Abstract
Biopolymers obtained from renewable resources are an interesting alternative to conventional polymers obtained from fossil resources, as they are sustainable and environmentally friendly. Poly(lactic acid) (PLA) is a biodegradable aliphatic polyester produced from 100% renewable plant resources and plays a key role in the biopolymer market, and is experiencing ever-increasing use worldwide. Unfortunately, this biopolymer has some usage limitations when compared with traditional polymers; therefore, blending it with other biopolymers, such as poly(butylene succinate) (PBS), poly(butylene succinate-co-butylene adipate) (PBSA), poly(butylene adipate-co-butylene terephthalate) (PBAT) and different poly(hydroxyalkanoates) (PHA), is considered an interesting method to improve it significantly, customize its properties and extend the range of its applications. The following review highlights, in its first part, the physico-chemical and mechanical properties of PLA in comparison to the other biopolymers listed above, highlighting the various drawbacks of PLA. The second part of the review deals with recent developments, results, and perspectives in the field of PLA-based blends.
1. Introduction
The exponential growth in global plastic manufacturing in recent years is a result of the rising world population, and is having a detrimental effect on the natural world and biodiversity [1]. As of 2020, there were 370 million tons of plastic items produced, up from a total of 1.5 million tons in 1950, and predictions show that by 2050, this percentage will have quadrupled [2,3].
Moreover, during the last 150 years, the industrial system has been based on a linear economic model involving the production of manufactured goods from fossil-based raw materials, their commercialization, utilization, and finally disposal as waste through incineration or landfill. As a result of the reduction in natural resources and the arising negative environmental impacts, this model is considered no longer sustainable for the future.
Nowadays, the production of most consumable items occurs using non-biodegradable traditional oil-based polymers, mainly polyolefins (polyethylene (PE), polypropylene (PP), polystyrene (PS)) and terephthalic-based polyesters (polyethylene terephthalate (PET)), leading to their accumulation in landfills or the Earth’s environment [4].
Back in 1970, the concept of the circular economy (CE) was introduced for the first time, as a useful industrial and political tool, for the reevaluation of global growth sustainability, changing the paradigm from a linear model, in which thinking is “produce-use-dispose”, to a new circular logic of “produce-use-reuse-recycle-value-reintegrate” [5].
This CE model involved two different types of recycling: biological and technical. Regarding biological recycling, a material of biological origin is used for anaerobic digestion, leading to benefits for the natural ecosystem. Hence, this organic material is considered a renewable resource to support the economy because this type of recycling leads to the regeneration of natural habitat characteristics of, for example, soil or marine environments. Instead, technical recycling allows the recovery of the material or product by exploiting strategies of reuse or repair until recycling is achieved [6]. The goal of this recycling type is to decrease the amount of plastic waste using destructive thermal treatment, such as combustion or pyrolysis [7].
The increasing use of polymeric materials in a wide range of industries makes it reasonable to develop an assessment to the life cycle of plastic products. Life cycle assessment (LCA) is one of the widely recognized and currently commercially available tools for assessing environmental impacts associated with all the stages of the life cycle of a commercial product [8,9]. It is based on a methodology governed by international standard 14040 and 14044 [10,11] and allows for quantifying and qualifying the relevant environmental impacts associated with the life cycle of a manufactured product, starting from preparing resources to obtain raw materials to the disposal of the manufactured product itself, through its processing, transportation, manufacturing, and end use. This review [12] reports on the large body of work analyzed to demonstrate that LCA is an important tool for evaluating how a preventive activity can reduce the environmental impact of such materials, and looks at the multiple perspectives that LCA can offer to different sectors (food packaging, agriculture, construction sector, and the automotive sector).
Also, from the perspective of sustainability, it is essential to conduct a life cycle cost analysis (LCC) with a life cycle impact assessment (LCIA). This is essential for building a techno-eco-efficient framework for comparing technical properties, environmental impacts and costs and making sure to maximize the utility and value of waste plastics in industrial applications. In the work of Jayawardane et al. [13], an LCIA and LCC evaluation was performed by comparing an artifact made by 3D printing from a PLA filament from virgin raw material and a filament derived from 100% recycled PLA. It was seen that the mechanical properties, density and hardness decreased dramatically with the content of fully recycled material compared to the virgin material. However, the manufactured product obtained from the fully recycled PLA was more eco-efficient. In this study, the evaluation of techno-eco-efficiency was limited to the PLA biopolymer, but this evaluation can be performed on all polymers and blend in future research and applications.
In this context, bioplastic materials have gained great interest in the plastic industry, with the resulting increment in the use of bioplastics derived from renewable sources leading to the reduction in plastic wasted in landfills, and promoting the use of renewable resources derived from agri-food scraps, which contributes to reducing the depletion of fossil resources to obtain plastic materials [14,15,16,17].
To clarify the meaning of biopolymer, the European Bioplastic Associations definition considers [18] as biopolymers both macromolecules synthesized starting from at least one monomer originating from sustainable resources and polymers that are biodegradable regardless of the origin of its raw materials [19].
In recent decades, biodegradable polymers, which can be based on either petroleum or renewable resources, are gaining attention as interesting alternatives, since through microbial degradation it is possible to reduce them into simple molecules, such as carbon dioxide (CO2), methane (CH4), water (H2O), biomass, and minerals [20]. The implementation of these new materials means that the CE model, if carefully applied, can be efficient at increasing resource efficiency, decreasing depletable or fossil raw materials, and minimizing the release of harmful substances into the environment [21].
Based on socio-economic pathways, it is necessary to formulate reduction strategies and implement policies to raise global awareness of greenhouse gas (GHG) emissions, as they are detrimental to air quality and ozone layer depletion. The Intergovernmental Panel on Climate Change in 2018 published a report proposing to limit global warming to 1.5 °C in order to avoid a range of global climate change impacts [22]. Unfortunately, GHG emissions occur at every stage of the life of plastics, including the extraction and transport of raw materials, plastic production, waste treatment and release into the environment [23]. Currently, recycling, incineration and landfill are used to manage most fossil-derived plastic waste. Data shown by Plastic Europe [24] state that the largest emissions from the incineration of plastic packaging waste were estimated at 16 million tons in 2015 and that this continued production of plastics will lead to an increase of 84 and 309 million tons by 2030 and 2050, respectively. According to the Intergovernmental Panel on Climate Change (IPCC) Working Group I [25], concentrations of CO2, CH4 and N2O have increased by 41%, 160% and 20%, respectively, since the Great Industrial Revolution, causing serious environmental imbalance problems. The GHGs released can have a negative impact on “carbon fixation” as they adversely affect the photosynthesis process carried out by “phytoplankton”, adversely affecting the natural habitat [26]. For this reason, the introduction of biobased and bio-degradable polymers, mostly of natural origin (deriving from agri-food or bacterial production waste) is essential, as they can reduce the impact of the global carbon footprint, reduce the transport of raw materials, and cancel the extraction process, as well as having a much shorter biodegradation time than conventional polymers and therefore lower GHG emissions. Indeed, if we think of the agricultural field, mulch films are frequently used for cultivation in the soil. According to a review by Somanathan et al. [27], several studies have shown that use of biodegradable polymers to make mulch films lead to rapid decomposition of the film, protection of the agricultural soil from both biotic and abiotic factors, increased yields, increased nutritional values to the soil, and maintenance of the bacterial population in the soil without polluting it.
Regarding biodegradable polymers, one of the most studied classes is aliphatic polyesters, due to their wide variety of properties and synthetic versatility. In this class, poly(lactic acid) (PLA), poly(butylene-succinate) (PBS), poly(butylene-succinate-co-adipate) (PBSA), and poly(butylene adipate-co-terephthalate) (PBAT) are included (Figure 1). Lately, an emerging class of biopolymers of great interest is bacterial-origin polymers such as the poly(hydroxy alkanoates) (PHAs) (Figure 2).
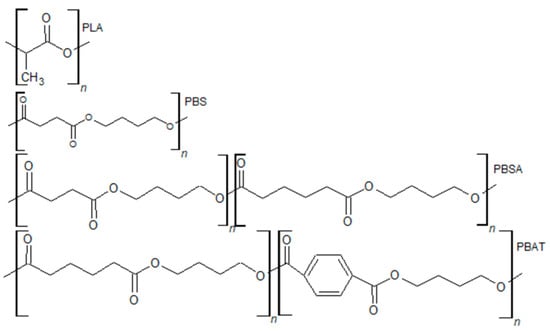
Figure 1.
Biodegradable aliphatic polyesters structures.
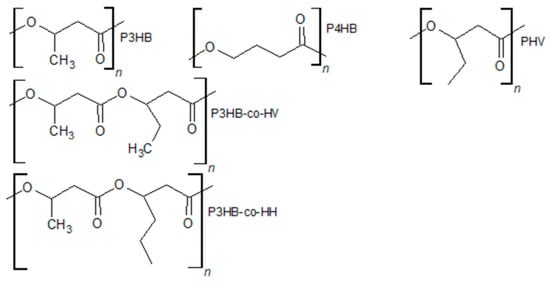
Figure 2.
Different PHA structures.
In this case, the polymers are prepared directly from biomass exploitation, for example, genetically modified microorganisms. PHAs are produced directly through fermentation of the carbon-containing substrate within the microorganism cells. The type of biomass used as an energy source depends on the microorganisms employed. For instance, it is well established in the literature that for E. coli, lipids and polysaccharides are generally used, while for R. eutropha, glucose is used as a carbon source [28]. PHA accumulates in granule form within the cell cytoplasm, acting as an energy reserve for the cell.
The issue with these biopolymers is that, when used alone as structural materials, they fail to meet the requirements of traditional polymers such as PP, PE, or PET.
Polymer blends are widely used in industry since they allow combining the properties of its components into a new material, without the need to design and develop a new polymer, which would be much more expensive and time consuming. Thus, by modifying the composition of blends, materials with properties suitable for various applications can be obtained [29,30].
As the preparation of sustainable PLA blends has been achieved in the last decade with significant results, detailed information on PLA blends with other biopolymers has been specifically summarized and analyzed. Therefore, this review is focused on the current developments in PLA blends produced by polymer blending methods. Different biopolymers commercially available today used in PLA blending are also discussed and systematically organized: PBS, PBAT, PBSA, PHA, Bio-PE and EVA. The aim is to provide as up-to-date a picture as possible of the different PLA-based blends currently accessible in the agri-food industrial packaging sector, highlighting their strengths and main critical issues.
2. Packaging Application of PLA and Its Blends
Most of the plastic packaging and containers are made with polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), polystyrene (PS), polyamides (PA) and polyvinyl chloride (PVC), derived from non-renewable and non-biodegradable petrochemical sources. In recent years, global plastic production has reached about 400 million tons per year and this represents an important source of waste [31]. More than 50% of this plastic packaging is used only one time before disposal [32]. Moreover, many traditional polymers are used to make multilayer, laminated, and mixed packaging in which aluminum foil or silica is inserted in the middle layer to improve barrier properties. At their end of life, only a small fraction of this waste is recycled as the multilayer separation process is expensive and a large part of plastic waste, especially from the food packaging sector, is disposed of in landfills. Furthermore, foodstuffs often contaminate the plastic containers, making their reuse or recycling process even less economically convenient and creating large quantities of waste at the end of its life, without energy and material recovery [33,34,35].
Currently, many biobased but non-biodegradable polymers are used in flexible and rigid packaging (e.g., bags and bottles) and are synthesized from renewable resources, such as Bio-PE, biopropylene (Bio-PP) or biopolyethylene terephthalate (Bio-PET) [36,37,38]. They have a greater application in the food industry than biodegradable and compostable biopolymers as they have the same properties and processing methods as conventional polymers and are therefore well known to the industry [39]. The problem with these bioplastics is that, even if they are derived from renewable resources, they are not biodegradable and therefore do not best meet the requirements of a sustainable circular economy. When considering the applications of short-life packaging (fruit, vegetables, and salad containers), disposable packaging for fresh food and ready meals and hygiene products, which do not require high barriers, these biobased but non-biodegradable bioplastics (Bio-PE, Bio-PP and Bio-PET) pose a major problem of management and disposal as they are used in large quantities in everyday life. One solution regarding the production of single-use and short-life packaging that is also eco-friendly are biodegradable and compostable bioplastics, which can play a crucial role in and have great potential for production from flexible films to rigid plastics, as they combine a renewable origin and the prerequisite of biodegradable end-of-life due to their susceptibility to microbial attack [40,41]. In food and non-food packaging applications, the most widely used biopolymers are PLA, PBS, PBSA, and PBAT, as well as the newly emerging class of PHAs.
The function of food packaging is not only to contain the food but also to protect it throughout its supply chain (from the place of production to the table) from external abiotic and biotic agents by preventing chemical or microbiological contamination that can cause adulteration, as well as to increase shelf life [42,43,44]. This has generated the need in the food industry to develop new packaging systems to maintain both the safety and quality of packaged food [45].
The essential prerogatives that packaging needs vary according to different applications and uses. Among the most common properties are barriers to gases (CO2, N2, O2, or volatile compounds) and moisture, useful mechanical properties (flexible or rigid), sealing and thermo-perforation, processability and heat stability, transparency or opacity depending on the application, anti-fog, printability, and resistance to light, acids and grease, and, as the last but not the least important factor, the cost [46,47]. The changes in mechanical properties will be covered in the review, so we will also mention the other prerogatives to emphasize the importance they have for an application.
As is well known, PLA has several benefits for these applications, as it is easy to process, transparent, has a high disintegration rate in compost, is nontoxic, and inexpensive, and has therefore been approved as a direct food contact material, and is currently being used to produce packaging for short-term applications. In addition, this biopolymer is in demand for a wide range of industries such as agricultural, medical, and pharmaceutical (e.g., drug delivery).
Studies have reported that currently PLA and the rest of the biopolymers are used more for packaging short-life products that do not require a high barrier to gases and water vapor. PLA has a low heat deflection temperature, which is an excellent prerequisite for making food packages stored in freezers under refrigerated conditions [48]. Due to this property of PLA, there is no deformation of the packaging, which is why currently PLA is also used to produce thermoformed trays and to transport fruits and organic products in a refrigerated condition.
However, this represents a limitation if one wants to broaden the range of applications in food packaging because, as explained, PLA has several problems compared to conventional polymers derived from oil resources that make it non-performing in several applications. As previously described, this biopolymer is characterized by low thermal, mechanical and gas barrier properties that are essential for food packaging to ensure its safety and preservation [49].
Therefore, an efficient and cost-effective solution to achieve desirable properties for films and packaging materials is blending with other biodegradable polymers, as it represents a valid strategy to increase and modulate their desired properties. More complex multilayer systems are still used on the market today, but they are largely made of conventional polymers, which pose a recycling and disposal problem [50]. The realization of a multilayer material consisting of only biodegradable or compostable biopolymers by means of an eco-friendly processing technology can lead to significant improvements not only in the final properties but also of the disposal benefits if the domestic compostability prerequisite is achieved, contributing to solve the problem of waste accumulation in landfills [51].
When considering barrier properties, an essential prerequisite for a food packaging material, it is known that most biodegradable biopolymers have lower moisture barrier properties than conventional plastics due to their chemical structure, but unlike them they exhibit similar oxygen barrier properties [52]. Therefore, the first applications to which this class of biopolymers is referred are food and short-life products. PLA exhibits low crystallinity and thus poor gas barrier properties [53], except for a lower oxygen transmission rate (OTR) than PET and PS [54]. If we think of moisture-sensitive products such as biscuits and bakery products, it is impossible to apply PLA-only packaging in this case [55]. Various methods are exploited in industry to improve the barrier properties of PLA, such as lamination with barrier materials, such as PP, PE and EVOH [55,56], or coating with silica dioxide (SiOx) and aluminum dioxide (AlOx) [56,57].
NatureWorks in collaboration with Metalvuoto have produced the first flexible, high-barrier PLA-based material that can be used for long-life foodstuffs. As a result, much academic and industrial effort has been put into increasing the crystallinity of PLA and making the material as eco-friendly as possible. Among the PHAs, PHB and PHBV have high crystallinity and thus good barrier properties towards O2, CO2 and moisture, similar to PE or PP [58,59,60]. Therefore, fusion with the PLA matrix can provide good gas barrier performance as well as adjusting its physical properties [61,62,63,64]. Furthermore, the production of PHAs is very expensive due to the fermentation and purification processes, so blending them with PLA can also lead to cost benefits [65,66]. From the point of view of barrier properties, Luzi et al. [67] formulated different mixtures of PLA/PBS (10, 20 and 30 wt%) to which cellulose nanocrystals (CNC) were added. The combination of both with PLA resulted in improved oxygen barrier properties, and the formulation with 77PLA/20PBS/3CNC was the best formulation, as it exhibited a 47% decrease in oxygen permeability. Furthermore, the migration levels of all composites produced were below European legislative limits and, therefore, they are useful as food packaging materials.
Still in the area of food packaging for active packaging in direct contact with food, Songtipya et al. [68] studied different PLA/PBAT formulations for packaging and containers in direct contact with ground coffee and tea leaves. The study showed that the total migration values were below the maximum migration limit (10 mg/dm2) imposed by European Union (EU) regulations. Therefore, these PLA- and PBAT-based materials may have a positive effect on food safety.
Hongsriphan and Sanga [69], after producing a PLA/PBS blend (90/10 wt%), developed an antibacterial food packaging towards E. coli and S. aureus by coating the PLA/PBS film with chitosan. It was observed that as the concentration of chitosan on the surface increased, from 0.25 to 2% w/v, bacterial growth decreased. The chitosan coating also decreased water vapor transmission rates compared to normal mixtures. This favorable phenomenon is due to the formation of intermolecular interactions between the chitosan and the polymer matrix.
The literature shows that biopolymers belonging to the PHA family were initially used for applications in cosmetics and articles for everyday use (such as vials, bottles, and containers) [70,71]. Today, they are also proposed for applications in short-term food packaging [59,72,73,74], medical devices and tissue regeneration, household goods, hygiene products and compostable bags due to their mechanical properties, biocompatibility, and biodegradability [75,76,77].
Food manufacturers such as Nestlé (Vevey, Switzerland) and PepsiCo (New York, NY, USA), in collaboration with Danimer Scientific (Bainbridge, NY, USA) and Kaneca (Tokyo, Japan) (leading manufacturers of biodegradable plastics), have developed PHA-based water bottles and flexible packaging for their snacks [78,79]. Concerning PepsiCo, the goal was to make the final artefact home-compostable by adding PHA to PLA and to improve certain properties, such as the sound of a bag of chips when opened. The Taghleef Industries company (Dubai, United Arab Emirates) is involved in the production of PLA-based films: for example the NATIVIA portfolio of PLA films is in the market for different packaging applications, like bread bags, coffee capsules, frozen food, labels, fashion and luxury products, and so on. But in recent years it has been collaborating with leading biopolymer manufacturers to make PLA-based films mixed with different biopolymers, and thus make the film home compostable rather than industrially compostable [80]. Also, in 2019 Danimer Scientific collaborated with UrthPact (Leominster, MA, USA) to produce edible straws using the biopolymer NodaxTM. In addition, PHAs are also used to produce waste bags in which PHA film is laminated with paper and other polymers such as PVC [77].
The company Rivoira (a fruit producer and distributor, Verzuolo, Italy) in cooperation with Bio-On (Bologna, Italy) have created the company Zeropack (Bologna, Italy) to produce 100% natural and biodegradable PHA-based films, containers, and holders for fruit and labels [81].
As reported by the authors of [51,82], mixing PLA with PHB at 25 wt% resulted in an improvement in the oxygen and water barrier properties of a film, while at the same time reducing the transparency of the final film (an intrinsic property of PLA).
The appearance and quality of the final packaging is a second essential prerequisite that can influence the buyer of a product during purchase, as it is essential to see through the packaging [47,49]. Since PLA is transparent, it is used as a single-layer packaging film, as biaxially oriented PLA (BOPLA) or in laminated films (barrier films) [55].
Another essential prerequisite is the cost of the final product. Regarding rigid packaging, PLA and PHA are stiffer than PS and therefore less plastic material would be required to produce the final manufactured product with the same stiffness, thus resulting in a lower final price [83].
In the agricultural sector, mulching is a widely used practice worldwide to maximize plant growth, allow water retention, regulate soil temperature, and protect agricultural soil from weeds. Normally, mulching films are made of PE and this has caused serious environmental problems as they are difficult to recycle and dispose of. This is why farmers prefer to leave the films in the fields and then burn them [84]. This procedure leads to negative effects that affect both crop growth, the environment and soil, as the accumulation of these films leads to a reduction in soil porosity as well as groundwater pollution [85]. Since biopolymers are non-toxic, biodegradable, and compostable, they are promising materials for mulching films as they can be degraded [86,87] and catabolized by the soil microbiota at their end of life [88]. Therefore, mulching films based on PHBH (NodaxTM) and PHB (MirelTM) have been produced [89,90]. In the work of Gao et al. [91], they produced mulching films based on PLA mixed with PBAT and directly field-tested these films on potato growth over a two-year period. They evaluated the effects on soil temperature dynamics and water retention and saw that crop yields did not change compared to the use of a PE-based film. They also evaluated the effects of the films during degradation in agricultural soils and the authors are very confident on the application of these films because in addition to improved crop yields, porosity and soil health also improved. BASF is promoting a PLA/PBAT blend, called Ecovio, for agricultural films [48].
In the work of Jandas et al. [92], they obtained mulching films by blending PLA with PHB and using maleic anhydride as a compatibilizer to improve polymer interaction. The addition of PHB to PLA not only improved the flexibility and impact strength of PLA, but also decreased the degradation time of PLA. Therefore, applied tests suggested that this type of film can be applied for short-term harvests (100–150 days).
Furthermore, PHA is biodegradable in water, which is why Havens et al. [93] patented a fishing gear made partly of PHA that can biodegrade in a marine environment.
In the biomedical field, these biopolymers and their blends have been extensively studied in various applications such as bone scaffolds and implants [94], sutures, drug delivery systems [95,96,97,98] and in tissue engineering [99,100,101]. This is due to their excellent properties such as biocompatibility, bioresorbability, biodegradability and non-toxicity. For example, polymer scaffolds provide sites for regeneration and functional restoration of tissues and for drug delivery. They require specific characteristics depending on the application and the tissue of interest. The key point of these biopolymer scaffolds is that once they have performed their restorative or structural function they biodegrade, eliminating the need for patient surgery [102]. By means of FDM printing and electrospinning, it is possible to produce different types of porous scaffolds useful for tissue regeneration due to the presence of pores that allow the transport of nutrients, oxygen and growth factors [103,104,105,106]. PLA has been blended with PHB to enhance its bioactivity and cell proliferation for medical use such as artificial muscles, wound dressings or drug delivery systems.
An important field of application for PLA-based blends is that of intelligent materials capable of responding to different stimuli. With PLA/PHB blends, a system called ‘non-woven fabric’, that showed a controllable, thermally induced shape memory effect, is realized [107]. He et al. [108] formulated various PLA/PHBH and PLA/PHBV blends for the fabrication of medical sutures. Their results showed that these blends can be used as medical sutures as they had high tensile strength, good elasticity, and biocompatibility.
3. PLA
In this regard, PLA or polylactide, a thermoplastic aliphatic polyester, is the most widespread and used among biopolymers, mainly made using the same bacterial fermentation that exploits sugar beet, corn, potatoes and other agricultural production wastes as a nutrient supply for lactic acid bacteria [109,110]. The phylum Firmicutes contains roughly twenty genera of lactic acid-producing bacteria, according to Reddy et al. [111]. Among them, the most important genus of lactic acid-producing bacteria is Lactobacillus, which has about 80 species and includes Lactobacillus casie, Lactobacillus bavaricus, Lactobacillus maltoromicus, and Lactobacillus salivarius [112]. At the same time, D-lactic acid and a combination of the two stereoisomers are produced by strains of Lactobacillus delbrueckii, Lactobacillus jensenii, and Lactobacillus acidophilus [113].
PLA can be obtained industrially by the polycondensation of D- or L-lactic acid (2-hydroxypropionic acid) or by the polymerization of lactide (the cyclic dimer of lactic acid) upon the ring-opening reaction [113,114,115]. Lactic acid, presenting a chiral center, comes in the form of two enantiomers, L-lactic acid (LLA) and D-lactic acid (DLA) (Figure 3). From the latter, it is possible to obtain two optically active cyclic stereoisomers (D,D-lactide and L,L-lactide) or two optically inactive stereoisomers (meso-lactide and the racemic mixture of LLA and DLA called rac-lactide).
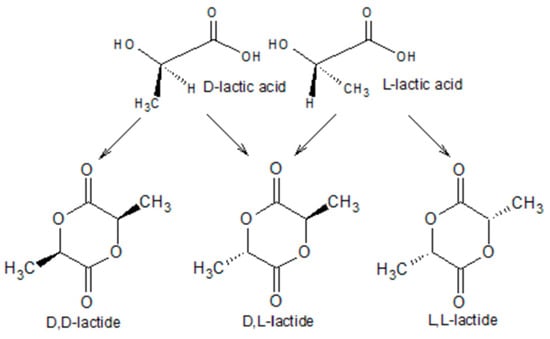
Figure 3.
The enantiomers L-lactic acid and D-lactic acid, from which to obtain the two optically active stereoisomers (D,D-lactide ad L,L-lactide) or two optically inactive stereoisomers (D,L-lactide).
The presence of chiral centers within the PLA biopolymer is of considerable importance, as it determines distinct polymer properties. Indeed, PLA biopolymers containing more than 90% of LLA tend to be semi-crystalline. On the other hand, increasing the concentration of the DLA stereoisomer tends to result in more amorphous PLA biopolymers. In addition, the increase in the DLA content results in a decrease in the melting (Tm) and glass transition (Tg) temperatures of PLA, and this is critical when a low activation temperature is required, as in the case of heat-sealable layers that make use of amorphous polymers [113].
This variety of structures highlights the importance of the choice of the PLA biopolymer matrix as it is reflected in the performance of the blends and its properties.
At present, PLA shows attractive mechanical properties that can be even superior to those of PET, PP and PE, with renewability, biodegradability, biocompatibility, a relatively low cost, and transparency [116]. Furthermore, a pure PLA polymer has a lower glass transition temperature (55–65 °C) than other fossil-based polymers, resulting in less energy (and subsequently processing costs) to transform and mold PLA. For this reason, PLA is one of the most abundant biopolymers on the market. Transparent bottles, food containers and wrappers can be produced using PLA, which is at the same time transparent, biodegradable and compostable. Furthermore, PLA is widely used for food service items, grocery bags and food waste, and coatings for paper and cardboard. In the biomedical field, it is used for sutures, prostheses, dialysis media and drug delivery devices [117]. This is due to its biocompatibility and safe degradation process. Once in contact with biological media, the polymer triggers an immune response as it is recognized as a “foreign body” [118,119]. The cells of the immune system (macrophages, neutrophils, and fibroblasts), by the secretion of enzymes, (such as acid phosphatase and lactate dehydrogenase) degrade the polymer matrix into lactic acid (LA) (a naturally occurring product and metabolic precursor of pyruvate in the Cori cycle) [120] or into carbon dioxide and water [121,122,123]. These products are subsequently metabolized intracellularly or excreted in the urine and airways.
These numerous applications are available because PLA can be processed using traditional and innovative transformation technologies used for processing plastic materials, respectively, extrusion (conventional, cast and blow film), injection molding, thermoforming, and additive manufacturing, such as fused deposition modeling [124,125,126,127].
Despite this, PLA suffer from major drawbacks. In fact, commercially available PLA has many inherent weaknesses, notably its brittleness, poor toughness, and low heat and impact resistance, which prevent its use for durable applications [128,129,130]. More importantly, PLA has a narrow processing window and a very slow crystallization rate that results in poor processability in the molten state [19,131,132,133]. Furthermore, another critical issue of bioplastics, which also affects PLA although to a lesser extent, is the higher cost of bioplastics compared to standard polymers, which limits the expansion of their market. Polymers of biological origin have a production cost that in many circumstances is 20% to 100% higher than conventional polymers. This is mainly due to the high costs of polymerization of polymers of biological origin, attributable to the fact that most technologies are still in the early stages of development and therefore have not yet achieved fully industrial production.
PLA is primarily degraded by hydrolysis through a two-step process. First, random chain cleavage of PLA’s ester groups reduces its molecular weight. The rate of chain cleavage depends on the pH value, temperature, and humidity levels of the environment [134]. Polymer embrittlement occurs as the molecular weight decreases. Secondly, microorganisms metabolize low-molecular-weight PLA, producing CO2, H2O, and humus [135]. Typically, a high rate and degree of crystallization is an important parameter because it enables applications where high mechanical performance is required. The degree of crystallization and thermal properties of PLA are influenced by its molecular weight (Mw), polymerization parameters and circumstances, as well as by its purity. In addition, the different lactide isomers can significantly influence Mw, Tg, Tm, the crystallization temperature (Tc) and the enthalpy of crystallization. In addition, PLA also has in general a relatively low Tg (55–65 °C), which can be a disadvantage for hot packing applications in which PS and PMMA are usually used. Moreover, it is essential to control the PLA degradation rate, mechanical and thermal properties as well as gas barrier properties, considering that for PLA, a lower permeability coefficient is reported if compared to PET.
To overcome these shortcomings regarding PLA, it is interesting to consider binary and ternary blends with other biodegradable polymers, including PBS, PBSA, and polymers of the PHA family, such as polyhydroxy butyrate (PHB), polyhydroxy valerate (PHV) and their co-polymers, which will be discussed later.
4. PBS
A biobased, biodegradable, and compostable material could be obtained by mixing PLA and PBS. This biopolymer has similar properties to PE and PP. PBS is a biodegradable aliphatic polyester that can be composted in accordance with ISO EN13432 [136] and is made through the polymerization of 1,4 butanediol (BDO) and succinic acid (SA) in a step-by-step process (Figure 4).
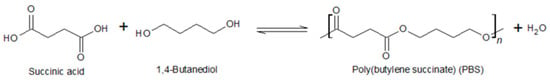
Figure 4.
Condensation reaction to obtain PBS.
The monomers are usually obtained from fossil-based raw materials (mainly maleic anhydride) as well as various biological sources [137,138,139], the latter providing improved sustainability. SA can be obtained using microbial fermentation or genetically modified yeasts that take advantage of different carbon sources, such as different carbohydrates (starch, cellulose, sucrose, etc.) [140,141,142,143,144,145]. These carbon sources can be obtained from potatoes, sugar beets, corn, wheat, lignin–cellulose [146,147]. In addition, glycerol has been seen to be an alternative route as a carbon source [148]. As far as BDO is concerned, since it is not present in nature, a green method has been developed by engineering Escherichia coli, using carbon sources derived from biomass [149,150].
PBS exhibits high flexibility, good thermal resistance, and chemical and physical stability, resulting in high compatibility with conventional processing techniques [117,151,152,153,154]. Its physical properties and biodegradation rate are strongly influenced by its molecular weight [155]. Details about the physical, chemical, and mechanical properties, as well as biodegradability of PBS have been the subject of several reviews [156,157,158].
PBS exhibits a Tm of around 100–115 °C, lower than PLA, estimated between 170–180 °C. The low Tm of PBS suggests that less energy is required for processing the melt. Furthermore, PBS has a high heat deflection temperature (HDT = 90–95 °C) compared to PLA, which has an HDT of 50–55 °C, and, therefore, PBS can be used in addition to contact with food (e.g., packaging material), and with hot liquid and solid aliments. The combination of its low melting temperature and its high thermal stability, greater than 300 °C, leads to the excellent processing properties of this biopolymer [151,153]. Tc of PBS ranges between 70 and 90 °C, while its Tg can be observed between −40 and −10 °C, depending on the supplier and the methods of fabrication [117,152].
Regarding the mechanical properties for PBS, an elastic modulus (E) of 300–350 MPa and elongation at break (ε) as a percentage, which can reach up to 650%, are observed. Its flexural modulus values have been measured around 650 MPa and its flexural strength about 40 MPa, although lower values have also been reported in the literature [159,160]. Its impact strength values have been recorded between 5 and 20 kJ/m2 [161], compared to the 1.5–2.5 kJ/m2 of PLA. These properties of PBS can go a long way in improving the poor brittleness, toughness, and ductility of PLA. Moreover, PBS is significantly more ductile than PLA, PHA, PHB, and PHV when the Mw is above 1.8 × 105 g/mol [158]. In fact, an Mw below 105 g/mol results in a polymer that is brittle and would not improve the properties of PLA. A higher molecular weight leads to a PBS with excellent properties, which can be easily processed through injection and extrusion techniques [158].
Some alternatives to improve the performance of PBS involves the use of bio-sourced plant fibers. Non-lignified fibers (flax and hemp) have been shown to exhibit higher interfacial forces with the PBS matrix [162], highlighting that the use of natural plant fibers can significantly not only improve the properties of PBS, but that this concept can be extended to other biopolymers such as PLA. The properties of fillers, such as polarity, particle size and water content, influence the final properties of the biocomposite material, as well as the quality of the interface between the matrix and filler and its processing. Therefore, in this same context, the use of plant fibers can affect the extrusion process depending on the polarity of the biopolymer matrix. Therefore, there is a trade-off between the improvement in the performance of the polymer’s properties and its subsequent processing using conventional techniques when bio-sourced plant fibers are embedded into the polymer matrix [163].
PLA/PBS Blends
Before describing the effects of different biopolymers mixed with PLA, it might be pertinent to remember that depending on how compatible the components are, polymer blends can be divided into either miscible or immiscible systems [164]. The compatibility between the components of the blend prevents the phase separation and, therefore, improves the performances of the resulting blend. The molecular interactions between the components of the blend are influenced by the Mw of the polymer chains, which in turn impacts their miscibility. When components in a miscible binary mixture are completely dissolved in each other, this results in a homogenous morphology at the nanoscale, which results in obtaining a mixture with improved properties compared to pure components [165,166,167]. On the other hand, if immiscible mixtures are produced, they exhibit a coarse morphology that is unevenly dispersed throughout the polymer matrix, commonly known as “droplet morphology”, and have weak interfacial adhesion. In this instance, the dispersed polymer phase often consists of spherical droplets with holes or spaces between them and the surrounding matrix. Therefore, in these situations, it is crucial to alter the interfacial characteristics of these mixes in order to have a reduction in interfacial tension and lower degrees of coalescence in order to have good final qualities.
For example, PBS with PLA can be miscible and/or partially miscible when used at low concentrations (5–20%), and in this case, PBS can assume the role of the plasticizing agent within the PLA polymer matrix [168,169,170,171]. Meanwhile, if PBS is used at high concentrations (20–40%), phase separation is observed and, thus, the blend performance drops compared to the pristine polymers [165].
There is some confusion in the literature regarding miscibility between the two polymers. PBS and PLA polymers were shown to be immiscible when PBS was over 20 wt% in the work of Bathia et al. [172], and when increasing the amount of PBS, phase separation took place. In addition, the average diameter of PBS domains was seen to increase when increasing the amount of PBS. Above the 50/50 wt% (PLA/PBS) mixture, the morphology reverses: when the concentration of PLA is below 50 wt%, PLA becomes the dispersed phase in PBS. The same immiscible behavior has been observed for PLA blends with PBSA by Lee and Lee [173].
According to the findings of Hassan et al. [168], PBS and PLA are only partially miscible when the PBS content is ≤10 wt%. This conclusion was supported by Jompang et al. [169], who used a SEM to examine the surface morphology of PBS/PLA blends. There are studies that, however, presume the immiscibility of PBS and PLA independent of the weight ratio, such as that of Yokohara and Yamaguchi [174].
To study PLA/PBS blends, Deng and Thomas [170] varied the PBS content between 0 and 100 wt%. According to reports, the degree of crystallinity in two different phases has increased as a result of a larger PBS concentration, but PLA has remained completely amorphous. Additionally, a continuous phase PLA-PBS with PBS contents between 10 and 40 wt% was disclosed [170]. This structure improved the elongation at break, increasing it in the range 270–340%.
PLA-PBS mixtures frequently show different mechanical properties. Ostrowska et al. [175] studied PLA/PBS blends with different concentrations. The mechanical properties of the blends were an average compared with the properties of pure polymers in the different phases.
In some cases, the reported results were higher for the mixture than for both individual components. In addition, previous studies indicate that PBS allows improvement of the thermal, mechanical, and biodegradation properties of PLA; in particular, the resulting blends appear to show a faster biodegradability [166,176].
Hassan et al. [168] confirmed that PBS/PLA blends were significantly more flexible than PLA, with the elongation at break reaching the value of 30% with a 40 wt% PBS content. However, Bhatia et al. [172] did not measure any increase in the ductility until reaching an amount of PBS up to 90%.
It is well recognized in the literature that stress may be transmitted from one component to another through the formation of “bridges” between polymer chains as a result of chemical or physical interactions, which can improve the properties of a polymer blend. This is possible through the use of a co-polymer (e.g., PLA-PBS) [177,178] and/or some compatibilizing agents such as fatty acids (lauric, palmitic and stearic acid) [179], maleic anhydride (MAH), acetyl tri-n-butyl citrate (ATBC) [180], isosorbide diester (IDE) [181], lysine tri-isocyanate (LTI), polyaryl-polymethylen-isocyanate (PAPI) [182], or triphenyl phosphite (TPP) [183]. The use of isocyanate as a reactive processing agent allowed the fabrication of a 10/90 wt% PBS/PLA blend with an up-to-four-fold improvement in impact strength over pure PLA [171]. In particular, reactive processing is a widely used compatibility strategy for the economic production of new polymer blends. During the processing of these polymeric materials, the high extrusion temperatures allow the reaction between the hydroxyl moieties of polymer and the isocyanate groups of the compatibilizer. The formation of more urethane bonds creates new cross-linking in the final blend.
Dicumyl peroxide (DCP), which was used by Ji et al. to study the compatibility of PLA/PBS blends, enabled the creation of PLA-co-PBS copolymers in the melt phase between the different polymer chains [184,185].
Bifunctional chemicals can, therefore, be used to improve interactions between blended polymers containing hydroxyl, carboxyl or amine functional groups, generating block copolymer structures at the interface of the two immiscible polymer chains. These chemicals include diacids, dibases hydroxy-acids or amino-acids.
As an example, applying two different types of compatibilizers such as the toluene di-isocyanate (TDI) and maleic anhydride-functionalized polylactide (MA-g-PLA), and two plasticizers such as the triethyl citrate (TEC) and tricresyl phosphate (TRP), Phetwarotai et al. [186] compatibilized PLA-PBS blends.
Some compatibilizers, such as ATBC and polyester adipate (PEA), can also be used as plasticizers. For example, introducing ATBC and IDE to PLA/PBS blends (PBS 20 wt%) allowed Fortunati et al. to study the plasticization of the mixtures [181]. It was demonstrated that ATBC is effective at plasticizing PLA (due to extremely comparable solubility parameters), but it is incompatible with PBS to the point ATBC levels of 30 wt% render PBS processing impossible. Despite having a different solubility parameter, IDE is also more effective on PLA, as shown by the decrease in the glass transition temperature (about 30 °C), cold crystallization temperature (around 35–45 °C), and melting temperature (up to 10 °C) of the PLA phase. With PBS, neither plasticizer works well.
Somsunan et al. [187] investigated the application of PEA to plasticize PLA/PBS mixtures by reducing intramolecular interactions and enhancing the mobility of the PLA and PBS chains. Due to the limited miscibility of the plasticizers in PBS, PEA does not have a plasticizing impact on PBS, deteriorating many of its mechanical properties. With 15% by weight of PEA in the mixture, PEA also causes a decrease in tensile strength and stiffness (76 and 38%, respectively), while increasing the elongation at break (up to 266%). Finally, PEA promotes the crystallization of pure PLA, barely changing its transparency.
However, high plasticizer concentrations excessively reduce the viscosity of the blend for flat die extrusion, requiring the addition of a copolymer (e.g., PLA-PBS) to increase the melt flow rate (MFR).
5. PBSA
PBSA is a biodegradable aliphatic polyester resulting, respectively, from the polycondensation of BDO with SA and adipic acid (AA) [188,189,190] (Figure 5).
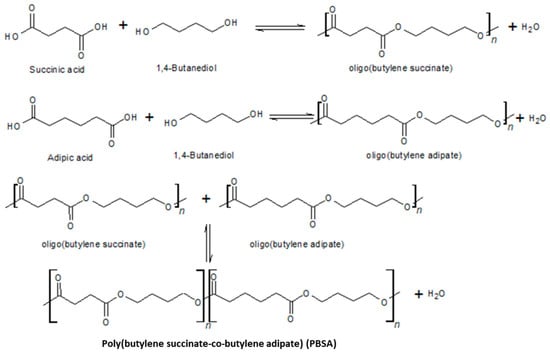
Figure 5.
Condensation reaction to obtain PBSA.
The nature of the oligomers used, butylene-succinate and butylene-adipate, influences the final properties of PBSA [191] as well as its biodegradation rates [192]. PBSA, compared to PBS, degrades much more rapidly, and shows a lower Tm and Tc [193]. Since the crystal fraction created is unstable and experience a melt–recrystallization–melt process during heating, PBSA exhibits two melting peaks between 80 °C and 100 °C. It exhibits a Tg that can be as low as −50 °C [194]. PBSA is highly flexible, processable, and highly resistant to impact, high temperatures and chemicals [173,195]. Its physical and mechanical properties, including density, tensile strength, and stiffness, are comparable with those of some flexible traditional fossil-based polymers [192], with a strength of around 20 MPa and a modulus of under 400 MPa, and with an elongation at break greater than 300% [196]. In addition to being somewhat less expensive than PBS [197], PBSA has been suggested for use in the production of films, notably for packaging and agricultural applications [133,198]. In addition, it is readily available thanks to the 100,000 tons/year amount produced. The tensile strength of this biopolymer declines as the amount of the AA component increases, and the same behavior is also found for its other physical properties [193].
PLA/PBSA Blends
According to the PBSA characteristics, the use of PBSA is very suitable for fabricating blends with PLA, since it increases its toughness and elongation at break [199,200,201]. Through blending PBSA with PLA, the resulting mixture’s ductility and toughness can be boosted while also enhancing its crystallization rate, transparency, workability, mechanical and barrier properties, and viscoelasticity [202]. Furthermore, the work of Gui et al. [203] showed that PLA/PBSA blends biodegrade faster than pure components because of the larger space in the microstructure, which facilitates their early degradation by microorganisms.
As a result, several research teams have lately emphasized the prospect of mixing PBSA with PLA to lessen PLA’s brittleness [204,205,206,207,208].
Nevertheless, PLA has been reported to be immiscible with PBSA due to unfavorable enthalpy mixing [209], thus obtaining a coarse morphology with poor compatibilization [210,211,212].
The blend’s composition, processing circumstances, rheological properties, and interfacial tension of the two elements all affect how a two-phase system evolves morphologically [213,214,215,216]. By adjusting the ratio of PLA to the rubbery polymer, several morphologies (droplet, co-continuous or double emulsion) may be produced, allowing the mechanical performance of the finished product to be managed [217,218,219]. In the work of Ojijo et al. [194] different blends of PLA/PBSA (100/0, 90/10, 70/30, 60/40, 50/50, 40/60, 30/70, 10/90, 0/100) were prepared and Fourier-transform infrared (FTIR) spectroscopy allowed them to assess the phase separation resulting from the absence of any significant interaction between the two components.
Since the mechanical properties of PLA/PBSA blends are poorer than those resulting from pure polymers alone, various attempts have been undertaken to increase their compatibility and give better interfacial adhesion [169,174,220,221].
Interestingly, chain extenders can be employed to reconnect the cleaved chains, resulting in an increase in the molecular weight [222,223,224]. Multifunctional epoxides [225], diisocyanate compounds [226], dianhydride [227], bis-oxazolines, tris(nonyl-phenyl) and phosphate (TNPP) [172] are a few examples of chain extenders that are also commercially available and have been the subject of in-depth research. Polymer degradation can also be better controlled and reduced thanks to the use of chain extenders during processing [222,223,228,229] while simultaneously favoring extrusion and injection molding [229,230]. In addition, chain extenders can react with both polymers to generate block copolymers, which are especially effective in the interfacial areas and boost the adhesion between the two phases of the polymer blend. In their study of PLA-PBSA blends (10-30 wt%) compatible with a chain extender, namely a styrene-acrylic oligomer with epoxy functionality (ESA) (0.5 phr), Lascano et al. [231] observed that compatibilization had an impact on the blend’s elongation at break. The researchers additionally detected a large improvement in impact strength (approximately 30%) and an order-of-magnitude increase in ductility. Similar results were obtained by Palai et al. [232] who showed that PLA/PBSA blends (5–20 wt%) using the identical chain extender ESA (added up to 3 wt%) could effectively make flexible films. In this work, morphological studies revealed positive polymer interaction thanks to the action of chain extenders, thus resulting in an enhancement of the mechanical properties of the blown film extruded. When compared to the PLA film, the blend’s rates of oxygen transmission (OTR) and water-vapor transmission (WVTR) were found to be 60% and 14% lower, respectively.
Ojijo and Ray [233] also prepared a PLA/PBSA blend using ESA through melt mixing. The chain extender was demonstrated to improve resilience, ductility, thermal stability, and crystallization. The same researcher demonstrated a similar effect when using triphenyl phosphate (TPP) in some blends containing, respectively, 30 and 10 wt% of PBSA [196]. Aliotta et al. [209] presented a study on PLA/PBSA blends (15–40 wt%) produced using twin-screw extrusion. The biggest increases in toughness (elongation at break and increase in impact strength) came from the 80-20 and 60-40 blends. The compatibilizer effect of an epoxy oligomer (EO) added up to 2% by weight was also investigated. Micromechanical analysis was performed on these mixtures, capable of going to record both axial and transverse elongation during tensile testing. The outcomes demonstrated that EO functions well as a compatibilizer, enhancing the compatibility of PBSA in a PLA matrix. The two blends vary morphologically in that the PBSA particles in the 80-20 blend are scattered throughout the PLA matrix, whereas the 60-40 blend exhibits a co-continuous microstructure. Although the reaction of the 60-40 blend is unaffected by the addition of EO, the response of the 80-20 blend is significantly altered, switching to deviatoric behavior as opposed to the basic blend, which displays cavitational behavior. Under cavitational behavior, the material, when tensile stressed, undergoes a volume increase in the area of interest as well as a change in shape while the deviatoric behavior results only in a change in shape and not volume [234]. This suggests that this response is likely due to a change in the morphology of the dispersed PBSA phase due to the addition of EO.
Eslami and Kamal [235] used a multifunctional epoxy-based chain extender to increase the melt strength of biodegradable polymer blends based on PLA and PBSA. The findings demonstrated that while the chain extender had no discernible impact on elastic modulus and strain at break of the blends, PBSA significantly improved the ductility of PLA/PBSA blends. They confirmed that such an approach results in a system with increased performance and processability since the combination of blending PLA with PBSA and including the chain extender gave the system both ductility and melt strength.
In the work of Yang et al. [236], a study was carried out involving both PLA-PBSA blends and blends in which PLA was functionalized with a fully biobased compatibilizer, namely crotonic acid (CA), subsequently coupled with PBSA (PLA-CA-PBSA) with a plasticizer function. Both blends were compared with pure PLA. The CA compatibilizer derives from the PHB recycling was coupled to PLA using a radical grafting reaction [237]. The analysis of differential scanning calorimetry (DSC) was used to assess its thermal properties. Tg dropped at around room temperature for PLA-CA-PBSA and PLA-PBSA. Tg for pure PLA was 44.3 °C, while it was 18.3 °C for the mixture with 20 wt% PBSA. Contrarily, PLA-CA-PBSA showed a Tg of 24.4 °C, which was greater than the Tg of PLA-PBSA but still 20 °C lower than the Tg of pure PLA. This reduction shows that adding PBSA to PLA, either by mixing or coupling, increased PLA’s chain mobility in each circumstance. This finding is of considerable significance when compared to a study in which PLA was mixed with PBS at different weight ratios and the mixture showed a reduction in Tg of only 4 °C [165,237]. Increases in the elongation at break of PLA from 14% to 165% and 460%, respectively, were found for PLA-CA-PBSA and PLA-PBSA.
Regarding the packaging application, gas permeability is an important property. Studies in the literature report that from simple PLA/PBSA binary blends, oxygen permeability increases slightly, in contrast to what is observed for blends in which a compatibilizer is used where oxygen permeability decreases [236].
6. PBAT
PBAT is an aliphatic–aromatic copolyester, produced currently from fossil resources, and consists of two types of comonomers, a rigid butylene terephthalate segment (BT) consisting of BDO and terephthalic acid (TPA) monomers, while the second butylene adipate segment (BA), is flexible and the section consists of BDO and AA [18] (Figure 6).
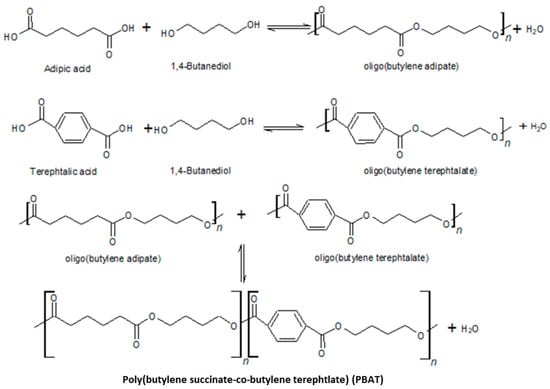
Figure 6.
Condensation reaction to obtain PBAT.
Although being so far completely fossil-based, biobased monomers are planned to be gradually introduced, as announced by manufacturers [238].
PBAT shows interesting properties at relatively low costs, which makes it suitable for various applications. To date, PBAT is applied for products such as shopping or rubbish bags, packaging materials, mulch films, cutlery, hygiene products, biomedical fields, and industrial composting [239,240].
It has good mechanical and physical properties, including high toughness, high ductility, resistance to moisture and water and is biodegradable and compostable [241]. The chemical composition and environmental conditions affecting degradation determine the speed at which PBAT biodegrades [19,242,243]. Witt et al. [191,244] published a study in 1996 that demonstrated how the amount of TPA in a polymer affects how quickly PBAT degrades: the chemical hydrolysis allows biodegrading even long aromatic oligomers in compost at high temperatures, while oligomers containing one or two terephthalates are easily and rapidly degraded.
The mechanical behavior of PBAT is similar to that of a thermoplastic elastomer due to its high elongation at break and low modulus of elasticity. As other biopolymers, the structural composition and molecular weight influences the mechanical properties of PBAT. In studies by Lee et al. and Herrera et al. it was reported that stiffness increases with an increasing BT unit content, while ductility decreases [245,246]. At the same time, tensile strength increases as the molecular weight increases in contrast to the elongation at break, which decreases. Considering these outcomes, the mechanical properties of PBAT can be changed by varying the reaction pressure and temperature, as both variables cause a reaction shift toward products and influence the molecular weight of PBAT.
According to the literature, low-density polyethylene (LDPE) and PBAT both have similar mechanical properties [247,248]. PBAT is a flexible material with flexural strength greater than 7 MPa and flexural modulus of about 130 MPa, as well as Young’s modulus of about 50 MPa, tensile strength between 20 and 35 MPa, and an elongation at break that can reach 700% [249,250].
PBAT shows a Tm that depends on the ratio of BA and BT units: as BT units increase, the melting temperature varies from 42 °C (10% mol BT) to 190 °C (80% mol BT) [251].
Owing to the high cost of manufacture and poor mechanical qualities of pure PBAT polymer products as compared to conventional plastics, the market has not yet accepted these materials for commercial products. In the future either the cost shall be reduced or the performance increased to allow for the development of a wide market for this type of polymer.
PLA/PBAT Blends
As seen for PBS, the properties of PLA may be considerably improved by combining PBAT with it, allowing for a greater range of applications in finished goods without compromising its biodegradability.
In the work of Jang et al. [132] PLA was blended with PBAT (5, 10, 14 and 20% by weight) in the molten state. This greatly improved the processability of PLA. During extrusion, pure PLA pellets led to high melt flow resistance, and this led to high screw torque and pressure during processing. PBAT has a lower melting point than PLA. Mixing it with PLA resulted in the reduction in screw torque and extrusion pressure as PBAT behaves as a lubricant at low extrusion/processing temperature. It was found that introducing PBAT accelerates the formation of crystals in the PLA but has no discernible impact on the final degree of crystallinity. The combination shows decreased tensile strength and Young’s modulus with increasing PBAT concentration (5–20% by weight), but enhanced elongation at break and toughness. Additionally, when the PBAT concentration increased, the specimen’s failure mode during the tensile test switched from brittle fracture to ductile fracture. Jiang et al. [132] showed that small amounts of PBAT added to the PLA matrix results in a significant and gradual increase in ductility, without significantly reducing its mechanical strength and stiffness.
Moreover, the addition of PBAT to PLA increases its toughness, as shown by the increase in the impact strength values from 21 J/m in pristine PLA to 50 J/m for a 75/25 PLA/PBAT blend [252].
Also, in the work of Gigante et al. [199] PLA/PBAT blends were prepared with an amount of 10 to 25% of PBAT, which results as dispersed phase within the PLA matrix. This is because it is known from the literature that, with an amount of 30–40% and over, PBAT would appear as a co-continuous morphology [253]. Additionally, it was noted how the quantity of PBAT affects the mechanical response of PLA. Data from further investigations [254,255,256] demonstrate that the elongation at break increases for mixes containing 20 and 25% PBAT up to 300 and 350%, respectively.
All the blends showed two distinct Tg values corresponding to the PLA and PBAT phases. This is evidence that the blends formed are thermodynamically immiscible. Signori et al. [257] observed similar findings for PLA/PBAT mixes having 75 wt% PLA. The addition of PBAT to PLA resulted in 20 °C drop in Tc, demonstrating the impact of PBAT on the rate of PLA crystallization in the combination, as previously described [132].
A review by Krishnan et al. [167] confirmed that the blending allows obtaining good toughness and flexibility, while maintaining biodegradability. Regarding processing, Quero et al. [258] reported a decrease in the final torque value since there are scattered PBAT domains in PLA matrix, while in an article by Gu et al. [259] it was noted that the linear viscoelastic limits of the molten PLA/PBAT blend were lower unlike those of pure PLA.
The immiscibility between PLA and PBAT remains a problem for optimizing the properties of blends based on these two polymers. It was shown by Ma et al. [260] that DCP functions as a free radical initiator for the in-situ compatibility of PLA/PBAT blends, reducing the domain size of the PBAT dispersion phase and increasing the toughness of the blend.
Chain extenders, which boost melt-state strength and thermal stability of polymer blends while also increasing polymer molecular weight, were recently developed [229,261]. Polyesters have made extensive use of multipurpose chain extenders with epoxy groups [229]. The hydroxyl and carboxyl groups in the polyester chain ends might react with the epoxy groups in the chain extender. This occurs as a result of a mechanism that also produces covalent bonds with the creation of hydroxyl and opens the epoxy group ring [224]. Thus, when compounding in the molten state, the production of copolymers is encouraged [262,263].
In their study, Arruda et al. [223] described the addition of various concentrations of Joncryl ADR4368 (BASF), a multifunctional epoxy chain extender utilized as a compatibilizer, to the PLA/PBAT blend. The compatibilizer-containing mixes exhibit an increase in viscosity. The correlation between the rise in complex viscosity and the increase in polymer molecular weight is brought on by the epoxy group of the chain extender interacting with both ends of the polymer chain [226]. Al-Itry et al. [256,264] also showed that the BASF product improved the stiffness, strain at break (up to 135%), and melt strength of PLA/PBAT blends. The addition of Joncryl and 1,6-hexanediol diglycidyl ether improved the compatibility of PLA and PBAT and increased the strain at break by up to 500% without noticeably reducing the strength, according to Dong et al. [265].
Another compatibilizer, glycidyl methacrylate (GMA), was employed by Kumar et al. [252] to enhance the interface regions of PLA-PBAT blends. The toughness and stiffness of the PLA matrix were seen to increase with increasing the amount of PBAT. In the presence of GMA and nano-clay, morphological images by SEM show increased interfacial adhesion between the PLA-PBAT blend. Compared to virgin PLA, DSC and TGA thermograms demonstrated superior thermal properties. Additionally, in the study of Zhang et al. [266], the introduction of GMA confirms the improvement in phase compatibility and, therefore, rheological properties due to the increased surface adhesion between the PLA and PBAT phases. Furthermore, the tensile strength was improved by up to 180% without compromising the strain at break.
Pan et al. [267] found that methylene diphenyl diisocyanate (MDI), as a responsive chain extender, can enhance the compatibility of PLA/PBAT. The strength of PLA/PBAT blends is increased by the formation of urethane bond between the chains of PLA and PBAT. However, due to the toxicity and volatility of diisocyanate compounds, which are reportedly gravely dangerous to humans according to the literature, the use of these blends is restricted.
Coltelli et al. [222] showed that including a radical initiator in PLA/PBAT blends during polymer mixing in the molten state improved compatibility while maintaining the Mw at similar values. In another work by Coltelli et al. [255], adding the plasticizer ATBC up to 30 wt% increased the elongation at break of the PLA/PBAT blend up to 300%. In another study, Dong et al. added phthalic anhydride (PA) and bioxazoline (BOZ) as compatibilizers to PLA/PBAT blends [268]. Due to the smaller domain size of PBAT, modest concentrations of PA or BOZ enhanced the elongation at break up to 515% without influencing the tensile strength. According to Nishida et al. [269], the application of 2,5-dimethyl 2,5-di(tert-butylperoxy) hexane (DMBPH) as a reactive compatibilizer decreased the domain size and therefore enhanced the elongation at break up to 30%, but more significantly, it enhanced the impact strength up to 30 times. Additionally, according to Coltelli et al. [222], the DMBPH affects the increase in mixture viscosity and the enhancement of elongation at break by up to 60%.
7. PHAs
Polymers of the PHA family have attracted growing interest since they are biocompatible, biodegradable, easy to process and show good mechanical properties [270]. PHAs are thermoplastic biopolymers produced directly by numerous microorganisms through the bacterial metabolism of carbohydrates or fatty acids. PHA macromolecules are accumulated by these bacteria as a water-insoluble inclusion that serve as carbon and energy storage components in their bodies [271,272]. Furthermore, it has been claimed that the variation in the amount and type of carbon sources fed to the bacteria affects the content and properties of the resulting polymer [100,273]. The most interesting feature of the PHA family is its complete biodegradability in compost, soil, and seawater, which makes it a suitable candidate for the development of environmentally friendly packaging. Additionally, the biocompatibility of PHAs is yet another crucial attribute that makes them appropriate for use in medical applications since they are not rejected by the body and their absorption generates nontoxic residues.
Regarding the chemical structure, PHAs are composed of aliphatic polyesters presenting a variable number of carbon atoms in the monomer unit. Depending on the number of monomers, they can be classified as “short-chain length PHA” (SCL-PHA) when obtained from C3-C5 units, or “medium-chain length PHA” (MCL-PHA) when made of C6-C14 monomers [274] (Figure 7). Monomeric units of SCL-PHA consist of 3-hydroxybutyric acid (3HB), 4-hydroxybutyric acid (4HB) or 3-hydroxyvaleric acid (HV), whereas those composing MCL-PHA are constituted by of 3-hydroxyhexanoic acid (HH), 3-hydroxyoctanoic acid (HO), 3-hydroxydecanoic acid (HD), 3-hydroxydodecanoic acid (HDD), 3-hydroxytetradecanoic acid (HTD) or even longer-chain co-monomeric units [275]. The physical properties of the resulting polymer, such as crystallinity, mechanical and processing properties, depend on the length of the side groups of the monomer units.
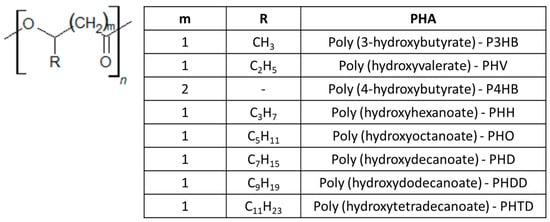
Figure 7.
Short-chain- and medium-chain-length PHA classification. In the table, the column “m” indicates the number of -CH2-(methylene group) repeats in the monomer unit, “R” indicates the side chain, and “PHA” stands for the type of polymer created by the monomer unit.
From the literature, it has been seen that SCL-PHAs exhibit a high degree of crystallinity and behave as rigid and brittle materials, while MCL-PHAs show a smaller elastic modulus thanks to lower crystallinity. The repeating units’ pendant group dimensions have a sizable impact on the physical properties of the resulting polymer [270].
Poly(3-hydroxybutyrate) (PHB), along with its copolymers poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH), represents the most commercially available and industrially produced microbial polyester thanks to its interesting mechanical properties, its thermoplastic behavior, and the various synthesis routes [276,277].
7.1. PHB
PHB can be obtained by various bacteria, as well as by cyanobacteria and/or microalgae. These species store PHB intracellularly as a source of reserve energy [278]. In addition to being a biopolymer, PHB is associated with the property of being biocompatible as low-molecular-weight oligomers of this biopolymer can be found in the bloodstream of humans. In addition, the biocompatibility of PHB is also related to its degradation product (3-hydroxybutyric acid), which is a common metabolite in living matter [279]. Therefore, due to its outstanding biodegradability and biocompatibility characteristics, PHB can find applications in various fields, such as packaging, agriculture, biomedicine, and cosmetics.
PHB (P3HB) has as its alkyl group -R a methyl group. It displays Tg between 0 and 5 °C, Tm between 170 and 180 °C, a degree of crystallization in the range of 60–80%, and a degradation temperature (Tdeg) of about 220 °C. The narrow window between Tm and Tdeg is critical since polymer decomposition may likely occur during processing. Thus, plasticizers are required to process PHB as well as to improve the strength and elasticity of the melt [280,281].
In particular, the elastic modulus and tensile strength of PHB are like PP values [282]. On the contrary, its ductility is much lower, making PHB an intrinsically brittle material, thus limiting its applications. Therefore, the addition of different monomeric hydroxyalkanoate co-units, such as hydroxyvalerate or hydroxyhexanoate, into PHB chains through microbial synthesis leads to an improvement in the mechanical properties of PHB [283]. All this leads to an expansion of the potential applications of these biopolymers.
Regarding homopolymer P4HB, it is more processable, flexible, and ductile than P3HB, thanks to its lower degree of crystallinity. This polymer has then found interesting applications in medicine to fabricate biomedical devices for repairing soft tissues [281].
7.2. PLA/PHB Blends
The research of PLA-PHB blends has been the subject of several studies [284,285,286,287,288,289]. These investigations showed that the molecular weight and fluidity index of the polymers affect the miscibility of PLA-PHB blends. The literature showed that PHB is only miscible with low-molecular-weight PLA, and the presence of PLLA affects PHB’s crystallization kinetics [286,290]. Likewise, in a melt containing up to 50 wt% of PHB, the PLA is also miscible with low-molecular-weight PHB but is not miscible with high-molecular-weight and commercial-grade PHB [287,288]. According to Ohkoshi et al. [288], PLA is only miscible with low molecular weight atactic PHB, combining PLLA with different molecular weights of atactic PHB. Moreover, the addition of PHB to PLA matrices could increase its crystallinity [291]. Given that crystallinity directly affects gas penetration, it is well recognized that raising the amount of crystallinity might enhance the usage of PLA as a packaging material [198]. In general, the barrier properties of PLA/PHB blends are generally better than PLA’s [51,292], but the strain at break values are still relatively low since improvements of less than 1% were found [82,293]. In agreement with this, Ni et al. [291] observed that introducing 3-hydroxybutyrate (HB) oligomers, at an amount less than 40 %, within the PLA biopolymer led to improved crystallinity. It is known from several studies that the PLA/PHB 75/25 blend shows significantly better mechanical properties than pure PLA: PHB crystals act as a filler and nucleating agent in PLA. Mixing PLA and PHB also leads to an improvement in PLA biodegradability as the PHB content increases, due to ester bonding, that can be broken [272] when exposed to biologically active conditions (soils, seawater or freshwater, aerobic and anaerobic composts, activated sludge, landfills). PHB also sinks in water, which speeds up the process of biodegradation in sediments [294].
Both PLA and PHB are semicrystalline polymers that, when exposed to room temperature, show excellent strength and stiffness but low fracture toughness and brittle behavior. The lack of ductility and flexibility limits their applications, such as film production. The brittleness results from the high Tg of PLA [295] and the high crystallinity degree and relatively large spherulites of PHB [296].
Tensile strength and elongation, fracture toughness, and impact strength are unquestionably the mechanical properties that packaging applications are most interested in [297,298]. As a result, PLA and PHB require the addition of plasticizers to increase their ductile properties and obtain the flexibility needed for film production. This is because PLA and PHB are naturally brittle.
The values of elongation at break reported for PLA/PHB (75:25 w/w) mixed with different plasticizers increase and are between 6% and 15% for poly(ethylene glycol) (PEG) [51,299], D-limonene [82] and Lapol 108 [293], and up to 90% for ATBC [34,35,43,300]. Furthermore, it has been demonstrated that ATBC works well to quicken the decomposition process while composting [43]. The application of PEG [301] and ATBC [302] does not create safety issues for food contact materials, according to the European Food Safety Authority (EFSA).
7.3. PHB Copolymers
The usefulness of PHB is limited due to its brittleness [303]. However, the addition of poly-3-hydroxyvalerate (PHV) to the PHB polymer chain can improve the ductility and processability of the polymer [304]. Regarding PHBV, the resulting copolymer structure consists of 3-hydroxybutyrate and 3-hydroxyvalerate repeating units randomly distributed in the polymer chain [305,306], adapting the polymer properties to specific applications as for conventional thermoplastics [307]. For biological applications, PHBV has been the topic of in-depth and ongoing study using different molar ratios of PHV units [308,309]. When the molar percentage of HV in the copolymers is increased, the processing temperature window can be widened and the processability of the melt is improved by lowering the Tm to 95 °C and the crystallinity to 25% without appreciably affecting the Tg and Tdeg [310,311]. Impact strength increases and tensile strength decreases with increasing HV units [312]. The degradation rate of PHBV is faster than PHB one. The degradation kinetics depends on the structure (copolymer or homopolymer) and crystallinity and, consequently, on the processing conditions [305].
These properties make PHBV a good candidate to substitute oil-based polyolefins in many areas [313], being able to process it by conventional techniques such as extrusion, injection, or compression molding [303]. The European Plastics Regulation (EU) No. 10/2011 authorizes the use of PHBV materials in food contact applications, and global migration tests have already been conducted to assess their inertness in liquid food simulants [314,315]. However, due to its high manufacturing costs [316,317], the broad application of PHBV is also constrained.
In the case of PHBH, the possibility of customizing its composition by combining highly crystalline (3HB) and elastomeric (3HH) units allows obtaining higher thermal stability, and better mechanical properties if compared to PHB and PHBV [318].
By raising the molar proportion of HH, the processing window may be opened up while minimizing thermal degradation, lowering the Tm for PHBH to 54 °C and reducing the crystallinity to 15% [319]. The Young’s modulus and elongation at break values for PHBV (0.5–3.5 GPa, 5–50%) and PHBH (0.1–0.5 GPa, 5–850%) given in the literature are typically lower than those recorded for PHBH (0.9–4.0 GPa, 5–20%).
This makes PHBH distinct from other PHAs and allows its usage in applications that require both flexibility and room-temperature compostability [320,321,322]. In this context, PHBH can be used for manufacturing processes such as injection molding [323,324], extrusion [325,326], thermoforming [327,328], foaming [329], non-woven fabrics and fiber production [330,331], 3D printing [332,333], as well as paper and fertilizer coating [334].
However, PHAs are currently too expensive to utilize on their own; thus, they are frequently combined with other, less expensive polymers that have complimentary features [19].
7.4. PLA/PHB Copolymer Blends
Studies have been performed regarding blends of PLA, the miscibility of two polymers, and the potential improvement in their physical properties [286,290,335].
Iannace and Huanc [336] studied the thermal and mechanical properties of a PLA/PHBV blend by means of a solvent melting process and reported a miscibility problem between the two polymer matrices, as also found in the work of Ferreira et al. [337].
Some authors also demonstrated that the addition of PHBV to the PLA polymer matrix caused a reduction in the crystallinity of PLA and its degradation rate [273,336].
On the other hand, it was also demonstrated that the dispersed phase of PHBV might serve as crystal nucleation sites, improving the crystallinity of PLA [338,339]. Partial miscibility with high compatibility or immiscibility has also been confirmed by other authors [271,340,341,342].
Additionally, it was demonstrated that PLA may prevent PHBV from crystallizing [340]. The rheological behavior of the PLA/PHBV blends revealed that PHBV has a very low thermal stability and measurements of melt viscosity suggested significant degradation [271].
In a study by Kanda et al. [343], PLA/PHBV mixes were made, and SEM images revealed evidence of interface cavities and limited interfacial interaction between the two polymers. Additionally, the mixtures all lacked co-continuous morphology. As the PHBV content rose, so did the average size and relative concentration of these cavities. With increasing PHBV concentration, both tensile strength and Young’s modulus dropped, reaching their maximum values at 10 wt% PHBV. The tensile strength and Young’s modulus were equivalent to or lower for samples of PLA/PHBV between 50/50 and 10/90 than for both pure polymers. However, Zembouai et al. [338,344,345] considered blends with 25, 50, and 75 wt% PHBV instead, and they found that the PHBV concentration increased Young’s modulus. Meanwhile, González-Ausejo et al. [346] claimed that mixes with a 75% PHBV content had superior tensile strength and Young’s modulus values than those of pure PLA.
The integration of 20 wt% PHBV with a high HV content resulted in a 230% increase in tensile strain at break, as demonstrated by Ma et al. in their study [347]. WVTR and OTR are also significantly improved by the presence of PHBV [345].
Nanda et al. [340] produced biodegradable polymer PLA/PHBV blends with a PLA content in the range 30–50 wt% using a twin-screw micro-compounder followed by injection molding. The incorporation of PLA into PHBV improved the strength and modulus of the blends [340]. A blend with high elongation, acceptable strength and modulus was formulated, with great potential for industrial use. They explained that the resulting increase in the blend strength and modulus was due to the PLA phase, resulting in effective stress transfer from one phase of the blend to the other.
When 10% PHA was added to PLA, Noda et al. [348] discovered that the blends’ ability to elongate greatly enhanced, and they hypothesized that this was because of the blend’s amorphous phase.
In the work of Richards et al. [349] and Nanda et al. [340], a reduction in the Tg of PLA from 60 to 45 °C was observed with increasing PHBV content in the polymer blend, as observed in the work of Kanda et al. [343], also observed two Tg values for PLA-PHBV blends, with the Tg of PLA decreasing with increasing PHBV content [271,338,340,344,345,346,349,350,351]. In contrast, the research of Ma et al. and Liu et al. revealed no significative change in Tg for blends with PHBV contents between 0 and 50 wt% and 5–30 wt%, respectively [339,347]. Based on these studies, the researchers concluded that these materials form an immiscible mixture [61,271,337,338,339,340,342,344,345,346,347,349,350,351].
To enhance the miscibility and the mechanical properties of PLA/PHBV blends, zinc acetate, a transesterification catalyst, can be added while the mixture is still liquid [352]; the transesterification reaction of PLA/PHBV inhibited the thermal degradation of PHBV. In the presence of three different kinds of diisocyanates, the compatibilization impact was further investigated [353]. The blend’s overall mechanical performance was enhanced by the compatibility, but the blend’s thermal stability was unaffected.
In the study of González et al. [353] sepiolite was used as compatibilizer in PLA/PHBV blends to increase the affinity between the biopolymers: reduced oxygen permeability and increased elongation at break was detected. In a different study, it is established that the addition of diisocyanates enhances the compatibility of the blend, resulting in an overall improvement in mechanical properties and primarily in a much higher complex viscosity at low frequencies than in the case of the identical system without diisocyanates [61]. Cellulose fibers were also mixed in a molten state within PLA/PHBV blends, simultaneously improving stiffness, ductility, and toughness [354].
Few studies have explored the behavior of PLA/PHBH blends [108,341,355], that were found to be immiscible with partial compatibility [350]. Even though the blends suffered fast physical aging that resulted in considerable toughness loss, it was found that the blend films’ toughness increased with 10 wt% PHBH [356]. Blends of PHBH and PLA were shown by Cheng et al. [331] to be immiscible at all mixing ratios. In this combination, PLA had no effect on how PHBH crystallized.
Another approach to increase the blend compatibility is through a reactive process during mixing in the molten state. Zhou et al. [356] used a reactive epoxy compatibilizer (REC), a copolymer of glycidyl methacrylate and styrene, to compatibilize the PHBH/PLA mixture (80/20 wt%). The epoxy group is believed to react with the hydroxyl group of PHBH on the one hand, and with the hydroxyl and carboxyl groups of PLA on the other hand. The result was a more uniform morphology with the PLA more evenly distributed in the matrix. An improvement in toughness with minimal loss of stiffness was observed, and the initial degradation temperature of the blends increased significantly from 256 °C with no REC to 293 °C at 4% REC. The addition of REC also seemed to improve elongation at break and impact strength, while tensile strength and elastic modulus did not appear to undergo significant changes.
A little quantity of Nodax, a ductile PHA that combines 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx) units, was combined with PLA by Noda et al. [348]. When less than 20 wt% of this ductile PHA was added, the mixes became tougher. PLA/Nodax melt blends were produced by Schreck and Hillmyer [357], who also investigated the use of a block copolymer as a compatibilizer. For blends containing 15 and 20 wt% Nodax, a considerable increase in impact strength was obtained, but the compatibilizer had no effect on the impact parameters.
8. Ternary Blends of PLA
The key problems with many binary blends (BBs) are the significant reductions in one property while increasing other properties, such as material strength and modulus.
With ternary blends (TBs), with proper balancing of the individual constituents, it is possible to obtain material properties that cannot be achieved with BBs or single components [358]. Compared to BB, the introduction of a third component usually enables the achievement of more balanced properties from combining different polymers with complementary characteristics [204].
Various morphologies can arise in ternary blend systems. Complete wetting in an A-B-C system arises when phases A and B, as well as phases B and C, completely wet each other. In the case of partial wetting, the most stable thermodynamic state is when there is three-phase contact, resulting in droplets of B located at the A-C interface, so that all three phases are in contact with one another. Possible microstructures of ternary polymer blends containing one major phase (polymer A) and two minor phases (polymers B and C), are, respectively, complete wetting, partial wetting and combined complete-partial wetting morphologies, as described in [359].
Zhang et al. [317] examined PLA-, PHBV-, and PBS-containing TBs using a primary phase that consisted of either PLA or PHBV at a concentration of 60% by weight. PBS and PHBV were added to PLA-based blends in alternating ratios of 10 and 30 wt% for each constituent. PLA and PBS were added to the PHBV-based blends as the main phase, again alternating the concentrations of 10 and 30 wt% for the two additional polymers. Only PHBV and PLA showed minimal miscibility, according to the results. After adding PHBV and PBS, blends with PLA as the primary polymer phase become more ductile and stronger. For blends containing 30 wt% PBS, the results indicated that the elongation at break was nearly double and impact strength increased by around 40%. Additionally, there is not much stiffness loss as compared to pure PLA. Blends based on PHBV were discovered to be more flexible, with an impact strength of roughly 30 J/m and an elongation at the break that was 80% greater. However, the stiffness of PHBV-based blends is lower than that and may be somewhat eliminated by adding 30 wt% PLA.
Ravati et al. [360] investigated the morphology and properties of TBs composed of PLA, PBAT, and PBS. The authors showed that in a blend of PBS/PLA/PBAT (33/33/33) [361], the morphology was highly continuous with a phase structure dominated by complete wetting dynamics, resulting in a tri-continuous structure. The system demonstrated significant values for the elongation at break (over 500%), Young’s modulus (1130 MPa), and impact strength (271 J/m). Moreover, the storage modulus (in DMA measurements) was approximately 50% greater than that of pristine PBS at room temperature.
In the work of Arrigo et al. [362], melt-blending was used to create PLA-based TBs with secondary phases of PHBH and PBS. The results show that the TBs improves toughness and impact strength compared to pure PLA. The PLA matrix responded favorably to the addition of the PBS and PHBH phases, with an improvement in its crystalline degree. Furthermore, compared to pure PLA, all formed TBs had a greater melt viscosity, providing the high melt strengths often needed for processing polymeric materials using industrial methods like thermoforming or film blowing.
The crystallization and mechanical behavior of the PLA and PBSA blend, using PBAT as an additive, were investigated by Sommai Pivsa-Art [221] and the PLA/PBSA blend (in a ratio of 4:1) in the presence of 20 wt% PBAT showed the highest impact strength. Weraporn Pivsa-Art [220] examined a comparable composition in terms of its use in the blown film sector and found that raising the proportion of PBAT lowered the melt flow index (MFI) and tensile strength while improving the elongation capacity.
Wang et al. [363] studied ternary blends of PLA/P3HB4HB/PBAT (where P3HB4HB is the poly(3-hydroxybutyrate-co-4-hydroxybutyrate)), which showed an excellent stiffness–toughness balance for the film with composition 55/10/35. In addition, with increasing P(3HB-co-4HB) content, crystallization in the molten and cold state of PLA was promoted.
The use of epoxidized vegetable oils as compatibilizers has been exploited by Garcia-Campo et al. in PLA, PHB, PBS or PBSA as alternative TBs [201]. Their use can give PLA and PHBH a significant increase in toughness, as well as being an economical solution. Due to the high reactivity of epoxy groups towards the hydroxyl end groups of polyester chains, these compatibilizers (epoxidized linseed oil and soybean oil) have been used for reactive extrusion with ternary blends based on PLA/PHB/PBS and PLA/PHBH/PBSA. Again, the incorporation of both flexible polyesters (PBS and PBSA) to the mixture consisting of PLA and PHBH resulted in an improvement in resilience.
9. Bio-PE
Polyolefins together with polyesters make up approximately 45% of most plastics produced today [364]. Of this family, polyethylene (PE) is the polymer most widely used in the world due to its versatile properties and low cost. The starting monomer used for the synthesis is ethylene, derived from petroleum raw materials. In recent years, the advent of new technological approaches has encourage the scientific communities to focus on the development of new emerging bioplastics obtained from renewable raw materials [365,366]. Among biopolymers, biopolyethylene (Bio-PE) has gained prominence as a technological breakthrough due to its reduced dependence on fossil materials [367,368]. To date, the most popular approach for obtaining this polymer is to synthesize the ethylene monomer through the dehydration of bioethanol, which in turn is obtained from glucose. The latter can be obtained from various agri-food wastes, such as sugar cane, sugar beet, wheat or cereal processing waste, and lignocellulosic materials. After obtaining the ethylene monomer, different types of Bio-PE can be obtained from its polymerization: Bio-High Density Polyethylene (Bio-HDPE) with a low degree of short-chain branching, Bio-Low Linear Density Polyethylene (Bio-LLDPE) with a high degree of short-chain branching and Bio-Low Density Polyethylene (Bio-LDPE) with a high degree of short-chain plus long-chain branching. The polymerization procedure of these biopolymers is the same as that used when using ethylene derived from petroleum slags, and furthermore the corresponding biopolymer is identical in properties.
PLA/Bio-PE Blends
Considering the increasing use of PLA in the packaging industry, PLA/BioPE blends could be an excellent solution to recycle both polymers. Many works have been reported in the literature in which PLA was blended with Bio-PE. The main disadvantage of mixing these two biopolymers lies in the fact of their different polarity, which results in poor miscibility. This difference in polarity is due to the different value of the solubility parameter (δ) of these polymers, as PE is a non-polar polymer and PLA is a polar polymer due to the presence of hydroxyl, ester, and carboxyl groups in its structure [369]. In fact, the typical δ value for PLA is 20 MPa [370], while for PE it is around 16 MPa [371].
To solve the problem of miscibility in a polymer blend, the use of compatibility agents is promoted, which contribute positively to reducing the size of dispersed phase domains and improve interfacial adhesion, thus improving the morphological stability and properties of the final blend. Compatibilizing agents used for PLA/BioPE blends that are reported in the literature include the PE-PLA block copolymer (PE-g-PLA) used by Wang et al. [372] and Anderson et al. [373].
In the work of Ferri et al. [374], they studied PLA/BioPE (80/20 wt%) blends, adding ethylene vinyl acetate (EVA), with a vinyl acetate content of 33%, polyvinyl alcohol (PVA) and dicumyl peroxide (DCP), as compatibilizers. Due to the poor miscibility, the binary PLA/Bio-PE mixture had poor mechanical properties, even worse than simple PLA. With the addition of these three compatibilizers, the interfacial interactions improved and consequently this led to an improvement in the mechanical properties. With the EVA compatibilizer, the highest increase in both elongation at break (86%) and impact energy absorption (63%) was achieved compared to the non-compatibilized mixture, due to an increase in polymer cohesion. In addition, the compatibilizing effect led to an increase in the peak of cold crystallization temperature and there was a decrease of approximately 50% in the crystallinity of the PLA/Bio-PE mixture in contrast to mixing the two polymers without the aid of a compatibilizer, since the Bio-PE acts as a nucleating agent for the PLA polymer.
In the work of Yoon et al. [375], they mixed PLA with EVA. The results showed a slight increase in elongation at break with a 70 wt% concentration of EVA, but when EVA was added at 90 wt%, a maximum elongation of 209% was achieved. As the concentration of EVA increased, there was a decrease in tensile strength and Young’s modulus.
In the work of Ferreira et al. [376], they evaluated the additional influence of different compatibilizers in the PLA/Bio-PE blend using poly(ethylene octene) and ethylene elastomer both grafted with glycidyl methacrylate (POE-g-GMA and EE-g-GMA), polyethylene grafted with maleic anhydride (PE-g-MA), polyethylene grafted with acrylic acid (PE-g-AA) and the block copolymer styrene–ethylene–butylene–styrene grafted with maleic anhydride (SEBS-g-MA). Again, after the addition of the different compatibilizing agents, there was an increase in the degree of crystallinity, the thermal stability, and the mechanical properties of the polymer blend compared to pure PLA. Thus, the added compatibilizers provided PLA/Bio-PE with higher performance than the simple blend.
In their work, Brito et al. [377] evaluated the effect of ethylene-glycidyl methacrylate (EGMA) copolymers and the terpolymer ethylene-methyl-acrylate-glycidyl methacrylate (EMA-GMA) on the mechanical and morphological properties of PLA/Bio-PE blends. The blends were prepared by extrusion followed by injection molding. From the results of the mechanical tests, MFI measurements together with FTIR analysis, it was found that the two compatibilizers promoted the compatibilization of the biopolymer blend through the in situ formation of copolymers due to the reaction between the hydroxyl and carboxyl functional groups of the PLA and the epoxy groups of the two copolymers (EGMA and EMA-GMA).
10. Conclusions and Future Perspectives
This paper reviews the main strategies for improving the problems and limitations related to the use of the biopolymer PLA. This has stimulated research and industries to investigate and seek improvements by blending with other biopolymers. Among them, the most promising biodegradable polymers are PBS, PBSA, PBAT, and different PHAs, as they represent a sustainable solution for reducing the environmental impact of traditional plastics in industrial, packaging, biomedical, and environmental applications. One of the main issues emerging from the literature regarding binary blends of PLA is the immiscibility between the constituent polymers. This immiscibility may lead to certain limitations and problems in the use of such blends as they may have disadvantages in terms of limited mechanical properties, low compatibility that may lead to phase separation, as well as the difficulty of processing the material.
To overcome these limitations, research is focusing on TBs of biodegradable polymers, where a third polymeric phase is introduced that can improve both the compatibility and the interaction between the different components. TBs offer the opportunity to combine polymers with complementary properties, allowing a better balance between strength, ductility, and thermal stability. The addition of a third phase can also favor the formation of more homogenous structures, improving the overall composite properties of the materials. However, even with ternary blends, it is important to carefully consider the formulations and proportions of the components to achieve optimal results.
For this, an additional prerequisite would be the use of fully biobased and biodegradable plasticizers and compatibilizers that can improve compatibility between polymers in both binary and ternary blends by optimizing the mechanical, thermal, and process properties of the materials. An important prerequisite is an increase in the degree of crystallinity of the final packaging, which can be useful in food packaging applications. Furthermore, another prerequisite is to obtain the maximum ductility of the final materials to achieve the ductility needed for various uses in the bioplastics industry. Today, many efforts are being made to implement environmentally friendly, natural-based plasticizers and compatibilizers that lead to improved biodegradation and properties of the final products, as well as solving toxicity issues.
Ultimately, continued research and innovation about the design of biodegradable plastics and PLA-based BBs and TBs are key to address the global challenges and push towards a more sustainable and responsible future for the plastics industry. However, further research and development are needed to realize the full potential of these materials and make them a more widespread and cost-effective choice for industry and consumers.
In a society that has set itself the objective of employing sustainable and ecologically friendly materials with the potential to translate the concepts of industrial ecology into practice, biodegradable polymers are anticipated to play an increasingly significant role.
For this reason, a pressing issue that needs to be urgently addressed, to evaluate the use of these formulations in the design of biodegradable and compostable films and packaging, is related to the lack of studies on the disintegration and compostability under domestic and industrial composting conditions as well as studies on migration in different environments to make the performance of innovative packaging as optimal as possible.
Finally, pure reliance on biodegradable plastic is insufficient to prevent environmental losses. In the end, it is crucial to concentrate on altering people’s behavior, especially by limiting the use of plastic and properly disposing of used plastic instead of throwing it in the trash. Additionally, it is essential to offer the facilities and resources required to promote these desirable habits, such as clear product labeling and the accessibility of separate collection containers nearby.
Author Contributions
Conceptualization, S.D.L., C.S. and D.M.; data curation, S.D.L.; writing—original draft preparation, S.D.L. and C.S.; writing—review and editing, C.S., D.M., D.G.-N. and A.C.; supervision, C.S. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support of the Project funded under the National Recovery and Resilience Plan (NRRP), Mission 04 Component 2 Investment 1.5-NextGenerationEU, Call for tender n. 3277 dated 30 December 2021, Award Number: 0001052 dated 23 June 2022.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Persson, L.; Carney Almroth, B.M.; Collins, C.D.; Cornell, S.; de Wit, C.A.; Diamond, M.L.; Fantke, P.; Hassellöv, M.; MacLeod, M.; Ryberg, M.W.; et al. Outside the Safe Operating Space of the Planetary Boundary for Novel Entities. Environ. Sci. Technol. 2022, 56, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Thew, C.X.E.; Lee, Z.S.; Srinophakun, P.; Ooi, C.W. Recent Advances and Challenges in Sustainable Management of Plastic Waste Using Biodegradation Approach. Bioresour. Technol. 2023, 374, 128772. [Google Scholar] [CrossRef] [PubMed]
- Goswami, M.; Rekhi, P.; Debnath, M.; Ramakrishna, S. Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds. Molecules 2021, 26, 860. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Carus, M.; Dammer, L. The Circular Bioeconomy-Concepts, Opportunities, and Limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- WEF_The_New_Plastics_Economy. World Economic Forum. Available online: https://www.weforum.org/publications/the-new-plastics-economy-rethinking-the-future-of-plastics/ (accessed on 1 June 2023).
- A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment Updated Bioeconomy Strategy. Available online: https://op.europa.eu/en/publication-detail/-/publication/edace3e3-e189-11e8-b690-01aa75ed71a1/language-en (accessed on 15 June 2023).
- Ingrao, C.; Lo Giudice, A.; Bacenetti, J.; Mousavi Khaneghah, A.; Sant’Ana, A.d.S.; Rana, R.; Siracusa, V. Foamy Polystyrene Trays for Fresh-Meat Packaging: Life-Cycle Inventory Data Collection and Environmental Impact Assessment. Food Res. Int. 2015, 76, 418–426. [Google Scholar] [CrossRef]
- Dassisti, M.; Intini, F.; Chimienti, M.; Starace, G. Thermography-Enhanced LCA (Life Cycle Assessment) for Manufacturing Sustainability Assessment. The Case Study of an HDPE (High Density Polyethylene) Net Company in Italy. Energy 2016, 108, 7–18. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management Life Cycle Assessment Principles and Framework. ISO (International Organization for Standardization): Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management Life Cycle Assessment Requirements and Guidelines. ISO (International Organization for Standardization): Geneva, Switzerland, 2006.
- Blanco, I.; Ingrao, C.; Siracusa, V. Life-Cycle Assessment in the Polymeric Sector: A Comprehensive Review of Application Experiences on the Italian Scale. Polymers 2020, 12, 1212. [Google Scholar] [CrossRef]
- Jayawardane, H.; Davies, I.J.; Gamage, J.R.; John, M.; Biswas, W.K. Additive Manufacturing of Recycled Plastics: A ‘Techno-Eco-Efficiency’ Assessment. Int. J. Adv. Manuf. Technol. 2023, 126, 1471–1496. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. From Monomers to Polymers from Renewable Resources: Recent Advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Wang, Z.; Ganewatta, M.S.; Tang, C. Sustainable Polymers from Biomass: Bridging Chemistry with Materials and Processing. Prog. Polym. Sci. 2020, 101, 101197. [Google Scholar] [CrossRef]
- Saxon, D.J.; Luke, A.M.; Sajjad, H.; Tolman, W.B.; Reineke, T.M. Next-Generation Polymers: Isosorbide as a Renewable Alternative. Prog. Polym. Sci. 2020, 101, 101196. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent Advances in Vegetable Oil-Based Polymers and Their Composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Carus, M.; Dammer, L.; Raschka, A.; Skoczinski, P.; vom Berg, C. Renewable Carbon—Key to a Sustainable and Future-Oriented Chemical and Plastic Industry. Greenhouse Gas Sci Technol 2020, 10, 488–505. [Google Scholar] [CrossRef]
- Greene, J. Marine Biodegradation of PLA, PHA, and Bio-Additive Polyethylene Based on ASTM D7081. Available online: www.academia.edu/9639333/Marine_Biodegradation_of_PLA_PHA_and_Bio_additive_Polyetylene_Based_on_ASTM_D7081 (accessed on 10 June 2023).
- IPCC. Special Report on Global Warming of 1.5 °C. 2018. Available online: https://www.ipcc.ch/sr15 (accessed on 10 June 2023).
- Royer, S.J.; Ferrón, S.; Wilson, S.T.; Karl, D.M. Production of Methane and Ethylene from Plastic in the Environment. PLoS ONE 2018, 13, e0200574. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics the Facts 2016. 2016. Available online: https://www.plasticseurope.org/en/resources/publications/3-plastics-facts-2016 (accessed on 1 July 2023).
- Working Group I of the IPCC. Climate Change 2013: The Physical Science Basis. Contr. Work 2013, 43, 866–871. Available online: https://www.ipcc.ch/report/ar5/wg1/ (accessed on 10 July 2023).
- Nolte, T.M.; Hartmann, N.B.; Kleijn, J.M.; Garnæs, J.; van de Meent, D.; Jan Hendriks, A.; Baun, A. The Toxicity of Plastic Nanoparticles to Green Algae as Influenced by Surface Modification, Medium Hardness and Cellular Adsorption. Aquat. Toxicol. 2017, 183, 11–20. [Google Scholar] [CrossRef]
- Somanathan, H.; Sathasivam, R.; Sivaram, S.; Mariappan Kumaresan, S.; Muthuraman, M.S.; Park, S.U. An Update on Polyethylene and Biodegradable Plastic Mulch Films and Their Impact on the Environment. Chemosphere 2022, 307, 135839. [Google Scholar] [CrossRef]
- Snell, K.D.; Peoples, O.P. Polyhydroxyalkanoate Polymers and Their Production in Transgenic Plants. Metab. Eng. 2002, 4, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Utracki, L.A. History of Commercial Polymer Alloys and Blends (From a Perspective of the Patent Literature). Polym. Eng. Sci. 1995, 35, 2–17. [Google Scholar] [CrossRef]
- Guo, Q. Polymer Morphology; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 464. [Google Scholar]
- Plastic in the Ocean Statistics. 2020. Available online: https://www.condorferries.co.uk/plastic-in-the-ocean-statistics (accessed on 15 July 2023).
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Gross, M. Our Planet Wrapped in Plastic. Curr. Biol. 2017, 27, R785–R788. [Google Scholar] [CrossRef]
- Briassoulis, D.; Dejean, C. Critical Review of Norms and Standards for Biodegradable Agricultural Plastics Part I. Biodegradation in Soil. J. Polym. Environ. 2010, 18, 384–400. [Google Scholar] [CrossRef]
- Avérous, L. Biodegradable Multiphase Systems Based on Plasticized Starch: A Review. J. Macromol. Sci. Polym. Rev. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Jordá-Vilaplana, A.; Balart, R.; Garcia-Sanoguera, D. Development and Characterization of Green Composites from Bio-Based Polyethylene and Peanut Shell. J. Appl. Polym. Sci. 2016, 133, 43940. [Google Scholar] [CrossRef]
- Briassoulis, D.; Giannoulis, A. Evaluation of the Functionality of Bio-Based Food Packaging Films. Polym. Test. 2018, 69, 39–51. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in Research and Development of Bioplastic for Food Packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef]
- European Bioplastics: Benefits of Biobased Rigid Packaging. Available online: https://docs.european-bioplastics.org/2016/publications/bp/EUBP_bp_Rigid_Packaing.pdf (accessed on 20 July 2023).
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of Bioplastics for Food Packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef]
- Stloukal, P.; Kalendova, A.; Mattausch, H.; Laske, S.; Holzer, C.; Koutny, M. The Influence of a Hydrolysis-Inhibiting Additive on the Degradation and Biodegradation of PLA and Its Nanocomposites. Polym. Test. 2015, 41, 124–132. [Google Scholar] [CrossRef]
- Castro López, M.D.M.; Dopico García, S.; Ares Pernas, A.; López Vilariño, J.M.; González Rodríguez, M.V. Effect of PPG-PEG-PPG on the Tocopherol-Controlled Release from Films Intended for Food-Packaging Applications. J. Agric. Food Chem. 2012, 60, 8163–8170. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite Films Based on Plasticized PLA-PHB/Cellulose Nanocrystal Blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in Food Packaging: Biodegradability and Potential Migration to Food-A Review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, H. Biodegradable Polylactic Acid Polymer with Nisin for Use in Antimicrobial Food Packaging. J. Food Sci. 2008, 73, M127–M134. [Google Scholar] [CrossRef]
- Weber, C.J. Biobased Packaging Materials for the Food Industry: Status and Perspectives, A European Concerted Action; KVL. 2000. Available online: http://www.biodeg.net/fichiers/Book%20on%20biopolymers%20(Eng).pdf (accessed on 20 July 2023).
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Sustainable Biopolymers: A BCC Research Outlooks; Global Sustainable Biopolymers Market: Growth Research Report; BCC Publishing: Wellesley, MA, USA, 2019; Available online: bccresearch.com (accessed on 1 October 2023).
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-Limonene Blends for Food Packaging Applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Lopez-Rubio, A. Nanotechnology for Bioplastics: Opportunities, Challenges and Strategies. Trends Food Sci. Technol. 2011, 22, 611–617. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic Acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, P.; Singh, V. Applications of bioplastics in Bulk Packaging: A Revolutionary and Sustainable Approach. Trends Food Sci. Technol. 2010, 32, 128–141. [Google Scholar]
- Arrieta, M.P. Films de PLA y PLA-PHB Plastificados para su Aplicacion en Envases de Alimentos. Caracterizacion y Analisis de los Procesos de Degradacion. Ph.D. Thesis, Universitat Politecnica de Valencia, Valencia, Spain, 2014. [Google Scholar]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Kumar, Y.; Shukla, P.; Singh, P.; Prabhakaran, P.P.; Tanwar, V.K.; Kumar, Y. Bio-Plastics: A Perfect Tool for Eco-Friendly Food Packaging: A Review. J. Food Prod. Dev. Packag. 2014, 1, 1–6. [Google Scholar]
- Best of Plastics: Barrier Packaging. Available online: http://exclusive.multibriefs.com/content/best-of-plastics-barrier-packaging/engineering (accessed on 20 July 2023).
- Naitove, M. Conference Report: Bioplastics are Breaking Out of Their “Green” Niche. Plast. Technol. 2012, 58, 13–17. [Google Scholar]
- Garcia-Garcia, D.; Rayón, E.; Carbonell-Verdu, A.; Lopez-Martinez, J.; Balart, R. Improvement of the Compatibility between Poly(3-Hydroxybutyrate) and Poly(ε-Caprolactone) by Reactive Extrusion with Dicumyl Peroxide. Eur. Polym. J. 2017, 86, 41–57. [Google Scholar] [CrossRef]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. Biodegradation and Physical Evaluation of PHB Packaging. Polym. Test. 2007, 26, 908–915. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.C. Effect of Different Plasticisers on the Mechanical and Barrier Properties of Extruded Cast PHBV Films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sánchez-Safont, E.; Lagarón, J.M.; Balart, R.; Cabedo, L.; Gámez-Pérez, J. Compatibilization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)–Poly(Lactic Acid) Blends with Diisocyanates. J. Appl. Polym. Sci. 2017, 134, 44806. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/Cellulose Based Films: Mechanical, Barrier and Disintegration Properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of Miscibility on Mechanical and Thermal Properties of Poly(Lactic Acid)/ Polycaprolactone Blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending Polylactic Acid with Polyhydroxybutyrate: The Effect on Thermal, Mechanical, and Biodegradation Properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Snell, K.D.; Peoples, O.P. PHA Bioplastic: A Value-Added Coproduct for Biomass Biorefineries. Biofuels Bioprod. Biorefin. 2009, 3, 456–467. [Google Scholar] [CrossRef]
- Levett, I.; Birkett, G.; Davies, N.; Bell, A.; Langford, A.; Laycock, B.; Lant, P.; Pratt, S. Techno-Economic Assessment of Poly-3-Hydroxybutyrate (PHB) Production from Methane—The Case for Thermophilic Bioprocessing. J. Environ. Chem. Eng. 2016, 4, 3724–3733. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Jiménez, A.; Puglia, D.; Pezzolla, D.; Gigliotti, G.; Kenny, J.M.; Chiralt, A.; Torre, L. Production and Characterization of PLA_PBS Biodegradable Blends Reinforced with Cellulose Nanocrystals Extracted from Hemp Fibres. Ind. Crops Prod. 2016, 93, 276–289. [Google Scholar] [CrossRef]
- Songtipya, L.; Limchu, T.; Phuttharak, S.; Songtipya, P.; Kalkornsurapranee, E. Poly(lactic acid)-Based Composites Incorporated with Spent Coffee Ground and Tea Leave for Food Packaging Application: A Waste to Wealth. IOP Conf. Ser. Mater. Sci. Eng. 2019, 553, 012047. [Google Scholar] [CrossRef]
- Hongsriphan, N.; Sanga, S. Antibacterial Food Packaging Sheets Prepared by Coating Chitosan on Corona-Treated Extruded Poly(lactic acid)/Poly(butylene succinate) Blends. J. Plast. Film Sheeting 2018, 34, 160–178. [Google Scholar] [CrossRef]
- Barrett, A. Frost & Sullivan Awards Bio-On for Best Cosmetic Innovation. 2018. Available online: https://bioplasticsnews.com/2018/10/08/frost-sullivan-awards-bio-best-cosmetic-innovation/ (accessed on 25 July 2023).
- Volova, T.G. Polyhydroxyalkanoates Plastic Materials of the 21st Century: Production, Properties and Application, 1st ed.; Nova Science Publishers: New York, NY, USA, 2004; pp. 206–207. [Google Scholar]
- Vandewijngaarden, J.; Wauters, R.; Murariu, M.; Dubois, P.; Carleer, R.; Yperman, J.; D’Haen, J.; Ruttens, B.; Schreurs, S.; Lepot, N.; et al. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)/Organomodified Montmorillonite Nanocomposites for Potential Food Packaging Applications. J. Polym. Environ. 2016, 24, 104–118. [Google Scholar] [CrossRef]
- Qiu, Y.; Fu, J.; Sun, B.; Ma, X. Sustainable Nanocomposite Films Based on SiO2 and Biodegradable Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBH) for Food Packaging. E-Polymers 2021, 21, 72–81. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent Advances in the Development of Biodegradable PHB-Based Toughening Materials: Approaches, Advantages and Applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, Y.; Chen, G.Q. Medical Application of Microbial Biopolyesters Polyhydroxyalkanoates. Artif. Cells Blood Substit. Biotechnol. 2009, 37, 1–12. [Google Scholar] [CrossRef]
- Chen, G.Q. A Microbial Polyhydroxyalkanoates (PHA) Based Bio- and Materials Industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Chang, H.M.; Wang, Z.H.; Luo, H.N.; Xu, M.; Ren, X.Y.; Zheng, G.X.; Wu, B.J.; Zhang, X.H.; Lu, X.Y.; Chen, F.; et al. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)- Based Scaffolds for Tissue Engineering. Braz. J. Med. Biol. Res. 2014, 47, 533–539. [Google Scholar] [CrossRef] [PubMed]
- PepsiCo, Danimer in PHA Collaboration. Available online: https://greenchemicalsblog.com/2017/03/01/pepsico-danimer-in-pha-collaboration/ (accessed on 1 October 2023).
- Kourmentza, K.; Kachrimanidou, V.; Psaki, O.; Pateraki, C.; Ladakis, D.; Koutinas, A. Competitive Advantage and Market Introduction of PHA Polymers and Potential use of PHA Monomers. In The Handbook of Polyhydroxyalkanoates; Koller, M., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 168–201. [Google Scholar]
- Nativia Project of Taghleef Industries. Available online: https://www.ti-films.com/brands/nativia/ (accessed on 1 October 2023).
- Bio-On and Rivoira Present Zeropack, Bioplastic for Food Packaging of Fruits and Vegetables. Available online: https://www.gualapack.com/sustainability-portfolio (accessed on 10 October 2023).
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA-PHB-Limonene Blends Intended for Biodegradable Food Packaging Applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Van Den Oever, M.; Molenveld, K.; Van Der Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics-Facts and Figures Focus on Food Packaging in the Netherlands; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2017. [Google Scholar]
- Sintim, H.Y.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Li, X.; Gonçalves, J.M.; Shi, H.; Tian, T.; Chen, N. Effects of Residual Plastic-Film Mulch on Field Corn Growth and Productivity. Sci. Total Environ. 2020, 729, 138901. [Google Scholar] [CrossRef] [PubMed]
- Sciancalepore, C.; Togliatti, E.; Giubilini, A.; Pugliese, D.; Moroni, F.; Messori, M.; Milanese, D. Preparation and Characterization of Innovative Poly(butylene adipate terephthalate)-Based Biocomposites for Agri-Food Packaging Application. J. Appl. Polym. Sci. 2022, 139, 52370. [Google Scholar] [CrossRef]
- Togliatti, E.; Milanese, D.; Pugliese, D.; Sciancalepore, C. Viscoelastic Characterization and Degradation Stability Investigation of Poly(butylene-adipate-co-terephthalate)—Calcium-Phosphate Glass Composites. J. Polym. Environ. 2022, 30, 3914–3933. [Google Scholar] [CrossRef]
- Bandopadhyay, S.; Martin-Closas, L.; Pelacho, A.M.; DeBruyn, J.M. Biodegradable Plastic Mulch Films: Impacts on Soil Microbial Communities and Ecosystem Functions. Front. Microbiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Barrett, A. Danimer Scientifiv and UrthPact Launch New Compostable Straw. 2019. Available online: https://bioplasticsnews.com/2019/10/30/danimer-scientific-and-urthpact-launch-new-compostable-straw/ (accessed on 10 October 2023).
- Mirel Bioplastics. Biobased and Biodegradable. 2018. Available online: https://www.miljodirektoratet.no/globalassets/publikasjoner/m1206/m1206.pdf (accessed on 10 October 2023).
- Gao, X.; Xie, D.; Yang, C. Effects of a PLA/PBAT Biodegradable Film Mulch as a Replacement of Polyethylene Film and Their Residues on Crop and Soil Environment. Agric. Water Manag. 2021, 255, 107053. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Sustainability, Compostability, and Specific Microbial Activity on Agricultural Mulch Films Prepared from Poly(Lactic Acid). Ind. Eng. Chem. Res. 2013, 52, 17714–17724. [Google Scholar] [CrossRef]
- Havens, K.J.; Bilkovic, D.M.; Stanhope, D.M.; Angstadt, K.T. Fishing Gear with Degradable Component. U.S. Patent 20150135580A1, 21 May 2015. [Google Scholar]
- Nasrin, R.; Biswas, S.; Rashid, T.U.; Afrin, S.; Jahan, R.A.; Haque, P.; Rahman, M.M. Preparation of Chitin-PLA Laminated Composite for Implantable Application. Bioact. Mater. 2017, 2, 199–207. [Google Scholar] [CrossRef]
- Qin, J.; Jiang, Y.; Fu, J.; Wan, Y.; Yang, R.; Gao, W.; Wang, H. Evaluation of Drug Release Property and Blood Compatibility of Aspirin-Loaded Electrospun PLA/RSF Composite Nanofibers. Iran. Polym. J. 2013, 22, 729–737. [Google Scholar] [CrossRef]
- Macha, I.J.; Ben-Nissan, B.; Santos, J.; Cazalbou, S.; Stamboulis, A.; Grossin, D.; Giordano, G. Biocompatibility of a New Biodegradable Polymer-Hydroxyapatite Composite for Biomedical Applications. J. Drug Deliv. Sci. Technol. 2017, 38, 72–77. [Google Scholar] [CrossRef]
- Tran, C.D.; Mututuvari, T.M. Cellulose, Chitosan, and Keratin Composite Materials. Controlled Drug Release. Langmuir 2015, 31, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, S.; Xiong, H.; Jing, X.; Xie, Z.; Chen, X.; Huang, Y. Electrospun PLA/MWCNTs Composite Nanofibers for Combined Chemo- and Photothermal Therapy. Acta Biomater. 2015, 26, 115–123. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent Progress in Development and Chemical Modification of Poly(hydroxybutyrate)-Based Blends for Potential Medical Applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, Synthesis and Medical Application of Bacterial Polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as Biomaterial for Electrospun Scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Gomes, M.E.; Ribeiro, A.S.; Malafaya, P.B.; Reis, R.L.; Cunha, A.M. A New Approach Based on Injection Moulding to Produce Biodegradable Starch-Based Polymeric Sca!Olds: Morphology, Mechanical and Degradation Behaviour. Biomaterials 2001, 22, 883–889. [Google Scholar] [CrossRef]
- Alam, F.; Varadarajan, K.M.; Kumar, S. 3D Printed Polylactic Acid Nanocomposite Scaffolds for Tissue Engineering Applications. Polym. Test. 2020, 81, 106203. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Zhao, R.; Jin, T.; Zhang, L.; Li, X. Chitosan Surface Modified Electrospun Poly(ε-Caprolactone)/Carbon Nanotube Composite Fibers with Enhanced Mechanical, Cell Proliferation and Antibacterial Properties. Int. J. Biol. Macromol. 2017, 104, 708–715. [Google Scholar] [CrossRef]
- Farajikhah, S.; Runge, A.F.J.; Boumelhem, B.B.; Rukhlenko, I.D.; Stefani, A.; Sayyar, S.; Innis, P.C.; Fraser, S.T.; Fleming, S.; Large, M.C.J. Thermally Drawn Biodegradable Fibers with Tailored Topography for Biomedical Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Sciancalepore, C.; Togliatti, E.; Marozzi, M.; Rizzi, F.M.A.; Pugliese, D.; Cavazza, A.; Pitirollo, O.; Grimaldi, M.; Milanese, D. Flexible PBAT-Based Composite Filaments for Tunable FDM 3D Printing. ACS Appl. Bio Mater. 2022, 5, 3219–3229. [Google Scholar] [CrossRef]
- Walczak, J.; Sobota, M.; Chrzanowski, M.; Krucinska, I. Application of the Melt-Blown Technique in the Production of Shape-Memory Nonwoven Fabrics from a Blend of Poly(L-Lactide) and Atactic Poly[(R,S)-3-Hydroxy Butyrate]. Text. Res. J. 2018, 88, 2141–2152. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Ren, M.; Ding, C.; Chen, P.; Gu, Q.; Wu, Q. Evaluation of PHBHHx and PHBV/PLA Fibers Used as Medical Sutures. J. Mater. Sci. Mater. Med. 2014, 25, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Vink, E.T.H.; Davies, S. Life Cycle Inventory and Impact Assessment Data for 2014 Ingeo® Polylactide Production. Ind. Biotechnol. 2015, 11, 167–180. [Google Scholar] [CrossRef]
- Yamanaka, T.; Ohme, H.; Inoue, T. Future Directions for the Research and Development of Polyesters: From High-Performance to Environmentally Friendly. Pure Appl. Chem. 2007, 79, 1541–1551. [Google Scholar] [CrossRef]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolytic Bacterial Lactic Acid Fermentation—A Review. Biotechnol. Adv. 2008, 26, 22–34. [Google Scholar] [CrossRef]
- Salminen, S.; Wright, A.V.; Ouwehand, A. Lactic Acid Bacteria: Microbiological and Functional Aspects; Mercel Dekker: New York, NY, USA, 2004; p. 629. [Google Scholar]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing Technologies for Poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New Emerging Trends in Synthetic Biodegradable Polymers—Polylactide: A Critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, J. Biodegradable and Biobased Polymers. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 127–143. ISBN 9780323390408. [Google Scholar]
- Kulkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic Acid for Surgical Implants LACTI C ACTIC Acid in Its Racemic or Optically. Available online: https://apps.dtic.mil/sti/tr/pdf/AD0636716.pdf (accessed on 10 October 2023).
- Schroeder, A.; Turjeman, K.; Schroeder, J.E.; Leibergall, M.; Barenholz, Y. Using Liposomes to Target Infection and Inflammation Induced by Foreign Body Injuries or Medical Implants. Expert. Opin. Drug Deliv. 2010, 7, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Yoshioka, T.; Lucarelli, M.; Hwanh, L.H.; Langer, R. Controlled Delivery Systems for Proteins Based on Poly(lactic/glycolic acid) Microspheres. Pharm. Res. 1991, 8, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Langer, R. Responsive Polymeric Delivery Systems. Adv. Drug Deliv. Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Goldbart, R.; Traitel, T.; Lapidot, S.A.; Kost, J. Enzymatically Controlled Responsive Drug Delivery Systems. Polym. Adv. Technol. 2002, 13, 1006–1018. [Google Scholar] [CrossRef]
- Schakenraad, J.M.; Hardonk, M.J.; Feijen, J.; Molenaar, I.; Nieuwenhuis, P. Enzymatic Activity toward Poly(L-lactic acid) Implants. J. Biomed. Mater. Res. 1990, 24, 529–545. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Seggiani, M. Thermo-Mechanical Properties of PLA/Short Flax Fiber Biocomposites. Appl. Sci. 2019, 9, 3797. [Google Scholar] [CrossRef]
- Nofar, M.; Maani, A.; Sojoudi, H.; Heuzey, M.C.; Carreau, P.J. Interfacial and Rheological Properties of PLA/PBAT and PLA/PBSA Blends and Their Morphological Stability under Shear Flow. J. Rheol. 2015, 59, 317–333. [Google Scholar] [CrossRef]
- Harris, A.M.; Lee, E.C. Improving Mechanical Performance of Injection Molded PLA by Controlling Crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for Environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Ramy-Ratiarison, R.; Paint, Y.; Raquez, J.M.; Dubois, P. Recent Advances in Production of Poly(lactic acid) (PLA) Nanocomposites: A Versatile Method to Tune Crystallization Properties of PLA. Nanocomposites 2015, 1, 71–82. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-Based Nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and Medical Applications of Polylactic Acid: A Review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.v.; Hirt, D.E. Poly(lactic acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of Biodegradable Polylactide/Poly(Butylene Adipate-Co-Terephthalate) Blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Pradeep, S.A.; Kharbas, H.; Turng, L.S.; Avalos, A.; Lawrence, J.G.; Pilla, S. Investigation of Thermal and Thermomechanical Properties of Biodegradable PLA/PBSA Composites Processed via Supercritical Fluid-Assisted Foam Injection Molding. Polymers 2017, 9, 22. [Google Scholar] [CrossRef]
- Taguchi, S. Current Advances in Microbial Cell Factories for Lactate-Based Polyesters Driven by Lactate-Polymerizing Enzymes: Towards the Further Creation of New LA-Based Polyesters. Polym. Degrad. Stab. 2010, 95, 1421–1428. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. Natural Fibers, Biopolymers, and Biocomposites; Taylor & Francis: Boca Raton, FL, USA, 2005; p. 896. ISBN 084931741X. [Google Scholar]
- Insight: The EN13432 Standard. Available online: https://www.european-bioplastics.org/en-13432-certified-bioplastics-performance-in-industrial-composting/ (accessed on 10 October 2023).
- Cheng, K.K.; Zhao, X.B.; Zeng, J.; Zhang, J.A. Biotechnological Production of Succinic Acid: Current State and Perspectives. Biofuels Bioprod. Biorefin. 2012, 6, 302–318. [Google Scholar] [CrossRef]
- Tsyskovskii, V.K.; Levina, M.I.; Prokof’ev, E.K.; Kostyleva, I.v.; Pyl’nikov, V.I.; Rudakova, V.I. Synthesis of Dicarboxylic Acids by Continuous oxidation of Paraffin Hydrocarbons with Air Under Pressure. Chem. Technol. Fuels Oils 1966, 2, 610–615. [Google Scholar] [CrossRef]
- Swann, S.; Wanderer, K.H.; Schaffer, H.J.; Streaker, W.A. The Electrolytic Reduction of Maleic Acid to Succinic Acid in Acid Solution. J. Electrochem. Soc. 1949, 96, 353. [Google Scholar] [CrossRef]
- Briki, A.; Kaboré, K.; Olmos, E.; Bosselaar, S.; Blanchard, F.; Fick, M.; Guedon, E.; Fournier, F.; Delaunay, S. Corynebacterium Glutamicum, a Natural Overproducer of Succinic Acid? Eng. Life Sci. 2020, 20, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.Y.; Liu, R.M.; Ma, J.F.; Chen, K.Q.; Jiang, M.; Wei, P. Increased Production of Succinic Acid in Escherichia Coli by Overexpression of Malate Dehydrogenase. Biotechnol. Lett. 2011, 33, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Lee, S.Y.; Hong, S.H.; Chang, H.N. Batch and Continuous Cultures of Mannheimia Succiniciproducens MBEL55E for the Production of Succinic Acid from Whey and Corn Steep Liquor. Bioprocess Biosyst. Eng. 2003, 26, 63–67. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, Y.S.; Lee, S.Y. Production of Succinic Acid by Metabolically Engineered Microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Zheng, P.; Sun, Z.H.; Ni, Y.; Dong, J.J.; Zhu, L.L. Economical Succinic Acid Production from Cane Molasses by Actinobacillus Succinogenes. Bioresour. Technol. 2008, 99, 1736–1742. [Google Scholar] [CrossRef]
- Lee, P.C.; Lee, W.G.; Kwon, S.; Lee, S.Y.; Chang, H.N. Succinic Acid Production by Anaerobiospirillum Succiniciproducens: Effects of the H2/CO2 Supply and 2 2 Glucose Concentration. Enzym. Microb. Technol. 1999, 24, 549–554. [Google Scholar] [CrossRef]
- Dorado, M.P.; Lin, S.K.C.; Koutinas, A.; Du, C.; Wang, R.; Webb, C. Cereal-Based Biorefinery Development: Utilisation of Wheat Milling By-Products for the Production of Succinic Acid. J. Biotechnol. 2009, 143, 51–59. [Google Scholar] [CrossRef]
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.v.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable Bio-Succinic Acid Production: Superstructure Optimization, Techno-Economic, and Lifecycle Assessment. Energy Environ. Sci. 2021, 14, 3542–3558. [Google Scholar] [CrossRef]
- Platnieks, O.; Gaidukovs, S.; Kumar Thakur, V.; Barkane, A.; Beluns, S. Bio-Based Poly (Butylene Succinate): Recent Progress, Challenges and Future Opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Reductive Catalytic Routes towards Sustainable Production of Hydrogen, Fuels and Chemicals from Biomass Derived Polyols. Renew. Sust. Energ. Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Forte, A.; Zucaro, A.; Basosi, R.; Fierro, A. LCA of 1,4-Butanediol Produced via Direct Fermentation of Sugars from Wheat Straw Feedstock within a Territorial Biorefinery. Materials 2016, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Gupta, R.K.; Bhattacharya, S.N.; Choi, H.J. An Investigation of Melt Rheology and Thermal Stability of Poly(Lactic Acid)/Poly(Butylene Succinate) Nanocomposites. J. Appl. Polym. Sci. 2009, 114, 2837–2847. [Google Scholar] [CrossRef]
- Rudnik, E. Compostable Polymer Properties and Packaging Applications. In Plastic Films in Food Packaging: Materials, Technology and Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 217–248. [Google Scholar] [CrossRef]
- Georgousopoulou, I.N.; Vouyiouka, S.; Dole, P.; Papaspyrides, C.D. Thermo-Mechanical Degradation and Stabilization of Poly(Butylene Succinate). Polym. Degrad. Stab. 2016, 128, 182–192. [Google Scholar] [CrossRef]
- Mizuno, S.; Maeda, T.; Kanemura, C.; Hotta, A. Biodegradability, Reprocessability, and Mechanical Properties of Polybutylene Succinate (PBS) Photografted by Hydrophilic or Hydrophobic Membranes. Polym. Degrad. Stab. 2015, 117, 58–65. [Google Scholar] [CrossRef]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and Properties of Poly(Butylene Succinate): Efficiency of Different Transesterification Catalysts. J. Polym. Sci. A Polym. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(butylene succinate)-Based Polyesters for Biomedical Applications: A Review in Memory of Our Beloved Colleague and Friend Dr. Lara Finelli. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-based Poly(butylene succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly(butylene succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X. Polybutylene Succinate/Cellulose Nanocrystals: Role of Phthalic Anhydride in Squeeze Oriented Bionanocomposites. Carbohydr. Polym. 2018, 196, 254–261. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, L.; Huang, H.; Zou, F.; Zhao, H.P. Fabrication and Properties of Poly(butylene succinate) Biocomposites Reinforced by Waste Silkworm Silk Fabric. Compos. Part. A Appl. Sci. Manuf. 2017, 95, 125–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Wang, J.; Tian, W.; Liew, K.M.; Zhang, Y.; Wang, B. Engineering Carbon Nanotubes Wrapped Ammonium Polyphosphate for Enhancing Mechanical and Flame Retardant Properties of Poly(butylene succinate). Compos. Part. A Appl. Sci. Manuf. 2018, 115, 215–227. [Google Scholar] [CrossRef]
- Marcuello, C.; Chabbert, B.; Berzin, F.; Bercu, N.B.; Molinari, M.; Aguié-Béghin, V. Influence of Surface Chemistry of Fiber and Lignocellulosic Materials on Adhesion Properties with Polybutylene Succinate at Nanoscale. Materials 2023, 16, 2440. [Google Scholar] [CrossRef] [PubMed]
- Berzin, F.; Lemkhanter, L.; Marcuello, C.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M.; Castellani, R.; Vergnes, B. Influence of the Polarity of the Matrix on the Breakage Mechanisms of Lignocellulosic Fibers during Twin-Screw Extrusion. Polym. Compos. 2020, 41, 1106–1117. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Park, J.W.; Im, S.S. Phase Behavior and Morphology in Blends of Poly(L-lactic acid) and Poly(butylene succinate). J. Appl. Polym. Sci. 2002, 86, 647–655. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, X.; Hua, K.; Duan, C.; Zhang, W.; Ji, J.; Yang, X. Enhanced Mechanical Properties and Degradability of Poly(butylene succinate) and Poly(lactic acid) Blends. Iran. Polym. J. 2013, 22, 267–275. [Google Scholar] [CrossRef]
- Krishnan, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Toughening of Polylactic Acid: An Overview of Research Progress. Polym.-Plast. Technol. Eng. 2016, 55, 1623–1652. [Google Scholar] [CrossRef]
- Hassan, E.; Wei, Y.; Jiao, H.; Muhuo, Y. Dynamic Mechanical Properties and Thermal Stability of Poly(lactic acid) and Poly(butylene succinate) Blends Composites. J. Fiber Bioeng. Inform. 2013, 6, 85–94. [Google Scholar] [CrossRef]
- Jompang, L.; Thumsorn, S.; On, J.W.; Surin, P.; Apawet, C.; Chaichalermwong, T.; Kaabbuathong, N.; O-Charoen, N.; Srisawat, N. Poly(lactic acid) and Poly(butylene succinate) Blend Fibers Prepared by Melt Spinning Technique. Energy Procedia 2013, 34, 493–499. [Google Scholar] [CrossRef]
- Deng, Y.; Thomas, N.L. Blending Poly(butylene succinate) with Poly(lactic acid): Ductility and Phase Inversion Effects. Eur. Polym. J. 2015, 71, 534–546. [Google Scholar] [CrossRef]
- Harada, M.; Ohya, T.; Iida, K.; Hayashi, H.; Hirano, K.; Fukuda, H. Increased Impact Strength of Biodegradable Poly(lactic acid)/Poly(butylene succinate) Blend Composites by Using Isocyanate as a Reactive Processing Agent. J. Appl. Polym. Sci. 2007, 106, 1813–1820. [Google Scholar] [CrossRef]
- Bhatia, A.; Gupta, R.; Bhattacharya, S.; Choi, H. Compatibility of Biodegradable Poly (lactic acid) (PLA) and Poly (Butylene Succinate) (PBS) Blends for Packaging Application. Korea Aust. Rheol. 2007, 19, 125–131. [Google Scholar]
- Lee, S.; Lee, J.W. Characterization and processing of Biodegradable polymer blends of poly(lactic acid) with poly(butylene succinate adipate). Korea Aust. Rheol. 2005, 17, 71–77. [Google Scholar]
- Yokohara, T.; Yamaguchi, M. Structure and Properties for Biomass-Based Polyester Blends of PLA and PBS. Eur. Polym. J. 2008, 44, 677–685. [Google Scholar] [CrossRef]
- Ostrowska, J.; Sadurski, W.; Paluch, M.; Tyński, P.; Bogusz, J. The Effect of Poly(butylene succinate) Content on the Structure and Thermal and Mechanical Properties of Its Blends with Polylactide. Polym. Int. 2019, 68, 1271–1279. [Google Scholar] [CrossRef]
- Krishnaswamy, R.K.; Padwa, A.R. Impact Modification of PLA Using Biobased, Biodegradable MirelTM PHB Copolymers. Available online: https://www.academia.edu/80481643/Impact_Modification_of_Pla_Using_Biobased_Biodegradable_Mirel_PHB_Copolymers (accessed on 10 October 2023).
- Srithep, Y.; Veang-In, O.; Pholharn, D.; Turng, L.S.; Morris, J. Improving Polylactide Toughness by Plasticizing with Low Molecular Weight Polylactide-Poly(butylene succinate) Copolymer. J. Renew. Mater. 2021, 9, 1267–1281. [Google Scholar] [CrossRef]
- Supthanyakul, R.; Kaabbuathong, N.; Chirachanchai, S. Poly(L-Lactide-b-Butylene Succinate-b-L-Lactide) Triblock Copolymer: A Multi-Functional Additive for PLA/PBS Blend with a Key Performance on Film Clarity. Polym. Degrad. Stab. 2017, 142, 160–168. [Google Scholar] [CrossRef]
- Ratsameetammajak, N.; Molloy, R.; Somsunan, R. Preparation and Property Testing of Polymer Blends of Poly(lactic acid) and Poly(butylene succinate) Plasticised with Long-Chain Fatty Acids. Plast. Rubber Compos. 2018, 47, 139–146. [Google Scholar] [CrossRef]
- Gigante, V.; Coltelli, M.B.; Vannozzi, A.; Panariello, L.; Fusco, A.; Trombi, L.; Donnarumma, G.; Danti, S.; Lazzeri, A. Flat Die Extruded Biocompatible Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate) (PBS) Based Films. Polymers 2019, 11, 1857. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Iannoni, A.; Terenzi, A.; Kenny, J.M.; Torre, L. Processing Conditions, Thermal and Mechanical Responses of Stretchable Poly (Lactic Acid)/Poly (Butylene Succinate) Films. Materials 2017, 10, 809. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, H.; Pan, H.; Zhang, H.; Ran, X. Heat Resistant and Mechanical Properties of Biodegradable Poly(Lactic Acid)/Poly(Butylene Succinate) Blends Crosslinked by Polyaryl Polymethylene Isocyanate. Polym. Plast. Technol. Eng. 2018, 57, 1882–1892. [Google Scholar] [CrossRef]
- Ma, M.; Xu, L.; Liu, K.; Chen, S.; He, H.; Shi, Y.; Wang, X. Effect of Triphenyl Phosphite as a Reactive Compatibilizer on the Properties of Poly(L-Lactic Acid)/Poly(Butylene Succinate) Blends. J. Appl. Polym. Sci. 2020, 137, 48646. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Z.; Lan, X.; Wu, F.; Xie, B.; Yang, M. Morphology, Rheology, Crystallization Behavior, and Mechanical Properties of Poly(Lactic Acid)/Poly(Butylene Succinate)/Dicumyl Peroxide Reactive Blends. J. Appl. Polym. Sci. 2014, 131, 39580. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Zhang, Y.; Wan, C.; Ma, P. Toughening Modification of PLLA/PBS Blends via in Situ Compatibilization. Polym. Eng. Sci. 2009, 49, 26–33. [Google Scholar] [CrossRef]
- Phetwarotai, W.; Maneechot, H.; Kalkornsurapranee, E.; Phusunti, N. Thermal Behaviors and Characteristics of Polylactide/Poly(Butylene Succinate) Blend Films via Reactive Compatibilization and Plasticization. Polym. Adv. Technol. 2018, 29, 2121–2133. [Google Scholar] [CrossRef]
- Somsunan, R.; Noppakoon, S.; Punyodom, W. Effect of G40 Plasticizer on the Properties of Ternary Blends of Biodegradable PLA/PBS/G40. J. Polym. Res. 2019, 26, 92. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M.; Okamoto, K. Structure and Properties of Nanocomposites Based on Poly(Butylene Succinate-Co-Adipate) and Organically Modified Montmorillonite. Macromol. Mater. Eng. 2005, 290, 759–768. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Compatibilized Polymer Blends for Packaging Applications: A Literature Review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Nikolic, M.S.; Djonlagic, J. Synthesis and Characterization of Biodegradable Poly(Butylene Succinate-Co-Butylene Adipate)s. Polym. Degrad. Stab. 2001, 74, 263–270. [Google Scholar] [CrossRef]
- Miiller, R.-J.; Witt, U.; Rantze, E.; Deckwer, W.-D. Architecture of Biodegradable Copolyesters Containing Aromatic Constituents. Polym. Degrad. Stab. 1998, 59, 203–208. [Google Scholar] [CrossRef]
- Fujimaki, T. Processability and Properties of Aliphatic Polyesters, “BIONOLLE”, Synthesized by Polycondensation Reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable Aliphatic Polyesters. Part I. Properties and Biodegradation of Poly(Butylene Succinate-Co-Butylene Adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Role of Specific Interfacial Area in Controlling Properties of Immiscible Blends of Biodegradable Polylactide and Poly[(Butylene Succinate)-Co-Adipate]. ACS Appl. Mater. Interfaces 2012, 4, 6690–6701. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.T.; Tsou, C.H.; Huang, C.Y.; Chen, K.N.; Wu, C.S.; Chai, W.L. Compatible and Crystallization Properties of Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blends. J. Appl. Polym. Sci. 2010, 116, 680–687. [Google Scholar] [CrossRef]
- Ojijo, V.; Sinha Ray, S.; Sadiku, R. Toughening of Biodegradable Polylactide/Poly(Butylene Succinate- Co -Adipate) Blends via in Situ Reactive Compatibilization. ACS Appl. Mater. Interfaces 2013, 5, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- Bioplastics Market Development, Update 2020. European Bioplastic Conference 2020. Available online: www.european-bioplastics.org (accessed on 10 October 2023).
- Bureepukdee, C.; Suttireungwong, S.; Seadan, M. A Study on Reactive Blending of (Poly Lactic Acid) and Poly (Butylene Succinate Co Adipate). IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012070. [Google Scholar] [CrossRef]
- Gigante, V.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Rubber Toughening of Polylactic Acid (PLA) with Poly(Butylene Adipate-Co-Terephthalate) (PBAT): Mechanical Properties, Fracture Mechanics and Analysis of Ductile-to-Brittle Behavior While Varying Temperature and Test Speed. Eur. Polym. J. 2019, 115, 125–137. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Heather, P.S.; Lazzeri, A. Rubber Toughening of Plastics. J. Mater. Sci. 1989, 16, 2255–2261. [Google Scholar] [CrossRef]
- Lazzeri, A.; Bucknall, C.B. Recent Developments in the Modeling of Dilatational Yielding in Toughened Plastics. ACS Symp. Ser. 2000, 759, 14–35. [Google Scholar] [CrossRef]
- Chaiwutthinan, P.; Leejarkpai, T.; Kashima, D.P.; Chuayjuljit, S. Poly(Lactic Acid)/Poly(Butylene Succinate) Blends Filled with Epoxy Functionalised Polymeric Chain Extender. Adv. Mater. Res. 2013, 664, 644–648. [Google Scholar] [CrossRef]
- Gui, Z.Y.; Wang, H.R.; Gao, Y.; Lu, C.; Cheng, S.J. Morphology and Melt Rheology of Biodegradable Poly(Lactic Acid)/Poly(Butylene Succinate Adipate) Blends: Effect of Blend Compositions. Iran. Polym. J. 2012, 21, 81–89. [Google Scholar] [CrossRef]
- Nofar, M.; Salehiyan, R.; Ciftci, U.; Jalali, A.; Durmuş, A. Ductility Improvements of PLA-Based Binary and Ternary Blends with Controlled Morphology Using PBAT, PBSA, and Nanoclay. Compos. B Eng. 2020, 182, 107661. [Google Scholar] [CrossRef]
- Meng, L.; Yu, L.; Khalid, S.; Liu, H.; Zhang, S.; Duan, Q.; Chen, L. Preparation, Microstructure and Performance of Poly (Lactic Acid)-Poly (Butylene Succinate-Co-Butyleneadipate)-Starch Hybrid Composites. Compos. B Eng. 2019, 177, 107384. [Google Scholar] [CrossRef]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Sanchez-Nacher, L.; Balart, R.; Montanes, N. High Toughness Poly(Lactic Acid) (PLA) Formulations Obtained by Ternary Blends with Poly(3-Hydroxybutyrate) (PHB) and Flexible Polyesters from Succinic Acid. Polym. Bull. 2019, 76, 1839–1859. [Google Scholar] [CrossRef]
- Supthanyakul, R.; Kaabbuathong, N.; Chirachanchai, S. Random Poly(Butylene Succinate-Co-Lactic Acid) as a Multi-Functional Additive for Miscibility, Toughness, and Clarity of PLA/PBS Blends. Polymer 2016, 105, 1–9. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate-Co-Adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef]
- Harrats, C.; Thomas, S.; Groeninckx, G. Micro- and Nanostructured Multiphase Polymer Blend Systems: Phase Morphology and Interfaces; Taylor & Francis Group: Boca Raton, FL, USA, 2006; p. 101. [Google Scholar]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. Characterization of Polymer Blends Miscibility, Morphology and Interfaces; John Wiley & Sons: Weinheim, Germany, 2014; p. 994. [Google Scholar]
- Cardinaels, R.; Moldenaers, P. Morphology Development in Immiscible Polymer Blends; Wiley Online Library: New York, NY, USA, 2016. [Google Scholar]
- Coiai, S.; Di Lorenzo, M.L.; Cinelli, P.; Righetti, M.C.; Passaglia, E. Binary Green Blends of Poly(Lactic Acid) with Poly(Butylene Adipate-Co-Butylene Terephthalate) and Poly(Butylene Succinate-Co-Butylene Adipate) and Their Nanocomposites. Polymers 2021, 13, 2489. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Coltelli, M.B.; Lazzeri, A. Volume Change during Creep and Micromechanical Deformation Processes in Pla–Pbsa Binary Blends. Polymers 2021, 13, 2379. [Google Scholar] [CrossRef]
- Makwakwa, D.; Ojijo, V.; Bandyopadhyay, J.; Sinha Ray, S. Flow Characteristics, Mechanical, Thermal, and Thermomechanical Properties, and 3d Printability of Biodegradable Polylactide Containing Boehmite at Different Loadings. Polymers 2021, 13, 2019. [Google Scholar] [CrossRef]
- Aliotta, L.; Canesi, I.; Lazzeri, A. Study on the Preferential Distribution of Acetyl Tributyl Citrate in Poly(Lactic) Acid-Poly(Butylene Adipate-Co-Terephthalate) Blends. Polym. Test. 2021, 98, 107163. [Google Scholar] [CrossRef]
- Puekpoonpoal, N.; Phattarateera, S.; Kerddonfag, N.; Aht-Ong, D. Morphology Development of PLAs with Different Stereo-Regularities in Ternary Blend PBSA/PBS/PLA Films. Polym.-Plast. Technol. Mater. 2021, 60, 1672–1685. [Google Scholar] [CrossRef]
- Wang, Y.; Mano, J.F. Biodegradable Poly(L-Lactic Acid)/Poly(Butylene Succinate-Co-Adipate) Blends: Miscibility, Morphology, and Thermal Behavior. J. Appl. Polym. Sci. 2007, 105, 3204–3210. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Zhang, Y. Morphology, Mechanical Properties, and Thermal Stability of Poly(L-Lactic Acid)/Poly(Butylene Succinate-Co-Adipate)/ Silicon Dioxide Composites. J. Appl. Polym. Sci. 2009, 113, 3630–3637. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Pavasupree, S.; O-Charoen, N.; Insuan, U.; Jailak, P.; Pivsa-Art, S. Preparation of Polymer Blends between Poly (L-Lactic Acid), Poly (Butylene Succinate-Co-Adipate) and Poly (Butylene Adipate-Co-Terephthalate) for Blow Film Industrial Application. Energy Procedia 2011, 9, 581–588. [Google Scholar] [CrossRef]
- Pivsa-Art, S.; Thumsorn, S.; Pavasupree, S.; O-Charoen, N.; Pivsa-Art, W.; Yamane, H.; Ohara, H. Effect of Additive on Crystallization and Mechanical Properties of Polymer Blends of Poly(Lactic Acid) and Poly[(Butylene Succinate)-Co-Adipate]. Energy Procedia 2013, 34, 563–571. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Bronco, S.; Chinea, C. The Effect of Free Radical Reactions on Structure and Properties of Poly(Lactic Acid) (PLA) Based Blends. Polym. Degrad. Stab. 2010, 95, 332–341. [Google Scholar] [CrossRef]
- Arruda, L.C.; Magaton, M.; Bretas, R.E.S.; Ueki, M.M. Influence of Chain Extender on Mechanical, Thermal and Morphological Properties of Blown Films of PLA/PBAT Blends. Polym. Test. 2015, 43, 27–37. [Google Scholar] [CrossRef]
- Corre, Y.M.; Duchet, J.; Reignier, J.; Maazouz, A. Melt Strengthening of Poly (Lactic Acid) through Reactive Extrusion with Epoxy-Functionalized Chains. Rheol. Acta 2011, 50, 613–629. [Google Scholar] [CrossRef]
- Han, C.D. Rheology and Processing of Polymeric Materials. Volume 1, Polymer Rheology; Oxford University Press: New York, NY, USA, 2007; p. 728. [Google Scholar]
- Meng, Q.; Heuzey, M.C.; Carreau, P.J. Control of Thermal Degradation of Polylactide/Clay Nanocomposites during Melt Processing by Chain Extension Reaction. Polym. Degrad. Stab. 2012, 97, 2010–2020. [Google Scholar] [CrossRef]
- Wu, C.S. Utilization of Peanut Husks as a Filler in Aliphatic-Aromatic Polyesters: Preparation, Characterization, and Biodegradability. Polym. Degrad. Stab. 2012, 97, 2388–2395. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Poly(Butylene Succinate) and Poly(Butylene Adipate-Co-Terephthalate) Blends: Reactive Extrusion and Performance Evaluation. J. Polym. Environ. 2014, 22, 336–349. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of Thermal Stability, Rheological and Mechanical Properties of PLA, PBAT and Their Blends by Reactive Extrusion with Functionalized Epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Pilla, S.; Kim, S.G.; Auer, G.K.; Gong, S.; Park, C.B. Microcellular Extrusion-Foaming of Polylactide with Chain-Extender. Polym. Eng. Sci. 2009, 49, 1653–1660. [Google Scholar] [CrossRef]
- Lascano, D.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Montanes, N. Toughened Poly (Lactic Acid)-PLA Formulations by Binary Blends with Poly(Butylene Succinate-Co-Adipate)-PBSA and Their Shape Memory Behaviour. Materials 2019, 12, 622. [Google Scholar] [CrossRef]
- Palai, B.; Mohanty, S.; Nayak, S.K. Synergistic Effect of Polylactic Acid(PLA) and Poly(Butylene Succinate-Co-Adipate) (PBSA) Based Sustainable, Reactive, Super Toughened Eco-Composite Blown Films for Flexible Packaging Applications. Polym. Test. 2020, 83, 106130. [Google Scholar] [CrossRef]
- Ojijo, V.; Ray, S.S. Super Toughened Biodegradable Polylactide Blends with Non-Linear Copolymer Interfacial Architecture Obtained via Facile in-Situ Reactive Compatibilization. Polymer 2015, 80, 1–17. [Google Scholar] [CrossRef]
- Naqui, S.I.; Robinson, I.M. Tensile Dilatornetric Studies of Deformation in Polymeric Materials and Their Composites. J. Mater. Sci. 1993, 28, 1421–1429. [Google Scholar] [CrossRef]
- Eslami, H.; Kamal, M.R. Effect of a Chain Extender on the Rheological and Mechanical Properties of Biodegradable Poly(Lactic Acid)/Poly[(Butylene Succinate)-Co-Adipate] Blends. J. Appl. Polym. Sci. 2013, 129, 2418–2428. [Google Scholar] [CrossRef]
- Yang, X.; Xu, H.; Odelius, K.; Hakkarainen, M. Poly(Lactide)-g-Poly(Butylene Succinate-Co-Adipate) with High Crystallization Capacity and Migration Resistance. Materials 2016, 9, 313. [Google Scholar] [CrossRef]
- Yang, X.; Odelius, K.; Hakkarainen, M. Microwave-Assisted Reaction in Green Solvents Recycles PHB to Functional Chemicals. ACS Sustain. Chem. Eng. 2014, 2, 2198–2203. [Google Scholar] [CrossRef]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, K.; Kiekens, P. Material Properties Biopolymers: Overview of Several Properties and Consequences on Their Applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- de Castro, J.G.; Rodrigues, B.V.M.; Ricci, R.; Costa, M.M.; Ribeiro, A.F.C.; Marciano, F.R.; Lobo, A.O. Designing a Novel Nanocomposite for Bone Tissue Engineering Using Electrospun Conductive PBAT/Polypyrrole as a Scaffold to Direct Nanohydroxyapatite Electrodeposition. RSC Adv. 2016, 6, 32615–32623. [Google Scholar] [CrossRef]
- Teamsinsungvon, A.; Ruksakulpiwat, Y.; Jarukumjorn, K. Preparation and Characterization of Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terepthalate) Blends and Their Composite. Polym.-Plast. Technol. Eng. 2013, 52, 1362–1367. [Google Scholar] [CrossRef]
- Witt, U.; Miiller, R.-J.; Deckwer, W.-D. Biodegradation Behavior and Material Properties of Aliphatic/Aromatic Polyesters of Commercial Importance. J. Environ. Polym. Degrad. 1997, 5, 81–89. [Google Scholar] [CrossRef]
- Mü, R.-J.; Kleeberg, I.; Deckwer, W.-D. Biodegradation of Polyesters Containing Aromatic Constituents. J. Biotechnol. 2001, 86, 87–95. [Google Scholar]
- Witt, U.; Miiller, R.-J.; Deckwer, W.-D. Evaluation of the Biodegradability of Copolyesters Containing Aromatic Compounds by Investigations of Model Oligomers. J. Environ. Polym. Degrad. 1996, 4, 9–20. [Google Scholar] [CrossRef]
- Herrera, R.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Characterization and Degradation Behavior of Poly(Butylene Adipate-Co-Terephthalate)s. J. Polym. Sci. A Polym. Chem. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.W.; Lee, K.H. Properties of Potentially Biodegradable Copolyesters of (Succinic Acid-1,4-Butanediol)/ (Dimethyl Terephthalate-1,4-Butanediol). Polym. Int. 1999, 48, 861–867. [Google Scholar] [CrossRef]
- Fukushima, K.; Wu, M.H.; Bocchini, S.; Rasyida, A.; Yang, M.C. PBAT Based Nanocomposites for Medical and Industrial Applications. Mater. Sci. Eng. C 2012, 32, 1331–1351. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.T.; Jung, C.H.; Kuk, I.S.; Choi, J.H.; Nho, Y.C. Electron Beam-Induced Crosslinking of Poly(Butylene Adipate-Co- Terephthalate). Nucl. Instrum. Methods Phys. Res. B 2010, 268, 3386–3389. [Google Scholar] [CrossRef]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-Biocomposites: Biodegradable Polyester/Nanoclay Systems. Prog. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Sustainable Green Composites: Value Addition to Agricultural Residues and Perennial Grasses. ACS Sustain. Chem. Eng. 2013, 1, 325–333. [Google Scholar] [CrossRef]
- Gan, Z.; Kuwabara, K.; Yamamoto, M.; Abe, H.; Doi, Y. Solid-State Structures and Thermal Properties of Aliphatic-Aromatic Poly(Butylene Adipate-Co-Butylene Terephthalate) Copolyesters. Polym. Degrad. Stab. 2004, 83, 289–300. [Google Scholar] [CrossRef]
- Kumar, M.; Mohanty, S.; Nayak, S.K.; Rahail Parvaiz, M. Effect of Glycidyl Methacrylate (GMA) on the Thermal, Mechanical and Morphological Property of Biodegradable PLA/PBAT Blend and Its Nanocomposites. Bioresour. Technol. 2010, 101, 8406–8415. [Google Scholar] [CrossRef]
- Shenoy, A.v.; Saini, D.R.; Nadkarni, V.M. Rheograms for Engineering Thermoplastics from Melt Flow Index. Rheol. Acta 1983, 22, 209–222. [Google Scholar] [CrossRef]
- Hongdilokkul, P.; Keeratipinit, K.; Chawthai, S.; Hararak, B.; Seadan, M.; Suttiruengwong, S. A Study on Properties of PLA/PBAT from Blown Film Process. IOP Conf. Ser. Mater. Sci. Eng. 2015, 87, 012112. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Maggiore, I.D.; Bertoldo, M.; Signori, F.; Bronco, S.; Ciardelli, F. Poly(Lactic Acid) Properties as a Consequence of Poly(Butylene Adipate-Co-Terephthalate) Blending and Acetyl Tributyl Citrate Plasticization. J. Appl. Polym. Sci. 2008, 110, 1250–1262. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A.; Billon, N.; Combeaud, C. Effect of the Simultaneous Biaxial Stretching on the Structural and Mechanical Properties of PLA, PBAT and Their Blends at Rubbery State. Eur. Polym. J. 2015, 68, 288–301. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.B.; Bronco, S. Thermal Degradation of Poly(Lactic Acid) (PLA) and Poly(Butylene Adipate-Co-Terephthalate) (PBAT) and Their Blends upon Melt Processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Quero, E.; Müller, A.J.; Signori, F.; Coltelli, M.-B.; Bronco, S. Isothermal Cold-Crystallization of PLA/PBAT Blends With and Without the Addition of Acetyl Tributyl Citrate. Macromol. Chem. Phys. 2012, 213, 48. [Google Scholar] [CrossRef]
- Gu, S.Y.; Zhang, K.; Ren, J.; Zhan, H. Melt Rheology of Polylactide/Poly(Butylene Adipate-Co-Terephthalate) Blends. Carbohydr. Polym. 2008, 74, 79–85. [Google Scholar] [CrossRef]
- Ma, P.; Cai, X.; Zhang, Y.; Wang, S.; Dong, W.; Chen, M.; Lemstra, P.J. In-Situ Compatibilization of Poly(Lactic Acid) and Poly(Butylene Adipate-Co-Terephthalate) Blends by Using Dicumyl Peroxide as a Free-Radical Initiator. Polym. Degrad. Stab. 2014, 102, 145–151. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of Chain Extension on the Properties of PLA/TPS Blends. J. Appl. Polym. Sci. 2011, 122, 134–141. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.; Ngouajio, M.; Fernandez, R.T. Biodegradation and Hydrolysis Rate of Aliphatic Aromatic Polyester. Polym. Degrad. Stab. 2010, 95, 2641–2647. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, M.; Zhang, H.; Zhang, X. Polylactide Toughening with Epoxy-Functionalized Grafted Acrylonitrile-Butadiene-Styrene Particles. J. Appl. Polym. Sci. 2011, 122, 2992–2999. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive Extrusion of PLA, PBAT with a Multi-Functional Epoxide: Physico-Chemical and Rheological Properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Dong, W.; Zou, B.; Yan, Y.; Ma, P.; Chen, M. Effect of Chain-Extenders on the Properties and Hydrolytic Degradation Behavior of the Poly(Lactide)/ Poly(Butylene Adipate-Co-Terephthalate) Blends. Int. J. Mol. Sci. 2013, 14, 20189–20203. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Q.; Ren, J.; Wang, L. Preparation and Properties of Biodegradable Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blend with Glycidyl Methacrylate as Reactive Processing Agent. J. Mater. Sci. 2009, 44, 250–256. [Google Scholar] [CrossRef]
- Pan, H.; Li, Z.; Yang, J.; Li, X.; Ai, X.; Hao, Y.; Zhang, H.; Dong, L. The Effect of MDI on the Structure and Mechanical Properties of Poly(Lactic Acid) and Poly(Butylene Adipate-Co-Butylene Terephthalate) Blends. RSC Adv. 2018, 8, 4610–4623. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zou, B.; Ma, P.; Liu, W.; Zhou, X.; Shi, D.; Ni, Z.; Chen, M. Influence of Phthalic Anhydride and Bioxazoline on the Mechanical and Morphological Properties of Biodegradable Poly(Lactic Acid)/Poly[(Butylene Adipate)-Co-Terephthalate] Blends. Polym. Int. 2013, 62, 1783–1790. [Google Scholar] [CrossRef]
- Nishida, M.; Ichihara, H.; Watanabe, H.; Fukuda, N.; Ito, H. Improvement of Dynamic Tensile Properties of Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Polymer Alloys Using a Crosslinking Agent and Observation of Fracture Surfaces. Int. J. Impact Eng. 2015, 79, 117–125. [Google Scholar] [CrossRef]
- Puppi, D.; Pecorini, G.; Chiellini, F. Biomedical Processing of Polyhydroxyalkanoates. Bioengineering 2019, 6, 108. [Google Scholar] [CrossRef]
- Gerard, T.; Budtova, T. Morphology and Molten-State Rheology of Polylactide and Polyhydroxyalkanoate Blends. Eur. Polym. J. 2012, 48, 1110–1117. [Google Scholar] [CrossRef]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P.J. Cyanobacterial Polyhydroxybutyrate for Sustainable Bioplastic Production: Critical Review and Perspectives. J. Environ. Chem. Eng. 2020, 8, 104007. [Google Scholar] [CrossRef]
- Lee, W.H.; Azizan, M.N.M.; Sudesh, K. Effects of Culture Conditions on the Composition of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Synthesized by Comamonas Acidovorans. Polym. Degrad. Stab. 2004, 84, 129–134. [Google Scholar] [CrossRef]
- Nomura, C.T.; Tanaka, T.; Gan, Z.; Kuwabara, K.; Abe, H.; Takase, K.; Taguchi, K.; Doi, Y. Effective Enhancement of Short-Chain-Length—Medium-Chain-Length Polyhydroxyalkanoate Copolymer Production by Coexpression of Genetically Engineered 3-Ketoacyl-Acyl-Carrier-Protein Synthase III (FabH) and Polyhydroxyalkanoate Synthesis Genes. Biomacromolecules 2004, 5, 1457–1464. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Q.; Wang, H.; Zhu, B.; Yu, F.; Chen, G.Q.; Inoue, Y. Polymorphic Crystallization of Fractionated Microbial Medium-Chain-Length Polyhydroxyalkanoates. Polymer 2009, 50, 4378–4388. [Google Scholar] [CrossRef]
- Rodriguez-Contreras, A. Recent Advances in the Use of Polyhydroyalkanoates in Biomedicine. Bioengineering 2019, 6, 82. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a Family of Natural Polymers, and Their Applications in Drug Delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Hiremath, L.; Kumar, N.S.; Angadi, S. Design, Screening and Microbial Synthesis of Bio-Polymers of Poly-Hydroxy-Butyrate (PHB) from Low Cost Carbon Sources. Int. J. Adv. Res. 2015, 3, 420–425. [Google Scholar]
- Doyle, V.; Pearson, R.; Lee, D.; Wolowacz, S.; Mctaggart, S. An Investigation of the Growth of Human Dermal Fibroblasts on Poly-L-Lactic Acid in Vitro. J. Mater. Sci. Mater. Med. 1996, 7, 381–395. [Google Scholar] [CrossRef]
- Leroy, E.; Petit, I.; Audic, J.L.; Colomines, G.; Deterre, R. Rheological Characterization of a Thermally Unstable Bioplastic in Injection Molding Conditions. Polym. Degrad. Stab. 2012, 97, 1915–1921. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological Polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Kunioka, M.; Doi, Y. Thermal Degradation of Microbial Copolyesters: Polu(3-hydroxybutyrate-co-3-hydroxyvalerate) and Poly(3-hydroxybutyrate-co-4hydroxybutyrate). Macromolecules 1990, 23, 1933–1936. [Google Scholar] [CrossRef]
- Unger, M.; Sato, H.; Ozaki, Y.; Fischer, D.; Siesler, H.W. Temperature-Dependent Fourier Transform Infrared Spectroscopy and Raman Mapping Spectroscopy of Phase-Separation in a Poly(3-Hydroxybutyrate)-Poly(L-Lactic Acid) Blend. Appl. Spectrosc. 2013, 67, 141–148. [Google Scholar] [CrossRef]
- Khasanah; Takahashi, I.; Reddy, K.R.; Ozaki, Y. Crystallization of Ultrathin Poly(3-Hydroxybutyrate) Films in Blends with Small Amounts of Poly(l-Lactic Acid): Correlation between Film Thickness and Molecular Weight of Poly(l-Lactic Acid). RSC Adv. 2017, 7, 52651–52660. [Google Scholar] [CrossRef]
- Bliimm, E.; Owen, A.J. Miscibility, Crystallization and Melting of Poly(3-hydroxybutyrate)/ Poly(L-Lactide) Blends. Polymer 1995, 36, 4077–4081. [Google Scholar] [CrossRef]
- Park, J.W.; Doi, Y.; Iwata, T. Uniaxial Drawing and Mechanical Properties of Poly[(R)-3-Hydroxybutyrate]/Poly(L-Lactic Acid) Blends. Biomacromolecules 2004, 5, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, I.; Abe, H.; Doi, Y. Miscibility and Solid-State Structures for Blends of Poly[(S)-Lactide] with Atactic Poly[(R,S)-3-Hydroxybutyrate]. Polymer 2000, 41, 5985–5992. [Google Scholar] [CrossRef]
- Zhang, J.; Tsuji, H.; Noda, I.; Ozaki, Y. Structural Changes and Crystallization Dynamics of Poly(L-Lactide) during the Cold-Crystallization Process Investigated by Infrared and Two-Dimensional Infrared Correlation Spectroscopy. Macromolecules 2004, 37, 6433–6439. [Google Scholar] [CrossRef]
- Zhang, J.; Sato, H.; Furukawa, T.; Tsuji, H.; Noda, I.; Ozaki, Y. Crystallization Behaviors of Poly(3-Hydroxybutyrate) and Poly(L-Lactic Acid) in Their Immiscible and Miscible Blends. J. Phys. Chem. B 2006, 110, 24463–24471. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Luo, R.; Xu, K.; Chen, G.Q. Thermal and Crystallinity Property Studies of Poly (L-Lactic Acid) Blended with Oligomers of 3-Hydroxybutyrate or Dendrimers of Hydroxyalkanoic Acids. J. Appl. Polym. Sci. 2009, 111, 1720–1727. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and Characterization of Plasticized PLA/PHB Blends for Biodegradable Multiphase Systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA-PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Urayama, H.; Kanamori, T.; Fukushima, K.; Kimura, Y. Controlled Crystal Nucleation in the Melt-Crystallization of Poly(L-Lactide) and Poly(L-Lactide)/Poly(D-Lactide) Stereocomplex. Polymer 2003, 44, 5635–5641. [Google Scholar] [CrossRef]
- Anderson, A.J.; Dawes, E.A. Occurrence, Metabolism, Metabolic Role, and Industrial Uses of Bacterial Polyhydroxyalkanoates. Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef]
- Iglesias Montes, M.L.; Cyras, V.P.; Manfredi, L.B.; Pettarín, V.; Fasce, L.A. Fracture Evaluation of Plasticized Polylactic Acid / Poly (3-HYDROXYBUTYRATE) Blends for Commodities Replacement in Packaging Applications. Polym. Test. 2020, 84, 106375. [Google Scholar] [CrossRef]
- Emblem, A. Plastics Properties for Packaging Materials. In Packaging Technology; Woodhead Publishing: Thorston, UK, 2012; pp. 287–309. [Google Scholar] [CrossRef]
- Parra, D.F.; Fusaro, J.A.; Ponce, P. Biodegradable Polymeric Films of PHB from Burkholderia Saccharia in Presence of Polyethyleneglycol. Pak. J. Biol. Sci. 2005, 8, 1041–1044. [Google Scholar] [CrossRef]
- Erceg, M.; Kovačić, T.; Klarić, I. Thermal Degradation of Poly(3-Hydroxybutyrate) Plasticized with Acetyl Tributyl Citrate. Polym. Degrad. Stab. 2005, 90, 313–318. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on the Safety Evaluation of the Active Substances, Iron, Polyethyleneglycol, Disodium Pyrophosphate, Monosodium Phosphate and Sodium Chloride for Use in Food Contact Materials. EFSA J. 2013, 11, 3245. [Google Scholar] [CrossRef]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Scientific Opinion on Flavouring Group Evaluation 10, Revision 3 (FGE.10Rev3): Aliphatic Primary and Secondary Saturated and Unsaturated Alcohols, Aldehydes, Acetals, Carboxylic Acids and Esters Containing an Additional Oxygenated Functional Group and Lactones from Chemical Groups 9, 13 and 30. EFSA J. 2012, 10, 2563. [Google Scholar] [CrossRef]
- Bassett, D.C. Developments in Crystalline Polymers; Springer: Dordrecht, The Netherlands, 1982. [Google Scholar] [CrossRef]
- Luzier, W.D. Materials Derived from Biomass/Biodegradable Materials. Proc. Natl. Acad. Sci. USA 1992, 89, 839–842. [Google Scholar] [CrossRef]
- Verhoogt, H.; Ramsay, B.A.; Favis, B.D. Polymer Review Polymer Blends Containing Poly(3-Hydroxyalkanoate)s. Polymer 1994, 35, 5155–5169. [Google Scholar] [CrossRef]
- Liu, Q.; Shyr, T.W.; Tung, C.H.; Deng, B.; Zhu, M. Block Copolymers Containing Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Poly (ɛ-Caprolactone) Units: Synthesis, Characterization and Thermal Degradation. Fibers Polym. 2011, 12, 848–856. [Google Scholar] [CrossRef]
- Zytner, P.; Pal, A.K.; Wu, F.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Morphology and Performance Relationship Studies on Poly(3-Hydroxybutyrate- Co-3-Hydroxyvalerate)/Poly(Butylene Adipate- Co-Terephthalate)-Based Biodegradable Blends. ACS Omega 2023, 8, 1946–1956. [Google Scholar] [CrossRef]
- Gogolewski, S.; Jovanovic, M.; Perren, S.M.; Dillon, J.G.; Hughes, M.K. Tissue Response and in Vivo Degradation of Selected Polyhydroxyacids: Polylactides (PLA), Poly(3-hydroxybutyrate) (PHB), and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA). J. Biomed. Mater. Res. 1993, 27, 1135–1148. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Properties of Blends and Composites Based on Poly(3-Hydroxy)Butyrate (PHB) and Poly(3-Hydroxybutyrate-Hydroxyvalerate) (PHBV) Copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, Biodegradable Polymers and Biocomposites: An Overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Wada, Y.; Mitobe, T.; Mitomo, H.; Seko, N.; Tamada, M. Effect of Hydrophilic and Hydrophobic Monomers Grafting on Microbial Poly(3-Hydroxybutyrate). J. Taiwan. Inst. Chem. Eng. 2009, 40, 413–417. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A Review of Biodegradable Polymers: Uses, Current Developments in the Synthesis and Characterization of Biodegradable Polyesters, Blends of Biodegradable Polymers and Recent Advances in Biodegradation Studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Tan, J.; Lu, T.; Li, R.; Zhang, S.; Liu, W.; Zhu, X.; Zhang, J.; Xin, J. Biodegradable Waste Frying Oil-Based Ethoxylated Esters as Highly Efficient Plasticizers for Poly(Lactic Acid). ACS Sustain. Chem. Eng. 2019, 7, 15957–15965. [Google Scholar] [CrossRef]
- Yu, H.; Sun, B.; Zhang, D.; Chen, G.; Yang, X.; Yao, J. Reinforcement of Biodegradable Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Cellulose Nanocrystal/Silver Nanohybrids as Bifunctional Nanofillers. J. Mater. Chem. B 2014, 2, 8479–8489. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(3-Hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef]
- Yang, F.; Niu, X.; Gu, X.; Xu, C.; Wang, W.; Fan, Y. Biodegradable Magnesium-Incorporated Poly(l-Lactic Acid) Microspheres for Manipulation of Drug Release and Alleviation of Inflammatory Response. ACS Appl. Mater. Interfaces 2019, 11, 23546–23557. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully Biodegradable and Biorenewable Ternary Blends from Polylactide, Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) and Poly(Butylene Succinate) with Balanced Properties. ACS Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Loo, C.Y.; Sudesh, K. Polyhydroxyalkanoates: Bio-Based Microbial Plastics and Their Properties. Malays. Polym. J. (MPJ) 2007, 2, 31–57. [Google Scholar]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; di Serio, M. In Vivo and Post-Synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Mo, W.; Yao, H.; Wu, Q.; Chen, J.; Chen, G.Q. Biodegradation Studies of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Polym. Degrad. Stab. 2004, 85, 815–821. [Google Scholar] [CrossRef]
- Weng, Y.X.; Wang, X.L.; Wang, Y.Z. Biodegradation Behavior of PHAs with Different Chemical Structures under Controlled Composting Conditions. Polym. Test. 2011, 30, 372–380. [Google Scholar] [CrossRef]
- Sashiwa, H.; Fukuda, R.; Okura, T.; Sato, S.; Nakayama, A. Microbial Degradation Behavior in Seawater of Polyester Blends Containing Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBHHx). Mar. Drugs 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB Packaging for the Storage of Food Products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Pachekoski, W.M.; Dalmolin, C.; Agnelli, J.A.M. The Influence of the Industrial Processing on the Degradation of Poly(Hidroxybutyrate)-PHB. Mater. Res. 2013, 16, 327–332. [Google Scholar] [CrossRef]
- Fischer, J.J.; Aoyagi, Y.; Enoki, M.; Doi, Y.; Iwata, T. Mechanical Properties and Enzymatic Degradation of Poly([R]-3-Hydroxybutyrate-Co-[R]-3-Hydroxyhexanoate) Uniaxially Cold-Drawn Films. Polym. Degrad. Stab. 2004, 83, 453–460. [Google Scholar] [CrossRef]
- Ino, K.; Sato, S.; Ushimaru, K.; Saika, A.; Fukuoka, T.; Ohshiman, K.; Morita, T. Mechanical Properties of Cold-Drawn Films of Ultrahigh-Molecular-Weight Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Produced by Haloferax Mediterranei. Polym. J. 2020, 52, 1299–1306. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Gámez-Pérez, J.; Cabedo, L. Biocomposites of Different Lignocellulosic Wastes for Sustainable Food Packaging Applications. Compos. B Eng. 2018, 145, 215–225. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Cabedo, L.; Gámez-Pérez, J. Study of the Compatibilization Effect of Different Reactive Agents in Phb/Natural Fiber-Based Composites. Polymers 2020, 12, 1967. [Google Scholar] [CrossRef]
- le Moigne, N.; Sauceau, M.; Benyakhlef, M.; Jemai, R.; Benezet, J.C.; Rodier, E.; Lopez-Cuesta, J.M.; Fages, J. Foaming of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Organo-Clays Nano-Biocomposites by a Continuous Supercritical CO2 Assisted Extrusion Process. Eur. Polym. J. 2014, 61, 157–171. [Google Scholar] [CrossRef]
- Kabe, T.; Hongo, C.; Tanaka, T.; Hikima, T.; Takata, M.; Iwata, T. High Tensile Strength Fiber of Poly[(R)-3-Hydroxybutyrate-Co-(R)-3-Hydroxyhexanoate] Processed by Two-Step Drawing with Intermediate Annealing. J. Appl. Polym. Sci. 2015, 132, 41258. [Google Scholar] [CrossRef]
- Cheng, M.L.; Chen, P.Y.; Lan, C.H.; Sun, Y.M. Structure, Mechanical Properties and Degradation Behaviors of the Electrospun Fibrous Blends of PHBHHx/PDLLA. Polymer 2011, 52, 1391–1401. [Google Scholar] [CrossRef]
- Giubilini, A.; Siqueira, G.; Clemens, F.J.; Sciancalepore, C.; Messori, M.; Nyström, G.; Bondioli, F. 3D-Printing Nanocellulose-Poly(3-Hydroxybutyrate- Co-3-Hydroxyhexanoate) Biodegradable Composites by Fused Deposition Modeling. ACS Sustain. Chem. Eng. 2020, 8, 10292–10302. [Google Scholar] [CrossRef]
- Mota, C.; Wang, S.Y.; Puppi, D.; Gazzarri, M.; Migone, C.; Chiellini, F.; Chen, G.Q.; Chiellini, E. Additive Manufacturing of Poly[(R)-3-Hydroxybutyrate-Co-(R)-3-Hydroxyhexanoate] Scaffolds for Engineered Bone Development. J. Tissue Eng. Regen. Med. 2017, 11, 175–186. [Google Scholar] [CrossRef]
- Sängerlaub, S.; Brüggemann, M.; Rodler, N.; Jost, V.; Bauer, K.D. Extrusion Coating of Paper with Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV)-Packaging Related Functional Properties. Coatings 2019, 9, 457. [Google Scholar] [CrossRef]
- Wang, S.; Ma, P.; Wang, R.; Wang, S.; Zhang, Y.; Zhang, Y. Mechanical, Thermal and Degradation Properties of Poly(d,l-Lactide)/Poly(Hydroxybutyrate-Co-Hydroxyvalerate)/Poly(Ethylene Glycol) Blend. Polym. Degrad. Stab. 2008, 93, 1364–1369. [Google Scholar] [CrossRef]
- Iannace, S.; Huanc, S.J. Poly(3-Hydroxybutyrate)-Co-(3-Hydroxyvalerate)/Poly-L-Lactide Blends: Thermal and Mechanical Properties. J. Appl. Polym. Sci. 1994, 54, 1525–1536. [Google Scholar] [CrossRef]
- Ferreira, B.M.P.; Zavaglia, C.A.C.; Duek, E.A.R. Films of PLLA/PHBV: Thermal, Morphological, and Mechanical Characterization. J. Appl. Polym. Sci. 2002, 86, 2898–2906. [Google Scholar] [CrossRef]
- Zembouai, I.; Bruzaud, S.; Kaci, M.; Benhamida, A.; Corre, Y.M.; Grohens, Y.; Taguet, A.; Lopez-Cuesta, J.M. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Polylactide Blends: Thermal Stability, Flammability and Thermo-Mechanical Behavior. J. Polym. Environ. 2014, 22, 131–139. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, C.; Zhang, H.; Deng, B. Blends of Polylactide and Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Low Content of Hydroxyvalerate Unit: Morphology, Structure, and Property. J. Appl. Polym. Sci. 2015, 132, 42689. [Google Scholar] [CrossRef]
- Nanda, M.R.; Misra, M.; Mohanty, A.K. The Effects of Process Engineering on the Performance of PLA and PHBV Blends. Macromol. Mater. Eng. 2011, 296, 719–728. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, S.; Kong, M.; Geng, W.; Li, R.K.Y.; Song, C.; Kong, D. Phase Morphology, Physical Properties, and Biodegradation Behavior of Novel PLA/PHBHHx Blends. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100 B, 23–31. [Google Scholar] [CrossRef]
- Modi, S.; Koelling, K.; Vodovotz, Y. Miscibility of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with High Molecular Weight Poly(Lactic Acid) Blends Determined by Thermal Analysis. J. Appl. Polym. Sci. 2012, 124, 3074–3081. [Google Scholar] [CrossRef]
- Kanda, G.S.; Al-Qaradawi, I.; Luyt, A.S. Morphology and Property Changes in PLA/PHBV Blends as Function of Blend Composition. J. Polym. Res. 2018, 25, 196. [Google Scholar] [CrossRef]
- Zembouai, I.; Bruzaud, S.; Kaci, M.; Benhamida, A.; Corre, Y.M.; Grohens, Y.; Lopez-Cuesta, J.M. Synergistic Effect of Compatibilizer and Cloisite 30B on the Functional Properties of Poly(3-Hydroxybutyrateco- 3-Hydroxyvalerate)/Polylactide Blends. Polym. Eng. Sci. 2014, 54, 2239–2251. [Google Scholar] [CrossRef]
- Zembouai, I.; Kaci, M.; Bruzaud, S.; Benhamida, A.; Corre, Y.M.; Grohens, Y. A Study of Morphological, Thermal, Rheological and Barrier Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Polylactide Blends Prepared by Melt Mixing. Polym. Test. 2013, 32, 842–851. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Gámez-Pérez, J.; Balart, R.; Lagarón, J.M.; Cabedo, L. Effect of the Addition of Sepiolite on the Morphology and Properties of Melt Compounded PHBV/PLA Blends. Polym. Compos. 2019, 40, E156–E168. [Google Scholar] [CrossRef]
- Ma, P.; Spoelstra, A.B.; Schmit, P.; Lemstra, P.J. Toughening of Poly (Lactic Acid) by Poly (β-Hydroxybutyrate-Co-β- Hydroxyvalerate) with High β-Hydroxyvalerate Content. Eur. Polym. J. 2013, 49, 1523–1531. [Google Scholar] [CrossRef]
- Noda, I.; Satkowski, M.M.; Dowrey, A.E.; Marcott, C. Polymer Alloys of Nodax Copolymers and Poly(Lactic Acid). Macromol. Biosci. 2004, 4, 269–275. [Google Scholar] [CrossRef]
- Richards, E.; Rizvi, R.; Chow, A.; Naguib, H. Biodegradable Composite Foams of PLA and PHBV Using Subcritical CO 2. J. Polym. Environ. 2008, 16, 258–266. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, Z.; Sun, X.; Turng, L.S.; Peng, X. Morphology and Properties of Injection Molded Solid and Microcellular Polylactic Acid/Polyhydroxybutyrate-Valerate (PLA/PHBV) Blends. Ind. Eng. Chem. Res. 2013, 52, 2569–2581. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Dynamic Mechanical Properties of PLA/PHBV, PLA/PCL, PHBV/PCL Blends and Their Nanocomposites with TiO2 as Nanofiller. Thermochim. Acta 2015, 613, 41–53. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, H.; Zhang, C.; Jiang, Q.; Zhao, Y.; Chen, P.; Wang, D. Transesterification Induced Mechanical Properties Enhancement of PLLA/PHBV Bio-Alloy. Polymer 2016, 83, 230–238. [Google Scholar] [CrossRef]
- González-Ausejo, J.; Sanchez-Safont, E.; Lagaron, J.M.; Olsson, R.T.; Gamez-Perez, J.; Cabedo, L. Assessing the Thermoformability of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly(Acid Lactic) Blends Compatibilized with Diisocyanates. Polym. Test. 2017, 62, 235–245. [Google Scholar] [CrossRef]
- Qiang, T.; Wang, J.; Wolcott, M.P. Facile Fabrication of 100% Bio-Based and Degradable Ternary Cellulose/PHBV/PLA Composites. Materials 2018, 11, 330. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Toughness Decrease of PLA-PHBHHx Blend Films upon Surface-Confined Photopolymerization. J. Biomed. Mater. Res. A 2009, 88, 1079–1086. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Huang, Z.G.; Diao, X.Q.; Weng, Y.X.; Wang, Y.Z. Characterization of the Effect of REC on the Compatibility of PHBH and PLA. Polym. Test. 2015, 42, 17–25. [Google Scholar] [CrossRef]
- Schreck, K.M.; Hillmyer, M.A. Block Copolymers and Melt Blends of Polylactide with NodaxTM Microbial Polyesters: Preparation and Mechanical Properties. J. Biotechnol. 2007, 132, 287–295. [Google Scholar] [CrossRef]
- Zhang, K.; Nagarajan, V.; Misra, M.; Mohanty, A.K. Supertoughened Renewable PLA Reactive Multiphase Blends System: Phase Morphology and Performance. ACS Appl. Mater. Interfaces 2014, 6, 12436–12448. [Google Scholar] [CrossRef]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.C. Poly (Lactic Acid) Blends: Processing, Properties and Applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Ravati, S.; Favis, B.D. Tunable Morphologies for Ternary Blends with Poly(Butylene Succinate): Partial and Complete Wetting Phenomena. Polymer 2013, 54, 3271–3281. [Google Scholar] [CrossRef]
- Ravati, S.; Beaulieu, C.; Zolali, A.M.; Favis, B.D. High Performance Materials Based on a Self-Assembled Multiple-Percolated Ternary Blend. AIChE J. 2014, 60, 3005–3012. [Google Scholar] [CrossRef]
- Arrigo, R.; D’Anna, A.; Frache, A. Fully Bio-Based Ternary Polymer Blends: Structural Characterization and Mechanical Behavior. Mater. Today Sustain. 2023, 21, 100314. [Google Scholar] [CrossRef]
- Wang, X.Y.; Pan, H.W.; Jia, S.L.; Cao, Z.W.; Han, L.J.; Zhang, H.L.; Dong, L.S. Mechanical Properties, Crystallization and Biodegradation Behavior of the Polylactide/Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate)/Poly(Butylene Adipate-Co-Terephthalate) Blown Films. Chin. J. Polym. Sci. 2020, 38, 1072–1081. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The Quest for High Glass Transition Temperature Bioplastics. J. Mater. Chem. A Mater. 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- van Beilen, J.B.; Poirier, Y. Plants as Factories for Bioplastics and Other Novel Biomaterials. In Plant Biotechnology and Agriculture: Prospects for the 21st Century; Elsevier: Amsterdam, The Netherlands, 2011; pp. 481–494. ISBN 9780123814661. [Google Scholar]
- Yoshiharu, K. SPSJ Mitsubishi Chemical Award Accounts Molecular, Structural, and Material Design of Bio-Based Polymers. Polym. J. 2009, 41, 797–807. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a Biocomposite Based on Green Polyethylene Biopolymer and Eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Spalding, M.A.; Chatterjee, A. Handbook of Industrial Polyethylene and Technology: Definitive Guide to Manufacturing, Properties, Processing, Applications and Markets; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Quiles-Carrillo, L.; Montanes, N.; Jorda-Vilaplana, A.; Balart, R.; Torres-Giner, S. A Comparative Study on the Effect of Different Reactive Compatibilizers on Injection-Molded Pieces of Bio-Based High-Density Polyethylene/Polylactide Blends. J. Appl. Polym. Sci. 2019, 136, 47396. [Google Scholar] [CrossRef]
- Dolores, S.M.; Patricia, A.M.; Santiago, F.; Juan, L. Influence of Biodegradable Materials in the Recycled Polystyrene. J. Appl. Polym. Sci. 2014, 131, 41161. [Google Scholar] [CrossRef]
- Hermes, H.E.; Higgins, J.S.; Bucknall, D.G. Investigation of the Melt Interface between Polyethylene and Polystyrene Using Neutron Reflectivity. Polymer 1997, 38, 985–989. [Google Scholar] [CrossRef]
- Wang, Y.; Hillmyer, M.A. Polyethylene-Poly(L-Lactide) Diblock Copolymers: Synthesis and Compatibilization of Poly(L-Lactide)/Polyethylene Blends. J. Polym. Sci. A Polym. Chem. 2001, 39, 2755–2766. [Google Scholar] [CrossRef]
- Anderson, K.S.; Hillmyer, M.A. The Influence of Block Copolymer Microstructure on the Toughness of Compatibilized Polylactide/Polyethylene Blends. Polymer 2004, 45, 8809–8823. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Rayón, E.; Samper, M.D.; Balart, R. Compatibilization and Characterization of Polylactide and Biopolyethylene Binary Blends by Non-Reactive and Reactive Compatibilization Approaches. Polymers 2020, 12, 1344. [Google Scholar] [CrossRef]
- Yoon, J.S.; Oh, S.H.; Kim, M.N.; Chin, I.J.; Kim, Y.H. Thermal and Mechanical Properties of Poly(L-Lactic Acid)-Poly (Ethylene-Co-Vinyl Acetate) Blends. Polymer 1999, 40, 2303–2312. [Google Scholar] [CrossRef]
- Ferreira, E.d.S.B.; Luna, C.B.B.; Siqueira, D.D.; Dos Santos Filho, E.A.; Araújo, E.M.; Wellen, R.M.R. Production of Eco-Sustainable Materials: Compatibilizing Action in Poly (Lactic Acid)/High-Density Biopolyethylene Bioblends. Sustainability 2021, 13, 12157. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Mélo, T.J.A. Mechanical and Morphological Properties of PLA/BioPE Blend Compatibilized with E-GMA and EMA-GMA Copolymers. Macromol. Symp. 2016, 367, 176–182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).