Stress-Induced Transcriptional and Epigenetic Plasticity of Astrocytes, Microglia and Oligodendrocytes in the Pathophysiology of Depression
Abstract
1. Introduction
2. Epigenetic Codes of Glial Identity and CNS Homeostasis
3. Astrocytic Gene Expression Landscape and Remodeling in Depressive Disorders
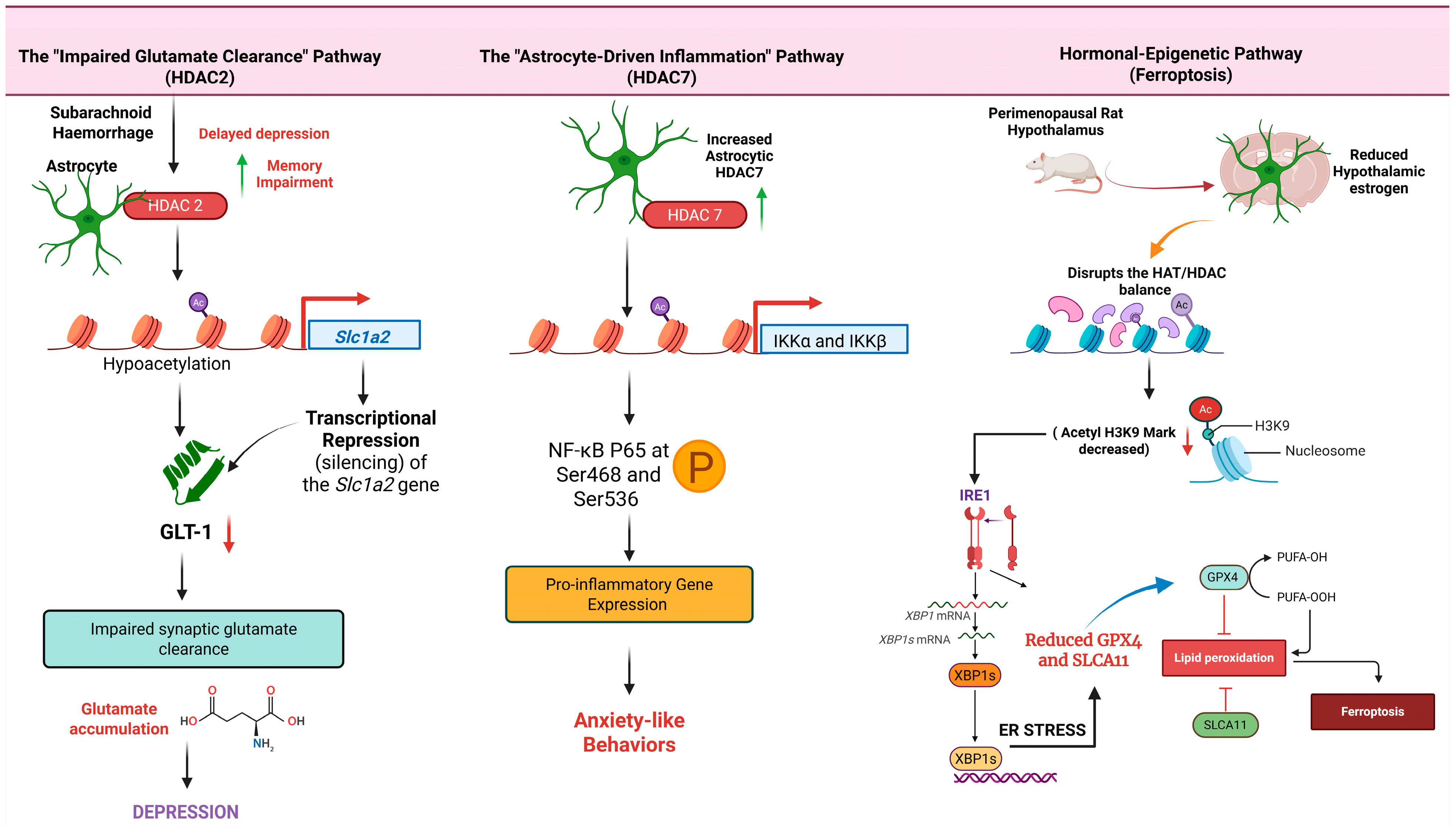
3.1. Histone Acetylation and Deacetylation
| Stress Paradigm/Model | Epigenetic Mechanism | Target Genes/Pathways | Observed Astrocyte Response | Behavioral/Functional Outcome | Therapeutic Implications/Interventions | Study Reference |
|---|---|---|---|---|---|---|
| Subarachnoid Haemorrhage (SAH) Mouse Model | Upregulation of astrocytic HDAC2 leads to histone hypoacetylation at the gene promoter. | Slc1a2 (encodes glutamate transporter GLT-1). | Transcriptional repression of Slc1a2, resulting in reduced GLT-1 protein levels and impaired glutamate homeostasis. | Delayed depression and memory impairment. | Pharmacological inhibition of HDAC2 rescued GLT-1 expression and ameliorated depressive-like behaviors. | [37] |
| Inflammatory Stimulus in Mice | Histone Deacetylation: HDAC7 physically binds to and deacetylates the IKK complex, leading to its activation. | NF-κB signaling pathway. | Activation of NF-κB and subsequent expression of pro-inflammatory genes specifically in astrocytes. | Induction of anxiety-like behaviors. | Implies that targeting HDAC7 could be a potential therapeutic strategy for astrocyte-mediated inflammation. | [39] |
| Chronic Social Defeat Stress (CSDS) Model | Histone Deacetylation (Class III): Activation of Sirtuin 1 (Sirt1), a class III HDAC. | SIRT1/NRF2/HO-1/GPX4 signaling cascade. | Mitigation of oxidative stress and ferroptosis in astrocytes within the hippocampus and medial prefrontal cortex. | Stress-induced astrocyte dysfunction and depressive behaviors. | The compound edaravone was found to mediate antidepressant effects by activating this pathway. | [40] |
| Perimenopausal Depression Rat Model | Histone Acetylation: Reduced estrogen levels disrupt the HAT/HDAC balance, decreasing histone 3 lysine 9 acetylation (acetyl-H3K9). | IRE1α/XBP1 endoplasmic reticulum stress pathway; Downregulation of GPX4 and SLC7A11. | Activation of ER stress in hypothalamic astrocytes, triggering ferroptosis, mitochondrial damage, and collapse of antioxidant defenses. | Depressive-like behaviors associated with perimenopause. | Quercetin was shown to alleviate depressive-like behavior by modulating the acetyl-H3K9 mediated ferroptosis pathway. | [43] |
| Human Post-Mortem MDD Tissue and Mouse Overexpression Model | Chromatin Remodeling: MDD risk variants are enriched in astrocyte open chromatin regions. ZBTB7A acts as a master regulator altering chromatin accessibility. | ZBTB7A and its downstream target genes. | Astrocyte-specific overexpression of ZBTB7A drives widespread changes in chromatin accessibility, promoting a state of stress susceptibility. | Induction of behavioral and molecular signatures of stress susceptibility. | Identifies the transcription factor ZBTB7A as a potential therapeutic target for modulating astrocyte-mediated stress responses. | [44] |
3.2. Histone Methylation
3.3. The Non-Coding Transcriptome and Regulation of Astroglial Responses
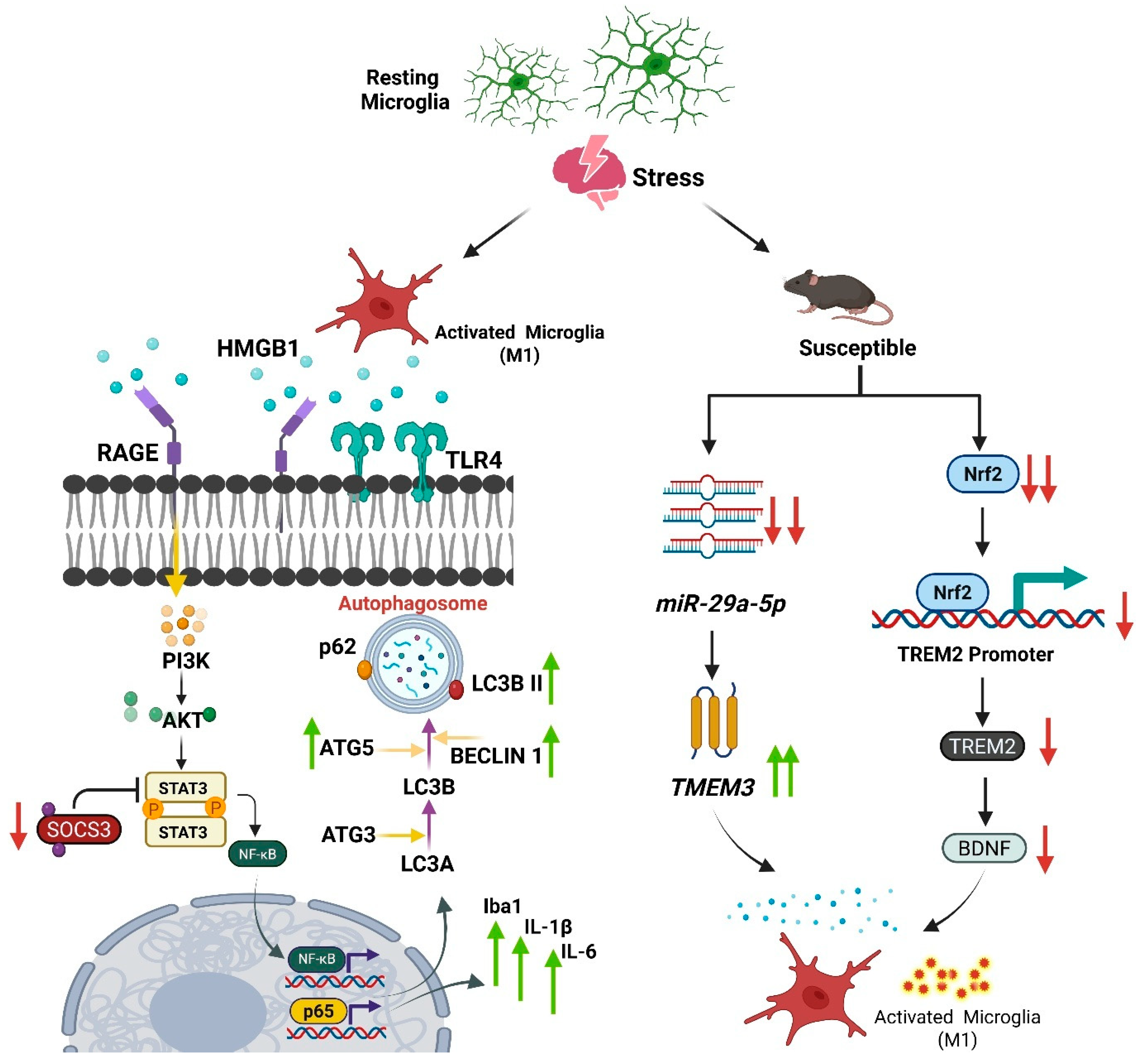
4. The Microglial Response to Stress
4.1. Stress-Induced Changes in Microglial Density, Morphology, and State
4.2. Divergent Microglial Phenotypes in Stress Susceptibility vs. Resilience
4.3. The Concept of Microglial “Priming” as an Epigenetic Memory
4.4. DNA Methylation as a Molecular Imprint of Stress in Microglia
4.5. Microglial Regulation via Histone Modifications and Non-Coding RNAs in Stress
4.5.1. The H3K27me3 Axis: EZH2 vs. Jmjd3
4.5.2. The H3K9me2 Axis: The G9a/GLP Complex
4.6. The Heterogeneous Microglial Transcriptome in Depression
4.7. Therapeutic Implications of Microglial Epigenetic Regulation in Stress
| Stress | Epigenetic Mechanism | Target Genes/Pathways | Observed Microglial Response | Behavioral/Functional Outcome | Therapeutic Implications/Interventions | Study Reference |
|---|---|---|---|---|---|---|
| Chronic Unpredictable Mild Stress (CUMS) in adolescent rats | Histone Demethylation (via KDM6B/JMJD3) | Increased Jmjd3 expression, decreased H3K27me3 levels. Upregulation of pro-inflammatory cytokines (IL-1β, TNF-α). | Microglial activation in the prefrontal cortex (PFC) and hippocampus (HIP). | Depressive-like behaviors (anhedonia, despair). Increased susceptibility to stress in adulthood. | Minocycline (microglial inhibitor) reversed microglial activation, normalized Jmjd3/H3K27me3 levels, and alleviated depressive-like behaviors. | [86] |
| Chronic Unpredictable Mild Stress (CUMS) in C57BL/6 and obese (ob/ob) mice | Histone Demethylation (via JMJD3) | Increased JMJD3 and NF-κB expression; decreased H3K27me3 levels. Increased pro-inflammatory cytokines. Downregulation of adiponectin (APN). | Microglial activation in PFC and HIP; excessive activation in ob/ob mice. | Depressive-like behaviors and memory impairment. Obese mice showed worse behavioral outcomes, suggesting increased susceptibility. | GSK-J4 (selective JMJD3 inhibitor) relieved depressive-like behaviors, memory impairment, microglial activation, and normalized epigenetic/inflammatory markers. | [82] |
| Chronic Unpredictable Mild Stress (CUMS) in mice | DNA Methylation (via DNMT1) | Downregulation of DNA methyltransferase 1 (DNMT1). | Suppression of CUMS-induced microglial activation and inflammatory response. | Reduction in depressive-like behavior (assessed by OFT, SPT, TST). | Nerolidol (a natural sesquiterpene) reduced DNMT1 levels and suppressed microglial activation. | [84] |
| Lipopolysaccharide (LPS)-induced depression model in mice | Histone Deacetylation (via HDAC11) | Inhibition of HDAC11 deacetylase function. | Suppression of LPS-induced microglial activation. Initiation of autophagy and inhibition of nitric oxide (NO) production. | Alleviation of depression-like behavior (reduced immobility, increased sucrose preference). | Novel selective HDAC11 inhibitor (Compound 5) alleviated depressive-like behavior by inhibiting microglial activation. | [83] |
| Human Post-Mortem Brain Tissue (Dorsolateral Prefrontal Cortex) from individuals with Major Depressive Disorder (MDD) | Chromatin Remodeling (Single-nucleus Assay for Transposase-Accessible Chromatin sequencing—snATAC-seq) | Decreased accessibility at binding sites for TFs known to regulate immune homeostasis. Disruption of TF binding sites linked to genes affecting synaptic communication. Key TF mentioned: NR4A2 (activity-dependent, stress-reactive). | A specific gray matter microglia cluster (termed Mic2) exhibited significantly decreased chromatin accessibility at key regulatory regions. | The study directly links these cell type specific epigenetic alterations to the clinical diagnosis of MDD in humans, providing strong evidence for real, measurable brain changes underlying the disorder. | Identification of a specific microglial subtype and regulatory mechanism opens pathways for developing highly targeted therapies | [87] |
| Gut microbiota-dysbiosis mouse model (germ-free mice colonized with microbiota from MDD patients vs. healthy controls). | Non-coding RNAs (lncRNA-miRNA-mRNA networks) | ceRNA networks involving lncRNAs (4930417H01Rik), miRNAs (mmu-miR-883b-3p), and mRNAs (Adcy1, Nr4a2). Axonal guidance and synaptogenesis pathways. | Dysregulation of microglial function via altered inflammatory signaling (IFNG pathway), contributing to decreased neuronal activity (Fos downregulation) | Dysregulated inflammatory response and neurodevelopment in the hippocampus | Identifies specific ncRNA networks in the gut–brain–epigenome axis as potential biomarkers and therapeutic targets for microbiota-based antidepressant strategies. | [88] |
| Chronic unpredictable mild stress (CUMS) | circRNA-mediated regulation | UBE2K (ubiquitin conjugating enzyme) downstream enrichment of TNF, IL-17, cytokine signaling. | Microglia show morphological activation (shorter branches, increased soma size, elevated Iba1 intensity), increased iNOS/CD68 and pro-inflammatory cytokines | Worsened depression-like behaviours; increased neuroinflammation and neuronal/synaptic damage. | Targeting circ-UBE2K (knockdown) or disrupting circ-UBE2K/HNRNPU axis reduced microglial activation and rescued depressive-like phenotypes suggests circRNA or HNRNPU as therapeutic targets/biomarkers | [85] |
| Lipopolysaccharide (LPS) systemic inflammation model in mice. | Histone demethylation: activation of JMJD3 (H3K27me3 demethylase). | TLR4, PI3K, AKT, NF-κB signaling; JMJD3-regulated pro-inflammatory gene transcription. | Microglial overactivation with elevated IL-1β, IL-6, TNF-α; reduced by Rd treatment. | LPS-induced depressive-like behavior with decreased sucrose preference and increased immobility; Rd rescues behavior and synaptic protein expression (PSD-95, SYP). | Ginsenoside Rd reduces JMJD3 expression and suppresses TLR4-PI3K-AKT-NF-κB signaling, leading to decreased microglial activation and behavioral improvement. | [81] |
| Postmortem brain tissue (4 regions) from donors with mood disorders (n = 13) vs. control (n = 8) donors. | DNA Methylation (Genome-wide methylation array) | Differentially Methylated Regions (DMRs) associated with mood disorder status, linked to genes involved in myeloid cell function and neuropsychiatric disorders. | Altered DNA methylation profiles in isolated microglia. Interindividual factors and age showed a larger effect on the methylome than brain regions. | Findings suggest the microglial methylome is highly responsive to individual-specific factors, contributing to cellular heterogeneity. | Identifies mood disorder-associated DMRs in microglia that could serve as biomarkers or targets for epigenetic drugs. | [74] |
5. The Oligodendrocytes: Myelin Plasticity and Vulnerability in Depression
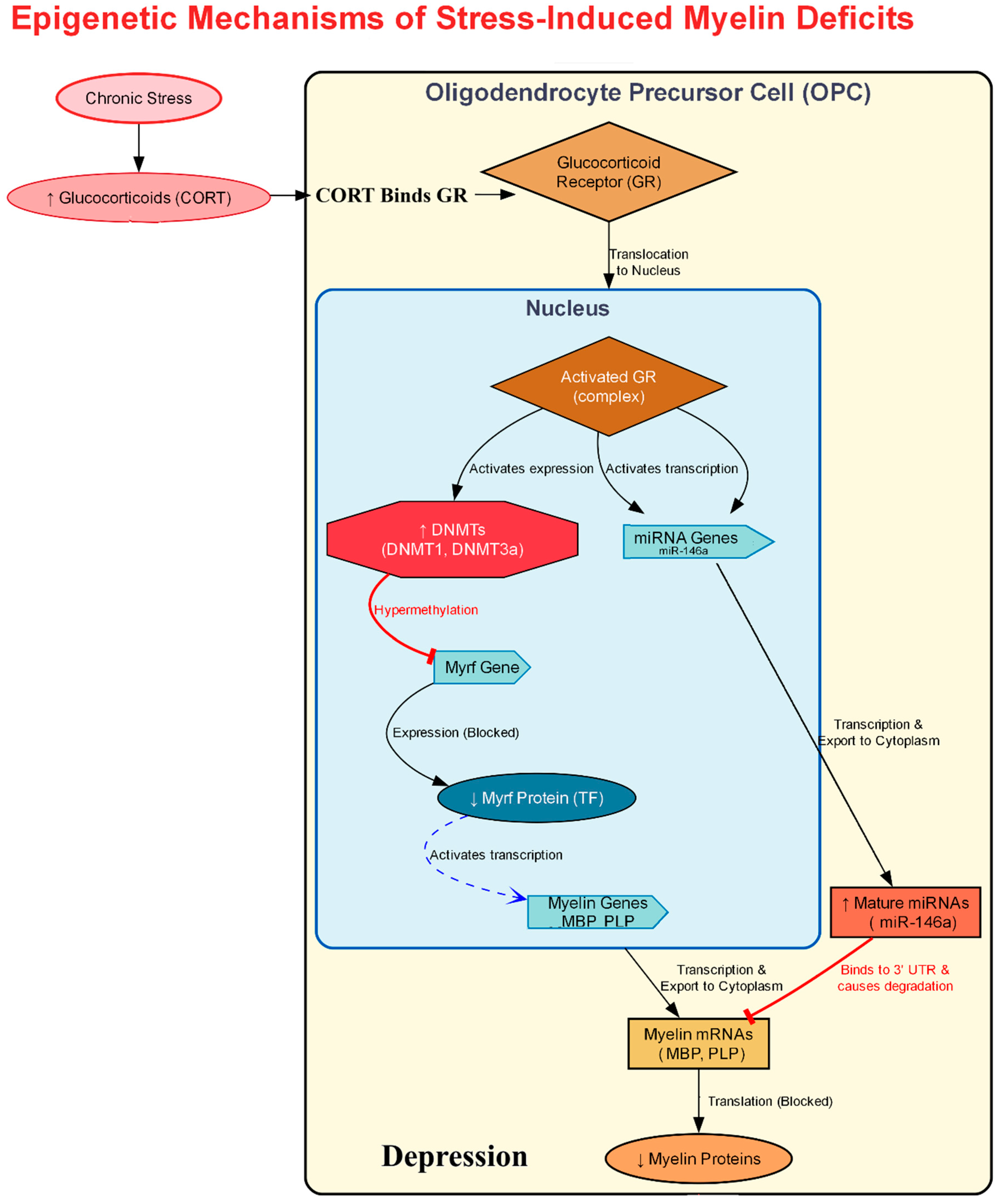
5.1. Transcriptional Control of Myelination and Its Disruption by Stress
5.2. Early-Life Stress Impairs Oligodendrocyte Developmental Trajectories
5.3. Linking Stress to Oligodendrocyte Transcriptional Dysfunction
5.4. MicroRNAs and Long Non-Coding RNAs in Oligodendrocyte Function
| Stress Paradigm/Model | Key Genes/Pathways Affected | Observed Oligodendrocyte Response | Behavioral/Functional Outcome | Study Reference |
|---|---|---|---|---|
| Repeated Social Defeat Stress (RSDS) Mouse Model | Oligodendrocyte Precursor Cells (OPCs) | Profound damage and reduction in the OPC population in the medial prefrontal cortex (mPFC). | Disruption of oligodendrogenesis, which is critical for brain plasticity, learning, and repair. | [92] |
| Major Depressive Disorder (MDD) (Post-mortem human prefrontal cortex) | Altered expression of long non-coding RNA MALAT1 | Greatest transcriptional dysregulation found in immature OPCs; MALAT1 expression in early OPCs (OPC2 stage) has high predictive power for distinguishing MDD cases. | Associated with the clinical diagnosis of MDD. | [119] |
| Human Post-Mortem MDD Tissue | PLP1, CNP, MOG, OLIG1, MOBP | Decreased expression of key genes related to myelin and oligodendrocyte function. MOBP decrease linked to childhood abuse. | Myelin deficits and impaired integrity of white matter tracts are considered core pathological features of MDD. | [93,94] |
| Early-Life Stress (ELS)/Maternal Separation Model | Wnt signaling pathway | An initial, premature differentiation of oligodendrocytes leads to the long-term depletion and exhaustion of the renewable OPC pool in the adult brain. | Impairs secondary development of astrocytes and leads to lasting white matter vulnerability. | [105,106] |
| Chronic Unpredictable Mild Stress (CUMS)/RSDS Models | Myelin Regulatory Factor (Myrf), Methyl-CpG binding protein 2 (Mecp2) | Widespread transcriptomic changes, with Myrf and Mecp2 identified as top upstream regulators linking the stress response to myelination machinery. | Shifts the genetic program away from myelination, contributing to stress-induced aversion and anxiety. | [102] |
| Chronic Unpredictable Mild Stress (CUMS) Mouse Model | Upregulation of miRNAs that target myelination-related mRNAs | A significant downregulation of mRNAs for myelination, GABAergic/dopaminergic synapses, and neuronal growth, inversely correlated with an upregulation of their targeting miRNAs. | Active dismantling of the transcriptional machinery required to maintain white matter integrity in the mPFC. | [116] |
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jesulola, E.; Micalos, P.; Baguley, I.J. Understanding the Pathophysiology of Depression: From Monoamines to the Neurogenesis Hypothesis Model-Are We There Yet? Behav. Brain Res. 2018, 341, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Guo, Z.; Ren, Z.; Feng, Y.; Fang, P.; Wang, T.; Chen, M. Bibliometric Insights into Astrocytic Roles in Depression and Treatment. Front. Cell. Neurosci. 2025, 18, 1521398. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J. Gliogenesis and Glial Pathology in Depression. CNS Neurol. Disord. Drug Targets 2008, 6, 219–233. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major Depressive Disorder: Hypothesis, Mechanism, Prevention and Treatment. Signal. Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Rajkowska, G.; Stockmeier, C. Astrocyte Pathology in Major Depressive Disorder: Insights from Human Postmortem Brain Tissue. Curr. Drug Targets 2013, 14, 1225–1236. [Google Scholar] [CrossRef]
- O’Leary, L.A.; Belliveau, C.; Davoli, M.A.; Ma, J.C.; Tanti, A.; Turecki, G.; Mechawar, N. Widespread Decrease of Cerebral Vimentin-Immunoreactive Astrocytes in Depressed Suicides. Front. Psychiatry 2021, 12, 640963. [Google Scholar] [CrossRef]
- Sreeja, V.; Jose, A.; Patel, S.; Menon, B.; Athira, K.V.; Chakravarty, S. Pharmacogenetics of Selective Serotonin Reuptake Inhibitors (SSRI): A Serotonin Reuptake Transporter (SERT)-Based Approach. Neurochem. Int. 2024, 173, 105672. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Wang, H.-Q.; Wang, Z.-Z.; Chen, N.-H. Linking Depression and Neuroinflammation: Crosstalk between Glial Cells. Eur. J. Pharmacol. 2025, 995, 177408. [Google Scholar] [CrossRef]
- Kushwaha, R.; Patel, S.; Yuvaraj, K.S.; Sharma, P.; Kumar, A.; Chakravarty, S. Investigating Molecular Mechanisms in Ischemic Preconditioning-Induced Resiliency to Severe Acute Global Cerebral Ischemia Using a Mouse Model of Chronic Cerebral Hypoperfusion. Cell. Mol. Neurobiol. 2025, 45, 27. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Moretti, A. Post-Stroke Depression: Mechanisms and Pharmacological Treatment. Pharmacol. Ther. 2018, 184, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Labonté, B.; Turecki, G. Epigenetic Effects of Childhood Adversity in the Brain and Suicide Risk; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Wang, R.; Wang, W.; Xu, J.; Liu, D.; Wu, H.; Qin, X.; Jiang, H.; Pan, F. Jmjd3 Is Involved in the Susceptibility to Depression Induced by Maternal Separation via Enhancing the Neuroinflammation in the Prefrontal Cortex and Hippocampus of Male Rats. Exp. Neurol. 2020, 328, 113254. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.G.M.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Li, N.; Du, J.; Yang, Y.; Zhao, T.; Wu, D.; Peng, F.; Wang, D.; Kong, L.; Zhou, W.; Hao, A. Microglial PCGF1 Alleviates Neuroinflammation Associated Depressive Behavior in Adolescent Mice. Mol. Psychiatry 2025, 30, 914–926. [Google Scholar] [CrossRef]
- Patel, S.; Kushwaha, R.; Chakravarty, S. Neurobiology of Depression-Associated Disability: Gender-Specific Perspectives. In The Palgrave Encyclopedia of Disability; Bennett, G., Goodall, E., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 1–18. ISBN 978-3-031-40858-8. [Google Scholar]
- Sierra, A.; Beccari, S.; Diaz-Aparicio, I.; Encinas, J.M.; Comeau, S.; Tremblay, M.-È. Surveillance, Phagocytosis, and Inflammation: How Never-resting Microglia Influence Adult Hippocampal Neurogenesis. Neural Plast. 2014, 2014, 610343. [Google Scholar] [CrossRef]
- Teter, B.; Rozovsky, I.; Krohn, K.; Anderson, C.; Osterburg, H.; Finch, C. Methylation of the Glial Fibrillary Acidic Protein Gene Shows Novel Biphasic Changes during Brain Development. Glia 1996, 17, 195–205. [Google Scholar] [CrossRef]
- Kremer, L.P.M.; Cerrizuela, S.; El-Sammak, H.; Al Shukairi, M.E.; Ellinger, T.; Straub, J.; Korkmaz, A.; Volk, K.; Brunken, J.; Kleber, S. DNA Methylation Controls Stemness of Astrocytes in Health and Ischaemia. Nature 2024, 634, 415–423. [Google Scholar] [CrossRef]
- Neal, M.; Richardson, J.R. Epigenetic Regulation of Astrocyte Function in Neuroinflammation and Neurodegeneration. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Ikezu, T. Transcriptional and Epigenetic Regulation of Microglia in Health and Disease. Trends Mol. Med. 2019, 25, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Cheray, M.; Joseph, B. Epigenetics Control Microglia Plasticity. Front. Cell. Neurosci. 2018, 12, 243. [Google Scholar] [CrossRef]
- Pietrowski, M.J.; Gabr, A.A.; Kozlov, S.; Blum, D.; Halle, A.; Carvalho, K. Glial Purinergic Signaling in Neurodegeneration. Front. Neurol. 2021, 12, 654850. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Copray, S.; Huynh, J.L.; Sher, F.; Casaccia-Bonnefil, P.; Boddeke, E. Epigenetic Mechanisms Facilitating Oligodendrocyte Development, Maturation, and Aging. Glia 2009, 57, 1579–1587. [Google Scholar] [CrossRef]
- Shen, S.; Li, J.; Casaccia-Bonnefil, P. Histone Modifications Affect Timing of Oligodendrocyte Progenitor Differentiation in the Developing Rat Brain. J. Cell Biol. 2005, 169, 577–589. [Google Scholar] [CrossRef]
- Li, J.F.; Hu, W.Y.; Chang, H.X.; Bao, J.H.; Kong, X.X.; Ma, H.; Li, Y.F. Astrocytes Underlie a Faster-Onset Antidepressant Effect of Hypidone Hydrochloride (YL-0919). Front. Pharmacol. 2023, 14, 1175938. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Varghese, S.M.; Patel, S.; Nandan, A.; Jose, A.; Ghosh, S.; Sah, R.K.; Menon, B.; KV, A.; Chakravarty, S. Unraveling the Role of the Blood-Brain Barrier in the Pathophysiology of Depression: Recent Advances and Future Perspectives. Mol. Neurobiol. 2024, 61, 10398–10447. [Google Scholar] [CrossRef] [PubMed]
- Dalbeyler, S.; Herud, A.; Zglinicki, B.; Ziuzia, P.; Piechota, M.; Hoinkis, D.; Bergauer, L.; Pando, C.M.; Soltys, Z.; Hanus, P. Glucocorticoid Receptors Mediate Reprogramming of Astrocytes in Depression. bioRxiv 2025. [Google Scholar] [CrossRef]
- Puentes-Orozco, M.; Albarracin, S.L.; Velásquez, M.M. Neuroinflammation and Major Depressive Disorder: Astrocytes at the Crossroads. Front. Cell. Neurosci. 2024, 18, 1504555. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Patel, S.; Kushwaha, R.; Holkar, A.; Dhaygude, S.S.; Kumar, A.; Chakravarty, S. Modeling Vicarious Social Defeat Stress in Rodents for Investigating Emotional Stress-Induced Depression. J. Psychiatr. Res. 2025, 190, 52–68. [Google Scholar] [CrossRef]
- Hao, T.; Du, X.; Yang, S.; Zhang, Y.; Liang, F. Astrocytes-Induced Neuronal Inhibition Contributes to Depressive-like Behaviors during Chronic Stress. Life Sci. 2020, 258, 118099. [Google Scholar] [CrossRef]
- Bansal, Y.; Codeluppi, S.A.; Banasr, M. Astroglial Dysfunctions in Mood Disorders and Rodent Stress Models: Consequences on Behavior and Potential as Treatment Target. Int. J. Mol. Sci. 2024, 25, 6357. [Google Scholar] [CrossRef]
- Lin, S.-S.; Zhou, B.; Liu, S.-L.; Ren, X.-Y.; Guo, J.; Tong, J.-L.; Chen, B.-J.; Jiang, R.-T.; Semyanov, A.; Yi, C. Astrocyte Ezrin Defines Resilience to Stress-Induced Depressive Behaviours in Mice. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chi, D.; Zhang, K.; Zhang, J.; He, Z.; Zhou, H.; Huang, W.; Liu, Y.; Huang, J.; Zeng, W.; Bai, X. Astrocytic Pleiotrophin Deficiency in the Prefrontal Cortex Contributes to Stress-Induced Depressive-like Responses in Male Mice. Nat. Commun. 2025, 16, 2528. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Cai, Q.; Zhang, X.; Zhu, L.; Liu, Z.; Li, F.; Wang, Q.; Liu, L.; Feng, D. Astrocytic Histone Deacetylase 2 Facilitates Delayed Depression and Memory Impairment after Subarachnoid Hemorrhage by Negatively Regulating Glutamate Transporter-1. Ann. Transl. Med. 2020, 8, 691. [Google Scholar] [CrossRef]
- Miguel-Hidalgo, J.J.; Waltzer, R.; Whittom, A.A.; Austin, M.C.; Rajkowska, G.; Stockmeier, C.A. Glial and Glutamatergic Markers in Depression, Alcoholism, and Their Comorbidity. J. Affect. Disord. 2010, 127, 230–240. [Google Scholar] [CrossRef]
- Ye, J.; Zhong, S.; Deng, Y.; Yao, X.; Liu, Q.; Wang, J.Z.; Xiao, S. HDAC7 Activates IKK/NF-ΚB Signaling to Regulate Astrocyte-Mediated Inflammation. Mol. Neurobiol. 2022, 59, 6141–6157. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone Ameliorates Depressive and Anxiety-like Behaviors via Sirt1/Nrf2/HO-1/Gpx4 Pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Sivalingam, K.; Doke, M.; Khan, M.A.; Samikkannu, T. Influence of Psychostimulants and Opioids on Epigenetic Modification of Class III Histone Deacetylase (HDAC)-Sirtuins in Glial Cells. Sci. Rep. 2021, 11, 21335. [Google Scholar] [CrossRef]
- Wu, X.; Chen, P.S.; Dallas, S.; Wilson, B.; Block, M.L.; Wang, C.-C.; Kinyamu, H.; Lu, N.; Gao, X.; Leng, Y. Histone Deacetylase Inhibitors Up-Regulate Astrocyte GDNF and BDNF Gene Transcription and Protect Dopaminergic Neurons. Int. J. Neuropsychopharmacol. 2008, 11, 1123–1134. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Z.; Yao, R.; Zhang, J.; Cui, W.; Dai, J.; Li, J.; Qian, H.; Zhao, X. Quercetin Alleviates Depressive-like Behavior by Modulating Acetyl-H3K9 Mediated Ferroptosis Pathway in Hypothalamus of Perimenopausal Depression Rat Model. Biomed. Pharmacother. 2024, 179, 117369. [Google Scholar] [CrossRef]
- Fulton, S.L.; Bendl, J.; Di Salvo, G.; Fullard, J.F.; Al-Kachak, A.; Lepack, A.E.; Stewart, A.F.; Singh, S.; Poller, W.F.; Bastle, R.M. Major-Depressive-Disorder-Associated Dysregulation of ZBTB7A in Orbitofrontal Cortex Promotes Astrocyte-Mediated Stress Susceptibility. Neuron 2025, 113, 2656–2672.e13. [Google Scholar] [CrossRef]
- Doke, M.; Pendyala, G.; Samikkannu, T. Psychostimulants and Opioids Differentially Influence the Epigenetic Modification of Histone Acetyltransferase and Histone Deacetylase in Astrocytes. PLoS ONE 2021, 16, e0252895. [Google Scholar] [CrossRef]
- Xiu, J.; Li, J.; Liu, Z.; Wei, H.; Zhu, C.; Han, R.; Liu, Z.; Zhu, W.; Shen, Y.; Xu, Q. Elevated BICD2 DNA Methylation in Blood of Major Depressive Disorder Patients and Reduction of Depressive-like Behaviors in Hippocampal Bicd2-Knockdown Mice. Proc. Natl. Acad. Sci. USA 2022, 119, e2201967119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-L.; Li, L.-D.; Lin, X.-Y.; Hu, J.; Wang, C.; Wang, Y.-J.; Zhou, Q.-G.; Zhang, J. Plasma-Derived Small Extracellular Vesicles MiR-182-5p Is a Potential Biomarker for Diagnosing Major Depressive Disorder. Mol. Neurobiol. 2025, 62, 11099–11111. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Dwivedi, Y. Integrated Long Noncoding RNA and Messenger RNA Expression Analysis Identifies Molecules Specifically Associated With Resiliency and Susceptibility to Depression and Antidepressant Response. Biol. Psychiatry Glob. Open Sci. 2024, 4, 100365. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Fuchs, U.; Gisa, V.; Pena, T.; Krüger, D.M.; Hempel, N.; Burkhardt, S.; Salinas, G.; Schütz, A.-L.; Delalle, I. PRDM16-DT Is a Novel LncRNA That Regulates Astrocyte Function in Alzheimer’s Disease. Acta Neuropathol. 2024, 148, 32. [Google Scholar] [CrossRef]
- Hu, N.; Zheng, Y.; Liu, X.; Jia, J.; Feng, J.; Zhang, C.; Liu, L.; Wang, X. CircKat6b Mediates the Antidepressant Effect of Esketamine by Regulating Astrocyte Function. Mol. Neurobiol. 2025, 62, 2587–2600. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, C. Navigating the Complex Terrain of Dysregulated Microglial Function in Depressive Disorders: Insights, Challenges and Future Directions. Aging Dis. 2024, 16, 1023. [Google Scholar] [CrossRef]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- González Ibáñez, F.; VanderZwaag, J.; Deslauriers, J.; Tremblay, M.-È. Ultrastructural Features of Psychological Stress Resilience in the Brain: A Microglial Perspective. Open Biol. 2024, 14, 240079. [Google Scholar] [CrossRef]
- Kokkosis, A.G.; Madeira, M.M.; Hage, Z.; Valais, K.; Koliatsis, D.; Resutov, E.; Tsirka, S.E. Chronic Psychosocial Stress Triggers Microglial-/Macrophage-induced Inflammatory Responses Leading to Neuronal Dysfunction and Depressive-related Behavior. Glia 2024, 72, 111–132. [Google Scholar] [CrossRef]
- Goodman, E.J.; DiSabato, D.J.; Sheridan, J.F.; Godbout, J.P. Novel Microglial Transcriptional Signatures Promote Social and Cognitive Deficits Following Repeated Social Defeat. Commun. Biol. 2024, 7, 1199. [Google Scholar] [CrossRef]
- Reemst, K.; Kracht, L.; Kotah, J.M.; Rahimian, R.; van Irsen, A.A.S.; Congrains Sotomayor, G.; Verboon, L.N.; Brouwer, N.; Simard, S.; Turecki, G.; et al. Early-Life Stress Lastingly Impacts Microglial Transcriptome and Function under Basal and Immune-Challenged Conditions. Transl. Psychiatry 2022, 12, 507. [Google Scholar] [CrossRef]
- Xu, K.; Wang, M.; Wang, H.; Zhao, S.; Tu, D.; Gong, X.; Li, W.; Liu, X.; Zhong, L.; Chen, J. HMGB1/STAT3/P65 Axis Drives Microglial Activation and Autophagy Exert a Crucial Role in Chronic Stress-Induced Major Depressive Disorder. J. Adv. Res. 2024, 59, 79–96. [Google Scholar] [CrossRef]
- Li, S.; Liao, Y.; Dong, Y.; Li, X.; Li, J.; Cheng, Y.; Cheng, J.; Yuan, Z. Microglial Deletion and Inhibition Alleviate Behavior of Post-Traumatic Stress Disorder in Mice. J. Neuroinflamm. 2021, 18, 7. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Shen, S.-Y.; Zhai, M.-Y.; Shen, Z.-Q.; Li, W.; Liang, L.-F.; Yin, S.-Y.; Han, Q.-Q.; Li, B.; Zhang, Y.-Q. Augmented Microglial Endoplasmic Reticulum-Mitochondria Contacts Mediate Depression-like Behavior in Mice Induced by Chronic Social Defeat Stress. Nat. Commun. 2024, 15, 5199. [Google Scholar] [CrossRef]
- Chen, J.; Huang, J.; Han, T.; Kojima, N. Chronic Stress Modulates Microglial Activation Dynamics, Shaping Priming Responses to Subsequent Stress. Brain Sci. 2025, 15, 534. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia Morphophysiological Diversity and Its Implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Han, Q.Q.; Gong, W.Q.; Pan, D.H.; Wang, L.Z.; Hu, W.; Yang, M.; Li, B.; Yu, J.; Liu, Q. Microglial Activation Mediates Chronic Mild Stress-Induced Depressive- and Anxiety-like Behavior in Adult Rats. J. Neuroinflamm. 2018, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, R.; Jinno, S. Identification of Hyper-Ramified Microglia in the CA1 Region of the Mouse Hippocampus Potentially Associated with Stress Resilience. Eur. J. Neurosci. 2022, 56, 5137–5153. [Google Scholar] [CrossRef]
- Yang, J.-C.; Zhao, J.; Chen, Y.-H.; Wang, R.; Rong, Z.; Wang, S.-Y.; Wu, Y.-M.; Wang, H.-N.; Yang, L.; Liu, R. MiR-29a-5p Rescues Depressive-like Behaviors in a CUMS-Induced Mouse Model by Facilitating Microglia M2-Polarization in the Prefrontal Cortex via TMEM33 Suppression. J. Affect. Disord. 2024, 360, 188–197. [Google Scholar] [CrossRef]
- He, L.; Zheng, Y.; Huang, L.; Ye, J.; Ye, Y.; Luo, H.; Chen, X.; Yao, W.; Chen, J.; Zhang, J. Nrf2 Regulates the Arginase 1+ Microglia Phenotype through the Initiation of TREM2 Transcription, Ameliorating Depression-like Behavior in Mice. Transl. Psychiatry 2022, 12, 459. [Google Scholar] [CrossRef]
- Kong, Y.; Zhang, X.; Li, L.; Zhao, T.; Huang, Z.; Zhang, A.; Sun, Y.; Jiao, J.; Zhang, G.; Liu, M. Microglia-Derived Vitamin D Binding Protein Mediates Synaptic Damage and Induces Depression by Binding to the Neuronal Receptor Megalin. Adv. Sci. 2025, 12, 2410273. [Google Scholar] [CrossRef]
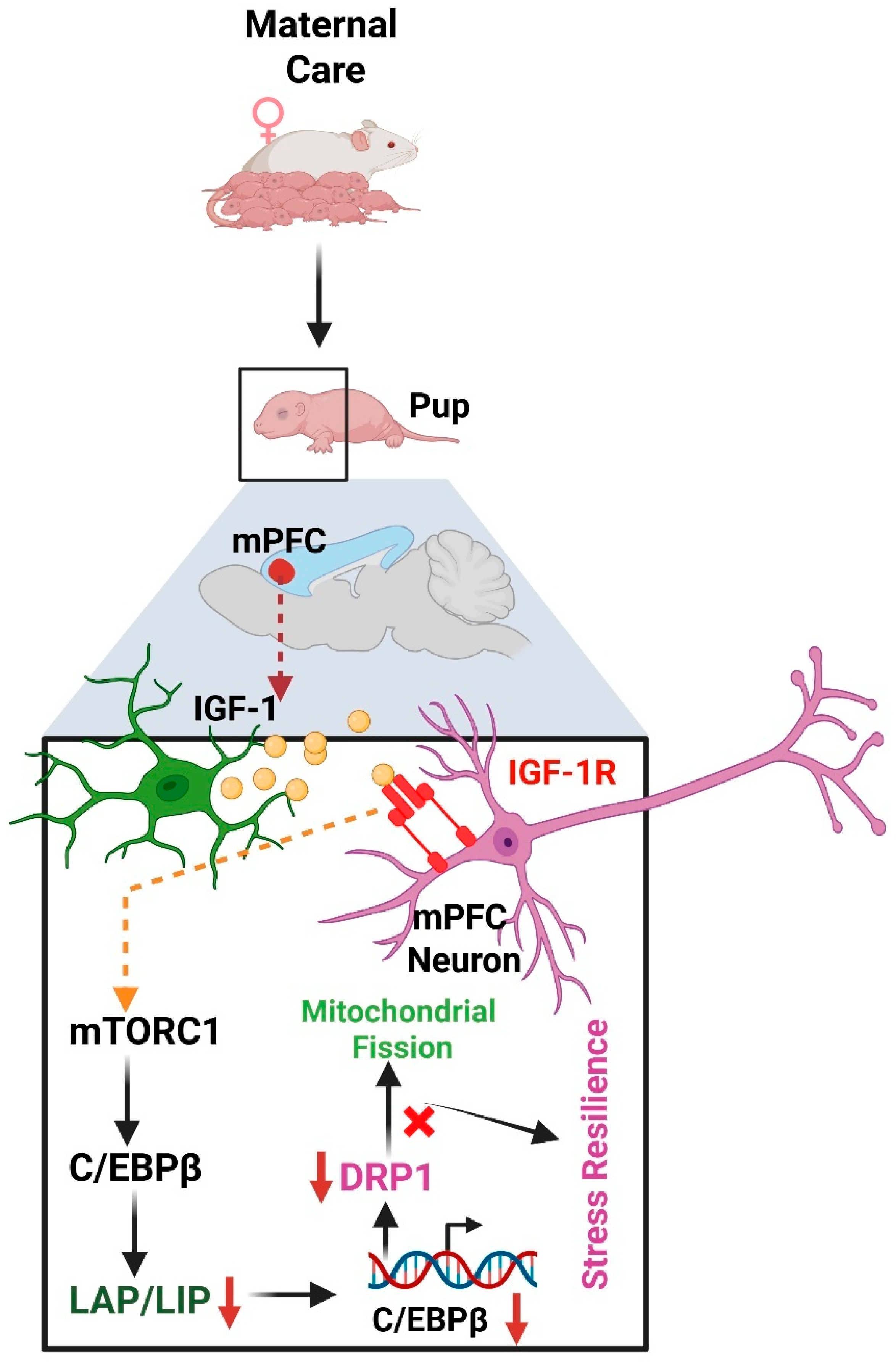
- Chen, H.; Xu, R.; Wang, J.; Gao, F.; Lv, Y.; Li, X.; Li, F.; Zhao, J.; Zhang, X.; Wang, J. Maternal Behavior Promotes Resilience to Adolescent Stress in Mice through a Microglia-Neuron Axis. Nat. Commun. 2025, 16, 2333. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Zheng, H.; Chen, J.; Gao, Y.; Ma, J.; Chen, D.; Li, Z.; Wu, C.; Lian, B.; Zhang, X. Social Hierarchy and Resilience Affect Stress-Induced PTSD via Uba7 Gene Expression and Subsequent Inflammation in Microglia of the MPFC. Mol. Psychiatry 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Malovic, E.; Ealy, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Microglial Immune Regulation by Epigenetic Reprogramming through Histone H3K27 Acetylation in Neuroinflammation. Front. Immunol. 2023, 14, 1052925. [Google Scholar] [CrossRef]
- Dantzer, R. From Stress Sensitization to Microglial Priming and Vice Versa: A New Era of Research in Biological Psychiatry. Biol. Psychiatry 2019, 85, 619–620. [Google Scholar] [CrossRef]
- Catale, C.; Bussone, S.; Lo Iacono, L.; Viscomi, M.T.; Palacios, D.; Troisi, A.; Carola, V. Exposure to Different Early-Life Stress Experiences Results in Differentially Altered DNA Methylation in the Brain and Immune System. Neurobiol. Stress. 2020, 13, 100249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Guo, Y.; Huang, A.; Shen, H.; Chen, Y.; Song, J.; Guan, A.; Wu, L.; Wang, H.; Deng, B. HDAC3-Regulated PGE2 Production by Microglia Induces Phobic Anxiety Susceptibility After Stroke and Pointedly Exploiting a Signal-Targeted Gamma Visual Stimulation New Therapy. Front. Immunol. 2022, 13, 845678. [Google Scholar] [CrossRef] [PubMed]
- Rasmi, Y.; Shokati, A.; Hassan, A.; Aziz, S.G.G.; Bastani, S.; Jalali, L.; Moradi, F.; Alipour, S. The Role of DNA Methylation in Progression of Neurological Disorders and Neurodegenerative Diseases as Well as the Prospect of Using DNA Methylation Inhibitors as Therapeutic Agents for Such Disorders. IBRO Neurosci. Rep. 2023, 14, 28–37. [Google Scholar] [CrossRef] [PubMed]
- de Witte, L.D.; Wang, Z.; Snijders, G.L.J.L.; Mendelev, N.; Liu, Q.; Sneeboer, M.A.M.; Boks, M.P.M.; Ge, Y.; Haghighi, F. Contribution of Age, Brain Region, Mood Disorder Pathology, and Interindividual Factors on the Methylome of Human Microglia. Biol. Psychiatry 2022, 91, 572–581. [Google Scholar] [CrossRef]
- Fan, C.; Li, Y.; Lan, T.; Wang, W.; Long, Y.; Yu, S.Y. Microglia Secrete MiR-146a-5p-Containing Exosomes to Regulate Neurogenesis in Depression. Mol. Ther. 2022, 30, 572–581. [Google Scholar] [CrossRef]
- Rodriguez-Zas, S.L.; Wu, C.; Southey, B.R.; O’Connor, J.C.; Nixon, S.E.; Garcia, R.; Zavala, C.; Lawson, M.; McCusker, R.H.; Romanova, E.V.; et al. Disruption of Microglia Histone Acetylation and Protein Pathways in Mice Exhibiting Inflammation-Associated Depression-like Symptoms. Psychoneuroendocrinology 2018, 97, 47–58. [Google Scholar] [CrossRef]
- Wang, D.; Kosowan, J.; Samsom, J.; Leung, L.; Zhang, K.; Li, Y.; Xiong, Y.; Jin, J.; Petronis, A.; Oh, G. Inhibition of the G9a/GLP Histone Methyltransferase Complex Modulates Anxiety-Related Behavior in Mice. Acta Pharmacol. Sin. 2018, 39, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Scheepstra, K.W.F.; Mizee, M.R.; van Scheppingen, J.; Adelia, A.; Wever, D.D.; Mason, M.R.J.; Dubbelaar, M.L.; Hsiao, C.-C.; Eggen, B.J.L.; Hamann, J. Microglia Transcriptional Profiling in Major Depressive Disorder Shows Inhibition of Cortical Gray Matter Microglia. Biol. Psychiatry 2023, 94, 619–629. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Huang, Y.; Liu, L.; Zhang, H.; Nagy, C.; Tan, X.; Cheng, K.; Liu, Y.; Pu, J. Integrating Spatial and Single-Nucleus Transcriptomic Data Elucidates Microglial-Specific Responses in Female Cynomolgus Macaques with Depressive-like Behaviors. Nat. Neurosci. 2023, 26, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Zhu, Y.; Sun, D.; Wang, S.; Weng, J.; Zhu, Y.; Peng, W.; Yu, B.; Jiang, Y. Microglia-Specific Transcriptional Repression of Interferon-Regulated Genes after Prolonged Stress in Mice. Neurobiol. Stress 2022, 21, 100495. [Google Scholar] [CrossRef]
- Chen, H.; Piao, J.; Geng, Z.; Han, X.; Li, R.; Cui, R.; Li, B. Ginsenoside Rd Alleviates LPS-Induced Neuroinflammation and Depressive-like Behaviors via Regulating TLR4-PI3K-NF-ΚB-JMJD3 Signaling. Int. Immunopharmacol. 2025, 162, 115071. [Google Scholar] [CrossRef]
- Wu, H.; Wang, R.; Qin, X.; Liu, D.; Wang, W.; Xu, J.; Jiang, H.; Pan, F. Effects of Chronic Stress on Depressive-like Behaviors and JMJD3 Expression in the Prefrontal Cortex and Hippocampus of C57BL/6 and Ob/Ob Mice. J. Psychiatr. Res. 2021, 133, 142–155. [Google Scholar] [CrossRef]
- Baek, S.Y.; Lee, J.; Kim, T.; Lee, H.; Choi, H.-S.; Park, H.; Koh, M.; Kim, E.; Jung, M.E.; Iliopoulos, D. Development of a Novel Histone Deacetylase Inhibitor Unveils the Role of HDAC11 in Alleviating Depression by Inhibition of Microglial Activation. Biomed. Pharmacother. 2023, 166, 115312. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, X.; Feng, Q.; Ke, W.; Pan, J.; Zhang, H.; Luan, Y.; Lei, B. Nerolidol Reduces Depression-like Behavior in Mice and Suppresses Microglia Activation by down-Regulating DNA Methyltransferase 1. Neuroreport 2024, 35, 457–465. [Google Scholar] [CrossRef]
- Cai, Y.; Ji, Y.; Liu, Y.; Zhang, D.; Gong, Z.; Li, L.; Chen, X.; Liang, C.; Feng, S.; Lu, J. Microglial Circ-UBE2K Exacerbates Depression by Regulating Parental Gene UBE2K via Targeting HNRNPU. Theranostics 2024, 14, 4058. [Google Scholar] [CrossRef]
- Wang, R.; Wang, W.; Xu, J.; Liu, D.; Jiang, H.; Pan, F. Dynamic Effects of Early Adolescent Stress on Depressive-like Behaviors and Expression of Cytokines and JMJD3 in the Prefrontal Cortex and Hippocampus of Rats. Front. Psychiatry 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Cakmakci, D.; Fiori, L.M.; Zang, W.; Maitra, M.; Yang, J.; Żurawek, D.; Frosi, G.; Rahimian, R.; Mitsuhashi, H. Single-Nucleus Chromatin Accessibility Profiling Identifies Cell Types and Functional Variants Contributing to Major Depression. Nat. Genet. 2025, 57, 1890–1904. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Li, W.; Rao, X.; Liu, Y.; Zhao, L.; Pu, J.; Gui, S.; et al. Integrative Analysis of Long Non-Coding RNAs, Messenger RNAs, and MicroRNAs Indicates the Neurodevelopmental Dysfunction in the Hippocampus of Gut Microbiota-Dysbiosis Mice. Front. Mol. Neurosci. 2022, 14, 745437. [Google Scholar] [CrossRef]
- Liu, J.; Dietz, K.; Hodes, G.E.; Russo, S.J.; Casaccia, P. Widespread Transcriptional Alternations in Oligodendrocytes in the Adult Mouse Brain Following Chronic Stress. Dev. Neurobiol. 2018, 78, 152–162. [Google Scholar] [CrossRef]
- Fields, R.D. A New Mechanism of Nervous System Plasticity: Activity-Dependent Myelination. Nat. Rev. Neurosci. 2015, 16, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Kokkosis, A.G.; Madeira, M.M.; Mullahy, M.R.; Tsirka, S.E. Chronic Stress Disrupts the Homeostasis and Progeny Progression of Oligodendroglial Lineage Cells, Associating Immune Oligodendrocytes with Prefrontal Cortex Hypomyelination. Mol. Psychiatry 2022, 27, 2833–2848. [Google Scholar] [CrossRef]
- Chen, H.; Kang, Z.; Liu, X.; Zhao, Y.; Fang, Z.; Zhang, J.; Zhang, H. Chronic Social Defeat Stress Caused Region-Specific Oligodendrogenesis Impairment in Adolescent Mice. Front. Neurosci. 2023, 16, 1074631. [Google Scholar] [CrossRef]
- Rajkowska, G.; Mahajan, G.; Maciag, D.; Sathyanesan, M.; Iyo, A.H.; Moulana, M.; Kyle, P.B.; Woolverton, W.L.; Miguel-Hidalgo, J.J.; Stockmeier, C.A. Oligodendrocyte Morphometry and Expression of Myelin–Related MRNA in Ventral Prefrontal White Matter in Major Depressive Disorder. J. Psychiatr. Res. 2015, 65, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Perlman, K.; Curto, E.; Barnett-Burns, S.; Davoli, M.-A.; Yerko, V.; Merza, R.; Papadakis, A.I.; Spatz, A.; Pryce, C.R.; Turecki, G. Characterizing Oligodendrocyte-Lineage Cells and Myelination in the Basolateral Amygdala: Insights from a Novel Methodology in Postmortem Human Brain. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xin, D.E.; Zhong, X.; Zhao, C.; Li, Z.; Zhang, L.; Dourson, A.J.; Lee, L.; Mishra, S.; Bayat, A.E. Small-Molecule-Induced Epigenetic Rejuvenation Promotes SREBP Condensation and Overcomes Barriers to CNS Myelin Regeneration. Cell 2024, 187, 2465–2484. [Google Scholar] [CrossRef]
- Li, Y.; Su, Z.; Zhai, J.; Liu, Q.; Wang, H.; Hao, J.; Tian, X.; Gao, J.; Geng, D.; Wang, L. Oligodendrocyte-Specific STAT5B Overexpression Ameliorates Myelin Impairment in Experimental Models of Parkinson’s Disease. Cells 2025, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wu, F.; Fang, Q.; Hu, Y.; Zhang, H.; Yuan, S.; Yang, C.; Shi, Y.; Luo, Y. Antidepressant Effect of Teriflunomide via Oligodendrocyte Protection in a Mouse Model. Heliyon 2024, 10, e29481. [Google Scholar] [CrossRef]
- Bu, J.; Liu, Y.; Zhao, Y.; Liu, L.; Shen, J.; Li, Y. Paroxetine Ameliorates Corticosterone-Induced Myelin Damage by Promoting the Proliferation and Differentiation of Oligodendrocyte Precursor Cells. Neuroscience 2025, 573, 344–354. [Google Scholar] [CrossRef]
- Ramsteijn, A.S.; Verkaik-Schakel, R.N.; Houwing, D.J.; Plösch, T.; Olivier, J.D.A. Perinatal Exposure to Fluoxetine and Maternal Adversity Affect Myelin-Related Gene Expression and Epigenetic Regulation in the Corticolimbic Circuit of Juvenile Rats. Neuropsychopharmacology 2022, 47, 1620–1632. [Google Scholar] [CrossRef]
- Brivio, E.; Kos, A.; Ulivi, A.F.; Karamihalev, S.; Ressle, A.; Stoffel, R.; Hirsch, D.; Stelzer, G.; Schmidt, M.V.; Lopez, J.P. Sex Shapes Cell-Type-Specific Transcriptional Signatures of Stress Exposure in the Mouse Hypothalamus. Cell Rep. 2023, 42, 112874. [Google Scholar] [CrossRef]
- Musaelyan, K.; Yildizoglu, S.; Bozeman, J.; Du Preez, A.; Egeland, M.; Zunszain, P.A.; Pariante, C.M.; Fernandes, C.; Thuret, S. Chronic Stress Induces Significant Gene Expression Changes in the Prefrontal Cortex alongside Alterations in Adult Hippocampal Neurogenesis. Brain Commun. 2020, 2, fcaa153. [Google Scholar] [CrossRef]
- Cathomas, F.; Azzinnari, D.; Bergamini, G.; Sigrist, H.; Buerge, M.; Hoop, V.; Wicki, B.; Goetze, L.; Soares, S.; Kukelova, D.; et al. Oligodendrocyte Gene Expression Is Reduced by and Influences Effects of Chronic Social Stress in Mice. Genes Brain Behav. 2019, 18, e12475. [Google Scholar] [CrossRef]
- Sharma, S.; Ma, W.; Ressler, K.J.; Anderson, T.; Li, D.C.; Jin, P.; Gourley, S.L.; Qin, Z. Dysregulation of Prefrontal Oligodendrocyte Lineage Cells Across Mouse Models of Adversity and Human Major Depressive Disorder Oligodendrocyte Dysregulation in Mouse Models of Stress and MDD. bioRxiv 2023. [Google Scholar] [CrossRef]
- Abraham, M.; Peterburs, J.; Mundorf, A. Oligodendrocytes Matter: A Review of Animal Studies on Early Adversity. J. Neural Transm. 2023, 130, 1177–1185. [Google Scholar] [CrossRef]
- Teissier, A.; Le Magueresse, C.; Olusakin, J.; Andrade da Costa, B.L.S.; De Stasi, A.M.; Bacci, A.; Imamura Kawasawa, Y.; Vaidya, V.A.; Gaspar, P. Early-Life Stress Impairs Postnatal Oligodendrogenesis and Adult Emotional Behaviour through Activity-Dependent Mechanisms. Mol. Psychiatry 2020, 25, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Y.; Yu, G.; Wang, X.; Chen, X.; Yu, B.; Cheng, Y.; Li, R.; Sáez, J.C.; Yi, C. Reduced Oligodendrocyte Precursor Cell Impairs Astrocytic Development in Early Life Stress. Adv. Sci. 2021, 8, 2101181. [Google Scholar] [CrossRef] [PubMed]
- Dean, D.C., III; Madrid, A.; Planalp, E.M.; Moody, J.F.; Papale, L.A.; Knobel, K.M.; Wood, E.K.; McAdams, R.M.; Coe, C.L.; Hill Goldsmith, H. Cord Blood DNA Methylation Modifications in Infants Are Associated with White Matter Microstructure in the Context of Prenatal Maternal Depression and Anxiety. Sci. Rep. 2021, 11, 12181. [Google Scholar] [CrossRef]
- Li, M.; Xiao, L.; Chen, X. Histone Acetylation and Methylation Underlie Oligodendroglial and Myelin Susceptibility in Schizophrenia. Front. Cell. Neurosci. 2022, 16, 823708. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Q.; D’ercole, A.J.; Ye, P. Histone Deacetylase 11 Regulates Oligodendrocyte-specific Gene Expression and Cell Development in OL-1 Oligodendroglia Cells. Glia 2009, 57, 1–12. [Google Scholar] [CrossRef]
- Peng, H.; Zhu, Y.; Strachan, E.; Fowler, E.; Bacus, T.; Roy-Byrne, P.; Goldberg, J.; Vaccarino, V.; Zhao, J. Childhood Trauma, DNA Methylation of Stress-Related Genes, and Depression: Findings from Two Monozygotic Twin Studies. Psychosom. Med. 2018, 80, 599–608. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, J.; Pillai, P.P.; Frost, E.E. Involvement of MeCP2 in Regulation of Myelin-Related Gene Expression in Cultured Rat Oligodendrocytes. J. Mol. Neurosci. 2015, 57, 176–184. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, F.; Zhou, S.; Qiu, M. MYRF: A Mysterious Membrane-Bound Transcription Factor Involved in Myelin Development and Human Diseases. Neurosci. Bull. 2021, 37, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, B.; Popko, B. Molecular Control of Oligodendrocyte Development. Trends Neurosci. 2019, 42, 263–277. [Google Scholar] [CrossRef]
- Dugas, J.C.; Cuellar, T.L.; Scholze, A.; Ason, B.; Ibrahim, A.; Emery, B.; Zamanian, J.L.; Foo, L.C.; McManus, M.T.; Barres, B.A. Dicer1 and MiR-219 Are Required for Normal Oligodendrocyte Differentiation and Myelination. Neuron 2010, 65, 597–611. [Google Scholar] [CrossRef]
- Ibrahim, P.; Denniston, R.; Mitsuhashi, H.; Yang, J.; Fiori, L.M.; Żurawek, D.; Mechawar, N.; Nagy, C.; Turecki, G. Profiling Small RNA From Brain Extracellular Vesicles in Individuals With Depression. Int. J. Neuropsychopharmacol. 2024, 27, pyae013. [Google Scholar] [CrossRef]
- Ma, K.; Guo, L.; Xu, A.; Cui, S.; Wang, J.H. Molecular Mechanism for Stress-Induced Depression Assessed by Sequencing MiRNA and MRNA in Medial Prefrontal Cortex. PLoS ONE 2016, 11, e0159093. [Google Scholar] [CrossRef]
- Vattathil, S.M.; Gerasimov, E.S.; Canon, S.M.; Lori, A.; Tan, S.S.M.; Kim, P.J.; Liu, Y.; Lai, E.C.; Bennett, D.A.; Wingo, T.S. Mapping the MicroRNA Landscape in the Older Adult Brain and Its Genetic Contribution to Neuropsychiatric Conditions. Nat. Aging 2025, 5, 306–319. [Google Scholar] [CrossRef]
- Tsarouchas, T.; Vacante, F.; Kazakou, N.-L.; Wagstaff, L.; Bennett, M.; Zoupi, L.; Gibson, E.M.; Baker, A.H.; Williams, A.C. The LncRNA MYRACL Regulates Human Oligodendrocyte Maturation and Myelination. Mol. Ther. 2025. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, L.; Wang, L.; Liu, T.; Zheng, Y.; Si, L.; Ge, H.; Xu, H.; Xiao, L.; Wang, G. Single-Nucleus Transcriptomic Analysis Reveals the Relationship between Gene Expression in Oligodendrocyte Lineage and Major Depressive Disorder. J. Transl. Med. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Kukharsky, M.S.; Ninkina, N.N.; An, H.; Telezhkin, V.; Wei, W.; de Meritens, C.R.; Cooper-Knock, J.; Nakagawa, S.; Hirose, T.; Buchman, V.L.; et al. Long Non-Coding RNA Neat1 Regulates Adaptive Behavioural Response to Stress in Mice. Transl. Psychiatry 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Javier Meana, J.; Callado, L.F.; Morentin, B. Do Post-Mortem Brain Studies Provide Useful Information for Psychiatry? Rev. Psiquiatr. Salud Ment. 2014, 7, 101–103. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.C.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely Hominid Features of Adult Human Astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Kushwaha, R.; Sinha, D.; Kumar, A.; Chakravarty, S. Stress-Induced Transcriptional and Epigenetic Plasticity of Astrocytes, Microglia and Oligodendrocytes in the Pathophysiology of Depression. Neuroglia 2025, 6, 42. https://doi.org/10.3390/neuroglia6040042
Patel S, Kushwaha R, Sinha D, Kumar A, Chakravarty S. Stress-Induced Transcriptional and Epigenetic Plasticity of Astrocytes, Microglia and Oligodendrocytes in the Pathophysiology of Depression. Neuroglia. 2025; 6(4):42. https://doi.org/10.3390/neuroglia6040042
Chicago/Turabian StylePatel, Shashikant, Roli Kushwaha, Debiprasad Sinha, Arvind Kumar, and Sumana Chakravarty. 2025. "Stress-Induced Transcriptional and Epigenetic Plasticity of Astrocytes, Microglia and Oligodendrocytes in the Pathophysiology of Depression" Neuroglia 6, no. 4: 42. https://doi.org/10.3390/neuroglia6040042
APA StylePatel, S., Kushwaha, R., Sinha, D., Kumar, A., & Chakravarty, S. (2025). Stress-Induced Transcriptional and Epigenetic Plasticity of Astrocytes, Microglia and Oligodendrocytes in the Pathophysiology of Depression. Neuroglia, 6(4), 42. https://doi.org/10.3390/neuroglia6040042





