Abstract
Under some circumstances, fires can be ignited by electric current. The two main mechanisms for this are arcing/sparking and hot surfaces. However, it has been viewed for a long time that this will not happen if the voltage, current, energy, or power are too low. The concept of a minimum ignition energy (MIE) characterizing the ignitability of flammable gas atmospheres is well established, and extensive published data are available. However, a corresponding ignition energy criterion for solids (minimum energy fluence) has been shown not to be valid. Some additional systematic experimental data (minimum voltage, current, power) have been collected for the spark ignition of gas atmospheres. However, it is found that the results are strongly dependent on the test conditions. Exceedingly scant data are available for the minimum electrical conditions for ignition of solid materials. Two concepts—intrinsic safety, and Class 2 or 3 power supplies—have long been available as safety measures against ignition from electrical circuit sources. However, ignition has been demonstrated to be possible with Class 2 power supplies. Ignition of solid material from a 1.2 V battery has been documented in the literature. Wide-ranging experimental research is urged to expand the knowledge base in this important area of electrical safety.
1. Introduction
Since electric current involves the flow of energy, under some circumstances this flow of energy can result in an unwanted or accidental fire being ignited [1]. The US national fire statistics [2] have some serious problems [3], but it is estimated that around 20% of structure fires are due to electrical causes [4]. In addition, a somewhat lower fraction of wildland fires is due to electrical causes [5]. Electric currents are also used in various circumstances for desired ignition, e.g., automotive spark plugs or glow plugs. The scope of this paper, however, is limited to conditions for unwanted ignition. Fires can also be ignited under certain circumstances from static electricity, which involves a situation where a continued source of power is not available. Static electricity ignitions were reviewed by Babrauskas [4,6] and will not be examined here.
There can be many reasons for endeavoring to exploit electrical ignition/non-ignition limits, notably the investigation of fire or explosion incidents [7]. However, this is not appropriate if such a limit does not exist, or if an incorrect value is ascribed to the limit. The concept that certain identifiable voltage, current, or power conditions must exist before there is danger of causing a fire or an explosion is utilized in a number of industries, where it becomes a design concept known as intrinsic safety, and this is typically applied in the context of explosion, rather than fire. In such cases, the definition of intrinsically safe equipment is commonly along the lines that a given circuit design will not result in an explosion of a certain fuel-gas/air mixture even under worst-case failure conditions. The field of intrinsic safety is complex, and we shall only refer to some general features for a background understanding. Instead, the focus will be on data that would be needed for investigations of fire or explosion incidents, under the assumption that specialized industrial-safety equipment (intrinsically safe, explosionproof, etc.) are not at issue and Classified Locations are not involved. However, a large volume of research exists on ignition in the context of intrinsically safe circuits or equipment. Thus, the literature will be reviewed from the standpoint of understanding its implications for the general ignition problem.
2. Ignition and Energy
The ignition of fire has been studied extensively and in significant depth. In the course of this, a great deal of research has been published; the present author [6] has published the Ignition Handbook on the topic. As an overview, what emerges is that ignition does not lend itself to simple characterization or useful rules of thumb. Instead, details of the experimental arrangement play a major role in determining the conditions needed for ignition. This is true for all states of matter, but more emphatically so for solids, compared with gases.
In fire science studies, a fundamental distinction is made between autoignition and piloted ignition. In autoignition, the temperature of a substance is progressively raised, until at a certain value (the autoignition temperature, AIT), the sample ignites. In piloted ignition, apart from any bulk heating, a small, localized flame, spark, or arc is provided. The piloted ignition temperature of a sample is generally lower than that for autoignition. Ignition temperatures of solid materials are normally measured empirically, often by the ASTM D1929 test [8], rather than being computed. Computation of ignition temperatures involves advanced modeling of a number of physical and chemical processes, including chemical kinetics, and cannot usefully be performed in any simplified way.
Experimental measurement of temperature is straightforward, and the tabulation of ignition temperatures is commonly undertaken since, for many types of ignition, the ignition temperature does not vary much as geometric or environmental conditions are changed. There are also theories [6] which suggest that this constancy should be expected.
Ignition has been systematically studied for four states of matter: gases, dust clouds, liquids, and solids. Ignition for most liquids comprises the ignition of their vapors, thus the problem becomes the same as for gases. Dust clouds behave more similarly to gaseous atmospheres than to solid matter. However, since they are of interest to only a few sectors of industry, they will not be considered further here, where attention will be limited to gases and solids.
Electric current may deliver energy to cause ignition in a number of ways [4], but here we shall limit our attention to the two primary ones: electric arcs or sparks, and hot surfaces. Ignition from ejecta is one of the specialized pathways for electrical ignitions, but this will not be reviewed here, since a recent study covered the mechanism in depth [4]. Ejecta are small, hot metal particles which can be ejected due to arcing or short circuiting.
The propensity for ignition of gas mixtures (an atmosphere consisting of air, or another oxidizer, plus a fuel gas) has been studied primarily by tabulating two quantities: the autoignition temperature, and the MIE (minimum ignition energy), with the latter being studied typically for the gas mixture at ambient temperature. The MIE is commonly measured by the ASTM E582 [9], although various other tests are also used.
For practical reasons, typically only the minimum ignition energy is tabulated; however, numerous variables, notably the fuel/air concentration (Figure 1) affect the energy needed for ignition. If the fuel/air ratio is not that required for worst-case conditions, the energy required for ignition may increase considerably, as indicated in the graph. Often, users may not be aware that the actual ignition energy required under their circumstances will be very different from the MIE.
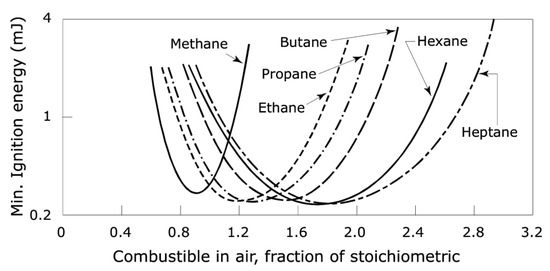
Figure 1.
The effect of fuel/air mixture on the MIE for alkane-series hydrocarbons [6,10].
3. Types of Electrical Ignition
The means by which electricity can ignite gases are primarily either by a spark or arc, or by a hot surface. A spark and an arc refer to the same phenomenon, but an arc involves a sustained delivery of current, whereas a spark is a transient event. Furthermore, the spark or arc can be delivered between fixed electrodes, or moving electrodes. The latter generally involves the making or breaking of contacts. A hot surface can be in various configurations, but it is found that larger hot-surface areas usually produce ignition under a wider range of circumstances [4,6,11] (Figure 2). The hot surfaces are often wires drawing a grossly excessive current for the wire size. However, hot-surface ignitions can also occur from overheated connections, where the inadequacy of ampacity is highly localized within the connection.
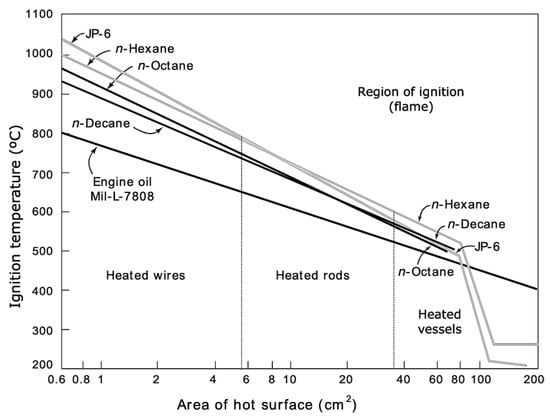
Figure 2.
Effect of a hot surface area on the ignition of several types of fuel vapors [6].
It is important to emphasize that, while both types of ignitions can be represented on a V–I diagram (if sufficient data are available), this does not in any way imply that the phenomena are interchangeable.
Solid materials can also be ignited by electric arcs/sparks or by hot surfaces. This is discussed in the final section of this paper.
4. Intrinsic Safety
In 1913, there was a large life-loss explosion at the Universal Colliery, in Senghenyddin, Wales [12]. The subsequent investigation suggested that the most likely ignition source was sparking at a battery-powered signal bell system [13]. Since this was the third mining disaster in two years attributed to sparking in low-voltage signaling systems [14], the UK government responded with a research program by Wheeler et al. [15,16,17], who developed the first version of a ‘break-flash’ testing apparatus. The intention was that the circuits which are qualified via that test apparatus will not be able to ignite flammable gas atmospheres. This work became the origin of what today is known as intrinsic safety.
NFPA 70, the National Electrical Code (NEC) [18] defines intrinsic safety as: “A circuit in which any spark or thermal effect is incapable of causing ignition of a mixture of flammable or combustible material in air under prescribed test conditions.” It then refers to UL 913 [19] for test requirements. Zborovszky [20] provides a more detailed explanation: “A circuit is considered intrinsically safe when any spark or thermal effect produced normally (that is, by operating the equipment in its correct operational manner to fulfill its purpose) or accidentally (caused by short circuit, earth fault, defective components, breaking the wiring, etc.) is incapable under prescribed test conditions of causing ignition of a prescribed gas mixture. The test conditions and gas mixture should be at least as hazardous as the true parameters of the environment where the circuit will be operated.” This exposition makes it clear that this is not a concept derived from some fundamental theory; instead, it is to be determined experimentally in the context of some agreed-upon test apparatus and test conditions. Magison [21] provides a detailed explanation of intrinsic safety and the means of achieving it. Such circuits normally exhibit the following characteristics:
- (1)
- Very low power consumption.
- (2)
- No electrical components which could store (and later discharge) a significant amount of energy. In practice, this means that values of capacitance and inductance must be kept very low. If higher values are needed, sometimes it is possible to arrange diode shunts which do not interfere with normal function, but which limit the amount of energy that could be made available in case of fault.
- (3)
- Protection circuits to limit the current that can flow into the device. Zener diodes placed across power supplies are a common way of meeting this requirement.
Devices with minimal capability of storing or generating energy can automatically qualify as intrinsically safe equipment. A device which cannot generate or store more than 1.2 V, 0.1 A, 20 mJ, nor 25 mW under normal or failure conditions qualifies without further testing [21]. Such a device is exemplified by a thermocouple, which generally produces less than 0.1 V. The limits are very tough to meet and devices other than transducers will rarely qualify. A device within these limits is sometimes referred to as a simple apparatus. The basis for the establishment of these minimum values is not known, and presumably represents committee action rather than laboratory research.
It is usually assumed that the break-flash apparatus (see below) presents sufficiently severe circuit conditions, so that testing with that apparatus suffices to establish voltage or current conditions such that ignition of flammable atmospheres is precluded. Thus, implications of this type of testing are discussed below.
5. Power-Limited Circuits
A milder form of safety concept is involved in the NEC descriptions of Class 1, Class 2, or Class 3 power-limited circuits (Sec. 725). The requirements for Class 1 circuits are so broad that there clearly is no basis for assuming any fire-safety effectiveness. Class 3 devices are only rarely encountered. Class 2 power-limited circuits or power supplies are common, however. The basic requirements for Class 2 and Class 3 power supplies are given in Tables 11(A) and 11(B) of the NEC, while additional details are provided in UL standards, there being at least eight different UL standards for this purpose [4]. Perhaps the most general of these is UL 1310 [22], for Class 2 power supplies. The NEC typically limits Class 2 power supplies to an output of 100 volt-amps, or less. Current output is limited to 8 A for voltage ratings of 30 V or lower, while power supplies providing 60–150 V, have the output is limited to 5 mA; however, there is not widespread usage of 60–150 V Class 2 power supplies.
Small and Vicars [23] studied some details of these power supplies and pointed out that designers using these supplies are sometimes unaware of the 8 A output potential and assume that for a 24 V, 40 V-A power supply only the steady-state output of 1.67 A will be present, thus not providing for safe handling of currents up to 8 A. Durham et al. [24] noted that Class 2 power supplies are sometimes assumed to be incapable of igniting fires, but neither the NEC nor the UL make such claims. They then conducted a series of tests with commercial Class 2 power supplies, and found that one in six trials led to a flaming fire. The test in question involved a Class 2 power supply rated for a 12 VDC, 900 mA output. The ignition mode was by hooking up grossly under-rated resistors to the output of the device. The power delivered was measured at 21.6 W. The authors also demonstrated that they could ignite paper with an 11.6 W output (not from a Class 2 power supply).
6. Thin Wires
The initial temperature rise of a bare copper wire subjected to an instantaneous application of a current I (amperes) is [4]:
where ΔT is the temperature rise (°C), A is the cross-sectional area (mm2), and t is the time (s). A simple expression is not available for intermediate intervals, but assuming that the surface heat transfer can be represented by a convective heat transfer coefficient h (W m−2 K−1), the steady-state expression is:
and where density of copper ρ = 8960 kg m−3. Note that no heat transfer coefficient is present in the first equation, since the initial temperature rise is assumed to be very rapid, making heat losses negligible during this time. Thus, for both short and long times, the temperature rise is proportional to I2, and inversely proportional to the conductor area, to the power of 3/2 or 2.
The above equations have an important implication: the temperature rise can be driven to any arbitrary, high value simply by making the conductor cross-sectional area small enough. There is a practical limit to this, however, even though no equations are available to quantify it. At high temperatures, the oxidation of copper becomes very rapid [4]. During this process, metallic copper is replaced by copper oxides, which are substances with low mechanical strength. The result is that a very thin wire is likely to crumble and fall apart. Nonetheless, there is the overt danger of igniting combustibles in propinquity, prior to ultimate failure of the wire.
This is the reason why small-diameter wires are generally not permitted for equipment with intrinsic safety. However, unintentionally small conductors can occur as a failure condition. This can often take the form of failed contacts or connection where the area over which current is carried in some location becomes very small. The above situation explains why a small design current may not be sufficient to assure no ignitions due to a high conductor temperature rise.
7. Minimum Conditions for Electric Arcs
When studying conditions for arcing, it is important to understand that two different limits may be involved. Some research has been undertaken to obtain the minimum values of voltage or current needed to establish an arc. In such experiments, ignition testing is not undertaken. However, other research has focused on incendive arcs, i.e., conditions under which an arc can actually ignite a target gas atmosphere. These two types of findings are not necessarily related.
Researchers studying electric arcs long ago concluded that a minimum voltage and a minimum current is needed for an electric arc to be possible. These values depend on the material of the electrodes, and for copper electrodes, the reported values [4] are 11.0 V and 0.45 A. At a lower voltage or current, a discharge can still be created, but it is generally classed as a spark [25], rather than an arc, due to the non-sustained nature.
Only limited research has been reported on studies of voltages or currents below the ‘minimum’ values. Zborovszky [26] conducted experiments showing that, for values below the arc minima, although spark discharges are possible, the energy delivery is so small that ignition will not occur. In addition, she further noted that, even above the minima values, for Imin < I < 2Imin and Vmin < V < 2Vmin, discharges tended to allow only low rates of power delivery. However, she noted that, under some circumstances, ignition could occur due to hot electrode surfaces, rather than the gas discharge. Her test rig, the IEC break-flash apparatus (see below), however, is not similar to most end-use conditions, due to the rotating electrode arrangement. Additionally, she noted that the limitations for operating below 2Vmin and 2Imin are due to specific heat losses in this test rig, and should not be taken as universal.
Unfortunately, Zborovszky’s conclusions do not appear to be conservative. Already by 1914, Wheeler [15] was able to ignite atmospheres of methane in air using a primitive break-flash apparatus running at 4.5 V. Additionally, in his 1915 report [16], he showed ignitions using only 0.30 A. Note that these results document incendive arcs, not just conditions where arcing is feasible. For the latter, in 1923, Nottingham [27] presented experimental results showing that steady arcs between copper electrodes can run at 6 V and hypothesized that the actual minimum voltage is 5 V. The disparity between these low values and present accepted values of 11.0 V and 0.45 A is presumably due to experimental differences, but this has not been satisfactorily elucidated. Some additional research also points to the poorly understood role of the experimental arrangements. If one multiplies the Vmin by the Imin, this indicates that, in a resistive circuit, 11.0 × 0.45 = 4.95 W would be the minimum power needed for an arc discharge. However, Pugh [28] conducted some experiments where she directly measured the minimum power needed to sustain an arc. Using a 2000–5000 VAC power supply, she found that, typically, 1 W sufficed, but in some cases, depending on the circuit resistance, as little as 0.2 W was sufficient. Even the 1 W value is substantially below the 4.95 W.
8. Electrode Materials
In the field of intrinsic safety research, some researchers [26] studied at length the role of the electrode materials in establishing ignition limits on a V–I plot. In turn, standards organizations (ISA [29] and UL [30]) ended up classifying electrode materials into two groups:
- Al, Cd, Mg, and Zn;
- Cu, Fe, and all others.
This grouping appears to have some relation to Im, but the details are not entirely clear. The values of Im for Cu are 0.45 A and for Fe 0.72 A. Meanwhile, Cd = 0.03 A and Zn = 0.03 A. However, Al = 0.4 A; thus, the basis for the constituency of the ‘sensitive’ group is unclear. Further research by Zborovszky [26] showed that, at least with some materials, surface condition also plays a major role. If arcing residues are allowed to accumulate, such conditions promote the initiation and continuation of a discharge.
9. The Role of Frequency
In early research (1924), Thornton [31] provided data showing that ignition from DC currents is easiest, and that progressively higher voltages or currents are required as the frequency is raised from 0 to 160 Hz. However, for 160–415 Hz there was a U-shaped response, although beyond 415 Hz frequency appears to have but little effect (Figure 3). Thornton considered that the initial increase in ignition difficulty with increasing frequency is due to heating of the electrodes, with more rapid polarity reversal leading to lower ability to provide sustained heating. The dip he considered to be due to competition between effects of ionization versus thermal heating. He also noted that the dip effect was not as pronounced with some other gases. Later research [32,33] indicates that actual performance seems to heavily depend on the test details, including parasitic reactances, and that DC conditions may or may not be more incendive than AC. The research by Allsop and Guénault [34] indicates that there may be a significant frequency effect if inductance is not minimized, but a negligible one in resistive circuits. Thus, this again is an area where guidance is inadequate, since research findings are limited and conflicted.
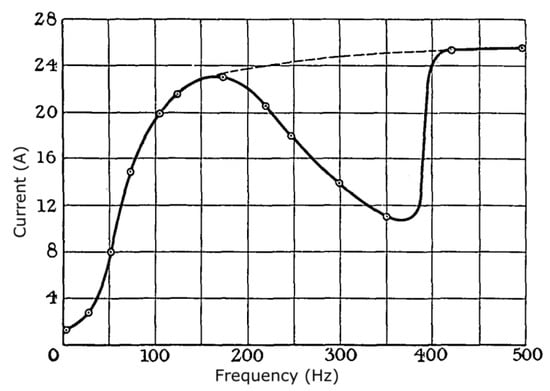
Figure 3.
The effect of frequency on the current needed to ignite a methane/air mixture with a 200 V power supply, as reported by Thornton [31].
10. Break-Flash Apparatus Testing
The earliest research on break-flash testing was in studies by Wheeler, discussed above, conducted during 1914–1916. In later decades, research of this type was most concertedly performed by the Bureau of Mines in the US, and by the Safety in Mines Research Establishment (SMRE) in the UK. In the course of their research, SMRE researchers developed several break-flash testing apparatuses. Meanwhile a different break-flash apparatus was developed by the German institute Physikalische-Technische Bundesanstalt (PTB). No published descriptions of this development work can be found, but eventually the method became standardized under IEC 60079-11 [35], and is often known as the IEC/PTB break-flash apparatus. This apparatus replaced the SMRE apparatuses, and was used in later SMRE research [6].
In the IEC/PTB test rig, a DC power supply, often 27–30 V, is applied to a set of rapidly opening contacts, where a break-spark (the spark created upon opening of the contacts) ignites, or does not ignite, a particular gas mixture. The standard electrodes are tungsten wires and a cadmium disk, these being chosen as representing worst-case conditions [36,37]. The independent variable is normally the available current; thus, tests in the apparatus determine the minimum igniting current (MIC), which, of course, is directly applicable only under conditions which are very similar to the test conditions. To determine the minimum values needed for ignition, it is necessary to consider the circuit. If a sizable capacitor or inductor exists in the circuit, then discharge of their stored energy may provide an ignition even in the absence of any power supply hookup. Thus, it is often desirable to study ignition conditions in a resistive circuit, i.e., with C → 0 and L→ 0. For such conditions, SMRE developed a set of ignition/non-ignition curves [38]. Figure 4 shows their results for methane atmospheres. Note that there is a large effect of electrode type; Cu electrodes lead to much greater currents required for ignition than those of Al or certain other metals.
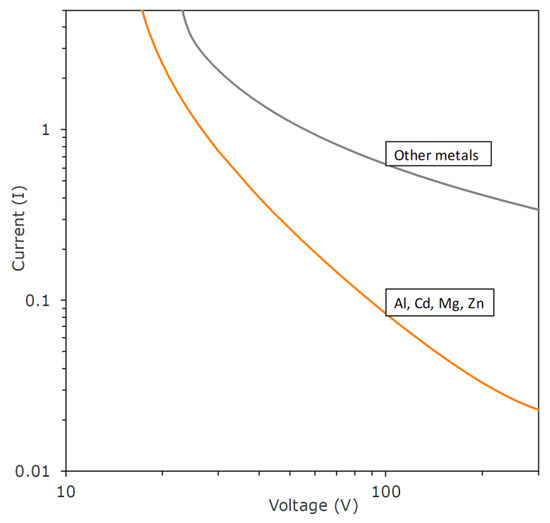
Figure 4.
Ignition curves for methane gas atmospheres in resistive circuits published by SMRE; curves give MIC as a function of voltage.
It must be emphasized that the ignition process is probabilistic, and that researchers compiling values of MIC have sometimes adopted definitions for MIC where they tabulate not the mean value of current needed to achieve ignition, but a value corresponding to a rare event. For example, Cawley [39] used a definition where the MIC corresponds to the current at which only 1 in 400 spark trials will product ignition, while Allsop [40] used 1 in 100 trials. Other researchers have used values ranging from 1 in 10, to 1 in 1000 [41]. Such definitions may be useful for regulatory activities, but are not helpful when trying to understanding actual incidents. Of even more concern, Bane [42] explains that many published data sources have unknown levels of probability, or erroneous ones.
Zborovszky [43] studied in detail the current needed to cause a break-spark ignition in a circuit with variable capacitance (Figure 5). There are four basic regimes of ignition from such a circuit. To the left of point A, the circuit acts as a purely resistive circuit. Between point A and point B, the charge on the capacitor contributes some energy, but the major part of the energy comes directly from the power supply. Between point B and point C, the bulk of the energy comes from the charge on the capacitor, while beyond point C, ignition energy is drawn only from the charge stored on the capacitor, and the energy flowing from the power supply is negligible. Observations also indicated that to the left of point B, ignition occurs at a break-spark, while to the right of point B at a make-spark.
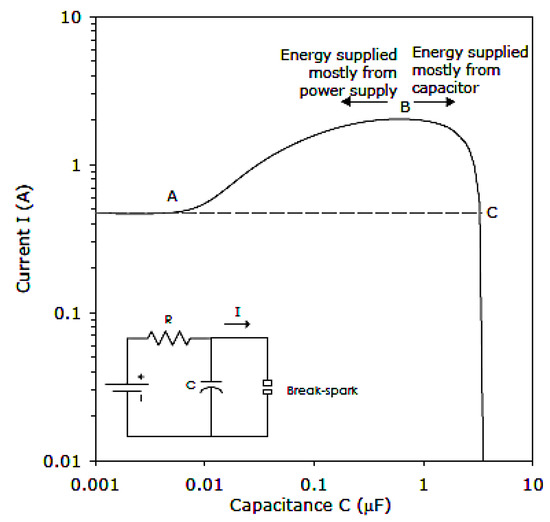
Figure 5.
Current needed for the ignition of an 8.3% CH4/air atmosphere using the IEC 60079-11 apparatus fed from a 30 V power supply. At lower supply voltages, the curve would move up and to the right.
Figure 6 shows some additional results obtained in the similar SMRE test rig [44]. The results should be viewed as indicative and not absolute, since different values are found using different test rigs [45,46] The 4 V value for minimum voltage is a very low value for this type of experiment and more commonly a lower limit of 8–15 V has been found in purely resistive circuits. In inductive circuits, however, ignitions have been reported at the 0.5 V level [47], and lower-yet values are not precluded. In Figure 6, there is an inverse relationship between voltage and current, suggesting that—under the test conditions considered—there may be, at least approximately, a power limit.
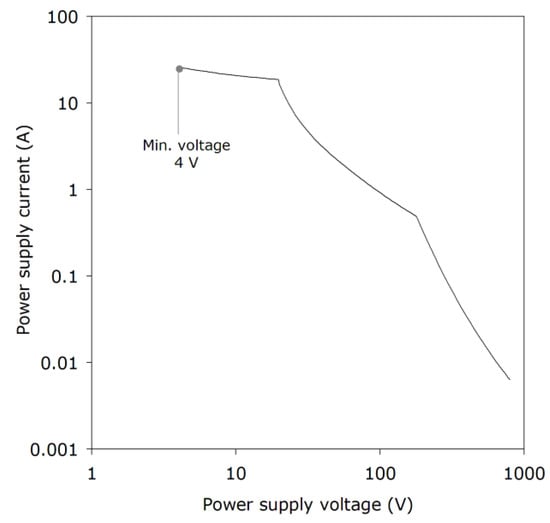
Figure 6.
Ignition of 8.3% methane/air mixtures with a resistive circuit: relation between voltage and MIC (SMRE Intermittent Break-flash test apparatus).
Note that these types of results are highly dependent on the test apparatus used. Widginton [47] examined seven different test arrangements, similar in principle, but different in details, and obtained results which show a great deal of disparity (Figure 7).
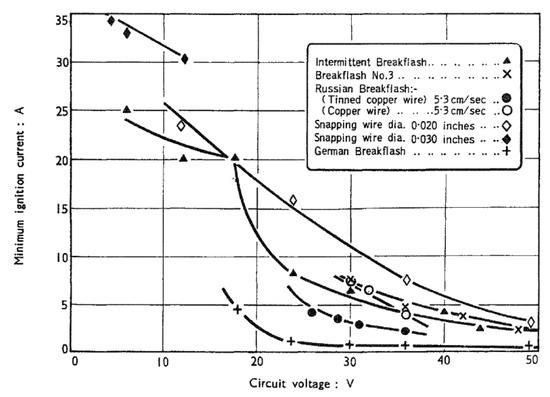
Figure 7.
Effect of apparatus differences on the I–V relationship for the ignition of 8.3% methane atmospheres in a resistive circuit (Widginton [48]).
The break-flash apparatus has the disadvantage in that the arc is surrounded by a large thermal mass. Oancea et al. [49] found that the energy required for ignition will be less from a spark between stationary electrodes, versus from the break-spark in the break-flash apparatus. Some use has been made of wire-break test rigs to reduce this heat loss effect [50], although the arrangement has not proven to be practical enough for standardized testing.
11. The Power Needed and the Role of Discharge Time
It appears that one of the reasons why researchers have found that the MIE concept is valid for gases is that, in ASTM E582 and in various other tests, the energy is delivered as the stored energy of a capacitor which is rapidly discharged. How fast is this discharge energy delivered? The actual spark duration varies with the test voltage, but is typically [51] in the range of 1–100 ms. Meanwhile, Takizawa et al. [52] found that if the duration time is <1 ms, the delivery is inefficient, and the MIE recorded will not be the true minimum. Gordon [53] found that a minimum duration of 17 μs was needed. Zborovszky [54] found that a minimum spark duration of around 3 ms was needed for successful ignition of gas mixture (Figure 8). Similar trends were found by Takizawa et al. [52], although with a great deal of scatter. Widginton [48], on the other hand, found that the effect was dependent on the electrode gap used. For a gap size roughly equal to the quenching distance, no effect was seen until about 300 μs, beyond which the MIE progressively rose. However, for a gap substantially smaller than the quenching distance, the energy needed rose directly with the increase in the discharge duration. The guidance to be taken from this set of research is very limited, unfortunately. It appears that experimental details control the outcome, and the effects of the different variables have not been adequately assessed.
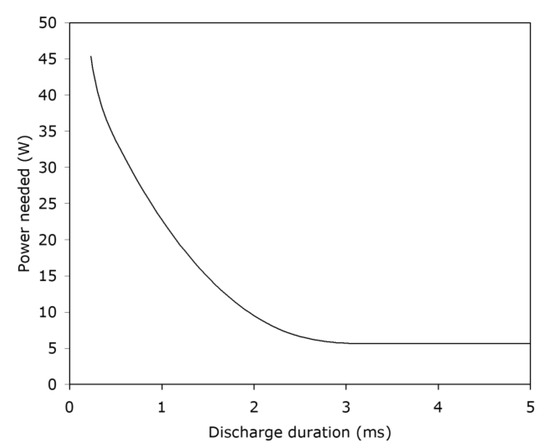
Figure 8.
Minimum power needed to ignite an 8.3% methane/air mixture using the IEC 60079-11 apparatus (27 VDC power supply, resistive circuit, spark at a tungsten wire separating from a cadmium plate).
The average power delivery can be computed by dividing the MIE by the spark duration time. This is only a crude measure, since, the energy delivery characteristic of capacitive discharge systems is typically a decaying exponential, not a steady-state delivery.
Basic physics suggests that if power is delivered at too slow a rate, no ignition will occur, despite an amount of energy being delivered which may be >>MIE. Combustion researchers have primarily studied the energy needed for ignition, and not the power. However, from an electrical safety point of view, quantifying both of these variables may be needed. One estimate of the minimum power needed for ignition can be derived from the V–I graph (Figure 4) by multiplying out P = VI at each data point. This gives the results shown in Figure 9. This shows a minimum value of 56 W for Cu and most other metals, along with 6.5 W for Al, Cd, Mg, or Zn.
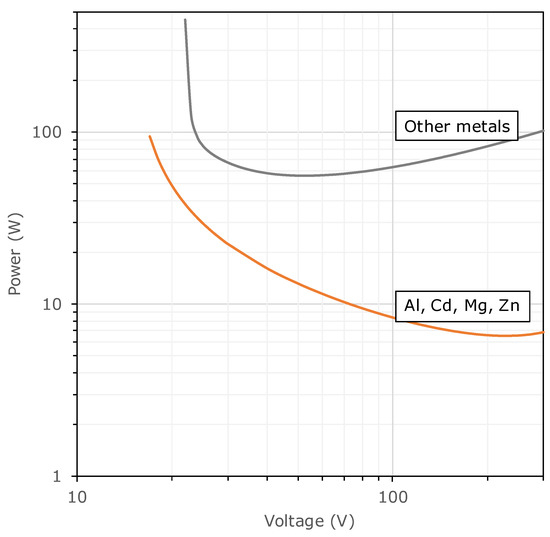
Figure 9.
Minimum power needed for ignition of methane atmospheres, derived from the SMRE V–I data.
In later research, Zborovszky [54] found that about 5 W is the minimum power needed to ignite methane–air mixtures (Figure 8). In another series of tests [26], she found that, for resistive circuits over a sizable range of voltages, conditions leading to ignition of atmospheres containing 8.3% methane in air entailed power levels varying over the range of 3.2–3.9 W. Consequently, her very early estimate [41] that 0.3 mW is the minimum power required for the ignition of methane/air atmospheres appears questionable.
The Bureau of Mines [55] showed that a 0.95 W incandescent lamp can cause an explosion when breaking the circuit within a flammable methane/air mixture, but one rated at 0.74 W could not. The 0.95 W value is very similar to the 1 W reported by Pugh, but note that Pugh was seeking to establish the minimum conditions for sustaining an arc, not for ignition of gases.
12. Ignition of Solids
Peacock and Vaishnav [56] designed a test method for testing solids that might be highly prone to electric arc ignition. Their apparatus used a 10 kV (AC) source to create an arc between two copper electrodes placed 5 mm apart and 3 mm above the specimen surface. Black powder and smokeless powder ignited in about 0.1 s, as did human hair. The head of a safety match ignited in 0.2 s, while common consumer goods such as paper, plastic and rubber took about 0.5–1.0 s. This study did not examine minimum arc conditions needed to ignite solid fuels, and it is noteworthy that no other study exists which would endeavor to do that.
Solids are more likely to be ignited from electrical failures by hot-surface heating, than by heating from arcs. Ignition of solids by hot surfaces, of course, is a general mechanism and electricity does not necessarily need to get involved. However, it is difficult to draw broad conclusions, since hot-surface heating involves more than a single variable. The temperature of the hot surface is the primary variable, but the size and shape of the surface are important, as are the thermophysicochemical properties of the hot body.
A theory exists for treating the situation where a spherical hot body is immersed into a granular material [6]. This can cover the situation where ejecta from electrical arcing are embedded into a granular material. Such incidents are not common, however. For a very few materials, e.g., steel balls in cellulose powder, sufficient data exist so that empirical guidance curves have been published [57]. These show that for a substance with an AIT = 250 °C, a hot-spot temperature of around 1200 °C would be needed for a 3 mm diameter body.
A small body of research exists where square or rectangular hot plates were used to ignite plastics [6]. The main conclusion that can be drawn is that the hot surface needs to be at a temperature substantially greater than the AIT of the ignitable solid. Conceptually, one can expect that curves similar to those shown in Figure 2 for gases would govern. However, detailed research is not available to actually create plots of this kind for solid materials. Most research on the ignitability of solids involve exposure of sizable areas, often on the order of 100 mm × 100 mm [58]. If the area of heat exposure is made progressively smaller, then increasing thermal attack (a higher heat flux) is needed to achieve ignition [5,59].
Perhaps the most important case of ignition of solid materials due to electrical failures involving hot surfaces is the ignition of wire insulation by excessive overcurrent flowing in wires. In [4], an equation is given for the overcurrent needed for ignition to occur, if thermophysical properties, along with the ignition temperature, are known. An example calculation indicates that a single AWG 14 copper wire, insulated with PVC insulation, would ignite at 86 A. In view of a rated ampacity of 15 A, this represents an overcurrent of 5.7×. This situation was also explored in several experimental programs. Significant scatter exists, but ignition generally can be expected at 3× to 8× of the rated current. This is a sufficiently broad range that focused experiments will generally be needed if reconstruction of an accident is necessary.
The Tokyo Fire Department [60] reported a case where a person’s pocket ignited when a Ni–Cd cell (1.2 V, 600 mAH) shorted out inside his pocket. Testing showed that, when short-circuited by an external metallic object, the cell was able to pass approximately 18–20 A through test clips and staples and heat them red-hot. This 1.2 V value is believed to be lowest reported value for the ignition of a solid substance.
13. Energy Criteria for the Ignition of Solids
The question of whether an energy criterion for the ignition of solids is appropriate has been studied [6]. Since solids can be of indefinitely large size, it is clear that a pure energy (mJ) criterion cannot possibly be valid, since the same amount of energy distributed over progressively larger bodies would lead to progressively lower temperature rise. Meanwhile, as noted above, under many conditions it is found that a fixed temperature rise criterion does serve to characterize the ignition process. For some explosives [61], it has been found that an energy fluence (defined as energy, divided by exposed face area, e.g., kJ/m2) can be valid. However, this concept is not valid for ordinary solid combustibles, as illustrated in Figure 10. If the fluence concept were valid, the data would be situated along horizontal lines, not downward-sloping curves.
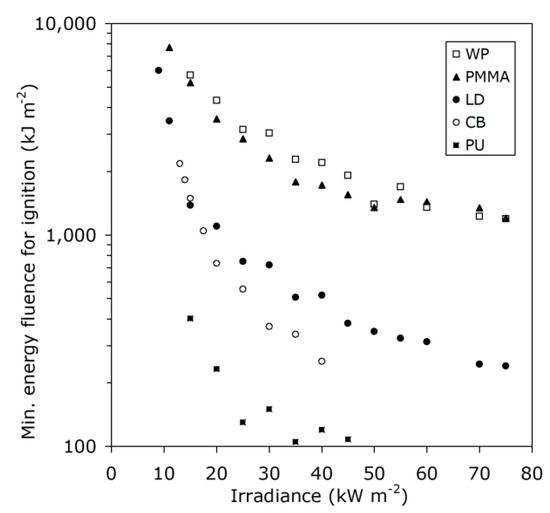
Figure 10.
Minimum energy fluence for some thermally thick solids [6] (WP, wood particleboard; PMMA, polymethylmethacrylate; LD, low-density fiberboard; CB, cardboard; PU, polyurethane foam).
14. Conclusions
No electrical ignition will occur under conditions of zero voltage, current, power, or energy. Conversely, ignitions can occur when substantial amounts of voltage, current, power, or energy are available. This leads one to consider that there may be some lower limits for ignition to be possible. This is consistent with the general observation that for ignition to occur of substances which are not self-heating or exothermically reacting, some finite amount of external energy must be provided. Due to the mass per volume of substance, it is much more difficult to ignite solids, compared with gases. However, for either state of matter, the minimum conditions needed will depend on the geometry and operating details of the experimental rig.
Minimum ignition energy (MIE) values for flammable gas mixtures have been studied for decades. Under ideal (worst-case) conditions, values of in the range of 0.2–2.0 mJ are found for many common fuel gases in air [6]. For solids, the corresponding energy criterion would be an energy fluence criterion. This is valid for certain explosives, but it has been shown not to be valid for common combustibles.
Conditions needed to cause ignitions of gas mixtures by sparks have been studied using break-flash apparatuses for over 100 years. This research began in 1914–1916 at the UK Home Office, then continued through the 1960s at the Safety in Mines Research Board (later, Safety in Mines Research Establishment; eventually Health and Safety Executive) in the UK. In the US, the Bureau of Mines sponsored extensive research by Zsuzsanna Zborovszky during 1972–1982. This was research at a single laboratory, and efforts were not undertaken to examine interlaboratory reproducibility of results. Research during the last 40 years has been nearly absent.
Despite over a century’s worth of research, guidance on the minimum voltage or the minimum current needed to create an arc is limited and conflicted. In circuits with substantial inductance or capacitance, creating of an arc (or igniting gases from an arc), can occur at indefinitely low current/voltage values, since substantive power can be delivered from the inductive or capacitive element. Thus, discussion of minimum values can be restricted to considerations of resistive circuits. The generally accepted values of 11.0 V and 0.45 A for arcs between copper electrodes are demonstrably unconservative, since arcs can be maintained at 6 V and possibly at 5 V. More modern research also indicates that an arc discharge may be possible using only 0.2 W of power, at least in some situations. Guidance as to the minimum conditions under which an arc can be incendive is also inadequate. Early research showed that flammable atmospheres demonstrably can be ignited with arcs between copper electrodes where 4.5 V, or 0.3 A is available; more recent research shows a 4.0 V value. However, there is no reason to believe that even these lower values are minimum values which would apply under varying experimental conditions.
No published research has been uncovered to justify the requirements for a ‘simple apparatus,’ nor for Class 2 or Class 3 power supplies.
A case history demonstrates that, under some circumstances, a 1.2 V voltage source can ignite a solid material. This should be viewed in the context that it is much more difficult to ignite solids, rather than gases.
It is urged that research be undertaken to determine if, under some specified conditions, values of voltage, current, or power can be established below which ignition is precluded. Research should also be undertaken to evaluate the reliability, or lack thereof, of the published criteria for intrinsic-safety-qualified equipment, and for Class 2 and Class 3 power supplies.
Many more fires occurs where electrical failures ignite solids materials, as opposed to gaseous atmospheres. However, existing research is inverted with regard to this need—most research has been on gaseous atmospheres. Significantly more research is needed to be able to give useful guidance on minimum circuit conditions needed for electrical ignitions of solid materials.
In this review of the subject, one of the notably lowest values (the 1.2 V ignition from a Ni–Cd cell) was documented because it was associated with a fire incident. This suggests that the state of knowledge could be improved if more use were made of case incidents. This paper may serve as motivation for the benefits to be seen from such publications.
Funding
No external funding was received.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Campbell, R.C. Home Electrical Fires; National Fire Protection Association: Quincy, MA, USA, 2019. [Google Scholar]
- The National Fire Data Center. Fire in the United States, 2008–2017, 20th ed.; U.S. Fire Administration: Emmitsburg, MD, USA, 2019.
- Icove, D.J.; Hargrove, T.K. Project Arson: Uncovering the True Arson Rate in the United States. In Proceedings of the International Symposium on Fire Investigation (ISFI 2014), Sarasota, FL, USA, 1 July 2014; pp. 283–292. [Google Scholar]
- Babrauskas, V. Electrical Fires and Explosions; Fire Science Publishers: New York, NY, USA, 2021. [Google Scholar]
- Ahrens, M. Brush, Grass, and Forest Fires; National Fire Protection Association: Quincy, MA, USA, 2018. [Google Scholar]
- Babrauskas, V. Ignition Handbook; Fire Science Publishers/Society of Fire Protection Engineers: Issaquah, WA, USA, 2003. [Google Scholar]
- Guide for Fire and Explosion Investigations (NFPA 921); National Fire Protection Association: Quincy, MA, USA, 2021.
- ASTM D1929; Standard Test Method for Determining Ignition Temperature of Plastics. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM E582; Standard Test Method for Minimum Ignition Energy and Quenching Distance in Gaseous Mixtures. ASTM International: West Conshohocken, PA, USA, 1999.
- Blanc, M.V.; Guest, P.G.; von Elbe, G.; Lewis, B. Ignition of Explosive Gas Mixtures by Electric Sparks. III. Minimum Ignition Energies and Quenching Distances of Mixtures of Hydrocarbons and Ether with Oxygen and Inert Gases. In Proceedings of the 3rd Symposium on Combustion and Flame and Explosion Phenomena; Williams & Wilkins: Philadelphia, PA, USA, 1949; pp. 363–367. [Google Scholar]
- Babrauskas, V. Ignition of Gases, Vapors, and Liquids by Hot Surfaces. Fire Technol. 2022, 58, 281–310. [Google Scholar] [CrossRef]
- Redmayne, R.A.S.; Williams, E.; Smillie, R. Causes and Circumstances Attending the Explosion Which Occurred at the Senghenydd Colliery on Tuesday, 14th October, 1913; Home Office, HMSO: London, UK, 1914. [Google Scholar]
- McMillan, A. Electrical Installations in Hazardous Areas; Butterworth-Heinemann: Oxford, UK, 1998. [Google Scholar]
- Lloyd, H.; Guénault, E.M. The Use of Break-Flash Apparatus No. 3 for Intrinsic Safety Testing (Research Report 33); Safety in Mines Research Establishment: Sheffield, UK, 1951. [Google Scholar]
- Wheeler, R.V. Wheeler’s Report on Experiments with Signalling Apparatus. In Causes and Circumstances Attending the Explosion Which Occurred at the Senghenydd Colliery on Tuesday, 14th October, 1913; HMSO: London, UK, 1914; pp. 36–40. [Google Scholar]
- Wheeler, R.V. Report of Battery-Bell Signalling Systems as Regards the Danger of Ignition of Firedamp-Air Mixtures by the Break-Flash at the Signal-Wires; HMSO: London, UK, 1915. [Google Scholar]
- Wheeler, R.V.; Thornton, W.M. Report on Electric Signalling with Bare Wires so far as Regards the Danger of Ignition of Inflammable Gaseous Mixtures by the Break-flash at the Signal Wires; HMSO: London, UK, 1916. [Google Scholar]
- National Fire Protection Association. National Electrical Code (NFPA 70); National Fire Protection Association: Quincy, MA, USA, 2017. [Google Scholar]
- Standard for Safety—Intrinsically Safe Apparatus and Associated Apparatus for Use in Class I, II, and III, Division 1, Hazardous (Classified) Locations (ANSI/UL 913) UL; Underwriters Laboratories Inc.: Northbrook, IL, USA, 1988.
- Zborovszky, Z.; Cotugno, L.A. A Comprehensive Study of Intrinsic Safety Criteria (Report DRI 2597; Bureau of Mines OFR 23–73); University of Denver Research Institute: Denver, CO, USA, 1972. [Google Scholar]
- Magison, E.C. Electrical Instruments in Hazardous Locations, 4th ed.; ISA: Research Triangle Park, NC, USA, 1998. [Google Scholar]
- Class 2 Power Units (UL 1310), UL.; Underwriters Laboratories Inc.: Northbrook, IL, USA, 2018.
- Small, J.E.; Vicars, R.J. Class 2 Transformers and Plastic Enclosed Printed Circuit Boards: A Potentially Perilous Combination, In Proceedings of the IEEE Symposium on Product Compliance Engineering, 2010.
- Durham, M.O.; Durham, R.A.; Ozment, C.I.; Coffin, J. Unraveling the Myths of Low Energy Electrical Ignition, Paper X. In Proceedings of the Frontiers in Power Conference 2009, Stillwater, OK, USA, 2009. [Google Scholar]
- Atalla, M.M. Arcing of Electrical Contacts in Telephone Switching Circuits. Part IV—Mechanisms of the Initiation of the Short Arc. Bell Syst. Tech. J. 1955, 34, 203–220. [Google Scholar] [CrossRef]
- Zborovszky, Z.; Cotugno, L.A. Evaluation of the Cadmium Disc Breakflash in Testing Electrical Circuits—Safety in Explosive Atmospheres—A Comprehensive Study of Intrinsic Safety Criteria (BuMines OFR 68–76); Bureau of Mines: Pittsburgh, PA, USA, 1974. [Google Scholar]
- Nottingham, W.B. A New Equation for the Static Characteristic of the Normal Electric Arc. J. AIEE 1923, 42, 12–19. [Google Scholar]
- Pugh, S.N. Forensic Evidence of Electric Arcs. Master’s Thesis, University of California, Davis, CA, USA, 2006. [Google Scholar]
- C22.2 No. 213-17; Nonincendive Electrical Equipment for Use in Class I and II, Division 2 and Class III, Divisions 1 and 2 Hazardous (Classified) Locations (ANSI/ISA-12.12.01–2000). The Instrumentation, Systems, and Automaton Society: Research Triangle Park, NC, USA, 2000.
- Intrinsically Safe Apparatus and Associated Apparatus for Use in Class I, II, and III, Division 1, Hazardous (Classified) Locations (UL 913), 6th ed.; note that ignition curves and other technical criteria were removed from subsequent editions; Underwriters Laboratories Inc.: Northbrook, IL, USA, 2002.
- Thornton, W.M. Some Researches on the Safe Use of Electricity in Mines. J. IEE 1924, 62, 481–491. [Google Scholar] [CrossRef]
- Wheeler, R.V. The Ignition of Gases. Part, V. Ignition by Inductance Sparks. Mixtures of the Paraffins with Air. J. Chem. Soc. Trans. 1925, 127, 14–26. [Google Scholar] [CrossRef]
- Morgan, J.D. The Thermal Theory of Gas Ignition by Electric Sparks. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1925, 49, 323–336. [Google Scholar] [CrossRef]
- Allsop, G.; Guénault, E.M. The Incendivity of Electric Sparks in Relation to the Characteristics of the Circuit. Proc. Combust. Inst. 1949, 3, 341–353. [Google Scholar] [CrossRef]
- IEC 60079-0; Electrical Apparatus for Explosive Gas Atmospheres. Part 11: Intrinsic Safety “i” (IEC 60079–11). International Electrotechnical Commission: Geneva, Switzerland, 2000.
- Zborovszky, Z. Study of Intrinsic Safety Basics and Testing Machines: A Comparison of Tungsten and Copper Hot Wire Ignition Capability and Discharge Duration in the Ignition Process of Explosive Atmospheres in Testing Apparatus (BuMines OFR 116–77); Bureau of Mines: Pittsburgh, PA, USA, 1976. [Google Scholar]
- Peterson, J.S. Influence of Electrode Material on Spark Ignition Probability (RI 9416); Bureau of Mines: Pittsburgh, PA, USA, 1992. [Google Scholar]
- Annual Report on Safety in Mines Research for 1966; Safety in Mines Research Establishment, HMSO: London, UK, 1966.
- Cawley, J.C. A Statistical Determination of Spark Ignition Safety Factors in Methane, Propane, and Ethylene Mixtures in Air (RI 9048); Bureau of Mines: Pittsburgh, PA, USA, 1986. [Google Scholar]
- Allsop, G. The Ignition of Explosive Atmospheres by Electric Sparks (Report G/T 128); Electrical Research Association: Leatherhead, UK, 1940. [Google Scholar]
- Zborovszky, Z. Ignition Criteria and Flame Kernel Development between Breakflash Electrodes in Explosive Gas Mixtures. Master’s Thesis, University of Surrey, Guildford, UK, 1969. [Google Scholar]
- Bane, S.P.M. Spark Ignition: Experimental and Numerical Investigation with Application to Aviation Safety. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 2010. [Google Scholar]
- Zborovszky, Z.K. New Concepts and Experimental Results on the Ignition Limits of CR Circuits. In Proceedings of the 3rd International Conference on Electrical Safety in Hazardous Environments, London, UK, 1982; pp. 123–128. [Google Scholar]
- Guénault, E.M. Intrinsic Safety—A Résumé of Recent Progress (Report 41); Safety in Mines Research Establishment: Sheffield, UK, 1952. [Google Scholar]
- Ikeda, T. Difference in Experimental Data from IEC Data in the Ignition Limit of Propane. Min. Technol. 1991, 73, 18–20. [Google Scholar]
- Thomas, V.M. Design of Intrinsically Safe Apparatus for Use in Coal Mines: A Review of Data and Techniques. Min. Electr. Mech. Eng. 1964, 44, 295–308, Part II, 321–329. [Google Scholar]
- Bartels, A.L.; Bradford, M.; Thompson, M.G. Incendivity of Electrical Sparking due to Circulating Currents in the Enclosure of Large Electrical Machines. In Proceedings of the 4th Intl. Conf. on Electrical Safety in Hazardous Areas (IEE Conf. Publ. 296); Institution of Electrical Engineers: London, UK, 1988; pp. 141–148. [Google Scholar]
- Widginton, D.W. Ignition of Methane by Electrical Discharges (Research Report 240); Safety in Mines Research Establishment: Sheffield, UK, 1966. [Google Scholar]
- Oancea, D.; Razus, D.; Munteanu, V.; Cojocea, I. High Voltage and Break Spark Ignition of Propylene/Air Mixtures at Various Initial Pressures. J. Loss Prev. Process Ind. 2003, 16, 353–361. [Google Scholar] [CrossRef]
- Berz, I. On Inductive Break Sparks of High Incendive Power. Combust. Flame 1959, 3, 131–132. [Google Scholar] [CrossRef]
- Takizawa, K.; Igarashi, N.; Takagi, S.; Tokuhashi, K.; Kondo, S. Quenching Distance Measurement of Highly to Mildly Flammable Compounds. Fire Saf. J. 2015, 71, 58–68. [Google Scholar] [CrossRef]
- Takizawa, K.; Tokuhashi, K.; Kondo, S. Flammability Assessments of CH2=CFCF3: Comparison with Fluoroalkenes and Fluoroalkanes. J. Haz. Mater. 2009, 172, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.L.; West, L.C.W.; Widginton, D.W. The Ignition of Methane-Air Mixtures by Arc Discharges of Controlled Duration. In Proceedings of the Flameproofing—Intrinsic Safety and Other Safeguards in Electrical Instrument Practice (IEE Conf. Report Series No. 3); Institution of Electrical Engineers: London, UK, 1962; pp. 15–20. [Google Scholar]
- Zborovszky, Z. Study of Intrinsic Safety Basics and Testing Machines: Study of the Effects of Discharge Duration, Electrode Materials and Surface Conditions on the Minimum Ignition Current (BuMines OFR 68–79); Bureau of Mines: Pittsburgh, PA, USA, 1977. [Google Scholar]
- Litchfield, E.L.; Kubala, T.A.; Schellinger, T.; Perzak, F.J.; Burgess, D. Practical Ignition Problems Related to Intrinsic Safety in Mine Equipment: Four Short-Term Studies (RI 8464); Bureau of Mines: Pittsburgh, PA, USA, 1980. [Google Scholar]
- Peacock, R.D.; Vaishnav, M.P. Flammability Testing of Solids Under the Federal Hazardous Substances Act (NBSIR 78-1580); National Bureau of Standards: Gaithersburg, MD, USA, 1980. [Google Scholar]
- Babrauskas, V. Smoldering Fires; Fire Science Publishers: New York, NY, USA, 2021. [Google Scholar]
- ASTM E1354; Standard Test Method for Heat and Visible Smoke Release Rates for Materials and Products using an Oxygen Consumption Calorimeter. ASTM International: West Conshohocken, PA, USA, 2022.
- Babrauskas, V. Unexposed-Face Temperature Criteria in Fire Resistance Tests: A Reappraisal. Fire Safety J. 2009, 44, 813–818. [Google Scholar] [CrossRef]
- Tokyo Fire Department. Fire Report: Fires of Small Battery. J. Jpn. Assn. Fire Sci. Eng. 1994, 44, 57–60. [Google Scholar]
- Verbeek, R.; Bouma, R.H.B. Evaluation of the Energy Fluence in the Small Gap Test. Propellants Explos. Pyrotech. 2011, 36, 16–21. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).