1. Introduction
The pathophysiology behind breathlessness with pleural effusions is complex. Significant hypoxaemia with pleural effusions is rare and should be investigated further, rather than pursuing aggressive drainage.
Pleural effusion is the accumulation of fluid in between the visceral and parietal pleura. In animal studies, 0.1 to 0.3 mL per kilogram (mL/kg) of pleural fluid has been found, and similar volumes are thought to exist in healthy human subjects. There are many different causes of pleural effusions, some of which are as follows: heart and renal failure; pneumonia; pleural infection; localised pleural cancers; metastatic disease; intra-abdominal pathologies, such as ascites; ovarian pathologies; pancreatitis; multi-system autoimmune conditions, such as IgG4 disease or rheumatoid arthritis [
1].
The epidemiological understanding of pleural effusions of any kind is increasing, and the trends in the United States have recently been published [
1,
2]. Mummadi et al. [
2] found that, in 2016, there were approximately 43,000 emergency room visits for pleural disease, and that 361,270 hospitalizations occurred; non-malignant pleural effusions accounted for 85.5% of the emergency room visits, alongside 63.5% of hospitalizations and 66.3% of 30-day readmissions. No such detailed evidence exists for the United Kingdom.
The main presenting symptom of pleural effusion is dyspnoea [
3,
4]. Dyspnoea, in itself, is a complex matter, and, by definition, it is the subjective awareness and feeling of not being able to breathe well. Social, physiological and pathological factors are at play, which are beyond the scope of this article. Broadly speaking, afferent signals, efferent signals, and central information processing contribute to dyspnoea [
5]. Afferent signals can come from airway, lung, or chest wall receptors, which are very sensitive to stimuli (for example, lung juxta-capillary receptors are sensitive to interstitial syndromes, and stretch receptors signal bronchoconstriction). Physiological dysregulation, through acid-sensing carotid body and medullary chemoreceptors, also sends afferent signals to the central respiratory drive. Efferent signals then travel to the respiratory muscles, mainly the diaphragm muscle. The specific mechanisms underlying dyspnoea in pleural effusions will be further elaborated.
Pleural effusions usually necessitate drainage, and all acute care physicians will be trained in thoracentesis and intercostal drain insertion. The former will usually be the first line of treatment in an acute medical unit. This intervention will usually have the following three aims: diagnostic (by performing well-established tests of protein, lactate dehydrogenase and glucose levels, as well as cytology and microbiology tests), therapeutic (relief of symptoms, notably dyspnoea), and preventative (in the case of a malignant effusion, pleurodesis might be offered) [
4].
The impact of pleural effusions on breathlessness is not well understood. Effusions do not usually lead to major cardio-respiratory compromise, and the presence of significant hypoxaemia with a pleural effusion (after adequate drainage) should alert clinicians to identify an alternative diagnosis. We describe a case to serve as a reminder of this important lesson. We also summarise the evidence of the lack of hypoxaemia in pleural effusions and recent evidence surrounding this important topic.
2. Case Report
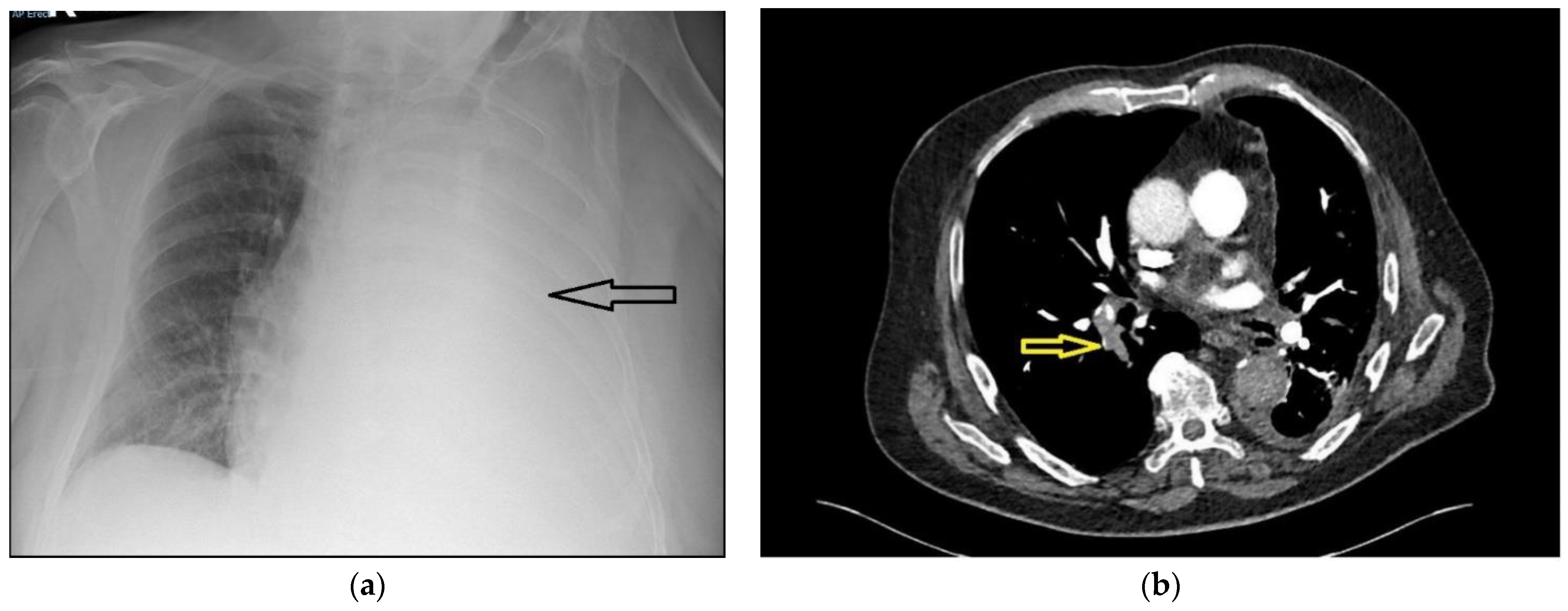
A 75-year-old male presented with a 5-day history of increasing dyspnoea. There were no red flag symptoms, and his past medical history only included treated hypertension. He was a life-long smoker of 62 pack-years. On assessment, he was normotensive, with a respiratory rate of 26 breaths per minute, pulse rate of 100 beats per minute, and saturations of 92% on 60% oxygen, measured using a Venturi mask. The arterial blood gas analysis showed type 1 respiratory failure (partial pressure of oxygen (PaO2) 8.1 Kilopascals, with a normal partial pressure of carbon dioxide (PaCO2)). A chest radiograph showed a large left pleural effusion (
Figure 1a). A thoracic point-of-care ultrasound showed a large echogenic pleural effusion with lung collapse and a flattened diaphragm with normal motion. After discussion with the respiratory team, a small-bore chest tube was inserted under ultrasound guidance, and 4.2 L of blood-stained fluid was drained over the next 12 h. The patient’s respiratory rate became normal, his symptoms improved, and a repeat radiograph showed significant re-expansion of the lung field.
The pleural fluid analysis was consistent with an exudative process (fluid protein count 56 g/litre, plasma protein count 76 g/litre, glucose levels 6.2 millimole/litre, pH 7.35, serum lactate dehydrogenase (LDH) 456 units/litre, fluid LDH 300 units/litre). However, the patient remained persistently hypoxemic, with saturations of 92% on 40% oxygen, measured using a Venturi mask. Further discussion with the pleural team prompted a computed tomography pulmonary angiogram (CTPA) scan. The D-dimer of the patient was 4 milligrams/litre fibrinogen-equivalent units (mg/L FEU). The local reference range for D-dimer levels for patients who are more than 50 years old is calculated by the age of the patient divided by 100. Hence, for this patient, the normal range would be <0.75 mg/L FEU.
Figure 1b shows a slice of the CTPA; this revealed a large right-sided pulmonary embolus and irregular pleural thickening on the left side, consistent with a pleural malignancy. Full-dose anticoagulation with tinzaparin was started and oxygenation improved over the next few days. Bilateral leg ultrasonography was performed and did not show any deep vein thrombosis. The chest drain was removed after cessation of drainage, and the patient was discharged with saturations of 93% on room air. Pleural fluid cytology yielded a predominantly lymphocytic effusion. The tumour cells were diffusely positive for thyroid transcription factor 1 (TTF1), and were consistent with primary lung adenocarcinoma.
3. Discussion
A brief understanding of the underlying pathophysiology helps to understand dyspnoea in pleural effusions.
Pleural effusions can cause dose-related hypoxaemia, which can be brought about by the instillation of saline into porcine pleural spaces. [
6] Eleven pigs were anaesthetised and then mechanically ventilated. Bilateral chest tubes were inserted and increasing numbers of pleural effusions were created by instilling saline. At the smallest pleural effusion, the arterial tension of oxygen was reduced by about 50% compared to baseline. The mechanism underlying this is most likely intrapulmonary shunting, as shown by Augusti et al. in a small group of nine patients with recent-onset pleural effusions of unknown aetiology [
7]. Pleural aspirations drained 693 +/− 424 mL of the fluid, and caused a significant fall in the mean pleural pressure and arterial oxygenation, and the amount of blood flow to the pulmonary circulation increased. Intra-pulmonary shunting also causes an increase in elastance (the pressure required for lung inflation), which, in turn, affects the peripheral mechanoreceptors and increases pleural compliance [
7]. There is some evidence to suggest that, with the development of pleural effusions, expansion of the thoracic cage and downward diaphragmatic displacement occur, which also contribute to the increase in respiratory elastance [
8]. However, it is unclear whether those factors cause significant hypoxaemia. Co-existing pulmonary pathologies, such as cardiac and pulmonary disease, are very common, and will contribute to symptom burden [
9]. Pleural pathologies, such as malignancy or fibrotic peels observed with asbestos-related pleuritis, will also cause extra-thoracic restriction, due to pleural visceral peels and the associated increase in pleural pressures with high pleural space elastance, in the so-called ‘trapped lungs’ [
10].
The above studies were small and not widely applicable, and those questions remained, as well as how dyspnoeic patients with pleural effusions are and whether pleural drainage helps. Psallidas et al. clearly validated patient-reported outcome measures in pleural effusions, and proved that dyspnoea was relieved with pleural drainage in the vast majority of patients studied [
3]. One hundred and fifty-eight patients undergoing pleural interventions were studied, and patient-reported outcome measures, using a Likert scale and a 100 mm visual analogue scale, were collated. Excluding diagnostic aspirations, nearly 86% of the patients benefitted from pleural fluid drainage, with a positive correlation found between the volume of fluid drained and symptom relief. This is corroborated by Muruganadan et al., who performed an elegant study of 145 patients with large, symptomatic pleural effusions, with a median volume drainage of just under 2 L [
11]. The breathlessness scores, assessed by the visual analogue scale, were high, and were improved by drainage. Abnormal diaphragm positions and movement were also implicated. However, it is clear from the study that oxygen saturations are unaffected by pleural drainage, hypoxaemia is not the main culprit in dyspnoea, and that
‘clinicians should consider searching for concurrent/alternative causes of hypoxaemia (if such exist) even in patients with a sizeable effusion’. Taylor et al. also reported another study that enrolled 502 patients undergoing thoracocentesis, which found no clinically significant change in oxygen saturations in the 24 h after a procedure, with an average drainage of 1.4 L [
12].
Thus, significant hypoxaemia with a pleural effusion is unusual. A PubMed search, using the terms (hypoxaemia) AND (pleural effusion) or (hypoxia) AND (pleural effusion), yielded over 500 results, but there were no cases describing alternative causes of hypoxaemia in patients with clinically significant effusions. Anecdotally, aggressive drainage is often pursued in the face of persisting hypoxaemia in a patient with a pleural effusion, which might not be the optimal course to follow. Our case highlights this point quite clearly.