K/Akt pathway. J. Ethnopharmacol. 2021, 268, 113568. [Google Scholar] [CrossRef]
- Shao, H.; Mi, Z.; Ji, W.G.; Zhang, C.H.; Zhang, T.; Ren, S.C.; Zhu, Z.R. Rhynchophylline protects against the amyloid beta-induced increase of spontaneous discharges in the hippocampal CA1 region of rats. Neurochem. Res. 2015, 40, 2365–2373. [Google Scholar] [CrossRef]
- Lu, Y.F.; Xie, X.L.; Wu, Q.; Wen, G.R.; Yang, S.F.; Shi, J.S. Effects of rhynchophylline on monoamine transmitter contents of striatum and hippocampus in cerebral ischemic rats. Chin. J. Pharmacol. Toxicol. 2004, 18, 253–258. [Google Scholar]
- Lai, T.; Chen, L.; Chen, X.; He, J.; Lv, P.; Ge, H. Rhynchophylline attenuates migraine in trigeminal nucleus caudalis in nitroglycerin-induced rat model by inhibiting MAPK/NF-kB signaling. Mol. Cell. Biochem. 2019, 461, 205–212. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, W.G.; Zhu, Z.R.; Wu, Y.L.; Zhang, Z.Y.; Qu, S.C. Rhynchophylline suppresses soluble Abeta1-42-induced impairment of spatial cognition function via inhibiting excessive activation of extrasynaptic NR2B-containing NMDA receptors. Neuropharmacology 2018, 135, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, Y.; Lv, X.; Yu, X.; Li, X.; Li, H.; Wang, Y.; Lu, D.; Qi, R.; Wang, H. Rhynchophylline prevents cardiac dysfunction and improves survival in lipopolysaccharide-challenged mice via suppressing macrophage I-kappaBalpha phosphorylation. Int. Immunopharmacol. 2012, 14, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.Y.; Tang, N.Y.; Hsiang, C.Y.; Hsieh, C.L. Uncaria rhynchophylla and rhynchophylline improved kainic acid-induced epileptic seizures via IL-1beta and brain-derived neurotrophic factor. Phytomedicine 2014, 21, 893–900. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Ho, T.Y.; Su, S.Y.; Lo, W.Y.; Liu, C.H.; Tang, N.Y. Uncaria rhynchophylla and Rhynchophylline inhibit c-Jun N-terminal kinase phosphorylation and nuclear factor-kappaB activity in kainic acid-treated rats. Am. J. Chin. Med. 2009, 37, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Tang, N.Y.; Liu, C.H.; Hsieh, C.L. Antiepileptic effect of Uncaria rhynchophylla and Rhynchophylline involved in the initiation of c-Jun N-terminal kinase phosphorylation of MAPK signal pathways in acute seizures of kainic acid-treated rats. Evid. Based Complement. Altern. Med. 2013, 2013, 961289. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, Y.; Li, C.; Peng, Q.; Fang, M.; Liu, W.; Kang, Q.; Lin, Y.; Yung, K.K.; Mo, Z. Inhibiting effects of rhynchophylline on zebrafish methamphetamine dependence are associated with amelioration of neurotransmitters content and down-regulation of TH and NR2B expression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 68, 31–43. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, W.; Li, J.; Chen, Z.J.; Li, C.; Zhou, Y.T.; Mo, Z.X. Rhynchophylline reverses methamphetamine-induced CPP by regulating GluR1 expression in zebrafish. Chin. Pharmacol. Bull. 2019, 35, 620–623. [Google Scholar]
- Liu, W.; Peng, Q.X.; Lin, X.L.; Luo, C.H.; Jiang, M.J.; Mo, Z.X.; Yung, K.K. Effect of rhynchophylline on the expression of p-CREB and sc-Fos in striatum and hippocampal CA1 area of methamphetamine-induced conditioned place preference rats. Fitoterapia 2014, 92, 16–22. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Mo, Z.X.; Zhou, S.W. Effect of rhynchophylline on central neurotransmitter levels in amphetamine-induced conditioned place preference rat brain. Fitoterapia 2010, 81, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tu, G.; Luo, C.; Guo, Y.; Fang, M.; Zhu, C.; Li, H.; Ou, J.; Zhou, Y.; Liu, W.; et al. Effects of rhynchophylline on the hippocampal miRNA expression profile in ketamine-addicted rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 379–389. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Peng, Q.; Jiang, M.; Luo, C.; Guo, Y.; Liu, Y.; Fang, M.; Mo, Z. Effect of rhynchophylline on conditioned place preference on expression of NR2B in methamphetamine-dependent mice. Biochem. Biophys Res. Commun. 2014, 452, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhong, R.; Xia, Z.; Song, J.; Feng, L. Neuroprotective effects of rhynchophylline against ischemic brain injury via regulation of the Akt/mTOR and TLRs signaling pathways. Molecules 2014, 19, 11196–11210. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Hsieh, C.L. Oral Uncaria rhynchophylla (UR) reduces kainic acid-induced epileptic seizures and neuronal death accompanied by attenuating glial cell proliferation and S100B proteins in rats. J. Ethnopharmacol. 2011, 135, 313–320. [Google Scholar] [CrossRef]
- Long, H.; Ruan, J.; Zhang, M.; Wang, C.; Huang, Y. Rhynchophylline attenuates Tourette Syndrome via BDNF/NF-kappaB pathway in vivo and in vitro. Neurotox Res. 2019, 36, 756–763. [Google Scholar] [CrossRef]
- Long, H.; Zhang, M.; Wang, C.; Hang, Y. Rhynchophylline attenuates neurotoxicity in Tourette Syndrome rats. Neurotox. Res. 2019, 36, 679–687. [Google Scholar] [CrossRef]
- Xian, Y.F.; Mao, Q.Q.; Wu, J.C.; Su, Z.R.; Chen, J.N.; Lai, X.P.; Ip, S.P.; Lin, Z.X. Isorhynchophylline treatment improves the amyloid-beta-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J. Alzheimers Dis. 2014, 39, 331–346. [Google Scholar] [CrossRef]
- Xian, Y.F.; Ip, S.P.; Li, H.Q.; Qu, C.; Su, Z.R.; Chen, J.N.; Lin, Z.X. Isorhynchophylline exerts antidepressant-like effects in mice via modulating neuroinflammation and neurotrophins: Involvement of the PI3K/Akt/GSK-3beta signaling pathway. FASEB J. 2019, 33, 10393–10408. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Zhao, Y.; Lv, C.; Zhou, G. Rhynchophylline attenuates allergic bronchial asthma by inhibiting transforming growth factor-beta1-mediated Smad and mitogen-activated protein kinase signaling transductions in vivo and in vitro. Exp. Ther. Med. 2019, 17, 251–259. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Dai, S.; Deng, W.; Wang, H.; Qin, W.; Yang, H.; Liu, H.; Yue, J.; Wu, D.; et al. Isorhynchophylline enhances Nrf2 and inhibits MAPK pathway in cardiac hypertrophy. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 203–212. [Google Scholar] [CrossRef]
- Lipscombe, D.; Helton, T.D.; Xu, W. L-type calcium channels: The low down. J. Neurophysiol. 2004, 92, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Chen, C.X.; Sim, S.M.; Kwan, C.Y. In vitro vasodilator mechanisms of the indole alkaloids rhynchophylline and isorhynchophylline, isolated from the hook of Uncaria rhynchophylla (Miquel). Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 232–238. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, G.X.; Huang, X.N. Effect of rhynchophylline on the contraction of rabbit aorta. Zhongguo Yao Li Xue Bao 1987, 8, 425–429. [Google Scholar] [PubMed]
- Wiera, G.; Nowak, D.; van Hove, I.; Dziegiel, P.; Moons, L.; Mozrzymas, J.W. Mechanisms of NMDA receptor- and voltage-gated L-Type calcium channel-dependent hippocampal LTP critically rely on proteolysis that is mediated by distinct metalloproteinases. J. Neurosci. 2017, 37, 1240–1256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; John, M.; Madhavan, M.; James, J.; Omkumar, R.V. Alteration in the phosphorylation status of NMDA receptor GluN2B subunit by activation of both NMDA receptor and L-type voltage gated calcium channel. Neurosci. Lett. 2019, 709, 134343. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat. Rev. Drug Discov. 2016, 15, 19–34. [Google Scholar] [CrossRef]
- Lacinova, L.; Moosmang, S.; Langwieser, N.; Hofmann, F.; Kleppisch, T. Cav1.2 calcium channels modulate the spiking pattern of hippocampal pyramidal cells. Life Sci. 2008, 82, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Nedergaard, S.; Andreasen, M. Intrinsic Ca2+-dependent theta oscillations in apical dendrites of hippocampal CA1 pyramidal cells in vitro. J. Neurophysiol. 2014, 112, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Plumbly, W.; Brandon, N.; Deeb, T.Z.; Hall, J.; Harwood, A.J. L-type voltage-gated calcium channel regulation of in vitro human cortical neuronal networks. Sci. Rep. 2019, 9, 13810. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Z.D.; Che, A.; Fischer, D.K.; Rice, R.C.; Rizzo, B.K.; Byrne, M.; Glass, M.J.; De Marco Garcia, N.V.; Rajadhyaksha, A.M. Rescue of impaired sociability and anxiety-like behavior in adult cacna1c-deficient mice by pharmacologically targeting eIF2alpha. Mol. Psychiatry 2017, 22, 1096–1109. [Google Scholar] [CrossRef]
- Ode, K.L.; Katsumata, T.; Tone, D.; Ueda, H.R. Fast and slow Ca(2+)-dependent hyperpolarization mechanisms connect membrane potential and sleep homeostasis. Curr. Opin. Neurobiol. 2017, 44, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Bazhenov, M.; Timofeev, I.; Steriade, M.; Sejnowski, T.J. Model of thalamocortical slow-wave sleep oscillations and transitions to activated States. J. Neurosci. 2002, 22, 8691–8704. [Google Scholar] [CrossRef] [PubMed]
- Sinnegger-Brauns, M.J.; Huber, I.G.; Koschak, A.; Wild, C.; Obermair, G.J.; Einzinger, U.; Hoda, J.C.; Sartori, S.B.; Striessnig, J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol. 2009, 75, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dedic, N.; Flachskamm, C.; Voule, S.; Deussing, J.M.; Kimura, M. Cacna1c (Cav1.2) modulates electroencephalographic rhythm and rapid eye movement sleep recovery. Sleep 2015, 38, 1371–1380. [Google Scholar] [CrossRef]
- Kantojarvi, K.; Liuhanen, J.; Saarenpaa-Heikkila, O.; Satomaa, A.L.; Kylliainen, A.; Polkki, P.; Jaatela, J.; Toivola, A.; Milani, L.; Himanen, S.L.; et al. Variants in calcium voltage-gated channel subunit Alpha1 C-gene (CACNA1C) are associated with sleep latency in infants. PLoS ONE 2017, 12, e0180652. [Google Scholar] [CrossRef]
- Schmutz, I.; Chavan, R.; Ripperger, J.A.; Maywood, E.S.; Langwieser, N.; Jurik, A.; Stauffer, A.; Delorme, J.E.; Moosmang, S.; Hastings, M.H.; et al. A specific role for the REV-ERBalpha-controlled L-Type Voltage-Gated Calcium Channel CaV1.2 in resetting the circadian clock in the late night. J. Biol. Rhythm 2014, 29, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Manis, P.B. Delayed rectifier and A-Type potassium channels. In Encyclopedia of Computational Neuroscience; Jaeger, D., Jung, R., Eds.; Springer: New York, NY, USA, 2015; pp. 971–985. [Google Scholar]
- Sheng, M.; Tsaur, M.L.; Jan, Y.N.; Jan, L.Y. Contrasting subcellular localization of the Kv1.2 K+ channel subunit in different neurons of rat brain. J. Neurosci. 1994, 14, 2408–2417. [Google Scholar] [CrossRef]
- Tsaur, M.L.; Sheng, M.; Lowenstein, D.H.; Jan, Y.N.; Jan, L.Y. Differential expression of K+ channel mRNAs in the rat brain and down-regulation in the hippocampus following seizures. Neuron 1992, 8, 1055–1067. [Google Scholar] [CrossRef]
- Espinosa, F.; Marks, G.; Heintz, N.; Joho, R.H. Increased motor drive and sleep loss in mice lacking Kv3-type potassium channels. Genes Brain Behav. 2004, 3, 90–100. [Google Scholar] [CrossRef]
- Douglas, C.L.; Vyazovskiy, V.; Southard, T.; Chiu, S.Y.; Messing, A.; Tononi, G.; Cirelli, C. Sleep in Kcna2 knockout mice. BMC Biol. 2007, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Deboer, T.; Rudy, B.; Lau, D.; Borbely, A.A.; Tobler, I. Sleep EEG in mice that are deficient in the potassium channel subunit K.v.3.2. Brain Res. 2002, 947, 204–211. [Google Scholar] [CrossRef]
- Douglas, C.L.; Vyazovskiy, V.; Southard, T.; Faraguna, U.; Cirelli, C.; Tononi, G. Voltage-dependent potassium channel Kv1.2: Effects on sleep and EEG power spectrum of intracortical injections of an anti-Kv1.2 antibody. Sleep 2006, 29, A36. [Google Scholar]
- Bushey, D.; Huber, R.; Tononi, G.; Cirelli, C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J. Neurosci. 2007, 27, 5384–5393. [Google Scholar] [CrossRef]
- Tatsuki, F.; Sunagawa, G.A.; Shi, S.; Susaki, E.A.; Yukinaga, H.; Perrin, D.; Sumiyama, K.; Ukai-Tadenuma, M.; Fujishima, H.; Ohno, R.; et al. Involvement of Ca(2+)-dependent hyperpolarization in sleep duration in mammals. Neuron 2016, 90, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Malinow, R.; Malenka, R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002, 25, 103–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wong, T.P.; Aarts, M.; Rooyakkers, A.; Liu, L.; Lai, T.W.; Wu, D.C.; Lu, J.; Tymianski, M.; Craig, A.M.; et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. 2007, 27, 2846–2857. [Google Scholar] [CrossRef]
- Xu, J.; Kurup, P.; Zhang, Y.; Goebel-Goody, S.M.; Wu, P.H.; Hawasli, A.H.; Baum, M.L.; Bibb, J.A.; Lombroso, P.J. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J. Neurosci. 2009, 29, 9330–9343. [Google Scholar] [CrossRef]
- Yin, D.; Dong, H.; Wang, T.X.; Hu, Z.Z.; Cheng, N.N.; Qu, W.M.; Huang, Z.L. Glutamate activates the histaminergic tuberomammillary nucleus and increases wakefulness in rats. Neuroscience 2019, 413, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Manfridi, A.; Brambilla, D.; Mancia, M. Stimulation of NMDA and AMPA receptors in the rat nucleus basalis of Meynert affects sleep. Am. J. Physiol. 1999, 277, R1488–R1492. [Google Scholar] [CrossRef]
- Datta, S.; Siwek, D.F. Excitation of the brain stem pedunculopontine tegmentum cholinergic cells induces wakefulness and REM sleep. J. Neurophysiol. 1997, 77, 2975–2988. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Spoley, E.E.; Patterson, E.H. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R752–R759. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.G.; Feinberg, I. NREM delta stimulation following MK-801 is a response of sleep systems. J. Neurophysiol. 1996, 76, 3714–3720. [Google Scholar] [CrossRef]
- Campbell, I.G.; Feinberg, I. Comparison of MK-801 and sleep deprivation effects on NREM, REM, and waking spectra in the rat. Sleep 1999, 22, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, I.; Shinno, H.; Ando, N.; Mori, T.; Nakamura, Y. The effect of memantine on sleep architecture and psychiatric symptoms in patients with Alzheimer’s disease. Acta Neuropsychiatr. 2016, 28, 157–164. [Google Scholar] [CrossRef]
- Kaushik, M.K.; Kumar, V.M.; Mallick, H.N. Glutamate microinjection at the medial preoptic area enhances slow wave sleep in rats. Behav. Brain Res. 2011, 217, 240–243. [Google Scholar] [CrossRef]
- Mukherjee, D.; Kaushik, M.K.; Jaryal, A.K.; Kumar, V.M.; Mallick, H.N. Glutamate microinjection in the medial septum of rats decreases paradoxical sleep and increases slow wave sleep. Neuroreport 2012, 23, 451–456. [Google Scholar] [CrossRef]
- Kocsis, B. State-dependent increase of cortical gamma activity during REM sleep after selective blockade of NR2B subunit containing NMDA receptors. Sleep 2012, 35, 1011–1016. [Google Scholar] [CrossRef]
- El Gaamouch, F.; Buisson, A.; Moustie, O.; Lemieux, M.; Labrecque, S.; Bontempi, B.; De Koninck, P.; Nicole, O. Interaction between alphaCaMKII and GluN2B controls ERK-dependent plasticity. J. Neurosci. 2012, 32, 10767–10779. [Google Scholar] [CrossRef]
- Krapivinsky, G.; Krapivinsky, L.; Manasian, Y.; Ivanov, A.; Tyzio, R.; Pellegrino, C.; Ben-Ari, Y.; Clapham, D.E.; Medina, I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 2003, 40, 775–784. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Li, D.; Li, M.; Wang, P.; Wen, J.; Liang, M.; Su, B.; Yin, Y. IGF-1 alleviates NMDA-induced excitotoxicity in cultured hippocampal neurons against autophagy via the NR2B/PI3K-AKT-mTOR pathway. J. Cell. Physiol. 2014, 229, 1618–1629. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat. Neurosci. 2007, 10, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Dolmetsch, R.E.; Pajvani, U.; Fife, K.; Spotts, J.M.; Greenberg, M.E. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 2001, 294, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.G.; Groth, R.D.; Ma, H.; Barrett, C.F.; Owen, S.F.; Safa, P.; Tsien, R.W. Ca(V)1 and Ca(V)2 channels engage distinct modes of Ca(2+) signaling to control CREB-dependent gene expression. Cell 2012, 149, 1112–1124. [Google Scholar] [CrossRef]
- Gale, N.W.; Baluk, P.; Pan, L.; Kwan, M.; Holash, J.; DeChiara, T.M.; McDonald, D.M.; Yancopoulos, G.D. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 2001, 230, 151–160. [Google Scholar] [CrossRef]
- Matsuo, K.; Otaki, N. Bone cell interactions through Eph/ephrin: Bone modeling, remodeling and associated diseases. Cell Adh. Migr. 2012, 6, 148–156. [Google Scholar] [CrossRef]
- Stark, D.A.; Karvas, R.M.; Siegel, A.L.; Cornelison, D.D. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development 2011, 138, 5279–5289. [Google Scholar] [CrossRef]
- Murai, K.K.; Pasquale, E.B. ‘Eph’ective signaling: Forward, reverse and crosstalk. J. Cell Sci. 2003, 116, 2823–2832. [Google Scholar] [CrossRef]
- Murai, K.K.; Pasquale, E.B. Eph receptors and ephrins in neuron-astrocyte communication at synapses. Glia 2011, 59, 1567–1578. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.A.; Murai, K.K.; Wang, L.; Roberts, A.J.; Pasquale, E.B. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc. Natl. Acad. Sci. USA 2009, 106, 12524–12529. [Google Scholar] [CrossRef]
- Fu, A.K.; Hung, K.W.; Fu, W.Y.; Shen, C.; Chen, Y.; Xia, J.; Lai, K.O.; Ip, N.Y. APC(Cdh1) mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat. Neurosci. 2011, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.Y.; Chen, Y.; Sahin, M.; Zhao, X.S.; Shi, L.; Bikoff, J.B.; Lai, K.O.; Yung, W.H.; Fu, A.K.; Greenberg, M.E.; et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 2007, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Freyburger, M.; Pierre, A.; Paquette, G.; Belanger-Nelson, E.; Bedont, J.; Gaudreault, P.O.; Drolet, G.; Laforest, S.; Blackshaw, S.; Cermakian, N.; et al. EphA4 is involved in sleep regulation but not in the electrophysiological response to sleep deprivation. Sleep 2016, 39, 613–624. [Google Scholar] [CrossRef]
- Freyburger, M.; Poirier, G.; Carrier, J.; Mongrain, V. Shorter duration of non-rapid eye movement sleep slow waves in EphA4 knockout mice. J. Sleep Res. 2017, 26, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, S.; O’Callaghan, E.K.; Freyburger, M.; Cermakian, N.; Mongrain, V. The cell adhesion molecule EphA4 is involved in circadian clock functions. Genes Brain Behav. 2018, 17, 82–92. [Google Scholar] [CrossRef]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef]
- Kang, H.; Schuman, E.M. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 1995, 267, 1658–1662. [Google Scholar] [CrossRef]
- Lu, B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef]
- Vigers, A.J.; Amin, D.S.; Talley-Farnham, T.; Gorski, J.A.; Xu, B.; Jones, K.R. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience 2012, 212, 1–18. [Google Scholar] [CrossRef]
- Ghosh, A.; Carnahan, J.; Greenberg, M.E. Requirement for BDNF in activity-dependent survival of cortical neurons. Science 1994, 263, 1618–1623. [Google Scholar] [CrossRef]
- Kowianski, P.; Lietzau, G.; Czuba, E.; Waskow, M.; Steliga, A.; Morys, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Li, W.; Keifer, J. BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK. Neurobiol. Learn. Mem. 2009, 91, 243–249. [Google Scholar] [CrossRef][Green Version]
- Ying, S.W.; Futter, M.; Rosenblum, K.; Webber, M.J.; Hunt, S.P.; Bliss, T.V.; Bramham, C.R. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J. Neurosci. 2002, 22, 1532–1540. [Google Scholar] [CrossRef]
- Yasuda, M.; Fukuchi, M.; Tabuchi, A.; Kawahara, M.; Tsuneki, H.; Azuma, Y.; Chiba, Y.; Tsuda, M. Robust stimulation of TrkB induces delayed increases in BDNF and Arc mRNA expressions in cultured rat cortical neurons via distinct mechanisms. J. Neurochem. 2007, 103, 626–636. [Google Scholar] [CrossRef]
- Blum, R.; Konnerth, A. Neurotrophin-mediated rapid signaling in the central nervous system: Mechanisms and functions. Physiology (Bethesda) 2005, 20, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Rahmani, F.; Rezaei, N. The brain-derived neurotrophic factor: Missing link between sleep deprivation, insomnia, and depression. Neurochem. Res. 2020, 45, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.C.; Monteiro, S.; Candida, M.; Adler, N.; Paes, F.; Rocha, N.; Nardi, A.E.; Murillo-Rodriguez, E.; Machado, S. Relationship between brain-derived neurotrofic factor (Bdnf) and sleep on depression: A critical review. Clin. Pract. Epidemiol. Ment. Health 2017, 13, 213–219. [Google Scholar] [CrossRef]
- Schmitt, K.; Holsboer-Trachsler, E.; Eckert, A. BDNF in sleep, insomnia, and sleep deprivation. Ann. Med. 2016, 48, 42–51. [Google Scholar] [CrossRef]
- Kushikata, T.; Fang, J.; Krueger, J.M. Brain-derived neurotrophic factor enhances spontaneous sleep in rats and rabbits. Am. J. Physiol. 1999, 276, R1334–R1338. [Google Scholar] [CrossRef]
- Deuschle, M.; Schredl, M.; Wisch, C.; Schilling, C.; Gilles, M.; Geisel, O.; Hellweg, R. Serum brain-derived neurotrophic factor (BDNF) in sleep-disordered patients: Relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. J. Sleep Res. 2018, 27, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.T.; Chen, W.H.; Shi, L.; Lin, X.; Tabarak, S.; Chen, S.J.; Que, J.Y.; Bao, Y.P.; Tang, X.D.; Shi, J.; et al. Objective sleep duration is associated with cognitive deficits in primary insomnia: BDNF may play a role. Sleep 2019, 42. [Google Scholar] [CrossRef]
- Watson, A.J.; Henson, K.; Dorsey, S.G.; Frank, M.G. The truncated TrkB receptor influences mammalian sleep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R199–R207. [Google Scholar] [CrossRef][Green Version]
- Faraguna, U.; Vyazovskiy, V.V.; Nelson, A.B.; Tononi, G.; Cirelli, C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J. Neurosci. 2008, 28, 4088–4095. [Google Scholar] [CrossRef]
- Bachmann, V.; Klein, C.; Bodenmann, S.; Schafer, N.; Berger, W.; Brugger, P.; Landolt, H.P. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep 2012, 35, 335–344. [Google Scholar] [CrossRef]
- Guindalini, C.; Mazzotti, D.R.; Castro, L.S.; D’Aurea, C.V.; Andersen, M.L.; Poyares, D.; Bittencourt, L.R.; Tufik, S. Brain-derived neurotrophic factor gene polymorphism predicts interindividual variation in the sleep electroencephalogram. J. Neurosci. Res. 2014, 92, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, N.; De Beaumont, L.; Gagnon, K.; Baril, A.A.; Mongrain, V.; Blais, H.; Montplaisir, J.; Gagnon, J.F.; Pelleieux, S.; Poirier, J.; et al. BDNF Val66Met polymorphism interacts with sleep consolidation to predict ability to create new declarative memories. J. Neurosci. 2016, 36, 8390–8398. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, H.; Xu, Z.P.; Lu, Y.; Fu, Q.; Wang, W.; Li, G.H.; Wang, Y.Y.; Yang, Y.T.; Mi, W.D. Activation of brain-derived neurotrophic factor signaling in the basal forebrain reverses acute sleep deprivation-induced fear memory impairments. Brain Behav. 2020, 10, e01592. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, H.; Xu, Z.; Ma, D.; Guo, R.; Yang, K.; Wang, Y. Paradoxical sleep deprivation aggravates and prolongs incision-induced pain hypersensitivity via BDNF signaling-mediated descending facilitation in rats. Neurochem. Res. 2018, 43, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.K.; Koul-Tiwari, R.; Garner, J.M.; Geist, P.A.; Datta, S. Activation of brain-derived neurotrophic factor-tropomyosin receptor kinase B signaling in the pedunculopontine tegmental nucleus: A novel mechanism for the homeostatic regulation of rapid eye movement sleep. J. Neurochem. 2017, 141, 111–123. [Google Scholar] [CrossRef]
- Datta, S.; Knapp, C.M.; Koul-Tiwari, R.; Barnes, A. The homeostatic regulation of REM sleep: A role for localized expression of brain-derived neurotrophic factor in the brainstem. Behav. Brain Res. 2015, 292, 381–392. [Google Scholar] [CrossRef]
- Giese, M.; Beck, J.; Brand, S.; Muheim, F.; Hemmeter, U.; Hatzinger, M.; Holsboer-Trachsler, E.; Eckert, A. Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. J. Psychiatr. Res. 2014, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.; Unternahrer, E.; Huttig, H.; Beck, J.; Brand, S.; Calabrese, P.; Holsboer-Trachsler, E.; Eckert, A. BDNF: An indicator of insomnia? Mol. Psychiatry 2014, 19, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bae, C.H.; Park, S.Y.; Lee, S.J.; Kim, Y. Uncaria rhynchophylla inhibits the production of nitric oxide and interleukin-1beta through blocking nuclear factor kappaB, Akt, and mitogen-activated protein kinase activation in macrophages. J. Med. Food 2010, 13, 1133–1140. [Google Scholar] [CrossRef]
- Cao, H.; Ren, W.H.; Zhu, M.Y.; Zhao, Z.Q.; Zhang, Y.Q. Activation of glycine site and GluN2B subunit of NMDA receptors is necessary for ERK/CREB signaling cascade in rostral anterior cingulate cortex in rats: Implications for affective pain. Neurosci. Bull. 2012, 28, 77–87. [Google Scholar] [CrossRef][Green Version]
- Mikhail, C.; Vaucher, A.; Jimenez, S.; Tafti, M. ERK signaling pathway regulates sleep duration through activity-induced gene expression during wakefulness. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gotoh, T.; Tsuji, K.; Lo, E.H.; Huang, S.; Feig, L.A. Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk and CREB. EMBO J. 2004, 23, 1567–1575. [Google Scholar] [CrossRef]
- Hu, X.; Paik, P.K.; Chen, J.; Yarilina, A.; Kockeritz, L.; Lu, T.T.; Woodgett, J.R.; Ivashkiv, L.B. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 2006, 24, 563–574. [Google Scholar] [CrossRef]
- Su, X.; Wang, C.; Wang, X.; Han, F.; Lv, C.; Zhang, X. Sweet dream liquid chinese medicine ameliorates learning and memory deficit in a rat model of paradoxical sleep deprivation through the ERK/CREB signaling pathway. J. Med. Food 2016, 19, 472–480. [Google Scholar] [CrossRef]
- Foltenyi, K.; Greenspan, R.J.; Newport, J.W. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat. Neurosci. 2007, 10, 1160–1167. [Google Scholar] [CrossRef]
- Dumoulin Bridi, M.C.; Aton, S.J.; Seibt, J.; Renouard, L.; Coleman, T.; Frank, M.G. Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci. Adv. 2015, 1, e1500105. [Google Scholar] [CrossRef] [PubMed]
- Graves, L.A.; Hellman, K.; Veasey, S.; Blendy, J.A.; Pack, A.I.; Abel, T. Genetic evidence for a role of CREB in sustained cortical arousal. J. Neurophysiol. 2003, 90, 1152–1159. [Google Scholar] [CrossRef]
- Wimmer, M.E.; Cui, R.; Blackwell, J.M.; Abel, T. Cyclic AMP response element-binding protein is required in excitatory neurons in the forebrain to sustain wakefulness. Sleep 2020, zsaa267. [Google Scholar] [CrossRef]
- Cirelli, C.; Tononi, G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J. Neurosci. 2000, 20, 9187–9194. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, C.; Pompeiano, M.; Tononi, G. Neuronal gene expression in the waking state: A role for the locus coeruleus. Science 1996, 274, 1211–1215. [Google Scholar] [CrossRef]
- Hendricks, J.C.; Williams, J.A.; Panckeri, K.; Kirk, D.; Tello, M.; Yin, J.C.; Sehgal, A. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat. Neurosci. 2001, 4, 1108–1115. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Alessi, D.R.; James, S.R.; Downes, C.P.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Li, Y.; Zhu, T.; Wu, J.; Guan, K.L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 2002, 4, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Coloff, J.L.; Mason, E.F.; Altman, B.J.; Gerriets, V.A.; Liu, T.; Nichols, A.N.; Zhao, Y.; Wofford, J.A.; Jacobs, S.R.; Ilkayeva, O.; et al. Akt requires glucose metabolism to suppress puma expression and prevent apoptosis of leukemic T cells. J. Biol. Chem. 2011, 286, 5921–5933. [Google Scholar] [CrossRef] [PubMed]
- del Peso, L.; Gonzalez-Garcia, M.; Page, C.; Herrera, R.; Nunez, G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 1997, 278, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Nesterova, A.; Sable, C.; Heidenreich, K.A.; Boxer, L.M.; Heasley, L.E.; Reusch, J.E. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 2000, 275, 10761–10766. [Google Scholar] [CrossRef]
- Lan, Y.L.; Zhou, J.J.; Liu, J.; Huo, X.K.; Wang, Y.L.; Liang, J.H.; Zhao, J.C.; Sun, C.P.; Yu, Z.L.; Fang, L.L.; et al. Uncaria rhynchophylla ameliorates Parkinson's Disease by inhibiting HSP90 expression: Insights from quantitative proteomics. Cell. Physiol. Biochem. 2018, 47, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Zhu, S.; Xu, T.; Lu, J.; Han, H.; Zhou, C.; Yan, J. The role of rhynchophylline in alleviating early brain injury following subarachnoid hemorrhage in rats. Brain Res. 2016, 1631, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Mines, M.A.; Song, L.; Jope, R.S. Glycogen synthase kinase-3 levels and phosphorylation undergo large fluctuations in mouse brain during development. Bipolar Disord. 2012, 14, 822–830. [Google Scholar] [CrossRef]
- Leroy, K.; Brion, J.P. Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J. Chem. Neuroanat. 1999, 16, 279–293. [Google Scholar] [CrossRef]
- Woodgett, J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990, 9, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Akt/GSK3 signaling in the action of psychotropic drugs. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 327–347. [Google Scholar] [CrossRef]
- Beaulieu, J.M.; Sotnikova, T.D.; Yao, W.D.; Kockeritz, L.; Woodgett, J.R.; Gainetdinov, R.R.; Caron, M.G. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA 2004, 101, 5099–5104. [Google Scholar] [CrossRef]
- Jain, A.K.; Jaiswal, A.K. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J. Biol. Chem. 2007, 282, 16502–16510. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.C.; Moinova, H.R.; Mulcahy, R.T. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J. Biol. Chem. 1999, 274, 33627–33636. [Google Scholar] [CrossRef]
- Nguyen, T.; Huang, H.C.; Pickett, C.B. Transcriptional regulation of the antioxidant response element. Activation by Nrf2 and repression by MafK. J. Biol. Chem. 2000, 275, 15466–15473. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Wan, Y.; Sun, X.; Zhang, X.; Gao, W.; Wu, W. Nicotinic mitigation of neuroinflammation and oxidative stress after chronic sleep deprivation. Front. Immunol. 2019, 10, 2546. [Google Scholar] [CrossRef] [PubMed]
- Ahnaou, A.; Drinkenburg, W.H. Disruption of glycogen synthase kinase-3-beta activity leads to abnormalities in physiological measures in mice. Behav. Brain Res. 2011, 221, 246–252. [Google Scholar] [CrossRef]
- Benedetti, F.; Dallaspezia, S.; Lorenzi, C.; Pirovano, A.; Radaelli, D.; Locatelli, C.; Poletti, S.; Colombo, C.; Smeraldi, E. Gene-gene interaction of glycogen synthase kinase 3-beta and serotonin transporter on human antidepressant response to sleep deprivation. J. Affect. Disord. 2012, 136, 514–519. [Google Scholar] [CrossRef]
- Benedetti, F.; Serretti, A.; Colombo, C.; Lorenzi, C.; Tubazio, V.; Smeraldi, E. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci. Lett. 2004, 368, 123–126. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Cirelli, C.; Pfister-Genskow, M.; Faraguna, U.; Tononi, G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat. Neurosci. 2008, 11, 200–208. [Google Scholar] [CrossRef]
- Bruning, F.; Noya, S.B.; Bange, T.; Koutsouli, S.; Rudolph, J.D.; Tyagarajan, S.K.; Cox, J.; Mann, M.; Brown, S.A.; Robles, M.S. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 2019, 366. [Google Scholar] [CrossRef] [PubMed]
- Khlghatyan, J.; Evstratova, A.; Bozoyan, L.; Chamberland, S.; Chatterjee, D.; Marakhovskaia, A.; Soares Silva, T.; Toth, K.; Mongrain, V.; Beaulieu, J.M. Fxr1 regulates sleep and synaptic homeostasis. EMBO J. 2020, 39, e103864. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Samalin, L.; Llorca, P.M.; Curis, E.; Bellivier, F. Influence of lithium on sleep and chronotypes in remitted patients with bipolar disorder. J. Affect. Disord. 2016, 204, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Sharpley, A.L.; Solomon, R.A.; Cowen, P.J. Lithium increases slow wave sleep: Possible mediation by brain 5-HT2 receptors? Psychopharmacology 1989, 98, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Perez, E.; Amici, R.; Luppi, M.; Baracchi, F.; Cerri, M.; Dentico, D.; Zamboni, G. Lithium affects REM sleep occurrence, autonomic activity and brain second messengers in the rat. Behav. Brain Res. 2008, 187, 254–261. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Gestwicki, J.E.; Murphy, L.O.; Klionsky, D.J. Potential therapeutic applications of autophagy. Nat. Rev. Drug Discov. 2007, 6, 304–312. [Google Scholar] [CrossRef]
- Tudor, J.C.; Davis, E.J.; Peixoto, L.; Wimmer, M.E.; van Tilborg, E.; Park, A.J.; Poplawski, S.G.; Chung, C.W.; Havekes, R.; Huang, J.; et al. Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci. Signal. 2016, 9, ra41. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Kielian, T. Overview of toll-like receptors in the CNS. Curr. Top. Microbiol. Immunol. 2009, 336, 1–14. [Google Scholar] [CrossRef]
- Cespuglio, R.; Amrouni, D.; Meiller, A.; Buguet, A.; Gautier-Sauvigne, S. Nitric oxide in the regulation of the sleep-wake states. Sleep Med. Rev. 2012, 16, 265–279. [Google Scholar] [CrossRef]
- Jewett, K.A.; Krueger, J.M. Humoral sleep regulation; interleukin-1 and tumor necrosis factor. Vitam. Horm. 2012, 89, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Hars, B. Endogenous nitric oxide in the rat pons promotes sleep. Brain Res. 1999, 816, 209–219. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Y.; Krueger, J.M. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. Am. J. Physiol. 1998, 274, R655–R660. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Patterson, E.H.; Siwek, D.F. Endogenous and exogenous nitric oxide in the pedunculopontine tegmentum induces sleep. Synapse 1997, 27, 69–78. [Google Scholar] [CrossRef]
- Opp, M.R.; Obal, F., Jr.; Krueger, J.M. Interleukin 1 alters rat sleep: Temporal and dose-related effects. Am. J. Physiol. 1991, 260, R52–R58. [Google Scholar] [CrossRef]
- Opp, M.R.; Krueger, J.M. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am. J. Physiol. 1991, 260, R453–R457. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, J.B.; Moldofsky, H.; Lue, F.A.; Hay, J.B. Intracerebroventricular injection of TNF-alpha promotes sleep and is recovered in cervical lymph. Am. J. Physiol. 1999, 276, R1018–R1022. [Google Scholar] [CrossRef]
- Kapas, L.; Shibata, M.; Kimura, M.; Krueger, J.M. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am. J. Physiol. 1994, 266, R151–R157. [Google Scholar] [CrossRef]
- Opp, M.R.; Krueger, J.M. Interleukin-1 is involved in responses to sleep deprivation in the rabbit. Brain Res. 1994, 639, 57–65. [Google Scholar] [CrossRef]
- Opp, M.R.; Krueger, J.M. Anti-interleukin-1 beta reduces sleep and sleep rebound after sleep deprivation in rats. Am. J. Physiol. 1994, 266, R688–R695. [Google Scholar] [CrossRef]
- Kapas, L.; Fang, J.; Krueger, J.M. Inhibition of nitric oxide synthesis inhibits rat sleep. Brain Res. 1994, 664, 189–196. [Google Scholar] [CrossRef]
- Dzoljic, M.R.; de Vries, R.; van Leeuwen, R. Sleep and nitric oxide: Effects of 7-nitro indazole, inhibitor of brain nitric oxide synthase. Brain Res. 1996, 718, 145–150. [Google Scholar] [CrossRef]
- Takahashi, S.; Kapas, L.; Seyer, J.M.; Wang, Y.; Krueger, J.M. Inhibition of tumor necrosis factor attenuates physiological sleep in rabbits. Neuroreport 1996, 7, 642–646. [Google Scholar] [CrossRef]
- Takahashi, S.; Tooley, D.D.; Kapas, L.; Fang, J.; Seyer, J.M.; Krueger, J.M. Inhibition of tumor necrosis factor in the brain suppresses rabbit sleep. Pflug. Arch. 1995, 431, 155–160. [Google Scholar] [CrossRef]
- Takahashi, S.; Fang, J.; Kapas, L.; Wang, Y.; Krueger, J.M. Inhibition of brain interleukin-1 attenuates sleep rebound after sleep deprivation in rabbits. Am. J. Physiol. 1997, 273, R677–R682. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.; Kapas, L. Day- and night time injection of a nitric oxide synthase inhibitor elicits opposite sleep responses in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R521–R531. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Jantos, H. Microinjection of the nitric oxide synthase inhibitor L-NAME into the lateral basal forebrain alters the sleep/wake cycle of the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 239–247. [Google Scholar] [CrossRef]
- Kubota, T.; Kushikata, T.; Fang, J.; Krueger, J.M. Nuclear factor-kappaB inhibitor peptide inhibits spontaneous and interleukin-1beta-induced sleep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R404–R413. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Kim, Y.; Karpova, S.A.; McCarley, R.W.; Strecker, R.E.; Gerashchenko, D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci. Lett. 2014, 580, 27–31. [Google Scholar] [CrossRef]
- Kalinchuk, A.V.; McCarley, R.W.; Porkka-Heiskanen, T.; Basheer, R. Sleep deprivation triggers inducible nitric oxide-dependent nitric oxide production in wake-active basal forebrain neurons. J. Neurosci. 2010, 30, 13254–13264. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Sollars, P.J.; Ogilvie, M.D.; Pack, A.I. Modulation of IL-1 beta gene expression in the rat CNS during sleep deprivation. Neuroreport 1996, 7, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gardi, J.; Kushikata, T.; Fang, J.; Krueger, J.M. Nuclear factor-kappaB-like activity increases in murine cerebral cortex after sleep deprivation. Am. J. Physiol. 1999, 276, R1812–R1818. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Gilligan, J.G.; Kapas, L. Systemic injection of a nitric oxide synthase inhibitor suppresses sleep responses to sleep deprivation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1048–R1056. [Google Scholar] [CrossRef]
- Shoham, S.; Davenne, D.; Cady, A.B.; Dinarello, C.A.; Krueger, J.M. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am. J. Physiol. 1987, 253, R142–R149. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Bargas, J.; Hemmings, H.C., Jr.; Nairn, A.C.; Greengard, P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron 1995, 14, 385–397. [Google Scholar] [CrossRef]
- Huang, X.Y.; Morielli, A.D.; Peralta, E.G. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell 1993, 75, 1145–1156. [Google Scholar] [CrossRef]
- Baghdoyan, H.A.; Rodrigo-Angulo, M.L.; McCarley, R.W.; Hobson, J.A. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984, 306, 39–52. [Google Scholar] [CrossRef]
- Niwa, Y.; Kanda, G.N.; Yamada, R.G.; Shi, S.; Sunagawa, G.A.; Ukai-Tadenuma, M.; Fujishima, H.; Matsumoto, N.; Masumoto, K.H.; Nagano, M.; et al. Muscarinic acetylcholine receptors Chrm1 and Chrm3 are essential for REM sleep. Cell Rep. 2018, 24, 2231–2247. [Google Scholar] [CrossRef]
- Gillin, J.C.; Sutton, L.; Ruiz, C.; Golshan, S.; Hirsch, S.; Warmann, C.; Shiromani, P. Dose dependent inhibition of REM sleep in normal volunteers by biperiden, a muscarinic antagonist. Biol. Psychiatry 1991, 30, 151–156. [Google Scholar] [CrossRef]
- Kurimoto, E.; Nakashima, M.; Kimura, H.; Suzuki, M. TAK-071, a muscarinic M1 receptor positive allosteric modulator, attenuates scopolamine-induced quantitative electroencephalogram power spectral changes in cynomolgus monkeys. PLoS ONE 2019, 14, e0207969. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Wang, L.; Li, N.; Barkai, E.; Zhang, X.; Lin, L.; Xu, J. The firing of theta state-related septal cholinergic neurons disrupt hippocampal ripple oscillations via muscarinic receptors. J. Neurosci. 2020, 40, 3591–3603. [Google Scholar] [CrossRef] [PubMed]
- Shirey, J.K.; Brady, A.E.; Jones, P.J.; Davis, A.A.; Bridges, T.M.; Kennedy, J.P.; Jadhav, S.B.; Menon, U.N.; Xiang, Z.; Watson, M.L.; et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J. Neurosci. 2009, 29, 14271–14286. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Kauer, J.A. Properties of carbachol-induced oscillatory activity in rat hippocampus. J. Neurophysiol. 1997, 78, 2631–2640. [Google Scholar] [CrossRef]
- Cea-del Rio, C.A.; Lawrence, J.J.; Tricoire, L.; Erdelyi, F.; Szabo, G.; McBain, C.J. M3 muscarinic acetylcholine receptor expression confers differential cholinergic modulation to neurochemically distinct hippocampal basket cell subtypes. J. Neurosci. 2010, 30, 6011–6024. [Google Scholar] [CrossRef] [PubMed]
- Fisahn, A.; Yamada, M.; Duttaroy, A.; Gan, J.W.; Deng, C.X.; McBain, C.J.; Wess, J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 2002, 33, 615–624. [Google Scholar] [CrossRef]
- Langmead, C.J.; Austin, N.E.; Branch, C.L.; Brown, J.T.; Buchanan, K.A.; Davies, C.H.; Forbes, I.T.; Fry, V.A.; Hagan, J.J.; Herdon, H.J.; et al. Characterization of a CNS penetrant, selective M1 muscarinic receptor agonist, 77-LH-28-1. Br. J. Pharmacol. 2008, 154, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Ursin, R. Serotonin and sleep. Sleep Med. Rev. 2002, 6, 55–69. [Google Scholar] [CrossRef]
- Ito, H.; Yanase, M.; Yamashita, A.; Kitabatake, C.; Hamada, A.; Suhara, Y.; Narita, M.; Ikegami, D.; Sakai, H.; Yamazaki, M.; et al. Analysis of sleep disorders under pain using an optogenetic tool: Possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol. Brain 2013, 6, 59. [Google Scholar] [CrossRef]
- Cape, E.G.; Jones, B.E. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J. Neurosci. 1998, 18, 2653–2666. [Google Scholar] [CrossRef] [PubMed]
- Horner, R.L.; Sanford, L.D.; Annis, D.; Pack, A.I.; Morrison, A.R. Serotonin at the laterodorsal tegmental nucleus suppresses rapid-eye-movement sleep in freely behaving rats. J. Neurosci. 1997, 17, 7541–7552. [Google Scholar] [CrossRef]
- Chowdhury, S.; Yamanaka, A. Optogenetic activation of serotonergic terminals facilitates GABAergic inhibitory input to orexin/hypocretin neurons. Sci. Rep. 2016, 6, 36039. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.C.; Maejima, T.; Nishitani, M.; Hasegawa, E.; Yanagawa, Y.; Mieda, M.; Sakurai, T. Monoamines inhibit GABAergic neurons in ventrolateral preoptic area that make direct synaptic connections to hypothalamic arousal neurons. J. Neurosci. 2018, 38, 6366–6378. [Google Scholar] [CrossRef] [PubMed]
- Linley, S.B.; Vertes, R.P. Serotonergic systems in sleep and waking. In Handbook of Behavioral Neuroscience; Dringenberg, H.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 30, pp. 101–123. [Google Scholar]
- Bjorvatn, B.; Fagerland, S.; Eid, T.; Ursin, R. Sleep/waking effects of a selective 5-HT1A receptor agonist given systemically as well as perfused in the dorsal raphe nucleus in rats. Brain Res. 1997, 770, 81–88. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H.; Monti, D. Increased REM sleep after intra-dorsal raphe nucleus injection of flesinoxan or 8-OHDPAT: Prevention with WAY 100635. Eur. Neuropsychopharmacol. 2002, 12, 47–55. [Google Scholar] [CrossRef]
- Portas, C.M.; Thakkar, M.; Rainnie, D.; McCarley, R.W. Microdialysis perfusion of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J. Neurosci. 1996, 16, 2820–2828. [Google Scholar] [CrossRef]
- Vertes, R.P.; Kinney, G.G.; Kocsis, B.; Fortin, W.J. Pharmacological suppression of the median raphe nucleus with serotonin1A agonists, 8-OH-DPAT and buspirone, produces hippocampal theta rhythm in the rat. Neuroscience 1994, 60, 441–451. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H. Effects of activation and blockade of 5-HT2A/2C receptors in the dorsal raphe nucleus on sleep and waking in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1189–1195. [Google Scholar] [CrossRef]
- Monti, J.M.; Leopoldo, M.; Jantos, H. The serotonin 5-HT7 receptor agonist LP-44 microinjected into the dorsal raphe nucleus suppresses REM sleep in the rat. Behav. Brain Res. 2008, 191, 184–189. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H.; Lagos, P. Activation of serotonin 5-HT(1B) receptor in the dorsal raphe nucleus affects REM sleep in the rat. Behav. Brain Res. 2010, 206, 8–16. [Google Scholar] [CrossRef]
- Monti, J.M.; Jantos, H. Effects of the serotonin 5-HT2A/2C receptor agonist DOI and of the selective 5-HT2A or 5-HT2C receptor antagonists EMD 281014 and SB-243213, respectively, on sleep and waking in the rat. Eur. J. Pharmacol. 2006, 553, 163–170. [Google Scholar] [CrossRef]
- Popa, D.; Lena, C.; Fabre, V.; Prenat, C.; Gingrich, J.; Escourrou, P.; Hamon, M.; Adrien, J. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J. Neurosci. 2005, 25, 11231–11238. [Google Scholar] [CrossRef] [PubMed]
- Dahan, L.; Astier, B.; Vautrelle, N.; Urbain, N.; Kocsis, B.; Chouvet, G. Prominent burst firing of dopaminergic neurons in the ventral tegmental area during paradoxical sleep. Neuropsychopharmacology 2007, 32, 1232–1241. [Google Scholar] [CrossRef]
- Eban-Rothschild, A.; Rothschild, G.; Giardino, W.J.; Jones, J.R.; de Lecea, L. VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci. 2016, 19, 1356–1366. [Google Scholar] [CrossRef]
- Lancel, M. Role of GABAA receptors in the regulation of sleep: Initial sleep responses to peripherally administered modulators and agonists. Sleep 1999, 22, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Winsky-Sommerer, R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur. J. Neurosci. 2009, 29, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Hannou, L.; Roy, P.G.; Ballester Roig, M.N.; Mongrain, V. Transcriptional control of synaptic components by the clock machinery. Eur. J. Neurosci. 2020, 51, 241–267. [Google Scholar] [CrossRef] [PubMed]
- Biello, S.M.; Bonsall, D.R.; Atkinson, L.A.; Molyneux, P.C.; Harrington, M.E.; Lall, G.S. Alterations in glutamatergic signaling contribute to the decline of circadian photoentrainment in aged mice. Neurobiol. Aging 2018, 66, 75–84. [Google Scholar] [CrossRef]
- Wang, L.M.; Schroeder, A.; Loh, D.; Smith, D.; Lin, K.; Han, J.H.; Michel, S.; Hummer, D.L.; Ehlen, J.C.; Albers, H.E.; et al. Role for the NR2B subunit of the N-methyl-D-aspartate receptor in mediating light input to the circadian system. Eur. J. Neurosci. 2008, 27, 1771–1779. [Google Scholar] [CrossRef]
- Bendova, Z.; Sladek, M.; Svobodova, I. The expression of NR2B subunit of NMDA receptor in the suprachiasmatic nucleus of Wistar rats and its role in glutamate-induced CREB and ERK1/2 phosphorylation. Neurochem. Int. 2012, 61, 43–47. [Google Scholar] [CrossRef]
- Bendova, Z.; Sumova, A.; Mikkelsen, J.D. Circadian and developmental regulation of N-methyl-d-aspartate-receptor 1 mRNA splice variants and N-methyl-d-aspartate-receptor 3 subunit expression within the rat suprachiasmatic nucleus. Neuroscience 2009, 159, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Coria-Lucero, C.D.; Golini, R.S.; Ponce, I.T.; Deyurka, N.; Anzulovich, A.C.; Delgado, S.M.; Navigatore-Fonzo, L.S. Rhythmic Bdnf and TrkB expression patterns in the prefrontal cortex are lost in aged rats. Brain Res. 2016, 1653, 51–58. [Google Scholar] [CrossRef]
- Cai, Y.; Ding, H.; Li, N.; Chai, Y.; Zhang, Y.; Chan, P. Oscillation development for neurotransmitter-related genes in the mouse striatum. Neuroreport 2010, 21, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Bi, Q.; Cui, H.; Lv, C.; Wang, M. Suppression of autophagy through JAK2/STAT3 contributes to the therapeutic action of rhynchophylline on asthma. BMC Complement. Med. Ther. 2021, 21, 21. [Google Scholar] [CrossRef]
- Boudreau, P.; Yeh, W.H.; Dumont, G.A.; Boivin, D.B. Circadian variation of heart rate variability across sleep stages. Sleep 2013, 36, 1919–1928. [Google Scholar] [CrossRef]
- Trinder, J.; Waloszek, J.; Woods, M.J.; Jordan, A.S. Sleep and cardiovascular regulation. Pflug. Arch. 2012, 463, 161–168. [Google Scholar] [CrossRef]
- Song, M.F.; Guan, Y.H.; Li, H.T.; Wei, S.G.; Zhang, L.X.; Zhang, Z.L.; Ma, X.J. The effects of genetic variation and environmental factors on rhynchophylline and isorhynchophylline in Uncaria macrophylla Wall. from different populations in China. PLoS ONE 2018, 13, e0199259. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).