Abstract
Archaeoentomology is the study of insects and other arthropods recovered from an archaeological site; they can be found in association with ancient human and animal remains, food, artefacts or they can be related to the environment and its changes throughout the time. Within archaeoentomology, the branch of “funerary archeoentomology” considers insects and other arthropods especially in association with human remains in funerary and burial contexts. The presence and the location of certain insect species closely associated with or nearby the remains, can be valuable in gathering information about the ecological situation at the time of burial and the changes that occurred in the environment up until the discovery of the body. Funerary archaeoentomology investigations have been carried out globally, primarily in countries like Italy, Peru, the United Kingdom and France. Similarly to forensic entomology contexts, the abundance and diversity of insects are affected by the type of burial, the macro and micro-environment of and surrounding the burial, the items associated with the cadaver, the post-mortem practices, and the time that has elapsed from the body deposition to the discovery and the excavation. While funerary archaeoentomology and forensic entomology remain two well-distinguished disciplines, the sampling practice, the insect identification process, and the analyses of the burial ecology in funerary archaeoentomology studies follow the best practices and the general guidelines of forensic entomology. In both disciplines, the correct identification of the insects is key to providing correct information. Various methods have proven effective for insect identification, i.e., morphological, molecular and chemical analysis. This review aims to collect the current knowledge in funerary archaeoentomology, discuss the strengths and weaknesses of insect identification methods in an archaeological context, and describe the groups of the most relevant insects and other arthropods found in association with ancient human remains worldwide. Furthermore, recommendations will be provided to advance the practices of archaeoentomology examinations.
1. Introduction
The decomposition of a cadaver (human or non-human) involves a nexus of spontaneous post-mortem changes that can occur soon after death, after days, months years or even after it becomes lithified [1,2]. It is a dynamic process that can have variable duration based on the cadaver itself (“intrinsic factors”, e.g., the size of the cadaver and the cause of death) and the depositional environment (“extrinsic factors”, e.g., the geographic location, the season and the type of burial). Generally, throughout the decomposition process macro and microorganisms consume the cadaver, as it represents a food source that does not resist being preyed upon [3,4].
There is a consensus across the literature that a natural decomposition of a complete cadaver in a temperate terrestrial environment consists of five stages of biochemical processes [2,5]. Starting with the ‘fresh’ stage promptly after death, autolytic processes result in cellular digestion [2]. As an effect of autolysis, bacteria induce a putrefactive process known as the ‘bloated’ phase, whereby gaseous odour production, colour change and abdominal bloating occur. Once the fluids and gasses produced during the bloated stage accumulate, the cadaver exhibits a rapid loss in mass as these putrefactive by-products escape and ferment in the ‘active decay’ stage [5]. At the point of ‘advanced decay’, most of the soft tissue has been stripped from the cadaver. The cadaver then progresses to the final ‘dry and remains’ stage, where desiccated skin, cartilage, bone, and hair are the only remaining cadaverous indicators [2]. Carrion insects are generally the organisms that drive the majority of the decomposition process, and under favourable environmental conditions, can colonise the cadaver soon after death. The relationship between insects and the cadaver is highly co-dependent and caused by a synanthropic motive: different families of insects colonise the cadaver at different stages of decomposition because they benefit from it in different ways [6]. Therefore, such insect families are divided into four ecological groupings: (1) necrophagous, that use the cadaver as a source of food for themselves and their offspring; (2) necrophilous, that arrive at the cadaver to predate on necrophagous species; (3) omnivorous, that engage in both necrophagous and necrophilic behaviour; and (4) opportunists, that use the cadaver as an extension of their habitat [7]. Generally, at the beginning of the decomposition process necrophages are the most present and active, but as time goes by, the colonisation becomes more complex and interrelated, with the presence of both active insects and the remains of insects that have completed their life cycle and have left the cadaver [8]. In the investigation of a suspicious death where the a body colonised by insects is found, the discipline of forensic entomology applies the knowledge of insect colonisation dynamics, alongside the insect ecology and growth rates to infer the approximate time of the insects’ arrival and departure, aiming to estimate the subject’s time since death (also known as the post-mortem interval, PMI, or the minimum post-mortem interval, minPMI) [9,10]. The best practice and the guidelines developed in forensic entomology are applied to the study of insects (and other arthropods) collected in an archaeological context (archaeoentomology), especially in association to skeletonised or mummified remains of archaeological interest. Insects can be found in archaeoentomological contexts thanks to the insects’ external part of the body (exoskeleton) made of chitin, a biopolymer that is able to resist in the environment for a long time [9,10,11].
Similar to a criminal case of forensic interest, in the context of an archaeological excavation insect specimens are collected from the remains, from the surrounding environment and from the artefacts connected to the remains, if present; the insects are then analysed morphologically, molecularly, or chemically to determine their species. The application of the two disciplines then diverges, as the estimation of the PMI in funerary archaeoentomology casework is not possible. However, in these contexts, the overall insect assemblage and the species-specific ecological tendencies can provide information on the environmental conditions at the time of death, insights regarding additional manipulation of remains post-mortem, the possible presence of drugs in the cadaver and, potentially, even the probable cause of death [12].
This literature review aims to address the topic of funerary archaeoentomology in determining the ability to generate knowledge on post-mortem dynamics, historical beliefs and funerary practices, through the analyses of the entomological assemblage associated with human remains of archaeological interest. This paper aims to collect the current knowledge about insect species colonising ancient human remains, highlight the research carried out globally, and address the pros and cons of the different analysis approaches. Finally, recommendations to advance the discipline and provide more information in the course of archaeological examinations are presented.
2. Funerary Archaeoentomology
Archaeoentomology is the study of synanthropic entomofauna observed and recovered from an archaeological site [13]. The discipline is highly connected with palaeoentomology (also known as “Quaternary archaeoentomology”), a scientific discipline that uses insects for the reconstruction of Quaternary environments (environments that began to develop 2.58 million years ago and continuing to the present day) [14]. The two disciplines differ as archaeoentomology considers insects and other arthropods collected from sites where human activity was present, while palaeoentomology from sites without the presence of human activity. Insects are present in almost any environment, and their remains made of a high content of chitin can be preserved even for thousands of years in both wet environments (e.g., peat, lake bottoms, etc.) and dry and protected environments (e.g., tombs), making them extremely valuable to obtain information on past environments and archaeological contexts. By virtue of their highly chitinised exoskeleton, it is almost exclusively beetles (Coleoptera) and fly pupal cases (Diptera) that are recovered from an archaeological setting, while fly larvae and adults are preserved only exceptionally, and especially in environments where remains are preserved on purpose (e.g., coffins). Taking advantage of this characteristic, archaeoentomology can specialise further in funerary archaeoentomology, where the insect assemblages recovered from historical graves and burial settings are used to infer information about past funerary practices [7,13,15,16]. While palaeoentomology is a well-established discipline with the foundation of techniques developed in the late 1950s, the term funerary archaeoentomology was established and defined by Huchet only in 1996 [7]. Currently, experts in the discipline are in a limited number; a dedicated conference has been held in 2015 (“1st International Conference Funerary Archaeoentomology, 2015 Huddersfield, UK”) and from 2017 meetings have been organised alongside the forensic entomology symposiums of the European Association of Forensic Entomology (EAFE).
Archaeoentomological investigations have been carried out across five of the seven continents. In Applendix 1, a table detailing the past research within the field is reported and divided per continent, country, region and city, where possible. The archaeological setting and a complete list of the recovered insects and other arthropods are indicated as in the cited publication. The table has been constructed considering research published between 1967–2022, gathered by searching the terms “archaeoentomology”, “funerary entomology”, and “funerary archaeoentomology” into the main scholarly search engines, as well as publications received from experts within the discipline. These publications document insects and other arthropods that have been collected exclusively on human cadavers, with the purpose of describing funerary rituals and palaeoecology concerning the deceased individual. The table excludes unpublished conference outputs and papers of palaeoecological investigations documenting the hygiene practices or living conditions in the absence of human remains [17].
As shown in Figure 1, the largest amount of archaeological work has been performed in Europe (58%), while fewer studies have been conducted in North and South America (respectively 19% and 15%), with even fewer in Africa and Asia (4% each). On a country-based level, the largest number of locations of archaeoentomological research has been in Italy (N = 14), followed by Peru (N = 5) and the United Kingdom (N = 4). Religious infrastructure such as churches, cathedrals and monasteries are common storage facilities in which mummified cadavers have been recovered within funerary urns, singular tombs and larger catacombs [18,19,20,21,22,23]. The disparity in the current archaeoentomology outputs from other regions of the world could be attributed to the locality where the researchers are based and working, a lack of funds for expeditions and limited access to certain locations due to ethical/religious/heritage reasons. Furthermore, several cases of interest are currently presented in non-peer-reviewed formats or consider non-human cadavers, e.g., ibis birds, dogs and llamas involved in ritualistic procedures in Egypt and Peru [24,25,26].
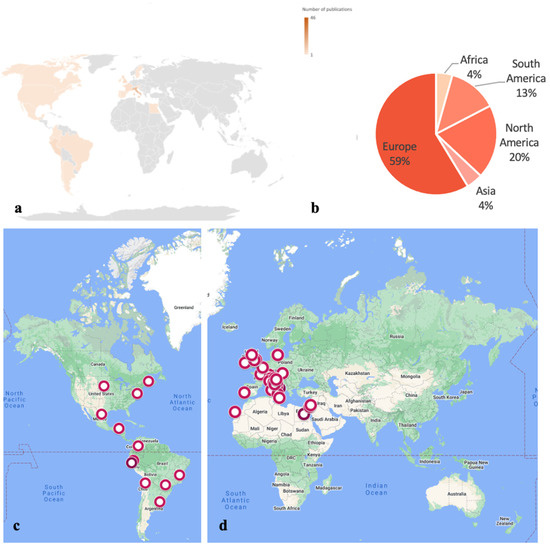
Figure 1.
World map showing the number (a) and percentage (b) of archeoentomology research published at a continent level (developed via Excel®) and the locations of the excavations at a country level in North and South America (c), compared to the rest of the world (d) (developed via GoogleMyMaps©). A gradation in hue has been used to represent the number of publications per area, i.e., the darker the hue, the more publications available in the current literature.
3. Insects of Archaeoentomological Interest
When a cadaver is left undisturbed and exposed on the ground surface in a temperate environment, necrophagous insects will be attracted to it in a short time (within minutes-hours in the warmer seasons), thanks to their olfactory system selected by the evolution for detecting the odours of decomposition [27]. During the early stages of decomposition, the insect activity is intense, with especially larvae of blow flies, flesh flies and house flies (Diptera: Calliphoridae, Sarcophagidae, Muscidae) actively feeding on the cadaver [6,28]. In the field of forensic entomology, the identification of the species and their age will be used to estimate the (min) PMI [9,10]. As decomposition continues, other species of insects and other arthropods are attracted to the cadaver, to feed directly on the remains or to pray or parasitise the carrion fauna already present; the cadaver thus becomes the base of a complex food chain made up of numerous species, active since the start or newly arrived (i.e., coffin flies, latrine flies, larder beetles, moths, spiders, mites and rather exceptionally false scorpions—respectively Diptera: Phoridae, Fanniidae; Coleoptera: Dermestidae; Lepidoptera; Arachnida: Araneae, Acariformes and Pseudoscorpiones) that coexist with residues of insects that have completed their life cycle and left the food source [29,30,31,32]. Insect residuals such as empty pupal cases of flies, beetle exoskeletons, legs and elytrae can persist in the environment for a long time because of their high chitin content [13,30]. In a late decomposition scenario, when the cadaver has gone undiscovered for months or years, it is the analyses of the whole colonisation assemblage made of active insects and insect residuals that can provide useful information [13,30]. Such information is based on the concept of “successional waves”, firstly proposed by Jean Pierre Mégnin in 1894 and then extensively referenced throughout the literature [8]. Successional waves are predictable groupings of insects specifically interested in subsequent stages of decomposition and the biological and chemical changes that result. The number of waves, their duration and the insect assemblage of each wave are strictly dependent on the location of the cadaver’s disposal and the micro and macro-environmental conditions [29]. Specifically, on cadavers exposed on the ground surface, authors have reported the occurrence of up to eight waves, while up to four in buried cadavers [8,29,33,34]. The duration of each wave is shortened in warm-hot seasons, and insect species diversity is larger in a natural environment compared to an apartment or a limited access environment [35,36]. Insects belonging to specific waves will leave the cadaver after completing their life cycle (non-reoccurring species), possibly leaving residuals of their presence (e.g., empty pupal cases); however, recurring species will reappear over time. One study examining 23 cadavers determined that 80% of all recovered insects persisted on the decomposing cadaver during a single period, while the remaining 20% would appear, leave and reappear over time [37].
Considering a cadaver exposed on the ground, in a temperate environment and without physical/chemical impediments to the colonization process, the first two successional waves occur during the initial autolytic process, when strictly necrophagous dipterous species colonise the remains [6]. Generally, species of blow flies are first-wave colonisers, while flesh flies arrive in the second wave [38]. The progression to putrefaction results in the oviposition and larval community of other species of blow flies, house flies and flesh flies with a focus towards natural orifices and wounds, to provide their offspring with a more accessible food source [39,40]. During this time, the third and fourth waves occur with different species of larder and rove beetles (Coleoptera: Staphylinidae) being active alongside species of latrine flies, cheese skipper flies (Diptera: Piophilidae) and true flies (Diptera: Sphaeroceridae) [29]. Once all soft tissue has been eliminated in advanced decay, larvae of Diptera migrate away from the cadaver and the beetle population significantly increases. The sixth wave includes the arrival of other arthropods like spiders (Arachnida) to the dry skin and bone, due to their necrophilic and opportunistic motive [32]. Larder beetles and moths (Lepidoptera) also colonise in the final seventh and eight waves, especially when clothing/textiles are on or around the cadaver [29]. Moreover, opportunistic species such as mites, can arrive at any stage of the decomposition [41].
Successional waves on a buried cadaver are highly affected by the depth of the burial and the type of soil [34]. Furthermore, some burial scenarios might see the addition of physical barriers like coffins or textile material wrapping the body [42]. Generally, blow flies cannot colonise a cadaver placed deep in the soil, while some species of sun flies (Diptera: Heleomyzidae), coffin flies and cheese skipper flies are abundant at greater depths [33]. Species-specific access to buried remains is also applicable to Coleoptera, with rove beetles, carrion beetles (Coleoptera: Silphidae), and clown beetles (Coleoptera: Histeridae) abundant in shallow depths, although only rove beetles are typically abundant at greater depths [33]. Research has shown an important colonisation reduction in cadaver samples deployed at less than 10cm, 10-30cm, 40-60cm, 90cm and 2m below the soil surface [33]. However, it must be taken into consideration that the burial can be secondary, and therefore, insects recovered from buried remains may provide information regarding the displacement of the cadaver post-mortem.
Successional waves are produced following studies with a duration of up to two years since the deployment of an experimental cadaver. To date, successional wave studies are limited in scenarios and geographical locations, and the understanding of the colonization process on cadavers concealed in limited access environments (e.g., suitcases and coffins), and extreme environments (e.g., caves and wells), is under-researched or based on case studies [35,42,43]. As a consequence, several investigative circumstances lack a baseline data set and for locations never studied might be impossible to reconstruct or predict the pattern of arrival of carrion species [34,36,44,45].
The ability to use the successional waves method for PMI estimation becomes weaker as the time since death extends into years and centuries due to the decrease or the total absence of live insect specimens, the alteration of insect residuals due to weathering or their association with clothes and crevices of bones, and the contamination by insects from other sources [46,47]. This is a common occurrence in archaeological excavations where cadavers are brought to light after hundreds of years. Furthermore, in extreme cases, the only indicator of insect activity are the damage and modifications produced by them on skeletal bone, i.e., burrows [48,49,50,51]. In these scenarios of poor/incomplete/contaminated insect collection, the typical forensic entomology-based PMI cannot be estimated [15].
4. Insect Species Identification with a Special Focus on Archaeological Settings
Within the standard guidelines for forensic archaeologists accepted by the Chartered Institute for Archaeologists, there is the acknowledgment of an archaeologist being able to advise the attendance of a forensic specialist, such as an entomologist, if beneficial for the investigation of the case [52]. However, if the entomologist is not required at the scene, or there is not one available, it is common practice for insect samples to be collected by other experts on site, during the excavation and/or while laboratory analyses are performed, with the intent to be sent to an entomologist for further investigation [53]. For correct collection and storage in the absence of an entomologist, it is possible to consult guideline papers, book chapters, and even smartphone apps (SmartInsects®) [11,54].
Practising under the internationally accepted guidelines for the best practice in forensic entomology, the insects present in an archaeological context will be carefully collected from the remains and the immediate surroundings (using forceps, spoons or even by hand), then stored in 70% ethanol (with hot water killing as a preliminary step if any living fly larva is present, although a rather seldom occurrence) or dry [11]. Entomologists will then be tasked with the taxonomic identification of the specimens to the furthest possible level. There has been great debate about the most objective, efficient, cost-effective and least time-consuming taxonomic identification method. This debate is due to the sheer number of insect species, the different morphologies in the subsequent stages of life (i.e., egg, different stages of larva, full and empty pupal cases, adult), and the easy occurrence of only partial/ruined insect remains. This conflict of ideas surrounds mostly the use of morphological methods versus a molecular diagnostic approach, but in the last few years it has also considered identification via chemical analyses (hydrocarbons) [55]. Each method’s pros and cons, as well as the limitations faced in an archaeoentomological setting are discussed in the following paragraphs. It is often in archaeological contexts for a combination of these approaches to be used in a multidisciplinary effort to overcome the barriers of such analyses [56,57]. It is important to note that although extensive research has already been conducted to identify species in the Diptera and Coleoptera orders, due to the sheer number of species and the different morphologies in the subsequent stages of life, there remain significant gaps in the documentation which require further research to aid investigations [44,58].
4.1. Morphological Identification of Insects
The foundation of morphological analysis is a non-destructive and inexpensive scoring-based method whereby dichotomous characteristics can be used to individualise one species from another [12]. These characteristics are commonly documented as taxonomic keys in written and pictorial formats and, in the last few years, have been translated into digital tools like Lucid Keys© [59]. The examination of insect specimens under a light microscope or a scanning electron microscope (SEM) has been commonly used for various stages of the insect’s life [60]. While SEM can visualise an abundance of fine detailed diagnostic characteristics, it cannot be easily applied to field studies or urgent identification and is less cost-efficient than light microscopy [61]. Furthermore, the preparation of the insects for SEM analyses may require their alteration, i.e., metal coating. Morphological identification requires dedicated training and extensive knowledge of specific characteristics in the different stages of life that is supported by both the availability of taxonomical keys and an established reference collection used for comparative purposes [12]. An extensive amount of diagnostic literature published in (forensic) entomology relies on the morphologies of adult Diptera specimens, therefore its success is often based on laboratory rearing of the immature specimens collected [62]. However, in an archaeoentomological context, where often only insect residuals are present, this is generally not possible.
In the last few years, automated approaches that utilise the principles of traditional morphology to identify insect species have been proposed [63,64]. The use of such computerised technology has been suggested to increase the identification efficiency, reduce the assessment time and eliminate the observer bias exhibited in traditional morphological approaches [63]. The first automated identification system was created using computer image analysis technology on a population of midges (Diptera: Chironomidae) [63]. To gather information for the digital automatic identification system (DAISY), the use of digital microscopy to photograph diagnostic features followed by a comprehensive training image analysis software allowed the accurate identification of 86% of the examined specimens, with the accuracy increasing when a larger number of training images are used. Like the DAISY system, the DAIIS automated computing technology provided similar results with an identification accuracy ranging between 94.5–97.7% for a population of owl flies (Neuroptera: Ascalaphidae). The development of similar systems for Diptera, Coleoptera, Lepidoptera and other relevant species in archaeological and forensic contexts would remove human bias and reduce the time needed for species identification and data analyses process.
4.2. Cleaning Procedures for Insect Samples Collected from Archaeological Settings
The morphological identification of insects in archaeological settings is affected by three main limits. Firstly, the limited number of taxonomic keys dedicated to pupal cases, which are often the most common samples available; secondly, the easy occurrence of partial and damaged insect remains; and lastly, the samples are often covered with decomposition residue and debris resulting from years spent within the burial environment in strict contact with the decomposing cadaver, making them unrecognisable (Figure 2) [65]. While chitin is a highly resistant material, it is not uncommon for the delicate posterior region and surface structures (i.e., anterior spiracles, respiratory horns and body protrusions) to be destroyed under extreme pressure, natural soil movement, excavation procedures or during aggressive mechanical cleaning, all of which can affect the morphological identification of the samples [66].
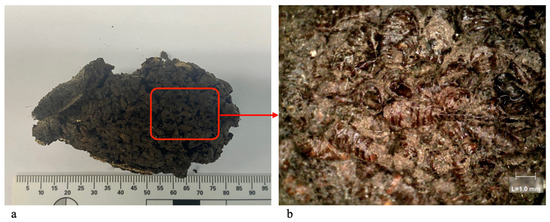
Figure 2.
Large pupal case cluster in original conditions recovered from Duomo Di Alba, Alba (North West of Italy) (a) and a magnified portion of the red square area (b).
A correct cleaning procedure of the samples represents an essential step towards the correct identification. Cleaning methods documented in the literature can be classified by two modes of action: mechanical removal of debris (using pins, dissecting needles, scalpels, mini brushes, and the air of an ultrasonic bath) and a solvent-based soaking system [67]. The cleaning method applied is chosen by the entomologist after evaluating the sample’s degree of preservation, the type, amount and texture of the debris, and sometimes a combination of both sets of factors. For a successful result, it is suggested that an initial sodium hydroxide-based clearing process followed by cleaning with warm water and soap in an ultrasonic bath prior to the examination of the sample via conventional light microscopy and SEM [66]. This is particularly important for posterior spiracles, respiratory horns and body protrusions which are areas of the larvae and pupal cases generally considered to contain the most definitive morphological characteristics. Due to their complex and varied structure composed of ridges, valleys and protrusions, debris of different sorts can easily accumulate in these areas and hide the real structure. With the application of this method, Giordani and collaborators were able to distinguish pupal cases of six different species of garbage flies, Hydrotaea sp. (Diptera: Muscidae) recovered from European and South American forensic and archaeological contexts, and laboratory breeding of material collected on carrion in Central-East Europe [66]. It was also possible to appreciate the shape of additional identification features, i.e., respiratory horns, the shape and position of posterior spiracle slits, the anal plate shape, the position and shape of anal papillae and intersegmental spines. These diagnostic areas have also been influential in identifying blow flies, house flies and flesh flies recovered from buried archaeological cadavers in Huaca de la Luna, Peru [68].
4.3. Molecular Identification of Insects
Molecular processes utilise the principles of traditional deoxyribonucleic acid (DNA) analysis, such as extraction, amplification and analysis of insect DNA via target sequence-specific primers. The sequence generated from the polymerase chain reaction (PCR) amplification process is then compared against DNA databases such as the National Centre of Biotechnology Information (NCBI) to determine similarity percentages between the unknown specimen sequence and known species sequences [69]. In the context of analysing old, partial or ruined insect samples, mitochondrial DNA (mtDNA) is highly desirable for molecular approaches due to the high copy number within cells and its increased resistance to degradation when compared to nuclear DNA (nuDNA) [70]. More specifically, the cytochrome c oxidase I (COI) region is the target sequence of the mtDNA used to taxonomically identify insect species [71,72,73,74,75]. Molecular approaches are subjected to rigorous scientific validation processes that yield specific dichotomous results and remove observer bias and expertise limitations commonly argued as a weakness of morphological approaches [76]. In particular, molecular analyses are highly suggested in cases of forensic entomological importance to confirm morphological observations that might be affected by human error and to intervene in cases in which the taxonomist is unable to provide an identification (i.e., ruined or partial samples and missing dichotomous keys for certain insects or stages of life). Larvae and adult insect specimens are an mtDNA-rich source compared empty pupal cases and other remnants like legs, elytrae and exuviae [74].
A limitation of the molecular approach is the deficits in insect species sequencing and identification [44,58]. Only a limited number of the species of forensic and archaeological interest are currently sequenced and available on NCBI compared to the number of documented living species [45]. For example, in a survey conducted in 2019, out of the 1520 known living blow fly species, only 515 were sequenced; 994 flesh fly species were sequenced compared to the 3079 known; 1345 house fly species were sequenced compared to the 5190 known; and 4030 coffin fly species were sequenced compared to the 4087 known [45].
Another common criticism of the molecular approach is its highly destructive extraction method that often reduces or prevents the ability to conduct repeat analyses [72]. In order to address this weakness, an experiment was conducted to preserve the morphological character of blow fly larvae and conduct biomolecular analyses [74]. Following hot water killing, larvae of blow flies, house flies, and coffin flies ranging from 6.5 mm to 17 mm were dissected. The internal tissues underwent COI analysis, and the remaining external cuticle was preserved for morphological identification. Sodium hydroxide/potassium hydroxide staining of the larval cuticle was used to increase the contrast of morphological characteristics without interfering with the sample’s integrity. This study’s results document the success of this combined protocol in obtaining correct species identification with molecular and morphological approaches.
Although the COI region is the most used target sequence, a collective of authors investigated the applicability and efficiency of the cytochrome c oxidase II (COII) sequence in determining percentage similarity of species [71]. Insect samples of forensic entomological importance in various life stages (i.e., larvae, full and empty pupal cases and adult flies) underwent mtDNA extraction, and the COI and COII regions were sequenced and compared to NCBI data. The results of this study included the identification of seven Diptera species with a percentage similarity range of 97-100%, which was explained by intraspecific and geographic variations [71]. The authors also noted some ambiguity in some species identification, with the analysis of the sole COI region identifying the common blue-bottle blow fly (Calliphora vicina Robineau-Desvoidy, Diptera: Calliphoridae) with a percentage similarity range of 100%, the sole COII at 99% similarity and the combined COI and COII at 100% similarity [71].
In order to verify if a specimen’s age affects the degradation of the molecular information within pupal cases, the combination of COI and COII analysis was conducted on empty pupal cases of varying ages (i.e., young, <5 years old; old, 5-20 years old; very old, >20 years old) [73]. The mtDNA extraction was conducted on whole pupal and pupal case fragments, resulting in the genotyping of 68.2% of the examined material. There was no mention of morphological analysis conducted on the specimens. For 77.4% of young specimens identified, there was no signs of mtDNA degradation detected. Comparatively, the old and very old specimens exhibited a greater abundance of shorter mtDNA fragments, indicative of mtDNA degradation. Based on these encouraging results, the authors suggest the mtDNA can be extracted and sequenced from very old empty pupal casings, albeit as shorter fragments [73] A limit of this research are the small sample sizes used, with a disproportionate representation favouring young pupal cases, and thus, they currently do not provide an upper age limit for the success of this technique.
4.4. Molecular Identification of Insects in Archaeological Settings
While the dual-process method proposed by Tuccia and collaborators is promising in its ability to overcome the destructive nature of mtDNA extraction and allow the potential for repeat morphological and molecular analyses, it is yet to be applied to empty pupal cases and old insect remains, which are typical of archaeological contexts [74]. Current research is focused on the adaptations to the standard practices of COI with the aim of improving the identification of antique specimens.
The first successful adaptation involves the cleaning of pupal cases prior to both morphological and molecular analyses. A study comparing the suitability of common cleaning methods on pupal cases based on their visual improvement for morphological analyses and compatibility with molecular analyses was conducted on empty pupal cases of blow flies, flesh flies, house flies, and small dung flies (Diptera: Sphaeroceridae) obtained from forensic and archaeological contexts [67]. The samples were subjected to the following cleaning methods: (a) warm water and soap solution, (b) ultrasonic bath, (c) glacial acetic acid, (d) sodium hydroxide solution, (e) hydrochloric acid/sodium bicarbonate, and (f) sodium hypochlorite. The warm water and soap, ultrasonic bath and sodium hydroxide methods proved to be the most efficient at removing debris whilst maintaining the integrity of the sample’s diagnostic morphologies. While the use of glacial acetic acid was corrosive to the thin empty pupal cases, it was proven by other authors to be an efficient cleaning method for beetle specimens thanks to their robust exoskeleton [65]. Samples treated with sodium hydroxide, warm water and soap, ultrasonic bath and brush application of glacial acetic acid produced positive mtDNA extraction results. In contrast, samples immersed in glacial acetic acid, hydrochloric acid, sodium hypochlorite and sodium bicarbonate failed to do so.
Another successful adaptation involves the application of nested PCR or 454 deep-sequencing techniques to extract and amplify ancient DNA (aDNA) from antique insect specimens [77]. The difference between traditional and nested PCR is the inclusion of an additional second round of amplification, where the first reaction product serves as a template for the second round [78]. The use of nested PCR aims to increase the product yield, which could be highly advantageous in the examination of degraded DNA from antique insect specimens [77]. Nested PCR and 454 deep-sequencing were applied in a study on empty pupal cases of blow flies aged 150–300 years old to evaluate their suitability as a standalone alternative molecular analysis technique [77]. For nested PCR, seven empty pupal cases were mechanically cleaned under a microscope before their aDNA was extracted by soaking, heating, freezing and grinding three fragments for each pupal case to a fine powder. Phylogenetic analysis was conducted after nested PCR on the product sequences to determine the species. The 454 deep-sequencing method, also known as the Roche 454, is a next generation sequencing (NGS) method and can simultaneously sequence millions of small DNA fragments from different sources of origin [79]. This reduces the assay run time and increases the sequencing efficiency [79]. Prior to 454 deep-sequencing, a sample of 100 pupal case fragments were assessed using qualitative PCR (qPCR) to determine the quality and quantity of aDNA analysed. The extraction product was then sequenced using the Roche 454 protocol referenced in the published thesis, and the respective sequences were compared against NCBI data [77]. The first COI amplification fragment (larger fragment size, 320 bp) assessed in nested PCR showed no visible amplification, which was expected due to the size of the fragment used and the absence of contamination was corroborated by the controls behaving as expected. The second COI fragment (shorter fragment size, 228 bp) detected amplification in 28.6% of the samples after nested-PCR. After purification, the products were deemed of high quality with sequencing identifying the sample’s phylogenetic position; one of close relatedness to C. vicina and the other of close relatedness to Chrysomya chloropyga (Wiedemann) (Diptera: Calliphoridae). The 454 deep-sequencing approach used on the pooled sample of 100 pupal case fragments generated a phylogenetic tree where a remarkable portion of the product sequences were from the Diptera order and included C. vicina and Ch. putoria (Wiedemann) (Diptera: Calliphoridae) (sister species to Ch. chloropyga). Three species of beetles and three species of moths were subsequently identified within these samples. The presence of beetles was then justified as the result of a recent contamination, possibly during storage; this issue must be taken into consideration especially by taxonomists performing the analyses of an insect assemblage, in particular when working on museum samples [80,81,82]. While nested PCR is inexpensive and does not require computer programming like 454 deep-sequencing, the samples used are highly susceptible to contamination because of the small starting template required (as few as three fragments). In relation to the 454 deep-sequencing approach, despite its ability to simultaneously sequence millions of samples, the financial burden of the technology has halted its widespread implementation. Nonetheless, these methods have shown promising results in amplifying, sequencing and identifying species from aDNA where traditional molecular approaches have fallen short.
4.5. Hydrocarbon Analyses for the Identification of Insects
During metamorphosis, insects undergo ecdysis, where the cuticular skin is moulted to facilitate growth [83]. The superficial lipid layer of the exuviated cuticle contains hydrocarbon compounds that protect the insect from desiccation before moulting [83,84]. As a result of researching alternative taxonomic identification and aging approaches other than morphology and DNA, the field of chemotaxonomy was developed [85]. The study of chemotaxonomy relies on cuticular hydrocarbons (CHCs), analysed by gas chromatography (GC) and mass spectrometry (MS) to determine the presence and abundance of CHCs [84]. Three main classes of hydrocarbons have been identified within insects, namely n-alkenes, olefins and methyl alkanes. Experimental research regarding CHCs and species identification revealed that the organic compounds are not only species-specific but also sex-specific in the type of hydrocarbons produced and their abundance, as well as connected to insect biogeography and the environmental temperature [84,86,87,88,89,90,91,92].
Cuticular hydrocarbon analysis has been developed for several species of blow flies and flesh flies at different stages of life with a specimen age range from a few weeks to over 100 years [93,94,95]. In an experiment using 185 museal dry-preserved adult flies, specimens were extracted with hexane before analysis via GC-MS. The use of CHCs in this study resulted in successful species identification for numerous specimens where gender differentiations were identifiable [95]. The application of CHCs on non-forensically relevant species has identified an abundance of unambiguous CHCs and provides an amplitude of identifiers to analyse [96]. Another noteworthy advantage of CHCs is their ability to separate highly similar insects, known as cryptic species, where morphological approaches fail to do so [89]. While these advantages make the use of CHCs in entomological analyses appealing, there is intra-species variation based on multiple factors, including sexual maturity, gender, age and temperature, that requires further investigation [89,97,98]. The boundary for how many CHCs define a species is still in question and requires further research to provide a standardised threshold for analyses [89]. To the authors’ knowledge, identification of the insects via CHC composition has never been tested for samples of archaeological interest. Research in this field could assist in providing further information alongside morphological analysis to eliminate or confirm species while lowering the costs associated with biomolecular analyses.
5. Information Provided by the Entomofauna Collected from an Archaeological Setting
If correctly identified and contextualised, the presence of an insect in an archaeological site and even sometimes the absence thereof in connection with a cadaver can be indicative of ecological, taphonomical, funeral, cultural and toxicological events regarding the deceased individual and their society [99,100].
5.1. Information regarding the Timeline of Death and Decomposition
The presence of an insect is strictly correlated with and co-dependent on the environmental factors (e.g., temperature, rainfall, presence of a food source) as well as the presence of other organisms (e.g., competitive, predatory, parasitical, commensal or pathogenic), both of which play a pivotal role in their development and reproductive success. The understanding of the relationship between an insect and its environment known as insect ecology can be used to determine information about the immediate surrounding environment and is applicable to describe present and past environmental dynamics [101]. The entomological remains associated with the cadaver can be used to approximate the season in which the events related to death (e.g., burial) have occurred [102,103,104].
In the recovery of a fragmentary right hemimandible with a partial mixed dentition in Upper Awash (Ethiopia), attributed to a Homo erectus estimated to be 2–3.5 years, it was noted the presence of perforations and characteristic damages possibly caused by dermestid beetles [105]. It was suggested that dermestid beetles may have fed and developed on the carcasses of animals in close proximity to the bony remains of the hominin bone, and possibly bore pupation chambers into it. The insects may have taken advantage of the pre-existing mental foramen, enlarging it and then substantially destroying the trabecular bone [105].
A successful inference of seasonality was determined in cadavers as old as 130-160 years exhumated in Arikara (South Dakota, USA) (Appendix A Table A1) [106]. The exhumation of the 278 Arikara burials yielded empty pupal cases of blow flies and flesh flies, but no active adults or immature forms to be reared in a laboratory. In that region, the species of flies found appear on decomposing cadavers by late March to ovi/larviposit and disappear by mid-October, therefore the season of the burial was dated in the period between late March to mid-October. This research provides limited details regarding the insect sampling, examination methods, or if any mechanical or chemical cleaning was performed on the insect remains to facilitate their identification. Differently to that, the description of the entomological assemblage collected from a First World war soldier buried in the Alps of North-Eastern Italy (Appendix A Table A1) was facilitated by applying pre-identification cleaning methods [15]. Numerous empty pupal cases were present on the soldier’s clothing, accessories, and environmental surroundings. Sodium hydroxide cleaning methods removed residual debris and assisted in the visualisation of hidden diagnostic morphologies. Based on the morphological analysis conducted, several blow fly species were identified (Protophormia terraenovae Robineau-Desvoidy and Phormia regina Meigen (Diptera: Calliphoridae), as well as a single empty pupal case of the latrine fly Fannia canicularis (Linneaus) (Diptera: Fanniidae). Specific to the Italian Alps, P. regina and F. cannicularis have been documented during early summer on cadavers, while P. terranovae is active during late spring through to winter [15]. Based on the taphonomical status of the remains, the habits and seasonality of the insect species and the environmental information of the region, the authors propose that the body was colonised by blow flies when it was exposed on the ground prior to the burial. At present, these species have not been listed in the most updated burial successions research, and the lack of adults can be explained by their ability to fly away once their life cycle is completed, an action that would be prevented if the cadaver was buried immediately after death [33,107]. Furthermore, since P. terranovae is active in late spring, it was inferred that the death and early colonisation occurred between late spring and early summer, with P. regina and F. canicularis as secondary colonisers. Efforts have been made to analyse the entomological assemblages recovered from other skeletonised Carspach soldiers, with the identification of Ophyra (Hydrotaea) capensis (Wiedemann) (Diptera: Muscidae) by Huchet; however, the assessment of the assemblage is still ongoing, with the aim to describe the post-mortem journey of the war veterans [108].
The collection of insects in archaeological settings can sometimes lead to the discovery of species currently not listed in the entomo-faunal checklist of the region being studied. Such deficits can be explained by a basic lack of biogeographical studies for that specific environment, a lack of instruments or knowledge to correctly identify the species, the effects of global trade, or the effect of geographical drift/climate change over time [109,110]. In the latter case, archaeoentomological findings become an important resource for paleoecology and palaeoentomology studies by providing a picture of old environmental settings [14,15,111]. An example in recent years of biogeographical change and the development of identification techniques/instruments is the sudden presence of Synthesiomyia nudiseta Van Der Wulp (Diptera: Muscidae) on cadavers discovered indoors throughout North-Western Italy [112]. The combined morphological and molecular protocol, the characteristics of the larval and pupal case specimens, alongside the presence of a silky white substance adhering to debris and textiles, allowed the identification of S. nudiseta as compared to other immature forms belonging to the subfamily Muscinae [74,112,113]. Prior to this observation S. nudiseta had not been listed in the Italian bioclimate, and its presence has only been noticed in recent years. The unanticipated retrieval of S. nudiseta pupal cases from the deceased remains, supports the distribution of the insect species and its interaction with the surrounding environment. It is important to note that a newly introduced species can present different developmental rates under new environmental conditions, compete with native entomofauna and be misidentified with similar species. This area of study is relatively underreported and requires the extension of further research to assist the interpretation of archaeoentomological evidence.
In some circumstances, a total absence of insects can be found in association with human remains. Generally, the complete absence of entomological evidence can lead to the assumption that the event of death likely occurred during winter, when insect migration and activity has ceased [114]. However, it could also suggest that some sort of chemical substance was associated with the body (e.g., pre-mortem use/abuse of toxicological substances or embalming) or a physical impediment for the insects to reach the body was present [35,36,42,115]. Furthermore, the presence of high moisture levels or a strong acid/basic soil pH and sun exposure can naturally degrade entomological evidence over time [102].
5.2. Information regarding Funerary Practices
The investigation of insect species and habits can yield important information about funerary practices and the post-mortem treatment of deceased individuals [13,68,81,116,117]. Practices such as delayed burial, secondary body manipulation and exhumation, alongside the degree of body exposure and methods of embalmment, can be inferred from the presence or absence of insect species [13,118,119,120,121].
The practice of primary and secondary burials can result in the incongruence of entomological evidence and provide important information on the case. Primary burials are used to sever contact with a deceased individual, while secondary burials provide additional contact with the body for ceremonial and spiritual purposes or simply to regain space in graveyard locations [20,42,122,123,124]. These two consecutive ceremonial practices have been documented in the investigation of camelid sacrifice (llamas) in Peru [26]. Entomological evidence was recovered from two contexts that originated from the excavation of the main room of a temple, one containing two incomplete juveniles and the other containing an almost complete skeleton of an adult. Insects were cleaned using an ultrasonic bath prior to being morphologically assessed for species identification. The examination of the anal plate and posterior spiracles identified specimens belonging to blow fly, flesh fly, latrine fly, coffin fly, house fly and cheese skipper fly families. Remains of beetle elytra were also recovered from the surrounding soil. While the collection of specific species of the cheese skipper fly on such buried remains has been found in burial environments only a few times and is considered atypical, the presence of cheese skipper flies in this archaeological setting was likely the result of a secondary burial where late colonisers like cheese skipper flies could colonise the camelid remains [33,125]. Similarly, subaerial insect species have been recovered and documented from hypogeal human remains [126,127].
Other examples of secondary burials of human cadavers have been documented in the Italian cities of Lucca and Naples and in the Sicily region, as part of outdated cultural practices [128,129]. In these regions of the South of Italy, cadavers were exhumed to check the state of decomposition, and once complete desiccation had been achieved, the relatives were allowed to wash and disinfect the remains with alcohol before their relocation to a remote area [130]. The development of funerary structures to facilitate the process of primary and secondary burials has been reported in the literature, and it still exists today to regain space in cemeteries [42,128,131]. One particular archaeoentomological investigation of a Franciscan monastery in the north of Italy revealed the presence of a hypogeal funerary structure containing a putridarium (a sitting chamber to facilitate the decomposition and mummification of a cadaver) [131]. The entomological assessment of the putridarium showed the presence of garbage flies (H. capensis, Diptera: Muscidae) and coffin flies (species non-identified due to a lack of dichotomous keys) (Appendix A Table A1). Hydrotaea capensis is known to be a carrion insect with omnivorous habits that colonise during advanced decomposition; however, in the absence of the typical first-wave colonisers, such as blow flies and flesh flies, they can appear as first colonisers in hypogeal cadavers [29]. Differently, coffin flies are known to be able to reach buried and/or confined remains [33,36]. In this case, the presence of two species with hypogeal habits, as well as the abundance of species that colonise exposed remains, supported the transition through complete decomposition within the putridarium, where the bodies were likely prepared and placed within the crypt immediately after death, to be displayed to family members at a later stage.
Insects recovered from a cadaver can also be useful to gain information about potential post-mortem intentional chemical treatments, such as embalmment, that are used to preserve the cadaver by inhibiting microbial growth and stall the production of putrid odours which attract the insects [132,133]. Various archaeological excavations have led to the retrieval of well-preserved cadavers, suggesting the cadavers were likely to have been treated before burial [134,135,136]. The archaeological investigation of 38 sarcophagi in the Basilica of San Domenico Maggiore (Naples, Italy) constitutes an instructive example of the interaction between embalmment and insect activity and relevant inferences that were achieved about the funerary practices of the Renaissance noblesse [137]. The sarcophagi housed the remains of 15th–18th century princes, princesses and aristocrats, one of whom was King Ferrante II of Aragona. Embalmment in this historical period was not uncommon and was linked to a hierarchical level with the purpose of preserving remains throughout the lengthy funerary practice [138]. Four different cadavers of varying degrees of preservation were investigated, and 842 entomological specimens (both intact and fragmented) were recovered (Appendix A Table A1). The microscopical investigation led to the identification of a large number of Diptera, followed by Lepidoptera and Coleoptera. The skeletonised cadaver exhibited the most abundant entomological presence (96.7%), followed by the burnt, disarticulated and well-preserved remains (1.5%, 1.2% and 0.6%, respectively). Insects like Fannia sp., H. capensis and Conicera tibialis Schmitz (Diptera: Phoridae) are late colonisers, and the absence of first-wave colonisers, such as blow flies and flesh flies, suggested that the remains were confined indoors for an extended period of time. Inside the sarcophagi, the abundance of empty pupal cases, remains of flies, adults and larval exuviae of larder beetles suggests that the cadavers were placed inside them after being colonised; inside the sarcophagi, the insects consumed the remains, but at the completion of their life cycle they were unable to depart due to the restricted environment. This insect assemblage was coherent with the practice of lengthy funeral processions where remains were kept in concealed indoor environments to undergo mummification. Furthermore, clothes moths (Lepidoptera: Tineidae) were also recovered in association with the cadavers’ ceremonial clothing [138]. The presence of clothes moths is typical of the final successional wave, in the presence of dry remains, hair and textiles of clothing or upholstery. The act of stuffing the clothes of a deceased individual with dry vegetable matter for presentation purposes has also been documented as a common funerary practice and can attribute to the attraction of other non-cadaverous insect species [139]. The presence of artefacts and their compositions have also been suggested to be a potential preservative of remains, as was in the case of the pre-historic graves in Canada [140].
5.3. Information regarding Causes of Death
Insects that feed on a cadaver incorporate information from it, in particular about substances to which the body had been exposed to or had consumed in life (e.g., recreative or medical drugs), and that, in some circumstances, could have led to the death (e.g., poison or overdose) [141]. In entomotoxicological analyses, the target matrix is the chitin in the insect’s exoskeleton. Chitin is also abundant in pupal cases, exuviated beetle skins and beetle faecal materials all of which are typical specimens recovered from archaeological settings [30,142]. The toxicological analysis of insect remains can take place even after centuries since the colonisation period, and the results can provide insights into the living habits and/or the cause and circumstances of death of the cadaver under examination [100,143]. Generally, entomotoxicological data provides information regarding the type of drugs or other chemical substances present in the body. The concentration of any chemical substance, including xenobiotics, measured in the insects does not directly reflect the exposure dose of the living individual, human or non-human. This is due to several factors, including the scarce knowledge of insect pharmacokinetics, the different metabolic pathways among taxa (e.g., insects and humans) and the effects that the parent chemical and its metabolite/s could exert on the different insect species colonising the cadaver [115]. Furthermore, the anatomical source, the specific substance and the stage of the insect’s development can influence the quantitation [141,143,144,145]. Lastly, the analytical techniques applied might be obsolete, not standardised or correctly validated, thus limiting the detection and quantification abilities of the instrument [146].
In the investigation of a mummified cadaver discovered at the deceased’s residence, antidepressants such as amitriptyline and nortriptyline were detected in both empty pupal cases of coffin flies and exuviated exoskeletons of larder beetles, with a higher quantity detected in the former [142]. The study of the food chain explained this difference, with flies feeding directly on the cadaver’s soft tissues, while beetles feed on tough outer layers or predated on flies. Overall, the entomotoxicological findings were used to support the determination of the cause of death as a multiple drug intoxication. The detection of cocaine and opiates has also been successfully quantitated from empty pupal cases where anatomical tissues and bodily fluids were no longer present at the time of discovery [147,148].
6. Conclusions
The correct collection, examination and identification of insect specimens in archaeological settings, if correctly correlated with the insect’s species ecology and habits, can provide a wealth of knowledge regarding the past environments, the funerary practices, the life habits and the causes/circumstances of death of the associated cadaver/s. When no other information is available on the cadaver in an archaeological context, insects have proven to be alternative witnesses because of their synanthropic relationship, the robustness of their body and their capability of storing chemo-toxicological data. The driving factor behind analysing archaeoentomological samples is to expand the knowledge of past cultural practices and the way human living has evolved. Currently, the published literature in this field is heavily skewed in favour of European countries such as Italy, while the literature in other countries remains scarce and underreported. This literature review emphasises the importance of the collaborative effort of archaeologists and entomologists to provide more information regarding the past of mankind and the environment.
Author Contributions
Conceptualization, P.A.M. and A.D.H.; Resources, P.A.M. and A.D.H.; Data curation P.A.M., A.D.H., E.E.G.; Supervision, P.A.M.; Project Administration, P.A.M.; Writing—Original Draft Preparation, A.D.H.; Writing—Review and Editing, P.A.M., A.D.H., E.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors acknowledge the contributions of Jean-Bernard Huchet in providing editorial advice and works of published funerary archaeoentomology casework. Furthermore, the authors thank Paul and Philip Buckland for their availability to provide us with manuscripts of difficult access and Alessandra Cinti and Francesco Merlo for providing the pupal case cluster pictured in Figure 2. Finally, we thank the three reviewers for the constructive comments to improve the paper and for suggesting additional research to be included for completeness.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Current published research reporting entomological assemblages recovered during archaeological excavations in relation to human cadavers. Reported locations have been alphabetised by continent. Insect and other arthropod species are reported as described in the published research. L = larvae unspecified stage, LII = larvae in second stage of life, LIII = larvae in third stage of life, P = pupae (full pupal case), Pu = puparia (empty pupal case), PP = parasitised pupal case, PC = pupation chamber, BD = bone damage, A = adult, FA = fragmented adult, C = cocoon, Ch = chrysalis, O = ootheca, USL = unprovided stage of life, NR = not recovered.
Table A1.
Current published research reporting entomological assemblages recovered during archaeological excavations in relation to human cadavers. Reported locations have been alphabetised by continent. Insect and other arthropod species are reported as described in the published research. L = larvae unspecified stage, LII = larvae in second stage of life, LIII = larvae in third stage of life, P = pupae (full pupal case), Pu = puparia (empty pupal case), PP = parasitised pupal case, PC = pupation chamber, BD = bone damage, A = adult, FA = fragmented adult, C = cocoon, Ch = chrysalis, O = ootheca, USL = unprovided stage of life, NR = not recovered.
| Continent | Country | Region/ State/ Province/ County | City/Town | Type of Study | Number of Bodies | Insect and Other Arthropod Species Identified | Citation | ||
|---|---|---|---|---|---|---|---|---|---|
| Diptera | Coleoptera | Other | |||||||
| Africa | Egypt | n.a. | n.a. | Museal mummified remains | 2 | NR | Attagenus sp. Latreille (Dermestidae)—FA | Genus? sp.? (Lepidoptera: Tineidae)—C Tinea pellionella Linnaeus (Lepidoptera: Tineidae)—C | [57] |
| n.a. | n.a. | Museal mummified remains | 1 | Genus? sp.? (Calliphoridae)—P Piophila casei (Linnaeus) (Piophilidae)—P | Genus? sp.? (Dermestes)—A Genus? sp.? (Staphylinidae)—L | NR | [56] | ||
| America (South) | Brazil | Minas Gerais | Itacambria | Mummified remains | 1 | Megaselia scalaris (Loew) (Phoridae)—Pu | NR | NR | [55] |
| Paranáb | Altonia | Funerary urn | 1 | Euxesta sp. (Ulidiidae)—A | Bembidion sp. (Carabidae)—A Corticaria sp. (Latridiidae)—A Cossonus sp. (Curculionidae)—A Genus? sp.? (Curculionidae)—FA Genus? sp.? (Latridiidae)—FA Genus? sp.? (Scarabidae)—FA Genus? sp.? (Scolytinae)—FA Lagria villosa (Fabricius) (Tenebrionidae)—A Nilio sp. (Tenebrionidae)—FA Osoriellus sp. (Staphylinidae)—A Xyleborus affinis (Eichhoff) (Curculionidae)—A | Campontus sp. (Hymenoptera: Formicidae)—A Genus? sp.? (Blattaria)—A Genus? sp.? (Hemiptera: Cicadellidae)—A Genus? sp.? (Lepidoptera: Tineidae)—L Hypoponera sp. (Hymenoptera: Formicidae)—A | [20] | ||
| Chile | Atacama | Calama | Funerary bundle | 7 | Genus? sp.? (Sarcophagidae)—Pu | Anthrenus sp. (Dermestidae)—L Dermestes peruvianus Laporte (Dermestidae)—USL | Genus? sp.? (Lepidoptera: Tineidae)—C | [80] | |
| Colombia | Andean region | Bogotá | La Candelaria cave | 2 | Genus? sp.? (Calliphoridae)—A Genus? sp.? (Muscidae)—Pu Genus? sp.? (Sarcophagidae)—Pu | Dermestes maculatus De Geer (Dermestidae)—A Genus? sp.? (Dermestidae)—L Genus? sp.? (Histeridae)—A, FA Genus? sp.? (Trogidae)—FA Lasioderma serricorne (Fabricius) (Ptinidae) | NR | [117] | |
| Ecuador | Napo | n.a. | Funerary urn | 1 | NR | NR | Genus? sp.? (Blattodea)—BD possibly caused by termites | [22] | |
| Argentina | Beunos Aires | La Plata | Buried remains | 1 | Fannia canicularis (Lineaus) (Fanniidae)—Pu Genus? sp.? (Sarcophagidae)—P Hydrotaea aenescens (Wiedemann) (Muscidae)—Pu, FA Megaselia scalaris (Loew) (Phoridae)—Pu Muscina stabulans Fallén (Muscidae)—Pu | Atheta sp. (Staphylinidae)—A Carpophilus sp. (Nitidulidae)—FA Genus? sp.? (Dermestidae)—L Genus? sp.? (Tenebrionidae)—L | Genus? sp.? (Acari: Gamasidae)—A Genus? sp.? (Blattodea)—O Genus? sp.? (Dermaptera: Anisolabididae)—FA Genus? sp.? (Juliformia)—A Genus? sp.? (Polydesmida)—A Tineola bisselliella (Hummel) (Lepidoptera: Tineidae)—Pu | [125] | |
| America (North) | Belize | n.a. | n.a. | Maya mortuary cave | 25 | NR | Genus? sp.? (Dermestidae)—PC | Genus? sp.? (Blattodea)—BD possibly caused by termites | [51] |
| Canada | New Brunswick | n.a. | Buried remains | NR | Cynomyopsis cadaverina (Robineau-Desvoidy) (Calliphoridae)—Pu Genus? sp.? (Heleomyzidae)—Pu Hydrotaea sp. (Muscidae)—Pu Muscina assimilis (Fallén) (Muscidae)—Pu Phormia regina (Meigen) (Calliphoridae)—Pu Protophormia terraenovae (Robineau-Desvoidy) (Calliphoridae)—Pu | NR | NR | [140] | |
| Mexico | Coahuila | n.a. | Funerary cave | 1 | Fannia sp. (Fanniidae)—Pu Genus? sp.? (Sarcophagidae)—Pu Synthesiomyia nudiseta Van der Wulp (Muscidae)—Pu | Dermestes carnivorous Fabricius (Dermestidae)—A Genus? sp.? (Staphylinidae)—USL Saprinus (s. str.) alienus J.L. Le Conte (Histeridae)—FA Niptus sp. (Anobiidae)—A Omorgus sp. (Trogidae)—FA Xerosaprinus (s. str.) coerulescens (J.L. Le Conte) (Histeridae)—FA Xerosaprinus (s. str.) vitiosus (J.L. Le Conte) (Histeridae) –A | Acromyrmex veriscolor (Pergande) (Hymenoptera: Formicidae)—A Genus? sp.? (Lepidoptera: Family?)—USL | [133] | |
| Peru | Amazonas | Chachapoyas | Mummy bundle | 1 | Genus? sp.? (Calliphoridae)—Pu | NR | Genus? sp.? (Hymenoptera)—A Genus? sp.? (Lepidoptera: Tineidae)—Pu, FA | [111] | |
| La Libertad | Trujillo | Entombed remains | 1 | Cochliomyia macellaria (Fabricius) (Calliphoridae)—Pu Compsomyops verena (Walker) (Calliphoridae)—Pu Hydrotaea aenescens (Wiedemann) (Muscidae)—Pu Sarcophaga sp. (Sarcophagidae)—Pu Synthesiomyia nudiseta Van der Wulp (Muscidae)—Pu | Omorgus suberosus (Fabricius) (Trogidae)—FA | Genus? sp.? (Hymenoptera: Pteromalidae?)—PP | [99] | ||
| Trujillo | Moche pyramids | 1 | Cochliomyia macellaria (Fabricius) (Calliphoridae)—Pu Compsomyiops verena (Walker) (Calliphoridae)—Pu Genus? sp.? (Sarcophagidae)—Pu Hydrotaea aenescens (Wiedemann) (Muscidae)—Pu Synthesiomyia nudiseta Van der Wulp (Muscidae)—Pu | Omorgus suberosus (Fabricius) (Trogidae)—FA | Muscidifurax or Sphalangia sp.? (Hymenoptera: Pteromalidae?)—PP | [68] | |||
| 1 | NR | NR | Genus? sp.? (Blattodea)—BD possibly caused by termites | [48] | |||||
| Lima | Lurin | Pachacamac site | n.a. | NR | NR | Genus? sp.? (Pseudoscorpiones: Cheiridiidae)—A | [31] | ||
| Untied States of America | South Dakota | n.a. | Buried remains | n.a. | Genus? sp.? (Calliphoridae)—P Genus? sp.? (Sarcophagidae)—P | NR | NR | [106] | |
| Asia | Israel | n.a. | n.a. | Munhata site | n.a. | NR | Dermestes sp. (Dermestidae)—PC | NR | [49] |
| Palestine | Jericho | Jericho site | n.a. | NR | Dermestes sp. (Dermestidae)—PC | NR | [49] | ||
| Europe | Austria | Niederösterreich | Lanzenkirhcen | Buried remains | 7 | Calliphora sp. (Calliphoridae)—USL Lucillia sp. (Calliphoridae)—USL Ophyra capensis (Wiedemann) (Muscidae)—P Ophyra sp. (Muscidae)—Pu Protophormia terranovae Robinaeu-Desvoidy (Calliphoridae)—USL | NR | NR | [126,127] |
| Vienna | Vienna | Skeletal remains | 3 | Calliphora sp. (Calliphoridae)—Pu Conicera tibialis Schmitz (Phoridae)—Pu Lucilia sp. (Calliphoridae)—Pu Sarcophaga sp. (Sarcophagidae)—Pu | NR | NR | [104] | ||
| France | Alsace | Carspach | Skeletal remains | n.a. | Ophyra capensis (Wiedeman) (Muscidae)—Pu | NR | NR | [107] | |
| Centre-Val de Loire | Clery-Saint-André | Sarcophagus | 1 | NR | Attagenus sp. (Dermestidae)—USL Bruchus sp. (Bruchidae)—USL Leistus spinibarbis (Dejean) (Carabidae: Nebriinae)—A Necrobia salina Fairmaire and Laboulbene (Carabidae: Nebriinae)—A Othius laeviusculus Stephens (Staphylinidae: Staphylininae)—USL Oxyomus sylvestris Scopoli (Aphodiidae)—USL Platystethus arenarius (Geoffroy) (Staphylinidae: Oxytelinae)—USL Ptinus sp. (Anobiidae: Ptininae)—USL Tasgius ater (Gravenhorst) (Staphylinidae: Staphylininae)—USL | NR | [21] | ||
| Hauts-de-France | Lille | Coffins | 22 | Calliphora vicina Robineau-Desvoidy (Calliphoridae)—Pu, A Conicera tibialis Schmitz (Phoridae)—L, P Fannia scalaris (Fabricius) (Fanniidae)—L Fannia manicata (Meigen) (Fanniidae)—L, A Hydrotaea capensis (Weidemann) (Muscidae)—LII, LIII P, A Hydrotaea sp. (Muscidae)—P Leptocera caenosa (Robdani) (Sphaeroceridae)—L, P, A Megaselia sp. (Phoridae)—L, P Megaselia rufipes (Meigen) (Phoridae)—P Phoridae sp. (Phoridae)—P Triphelba hyalinata (Meigen) (Phoridae)—A | Omalium rivulare (Paykull) (Staphylinidae)—A Philonthus sp. (Staphylinidae)—A Staphylinidae sp. (Staphylinidae)—L | NR | [103] | ||
| Ireland | Kildimo | Limerick | Cemeterial buried remains | 6 | Calliphora vicina Robineau-Desvoidy (Calliphoridae)—P, FA Calliphora vomitoria (Linneaus) (Calliphoridae)—P, FA Genus? sp.? (Phoridae)—P | NR | NR | [121] | |
| Italy | Campania | Naples | Church | 4 | Coincera cfr tibialis Schmitz (Phoridae)—Pu Fannia sp. (Fanniidae)—Pu Hydrotaea capensis (Wiedemann) (Muscidae)—Pu, FA | Genus? sp.? (Dermestidae)—L Ptinus sp. (Ptinidae)—FA | Genus? sp.? (Tineidae)—L, C | [137] | |
| Monastery | 1 | Hermetia illucens (Linnaeus) (Stratiomyidae)—L | NR | NR | [109] | ||||
| Lombardia | Azzio | Crypt | n.a.. | Genus? sp.? (Phoridae)—Pu Hydrotaea capensis (Wiedemann) (Muscidae)—Pu | Cryptophagus montanus C.Bristout de Barneville (Cryptophagidae)—L Genus? sp.?—A Quedius sp. (Staphylinidae) –A | Genus? sp.? (Acarina)—A | [131] | ||
| Lazio regione | Rome | Catacomb | 2 | Calliphora sp. (cf. vicina) (Calliphoridae)—Pu Hydrotaea capensis (Wiedemann) (Muscidae)—Pu | Ablattaria laevigata (Fabricius) (Silphidae)—FA Alphitiobius diaperinus Panzer (Tenebrionidae)—USL Carabus (Archicarabus) alysidotus Illiger (Carabidae)—FA Dermestes sp. (Dermestidae)—L, FA Geotrupes spiniger (Marsham) (Geotrupidae)—FA Jekelius intermedius (Costa) (Geotrupidae)—FA Necrobia rufipes (De Geer) (Cleridae)—FA | NR | [23] | ||
| Marche | Urbino | Church | 1 | Hydrotaea leucostoma (Wiedemann) (Muscidae)—P, FA | Genus? sp.? (Dermestidae)—L, FA | Cydia splendana (Hübner) (Lepidoptera: Torticidae)—Ch | [116] | ||
| Pieddmont | Fossano | Monastery | 1 | Hydrotaea capensis (Wiedemann) (Musdicae)—Pu, A | Anthrenus verbascii Linneaus (Dermestidae)—A Genus? sp.? (Carabidae)—A Langelandia anophtalma Aubé (Zopheridae)—A Mycetaea subterranean (Fabricius)—A | Genus? sp.? (Aranea)—FA Messor sp. (Hymenoptera: Formicidae)—A | [124] | ||
| Sardinia | n.a. | Cathedral | 2 | Calliphora vicina Robineau-Desvoidy (Calliphoridae)—Pu Genus? sp.?—FA Hydrotaea capensis (Wiedemann) (Muscidae)—Pu Phormia regina Meigen (Calliphoridae)—Pu Sarcophaga sp. (Sarcophagidae)—Pu | Genus? sp.? (Tenebrionidae)—FA Saprinus semistriatus (Scriba) (Histeridae)—FA | Gen? sp.? (Tineidae)—C | [110] | ||
| Sicily | Palermo | Catacomb | 667 | Coincera tibialis Schmitz (Phoridae)—FA Fannia scalaris (Fabricius) (Fanniidae) -USL Hydrotaea ignava (Harris) (Muscidae)—USL Leptocera sp. (Sphaeroceridae)—USL | Gibbium psylloides Czenpiński (Ptinidae)—A Necrobia rufipes (De Geer) (Cleridae)—USL Oryzaephilus surinamensis (Linneaus) (Silvanidae)—A | Genus? sp.? (Arachnida: Pseudoscorpions)—FA Genus? sp.? (Hymenoptera: Braconidae)—USL Tinella Pellionella Linneaus (Tineidae)—USL | [140] | ||
| Trentino-Alto Adige | Laghi | Buried remains | 1 | Fannia canicularis (Linneaus) (Fanniidae)—Pu Phormia regina Meigen (Calliphoridae)—Pu Protophormia terraenovae (Robineau-Desvoidy) (Calliphoridae)—Pu | NR | NR | [15] | ||
| Tuscany | Florence | Embalming jars | 10 jars containing soft tissue | Conicera tibialis Schmitz (Phoridae)—Pu Hydrotaea capensis (Wiedemann) (Muscidae)—Pu | Ptinus dibius Sturm (Ptinidae)—FA Ptinus subpilosus Sturm (Ptinidae)—FA | NR | [119] | ||
| Lucca | Entombed remains | 1 | Conicera sp. (Phoridae)—Pu Hydrotaea capensis (Wiedemann) (Muscidae)—Pu Muscina sp. (Muscidae)—Pu | Anobium punctatum (Ptinidae)—FA Gnathoncus sp. (Histeridae)—FA Necrobia sp. (Cleridae)—FA Sitophilus granarius (Curculionidae)—FA Trox scaber (Trogidae)—FA | Genus? sp.? (Hymenoptera)—FA Genus? sp.? (Ichneumonidae: Julida)—FA Genus? sp.? (Ichneumonidae: Scorpiones)—FA Genus? sp.? (Lepidoptera: Tineidae)—C Genus? sp.? (Lepidoptera: Pyralidae)—C | [129] | |||
| Monticiano | Entombed remains | 1 | Hydrotaea capensis (Wiedemann) (Muscidae)—Pu | NR | Genus? sp.? (Lepidoptera: Tineidae)—FA | [81] | |||
| Veneto | Venice | Monastery | 1 | Chrysomya albiceps (Wiedemann) (Calliphoridae)—P, Pu | Anthrenus sp. (Dermestidae)—L Anthrenus (Nathrenus) verbasci (Linneaus) (Dermestidae)—L, A Attagenus (s. str.) unicolor (Brahm) (Dermestidae)—L Necrobia rufipes (De Geer) (Cleridae)—A | Genus? sp.? (Arachnida: Pseudoscorpions)—USL | [120] | ||
| n.a. | Mass grave | 7 | Protophormia terraenovae Robinaeu-Desvoidy (Calliphoridae)—Pu | NR | NR | [107] | |||
| Malta | Northern region of Malta | St Pauls Bay | Xemxija tombs | 15,000 fragmented bones | NR | Genus? sp.? (Dermestidae)—PC | NR | [50] | |
| Portugal | Lisboa region | Lisbon | Crypt | 1 | Ophyra capensis (Wiedemann) (Muscidae)—Pu, FA | NR | Genus? sp. (Diplopoda) | [39,40] | |
| Spain | Macaronesia | Las Palmas de Gran Canaria | Mummified remains | 3 | Chrysomya albiceps (Wiedemann) (Calliphoridae)—Pu Genus? sp.? (Fanniidae)—USL | Dermestes maculatus De Geer (Dermestidae)—A, FA Mezium americanum Laporte de Castelnau (Anobiidae)—A Necrobia rufipes (De Geer) (Cleridae)—A Stegobium paniceum (Linneaus) (Anobiidae)—A | Genus? sp.? (Tineidae)—USL Pheidole sp. (Hymenoptera: Formicidae)—FA Nosopsyllus fasciatus (Siphonaptera: Ceratophyllidae)—FA | [136] | |
| Sweden | Scania | Lund | Coffin | 1 | Chloromyia formosa (Scopoli) (Stratiomyidae)—USL Genus? sp.?—USL Genus? sp.? (Anisopodidae)—USL Genus? sp.? (Calliphoridae)—USL Genus? sp.? (Chaoboridae)—USL Genus? sp.? (Heleomyzidae)—USL Genus? sp.? (Rhagionidae)—USL Mochlonyx sp. (Chaoboridae)—USL | Amara ovata (Fabricius) (Carabidae)—USL Anobium punctatum De Geer (Anobiidae)—USL Atomaria munda Erichson (Cryptophagidae)—USL Atomaria nigripennis Kugelann (Cryptophagidae)—A Attagenus pellio (Linneaus) (Dermestidae)—USL Corticaria fulva (Comolli) (Latridiidae)—USL Cryptophagus dentatus (Herbst) (Cryptophagidae)—USL Cryptophagus cf. distinguendus Strum (Cryptophagidae)—USL Cryptophagus saginatus Strum (Cryptophagidae)—USL Cryptophagus cellaris (Scopoli) (Cryptophagidae)—USL Dermestes lardarius Linneaus (Dermestidae)—USL Epauloecus unicolor (Piller & Mitterpacher) (Ptinidae)—A Ernobius mollis (Linneaus) (Anobiidae)—USL Latridius minutus (Linneaus) (Latridiidae)—USL Meligethes cf. aeneus (Fabricius) (Nitidulidae)—USL Mycetaea subterranea (Fabricius) (Endomychidae)—USL Ocys quinquestriatus (Gyllenhal) (Carabidae)—USL Orthoperus sp. (Corylophidae)—USL Phyllodrepa puberula (Bernhauer) (Staphylinidae)—USL Polydrusus cf. flavipes (De Geer) (Curculionidae)—USL Protapion sp. (Apionidae) –USL Ptinus fur (Linneaus) (Ptinidae)—USL Stegobium paniceum (Linneaus) (Anobiidae)—USL Typhaea sp. (Mycetophagidae)—USL | Aphidoidea sp. (Hemiptera: Aphididae)—USL Cheyletus sp. (Trombidformes: Cheyletidae)—USL Cimex lectularius Linneaus (Hemiptera: Cimicidae)—USL Coleophora sp. (Lepidoptera: Coleophoridae)—USL Dahlicini sp. (Lepidoptera: Psychidae)—USL Eulaelaps stabularis (C.L. Koch) (Mesostigmata: Haemogamasidae)—A Eulohmannia ribagai (Berlese) (Sarcoptiformes: Eulohmannidae)—USL Eupteryx aurata (Linneaus) (Hemiptera: Cicadellidae)—USL Forficula auricularia Linneaus (Dermaptera: Forficulidae)—USL Genus? sp.? (Araneae)—USL Genus? sp.? (Hymenoptera)—USL Genus? sp.? (Hymenoptera: Pteromalidae)—USL Hemannia sp. (Oribatida: Hermanniidae)—USL Hypoaspis sp. (Mesostigmata: Laelapidae)—USL Lasius niger (Linneaus) (Hymenoptera: Formicidae)—USL Lygaeidae sp. (Hemiptera: Lygaeidae)—USL Macrosiphoniella cf. abrotani (Walker) (Hemiptera: Aphididae)—FA Parasitus sp. (Mesostigmata: Parasitidae)—USL Phorodon humuli (Schrank) (Hemiptera: Aphididae)—USL Picrostigeus/Batakomacrus sp. (Hymenoptera: Ichneumonidae)—USL Scotophaeus cf. scutulatus (L. Koch) (Araneae: Gnaphosidae)—USL Steatoda cf. bipunctata (Linneaus) (Araneae: Theridiidae)—USL Tegenaria domestica (Clerck) (Araneae: Agelenidae)—USL Tinea pellionella (Linneaus) (Lepidoptera: Tineidae)—USL | [135] | |
| UK | England | Manchester | Museal mummified remains | 4 | Genus? sp.? (Chrysomya)—L, Pu Genus? sp.? (Piophilidae)—Pu, A Musca domestica (Linnaeus) (Muscidae)—Pu | Genus? sp.? (Carabidae)—FA Gibbium psylloides Czenpiński (Ptinidae)—FA Mesotsenopa sp. (Tenebrionidae)—FA Necrobia rufipes (Fabricius) (Cleridae)—A | Blatta orientalis Linnaeus (Dictyoptera: Blattodea)—O | [118] | |
| North Yorkshire | n.a. | Cathedral | 1 | Genus? sp.? (Phoridae)—Pu Genus? sp.? (Sphaeroceridae)—Pu | Aleocharinae sp. (Staphylinidae)—USL Cryptophagus sp. (Cryptophagidae)—FA Mycetaea subterranea (Marsham) (Endomycidae)—FA Phyllodrpa floralis (Paykull) (Staphylinidae)—USL Quedius mesomelinus (Marsham) (Staphylinidae)—FA Rhizophagus parallelocollis Gyllenhal (Monotomidae)—FA | NR | [16] | ||
| Scotland | Black Burn | Monastery | 3 | Fannia sp. (Fannidae)—Pu Genus? sp.? (Heleomyzidae)—Pu Genus? sp.? (Leptoceridae)—FA Genus? sp.? (Muscidae)—Pu Genus? sp.? (Phoridae)—Pu Genus? sp.? (Pscyhodidae)—Pu Terrilmosina racovitzai (Bezzi) (Sphareoceridae)—P Trichocera sp. (Trichoceridae)—L | Acrolocha sulcula (Stephens) (Staphylinidae)—USL Aleochara sp. (Staphylinidae)—USL Genus? sp.? (Staphylinidae: Aleocharinae)—USL Aphodius rufipes (Linneaus) (Scarabaeidae)—USL Atomaria sp. (Cryptophagidae)—USL Catops sp. (Leiodidae)—USL Corticaria sp. (Cryptophagidae)—USL Cryptophagus scutellatus (Newman) (Cryptophagidae)—USL Cryptophagus sp. (Cryptophagidae)—USL Lathridius minutus (Linnaeus) (Lathridiidae)—USL Ootypus globosus (Waltl) (Cryptophagidae)—USL Philonthus sp. (Staphylinidae)—USL Ptenidium sp. (Ptiliidae) –USL Quedius mesomelinus (Marsham) (Staphylinidae)—USL Rhizophagus parallelocollis (Gyllenhal) (Rhizophagidae)—USL Tipnus unicolor (Piller and Mitterpacher) (Ptinidae)—USL Trichocellus sp. (Carabidae)—FA Xylodromus concinnus (Marsham) (Staphylinidae)—USL | NR | [19] | ||
| Glasgow | Cathedral | 1 | NR | Rhizophagus parallelocollis Gyllenhal (Monotomidae)—USL | NR | [18] | |||
References
- Whitworth, T. Fossilized human remains from northern Kenya. Nature 1960, 185, 947–948. [Google Scholar] [CrossRef]
- Dent, B.B.; Forbes, S.L.; Stuart, B.H. Review of human decomposition processes in soil. Environmental 2004, 45, 576–585. [Google Scholar] [CrossRef]
- Zumpt, F. Myiasis in Man and Animals in the Old World: A Textbook for Physicians, Veterinarians and Zoologists; Butterworth: London, UK, 1965. [Google Scholar]
- Voss, S.C.; Spafford, H.; Dadour, I.R. Annual and seasonal patterns of insect succession on decomposing remains at two locations in Western Australia. Forensic Sci. Int. 2009, 193, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Vass, A.A. Beyond the grave-understanding human decomposition. Microbiol. Today 2001, 28, 190–193. [Google Scholar]
- Goff, M.L. Early postmortem changes and stages of decomposition. In Current Concepts in Forensic Entomology, 1st ed.; Amendt, J., Campobasso, C., Goff, M., Grassberger, M., Eds.; Springer Dordrecht: Dordrecht, The Netherlands, 2009; pp. 1–24. [Google Scholar]
- Huchet, J.B. L’Archéoentomologie funéraire: Une approche originale dans l’interprétation des sépultures. Bull. Mem. Soc. Anthropol. Paris 1996, 8, 299–311. [Google Scholar] [CrossRef]
- Mégnin, P. La Faune des Cadavres: Application de l’entomologie à la Médecine Légale; G. Masson: Paris, France, 1894. [Google Scholar]
- Wells, J.D. A forensic entomological analysis can yield an estimate of postmortem interval, and not just a minimum postmortem interval: An explanation and illustration using a case. J. Forensic Sci. 2019, 64, 634–637. [Google Scholar] [CrossRef]
- Matuszewski, S. Post-mortem interval estimation based on insect evidence: Current challenges. Insects 2021, 12, 314. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M. Best practice in forensic entomology—Standards and guidelines. Int. J. Leg. Med 2007, 121, 90–104. [Google Scholar] [CrossRef]
- Giordani, G. Non-Invasive Approaches to Morphological and Molecular Identification of Insects from Museum, Archaeo-funerary and Forensic Contexts. Ph.D. Thesis, University of Huddersfield, Huddersfield, UK, 2019. [Google Scholar]
- Huchet, J.B. Insect remains and their traces: Relevant fossil witnesses in the reconstruction of past funerary practices. Anthropologie 2014, 52, 329–346. [Google Scholar]
- Buckland, P.I.; Buckland, P.C.; Olsson, F. Paleoentomology: Insects and other arthropods in environmental archaeology. In Encyclopedia of Global Archaeology, 2nd ed.; Smith, C., Ed.; Springer: New York, NY, USA, 2020; pp. 8291–8312. [Google Scholar]
- Vanin, S.; Turchetto, M.; Galassi, A.; Cattaneo, C. Forensic entomology and the archaeology of war. J. Confl. Archaeol. 2009, 5, 127–139. [Google Scholar] [CrossRef]
- Panagiotakopulu, E.; Buckland, P.C. Forensic archaeoentomology—An insect fauna from a burial in York Minster. Forensic Sci. Int. 2012, 221, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Buckland, P.C.; Cooper, G.R.; Sadler, J.P. A Bibliography of Quaternary Entomology. Trans. Ent. Soc. 2004, 116, 329–346. [Google Scholar]
- Buckland, P.C. Insect fauna from within the cranium of skeleton 570. In Excavations at Glasgow Cathedral; Routledge: Abingdon, UK, 1988; Volume 1997, p. 157. [Google Scholar]
- McCormick, F.; Buckland, P.; Carter, S.; Cerón-Carrasco, R.; Fawcett, R.; Ford, B.; Flavell, D.A.; Hamilton-Dyer, S.; Lorimer, D.H.; Mills, C. Excavations at Pluscarden Priory, Moray; Society of Antiquaries of Scotland: Edinburgh, Scotland, 1995. [Google Scholar]
- Macari, B.P. Arqueoentomologia: Um estudo de Caso Tupiguarani, Altônia, Paraná, Brasil; Federal University of Paraná: Paraná, Brasil, 2013. [Google Scholar]
- Huchet, J.B. Les sépultures prestigieuses de l’église Notre-Dame de Cléry-saint-André (Loiret): Etude pluridisciplinaire du caveau de Louis XI. In Les Sépultures Prestigieuses de l’église Notre-Dame de Cléry-Saint-André (Loiret); Georges-Zimmermann, P., Ed.; L’Harmattan: Paris, France, 2015; pp. 172–183. [Google Scholar]
- Jastremski, N.A.; Sánchez-Polo, A. Human Skeletal Remains Recovered from a Napo Funerary Urn in the Ecuadorian Amazon: A Taphonomic and Mortuary Assessment. Bioarchaeol. Int 2021, 5, 143. [Google Scholar] [CrossRef]
- Huchet, J.B.; Castex, D. The walking dead-life after death—Archaeoentomological evidence in a Roman catacomb: (Saints Marcellinus and Peter, central area, 1st–3rd century AD). In The Routledge Handbook of Archaeothanatology, 1st ed.; Knüsel, C.J., Schotsman, E.M.J., Eds.; Routledge: Oxforshire, UK, 2022; pp. 481–498. [Google Scholar]
- Hope, F.W. Observations on some mummified beetles taken from the inside of a mummified ibis. Trans. Ent. Soc. 1842, 3, 191–193. [Google Scholar]
- Huchet, J.B.; Callou, C.; Lichtenberg, R.; Dunand, F. The dog mummy, the ticks and the louse fly: Archaeological report of severe ectoparasitosis in Ancient Egypt. Int. J. Paleopathol. 2013, 3, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Giordani, G.; Erauw, C.; Eeckhout, P.A.; Owens, L.S.; Vanin, S. Patterns of camelid sacrifice at the site of Pachacamac, Peruvian Central Coast, during the Late Intermediate Period (AD1000–1470): Perspectives from funerary archaeoentomology. J. Archaeol. Sci. 2020, 114, 105065. [Google Scholar] [CrossRef]
- Han, H.; Liu, Z.; Meng, F.; Jiang, Y.; Cai, J. Identification of olfactory genes of a forensically important blow fly, Aldrichina grahami (Diptera: Calliphoridae). PeerJ 2020, 8, e9581. [Google Scholar] [CrossRef]
- Barton, P.S.; Archer, M.S.; Quaggiotto, M.M.; Wallman, J.F. Invertebrate succession in natural terrestrial environments. In Forensic Entomology: The Utility of Arthropods in Legal Investiations, 3rd ed.; Byrd, J.H., Tomberlin, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 141–153. [Google Scholar]
- Smith, K.G. A Manual of Forensic Entomology, 1st ed.; Cornell University Press: New York, NY, USA, 1986. [Google Scholar]
- Magni, P.A.; Voss, S.C.; Testi, R.; Borrini, M.; Dadour, I.R. A biological and procedural review of forensically significant Dermestes species (Coleoptera: Dermestidae). J. Med. Entomol. 2015, 52, 755–769. [Google Scholar] [CrossRef]
- Morrow, J.J.; Taylor, L.; Peck, L.; Elowsky, C.; Owens, L.S.; Eeckhout, P.; Reinhard, K.J. Pseudoscorpions of the family Cheiridiidae (Arachnida: Pseudoscorpiones) recovered from burial sediments at Pachacamac (500–1500 CE), Perú. J. Arachnol. 2017, 45, 370–375. [Google Scholar] [CrossRef]
- Kadej, M.; Szleszkowski, Ł.; Thannhäuser, A.; Jurek, T. A mummified human corpse and associated insects of forensic importance in indoor conditions. Int. J. Leg. Med. 2020, 134, 1963–1971. [Google Scholar] [CrossRef]
- Gaudry, E. The insects colonisation of buried remains. In Current Concepts in Forensic Entomology, 1st ed.; Springer Dordrecht: Dordrecht, The Netherlands, 2009; pp. 273–311. [Google Scholar]
- Pastula, E.C.; Merritt, R.W. Insect arrival pattern and succession on buried carrion in Michigan. J. Med. Entomol. 2013, 50, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Lutz, L.; Moreau, G.; Czuprynski, S.; Bernhardt, V.; Amendt, J. An empirical comparison of decomposition and fly colonisation of concealed carcasses in the Old and New World. Int. J. Leg. Med. 2019, 133, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.A.; Petersen, C.; Georgy, J.; Dadour, I.R. The effect of suitcase concealment on insect colonization: A pilot study in Western Australia. GJFSM 2019, 1, 513. [Google Scholar] [CrossRef]
- Schoenly, K. A statistical analysis of successional patterns in carrion-arthropod assemblages: Implications for forensic entomology and determination of the postmortem interval. J. Forensic Sci. 1992, 37, 1489–1513. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Galal, F.H.; Seufi, A.M.; Elhefnawy, A.A. Insect succession associated with corpse’s decomposition of the guinea pig Cavia porcellus in Benha city, Egypt. Egypt. Acad. J. Biol. 2013, 5, 1–20. [Google Scholar] [CrossRef][Green Version]
- Couri, M.S.; de Souza, S.M.F.M.; Cunha, A.M.; Pinheiro, J.; Cunha, E. Diptera Brachycera found inside the esophagus of a mummified adult male from the early XIX century, Lisbon, Portugal. Mem. Inst. Oswaldo Cruz 2008, 103, 211–213. [Google Scholar] [CrossRef]
- Couri, M.S.; Cunha, A.M.; de Souza, S.M.F.M.; Laeta, M. Ophyra capensis (Wiedemann)(Diptera, Muscidae) found inside the esophagus of a mummy in Lisbon (Portugal). Pap. Avulsos Zool. 2009, 49, 87–91. [Google Scholar] [CrossRef][Green Version]
- Magni, P.A.; Guareschi, E.E. After the flood: A multidisciplinary investigation of human remains found in a floodplain and first record of raft spiders colonizing a corpse. JCHS 2021, 6, 68–75. [Google Scholar]
- Guareschi, E.; Dadour, I.R.; Magni, P.A. A taphonomic examination of inhumed and entombed remains in Parma cemeteries, Italy. GJFSM 2019, 1, 1–8. [Google Scholar]
- Magni, P.A.; Borrini, M.; Dadour, I.R. Human remains found in two wells: A forensic entomology perspective. Forensic Sci. Med. Pathol. 2013, 9, 413–417. [Google Scholar] [CrossRef]
- Byrd, J.H.; Castner, J.L. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Gemmellaro, M.D.; Bucolo, C.; Musumeci, E.; Hamilton, G.C.; Weidner, L.M. First observations of initial blow fly (Diptera: Calliphoridae) activity on lava fields and in subterranean environments in Sicily in cool temperatures. J. Med. Entomol. 2018, 55, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L. Estimation of postmortem interval using arthropod development and successional patterns. Forensic Sci. Rev. 1993, 5, 81. [Google Scholar] [PubMed]
- Kenward, H.; Large, F. Recording the preservational condition of archaeological insect fossils. Environ. Archaeol 1998, 2, 49–60. [Google Scholar] [CrossRef]
- Huchet, J.B.; Deverly, D.; Gutierrez, B.; Chauchat, C. Taphonomic evidence of a human skeleton gnawed by termites in a Moche-civilisation grave at Huaca de la Luna, Peru. Int. J. Osteoarchaeol. 2011, 21, 92–102. [Google Scholar] [CrossRef]
- Huchet, J.B.; Le Mort, F.; Rabinovich, R.; Blau, S.; Coqueugniot, H.; Arensburg, B. Identification of dermestid pupal chambers on Southern Levant human bones: Inference for reconstruction of Middle Bronze Age mortuary practices. J. Archaeol. Sci. 2013, 40, 3793–3803. [Google Scholar] [CrossRef]
- Thompson, J.E.; Martin-Vega, D.; Buck, L.T.; Power, R.K.; Stoddart, S.; Malone, C. Identification of dermestid beetle modification on Neolithic Maltese human bone: Implications for funerary practices at the Xemxija tombs. J. Archaeol. Sci. Rep. 2018, 22, 123–131. [Google Scholar] [CrossRef]
- Wrobel, G.D.; Biggs, J. Osteophageous insect damage on human bone from Je’reftheel, a Maya mortuary cave site in west-central Belize. Int. J. Osetoarchaeol. 2018, 28, 745–756. [Google Scholar] [CrossRef]
- Powers, N.; Sibun, L. Standards and Guidance for Forensic Archaeologists; Institute for Archaeology: Earley, Reading, UK, 2016. [Google Scholar]
- Magni, P.A.; Guercini, S.; Leighton, A.; Dadour, I. Forensic entomologists: An evaluation of their status. J. Insect Sci. 2013, 13, 78. [Google Scholar] [CrossRef]
- Sanford, M.R.; Byrd, J.H.; Tomberlin, J.K.; Wallace, J.R. Entomological evidence collection methods: American board of forensic entomology approved protocols. In Forensic Entomology: The Utility of Arthropods in Legal Investigations, 3rd ed.; Byrd, J.H., Tomberlin, J.K., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 63–85. [Google Scholar]
- Braga, M.V.; Mendonça, P.M.; Barbosa, R.R.; Blomquist, G.J.; Novo, S.P.C.; Dutra, J.M.F.; de Souza, S.M.; Queiroz, M.M.C. Identification of Megaselia scalaris (Loew, 1866)(Diptera: Phoridae) in mummified human body from Itacambira (MG), Brazil, using scanning electron microscopy and cuticular hydrocarbons. J. Nat. Hist. 2016, 50, 1381–1388. [Google Scholar] [CrossRef]
- Cockburn, A.; Barraco, R.A.; Reyman, T.A.; Peck, W.H. Autopsy of an Egyptian mummy: A mummy can be a scientific treasure chest; to unlock its secrets, a multidisciplinary approach is needed. Science 1975, 187, 1155–1160. [Google Scholar] [CrossRef]
- Oras, E.; Anderson, J.; Tõrv, M.; Vahur, S.; Rammo, R.; Remmer, S.; Mölder, M.; Malve, M.; Saag, L.; Saage, R. Multidisciplinary investigation of two Egyptian child mummies curated at the University of Tartu Art Museum, Estonia (Late/Graeco-Roman Periods). PLoS ONE 2020, 15, e0227446. [Google Scholar] [CrossRef] [PubMed]
- Raguso, R.A. Don’t forget the flies: Dipteran diversity and its consequences for floral ecology and evolution. Appl. Entomol. Zool. 2020, 55, 1–7. [Google Scholar] [CrossRef]
- Lucidcentral: Identification and Diagnostic Tools. Available online: https://www.lucidcentral.org (accessed on 26 August 2022).
- Szpila, K.; Richet, R.; Pape, T. Third instar larvae of flesh flies (Diptera: Sarcophagidae) of forensic importance—Critical review of characters and key for European species. Parasitol. Res. 2015, 114, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.; Sukontason, K.L.; Piangjai, S.; Boonchu, N.; Kurahashi, H.; Hope, M.; Olson, J.K. Identification of forensically important fly eggs using a potassium permanganate staining technique. Micron 2004, 35, 391–395. [Google Scholar] [CrossRef]
- Byrd, J.H. Laboratory rearing of forensic insects. In Forensic Entomology: The Utility of Arthropods in Legal Investigations, 2nd ed.; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 177–200. [Google Scholar]
- Weeks, J.D.; O’Neill, M.A.; Gaston, K.J.; Gauld, I.D. Automating insect identification: Exploring the limitations of a prototype system. J. Appl. Entomol. 1999, 123, 1–8. [Google Scholar] [CrossRef]
- Yang, H.P.; Ma, C.S.; Wen, H.; Zhan, Q.B.; Wang, X.L. A tool for developing an automatic insect identification system based on wing outlines. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Nelson, H.G. A method of cleaning insects for study. Coleoptera. Bull. 1949, 3, 89–92. [Google Scholar]
- Giordani, G.; Grzywacz, A.; Vanin, S. Characterization and identification of puparia of Hydrotaea Robineau-Desvoidy, 1830 (Diptera: Muscidae) from forensic and archaeological contexts. J. Med. Entomol. 2019, 56, 45–54. [Google Scholar] [CrossRef]
- Pradelli, J.; Tuccia, F.; Giordani, G.; Vanin, S. Puparia cleaning techniques for forensic and archaeo-funerary studies. Insects 2021, 12, 104. [Google Scholar] [CrossRef]
- Huchet, J.B.; Greenberg, B. Flies, Mochicas and burial practices: A case study from Huaca de la Luna, Peru. J. Archaeol. Sci. 2010, 37, 2846–2856. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information: Taxonomy. Available online: https://www.ncbi.nlm.nih.gov/taxonomy (accessed on 26 August 2022).
- Merheb, M.; Matar, R.; Hodeify, R.; Siddiqui, S.S.; Vazhappilly, C.G.; Marton, J.; Azharuddin, S.; Al Zouabi, H. Mitochondrial DNA, a powerful tool to decipher ancient human civilization from domestication to music, and to uncover historical murder cases. Cells 2019, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, F.; Mazzanti, M.; Onofri, V.; Turchi, C.; Tagliabracci, A. MtDNA analysis for genetic identification of forensically important insects. Forensic Sci. Int. Genet. 2008, 1, 584–585. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Elias, S.; Gilbert, M.T.; Haile, J.; Munch, K.; Kuzmina, S.; Froese, D.G.; Sher, A.; Holdaway, R.N.; Willerslev, E. Non-destructive sampling of ancient insect DNA. PLoS ONE 2009, 4, e5048. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, M.; Alessandrini, F.; Tagliabracci, A.; Wells, J.D.; Campobasso, C.P. DNA degradation and genetic analysis of empty puparia: Genetic identification limits in forensic entomology. Forensic Sci. Int. 2010, 195, 99–102. [Google Scholar] [CrossRef]
- Tuccia, F.; Giordani, G.; Vanin, S. A combined protocol for identification of maggots of forensic interest. Sci. Justice 2016, 56, 264–268. [Google Scholar] [CrossRef]
- Iancu, L.; Junkins, E.N.; Necula-Petrareanu, G.; Purcarea, C. Characterizing forensically important insect and microbial community colonization patterns in buried remains. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Knapp, M.; Hofreiter, M. Next generation sequencing of ancient DNA: Requirements, strategies and perspectives. Genes 2010, 1, 227–243. [Google Scholar] [CrossRef]
- Parrott, J.J. A molecular Study of Contemporary and Museum Calliphoridae of Forensic Importance. Doctoral Dissertation, University of Portsmouth, Portsmouth, UK, 2013. [Google Scholar]
- Snounou, G.; Singh, B. Nested PCR analysis of Plasmodium parasites. In Malaria Methods and Protocols, 1st ed.; Doolan, D.L., Ed.; Humana Press: Totowa, NJ, USA, 2002; pp. 189–203. [Google Scholar]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. 2013, 98, 236–238. [Google Scholar] [CrossRef]
- Squella, D.J. Insectos hallados en fardos funerarios provenientes del cementerio arqueológico de Topater (Región de Atacama, Chile). Acta Entomol. Chil. 2007, 31, 31–34. [Google Scholar]
- Morrow, J.J.; Baldwin, D.A.; Higley, L.; Piombino-Mascali, D.; Reinhard, K.J. Curatorial implications of Ophyra capensis (order Diptera, family Muscidae) puparia recovered from the body of the Blessed Antonio Patrizi, Monticiano, Italy (Middle Ages). J. Forensic Leg. Med. 2015, 36, 81–83. [Google Scholar] [CrossRef]
- Vanin, S.; Azzoni, M.; Giordani, G.; Belcastro, M.G. Bias and potential misinterpretations in the analysis of insects collected from human remains of archaeological interest. Archaeol. Anthropol. Sci. 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Lockey, K.H. Insect hydrocarbon classes: Implications for chemotaxonomy. Insect Biochem. 1991, 21, 91–97. [Google Scholar] [CrossRef]
- Singer, T.L. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 1998, 38, 394–405. [Google Scholar] [CrossRef]
- Moore, H. Cuticular hydrocarbon analysis in forensic entomology: A Review. AEFS 2017, 1, 127–138. [Google Scholar] [CrossRef]
- Byrne, A.L.; Camann, M.A.; Cyr, T.L.; Catts, E.P.; Espelie, K.E. Forensic implications of biochemical differences among geographic populations of the black blow fly, Phormia regina (Meigen). J. Forensic Sci. 1995, 40, 372–377. [Google Scholar] [CrossRef]
- Bernier, U.R.; Carlson, D.A.; Geden, C.J. Gas chromatography/mass spectrometry analysis of the cuticular hydrocarbons from parasitic wasps of the genus Muscidifurax. J. Am. Soc. Mass Spectrom 1998, 9, 320–332. [Google Scholar] [CrossRef]
- Anyanwu, G.I.; Molyneux, D.H.; Phillips, A. Variation in cuticular hydrocarbons among strains of the Anopheles gambiae sensu stricto by analysis of cuticular hydrocarbons using gas liquid chromatography of larvae. Mem. Inst. Oswaldo Cruz 2000, 95, 295–300. [Google Scholar] [CrossRef]
- Kather, R.; Martin, S.J. Cuticular hydrocarbon profiles as a taxonomic tool: Advantages, limitations and technical aspects. Physiol. Entomol. 2012, 37, 25–32. [Google Scholar] [CrossRef]
- Braga, M.V.; Pinto, Z.T.; de Carvalho Queiroz, M.M.; Matsumoto, N.; Blomquist, G.J. Cuticular hydrocarbons as a tool for the identification of insect species: Puparial cases from Sarcophagidae. Acta Trop. 2013, 128, 479–485. [Google Scholar] [CrossRef]
- Kula, C.; Moore, H.E. The Temperature Effect on the Cuticular Chemical Profile of Lucilia Sericata Blowfly Larvae. Ph.D. Thesis, Turkish Ministry of National Education, Ankara, Turkey, 2020. [Google Scholar]
- Moore, H.; Lutz, L.; Bernhardt, V.; Drijfhout, F.P.; Cody, R.B.; Amendt, J. Cuticular hydrocarbons for the identification and geographic assignment of empty puparia of forensically important flies. Int. J. Leg. Med. 2022, 136, 1791–1800. [Google Scholar] [CrossRef]
- Moore, H.E.; Adam, C.D.; Drijfhout, F.P. Identifying 1st instar larvae for three forensically important blowfly species using “fingerprint” cuticular hydrocarbon analysis. Forensic Sci. Int. 2014, 240, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.E.; Pechal, J.L.; Benbow, M.E.; Drijfhout, F.P. The potential use of cuticular hydrocarbons and multivariate analysis to age empty puparial cases of Calliphora vicina and Lucilia sericata. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Moore, H.E.; Hall, M.; Drijfhout, F.P.; Cody, R.B.; Whitmore, D. Cuticular hydrocarbons for identifying Sarcophagidae (Diptera). Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Wagner, D.; Tissot, M.; Gordon, D. Task-related environment alters the cuticular hydrocarbon composition of harvester ants. J. Chem. Ecol. 2001, 27, 1805–1819. [Google Scholar] [CrossRef]
- Martin, S.J.; Helanterae, H.; Drijfhout, F.P. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linn. Soc. 2008, 95, 131–140. [Google Scholar] [CrossRef]
- Huchet, J.B. Flies, the Dead and Offerings: Archaeoentomology of Mochica graves from the Pyramid of the Moon, Peru. Rech. Amerindien. Que. 2017, 47, 23–36. [Google Scholar]
- Vanin, S.; Huchet, J.B. Forensic entomology and funerary archaeoentomology. In Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment, 1st ed.; Schotsmans, E.M.J., Márquez-Grant, N., Forbes, S.L., Eds.; Wiley: Oxford, UK, 2017; pp. 167–186. [Google Scholar]
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. Insect Ecology: Behavior, Populations and Communities; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Anderson, G.S. Insect succession on carrion and its relationship to determining time of death. In Forensic Entomology: The Utility of Arthropods in Legal Investigations, 1st ed.; Byrd, J.H., Castner, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 143–175. [Google Scholar]
- Bourel, B.; Tournel, G.; Hédouin, V.; Gosset, D. Entomofauna of buried bodies in northern France. Int. J. Leg. Med. 2004, 118, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Waitzbauer, W. Dipterenreste aus Awarengräbern im Wiener Ortsgebiet. Verh. Zool.-Bot. Ges. Osterreich 1984, 122, 35–42. [Google Scholar]
- Le Cabec, A.; Colard, T.; Charabidze, D.; Chaussain, C.; Di Carlo, G.; Gaudzinski-Windheuser, S.; Hublin, J.J.; Melis, R.T.; Pioli, L.; Ramirez-Rozzi, F. Insights into the palaeobiology of an early Homo infant: Multidisciplinary investigation of the GAR IVE hemi-mandible, Melka Kunture, Ethiopia. Sci. Rep. 2021, 11, 1–14. [Google Scholar]
- Gilbert, B.M.; Bass, W.M. Seasonal dating of burials from the presence of fly pupae. Am. Antiq. 1967, 32, 534–535. [Google Scholar] [CrossRef]
- Gaudio, D.; Betto, A.; Vanin, S.; De Guio, A.; Galassi, A.; Cattaneo, C. Excavation and study of skeletal remains from a World War I mass grave. Int. J. Osteoarchaeol. 2015, 25, 585–592. [Google Scholar] [CrossRef]
- Huchet, J.B. L’archéo-entomologie: Les insectes nécrophages associés aux soldats de Carspach. A l’Est du Nouveau 2013, 109–110. [Google Scholar]
- Benelli, G.; Canale, A.; Raspi, A.; Fornaciari, G. The death scenario of an Italian Renaissance princess can shed light on a zoological dilemma: Did the black soldier fly reach Europe with Columbus? J. Archaeol. Sci. 2014, 49, 203–205. [Google Scholar] [CrossRef]
- Giordani, G.; Tuccia, F.; Floris, I.; Vanin, S. First record of Phormia regina (Meigen, 1826) (Diptera: Calliphoridae) from mummies at the Sant’Antonio Abate Cathedral of Castelsardo, Sardinia, Italy. PeerJ 2018, 6, e4176. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, K.C.; Goff, A.; Goff, M.L. Mortuary behaviour reconstruction through palaeoentomology: A case study from Chachapoya, Perú. Int. J. Osteoarchaeol. 2005, 15, 175–185. [Google Scholar] [CrossRef]
- Pinto, S.L.; Giordani, G.; Tuccia, F.; Ventura, F.; Vanin, S. First records of Synthesiomyia nudiseta (Diptera: Muscidae) from forensic cases in Italy. Forensic Sci. Int. 2017, 276, 1–7. [Google Scholar] [CrossRef]
- Velásquez, Y.; Ivorra, T.; Grzywacz, A.; Martínez-Sánchez, A.; Magaña, C.; García-Rojo, A.; Rojo, S. Larval morphology, development and forensic importance of Synthesiomyia nudiseta (Diptera: Muscidae) in Europe: A rare species or just overlooked? Bull. Entomol. Res 2013, 103, 98–110. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef]
- Magni, P.A.; Pazzi, M.; Vincenti, M.; Alladio, E.; Brandimarte, M.; Dadour, I.R. Development and validation of a GC–MS method for nicotine detection in Calliphora vomitoria (L.)(Diptera: Calliphoridae). Forensic Sci. Int. 2016, 261, 53–60. [Google Scholar] [CrossRef]
- Masetti, M.; Menconi, M.; Fornaciari, G. Insect remains associated with the mummy of Cardinal Giulio della Rovere, Archbishop of Ravenna (1533–1578). In Proceedings of the VI World Congress on Mummy Studies, Teguise, Lanzarote, 20–24 February 2008; pp. 379–385. [Google Scholar]
- Vergara-Pineda, S.; Rojas-Chávez, J.M.; Mansilla, J.; Campos-de-la-Rosa, T. Fragmentos de insectos asociados a momias prehispánicas. Entomol. Mex 2009, 8, 798–801. [Google Scholar]
- Curry, A. The insects associated with the Manchester mummies. In The Manchester Museum Mummy Project; Curry, A. Manchester Museum: Manchester, UK, 1979; pp. 113–118. [Google Scholar]
- Morrow, J.J.; Myhra, A.; Piombino-Mascali, D.; Lippi, D.; Roe, A.; Higley, L.; Reinhard, K.J. Archaeoentomological and archaeoacarological investigations of embalming jar contents from the San Lorenzo Basilica in Florence, Italy. J. Archaeol. Sci. Rep. 2016, 10, 166–171. [Google Scholar] [CrossRef]
- Huchet, J.B. Archaeoentomological study of the insects remains found within the mummy of Namenkhet Amon, San Lazzaro Armenian Monastery,(Venice/Italy). Adv. Egyptol. 2010, 1, 59–80. [Google Scholar]
- Lynch, L.G.; Reilly, E. Early medieval human burials and insect remains from Kildimo, Co. Limerick. J. Ir. Archaeol. 2011, 20, 65–76. [Google Scholar]
- Miles, D. Socio-economic aspects of secondary burial. Oceania 1965, 35, 161–174. [Google Scholar] [CrossRef]
- Guareschi, E.E.; Magni, P.A. Preliminary Taphonomical Comparison of the Decomposition Process in Simple Burials, Traditional Tombs and Aerated Tombs in an Urban Cemetery in Northern Italy. Forensic Sci. 2022, 2, 505–515. [Google Scholar] [CrossRef]
- Vanin, S.; Boano, R.; Giordani, G.; Carta, G.; Fulcheri, E. Description of the entomofauna associated with the remains of the Cistercian nun Angela Veronica Bava (1591–1637). Med. Hist. 2022, 6, e2022006. [Google Scholar]
- Mariani, R.; García-Mancuso, R.; Varela, G.L.; Inda, A.M. Entomofauna of a buried body: Study of the exhumation of a human cadaver in Buenos Aires, Argentina. Forensic Sci. Int. 2014, 237, 19–26. [Google Scholar] [CrossRef]
- Scharrer-Liška, G.; Grassberger, M. Archäoentomologische untersuchungen von grab 34 awarischen Gräberfeldes von Frohsdorf, Niederösterreich. Archaol. Korresp. 2005, 35, 531–544. [Google Scholar]
- Scharrer-Liška, G.; Grassberger, M. Aussagemöglichkeiten der Archäoentomologie anhand des awarischen Gräberfeldes von Frohsdorf, Niederösterreich. In Proceedings of the Erster Österreichischer Archäometriekongress, Salzburg, Austria, 15–17 May 2009. [Google Scholar]
- Fornaciari, A.; Giuffra, V.; Pezzini, F. Secondary burial and mummification practices in the Kingdom of the two Sicilies. Mortality 2010, 15, 223–249. [Google Scholar] [CrossRef]
- Loni, A.; Vanin, S.; Fornaciari, A.; Tomei, P.E.; Giuffra, V.; Benelli, G. Back to the Middle Ages: Entomological and Botanical Elements Reveal New Aspects of the Burial of Saint Davino of Armenia. Insects 2022, 13, 1113. [Google Scholar] [CrossRef]
- Pardo, I. L’elaborazione del lutto in un quartiere tradizionale di Napoli. (L’élaboration du deuil dans un quartier traditionnel de Naples). Ras. Ital. Soc. 1982, 23, 535–569. [Google Scholar]
- Pradelli, J.; Rossetti, C.; Tuccia, F.; Giordani, G.; Licata, M.; Birkhoff, J.M.; Verzeletti, A.; Vanin, S. Environmental necrophagous fauna selection in a funerary hypogeal context: The putridarium of the Franciscan monastery of Azzio (northern Italy). J. Archaeol. Sci. Rep. 2019, 24, 683–692. [Google Scholar] [CrossRef]
- Biswadeep, P.; Patowary, A. Embalming: The Art of Preserving the Dead. IJHRMLP 2016, 2, 10. [Google Scholar]
- Barnes, K.M.; Whiffin, A.L.; Bulling, M.T. A preliminary study on the antibacterial activity and insect repellent properties of embalming fluids from the 18th Dynasty (1550–1292 BCE) in ancient Egypt. J. Archaeol. Sci. Rep. 2019, 25, 600–609. [Google Scholar] [CrossRef]
- Huchet, J.B.; Pereira, G.; Gomy, Y.; Philips, T.K.; Alatorre-Bracamontes, C.E.; Vásquez-Bolaños, M.; Mansilla, J. Archaeoentomological study of a pre-Columbian funerary bundle (mortuary cave of Candelaria, Coahuila, Mexico). Ann. Soc. Entomol. Fr. 2013, 49, 277–290. [Google Scholar] [CrossRef]
- Fägerström, C.; Buckland, P.I.; Lemdahl, G.; Karsten, P.; Lagerås, P.; Manhag, A. Insects and other invertebrate remains from the coffin of a 17th century bishop in Lund Minster, S Sweden. J. Archaeol. Sci. Rep. 2020, 31, 102299. [Google Scholar] [CrossRef]
- López-Dos Santos, N.; Martínez, C.P.; Darias, T.D.; Barroso, V.A.; Vázquez, J.V. Archaeoentomology applied to the Gran Canaria mummies: First results. In Proceedings of the Extraordinary World Congress on Mummy Studies, Tenerife, Spain, 5–9 September 2018. [Google Scholar]
- Loni, A.; Fornaciari, A.; Canale, A.; Giuffra, V.; Vanin, S.; Benelli, G. Insights on Funeral Practices and Insects Associated With the Tombs of King Ferrante II d’Aragona and Other Renaissance Nobles. J. Med. Entomol. 2019, 56, 1582–1589. [Google Scholar] [CrossRef]
- Giuffra, V.; Fornaciari, A.; Minozzi, S.; Vitiello, A.; Fornaciari, G. Autoptic practices in 16th–18th century Florence: Skeletal evidences from the Medici family. Int. J. Paleopathol. 2016, 15, 21–30. [Google Scholar] [CrossRef]
- Baumjohann, K.; Benecke, M. Insect Traces and the Mummies of Palermo—A Status Report. Entomol. Heute 2019, 31, 73–93. [Google Scholar]
- Teskey, H.J.; Turnbull, C. Diptera puparia from pre-historic graves. Can. Entomol. 1979, 111, 527–528. [Google Scholar] [CrossRef]
- Chophi, R.; Sharma, S.; Sharma, S.; Singh, R. Forensic entomotoxicology: Current concepts, trends and challenges. J. Forensic Leg. Med. 2019, 67, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.L.; Lord, W.D.; Goff, M.L.; Donnelly, B.; McDonough, E.T.; Alexis, J.C. Isolation of amitriptyline and nortriptyline from fly puparia (Phoridae) and beetle exuviae (Dermestidae) associated with mummified human remains. J. Forensic Sci. 1994, 39, 1305–1313. [Google Scholar] [CrossRef]
- Gagliano-Candela, R.; Aventaggiato, L. The detection of toxic substances in entomological specimens. Int. J. Leg. Med. 2001, 114, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L.; Brown, W.A.; Hewadikaram, K.A.; Omori, A.I. Effect of heroin in decomposing tissues on the development rate of Boettcherisca peregrina (Diptera, Sarcophagidae) and implications of this effect on estimation of postmortem intervals using arthropod development patterns. J. Forensic Sci. 1991, 36, 537–542. [Google Scholar] [CrossRef]
- Hédoui, V.; Bourel, B.; Martin-Bouyer, L.; Bécart, A.; Tournel, G.; Deveaux, M.; Gosset, D. Morphine perfused rabbits: A tool for experiments in forensic entomotoxicology. J. Forensic Sci. 1999, 44, 347–350. [Google Scholar] [CrossRef]
- Magni, P.A.; Pazzi, M.; Droghi, J.; Vincenti, M.; Dadour, I.R. Development and validation of an HPLC-MS/MS method for the detection of ketamine in Calliphora vomitoria (L.)(Diptera: Calliphoridae). J. Forensic Leg. Med. 2018, 58, 64–71. [Google Scholar] [CrossRef]
- Nolte, K.B.; Pinder, R.D.; Lord, W.D. Insect larvae used to detect cocaine poisoning in a decomposed body. J. Forensic Sci. 1992, 37, 1179–1185. [Google Scholar] [CrossRef]
- Introna, F.; Di Vella, G.; Gagliano Candela, R. Identification d’opiacés sur les pupes vides: Contribution expérimentale. J. Med. Leg. Droit. Med. 2001, 44, 211–215. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).