Abstract
Water is an essential element for human survival, yet many individuals still lack access to treated water to meet their basic needs. To mitigate this situation, alternative water treatment technologies that are accessible and easy to handle are being explored. Among these, the use of Moringa oleifera seeds as a natural coagulant and the application of a helically coiled tube as a flocculation unit have been studied. In this context, this study aimed to evaluate the turbidity removal efficiency using two different coagulants (Moringa oleifera and aluminum sulfate) in an alternative water clarification system. The system consists of a helically coiled tube flocculator (HCTF) coupled with a conventional decantation unit. It was observed that the coagulant solution from shelled seeds required a lower dosage to achieve efficiencies above 90% compared to the coagulant solution from seeds with shells. The optimal dosage was 30 mL/L of the coagulant solution from shelled seeds. This dosage resulted in high turbidity-removal efficiencies, ranging from 92% to 100%. The processing method of the seeds that yielded the highest efficiency in turbidity removal was the mortar and pestle, as opposed to a blender. The optimal configuration of the alternative water clarification system comprised using the lower HCTF in a horizontal orientation. The use of the alternative water clarification system, along with the natural coagulant, proves to be a promising alternative clean technology for water clarification in locations without access to conventional treatment, being efficient in turbidity removal.
1. Introduction
Water is an essential and irreplaceable resource, crucial for fulfilling the basic needs of human life. It is essential for survival and well-being and plays a significant role in sustainable development [1]. It must, therefore, be accessible to the population in sufficient quantities and be of satisfactory quality. However, an analysis of data from the Brazilian National Sanitation Information System reveals that approximately 15.9% of Brazil’s population lacks access to the water supply network [2]. This deficiency exposes a significant segment of the population to the risk of consuming untreated water, potentially leading to diseases transmitted through water. Ensuring the availability of clean and safe water is therefore not only a matter of public health but also a critical component of social equity and environmental sustainability.
In response to global challenges, including poverty, climate change, and the universal aspiration for peace and prosperity, the United Nations (UN) formulated the 2030 Agenda for Sustainable Development. This strategic blueprint is committed to fostering a more sustainable and resilient world by the year 2030, articulated through 17 Sustainable Development Goals (SDGs) accompanied by 169 specific targets. Prominently featured within this suite of objectives is Goal 6: Clean Water and Sanitation. This goal is dedicated to guaranteeing universal access to safe and sustainably managed water and sanitation services by 2030 [3].
To address the needs of populations without access to water of suitable quality and quantity for basic use, the development and implementation of alternative, accessible, sustainable, and user-friendly water treatment and supply technologies is imperative. These technologies are essential for ensuring that clean water, a fundamental human necessity, is available to all segments of society [4].
In Brazil, the vast majority of water treatment plants employ conventional treatment methods, encompassing stages such as coagulation, flocculation, sedimentation or flotation, filtration, and disinfection [5]. These processes are designed to effectively reduce turbidity, remove organic matter, eliminate toxic substances, and mitigate odor and taste, in addition to eradicating a wide range of microorganisms [6]. The water clarification phase, incorporating coagulation, flocculation, and sedimentation/flotation, aims to produce an effluent with reduced turbidity and enhanced quality for subsequent treatment stages.
The coagulation and flocculation processes are facilitated by the introduction of either natural or chemical coagulants [7]. While chemical coagulants are effective in their role, it is crucial to note their need for pH adjustments, their production of significant quantities of non-biodegradable sludge, and the association of their residues in water with neurological disorders, including Alzheimer’s disease [8,9,10].
Conversely, natural coagulants present a safer option, typically devoid of toxic substances and biodegradable, thereby offering a more sustainable solution when completely or partially substituting for conventional coagulants [7,11]. The adoption of natural coagulants marks notable progress, originating from renewable resources and having a direct impact on enhancing the quality of life within underprivileged communities [12].
Considering this, numerous natural coagulants are under investigation as viable substitutes for chemical coagulants. Among these, the Moringa oleifera seeds have garnered attention for their high efficiency in turbidity removal, low cost, and minimal toxicity, with the added advantage of on-site preparation feasibility [8,13,14,15].
The primary goals of pursuing sustainable water treatment technologies encompass enhancing the efficiency of treatment processes and minimizing operational inputs. This reduction targets the consumption of chemicals and electrical energy, as well as the expenses associated with the construction and upkeep of treatment facilities [16].
From this standpoint, the helically coiled tube flocculator (HCTF) emerges as an innovative clean technology for facilities employing coagulation/flocculation methods. Characterized by its reduced flocculation duration, the HCTF obviates the necessity for mechanical or electrical energy, boasts a compact form factor, low construction expenses, and ease of maintenance, and has proven effective in turbidity elimination, particularly when integrated with a sedimentation unit [16,17,18].
In [19], the authors demonstrate that integrating the natural coagulant obtained from Moringa oleifera seeds with HCTF technology offers considerable potential for water clarification. Nonetheless, the research indicates a need for additional studies, particularly concerning the exploration of various geometries of the flocculation unit, which have yet to be examined.
Consequently, the objective of this study is to assess the efficiency of employing the natural coagulant extracted from Moringa oleifera seeds within an innovative water clarification clean technology, which integrates an HCTF and a standard decanter. This approach endeavors to offer a sustainable and attainable solution for enhancing the quality of water accessible for consumption.
2. Materials and Methods
The research methodology employed to fulfill the overarching goal was structured into four distinct phases. The initial phase entailed the execution of preliminary activities essential for facilitating the progression of later stages. The second phase was dedicated to conducting experimental tests via the jar test method. Subsequently, the third phase involved experimental testing with the alternative water clarification system. In the concluding fourth phase, a comprehensive data analysis was undertaken.
2.1. Preliminary Activities
The preliminary activities aimed to define the necessary parameters for conducting the jar testing and the alternative water clarification system.
2.1.1. Coagulant Definition
To identify the natural coagulant for this study, a comprehensive literature review was carried out across four search platforms. Google Scholar was first explored using the keywords “natural coagulant” and “water treatment”. The Scielo database search included “water treatment” and “natural coagulant”. For the CAPES Periodicals Portal and the Science Direct database, searches were performed with “water treatment” and “natural coagulant”, specifically filtering for peer-reviewed articles on the CAPES Portal. The timeframe for this search extended from 2008 to 2023. Subsequently, the selected articles were systematically arranged by publication date in ascending order using Microsoft Excel®. This process led to the selection of Moringa oleifera tree seeds as the natural coagulant due to their prominent presence in the water treatment literature, where they have been recognized for their effective turbidity removal. Notably, the literature discusses the use of these seeds in both shelled and unshelled forms. Hence, this research will examine the efficiency of Moringa oleifera seeds as a natural coagulant in both these conditions.
In the scientific literature, a variety of processing techniques for Moringa oleifera seeds to obtain the natural coagulant are described. To ascertain the most effective processing method, two accessible and cost-efficient devices were chosen: a blender and a mortar and pestle.
Aluminum sulfate was selected as the chemical coagulant for comparison purposes with Moringa oleifera, given its prevalent mention in scholarly articles and common use in water treatment facilities.
Employing aluminum sulfate as a coagulant leads to a decrease in the pH level of water. To adjust this parameter, sodium hydroxide was applied as an alkalizing agent, in line with what is presented in [17]. The chemical substances employed in this study were analytical grade aluminum sulfate and sodium hydroxide.
The aluminum sulfate dosage applied was 0.249 g/L, in accordance with the coagulation diagram presented in [17]. To ensure the pH levels ranged between 6 and 7 throughout the experiments, 0.050 g/L of sodium hydroxide was introduced.
2.1.2. Definition and Preparation of Synthetic Water
To enhance the control of water turbidity, laboratory preparation was employed [17,20]. A turbidity level of 50 UT was selected for the study, aiming to adhere to the aluminum sulfate dosage recommended in [17]. It should be noted that this turbidity value is commonly observed in Brazilian water resources.
Synthetic water for the jar test was formulated using bentonite clay and tap water. The formulation approach drew upon methodologies outlined in [21,22]. For each jar test container, 2.0 L of tap water and 30.0 g of bentonite were combined. The mixture was then agitated at 500 rpm for 30 min. Following agitation, the suspension was allowed to settle for 24 h. The supernatant was subsequently decanted until the volume was reduced to 1.0 L, carefully avoiding the resuspension of the settled solids. This procedure resulted in what is referred to as “prepared water”. Stored in plastic containers, the prepared water was segregated, with excess water from the jars being discarded. The preparation sequence is depicted in Figure 1.

Figure 1.
Synthetic water preparation process in the jar test. Source: Authors (2023).
The prepared water demonstrated a turbidity level exceeding 50 UT. To ascertain the precise volume of prepared water required to achieve a turbidity of 50 UT in the jar test apparatus with 2.0 L of tap water, a calibration test was undertaken. The procedure encompassed the following steps: (a) varying volumes of the prepared water (60.0 mL, 50.0 mL, 40.0 mL, 30.0 mL, 20.0 mL, and 10.0 mL) were dispensed into each jar; (b) the volume in each jar was then topped up with tap water to achieve a total of 2.0 L; (c) the contents were mixed at 500 rpm for 30 min; (d) following this agitation period, turbidity readings were taken; (e) the data collected facilitated the construction of a linear regression graph to determine the optimal volume of prepared water for achieving the target turbidity.
Upon data analysis, it was established that to achieve 1.0 L of synthetic water with a median turbidity of 50 UT, the required volume of prepared water was approximately 23.0 mL/L. Therefore, the methodology for synthesizing the water for this investigation entailed (a) dispensing 2.0 L of tap water into the jar test vessels; (b) adding 46.0 mL of prepared water; (c) agitating at 500 rpm for a duration of 30 min; and (d) conducting turbidity measurements. Where necessary, adjustments were made by adding either more tap water or prepared water to attain a turbidity level proximate to 50 UT.
2.1.3. Definition and Assembly of the HCTFs
To develop a low-cost and accessible alternative water clarification system, the HCTF, constructed from flexible and transparent PVC tubing wrapped around a rigid PVC pipe, was selected for use.
This study evaluated two distinct HCTF models, referred to as HCTF 1 and HCTF 2, differing in length. These models were fabricated following the guidelines established in [17]. HCTF 1, measuring 11.84 m, was chosen because its length was the shortest within the optimal range identified in [17]. Conversely, HCTF 2 was selected for its 2.96 m length, the minimum tested in [17], which not only requires less space but also offers reduced assembly costs.
Both HCTF models feature a 10.0 cm coiling diameter and a hose diameter of 1/2”. Figure 2 displays the constructed HCTFs.

Figure 2.
(a) Assembled HCTF 1 and (b) assembled HCTF 2. Source: Authors (2023).
2.1.4. Definition and Assembly of the Decanter
The decanter was dimensioned as follows: 25.0 cm wide (B), 62.5 cm long (L), and 30.0 cm deep (H). This ensured compliance with the ratios proposed by the authors, achieving a length-to-width ratio (L/B) of 2.5 and a length-to-depth ratio (L/H) of 2.1.
The decanter was fabricated using high-density polyethylene sheets, 0.3 mm thick. After the cutting process, the sheets were assembled with adhesive. Figure 3 displays the fully assembled decanter.

Figure 3.
Decanter used in the alternative water clarification system. Source: Authors (2023).
2.1.5. Assembly of the Alternative Water Clarification System
To facilitate gravity-based operation of the system, thus eliminating the need for pumps, a significant height differential was required between the water reservoir and the flocculation and decantation units. A steel shelving unit, measuring 198.0 cm in height, 92.5 cm in length, and 27.0 cm in width, was employed to achieve this, as illustrated in Figure 4.
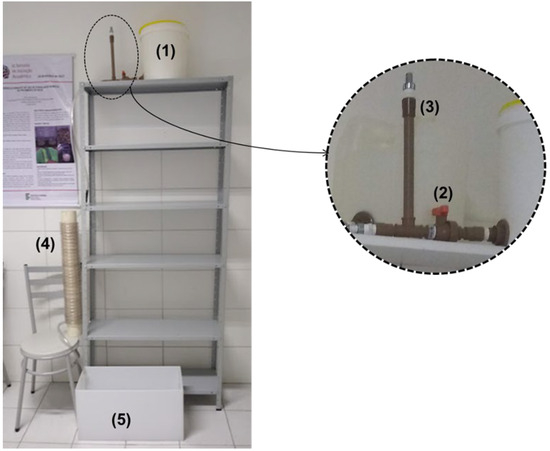
Figure 4.
Assembled alternative water clarification system. Source: Authors (2023).
The depicted alternative water clarification system in Figure 4 comprises (1) a raw water reservoir with a 15 L capacity, into which the coagulant was introduced; (2) a valve to hold the water within the reservoir until reaching the desired volume; (3) a vent for expelling air from the piping; (4) an HCTF; and (5) a decanter. Subsequent to the vent, a 1/2” hose was affixed to supply the flocculator with water from the reservoir.
The alternative water clarification system functioned in a batch mode, as follows: (a) the valve was closed; (b) synthetic water was introduced; (c) the coagulant was added; (d) the valve was then opened, facilitating the passage of the fluid through the flocculator towards the decanter; (e) the process continued until the decanter’s volume reached 15.0 L, aligning with the water reservoir’s maximum capacity; (f) subsequently, the valve was closed; and (g) the collection of decanted water samples commenced for turbidity analysis, reflective of the sedimentation duration for each solution evaluated. Upon the completion of sample gathering, the decanter underwent emptying and cleansing.
2.1.6. Turbidity Removal Efficiency Analysis
The assessment of the clarification system’s performance was directly associated with its turbidity removal efficiency. During the experimental tests, the effectiveness of turbidity removal was evaluated, encompassing both the jar test apparatus and the alternative water clarification system. This evaluation sought to establish a correlation between the proportion of flocculated and settled particles to the initial total solid particle count at the process commencement. Turbidity removal efficiency was quantified in accordance with Equation (1).
For turbidity measurement, a nephelometric turbidimeter was utilized. This technique relies on the scattering of light beams by a sample under examination. The device assesses the scattered light intensity against a calibration standard, indicating that a higher scattered light intensity signifies increased sample turbidity [17]. For this study, a turbidimeter from the Akso brand, model TU 430, was employed.
2.1.7. Preparation of Coagulant Solutions
In this research, Moringa oleifera seeds were employed to create coagulant solutions, processed in two variations: shelled and unshelled. The equipment selected for seed processing included (a) a mortar and pestle, chosen due to availability in the laboratory, and (b) an industrial blender, as shown in Figure 5.

Figure 5.
Processing equipment for Moringa oleifera seeds: (a) mortar and pestle; (b) industrial blender. Source: Authors (2023).
This approach yielded four distinct coagulant solutions, depicted in Figure 6.
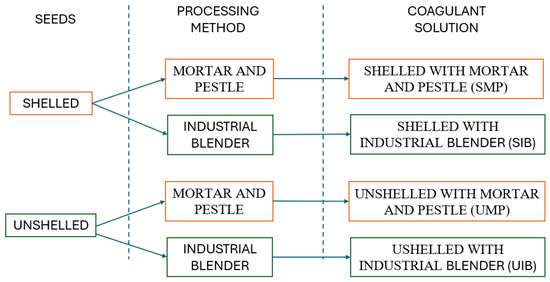
Figure 6.
Derived coagulant solutions from two processing techniques. Source: Authors (2023).
The preparation of the SMP and UMP solutions commenced with grinding the seeds. For the SMP coagulant solution, seeds were manually shelled before being ground to a powder, as illustrated in Figure 7.

Figure 7.
Grinding process for shelled Moringa oleifera seeds. Source: Authors (2023).
For the UMP coagulant solution, unshelled seeds were ground to a powder using a mortar and pestle, as shown in Figure 8.

Figure 8.
Grinding process for unshelled Moringa oleifera seeds. Source: Authors (2023).
Subsequent to grinding, the procedure for formulating both the SMP and UMP solutions involved (a) weighing 1 g of the ground seeds; (b) adding the seed powder to a beaker—with shelled seed powder for SMP and unshelled seed powder for UMP—combined with 100 mL of tap water; (c) stirring the mixture with a magnetic stirrer for 10 min at 500 rpm; and (d) filtering the solution through filter paper. Figure 9 depicts the coagulant solution preparation using ground Moringa oleifera seed powder.

Figure 9.
Coagulant solution preparation process using ground Moringa oleifera seed powder: (a) weighing, (b) stirring, and (c) filtering. Source: Authors (2023).
To streamline the process, the SIB and UIB coagulant solutions were formulated directly in the industrial blender, bypassing the initial grinding phase. The process for both involved (a) weighing 1 g of seeds; (b) adding the seeds to 100 mL of tap water in the blender—for SIB, shelled seeds, and for UIB, unshelled seeds; (c) blending for 1 min; and (d) filtering through filter paper. Figure 10 showcases the preparation of coagulant solutions using a blender.
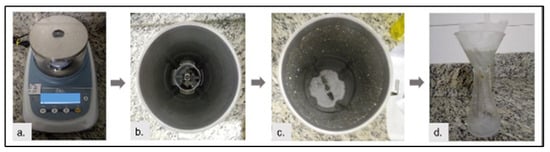
Figure 10.
Coagulant solution preparation using a blender: (a) weighing, (b) adding water and seeds, (c) blending, and (d) filtering. Source: Authors (2023).
The shelling process of the Moringa oleifera seeds for the SIB coagulant solution was performed manually. The coagulant solutions were freshly prepared before each experimental tests, following the methodology described in [8].
2.2. Jar Test Experiments
The aim of the jar test experiments was to evaluate the effectiveness of turbidity removal using Moringa oleifera seeds, in both unshelled and shelled forms, in comparison with a chemical coagulant. Preliminary tests were carried out to establish the appropriate sedimentation time for this research. The jar test apparatus employed was the JT—303M/6 model by LABOR, consisting of six jars, each with a 2.0 L capacity.
The operational parameters were set in accordance with [17], specifying an average velocity gradient of 250 s−1 and a flocculation duration of 90 s, which specifically apply to the HCTF 1 setup.
2.2.1. Experimental Aspects to Define the Sedimentation Time
The objective of these experiments was to establish the optimal sedimentation time for the natural coagulant derived from Moringa oleifera seeds, evaluated in both unshelled and shelled forms.
The initial coagulant dosage from Moringa oleifera was identified through a comprehensive literature review, aimed at determining the effective quantity of coagulant necessary to achieve a 50 UT reduction in turbidity. This review led to the discovery presented in [23], which reported a 78% turbidity reduction using an optimal dosage of 32.0 mL/L after 60 min of sedimentation with a coagulant solution made from shelled Moringa oleifera seeds. The research utilized synthetic water with a turbidity of 50 UT, created using kaolin and tap water, setting this dosage as the benchmark for our experiments.
The selection of sedimentation times was guided by the research conducted in [19]; in this paper, the authors explored a range of sedimentation durations between 10 and 45 min. Drawing on their findings, sedimentation times of 30, 40, and 50 min were chosen for testing solutions derived from both types of seeds.
Preliminary analyses revealed that solutions from unshelled seeds attained turbidity removal efficiencies of 57.7%, 64.5%, and 67.9% for the 30, 40, and 50 min intervals, respectively, demonstrating a progressive increase in removal efficiency over time. Therefore, it was resolved to further assess the turbidity removal capacity of unshelled seed solutions across these durations. Conversely, solutions derived from shelled seeds reached 100.0% efficiency in turbidity removal for all evaluated times, prompting an investigation into shorter sedimentation intervals of 10 and 20 min.
This exploration yielded turbidity removal efficiencies of 100.0% and 99.7% for the 10 and 20 min marks, respectively. As a result, the 10 min period was selected for further experiments with shelled seed solutions, aiming to minimize operational time.
2.2.2. Experimental Aspects with Natural and Chemical Coagulants
The objective of the jar test experiments involving the natural coagulant was to ascertain the turbidity removal capabilities of four Moringa oleifera coagulant formulations, SMP, SIB, UMP, and UIB, to identify the most effective coagulant dosage. Concurrently, experiments with the aluminum sulfate chemical coagulant were designed to assess its turbidity removal performance. All experiments were conducted in triplicate to ensure reliability.
During the sedimentation time determination experiments, a 32 mL/L dosage of the solution from the shelled seeds achieved a 100% efficiency in turbidity removal. Given this maximum efficiency, adjacent dosages were tested for shelled seed solutions (SMP and SIB), with adjustments made in 0.5 mL/L increments, aiming to minimize coagulant use while maintaining high efficiency. Dosages of 32.0 mL/L, 31.5 mL/L, 31.0 mL/L, 30.5 mL/L, 30.0 mL/L, and 29.5 mL/L were evaluated, with a sedimentation period of 10 min.
For unshelled seed solutions and using a 32 mL/L dosage, observed efficiencies varied between 57.7% and 67.9%, lower than those for shelled seeds. In [8], the authors made similar observations, noting improved outcomes for shelled seeds. To achieve 90.0% efficiency with both types, it was necessary to increase the coagulant dosage for unshelled seeds compared to shelled seeds.
Thus, dosages exceeding 32 mL/L were tested for unshelled seed solutions (UMP and UIB) to enhance turbidity removal efficiency. Dosages evaluated included 32.0 mL/L, 36.0 mL/L, 40.0 mL/L, and 48.0 mL/L, with sedimentation times set at 30, 40, and 50 min to monitor efficiency trends.
Subsequent testing of the UMP solution revealed that a 48.0 mL/L dosage surpassed the 90% efficiency threshold in turbidity removal. To further explore this range, 47.5 mL/L and 48.5 mL/L dosages were examined. Sample collection occurred at 30, 40, and 50 min to evaluate time-dependent efficiency.
Upon reviewing the outcomes from all four coagulant solutions, the 30.0 mL/L dosage of the SMP solution was found to be the most efficient in surpassing 90.0% turbidity removal and was thereby selected for further investigation in the alternative water clarification system experiments.
Experiments with the chemical coagulant sought to compare the turbidity removal efficiency of aluminum sulfate against the optimal natural coagulant dosage (30.0 mL/L of the SMP solution) in synthetic water.
Following the completion of experimental tests using both coagulant types and determining the superior natural coagulant dosage alongside the chemical coagulant’s turbidity removal efficiency, the study progressed to experiments with the alternative water clarification system.
2.3. Experiments with the Alternative Water Clarification System
The objective of these experiments was to pinpoint the most effective setup of the alternative water clarification system for reducing turbidity, utilizing the ideal dosage of the natural coagulant identified through jar test experiments. Moreover, assessments were carried out using a chemical coagulant to gauge its efficiency in turbidity reduction.
Determining the Most Effective Configuration of the Alternative Water Clarification System
The determination of the system’s most effective configuration was guided by the achievement of the highest turbidity removal efficiencies following the decantation phase. The system comprised three primary components: a water reservoir, a flocculator (either HCTF 1 or HCTF 2), and a decanter. Experiments were devised to test the impact of the flocculator’s orientation on turbidity removal efficiency, exploring both horizontal and vertical positions for HCTF 1 and HCTF 2. This approach yielded four distinct configurations, showcased in Figure 11 (schematic hydraulic circuit) and Figure 12 (photos of the actual system).
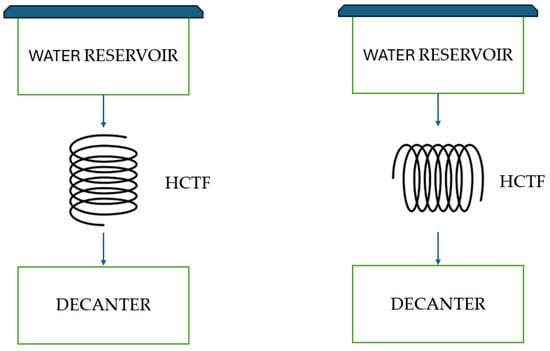
Figure 11.
Schematic of the alternative water clarification system with HCTF in vertical and horizontal orientation. Source: Authors (2023).

Figure 12.
The alternative water clarification system with HCTF 1 in (a) vertical and (b) horizontal orientation and HCTF 2 in (c) vertical and (d) horizontal orientation. Source: Authors (2023).
The experiments utilized 15 L of synthetic water, equivalent to the water reservoir’s full capacity. A 30.0 mL/L dosage of the natural coagulant was used. Sample collection from the decanter was consistently performed at the same level, following a 10 min sedimentation period. Each flocculator orientation was tested in triplicate.
Further evaluations were then conducted using the chemical coagulant in the system’s identified optimal configuration to analyze its effectiveness in turbidity removal.
3. Results
This section details the outcomes pertaining to the synthesis of artificial water, insights derived from jar test experiments, and the performance of the alternative water clarification system.
3.1. Synthesis of Synthetic Water
Aimed at enhancing the efficiency of synthetic water preparation, an experiment was designed to pinpoint the precise volume of prepared water required to achieve a target turbidity of 50 UT. The measured volume of prepared water added to each jar and its subsequent turbidity post-stirring is detailed in Table 1.

Table 1.
Volume of prepared water and resultant turbidity for each jar.
Leveraging the data from Table 1, a linear regression analysis was performed to establish a relationship between turbidity and the volume of prepared water. The analysis revealed a coefficient of determination (R²) of 0.9972, signifying that nearly 99.72% of turbidity variation is attributable to the addition of specific volumes of prepared water. Additionally, the standard error of the regression was found to be 1.36, indicating that, on average, the model’s predictions deviate from actual turbidity values by 1.36 units. Based on the linear regression equation, it was determined that to achieve synthetic water with an approximate turbidity of 50 UT, 46.0 mL of prepared water should be added to each 2.0 L capacity jar in the jar test setup.
3.2. Jar Test Experimental Outcomes
This segment delineates the results obtained from the jar test experiments employing the natural coagulant to evaluate its effectiveness in turbidity removal and establish the optimal coagulant concentration. It also encompasses outcomes from the utilization of a chemical coagulant to ascertain its turbidity removal capabilities. Preliminary to these findings, the document elaborates on the outcomes from sedimentation time determination tests.
3.2.1. Sedimentation Time Determination Outcomes
This section initially sheds light on the turbidity removal efficiencies achieved with sedimentation durations of 30, 40, and 50 min using the coagulant solution derived from Moringa oleifera seeds, both shelled and unshelled. It then proceeds to detail the turbidity removal efficiencies for the shelled seed coagulant solution across shortened sedimentation intervals of 10 and 20 min, employing a consistent coagulant dosage of 32.0 mL/L throughout all experiments.
The effectiveness in reducing turbidity at sedimentation times of 30, 40, and 50 min for unshelled Moringa oleifera seed coagulant solutions was 57.7%, 64.5%, and 67.9%, respectively. On the other hand, the values for shelled Moringa oleifera seed coagulant solutions were maintained constant at 100.0%.
It was discerned that the duration of sedimentation notably influenced the turbidity removal performance of the unshelled seed coagulant solution, showcasing an increment in efficiency over time. The peak efficiency recorded was 67.9%, following a sedimentation period of 50 min, the lengthiest duration explored. Consequently, shortening the sedimentation interval was deemed impractical, prompting the decision to maintain sedimentation durations of 30, 40, and 50 min for subsequent experimental evaluation involving unshelled seed coagulant solutions.
Conversely, the shelled seed coagulant solution exhibited a consistent turbidity removal efficiency of 100.0% across all examined sedimentation times. Given the maximal efficiency observed at every sedimentation interval, further testing was undertaken with reduced times of 10 and 20 min. Both evaluated durations yielded almost identical efficiencies: 100% for a 10 min interval and 99.7% for a 20 min interval, indicating that the shelled seed coagulant solution achieved 100.0% efficiency for sedimentation times of 10, 30, 40, and 50 min. This permits a feasible reduction of the water clarification process duration to 10 min within the scope of this research.
Referencing the study presented in [23], which served as the coagulant dosage benchmark for this investigation, the authors documented a 78.0% decrease in turbidity following a 60 min sedimentation using a 32.0 mL/L dosage of a shelled seed coagulant solution. Contrarily, the current study realized a 100.0% turbidity removal efficiency with just 10 min of sedimentation. Nonetheless, it is noteworthy that the synthetic water employed by the authors was concocted using kaolin, unlike the bentonite-utilized synthetic water in this study.
In research conducted in [24], the effect of sedimentation duration on turbidity removal efficiency using both unshelled and shelled Moringa oleifera seeds was examined. With an initial turbidity of 70 UT in synthetic water, sedimentation intervals of 60, 90, and 120 min were evaluated. The authors reported enhanced turbidity removal efficiencies at the extended sedimentation time for both seed types.
This aligns with the turbidity reduction efficiencies observed in this study for the unshelled seed coagulant solution, where the most significant efficiency improvements were also noted at the maximum sedimentation duration of 50 min. However, distinct from the findings observed in [24], the shelled seed coagulant solution demonstrated uniform efficiencies for both the minimal (10 min) and maximal (50 min) durations assessed.
Following the compilation of experiment outcomes and the establishment of sedimentation times (30, 40, and 50 min for coagulant solutions derived from unshelled seeds and 10 min for those from shelled seeds), the investigation proceeded with experiments involving both natural and chemical coagulants to evaluate their turbidity removal efficiencies.
3.2.2. Findings from Investigations with Natural and Chemical Coagulants
This section reports on the turbidity removal efficiency of the natural coagulant derived from Moringa oleifera seeds compared to the efficiency of the chemical coagulant, aluminum sulfate.
The initial findings relate to the natural coagulant, specifically focusing on solutions derived from shelled seeds (SMP and SIB). Results for the unshelled seeds’ coagulant solutions (UMP and UIB) are subsequently discussed.
The effectiveness of turbidity removal for the shelled seed solutions, SMP and SIB, is depicted in Figure 13. These findings stem from utilizing six different dosages with a predefined sedimentation period of 10 min. The pH values were measured and, in all tests, remained within the working range between 6 and 8.
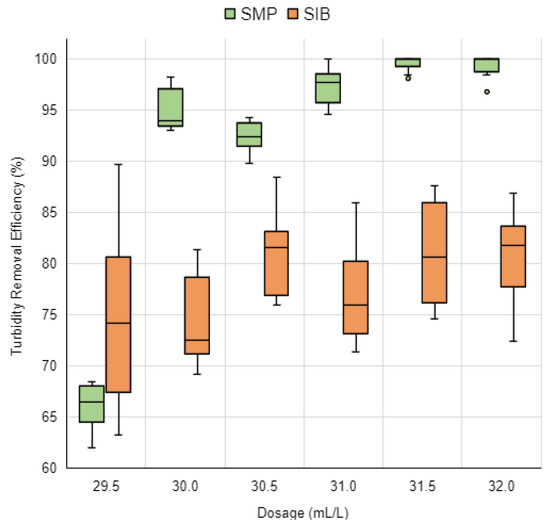
Figure 13.
Turbidity removal efficiency graph for SMP and SIB coagulant solutions across six dosages with a 10 min sedimentation period. Source: Authors (2023).
The SMP solution’s tests yielded turbidity removal efficiencies ranging from 62.0% to 100.0%. It was noted that the SMP coagulant solution consistently produced higher efficiency levels in comparison to the SIB solution, except at a dosage of 29.5 mL/L, which recorded lower efficiency levels.
For the SMP solution dosages of 30.0 mL/L, 31.0 mL/L, 31.5 mL/L, and 32.0 mL/L, turbidity removal efficiencies exceeded 90.0%, with a range between 93.0% and 100.0%. Remarkably, the 30.0 mL/L dosage, being the smallest coagulant amount used, achieved substantial turbidity removal efficiencies between 93.0% and 98.2%, highlighting a cost-effective advantage in the water clarification process.
Contrastingly, the SIB coagulant solution’s turbidity removal efficiencies were below 90.0% across all tested dosages, varying between 63.2% and 89.7%.
In [25], the authors also explored the effects of using a mortar and pestle versus a blender for processing shelled Moringa oleifera seeds. Their synthetic water, made with bentonite and initially presenting a turbidity of 105 UT, showed that the mortar and pestle processing method resulted in a higher turbidity removal efficiency (86.1%) compared to the blender method (76.9%). This outcome is in line with the findings of this research, suggesting that the mortar and pestle method, which partly removes the oil present in the seeds, yields a more efficient turbidity reduction than blending.
Advancing to the results from the coagulant solutions derived from unshelled Moringa oleifera seeds, the findings for the UMP coagulant solution are first examined, followed by those for the UIB solution. Each test set utilized sedimentation times of 30, 40, and 50 min.
Initially, turbidity removal efficiency outcomes for the UMP solution are discussed for dosages of 32.0 mL/L, 36.0 mL/L, 40.0 mL/L, and 48.0 mL/L. The results for subsequent dosages of 47.5 mL/L and 48.5 mL/L are also examined.
The turbidity removal efficiency outcomes for the UMP coagulant solution, across the stated dosages, are illustrated in Figure 14.
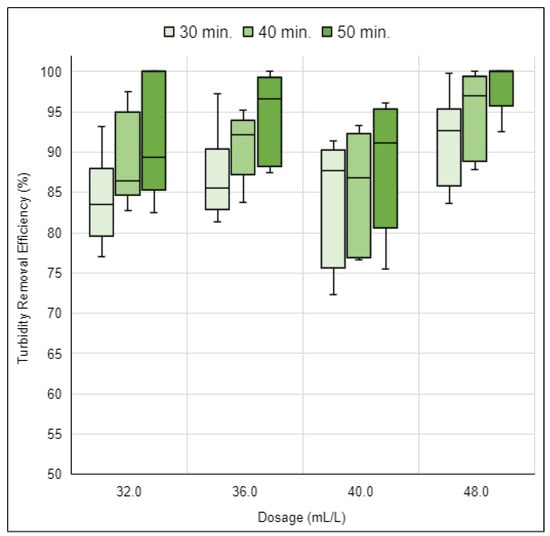
Figure 14.
Turbidity removal efficiency of the UMP coagulant solution for four dosages over 30, 40, and 50 min of sedimentation. Source: Authors (2023).
Experimental tests conducted with the UMP coagulant solution demonstrated turbidity removal efficiencies ranging from 72.3% to 100.0%. It was observed that the 48.0 mL/L dosage achieved the best efficiency results across all three studied sedimentation times. With a 30 min sedimentation period, efficiencies ranged between 83.6% and 99.8%. For 40 min of sedimentation, efficiencies varied between 87.9% and 100.0%. Notably, with 50 min of sedimentation, efficiency results exceeded 90.0%, ranging from 92.5% to 100.0%.
After identifying that only the 48.0 mL/L dosage of the UMP coagulant solution achieved efficiencies above 90.0% with a 50 min sedimentation time, it was decided to investigate two additional dosages: a lower one, 47.5 mL/L, and a higher one, 48.5 mL/L. The turbidity removal efficiency results for these new dosages are shown in Figure 15, which also includes the results of the 48.0 mL/L dosage for easier data visualization.
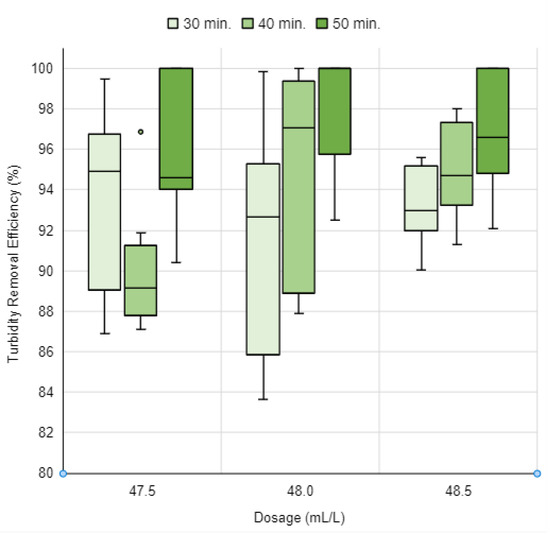
Figure 15.
Turbidity removal efficiency of the UMP coagulant solution as a function of dosages of 47.5 mL/L, 48.0 mL/L, and 48.5 mL/L, with 30, 40, and 50 min of sedimentation. Source: Authors (2023).
The observed turbidity removal efficiencies for the dosage of 47.5 mL/L ranged from 86.9% to 100.0%. A dosage of 48.0 mL/L achieved efficiency values fluctuating between 83.6% and 100.0%. For the 48.5 mL/L dosage, efficiency outcomes varied from 90.1% to 100.0%. Remarkably, dosages of 47.5 mL/L and 48.0 mL/L attained turbidity removal efficiencies exceeding 90.0% with just 50 min of sedimentation. Further scrutiny of the 47.5 mL/L dosage revealed a turbidity removal efficiency ranging from 90.4% to 100.0%. Meanwhile, the 48.0 mL/L dosage demonstrated efficiency values between 92.5% and 100.0% in turbidity removal.
However, upon increasing the dosage to 48.5 mL/L, it was observed that the turbidity removal efficiency for all three tested durations exceeded 90.0%. Achieving efficiencies over 90.0% for sedimentation times of 30 and 40 min had not been previously reported for other tested dosages of the UMP coagulant solution.
The turbidity removal efficiency results for a dosage of 48.5 mL/L with 30 min of sedimentation ranged from 90.1% to 95.6%. Efficiencies observed with a sedimentation time of 40 min varied between 91.3% and 98.0%. With 50 min of sedimentation, the efficiency results achieved spanned from 92.1% to 100.0%.
Thus, the UMP coagulant solution can be utilized according to the specific needs within the water clarification process. If the goal is to use the least amount of coagulant while achieving efficiencies above 90.0%, the most efficient dosage is 47.5 mL/L, attaining these results with a sedimentation time of 50 min. Conversely, if the objective is to minimize the process time to 30 min while maintaining turbidity removal efficiencies above 90.0%, a higher dosage such as 48.5 mL/L can be selected.
Following the presentation of turbidity removal efficiency results for the UMP coagulant solution, the results pertaining to the UIB solution are presented. Figure 16 illustrates the turbidity removal efficiency for dosages of 32.0 mL/L, 36.0 mL/L, 40.0 mL/L, and 48.0 mL/L.
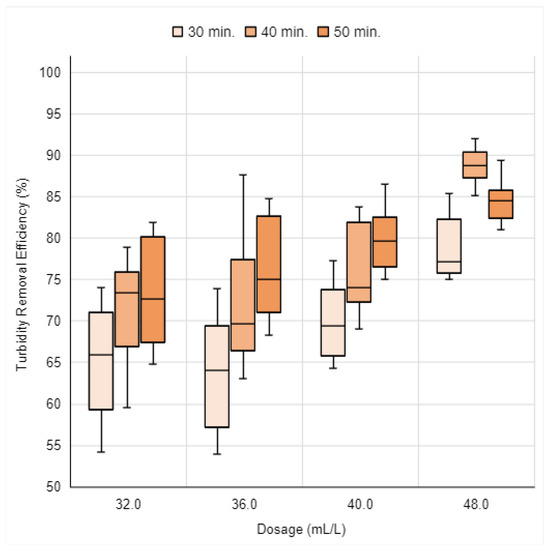
Figure 16.
Turbidity removal efficiency of the UIB coagulant solution as a function of 4 dosages over 30, 40, and 50 min of sedimentation. Source: Authors (2023).
The turbidity removal efficiencies achieved using the UIB coagulant solution dosages ranged from 53.9% to 92.0%. It is noteworthy that the highest efficiencies were recorded for the 48.0 mL/L dosage across all three sedimentation times studied. At this dosage, with 30 min of sedimentation, the efficiencies varied from 75.1% to 85.4%. The best efficiency values were found at 40 min of sedimentation, ranging from 85.1% to 92.0%. With 50 min of sedimentation, the efficiency results were between 81.0% and 89.4%.
For none of the dosages of the UIB solution studied were all turbidity removal efficiency results observed to be above 90.0%. When comparing the turbidity removal efficiencies obtained by the same dosages of the UMP and UIB coagulant solutions, as shown in Figure 14 and Figure 16, it is evident that the use of the UMP coagulant solution resulted in higher turbidity removal efficiencies, ranging from 72.3% to 100.0%. In contrast, the UIB coagulant solution exhibited efficiencies ranging from 53.9% to 92.0%. Therefore, for the Moringa oleifera seeds with husk, the processing method that resulted in the highest efficiencies was that utilizing the pestle.
After obtaining the turbidity removal efficiency results from the four coagulant solutions derived from Moringa oleifera seeds, it was noted that the lowest dosage achieving high turbidity removal efficiencies, over 90.0%, was 30.0 mL/L, using the SMP coagulant solution. Therefore, this dosage was selected as the optimal dosage.
In this study, coagulant solutions derived from shelled Moringa oleifera seeds demonstrated higher efficiency in turbidity removal, as they required lower dosages to achieve efficiencies above 90.0% and had shorter sedimentation times compared to coagulant solutions from seeds with husks.
The research conducted in [8], which compared coagulant solutions from Moringa oleifera seeds with and without husks using synthetic water (produced with kaolin) with an initial turbidity of 105 UT, concluded that both shelled and unshelled seeds can be used as coagulants. However, the shelled seeds showed greater efficiency in turbidity removal, requiring a lower dosage. According to the authors, shelled seeds contained a higher amount of protein compared to seeds with husks.
According to [26], planting conditions such as climate, soil, fertilization, etc., can lead to variations in the characteristics of Moringa oleifera seeds. The author suggests that these variations may influence the efficiency of the seeds in coagulation and flocculation processes during water treatment.
In the study presented in [24], both shelled and unshelled Moringa oleifera seeds were used to evaluate the efficiency of turbidity removal in the water treatment process, from coagulation to filtration. The water used in the research was synthetic and had an initial turbidity of 70 UT. The results indicated that the turbidity removal efficiency was similar for Moringa oleifera seeds with and without husks. However, the highest efficiencies were achieved with the coagulant derived from shelled seeds. Furthermore, the authors note that using shelled seeds simplifies and practicalizes the process, since it eliminates the need to dehusk the seeds.
In [27], the authors assessed the turbidity removal efficiency of coagulants derived from shelled and unshelled Moringa oleifera seeds up to the filtration stage in natural water with turbidity ranging from 17 UT to 27 UT. The highest efficiencies recorded were 90.9% for unshelled seeds and 92.7% for shelled seeds. The authors suggest that the increased efficiency for shelled seeds may be attributed to the higher protein content provided by the seed husk. In contrast, the study conducted in [28], which tested the husks of Moringa oleifera seeds for turbidity reduction, did not demonstrate effective turbidity removal.
In experiments employing aluminum sulfate as a chemical coagulant to evaluate turbidity removal efficiency, it was noted that turbidity was effectively eliminated within 10 min of sedimentation, achieving a consistent 100.0% efficiency across all tests. These efficiency values were higher than those found for the best dosage of the natural coagulant, 30.0 mL/L of the SMP solution, with efficiency ranging from 93.0% to 98.2%.
Following the jar testing with the natural and chemical coagulant, experiments commenced with an alternative water clarification system using a 30.0 mL/L dosage of the SMP solution and a sedimentation time of 10 min.
3.2.3. Findings from Experiments with the Alternative Water Clarification System
This section presents the results of the experimental tests conducted with the alternative water clarification system using the natural coagulant, aiming to determine the optimal configuration. Additionally, it includes the turbidity removal efficiency results when using the chemical coagulant in the best configuration of the alternative water clarification system.
To establish the optimal configuration of the alternative water clarification system, tests were carried out using HCTF 1 and HCTF 2, in both vertical and horizontal orientations. In these experiments, a dosage of 30.0 mL/L of the SMP solution was used, with a sedimentation time of 10 min, as determined from the jar testing. The pH values were measured and, in all tests, remained within the working range between 6 and 8.
First, the results of the tests conducted with HCTF 1 are presented, followed by those with HCTF 2. The turbidity removal efficiency of the alternative water clarification system using HCTF 1, in both vertical and horizontal orientations, is shown in Figure 17.
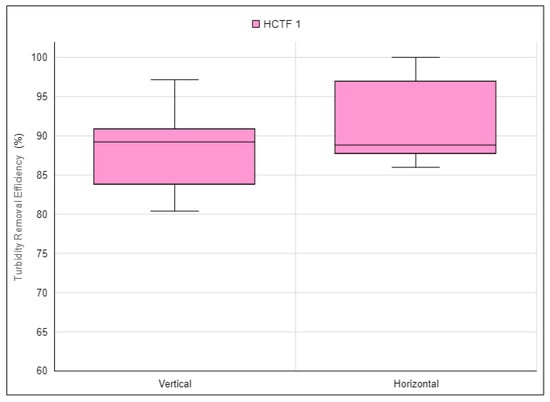
Figure 17.
Turbidity removal efficiency of the alternative water clarification system using HCTF 1 in vertical and horizontal orientations. Source: Authors (2023).
It was observed that the highest turbidity removal efficiency results for the alternative water clarification system were achieved using HCTF 1 in the horizontal orientation, with efficiencies ranging from 86.0% to 100.0%. When employing HCTF 1 in the vertical orientation, efficiencies varied from 80.4% to 97.2%. It is noteworthy that, in the alternative water clarification system utilizing HCTF 1, it was not possible to achieve efficiencies above 90% in all tests.
The turbidity removal efficiency results of the alternative water clarification system using HCTF 2, in both vertical and horizontal orientations, are depicted in Figure 18.
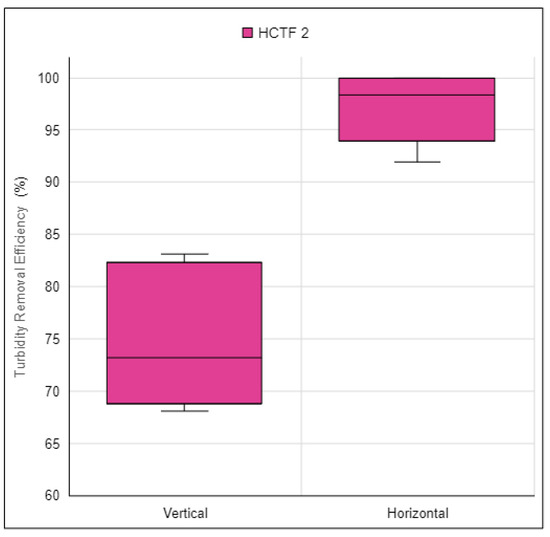
Figure 18.
Turbidity removal efficiency of the alternative water clarification system using HCTF 2 in vertical and horizontal orientations. Source: Authors (2023).
It was found that the highest turbidity removal efficiency results for the alternative water clarification system were achieved using HCTF 2 in the horizontal orientation. In this configuration, all efficiencies were above 90.0%, ranging from 92.0% to 100.0%. However, when using HCTF 2 in the vertical orientation, efficiencies varied from 68.1% to 83.1%.
Therefore, the configuration of the alternative water clarification system that achieved the highest efficiencies, above 90.0%, was that using HCTF 2 in the horizontal orientation. Consequently, this configuration was selected as the best due to its lower construction cost attributed to the reduced length of HCTF.
With this configuration of the alternative water clarification system, experimentation began utilizing the chemical coagulant aluminum sulfate. The trial results are displayed in Figure 19, which also includes results from the use of the natural coagulant for easier comparison.
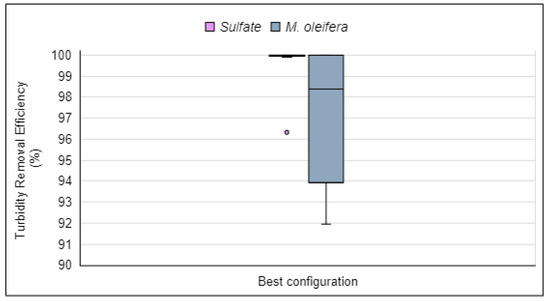
Figure 19.
Turbidity removal efficiency of aluminum sulfate and Moringa oleifera in the optimal configuration of the alternative water clarification system. Source: Authors (2023).
With the optimal configuration of the alternative water clarification system, aluminum sulfate demonstrated turbidity removal efficiencies ranging from 99.6% to 100%, with a single outlier result of 96.3%. The efficiencies obtained with the natural coagulant varied from 92.0% to 100.0%. Notably, the coagulant derived from Moringa oleifera seeds achieved high turbidity removal efficiencies, although slightly lower compared to aluminum sulfate. However, its use does not require the addition of chemical elements based on aluminum salts.
The alternative water clarification system proved effective in removing turbidity using both natural and chemical coagulants, offering an option for those without access to conventional water treatment. Its ability to operate in locations without electricity represents a significant advantage. These findings have the potential to enhance water treatment quality in resource-limited areas, contributing towards achieving the sixth Sustainable Development Goal. The results demonstrate the efficiency of the proposed clarification system in eliminating turbidity, with minimal processing time, cost-effectiveness, and independence from chemical reagents for efficacy. This eco-friendly technology offers a viable solution for communities facing water scarcity, ensuring an alternative, accessible, sustainable, and user-friendly approach to water treatment and supply.
4. Conclusions
This study aimed to evaluate an alternative clean technology based on the initial steps of water treatment. An alternative low-cost clarification system consisting of a helically coiled tube as a flocculation unit and a conventional decanter was tested, and the efficiency of turbidity removal was analyzed using the natural coagulant Moringa oleifera and the chemical coagulant aluminum sulfate. To achieve this general objective, experiments were first conducted using the jar test and subsequently the alternative water clarification system. The results obtained show that the proposed clarification system is efficient in removing turbidity, has a low processing time, is cost-effective, and involves operation that does not depend on chemical reagents for effectiveness. This clean technology can be used by populations lacking access to water of appropriate quality and quantity for basic use, ensuring an alternative, accessible, sustainable, and user-friendly water treatment and supply solution.
Hence, the detailed conclusions are presented as follows. The conclusions from the jar test simulation are as follows:
- A difference was observed in the sedimentation times between solutions derived from shelled and unshelled seeds. Solutions from shelled seeds showed shorter sedimentation times compared to solutions containing shells. This result suggests that using solutions from shelled seeds as a coagulant allows for a shorter water clarification process time.
- The highest turbidity removal efficiencies were achieved using the mortar and pestle processing method for both shelled seeds and seeds with shells, except for the dosage of 29.5 mL/L from shelled seeds.
- Dosages of coagulant solutions from shelled seeds required a smaller amount of coagulant compared to solutions from seeds with shells for turbidity removal.
- The optimal dosage of the natural coagulant was 30.0 mL/L of the SMP solution for the synthetic water with 50 UT used in this research, as it represented the lowest amount of coagulant that achieved the best turbidity removal efficiencies, above 90.0%.
- Experiments with aluminum sulfate exhibited a maximum efficiency of 100.0%, with no variation among the results.
The findings from the alternative water clarification system led to the following conclusions:
- The highest turbidity removal efficiencies in the alternative water clarification system were achieved when using the flocculators in the horizontal orientation with the natural coagulant.
- The optimal configuration of the alternative water clarification system was with HCTF 2 in the horizontal orientation, as it achieved the highest turbidity removal efficiencies, above 90.0%, when the natural coagulant was used. Furthermore, HCTF 2 is associated with lower construction costs.
- The alternative water clarification system exhibited efficiencies above 90.0% when using both the natural and chemical coagulants.
- The alternative water clarification system, combined with the natural coagulant derived from shelled Moringa oleifera seeds, proved to be efficient in turbidity removal, presenting a viable option for those without access to conventional water treatment.
Author Contributions
Conceptualization, J.R.S. and D.S.O.; methodology, J.R.S. and D.S.O.; validation, J.R.S. and D.S.O.; formal analysis, J.R.S. and D.S.O.; investigation, J.R.S. and D.S.O.; writing—original draft preparation, D.S.O.; writing—review and editing, D.S.O.; supervision, D.S.O.; project administration, D.S.O.; funding acquisition, D.S.O. All authors have read and agreed to the published version of the manuscript.
Funding
APC was supported by the Federal Institute of Espírito Santo (IFES, notice 08/2024) and National Council for Scientific and Technological Development (CNPq). The experimental apparatus was funded by Foundation for Research Support of Espírito Santo (FAPES), Federal Institute of Espírito Santo (IFES) and National Council for Scientific and Technological Development (CNPq).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the Foundation for Research Support of Espírito Santo (FAPES), Federal Institute of Espírito Santo (IFES) and National Council for Scientific and Technological Development (CNPq).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qtaishat, Y.; Hofman, J.; Adeyeye, K. Circular Water Economy in the EU: Findings from Demonstrator Projects. Clean Technol. 2022, 4, 865–892. [Google Scholar] [CrossRef]
- SNIS. Thematic Diagnosis Water and Sewage Services: Technical Water Management; SNS/MDR: Brasília, Brazil, 2022; p. 56. (In Portuguese) [Google Scholar]
- BRASIL. Agenda 2030 for Sustainable Development. 2022. Available online: https://www.gov.br/mre/pt-br/delbrasonu/desenvolvimento-sustentavel-e-meio-ambiente/agenda-2030-para-o-desenvolvimento-sustentavel (accessed on 5 June 2023). (In Portuguese)
- Thomas, B.; Vinka, C.; Pawan, L.; David, S. Sustainable groundwater treatment technologies for underserved rural communities in emerging economies. Sci. Total Environ. 2022, 813, 152633. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Aquino, D.S.; Cordeiro, L.L. Evaluation of Aloe arborescens as a coagulant for color and turbidity removal in conventional water treatment. Ing. Agua 2020, 24, 81–88. (In Portuguese) [Google Scholar] [CrossRef]
- Libânio, M. Fundamentals of Water Quality and Treatment; Átomo: Campinas, Brazil, 2010. (In Portuguese) [Google Scholar]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Ramanan, R.N. Utilization of plant-based natural coagulants as future alternatives towards sustainable water clarification. J. Environ. Sci. 2014, 26, 2178–2189. [Google Scholar] [CrossRef]
- Ndabigengesere, A.; Narasiah, K.S. Use of Moringa oleifera Seeds as a Primary Coagulant in Wastewater Treatment. Environ. Technol. 1998, 19, 789–800. [Google Scholar] [CrossRef]
- Vaz, L.G.d.L. The performance of the coagulation/flocculation process in treating the liquid effluent generated in electroplating. In Postgraduate Program in Chemical Engineering; State University of Western Paraná: Toledo, Brazil, 2009; p. 83. (In Portuguese) [Google Scholar]
- Camacho, F.P.; Sousa, V.S.; Bergamasco, R.; Ribau Teixeira, M. The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Buenaño, B.; Vera, E.; Aldás, M.B. Study of coagulating/flocculating characteristics of organic polymers extracted from biowaste for water treatment. Ing. Investig. 2019, 39, 24–35. [Google Scholar]
- Yin, C.-Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef]
- Gali Aba Lulesa, T.; Beyene, D.; Ebba, M.; Kenea, G. Water Treatment Using Natural Coagulant and Electrocoagulation Process: A Comparison Study. Int. J. Anal. Chem. 2022, 2022, 4640927. [Google Scholar] [CrossRef]
- da Conceição, V.M.; Yamaguchi, N.U.; de Jesus Bassetti, F.; Bergamasco, R. Process Performance Combining Natural Coagulant Moringa oleifera Lam and Ultrafiltration for Groundwater Defluoridation. Water Air Soil Pollut. 2021, 232, 222. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Mishra, R.; Singh, S.K. Elucidation of the potential of Moringa oleifera leaves extract as a novel alternate to the chemical coagulant in water treatment process. Water Environ. Res. A Res. Publ. Water Environ. Fed. 2020, 92, 1051–1056. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Teixeira, E.C. Hydrodynamic characterization and flocculation process in helically coiled tube flocculators: An evaluation through streamlines. Int. J. Environ. Sci. Technol. 2017, 14, 2561–2574. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Teixeira, E.C. Experimental evaluation of helically coiled tube flocculators for turbidity removal in drinking water treatment units. Water SA 2017, 43, 378–386. [Google Scholar] [CrossRef]
- Carissimi, E.; Rubio, J. The flocs generator reactor—FGR: A new basis for flocculation and solid–liquid separation. Int. J. Miner. Process. 2005, 75, 237–247. [Google Scholar] [CrossRef]
- Armeloni, J.P.N.; Oliveira, D.S.d.; Donadel, C.B. Natural agents as auxiliaries in water clarification: Literature review and experimental evaluation. Acta Sci. Technol. 2020, 42, e44800. [Google Scholar] [CrossRef]
- Oliveira, D.S.; Donadel, C.B. Mathematical modelling and analysis of the flocculation process in low retention time hydraulic flocculators. Water SA 2019, 45, 1–11. [Google Scholar] [CrossRef]
- Arantes, C.C.; Paterniani, J.E.S.; Rodrigues, D.S.; Hatori, P.S.; Pires, M.S.G. Different forms of application of Moringa oleifera seeds in water treatment. Braz. J. Agric. Environ. Eng. 2015, 19, 266–272. (In Portuguese) [Google Scholar]
- Lopes, A.M.B. Development of a new self-cleaning design in the internal configuration of high-rate horizontal tubular settlers. In Technological Center; Federal University of Santa Catarina: Florianópolis, Brazil, 2020; p. 240. (In Portuguese) [Google Scholar]
- Okuda, T.; Baes, A.U.; Nishijima, W.; Okada, M. Improvement of extraction method of coagulation active components from Moringa oleifera seed. Water Res. 1999, 33, 3373–3378. [Google Scholar] [CrossRef]
- Muniz, G.L.; Duarte, F.V.; Oliveira, S.B.d. Use of Moringa oleifera seeds for the removal of turbidity of water supply. Environ. Water J. 2015, 10, 454–463. (In Portuguese) [Google Scholar]
- Arantes, C.C.; Ribeiro, T.A.P.; Paterniani, J.E.S. Processing of Moringa oleifera seeds using different equipments to obtain coagulant solution. Braz. J. Agric. Environ. Eng. 2012, 16, 661–666. (In Portuguese) [Google Scholar]
- Madrona, G.S.; Branco, I.G.; Seolin, V.J.; Filho, B.d.A.A.; Fagundes-Klen, M.R.; Bergamasco, R. Evaluation of extracts of Moringa oleifera Lam seeds obtained with NaCl and their effects on water treatment. Acta Sci. Technol. 2012, 34, 289–293. [Google Scholar] [CrossRef]
- Michelan, D.C.d.G.S.; Santos, W.N.d.A.; Rosa, T.S.; Santos, D.d.G.; Jesus, R.d.C.S.d. Use of emergent moringa-based coagulant/flocculant for water treatment with verification of composition and toxicity of the produced sludge: Water treatment with Moringa and toxicity of the sludge. Sanit. Environ. Eng. J. 2021, 26, 955–963. (In Portuguese) [Google Scholar]
- Silva, C.A. Studies applied to the use of Moringa oleifera as a natural coagulant for improving water quality. In Institute of Chemistry; Federal University of Uberlândia: Uberlândia, Brazil, 2005; p. 91. (In Portuguese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).