Reelin Signaling by the Prime Neurogenic Niche of the Adult Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Tamoxifen and Bromodeoxyuridine Administration
2.3. Alkaline Phosphatase-Reelin in Situ Staining
2.4. Immunohistochemistry
2.5. Production of Reelin
2.6. Dab1 Phosphorylation Assay
2.7. Ultrastructural Analysis
2.8. Intraventricular Infusion of Reelin
3. Results
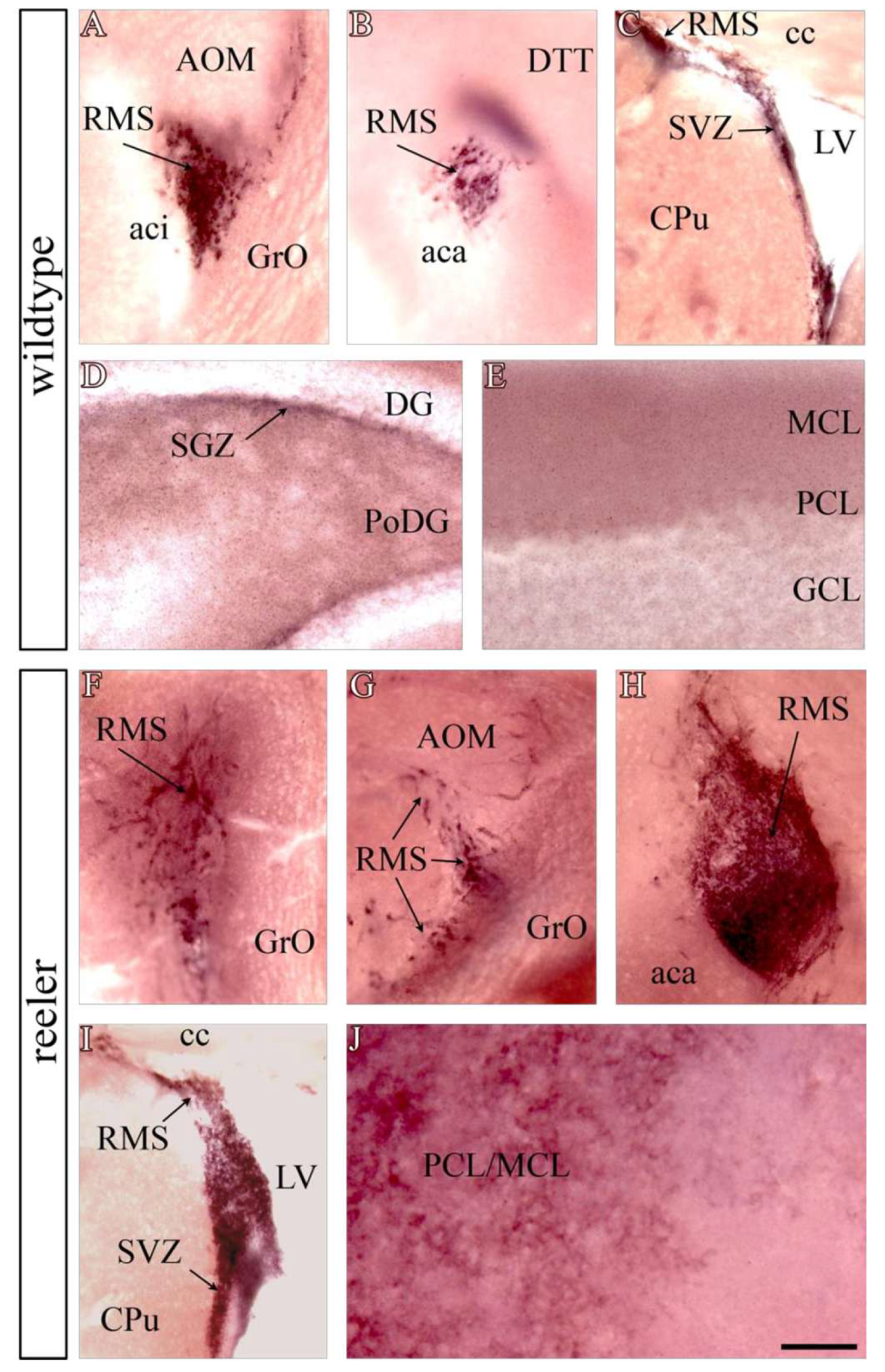
3.1. The EZ/SEZ and RMS Contain the Highest Density of Functional Reelin Receptors in the Adult Mouse Brain
3.2. Functional ApoER2 Is Present in the Main Cellular Compartments of the EZ/SEZ/RMS Neurogenic Axis
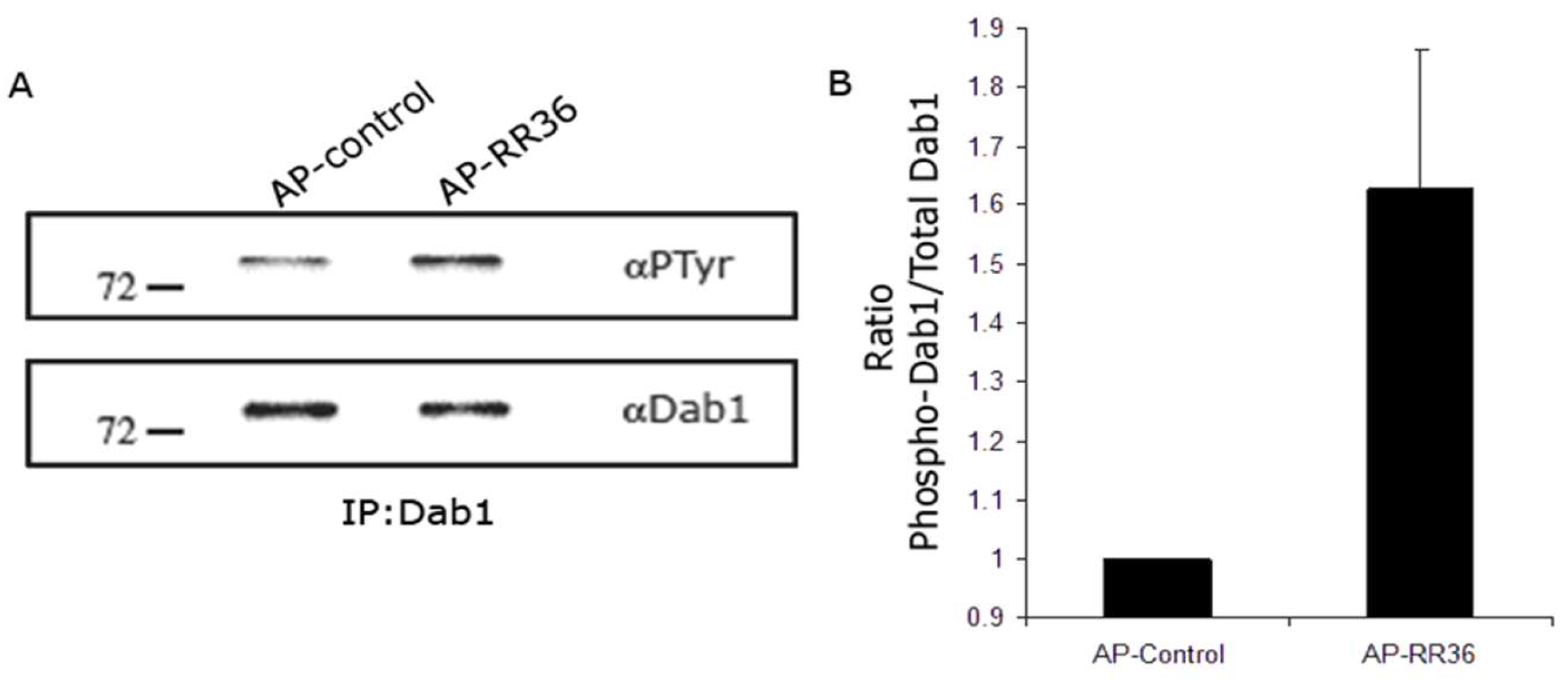
3.3. Reelin Signaling Remains Active in the EZ/SEZ
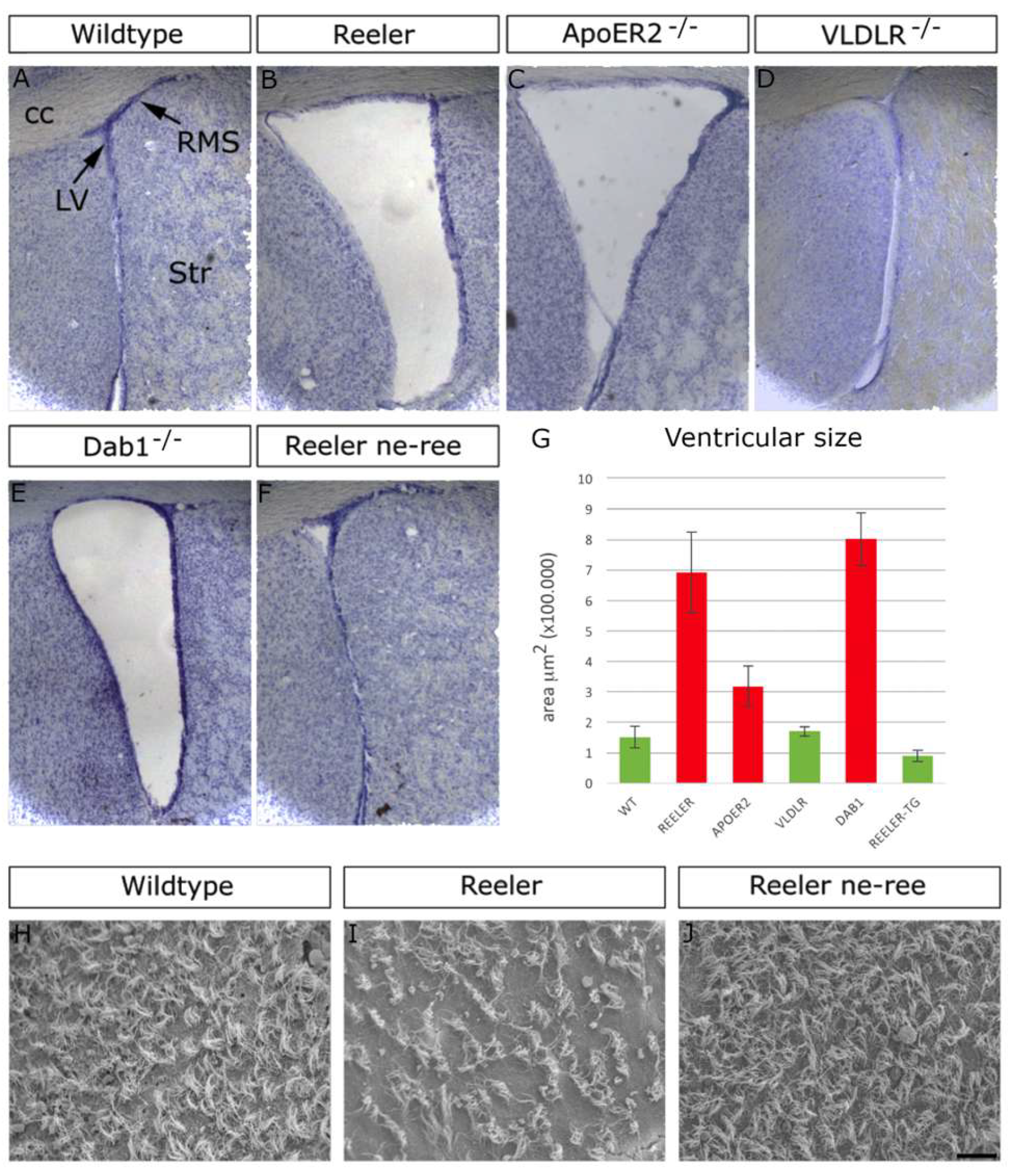
3.4. Lack of Function of Reelin, ApoER2 or Dab1, but Not of VLDLR, Causes Analogous Phenotypes in the Lateral Ventricles and RMS
3.5. Partial Rescue of Lateral Ventricle Integrity and Neuroblast Migration by Ectopic Expression of Reelin in NPCs of Reeler Mutants
3.6. Conditional Ablation Dab1 in NPCs Impairs Neuroblast Clearance from the SVZ
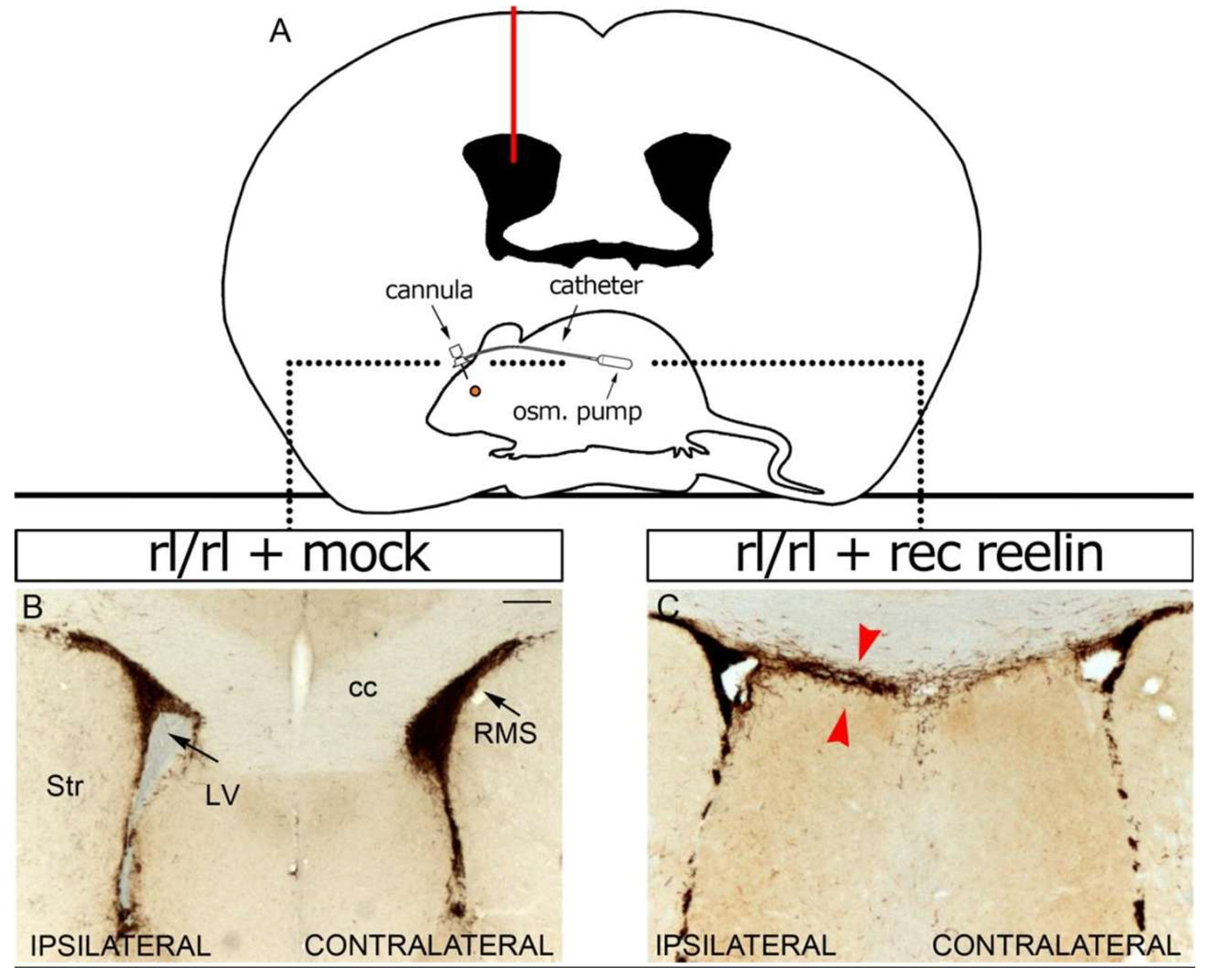
3.7. Intraventricular Infusion of Recombinant Reelin Alters the Migration of Neuroblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kriegstein, A.; Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 2009, 32, 149–184. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Ihrie, R.A.; Alvarez-Buylla, A. Lake-front property: A unique germinal niche by the lateral ventricles of the adult brain. Neuron 2011, 70, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Lois, C.; Alvarez-Buylla, A. Long-distance neuronal migration in the adult mammalian brain. Science 1994, 264, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Caillé, I.; Lim, D.A.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Carleton, A.; Petreanu, L.T.; Lansford, R.; Alvarez-Buylla, A.; Lledo, P.M. Becoming a new neuron in the adult olfactory bulb. Nat. Neurosci. 2003, 6, 507–518. [Google Scholar] [CrossRef]
- Brill, M.S.; Ninkovic, J.; Winpenny, E.; Hodge, R.D.; Ozen, I.; Yang, R.; Lepier, A.; Gascón, S.; Erdelyi, F.; Szabo, G.; et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 2009, 12, 1524–1533. [Google Scholar] [CrossRef]
- Mercier, F.; Kitasako, J.T.; Hatton, G.I. Anatomy of the brain neurogenic zones revisited: Fractones and the fibroblast/macrophage network. J. Comp. Neurol. 2002, 451, 170–188. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef]
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3, 279–288. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Merkle, F.T.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 2008, 3, 265–278. [Google Scholar] [CrossRef]
- Sawamoto, K.; Wichterle, H.; Gonzalez-Perez, O.; Cholfin, J.A.; Yamada, M.; Spassky, N.; Murcia, N.S.; Garcia-Verdugo, J.M.; Marin, O.; Rubenstein, J.L.; et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 2006, 311, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Song, H.; Ming, G.L. Regulation of Adult Neurogenesis by Neurotransmitters. In Adult Neurogenesis; Gage, F.H., Kempermann, G., Song, H., Eds.; CSHL Press: Cold Spring Harbor, NY, USA, 2008; pp. 397–423. [Google Scholar]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Rice, D.S.; Curran, T. Role of the reelin signaling pathway in central nervous system development. Annu. Rev. Neurosci. 2001, 24, 1005–1039. [Google Scholar] [CrossRef]
- Tissir, F.; Goffinet, A.M. Reelin and brain development. Nat. Rev. Neurosci. 2003, 4, 496–505. [Google Scholar] [CrossRef]
- Luque, J.M. Puzzling out the reeler brainteaser: Does reelin signal to unique neural lineages? Brain Res. 2007, 1140, 41–50. [Google Scholar] [CrossRef]
- Cooper, J.A. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008, 31, 113–119. [Google Scholar] [CrossRef]
- Magdaleno, S.; Keshvara, L.; Curran, T. Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron 2002, 33, 573–586. [Google Scholar] [CrossRef]
- Lakomá, J.; Garcia-Alonso, L.; Luque, J.M. Reelin sets the pace of neocortical neurogenesis. Development 2011, 138, 5223–5234. [Google Scholar] [CrossRef]
- Hashimoto-Torii, K.; Torii, M.; Sarkisian, M.R.; Bartley, C.M.; Shen, J.; Radtke, F.; Gridley, T.; Sestan, N.; Rakic, P. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 2008, 60, 273–284. [Google Scholar] [CrossRef]
- Pérez-Martínez, F.J.; Luque-Río, A.; Sakakibara, A.; Hattori, M.; Miyata, T.; Luque, J.M. Reelin-dependent ApoER2 downregulation uncouples newborn neurons from progenitor cells. Biol. Open. 2012, 1, 1258–1263. [Google Scholar] [CrossRef]
- Kim, H.M.; Qu, T.; Kriho, V.; Lacor, P.; Smalheiser, N.; Pappas, G.D.; Guidotti, A.; Costa, E.; Sugaya, K. Reelin function in neural stem cell biology. Proc. Natl. Acad. Sci. USA 2002, 99, 4020–4025. [Google Scholar] [CrossRef]
- Hack, I.; Bancila, M.; Loulier, K.; Carroll, P.; Cremer, H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat. Neurosci. 2002, 5, 939–945. [Google Scholar] [CrossRef]
- Won, S.J.; Kim, S.H.; Xie, L.; Wang, Y.; Mao, X.O.; Jin, K.; Greenberg, D.A. Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp. Neurol. 2006, 198, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chai, X.; Frotscher, M. Balance between neurogenesis and gliogenesis in the adult hippocampus: Role for reelin. Dev. Neurosci. 2007, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, C.; Nitta, N.; Flubacher, A.; Müller, M.; Fahrner, A.; Kirsch, M.; Freiman, T.; Suzuki, F.; Depaulis, A.; Frotscher, M.; et al. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J. Neurosci. 2006, 26, 4701–4713. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Komnenovic, V.; Blake, S.M.; Jossin, Y.; Howell, B.; Goffinet, A.; Schneider, W.J.; Nimpf, J. ApoER2/VLDL receptor and Dab1 in the rostral migratory stream function in postnatal neuronal migration independently of Reelin. Proc. Natl. Acad. Sci. USA 2007, 104, 8508–8513. [Google Scholar] [CrossRef]
- Gong, C.; Wang, T.W.; Huang, H.S.; Parent, J.M. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J. Neurosci. 2007, 27, 1803–1811. [Google Scholar] [CrossRef]
- Simó, S.; Pujadas, L.; Segura, M.F.; La Torre, A.; Del Río, J.A.; Ureña, J.M.; Comella, J.X.; Soriano, E. Reelin induces the detachment of postnatal subventricular zone cells and the expression of the Egr-1 through Erk1/2 activation. Cereb. Cortex 2007, 17, 294–303. [Google Scholar] [CrossRef][Green Version]
- Blake, S.M.; Strasser, V.; Andrade, N.; Duit, S.; Hofbauer, R.; Schneider, W.J.; Nimpf, J. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J. 2008, 27, 3069–3080. [Google Scholar] [CrossRef]
- Massalini, S.; Pellegatta, S.; Pisati, F.; Finocchiaro, G.; Farace, M.G.; Ciafrè, S.A. Reelin affects chain-migration and differentiation of neural precursor cells. Mol. Cell Neurosci. 2009, 42, 341–349. [Google Scholar] [CrossRef][Green Version]
- Pujadas, L.; Gruart, A.; Bosch, C.; Delgado, L.; Teixeira, C.M.; Rossi, D.; de Lecea, L.; Martínez, A.; Delgado-García, J.M.; Soriano, E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci. 2010, 30, 4636–4649. [Google Scholar] [CrossRef] [PubMed]
- Courtès, S.; Vernerey, J.; Pujadas, L.; Magalon, K.; Cremer, H.; Soriano, E.; Durbec, P.; Cayre, M. Reelin controls progenitor cell migration in the healthy and pathological adult mouse brain. PLoS ONE 2011, 6, e20430. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, C.M.; Kron, M.M.; Masachs, N.; Zhang, H.; Lagace, D.C.; Martinez, A.; Reillo, I.; Duan, X.; Bosch, C.; Pujadas, L.; et al. Cell-Autonomous Inactivation of the Reelin Pathway Impairs Adult Neurogenesis in the Hippocampus. J. Neurosci. 2012, 32, 12051–12065. [Google Scholar] [CrossRef]
- Trommsdorff, M.; Gotthardt, M.; Hiesberger, T.; Shelton, J.; Stockinger, W.; Nimpf, J.; Hammer, R.E.; Richardson, J.A.; Herz, J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor. Cell 1999, 97, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Balordi, F.; Fishell, G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J. Neurosci. 2007, 27, 14248–14259. [Google Scholar] [CrossRef]
- Howell, B.W.; Hawkes, R.; Soriano, P.; Cooper, J.A. Neuronal position in the developing brain is regulated by mouse disabled. Nature 1997, 389, 733–737. [Google Scholar] [CrossRef]
- Pramatarova, A.; Chen, K.; Howell, B.W. A genetic interaction between the APP and Dab1 genes influences brain development. Mol. Cell. Neurosci. 2008, 37, 178–186. [Google Scholar] [CrossRef]
- Uchida, T.; Baba, A.; Pérez-Martínez, F.J.; Hibi, T.; Miyata, T.; Luque, J.M.; Nakajima, K.; Hattori, M. Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages. J. Neurosci. 2009, 29, 10653–10662. [Google Scholar] [CrossRef]
- Heimer, L.; Robards, M.J. Neuroanatomical Tract-Tracing Methods; Springer: Boston, MA, USA, 1981. [Google Scholar]
- Howell, B.W.; Gertler, F.B.; Cooper, J.A. Mouse disabled (mDab1): A Src binding protein implicated in neuronal development. EMBO J. 1997, 16, 121–132. [Google Scholar] [CrossRef]
- Luque, J.M.; Morante-Oria, J.; Fairén, A. Localization of ApoER2, VLDLR and Dab1 in radial glia: Groundwork for a new model of reelin action during cortical development. Dev. Brain Res. 2003, 140, 195–203. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, G.; Nakajima, K.; Miyata, T.; Ogawa, M.; Mikoshiba, K.; Curran, T. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 1997, 17, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Valero, J.; Costell, M.; Sjögren, M.; Andreasen, N.; Blennow, K.; Luque, J.M. Altered levels of cerebrospinal fluid reelin in frontotemporal dementia and Alzheimer’s disease. J. Neurosci. Res. 2003, 72, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Howell, B.W.; Herrick, T.M.; Cooper, J.A. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 1999, 13, 643–648. [Google Scholar] [CrossRef]
- Wichterle, H.; Garcia-Verdugo, J.M.; Herrera, D.G.; Alvarez-Buylla, A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat. Neurosci. 1999, 2, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Wichterle, H.; Turnbull, D.H.; Nery, S.; Fishell, G.; Alvarez-Buylla, A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 2001, 128, 3759–3771. [Google Scholar] [CrossRef] [PubMed]
- Menezes, J.R.; Smith, C.M.; Nelson, K.C.; Luskin, M.B. The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol. Cell. Neurosci. 1995, 6, 496–508. [Google Scholar] [CrossRef]
- Jossin, Y.; Ignatova, N.; Hiesberger, T.; Herz, J.; Lambert de Rouvroit, C.; Goffinet, A.M. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J. Neurosci. 2004, 24, 514–521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef]
- Ignatova, N.; Sindic, C.J.; Goffinet, A.M. Characterization of the various forms of the Reelin protein in the cerebrospinal fluid of normal subjects and in neurological diseases. Neurobiol. Dis. 2004, 15, 326–330. [Google Scholar] [CrossRef]
- Guldbrandsen, A.; Vethe, H.; Farag, Y.; Oveland, E.; Garberg, H.; Berle, M.; Myhr, K.M.; Opsahl, J.A.; Barsnes, H.; Berven, F.S. In-depth characterization of the cerebrospinal fluid (CSF) proteome displayed through the CSF proteome resource (CSF-PR). Mol. Cell Proteomics 2014, 13, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Macron, C.; Lane, L.; Núñez Galindo, A.; Dayon, L. Identification of Missing Proteins in Normal Human Cerebrospinal Fluid. J. Proteome Res. 2018, 17, 4315–4319. [Google Scholar] [CrossRef] [PubMed]
- Iliff, J.J.; Wang, M.; Zeppenfeld, D.M.; Venkataraman, A.; Plog, B.A.; Liao, Y.; Deane, R.; Nedergaard, M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013, 33, 18190–18199. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS 2014, 11, 26. [Google Scholar] [CrossRef]
- Worthington, W.C., Jr.; Cathcart, R.S., 3rd. Ciliary currents on ependymal surfaces. Ann. N. Y. Acad. Sci. 1966, 130, 944. [Google Scholar] [CrossRef]
- Cifuentes, M.; Rodríguez, S.; Pérez, J.; Grondona, J.M.; Rodríguez, E.M.; Fernández-Llebrez, P. Decreased cerebrospinal fluid flow through the central canal of the spinal cord of rats immunologically deprived of Reissner’s fibre. Exp. Brain Res. 1994, 98, 431–440. [Google Scholar] [CrossRef]
- Siyahhan, B.; Knobloch, V.; de Zélicourt, D.; Asgari, M.; Schmid Daners, M.; Poulikakos, D.; Kurtcuoglu, V. Flow induced by ependymal cilia dominates near-wall cerebrospinal fluid dynamics in the lateral ventricles. J. R. Soc. Interface 2014, 11, 20131189. [Google Scholar] [CrossRef]
- Jiménez, A.J.; Domínguez-Pinos, M.D.; Guerra, M.M.; Fernández-Llebrez, P.; Pérez-Fígares, J.M. Structure and function of the ependymal barrier and diseases associated with ependyma disruption. Tissue Barriers 2014, 2, e28426. [Google Scholar] [CrossRef]
- Ohata, S.; Alvarez-Buylla, A. Planar Organization of Multiciliated Ependymal (E1) Cells in the Brain Ventricular Epithelium. Trends Neurosci. 2016, 39, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, M.K.; Walsh, C.A. Neurogenesis at the brain-cerebrospinal fluid interface. Annu. Rev. Cell Dev. Biol. 2011, 27, 653–679. [Google Scholar] [CrossRef] [PubMed]
- Zappaterra, M.D.; Lisgo, S.N.; Lindsay, S.; Gygi, S.P.; Walsh, C.A.; Ballif, B.A. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J. Proteome Res. 2007, 6, 3537–3548. [Google Scholar] [CrossRef] [PubMed]
- Fuentealba, L.C.; Obernier, K.; Alvarez-Buylla, A. Adult neural stem cells bridge their niche. Cell Stem Cell 2012, 10, 698–708. [Google Scholar] [CrossRef]
- Whish, S.; Dziegielewska, K.M.; Møllgård, K.; Noor, N.M.; Liddelow, S.A.; Habgood, M.D.; Richardson, S.J.; Saunders, N.R. The inner CSF-brain barrier: Developmentally controlled access to the brain via intercellular junctions. Front. Neurosci. 2015, 9, 16. [Google Scholar] [CrossRef]
- Marzesco, A.M.; Janich, P.; Wilsch-Bräuninger, M.; Dubreuil, V.; Langenfeld, K.; Corbeil, D.; Huttner, W.B. Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J. Cell Sci. 2005, 118, 2849–2858. [Google Scholar] [CrossRef]
- Huttner, H.B.; Janich, P.; Köhrmann, M.; Jászai, J.; Siebzehnrubl, F.; Blümcke, I.; Suttorp, M.; Gahr, M.; Kuhnt, D.; Nimsky, C.; et al. The stem cell marker prominin-1/CD133 on membrane particles in human cerebrospinal fluid offers novel approaches for studying central nervous system disease. Stem Cells 2008, 26, 698. [Google Scholar] [CrossRef]
- Aasebø, E.; Opsahl, J.A.; Bjørlykke, Y.; Myhr, K.M.; Kroksveen, A.C.; Berven, F.S. Effects of blood contamination and the rostro-caudal gradient on the human cerebrospinal fluid proteome. PLoS ONE 2014, 9, e90429. [Google Scholar] [CrossRef]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 4457124, 168. [Google Scholar] [CrossRef]
- Johansson, P.A.; Irmler, M.; Acampora, D.; Beckers, J.; Simeone, A.; Götz, M. The transcription factor Otx2 regulates choroid plexus development and function. Development 2013, 140, 1055–1066. [Google Scholar] [CrossRef]
- Johansson, P.A. The choroid plexuses and their impact on developmental neurogenesis. Front. Neurosci. 2014, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Silva-Vargas, V.; Maldonado-Soto, A.R.; Mizrak, D.; Codega, P.; Doetsch, F. Age-Dependent Niche Signals from the Choroid Plexus Regulate Adult Neural Stem Cells. Cell Stem Cell 2016, 19, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Agassandian, K.; Grobe, J.L.; Liu, X.; Agassandian, M.; Thompson, A.P.; Sigmund, C.D.; Cassell, M.D. Evidence for intraventricular secretion of angiotensinogen and angiotensin by the subfornical organ using transgenic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R973–R981. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.M.; González, C.; Caprile, T.; Jara, M.; Vío, K.; Muñoz, R.I.; Rodríguez, S.; Rodríguez, E.M. Understanding How the Subcommissural Organ and Other Periventricular Secretory Structures Contribute via the Cerebrospinal Fluid to Neurogenesis. Front. Cell Neurosci. 2015, 9, 480. [Google Scholar] [CrossRef]
- Adams, J.C.; Tucker, R.P. The thrombospondin type 1 repeat (TSR) superfamily: Diverse proteins with related roles in neuronal development. Dev. Dyn. 2000, 218, 280–299. [Google Scholar] [CrossRef]
- Ranaivoson, F.M.; von Daake, S.; Comoletti, D. Structural Insights into Reelin Function: Present and Future. Front. Cell Neurosci. 2016, 10, 137. [Google Scholar] [CrossRef]
- Hoe, H.S.; Wessner, D.; Beffert, U.; Becker, A.G.; Matsuoka, Y.; Rebeck, G.W. F-spondin interaction with the apolipoprotein E receptor ApoEr2 affects processing of amyloid precursor protein. Mol. Cell. Biol. 2005, 25, 9259–9268. [Google Scholar] [CrossRef]
- Meiniel, A. The secretory ependymal cells of the subcommissural organ: Which role in hydrocephalus? Int. J. Biochem. Cell Biol. 2007, 39, 463. [Google Scholar] [CrossRef]
- Furey, C.G.; Choi, J.; Jin, S.C.; Zeng, X.; Timberlake, A.T.; Nelson-Williams, C.; Mansuri, M.S.; Lu, Q.; Duran, D.; Panchagnula, S.; et al. De Novo Mutation in Genes Regulating Neural Stem Cell Fate in Human Congenital Hydrocephalus. Neuron 2018, 99, 302–314.e4. [Google Scholar] [CrossRef]
- Roegiers, F.; Jan, Y.N. Asymmetric cell division. Curr. Opin. Cell Biol. 2004, 16, 195–205. [Google Scholar] [CrossRef]
- Engler, A.; Rolando, C.; Giachino, C.; Saotome, I.; Erni, A.; Brien, C.; Zhang, R.; Zimber-Strobl, U.; Radtke, F.; Artavanis-Tsakonas, S.; et al. Notch2 Signaling Maintains NSC Quiescence in the Murine Ventricular- Subventricular Zone. Cell Rep. 2018, 22, 992–1002. [Google Scholar] [CrossRef]
- Imayoshi, I.; Sakamoto, M.; Yamaguchi, M.; Mori, K.; Kageyama, R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010, 30, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Rubio, M.E.; Gallo, V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 2010, 4677313, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Irvin, D.K.; Nakano, I.; Paucar, A.; Kornblum, H.I. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J. Neurosci. Res. 2004, 75, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.T.; Mirzadeh, Z.; Soriano-Navarro, M.; Rasin, M.; Wang, D.; Shen, J.; Sestan, N.; Garcia-Verdugo, J.; Alvarez-Buylla, A.; Jan, L.Y.; et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 2006, 127, 1253–1264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carlén, M.; Meletis, K.; Göritz, C.; Darsalia, V.; Evergren, E.; Tanigaki, K.; Amendola, M.; Barnabé-Heider, F.; Yeung, M.S.; Naldini, L.; et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 2009, 12, 259–267. [Google Scholar] [CrossRef]
- Li, Y.; Hibbs, M.A.; Gard, A.L.; Shylo, N.A.; Yun, K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by NotchStem. Cells 2012, 30, 741–752. [Google Scholar] [CrossRef]
- Yap, C.C.; Winckler, B. Adapting for endocytosis: Roles for endocytic sorting adaptors in directing neural development. Front. Cell Neurosci. 2015, 9, 119. [Google Scholar] [CrossRef]
- Piccin, D.; Yu, F.; Morshead, C.M. Notch signaling imparts and preserves neural stem characteristics in the adult brain. Stem. Cells Dev. 2013, 22, 1541–1550. [Google Scholar] [CrossRef]
- Givogri, M.I.; de Planell, M.; Galbiati, F.; Superchi, D.; Gritti, A.; Vescovi, A.; de Vellis, J.; Bongarzone, E.R. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev. Neurosci. 2006, 28, 81–91. [Google Scholar] [CrossRef]
- Weinhold, B.; Seidenfaden, R.; Röckle, I.; Mühlenhoff, M.; Schertzinger, F.; Conzelmann, S.; Marth, J.D.; Gerardy-Schahn, R.; Hildebrandt, H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J. Biol. Chem. 2005, 280, 42971. [Google Scholar] [CrossRef]
- Petridis, A.K.; El-Maarouf, A.; Rutishauser, U. Polysialic acid regulates cell contact-dependent neuronal differentiation of progenitor cells from the subventricular zone. Dev. Dyn. 2004, 230, 675–684. [Google Scholar] [CrossRef]
- Battista, D.; Rutishauser, U. Removal of polysialic acid triggers dispersion of subventricularly derived neuroblasts into surrounding CNS tissues. J. Neurosci. 2010, 30, 3995–4003. [Google Scholar] [CrossRef]
- Sato, Y.; Mii, A.; Hamazaki, Y.; Fujita, H.; Nakata, H.; Masuda, K.; Nishiyama, S.; Shibuya, S.; Haga, H.; Ogawa, O.; et al. Heterogeneous fibroblasts underlie age-dependent tertiary lymphoid tissues in the kidney. JCI Insight 2016, 1, e87680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larios, J.A.; Jausoro, I.; Benitez, M.L.; Bronfman, F.C.; Marzolo, M.P. Neurotrophins regulate ApoER2 proteolysis through activation of the Trk signaling pathway. BMC Neurosci. 2014, 15, 108. [Google Scholar] [CrossRef] [PubMed]
- Horstkorte, R.; Schachner, M.; Magyar, J.P.; Vorherr, T.; Schmitz, B. The fourth immunoglobulin-like domain of NCAM contains a carbohydrate recognition domain for oligomannosidic glycans implicated in association with L1 and neurite outgrowth. J. Cell Biol. 1993, 121, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Lutz, D.; Sharaf, A.; Drexler, D.; Kataria, H.; Wolters-Eisfeld, G.; Brunne, B.; Kleene, R.; Loers, G.; Frotscher, M.; Schachner, M. Proteolytic cleavage of transmembrane cell adhesion molecule L1 by extracellular matrix molecule Reelin is important for mouse brain development. Sci. Rep. 2017, 7, 15268. [Google Scholar] [CrossRef]
- Redmond, S.A.; Figueres-Oñate, M.; Obernier, K.; Nascimento, M.A.; Parraguez, J.I.; López-Mascaraque, L.; Fuentealba, L.C.; Alvarez-Buylla, A. Development of Ependymal and Postnatal Neural Stem Cells and Their Origin from a Common Embryonic Progenitor. Cell Rep. 2019, 27, 429–441. [Google Scholar] [CrossRef]
- Jossin, Y.; Goffinet, A.M. Reelin signals through phosphatidylinositol 3- kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell Biol. 2007, 27, 7113–7124. [Google Scholar] [CrossRef]
- Foerster, P.; Daclin, M.; Asm, S.; Faucourt, M.; Boletta, A.; Genovesio, A.; Spassky, N. mTORC1 signaling and primary cilia are required for brain ventricle morphogenesis. Development 2017, 144, 201–210. [Google Scholar] [CrossRef]
- Magri, L.; Cominelli, M.; Cambiaghi, M.; Cursi, M.; Leocani, L.; Minicucci, F.; Poliani, P.L.; Galli, R. Timing of mTOR activation affects tuberous sclerosis complex neuropathology in mouse models. Dis. Model Mech. 2013, 6, 1185–1197. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Martínez, F.J.; Cifuentes, M.; Luque, J.M. Reelin Signaling by the Prime Neurogenic Niche of the Adult Brain. Neuroglia 2025, 6, 43. https://doi.org/10.3390/neuroglia6040043
Pérez-Martínez FJ, Cifuentes M, Luque JM. Reelin Signaling by the Prime Neurogenic Niche of the Adult Brain. Neuroglia. 2025; 6(4):43. https://doi.org/10.3390/neuroglia6040043
Chicago/Turabian StylePérez-Martínez, Francisco Javier, Manuel Cifuentes, and Juan M. Luque. 2025. "Reelin Signaling by the Prime Neurogenic Niche of the Adult Brain" Neuroglia 6, no. 4: 43. https://doi.org/10.3390/neuroglia6040043
APA StylePérez-Martínez, F. J., Cifuentes, M., & Luque, J. M. (2025). Reelin Signaling by the Prime Neurogenic Niche of the Adult Brain. Neuroglia, 6(4), 43. https://doi.org/10.3390/neuroglia6040043





