A Comparative Analysis of Different Hydrogen Production Methods and Their Environmental Impact
Abstract
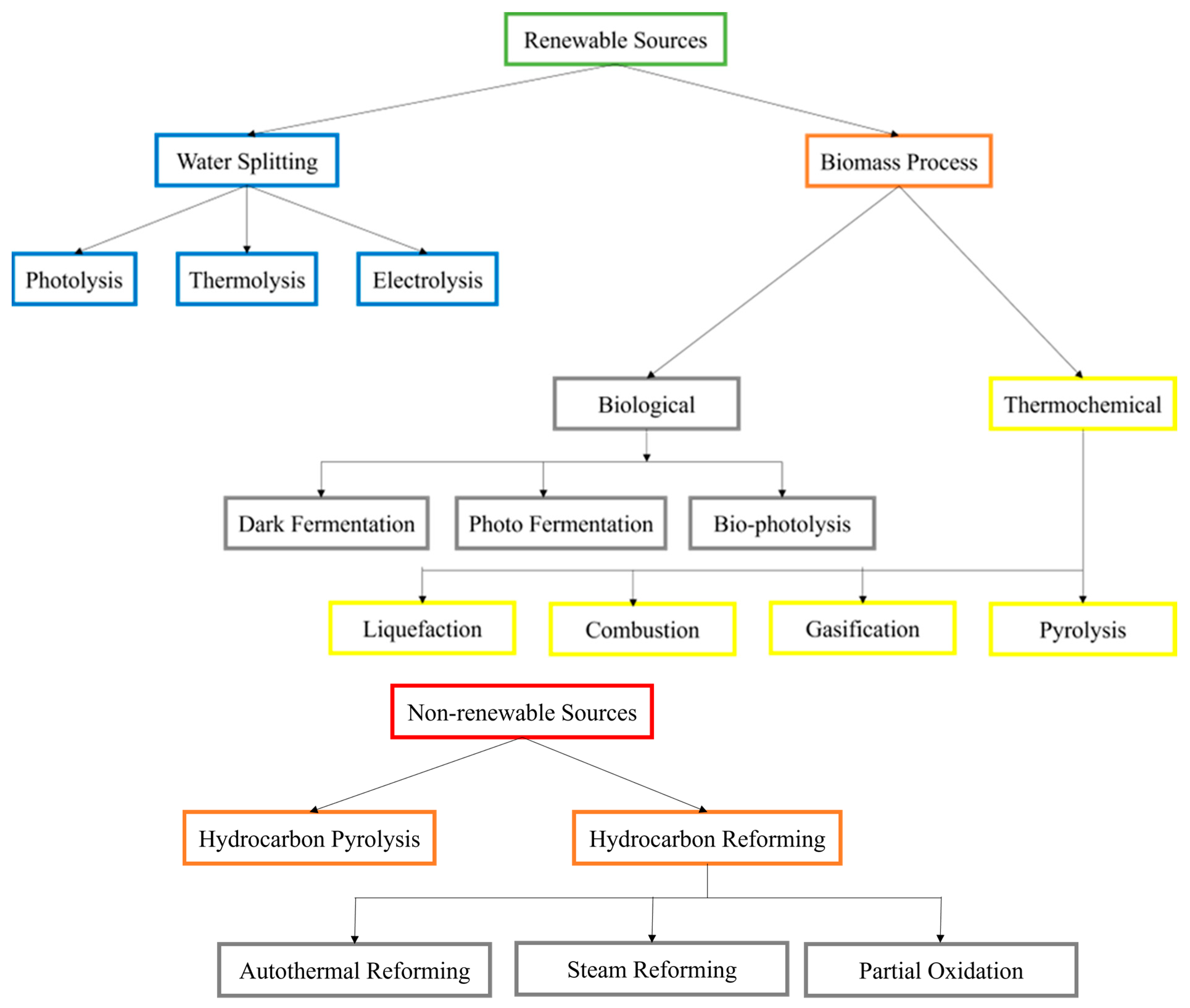
:1. Introduction
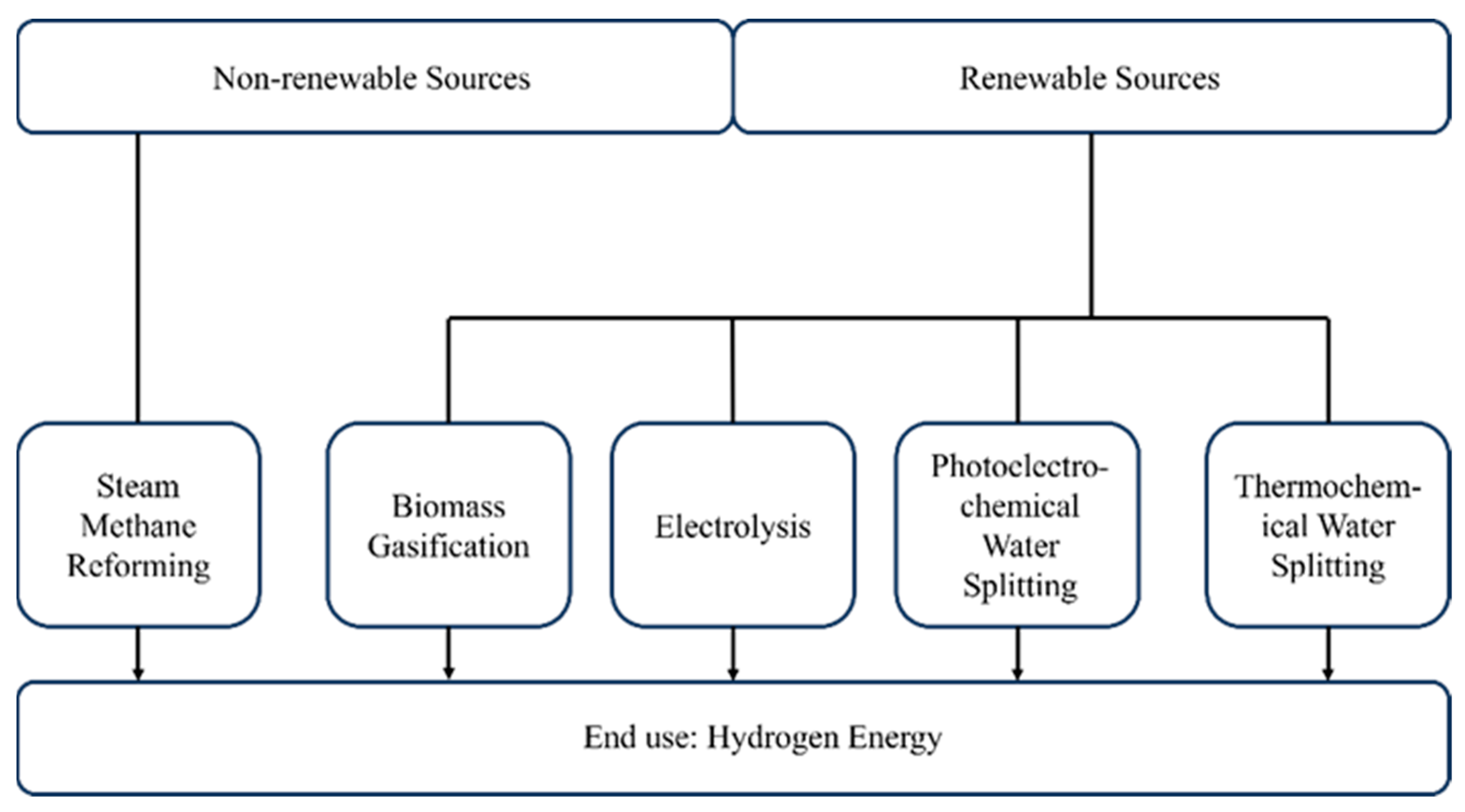
2. Hydrogen Production Methods
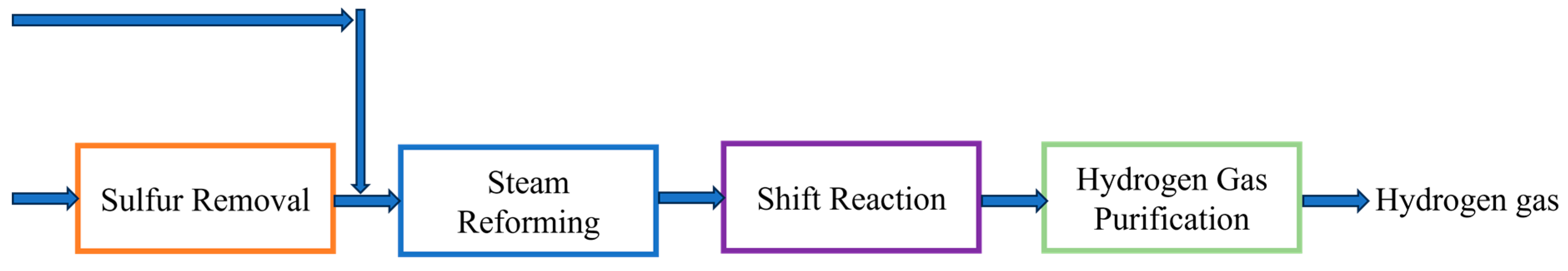
2.1. Steam Methane Reforming
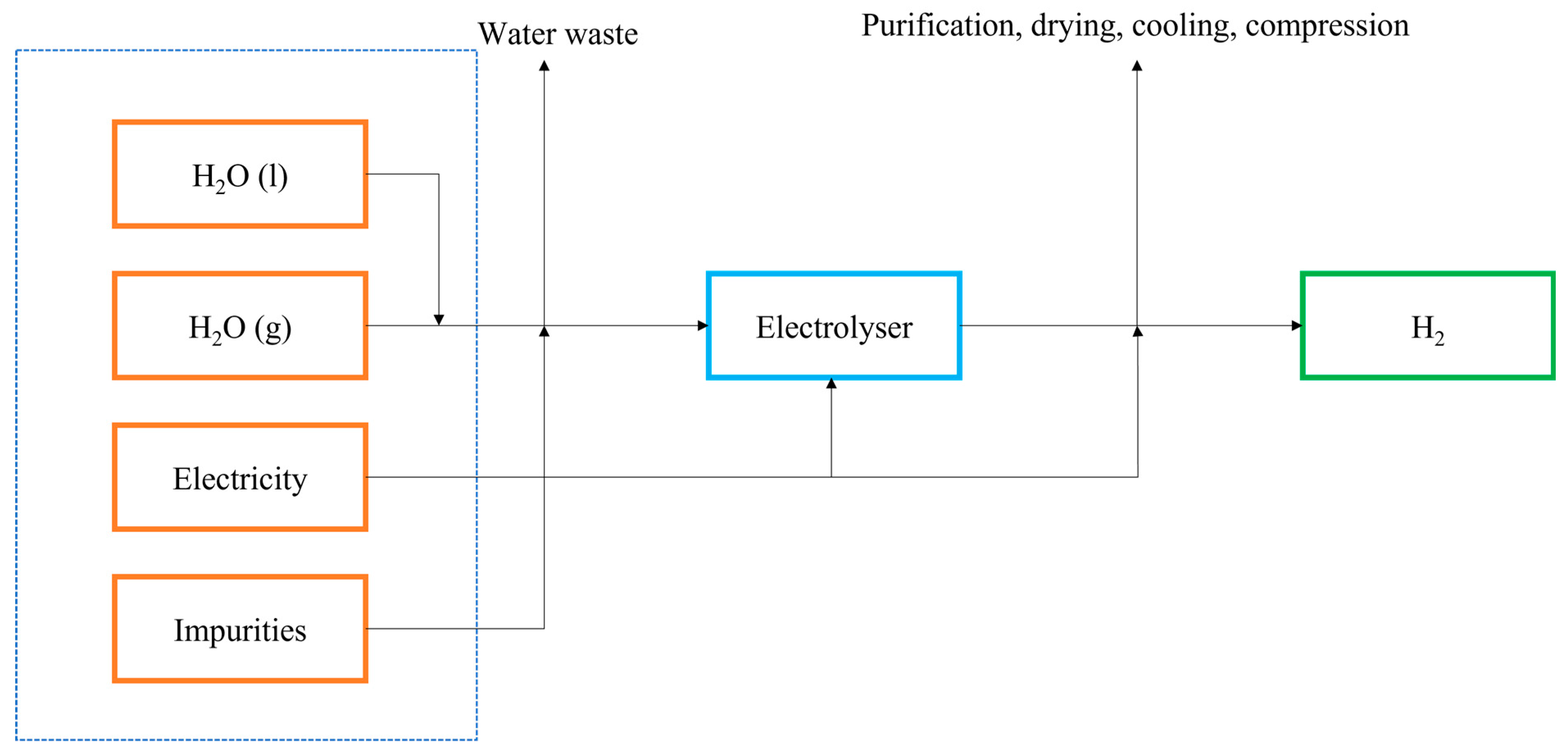
2.2. Electrolysis

| Specification | SOE | AWE | PEM |
|---|---|---|---|
| Cell temperature, °C | 900–1000 | 60–80 | 50–80 |
| System lifetime, yr | - | 20–30 | 10–20 |
| Hydrogen purity, % | - | >99.8 | 99.999 |
| Cold start-up time, min | >60 | 15 | <15 |
| Specific system energy consumption, kWh/Nm2 | 2.5–3.5 | 4.5–7.0 | 4.5–7.5 |
| Cell pressure, psi | <30 | <30 | <30 |
| Current density, A/cm2 | 0.3–1.0 | 0.2–0.4 | 0.6–2.0 |
| Hydrogen production, Nm2/h | - | <760 | <30 |
| Stack lifetime, h | <40,000 | <90,000 | <20,000 |
| Cell voltage, V | 0.95–1.3 | 1.8–2.4 | 1.8–2.2 |
| Power density, W/cm2 | - | Up to 1.0 | Up to 4.4 |
| Voltage efficiency, % | 81–86 | 62–82 | 67–82 |
| Partial load range, % | - | 20–40 | 0–10 |
| Cell area, m2 | - | <4 | <300 |
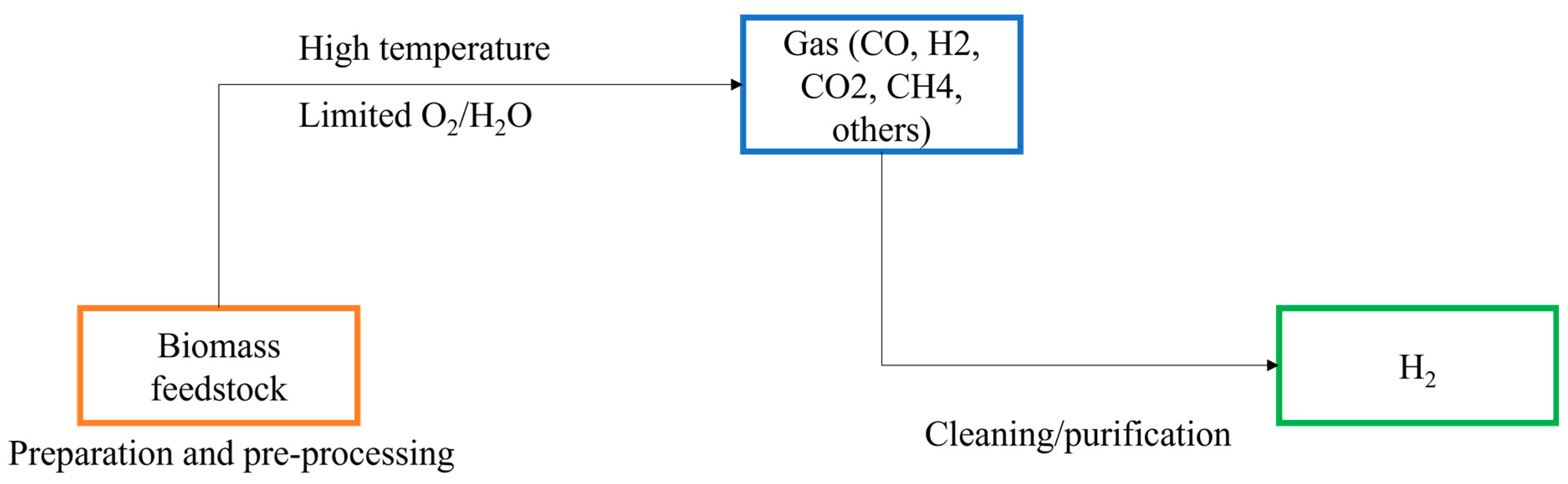
2.3. Biomass Gasification
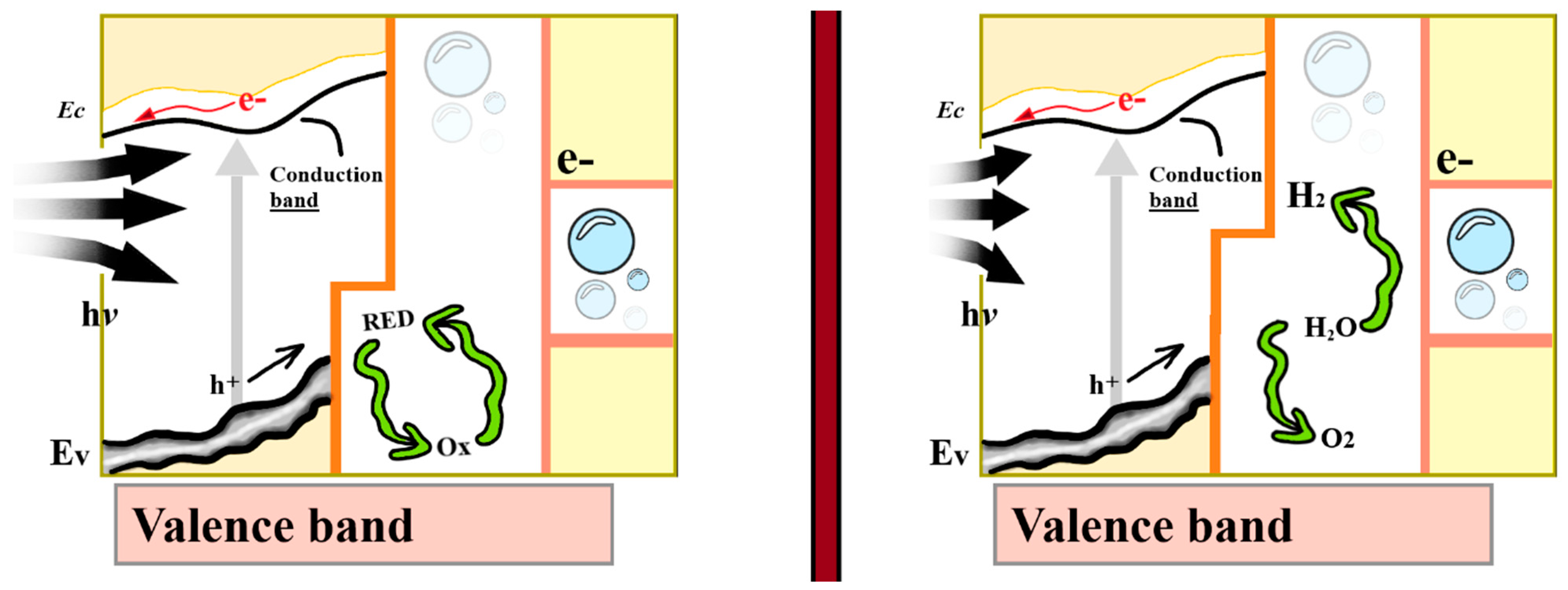
2.4. Photoelectrochemical Water Splitting
2.5. Thermochemical Water Splitting
3. Environmental Impact of Hydrogen Production Methods
- Rising Energy Demand: According to the International Energy Agency (IEA), GED is expected to rise by more than 50% by 2040 due to global population growth and industrialisation.
- Energy sources and sustainability: Fossil fuels dominate the contemporary energy environment, creating challenges such as greenhouse gas emissions, air pollution, and limited supplies. It is critical to transition to more sustainable, cleaner energy sources in order to prevent climate change and protect the environment.
- Hydrogen energy’s role: Hydrogen is a versatile energy carrier that may be produced from natural gas, water, and biomass via processes such as steam methane reforming and electrolysis. It may be used in a variety of applications, such as car fuel cells, industrial operations, and power generation. Green hydrogen, derived from renewable sources, is a clean, long-term alternative.
- Managing energy issues: Hydrogen can effectively store and transfer excess renewable energy, acting as an energy buffer to meet peak demand. It also provides a low-carbon alternative for sectors that are difficult to electrify directly, such as heavy industrial and long-distance transportation, by replacing hydrogen fuel cells with fossil fuels.
- Hydrogen production and costs: The cost of producing hydrogen varies by technique, with steam methane reforming being the most cost-effective but generating carbon without carbon capture and storage. Green hydrogen, created using renewable energy-powered electrolysis, is more expensive but provides environmental advantages, with cost reductions projected as renewable energy becomes more inexpensive.
- Infrastructure and limitations: Establishing a full hydrogen infrastructure, including production, storage, and delivery, presents considerable hurdles, needing significant expenditures and technological developments.
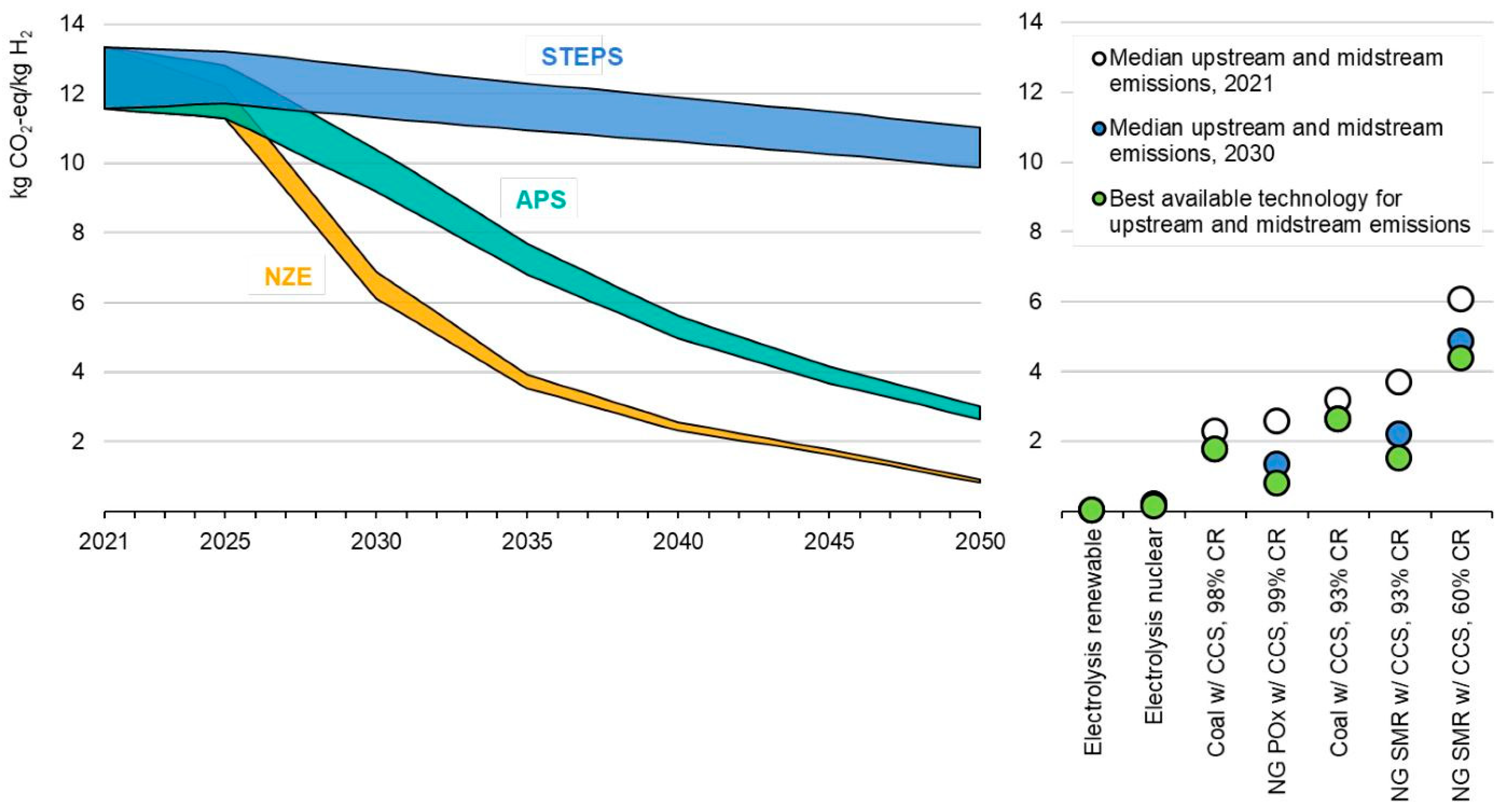
3.1. Greenhouse Gas Emissions
3.2. Water Usage
3.3. Energy and Exergetic Efficiency
3.3.1. Energy Efficiency
3.3.2. Exergetic Efficiency
3.4. Air Pollution
3.5. Land Use
4. Comparative Analysis of Hydrogen Production Methods
4.1. Comparison of Environmental Impact
4.2. Comparison of Energy Efficiency
4.3. Comparison of Economic Viability
4.4. Comparison of Technological Maturity
5. The Influence of Hydrogen Safety on the Economy of Hydrogen Energy
5.1. The H2 Economy
5.2. Hydrogen Storage
5.3. Hydrogen Transportation
5.4. Safety Concerns with the Transportation and Storage of Hydrogen
6. Conclusions and Recommendations
- Research and development: To increase the effectiveness and environmental sustainability of hydrogen production techniques, more research and development are required. To circumvent their present restrictions and improve their economic viability, emphasis should be placed on thermochemical, biomass gasification, and electrolysis methods.
- Integration of renewable energy sources: Regulations and financial incentives ought to be put in place to promote the incorporation of renewable energy sources, such as solar and wind power, into the methodologies used to produce hydrogen. As a result, carbon dioxide emissions linked to the production of hydrogen will be decreased, and the overall sustainability of the energy system will be improved.
- Technological innovation: For the advancement of hydrogen production techniques, investments in new technologies and pilot initiatives are crucial. Partnerships between governments, businesses, and academia can hasten the creation of new, environmentally friendly methods for producing hydrogen.
- Life cycle evaluation: Performing thorough life cycle analyses of various hydrogen production techniques can give us in-depth knowledge of the effects they have on the environment. To discover and tackle prospective environmental hotspots, this assessment should take into account every step, from the extraction of raw materials to the final product.
- Policy encouragement: To encourage the implementation of environmentally friendly hydrogen production methods, policymakers and governments ought to develop enabling policies, rules, and rewards. To encourage the switch to more environmentally friendly hydrogen production techniques, this involves pricing carbon processes, funding for research, and tax incentives.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | Autothermal Reforming |
| AP | Acidification Potential |
| ADP | Abiotic Depletion Potential |
| CCU | Carbon Capture and Utilisation |
| CCS | Carbon Capture and Storage |
| CO2 | Carbon dioxide |
| Cu–Cl | Copper–chlorine |
| GHG | Greenhouse Gas |
| GWP | Global Warming Potential |
| EP | Eutrophication Potential |
| FID | Final Investment Decision |
| H2 | Hydrogen |
| HHV | High Heating Value |
| GED | Global Energy Demand |
| CG | Coal Gasification |
| HTP | Human Toxicity Potential |
| IPCC | Intergovernmental Panel on Climate Change |
| Fe | Iron |
| FCEVs | Fuel Cell Electric Vehicles |
| IPHE | International Partnership for Hydrogen and fuels cells in the Economy |
| LCA | Life Cycle Assessment |
| LHV | Lower Heating Value |
| SMR | Steam Methane Reformer |
| TWD | Thermochemical Water Decomposition |
| AWE | Alkaline Water Electrolysis |
| SOE | Solid Oxide Electrolysis |
| NOx | Nitrogen Oxides |
| Ni | Nickel |
| NZE | Net Zero Emission |
| PEM | Proton Exchange Membrane |
| PEC | Photoelectrochemical |
| SO2 | Sulfur Dioxide |
| KOH | Potassium hydroxide |
| PM | Particulate Matter |
| NaCl | Sodium Chloride |
| VOCs | Volatile Organic Compounds |
| H2O | Water |
References
- Althabaiti, S.A.; Khan, Z.; Bawaked, S.M.; Al-Sheheri, S.Z.; Mokhtar, M.; Malik, M.A.; Narasimharao, K. PtOx Deposited Fe3O4-ZnO/TiO2 Nanocomposites for Photocatalytic H2 Production under Visible Light. J. Environ. Chem. Eng. 2023, 11, 110615. [Google Scholar] [CrossRef]
- Ling, B.; Wang, Z.; Zhang, J.; He, Y.; Zhu, Y.; Cen, K. Comprehensive Comparative Analysis of Open-Loop and Closed-Loop Iodine-Sulfur Thermochemical Cycle for Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 14941–14953. [Google Scholar] [CrossRef]
- Sadeghi, S.; Ghandehariun, S. Environmental Impacts of a Standalone Solar Water Splitting System for Sustainable Hydrogen Production: A Life Cycle Assessment. Int. J. Hydrogen Energy 2023, 48, 19326–19339. [Google Scholar] [CrossRef]
- Weidner, T.; Tulus, V.; Guillén-Gosálbez, G. Environmental Sustainability Assessment of Large-Scale Hydrogen Production Using Prospective Life Cycle Analysis. Int. J. Hydrogen Energy 2023, 48, 8310–8327. [Google Scholar] [CrossRef]
- Razi, F.; Hewage, K.; Sadiq, R. A Comparative Exergoenvironmental Evaluation of Chlorine-Based Thermochemical Processes for Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 37108–37123. [Google Scholar] [CrossRef]
- Ozbilen, A.; Dincer, I.; Rosen, M.A. Life Cycle Assessment of Hydrogen Production via Thermochemical Water Splitting Using Multi-Step Cu-Cl Cycles. J. Clean. Prod. 2012, 33, 202–216. [Google Scholar] [CrossRef]
- Norouzi, N. Hydrogen Production in the Light of Sustainability: A Comparative Study on the Hydrogen Production Technologies Using the Sustainability Index Assessment Method. Nucl. Eng. Technol. 2022, 54, 1288–1294. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A Review and Comparative Evaluation of Thermochemical Water Splitting Cycles for Hydrogen Production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Suleman, F.; Dincer, I.; Agelin-Chaab, M. Environmental Impact Assessment and Comparison of Some Hydrogen Production Options. Int. J. Hydrogen Energy 2015, 40, 6976–6987. [Google Scholar] [CrossRef]
- Karaca, A.E.; Dincer, I. Development of a New Photoelectrochemical System for Clean Hydrogen Production and a Comparative Environmental Impact Assessment with Other Production Methods. Chemosphere 2023, 337, 139367. [Google Scholar] [CrossRef]
- Wu, H.; Alkhatami, A.G.; Farhan, Z.A.; AbdalSalam, A.G.; Hamadan, R.; Aldarrji, M.Q.; Izzat, S.E.; Yosif, A.A.; Hadrawi, S.K.; Riyahi, Y.; et al. Recent Developments in the Production of Hydrogen: Efficiency Comparison of Different Techniques, Economic Dimensions, Challenges and Environmental Impacts. Fuel Process. Technol. 2023, 248, 107819. [Google Scholar] [CrossRef]
- Mannaa, M.A.; Qasim, K.F.; Alshorifi, F.T.; El-Bahy, S.M.; Salama, R.S. Role of NiO Nanoparticles in Enhancing Structure Properties of TiO2and Its Applications in Photodegradation and Hydrogen Evolution. ACS Omega 2021, 6, 30386–30400. [Google Scholar] [CrossRef]
- Althabaiti, S.A.; Khan, Z.; Narasimharao, K.; Bawaked, S.M.; Al-Sheheri, S.Z.; Mokhtar, M.; Malik, M.A. Selective Thermal and Photocatalytic Decomposition of Aqueous Hydrazine to Produce H2 over Ag-Modified TiO2 Nanomaterial. Nanomaterials 2023, 13, 2076. [Google Scholar] [CrossRef]
- Hren, R.; Vujanović, A.; Van Fan, Y.; Klemeš, J.J.; Krajnc, D.; Čuček, L. Hydrogen Production, Storage and Transport for Renewable Energy and Chemicals: An Environmental Footprint Assessment. Renew. Sustain. Energy Rev. 2023, 173, 113113. [Google Scholar] [CrossRef]
- Aydin, M.I.; Dincer, I. A Life Cycle Impact Analysis of Various Hydrogen Production Methods for Public Transportation Sector. Int. J. Hydrogen Energy 2022, 47, 39666–39677. [Google Scholar] [CrossRef]
- Tan, K.C.; Chua, Y.S.; He, T.; Chen, P. Strategies of Thermodynamic Alternation on Organic Hydrogen Carriers for Hydrogen Storage Application: A Review. Green Energy Resour. 2023, 1, 100020. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Xia, X.; Zhou, H.; Zhang, Y.; Jiang, H. Innovative Steam Methane Reforming for Coproducing CO-Free Hydrogen and Syngas in Proton Conducting Membrane Reactor. AIChE J. 2019, 65, e16740. [Google Scholar] [CrossRef]
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative Assessment of Blue Hydrogen from Steam Methane Reforming, Autothermal Reforming, and Natural Gas Decomposition Technologies for Natural Gas-Producing Regions. Energy Convers. Manag. 2022, 254, 115245. [Google Scholar] [CrossRef]
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 07, 107–154. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Ugbeh-Johnson, J.; Okeke, N.E.; Ogbonnaya, C. Present and Projected Developments in Hydrogen Production: A Technological Review∗. Carbon Capture Sci. Technol. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Susmozas, A.; Iribarren, D.; Dufour, J. Life-Cycle Performance of Indirect Biomass Gasification as a Green Alternative to Steam Methane Reforming for Hydrogen Production. Int. J. Hydrogen Energy 2013, 38, 9961–9972. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Oko, E.; Kuang, B.; Bello, A.; Onwualu, A.P.; Oyagha, S.; Whidborne, J. The Prospects of Hydrogen in Achieving Net Zero Emissions by 2050: A Critical Review. Sustain. Chem. Clim. Action 2023, 2, 100024. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Cuihua Xuebao/Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Folgado, F.J.; González, I.; Calderón, A.J. Simulation Platform for the Assessment of PEM Electrolyzer Models Oriented to Implement Digital Replicas. Energy Convers. Manag. 2022, 267, 115917. [Google Scholar] [CrossRef]
- Ma, Z.; Witteman, L.; Wrubel, J.A.; Bender, G. A Comprehensive Modeling Method for Proton Exchange Membrane Electrolyzer Development. Int. J. Hydrogen Energy 2021, 46, 17627–17643. [Google Scholar] [CrossRef]
- Zhou, Z.; Zholobko, O.; Wu, X.F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2021, 14, 135. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. Environmental Impact Assessment of Hydrogen Production via Steam Methane Reforming Based on Emissions Data. Energy Rep. 2022, 8, 13585–13595. [Google Scholar] [CrossRef]
- Ursúa, A.; Gandía, L.M.; Sanchis, P. Hydrogen Production from Water Electrolysis: Current Status and Future Trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Galyamin, D.; Torrero, J.; Elliott, J.D.; Rodríguez-García, I.; Sánchez, D.G.; Salam, M.A.; Gago, A.S.; Mokhtar, M.; Gómez de la Fuente, J.L.; Bueno, S.V.; et al. Insights into the High Activity of Ruthenium Phosphide for the Production of Hydrogen in Proton Exchange Membrane Water Electrolyzers. Adv. Energy Sustain. Res. 2023, 4, 2300059. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. Recent Advances in Hydrogen Production through Proton Exchange Membrane Water Electrolysis—A Review. Sustain. Energy Fuels 2023, 7, 3560–3583. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Zamfirescu, C.; Gabriel, K.S. Analysis of Copper Chlorine Electrolysis for Large-Scale Hydrogen Production. Int. J. Hydrogen Energy 2022, 48, 22720–22733. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, T.; Bauer, C.; McKenna, R.; Mazzotti, M. Large-Scale Hydrogen Production via Water Electrolysis: A Techno-Economic and Environmental Assessment. Energy Environ. Sci. 2022, 15, 3583–3602. [Google Scholar] [CrossRef]
- Tashie-Lewis, B.C.; Nnabuife, S.G. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. Chem. Eng. J. Adv. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Tahir, F.; Saeed, M.A.; Ali, U. Biomass Energy Perspective in Pakistan Based on Chemical Looping Gasification for Hydrogen Production and Power Generation. Int. J. Hydrogen Energy 2023, 48, 18211–18232. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and Evaluation of Hydrogen Production Options for Better Environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical Cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Razi, F.; Hewage, K.; Sadiq, R. A Comparative Assessment of Thermodynamic and Exergoeconomic Performances of Three Thermochemical Water-Splitting Cycles of Chlorine Family for Hydrogen Production. Energy Convers. Manag. 2022, 271, 116313. [Google Scholar] [CrossRef]
- Su, B.; Wang, Y.; Xu, Z.; Han, W.; Jin, H.; Wang, H. Novel Ways for Hydrogen Production Based on Methane Steam and Dry Reforming Integrated with Carbon Capture. Energy Convers. Manag. 2022, 270, 116199. [Google Scholar] [CrossRef]
- Andrews, J.W. Hydrogen Production and Carbon Sequestration by Steam Methane Reforming and Fracking with Carbon Dioxide. Int. J. Hydrogen Energy 2020, 45, 9279–9284. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, H.; He, H.; Wei, W. Efficiency and Consistency Enhancement for Alkaline Electrolyzers Driven by Renewable Energy Sources. Commun. Eng. 2023, 2, 22. [Google Scholar] [CrossRef]
- Li, D.; Motz, A.R.; Bae, C.; Fujimoto, C.; Yang, G.; Zhang, F.Y.; Ayers, K.E.; Kim, Y.S. Durability of Anion Exchange Membrane Water Electrolyzers. Energy Environ. Sci. 2021, 14, 3393–3419. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and Innovative Solid Oxide Electrolysis Cell Materials: A Short Review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Nunes, L.J.R. Biomass Gasification as an Industrial Process with Effective Proof-of-Concept: A Comprehensive Review on Technologies, Processes and Future Developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Erdiwansyah; Gani, A.; Zaki, M.; Mamat, R.; Nizar, M.; Rosdi, S.M.; Yana, S.; Sarjono, R.E. Analysis of Technological Developments and Potential of Biomass Gasification as a Viable Industrial Process: A Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100439. [Google Scholar] [CrossRef]
- Yu, J.M.; Lee, J.; Kim, Y.S.; Song, J.; Oh, J.; Lee, S.M.; Jeong, M.; Kim, Y.; Kwak, J.H.; Cho, S.; et al. High-Performance and Stable Photoelectrochemical Water Splitting Cell with Organic-Photoactive-Layer-Based Photoanode. Nat. Commun. 2020, 11, 5509. [Google Scholar] [CrossRef]
- Kumar, M.; Meena, B.; Subramanyam, P.; Suryakala, D.; Subrahmanyam, C. Recent Trends in Photoelectrochemical Water Splitting: The Role of Cocatalysts. NPG Asia Mater. 2022, 14, 88. [Google Scholar] [CrossRef]
- Oudejans, D.; Offidani, M.; Constantinou, A.; Albonetti, S.; Dimitratos, N.; Bansode, A. Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies 2022, 15, 3044. [Google Scholar] [CrossRef]
- Wexler, R.B.; Stechel, E.B.; Carter, E.A. Materials Design Directions for Solar Thermochemical Water Splitting. In Solar Fuels; Wiley: Hoboken, NJ, USA, 2023; pp. 1–63. [Google Scholar]
- Lee, J.E.; Shafiq, I.; Hussain, M.; Lam, S.S.; Rhee, G.H.; Park, Y.K. A Review on Integrated Thermochemical Hydrogen Production from Water. Int. J. Hydrogen Energy 2022, 47, 4346–4356. [Google Scholar] [CrossRef]
- Derwent, R.G. Global Warming Potential (GWP) for Hydrogen: Sensitivities, Uncertainties and Meta-Analysis. Int. J. Hydrogen Energy 2023, 48, 8328–8341. [Google Scholar] [CrossRef]
- Althabaiti, S.A.; Khan, Z.; Malik, M.A.; Bawaked, S.M.; Al-Sheheri, S.Z.; Mokhtar, M.; Siddiqui, S.I.; Narasimharao, K. Biomass-Derived Carbon Deposited TiO2 Nanotube Photocatalysts for Enhanced Hydrogen Production. Nanoscale Adv. 2023, 5, 3671–3683. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, K.; Yang, B.; Liu, F.; Weng, Y.; Liu, Z.; Fang, Y. Life Cycle Analysis of a Coal to Hydrogen Process Based on Ash Agglomerating Fluidized Bed Gasification. Energy 2019, 174, 638–646. [Google Scholar] [CrossRef]
- Verhoef, L.A.; Budde, B.W.; Chockalingam, C.; García Nodar, B.; van Wijk, A.J.M. The Effect of Additive Manufacturing on Global Energy Demand: An Assessment Using a Bottom-up Approach. Energy Policy 2018, 112, 349–360. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of Hydrogen Safety during Storage, Transmission, and Applications Processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Davis, M.; Okunlola, A.; Di Lullo, G.; Giwa, T.; Kumar, A. Greenhouse Gas Reduction Potential and Cost-Effectiveness of Economy-Wide Hydrogen-Natural Gas Blending for Energy End Uses. Renew. Sustain. Energy Rev. 2023, 171, 112962. [Google Scholar] [CrossRef]
- Anika, O.C.; Nnabuife, S.G.; Bello, A.; Okoroafor, E.R.; Kuang, B.; Villa, R. Prospects of Low and Zero-Carbon Renewable Fuels in 1.5-Degree Net Zero Emission Actualisation by 2050: A Critical Review. Carbon Capture Sci. Technol. 2022, 5, 100072. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, S.; Liu, R.E.; Roda-Stuart, D.J.; Ravikumar, A.P.; Bradley, A.; Masnadi, M.S.; Brandt, A.R.; Bergerson, J.; Bi, X.T. Greenhouse-Gas Emissions of Canadian Liquefied Natural Gas for Use in China: Comparison and Synthesis of Three Independent Life Cycle Assessments. J. Clean. Prod. 2020, 258, 120701. [Google Scholar] [CrossRef]
- Bradbury, J.; Clement, Z.; Down, A. Greenhouse Gas Emissions and Fuel Use within the Natural Gas Supply Chain—Sankey Diagram Methodology; Special Report; US Department of Energy: Washington, DC, USA, 2015; pp. 1–22.
- Sun, P.; Young, B.; Elgowainy, A.; Lu, Z.; Wang, M.; Morelli, B.; Hawkins, T. Criteria Air Pollutants and Greenhouse Gas Emissions from Hydrogen Production in U.S. Steam Methane Reforming Facilities. Environ. Sci. Technol. 2019, 53, 7103–7113. [Google Scholar] [CrossRef]
- Li, G.; Cui, P.; Wang, Y.; Liu, Z.; Zhu, Z.; Yang, S. Life Cycle Energy Consumption and GHG Emissions of Biomass-to-Hydrogen Process in Comparison with Coal-to-Hydrogen Process. Energy 2020, 191, 116588. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Singh, K.K.; Bhanja, K.; Grover, R.B. Assessing Techno-Economic Uncertainties in Nuclear Power-to-X Processes: The Case of Nuclear Hydrogen Production via Water Electrolysis. Int. J. Hydrogen Energy 2023, 48, 14149–14169. [Google Scholar] [CrossRef]
- Cho, H.H.; Strezov, V.; Evans, T.J. A Review on Global Warming Potential, Challenges and Opportunities of Renewable Hydrogen Production Technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar] [CrossRef]
- Nasser, M.; Megahed, T.F.; Ookawara, S.; Hassan, H. A Review of Water Electrolysis–Based Systems for Hydrogen Production Using Hybrid/Solar/Wind Energy Systems. Environ. Sci. Pollut. Res. 2022, 29, 86994–87018. [Google Scholar] [CrossRef] [PubMed]
- Suleman, F.; Dincer, I.; Agelin-Chaab, M. Comparative Impact Assessment Study of Various Hydrogen Production Methods in Terms of Emissions. Int. J. Hydrogen Energy 2016, 41, 8364–8375. [Google Scholar] [CrossRef]
- Maduta, C.; Melica, G.; D’Agostino, D.; Bertoldi, P. Towards a Decarbonised Building Stock by 2050: The Meaning and the Role of Zero Emission Buildings (ZEBs) in Europe. Energy Strategy Rev. 2022, 44, 101009. [Google Scholar] [CrossRef]
- Jin, Y.; Behrens, P.; Tukker, A.; Scherer, L. Water Use of Electricity Technologies: A Global Meta-Analysis. Renew. Sustain. Energy Rev. 2019, 115, 109391. [Google Scholar] [CrossRef]
- Kegler, S.R.; Simon, T.R.; Zwald, M.L.; Chen, M.S.; Mercy, J.A.; Jones, C.M.; Mercado-Crespo, M.C.; Blair, J.M.; Stone, D.M.; Ottley, P.G.; et al. Vital Signs: Changes in Firearm Homicide and Suicide Rates—United States, 2019–2020. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 656–663. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Beswick, R.R.; Yan, Y. A Green Hydrogen Economy for a Renewable Energy Society. Curr. Opin. Chem. Eng. 2021, 33, 100701. [Google Scholar] [CrossRef]
- Winter, L.R.; Cooper, N.J.; Lee, B.; Patel, S.K.; Wang, L.; Elimelech, M. Mining Nontraditional Water Sources for a Distributed Hydrogen Economy. Environ. Sci. Technol. 2022, 56, 10577–10585. [Google Scholar] [CrossRef]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Yildiz, B.; Kazimi, M.S. Efficiency of Hydrogen Production Systems Using Alternative Nuclear Energy Technologies. Int. J. Hydrogen Energy 2006, 31, 77–92. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2014, 40, 11094–11111. [Google Scholar] [CrossRef]
- Ghorbani, B.; Zendehboudi, S.; Afrouzi, Z.A. Multi-Objective Optimization of an Innovative Integrated System for Production and Storage of Hydrogen with Net-Zero Carbon Emissions. Energy Convers. Manag. 2023, 276, 116506. [Google Scholar] [CrossRef]
- Qi, X.; Kochan, O.; Ma, Z.; Siarry, P.; Królczyk, G.; Li, Z. Energy, Exergy, Exergoeconomic and Exergoenvironmental Analyses of a Hybrid Renewable Energy System with Hydrogen Fuel Cells. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Abánades, A. Comparative Analysis of Energy and Exergy Performance of Hydrogen Production Methods. Entropy 2020, 22, 1286. [Google Scholar] [CrossRef]
- Naeiji, E.; Noorpoor, A.; Ghanavati, H. Energy, Exergy, and Economic Analysis of Cryogenic Distillation and Chemical Scrubbing for Biogas Upgrading and Hydrogen Production. Sustainability 2022, 14, 3686. [Google Scholar] [CrossRef]
- Omidvar, M.R.; Khanmohammadi, S.; Shababi, Z.; Kumar, R. Performance Assessment and Exergy Analysis of Hydrogen Production from Natural Gas in a Petrochemical Unit (A Real Case Study). Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Dincer, I.; Abu-Rayash, A. Chapter 6—Sustainability Modeling. In Energy Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–164. ISBN 978-0-12-819556-7. [Google Scholar]
- Van Ruijven, B.; Lamarque, J.F.; Van Vuuren, D.P.; Kram, T.; Eerens, H. Emission Scenarios for a Global Hydrogen Economy and the Consequences for Global Air Pollution. Glob. Environ. Chang. 2011, 21, 983–994. [Google Scholar] [CrossRef]
- Yang, B.; Mane, M.; Crossley, W.A. An Approach to Evaluate Fleet Level CO2 Impact of Introducing Liquid Hydrogen Aircraft to a World-Wide Network. In Proceedings of the 2022 AIAA Aviation and Aeronautics Forum and Exposition (AIAA AVIATION Forum), Chicago, IL, USA, 27 June–1 July 2022. Virtual. [Google Scholar]
- Acar, C.; Beskese, A.; Temur, G.T. Sustainability Analysis of Different Hydrogen Production Options Using Hesitant Fuzzy AHP. Int. J. Hydrogen Energy 2018, 43, 18059–18076. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Life Cycle Sustainability Assessment of Hydrogen from Biomass Gasification: A Comparison with Conventional Hydrogen. Int. J. Hydrogen Energy 2019, 44, 21193–21203. [Google Scholar] [CrossRef]
- Honnery, D.; Moriarty, P. Estimating Global Hydrogen Production from Wind. Int. J. Hydrogen Energy 2009, 34, 727–736. [Google Scholar] [CrossRef]
- Karaca, A.E.; Dincer, I.; Gu, J. Life Cycle Assessment Study on Nuclear Based Sustainable Hydrogen Production Options. Int. J. Hydrogen Energy 2020, 45, 22148–22159. [Google Scholar] [CrossRef]
- Mehmeti, A.; Angelis-Dimakis, A.; Arampatzis, G.; McPhail, S.J.; Ulgiati, S. Life Cycle Assessment and Water Footprint of Hydrogen Production Methods: From Conventional to Emerging Technologies. Environments 2018, 5, 24. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W. Comparative Life Cycle Energy Consumption, Carbon Emissions and Economic Costs of Hydrogen Production from Coke Oven Gas and Coal Gasification. Int. J. Hydrogen Energy 2020, 45, 27979–27993. [Google Scholar] [CrossRef]
- Onwuemezie, L.; Gohari Darabkhani, H.; Moghimi Ardekani, M. Integrated Solar-Driven Hydrogen Generation by Pyrolysis and Electrolysis Coupled with Carbon Capture and Rankine Cycle. Energy Convers. Manag. 2023, 277, 116641. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Wang, L.; Zhang, P.; Chen, S. Preparation and Characterization of Hollow Carbon Sphere Supported Catalysts (M@HCS [M=Pt, Ir, Ni]) for HI Decomposition in the Iodine–Sulfur Cycle for Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 14270–14276. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jianu, O.A. Novel Thermoelectric Generator Heat Exchanger for Indirect Heat Recovery from Molten CuCl in the Thermochemical Cu–Cl Cycle of Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 5001–5017. [Google Scholar] [CrossRef]
- Mei, D.; Qiu, X.; Liu, H.; Wu, Q.; Yu, S.; Xu, L.; Zuo, T.; Wang, Y. Progress on Methanol Reforming Technologies for Highly Efficient Hydrogen Production and Applications. Int. J. Hydrogen Energy 2022, 47, 35757–35777. [Google Scholar] [CrossRef]
- Chen, W.H.; Biswas, P.P.; Ubando, A.T.; Park, Y.K.; Ashokkumar, V.; Chang, J.S. Design of Experiment for Hydrogen Production from Ethanol Reforming: A State-of-the-Art Review. Fuel 2023, 342, 127871. [Google Scholar] [CrossRef]
- Khojasteh Salkuyeh, Y.; Saville, B.A.; MacLean, H.L. Techno-Economic Analysis and Life Cycle Assessment of Hydrogen Production from Natural Gas Using Current and Emerging Technologies. Int. J. Hydrogen Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- Sharma, M.; Kaushik, A. Biohydrogen Economy: Challenges and Prospects for Commercialization. In Biohydrogen Production: Sustainability of Current Technology and Future Perspective; Springer: Berlin/Heidelberg, Germany, 2016; pp. 253–267. ISBN 9788132235774. [Google Scholar]
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Md. Jahim, J. Biohydrogen Production through Photo Fermentation or Dark Fermentation Using Waste as a Substrate: Overview, Economics, and Future Prospects of Hydrogen Usage. Biofuels Bioprod. Biorefining 2013, 7, 334–352. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Rosen, M.A.; Koohi-Fayegh, S. The Prospects for Hydrogen as an Energy Carrier: An Overview of Hydrogen Energy and Hydrogen Energy Systems. Energy Ecol. Environ. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, F. Photocatalytic Water Splitting for Hydrogen Production. Acta Chim. Sin. 2022, 80, 827–838. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Singh, K.K.; Grover, R.B.; Bhanja, K. Nuclear Hydrogen Production for Industrial Decarbonization: Creating the Business Case for the near Term. Int. J. Energy Res. 2022, 46, 6929–6943. [Google Scholar] [CrossRef]
- Balat, M. Potential Importance of Hydrogen as a Future Solution to Environmental and Transportation Problems. Int. J. Hydrogen Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Lata, D.B.; Misra, A. Theoretical and Experimental Investigations on the Performance of Dual Fuel Diesel Engine with Hydrogen and LPG as Secondary Fuels. Int. J. Hydrogen Energy 2010, 35, 11918–11931. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Beasy, K.; Lodewyckx, S.; Mattila, P. Industry Perceptions and Community Perspectives on Advancing a Hydrogen Economy in Australia. Int. J. Hydrogen Energy 2023, 48, 8386–8397. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Zou, X.; Li, S.; Shen, H.; Jian, J. Energy Taxes, Carbon Dioxide Emissions, Energy Consumption and Economic Consequences: A Comparative Study of Nordic and G7 Countries. Sustainability 2019, 11, 6100. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of Hydrogen Storage Techniques for on Board Vehicle Applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Chanchetti, L.F.; Leiva, D.R.; Lopes de Faria, L.I.; Ishikawa, T.T. A Scientometric Review of Research in Hydrogen Storage Materials. Int. J. Hydrogen Energy 2020, 45, 5356–5366. [Google Scholar] [CrossRef]
- Chantre, C.; Andrade Eliziário, S.; Pradelle, F.; Católico, A.C.; Branquinho Das Dores, A.M.; Torres Serra, E.; Campello Tucunduva, R.; Botelho Pimenta Cantarino, V.; Leal Braga, S. Hydrogen Economy Development in Brazil: An Analysis of Stakeholders’ Perception. Sustain. Prod. Consum. 2022, 34, 26–41. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Ozarslan, A. Large-Scale Hydrogen Energy Storage in Salt Caverns. Int. J. Hydrogen Energy 2012, 37, 14265–14277. [Google Scholar] [CrossRef]
- Laadel, N.E.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation Barriers for Hydrogen Embrittlement Prevention in Metals—A Review on Mechanisms, Materials Suitability and Efficiency. Int. J. Hydrogen Energy 2022, 47, 32707–32731. [Google Scholar] [CrossRef]
- Gahleitner, G. Hydrogen from Renewable Electricity: An International Review of Power-to-Gas Pilot Plants for Stationary Applications. Int. J. Hydrogen Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Parra, D.; Valverde, L.; Pino, F.J.; Patel, M.K. A Review on the Role, Cost and Value of Hydrogen Energy Systems for Deep Decarbonisation. Renew. Sustain. Energy Rev. 2019, 101, 279–294. [Google Scholar] [CrossRef]
- Chandrakumar, K.R.S.; Ghosh, S.K. Alkali-Metal-Induced Enhancement of Hydrogen Adsorption in C60 Fullerene: An Ab Initio Study. Nano Lett. 2008, 8, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Feaver, A.; Sepehri, S.; Shamberger, P.; Stowe, A.; Autrey, T.; Cao, G. Coherent Carbon Cryogel-Ammonia Borane Nanocomposites for H2 Storage. J. Phys. Chem. B 2007, 111, 7469–7472. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A Review on the Current Progress of Metal Hydrides Material for Solid-State Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Ohaeri, E.; Eduok, U.; Szpunar, J. Hydrogen Related Degradation in Pipeline Steel: A Review. Int. J. Hydrogen Energy 2018, 43, 14584–14617. [Google Scholar] [CrossRef]
- Guo, L.; Su, J.; Wang, Z.; Shi, J.; Guan, X.; Cao, W.; Ou, Z. Hydrogen Safety: An Obstacle That Must Be Overcome on the Road towards Future Hydrogen Economy. Int. J. Hydrogen Energy 2023. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Liu, Y.; Shao, Y.; Nan, Z.; Teng, L.; Peng, W.; Bian, J. Safety of Hydrogen Storage and Transportation: An Overview on Mechanisms, Techniques, and Challenges. Energy Rep. 2022, 8, 6258–6269. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Zhang, R.; Li, X.; Xie, J.; Chen, J. Numerical Investigation of the Leakage and Explosion Scenarios in China’s First Liquid Hydrogen Refueling Station. Int. J. Hydrogen Energy 2022, 47, 18786–18798. [Google Scholar] [CrossRef]


| Source | Studies | Results |
|---|---|---|
| [9] | The environmental impacts associated with different hydrogen-generating processes are analysed and compared. The techniques under consideration are classified according to their energy sources, which include renewables and fossil fuels. Steam methane reforming (SMR) of natural gas was investigated for the synthesis of hydrogen from fossil fuels. Electrolysis utilising the sodium chloride cycle is one method of producing hydrogen from renewable sources. Electrolytic hydrogen generation is also compared using several types of cells, including diaphragm, membrane, and mercury cells. | According to the results of the environmental implications of the hydrogen generation methods, SMR of natural gas exhibits the greatest adverse effects in terms of global warming potential, abiotic depletion, and other impact categories. The abiotic depletion for SMR is 0.131 kg Sb eq, which is the highest among all technologies, including renewable hydrogen generation. The electrolysis utilising the mercury cell produces the second highest abiotic depletion value, 0.00786 kg Sb eq. |
| [10] | The study evaluates the life cycle of a newly created photoelectrochemical reactor’s hydrogen-producing process. The proposed hydrogen photoelectrochemical generation system was the subject of extensive research for a cradle-to-gate life cycle evaluation. | The suggested photoelectrochemical cell’s ability to create hydrogen is estimated to have a global warming potential of 1.052 kg of CO2 equivalent per kilogramme of produced hydrogen. According to the results of the normalised comparative life cycle evaluation, the PEC-based hydrogen generation method is the most sustainable choice among the paths taken into consideration. |
| [5] | This study was primarily concerned with analysing the exergoenvironmental effects of the magnesium–chlorine, copper–chlorine, and iron–chlorine thermochemical hydrogen production processes. An exergoenvironmental comparison of the three processes was carried out in this work. Based on exergy destruction environmental impact rates, cumulative environmental impact rates, component-related environmental impact rates, and exergoenvironmental variables, the effectiveness of the different methods was evaluated. | The findings imply that, in comparison to the environmental effect rates associated with the components of all activities, the rates of energy destruction are generally significantly greater. For all thermochemical cycles taken into consideration, the hydrolysis phase also produces the greatest component-associated environmental impact rate. Additionally, of the three cycles, the iron–chlorine cycle has the largest component-related environmental effect rate, whereas the magnesium–chlorine cycle results in the highest rate of energy destruction. Additionally, for a number of electrical sources, the magnesium—chlorine cycle has a considerably larger global warming potential than the copper–chlorine cycle. |
| [3] | The integrated solar Cu–Cl fuel production plant for large-scale hydrogen generation is investigated here using the life cycle assessment (LCA) approach. The effects of altering key input factors, such as plant lifespan, radiation level, and solar-to-hydrogen efficiency, on a variety of environmental effects are then examined. | Results compared with earlier thermochemical-based research reveal that the new integrated system’s GWP is 7% lower than that of a solar sulphur–iodine thermochemical cycle. |
| [11] | The pros and cons of various H2 generation systems are thoroughly reviewed in the study. Additionally, the research aimed to analyse the economic aspects of each approach as well as the function of nanotechnology in the manufacturing of H2. | According to the review study, steam reforming of natural gas has been identified as the most effective method of producing hydrogen due to its excellent performance in producing hydrogen (70–85%), low capital (USD 3.4 M), and production costs (USD 2.42 M/kg). |
| Technologies | Process Phase/Unit | Main Sources of Emissions | Alternative Sources of Emissions |
|---|---|---|---|
| Electrolysis | production | Electricity for the electrolyser unit | Steam, solid, liquid, and gaseous fuel combustion for the production of steam |
| cooling, compression, drying, and purification | Units’ electricity | Steam production, liquid, solid, and units’ combustions of gaseous fuel | |
| Autothermal reforming (ATR) with CCS | Natural gas (NG) recovery | /methane from transport and extraction of NG | Venting and flaring |
| compression and transportation | emissions | ||
| Separation of air | air to feed reformer | ||
| Biomass/CCS | Gasification | Burning of dry biomass inside the biogenic (gasifier) | |
| Transportation of biomass materials | Leakage of biomethane. Electricity or combustion of liquid fuel for feedstocks movement | ||
| Feedstock (organic) processing | Fuel or electricity usage for the movement, extraction, and treatment of the feedstocks | ||
| storage | The electricity for transformation or injection | Escaped carbon dioxide from a storage permanent area | |
| storage and compression | Storage and compression electricity | ||
| SMR/CCS | enrichment | enrichment | Venting and flaring |
| storage and compression | Electricity for storage and compression maintenance | H2 | |
| Gasification of Coal/CCS | Coal processing and mining | Electricity/combustion of liquid fuel for materials movement and extraction | Explosives used in the mining of coal |
| Coal processing | Electricity for unloading and loading of coal | Chemical deployment for coal handling | |
| Gasification | Burning of coal inside the gasifier | ||
| storage and compression | Electricity for storage and compression maintenance |
| Production Process | Water Consumption Factor (gal/mmBtu of H2) |
|---|---|
| Central SMR | 27.2–31.6 |
| Forecourt SMR | 50.9 |
| Central electrolysis | 70.2 |
| Forecourt electrolysis | 59.6 |
| Biomass gasification | 38.1 |
| Production Technique | Energy Efficiency |
|---|---|
| Electrolysis | 5.30 |
| Biomass gasification | 6.50 |
| Photoelectrochemical method | 0.70 |
| Thermochemical water splitting | 4.20 |
| Compounds | Standard Mole Chemical Exergy (kJ/mol) |
|---|---|
| O2 | 3.87 |
| H2O | 9.50 |
| CO2 | 19.87 |
| H2 | 236.10 |
| CO | 275.10 |
| C (s) | 410.00 |
| CH4 | 831.65 |
| Autothermal reforming of methane | 10.92 | 11.66 | 0.75 | 89.08 |
| Electrolysis | 12.08 | 12.81 | 0.73 | 87.92 |
| Steam reforming of methane | 21.13 | 21.39 | 0.26 | 78.87 |
| Partial oxidation of methane | 41.65 | 47.16 | 5.51 | 58.35 |
| Coal gasification | 49.08 | 55.55 | 6.45 | 50.92 |
| Dry reforming of methane | 52.03 | 53.50 | 1.46 | 47.97 |
| Natural gas pyrolysis | 53.12 | 93.82 | 40.70 | 46.88 |
| Pollutants | Average Emissions Kg/KgH2 (Std) |
|---|---|
| CO | 0.27 (±1.51) |
| NOX | 1.68 × 10−3 (±3.29 × 10−3) |
| SO2 | 1.00 × 10−4 (±5.46 × 10−4) |
| PM2.5 | 4.44 × 10−4 (±1.53 × 10−3) |
| PM10 | 5.35 × 10−4 (±1.55 × 10−3) |
| VOC | 9.01 × 10−4 (±4.05 × 10−3) |
| Lead | 5.07 × 10−8 (±2.21 × 10−7) |
| Production Process | eq) | eq) | ||||
|---|---|---|---|---|---|---|
| Average | Min. | Max. | Average | Min. | Max. | |
| SMR involving CCS [22] | 3.70 | 3.90 | 3.70 | |||
| SMR [88] | 15.2 | 8.4 | 28.9 | 11.98 | 10.56 | 13.80 |
| Coal Gasification (CG) involving CCS [62] | 4.87 | 4.14 | 7.14 | |||
| CG [89] | 59.7 | 11.0 | 139.0 | 22.99 | 19.42 | 25.28 |
| BG (biomass gasification) [53] | 22.5 | 14.5 | 37.1 | 3.54 | 2.67 | 4.40 |
| Electrolysis (via wind) [53] | 4.3 | 0.2 | 11.8 | 1.08 | 0.03 | 2.21 |
| Electrolysis (via biomass) [53] | 29.0 | 2.70 | 2.40 | 3.00 | ||
| Electrolysis (via solar) [90] | 6.1 | 2.1 | 8.1 | 1.82 | 0.37 | 2.50 |
| Electrolysis (high temp. via nuclear) [63] | 4.4 | 3.4 | 4.8 | 1.24 | 0.42 | 2.00 |
| Sulphur–iodine (S–I) cycle (via nuclear) [91] | 3.4 | 2.4 | 4.3 | 0.64 | 0.41 | 0.86 |
| Copper–chlorine (Cu–Cl) via grid [92] | 91.7 | 76.6 | 99.5 | 14.67 | 12.30 | 15.90 |
| Copper–chlorine (Cu–Cl) via nuclear [32] | 6.2 | 2.8 | 9.6 | 0.92 | 0.56 | 1.35 |
| Methanol reforming [93] | 17.0 | 17.90 | ||||
| Ethanol reforming [94] | 32.0 | 12.20 | ||||
| Methods | Cost of Production (USD per kg) | Source | References |
|---|---|---|---|
| Photo-catalytic splitting | 5.0 | Solar | [100] |
| Steam reforming | 0.75 | Methane | [64] |
| Centralised biomass gasification | 1.2 to 2.4 | Biomass | [85] |
| Gasification without sequestration | 0.92 | Coal | [36] |
| Electrolysis | 2.6 to 3.0 | Nuclear | [34] |
| splitting | 1.4 to 2.3 | Nuclear | [101] |
| Technology | Method of Production | Technology Maturity | Feedstock | Efficiency (%) | Temperature (°C) |
|---|---|---|---|---|---|
| Fossil fuel-based | reforming | Near team | 28.3 | 800–900 | |
| Aqueous reforming | Medium term | Carbohydrate | 35–55 | 220–270 | |
| Plasma reforming | Long term | Hydrocarbon | 9–85 | 900–1300 | |
| Pyrolysis | Near term | - | 51 | 1000–1400 | |
| POX | Commercial | - | 60–75 | 800–1000 | |
| ATR | Near term | - | 700–1000 | ||
| SMR | Commercial | - | 74–85 | ||
| Renewable | Photolysis | Long-term | and sunlight | 0.5 | Ambient |
| Photo-fermentation | - | Sunlight and biomass | 1.9 | - | |
| Dark fermentation | - | Biomass | 60–80 | - | |
| MEC | - | Electricity and biomass | 78 | - | |
| Biomass gasification | Commercial | Biomass | 35–50 | 800–1000 | |
| SOEC | Medium term | and electricity | <110 a | 700–1000 | |
| Alkaline electrolysis | Commercial | 62–82 a | 40–90 | ||
| PEM electrolysis | 20–100 | ||||
| Photo electrolysis/PEC | Long term | 12.4 | Ambient | ||
| splitting | - | 20–45 | 500–1000+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nnabuife, S.G.; Darko, C.K.; Obiako, P.C.; Kuang, B.; Sun, X.; Jenkins, K. A Comparative Analysis of Different Hydrogen Production Methods and Their Environmental Impact. Clean Technol. 2023, 5, 1344-1380. https://doi.org/10.3390/cleantechnol5040067
Nnabuife SG, Darko CK, Obiako PC, Kuang B, Sun X, Jenkins K. A Comparative Analysis of Different Hydrogen Production Methods and Their Environmental Impact. Clean Technologies. 2023; 5(4):1344-1380. https://doi.org/10.3390/cleantechnol5040067
Chicago/Turabian StyleNnabuife, Somtochukwu Godfrey, Caleb Kwasi Darko, Precious Chineze Obiako, Boyu Kuang, Xiaoxiao Sun, and Karl Jenkins. 2023. "A Comparative Analysis of Different Hydrogen Production Methods and Their Environmental Impact" Clean Technologies 5, no. 4: 1344-1380. https://doi.org/10.3390/cleantechnol5040067
APA StyleNnabuife, S. G., Darko, C. K., Obiako, P. C., Kuang, B., Sun, X., & Jenkins, K. (2023). A Comparative Analysis of Different Hydrogen Production Methods and Their Environmental Impact. Clean Technologies, 5(4), 1344-1380. https://doi.org/10.3390/cleantechnol5040067









