Abstract
In recent works, many industrial by-products were employed as solid precursors for the synthesis of alkali-activated binders and as alternatives to Portland cement for the immobilization of hazardous, toxic and nuclear wastes. Among industrial wastes, alkali-activated brick was found to be an interesting porous composite for removing very toxic heavy metals (Pb2+, Cd2+, Co2+) and radio-nuclides (Sr2+, Cs+, Rb+) from aqueous solutions. The starting material is very attractive due to the presence of metakaolinite as a geo-polymer precursor and silica for increasing material permeability and facilitating water filtration. The alkaline reaction gave rise to geo-polymerization followed by partial zeolitization. Elemental surface micro-analysis was performed by Scanning Electron Microscopy (SEM) equipped with an Energy-Dispersive X-ray Spectrometer (EDS). The formation of crystalline phases was corroborated by X-ray diffraction (XRD) analysis. Information about 29Si, 27Al and 1H nuclei environments in crystallized and amorphous aluminosilicates was obtained by 29Si, 27Al and 1H MAS NMR. 27Al–1H dipolar-mediated correlations were investigated by employing dipolar hetero-nuclear multiple quantum coherence (D-HMQC) NMR, highlighting Al–O–H bonds in bridging hydroxyl groups (Si–OH–Al) that are at the origin of adsorptive properties. Aqueous structural stability and cationic immobilization characteristics before and after material calcination were investigated from acid-leaching experiments.
1. Introduction
Human activities, such as fossil fuel burning, deforestation and cement production, have contributed strongly to the excessive emission of carbon dioxide into the atmosphere and the acceleration of global-warming phenomena. In order to limit climate change, more efficient policy actions towards decarbonization must be undertaken urgently [1]. Among them, a better efficiency/control of extraction procedures and management of non-renewable natural resources and incentive re-using/recycling and valorising of industrial (by-)products have to be encouraged to diminish substantially environmental degradation and ensure environmental preservation and sustainability.
Portland cement is an industrial low-cost product that has been used for many years as a stabilization/solidification matrix for immobilizing low- and intermediate-level radioactive liquid wastes [2]. However, radio-nuclides are found to be generally difficult to stabilize within cement matrices [3], because these latter are prone to have structural and/or chemical degradations in contact with aggressive media or at high temperatures. The generation of micro-cracks in solidification matrices (concretes) may cause, with time, radioactive leaching and severe contamination in disposal sites, thereby preventing safe land disposal. To improve the physical and chemical stability of the matrix and limit radioactive leaching in aqueous aggressive media, cementitious materials, such as blast furnace slag, fly ash and metakaolin, have been proposed as promising alternatives to Portland cement for the immobilization of hazardous, toxic and nuclear radio-elements, such as cesium, strontium, cobalt and europium [4,5,6,7,8,9,10,11,12,13,14], and by employing them either alone or combined with other solid aluminosilicate precursors [3]. It is worth noting that, contrary to normal concrete, alternative cementing systems based on aluminosilicate polymers or geo-polymers are characterized by a lack of calcium and, thereby, would not suffer from effects such as carbonation or acid leaching after stabilization/solidification [15].
In order to enrich or separate radio-nuclides, the adsorption and ion exchange method remains the best (efficiently and economically) remediation technique due to a facile large-scale production, much lower operating costs and, above all, easier industrial operability. Many inorganic ions exchange materials and multi-functional core-shell adsorbents exhibit high adsorption capacity and excellent selectivity in the removal of nuclides from solutions [16,17]; but, these materials presented relevant disadvantages and difficulties in practical/industrial applications, such as (i) products costs; (ii) complicated synthesis processes consisted of two or more reaction steps, rending adsorbent preparation relatively costly and time-consuming; and mostly, (iii) small particulate sizes which are not suitable for carrying out large-scale water treatment (in dynamic adsorption) or separating powdered particles from the liquid phase. In contrast, the heterogeneous surface behavior of mixed materials (or composites) contributed to improving adsorption capacity, permeability and filtration, especially owing to the possibility of obtaining larger particle sizes which are more adapted to column experiments [18,19]. Recent studies thus revealed remarkable advantages of using geo-polymer-zeolite-A composites for immobilizing radioactive wastes [20]. Analogically with previous works on the synthesis of zeolite-geo-polymer composites using industrial solid wastes [21], a zeolite-geo-polymer composite was prepared in the lab by proceeding to an alkaline activation of a metakaolinite-rich brick.
The aim of this paper was to review the chemical, mineralogical and micro-structural properties of alkali-activated brick as a promising matrix for radio-nuclides immobilization in the perspective of large-scale applications.
In the first part of this work, a few comments were made about the different reaction steps of the “brick-composite” synthesis.
In the second part, chemical and mineralogical analyses of alkali-activated brick aggregates were performed by using an environmental scanning electron microscope (ESEM) equipped with an energy-dispersive X-ray spectrometer (EDS), and their crystalline characteristics were determined by using X-ray diffraction.
In the third part, the generation of crystallized and amorphous aluminosilicates was evidenced and quantified by means of 29Si and 27Al MAS NMR spectroscopy. Information about 1H nuclei environments was obtained by 1H MAS NMR. 27Al–1H dipolar-mediated correlations were investigated by employing dipolar hetero-nuclear multiple quantum coherence (D-HMQC) NMR with the aim of highlighting the occurrence of Al–O–H bonds and their implication in bridging hydroxyl groups (Si–OH–Al).
In the fourth part, the aqueous structural stability and cationic immobilization characteristics of the brick-based composite before and after its calcination were investigated from acid-leaching experiments and analytical data were discussed.
2. Materials and Methods
2.1. Materials Preparation and Making Cost
The raw material used in the experiments was obtained from a brick made locally by craftsmen in the Bangui region (Central African Republic). This brick contains mainly quartz (60–65 w%) and metakaolinite (20–26 w%) and, to a lesser extent, iron oxide/hydroxide, illite and titanium dioxide [22]. Before alkaline treatment, the brick was broken into grains and sieved with sizes ranging from 0.7 to 1.0 mm. Second, brick pellets were treated with sodium hydroxide according to the following optimized synthesis conditions [23]: 10 g of Bangui brick reacted in 40 mL of a diluted NaOH solution (0.6 mol·L−1) at room temperature for one night under a slow shaking at a speed of 120 rpm. This procedure was afterward followed by a fixed-temperature increase of the mixture at 90 °C for a constant reaction time of six days. Finally, the recovered grains were rinsed several times with MilliQ water and dried at 90 °C for 24 h.
Making cost of synthesized composite: Briefly, a raw brick which was provided by African craftsmen (in the Central African Republic) and used here as starting material weighed about 5 kg, and its price is 75 CFA (i.e., 0.11 Euro; 1 CFA = ~0.0015 Euro); while solid sodium hydroxide which was provided by local soap factories and used here as reagent cost 30,000 CFA/25 kg (i.e., 45 Euros). Knowing that 4 liters of a NaOH solution at a concentration of ~0.6 mol·L−1 were necessary to treat 1 kg of ground brick, the authors estimated the cost for synthesizing 1 kg of adsorbent at ca 0.20 Euro (without taking into account labor costs, which are very low in this country). Hence, the low making cost of the brick-based composite should enable its use as a good low-cost adsorbent for radioactive wastewater treatment.
As for the NH4+-saturated alkali-brick, an ion-exchange reaction between Na+ ions in brick frameworks and NH4+ ions in the aqueous phase was carried out by mixing 1 g of alkali-brick grains (diameter 0.7–1.0 mm) with 100mL of an NH4Cl solution (2 mol·L−1). The suspension was shaken gingerly for 24 h in a thermostatic orbital shaker. The resulting brick grains were afterward filtered, and the filtration operation was repeated three times with the aim of ensuring a total exchange of extra-framework sodium with ammonium. Finally, brick grains were rinsed several times with Milli-Q water and dried at 70 °C for 24 h.
2.2. Chemicals
All chemicals employed in the experiments were analytical grades. Sodium hydroxide, ammonium chloride and hydrochloride acid were supplied by DISLAB (Paris, France).
2.3. Electron Microscopy Analysis
Micrographs of representative specimens of the brick before and after chemical treatment were recorded by using an environmental scanning electron microscope equipped with an Energy Dispersive X-Ray Spectrometer EDS X flash 3001 (ThermoFisher Scientific, Courtaboeuf, France). EDS measurements were carried out at 20 kV at low vacuum (1.00 Torr), and the maximum pulse throughput was 20 kcps. Different surface areas ranging from 0.12 to 0.50 mm2 were targeted on alkali-brick grains and examined by ESEM/EDS. For that, a narrow beam scanned selected areas of brick pellets for chemical analysis. Atomic quantifications and mathematical treatments were undertaken using QUANTA-400 software in order to determine the averaged elemental composition of the surface brick and to detect chemical/elemental variability.
2.4. X-ray Diffraction Analysis
XRD patterns were conducted at room temperature in a Bruker D8 Advance diffractometer (Evry, France) using Ni-filtered CuKα radiation (40 kV, 40 mA). Samples were scanned with a step size of 0.02° and a counting time of 0.5 s per step.
2.5. MAS NMR Analysis
The 29Si MAS-NMR experiments were acquired at 79.5 MHz on a Bruker 9.4 T spectrometer (Palaiseau, FR) equipped with a Bruker 7.0 mm MAS probe operating at a spinning frequency (νrot) of 4 kHz. The 29Si MAS-NMR spectra were recorded with a pulse length of 5 µs (π/2 flip angle), 256 transients, and a recycle delay (rd) of 30 s. This short rd value was possible because of the presence of paramagnetic species (as iron(III) oxides/hydroxides) homogeneously distributed within the brick matrix. The 29Si MAS-NMR spectra were decomposed using the Gaussian/Lorentzian model of the dmfit software (version 2.0) [24]. The 1H, 23Na and 27Al MAS-NMR spectra were recorded at 800, 211.7 and 208.5 MHz, respectively, on a Bruker 18.8 T spectrometer equipped with a Bruker 3.2 mm CP-MAS probe operating at a νrot of 20 kHz. The 23Na (I = 3/2) MAS-NMR experiments were recorded with a pulse length of 1 µs (~π/5 flip angle), 1024 transients, and a recycle delay of 2 s. The 27Al (I = 5/2) MAS-NMR spectra were obtained with a pulse length of 1 µs (~π/10 flip angle), 1024 transients, and a recycle delay of 2 s. The 1H MAS-NMR experiments were recorded with a π/2 pulse length of 3.5 µs, 128 transients and a 5s rd using the DEPTH sequence in order to suppress the signal coming from the measurement probe. The presence of Al–O–H bonds was investigated by correlation NMR with the 27Al(1H) dipolar heteronuclear multiple quantum coherence (D-HMQC) NMR technique [25,26]. The 2D maps were recorded under rotor-synchronized conditions at 18.8 T with 2048 × 40 acquisition points. Each direct slice was recorded with 27Al and 29Si π pulses of 16 and 10 µs, 1024 transients, a rd of 2 s and a 2 ms SR421 re-coupling scheme. All the 29Si, 1H, 23Na and 27Al chemical shifts were referenced as 0 ppm to TMS, TMS, NaCl solution (1 M), and Al(NO3)3 solution (1 mol.L−1), respectively. It is noteworthy that the spectra acquired at high field (1H, 23Na and 27Al) were recorded on similar sample mass (40 mg) in order to compare the peak intensities on the different NMR spectra.
2.6. Acid Leaching Tests
Leaching of different elements (Si, Al and Na) was carried out on alkali-brick samples (mass of composite: 500 mg) in different HCl solutions (volume of leachant: 50 mL) with pH values ranging from 0.39 to 4.83 at room temperature. Under similar experimental conditions, leaching of radioactive elements (Cs and Rb) and Si, Al and Na was carried out on Cs (or Rb)-doped alkali-brick in a 0.01 M HCl solution (pH~2). Each collected leachate was vacuum filtered through a 0.45 μm membrane filter. The concentrations of leached elements present in these leachates were determined by using an inductively coupled plasma optical emission spectrometer (ICP–OES, model 5110 VDV, Agilent Technologies).
3. Results and discussion
3.1. Synthesis Process
During the reaction course of brick-composite synthesis, the metakaolinite mineral initially present in the raw brick was dissolved in an alkali medium by forming sodium poly/mono silicates and sodium aluminates intermediately; these ionic species were subsequently polymerized into gel-state sodium alumino-silicate(s) which impregnated insoluble brick pellets. This polymerization/impregnation was followed by an induction period during which nucleation/crystallization took place at the surfaces of unreacted brick grains (mainly quartz) [27,28]. These authors [27,28] also stated that the presence of a high amount of quartz (60–65 w%) as a “secondary matrix” in brick composite contributed to facilitating the flow of inlet solutions through fixed-bed columns. Note that alkali brick had recently been assimilated to an adsorptive membrane prepared from blended zeolites into a layered geo-polymer-quartz matrix [29]. This mixed material might be considered as a surface composite membrane possessing a dual function of adsorption and filtration: with zeolitic/geo-polymeric aggregates favoring adsorption capacity, while quartz matrix allowing mostly low operating pressure and high permeability flux [29].
3.2. Microscopic and Crystalline Studies
3.2.1. Microscopic (ESEM/EDS) Analysis
ESM analysis of raw brick revealed the presence of porous and non-textural surfaces with major fissures and cracks, except for quartz crystals [22,30]. In addition, elemental (ESEM/EDS) analysis indicated the presence of: (i) silicon (Si), aluminum (Al), oxygen (O) and iron (Fe) as major elements; and (ii) titanium (Ti), magnesium (Mg), potassium (K), and calcium (Ca) as minor elements. Some of these elements were found to be distributed across brick surfaces with apparent localization, evidencing the existence of mainly SiO2 (as quartz) and alumino-silicate (as metakaolinite) and, to a lesser extent, iron oxides/hydroxides and rutile [22].
As for alkali-activated brick aggregates, ESEM analysis revealed that the morphological aspect of the treated brick was changed significantly into aggregated cubic and spherical shapes (Figure 1). The size of these new specimens varied from 7 µm to 10 µm for cubic ones and from 3 µm to 6 µm for spherical ones. Cubic crystals were found to be comparable with those observed previously for the A-type zeolite [31,32], while spherical shape crystals were morphologically similar to those reported in the literature for zeolite NaP [33,34,35].
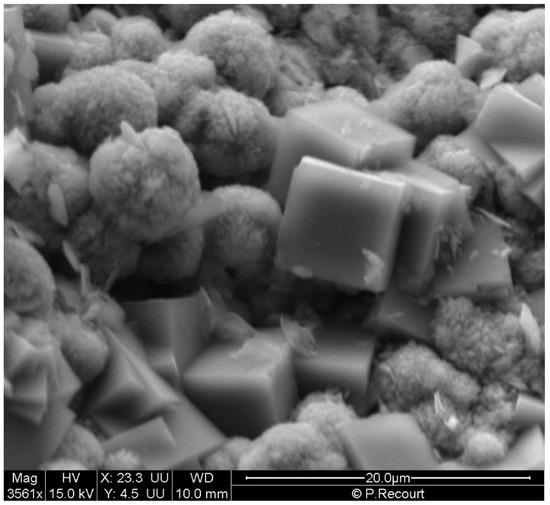
Figure 1.
ESEM image of brick-composite specimens.
The spatial distribution of the framework elements Al, Fe, Na and Si is displayed in Figure 2. The ESEM/EDS mapping procedure gives the color overlay shown in this Figure, where the elemental distributions for Al, Fe, Na and Si are represented in red, green, yellow, and blue, respectively. Element distribution images indicate a positive correlation between Al, Si and Na (Figure 2B) due to the presence of sodic alumino-silicates as Na-zeolites. Conversely, there is a negative correlation between sodium and silicon in Si-rich zones which are composed of quartz crystals (in blue regions of the reconstituted image; see Figure 2A). Note as well the presence of micro-specimens of TiO2 (rutile) in the elemental distribution for titanium; see Figure 2B.
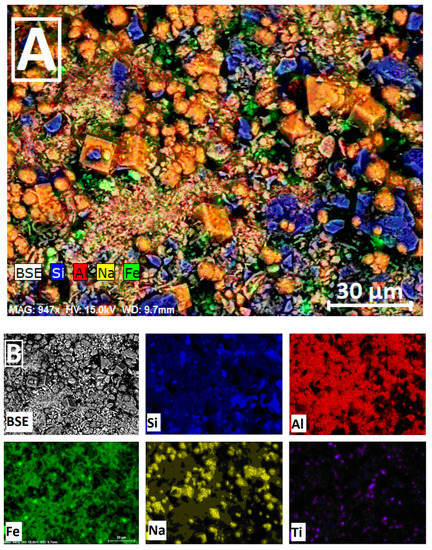
Figure 2.
Reconstituted ESEM/EDS mapping image of alkali-activated brick aggregates (A); Spatial distribution of the framework elements Al, Fe, Na, Si and Ti (B). BSE: Energy Selected Backscatter.
Quantitative ESEM/EDS analysis permitted us to show that the elemental composition of cubic and spherical specimens corresponded well to those of low-silica zeolites with atomic ratios of Si/Al close to 1. Indeed, the atomic percentage of aluminum and silicon were 14.97 ± 2.05 atomic % and 16.12 ± 1.46 atomic %, respectively, which led to an averaged atomic ratio Si/Al of 1.08 (or Al/Si = 0.926). As for sodium, we found: 14.77 ± 2.67 atomic %. The presence of high amounts of sodium on the surfaces of cubic and spherical particles confirmed the presence of sodic zeolites.
3.2.2. X-ray Diffraction (XRD) Analysis
Figure 3a displays the XRD patterns of raw-brick powder. XRD analysis revealed that the material contains quartz as the principal crystalline mineral and, to a lesser extent, illite and rule. In what follows, minerals were identified on the basis of ‘2θ’ reflection angles and “hkl” Miller indices (“hkl” indices are given in the parenthesis).
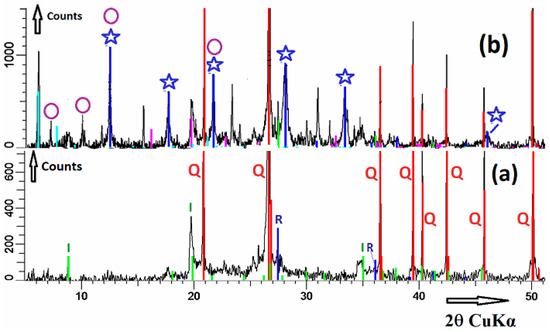
Figure 3.
XRD patterns of raw brick (a) and alkali brick (b): I, illite; Q, quartz; R, rutile; (✩), NaP zeolite; (ο), LTA zeolite.
Quartz: 20.9° (100); 26.6° (011); 36.5° (110); 39.5° (102); 40.3° (111); 42.4° (200); 45.8° (201); and 50.1° (112) [ICSD Collection Code: 89276]. Illite: 8.8° (001), 17.9° (004), 19.8° (021), and 34.3° (034) [ICDD (International Centre for Diffraction data): 00-009-0343]. Rutile: 27.4° (110) and 36.1° (101) [ICSD Collection Code: 168140].
Figure 3b displays the XRD patterns of alkali-brick powder. Compared to the XRD patterns of raw brick, one can observe new XRD signals in addition to those ascribed to quartz, illite and rutile. These reflections were attributed to zeolites LTA and NaP.
LTA: 7.2° (200), 10.2° (220), 12.5° (222) and 21.7° (600 and 442) [36]. NaP: 12.5° (101 and 110), 17.7° (200 and 002), 21.7° (211, 112 and 121), 28.1° (310, 301, and 103), 33.4° (132, 123, 231, 213, 312, and 321) and 46.1° (134) [36].
3.3. Solid MAS MNR Study
In the past, Magic Angle Spinning (MAS) Nuclear Magnetic Resonance (NMR) spectroscopy was successfully used for providing relevant information about structure changes, chain lengths and elements ratios of (three-dimensional) geo-polymeric gels present in alkali slag and fly ash as aluminosilicates dominated (Si + Al) industrial by-products [37]. This led us to undertake detailed NMR analyses of alkali brick to gain a comprehensive insight into the different 29Si, 27Al, and 1H atomic environments constituting the heterogeneous nature of alkali-activated brick. Ammonium chloride (NH4Cl) was used as a probe for bringing information about the characteristics and mobility in brick-composite frameworks by means of 1H MAS NMR [1D 1H and 2D 27Al(1H) D-HMQC].
Moreover, before undertaking the analysis of NMR data, it was important to mention that it was observed no noticeable broadenings of NMR peaks related to paramagnetic NMR effects arising from the interactions of nuclear spins (1H, 23Na, 29Si or 27Al atoms) with unpaired d electrons (Fe(III). This could result from the presence of too-low Fe contents detected in alkali-brick samples by ESEM/EDS (ranging from 0.2% to 0.7%), suggesting that resonating nuclei detected were certainly located far from paramagnetic centers.
3.3.1. 29Si MAS NMR
Figure 4 displays solid-state MAS NMR data for 29Si nuclei in alkali brick before and after different chemical treatments. The 29Si MAS NMR spectrum of an alkali-brick sample indicated several signals (ranging from −70 ppm to ~−135 ppm), which characterized the resonances of framework silicon atoms in Q4(mAl) type environments with m varies between 0 and 4. Note globally that in the 29Si MAS NMR spectra, the relatively high intensity of resonances in the range (−84)–(−90) ppm reflected the importance of Al-rich structural units inside brick frameworks.
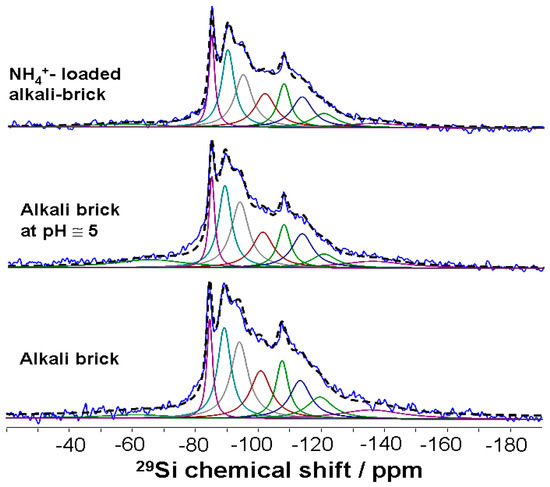
Figure 4.
29Si MAS NMR spectra of alkali brick before and after different chemical treatments. De-convoluted spectra were obtained by employing the dmfit software [24].
In order to facilitate the interpretation of 29Si MAS NMR data, the experimental 29Si MAS NMR spectra were de-convoluted (Figure 4). Components were identified through the de-convolution of the spectra into individual Gaussian/Lorentzian peaks representing different silicon centers: Qn(mAl) with n and m capable of varying from 0 to 4. The de-convolution criterion followed at best published 29Si NMR results relative to the different chemical environments surrounding the silicon nucleus belonging to the following compounds [38,39,40,41,42]: (i) zeolites NaA and NaP; (ii) aluminosilicate gels produced during the alkali activation of metakaolin; and (iii) quartz as an unreacted mineral present in the starting material. It is noteworthy to mention that other un-reacted starting materials such as illite and biotite (which are nevertheless present at low levels in the analyzed material) would also contribute to the resonances detected and, as a consequence, lead to some degree of error in the decomposition of the 29Si MAS NMR spectra.
The de-convolution of the 29Si MAS NMR spectrum of alkali brick revealed a series of peaks at −84.5, −89.2, −94.0, −100.7, −107.5, −113.3 and −119.5 ppm (±0.3 ppm), see Figure 4 and Table 1. The NMR line at −89.2 ppm corresponded well to the chemical shift of Si atoms in Q4(4Al) sites in NaA zeolite [39] and NaP zeolite [41]. The NMR resonances at −94.0 and −100.7 ppm were close enough to those detected for NaP zeolites by Criado and co-workers [41]: These authors ascribed them to silicon tetrahedra surrounded by a certain coordination number of 27Al atoms ranging from 3 to 1, respectively (Q4(1–3Al)). As a whole, the NMR peaks at −94.0 and −100.7 ppm might certainly be due to Si(OSi)(OAl)3, Si(OSi)2(OAl)2 and/or Si(OSi)3(OAl)1, respectively. As for the particular NMR peak at −84.5 ppm, it was associated with the presence of sodalite-type Q4(4Al) units in brick gel [41,43]. The −84.5 ppm signal was comparable enough to that observed previously for alkaline inorganic polymers generated through the alkaline activation of pure metakaolinite [40]. Such polymers exhibited additional peaks at around −87/−89 ppm and −94 ppm, which were superimposed on those of NaA and NaP zeolites [40]. These resonances were, therefore, assigned partly to tetrahedrally coordinated and fully polymerized (Q4) silicon nuclei surrounded by m AlO4 tetrahedra and associated with the chemical 29Si environments in alkali-activated metakaolin. Indeed, according to Fernandez-Jiménez and co-workers [40], the 29Si MAS-NMR spectrum of metakaolin illustrating its chemical transformation into an alumino-silicate gel during alkaline activation exhibited three well-defined signals at around −84.6, −89, and −94 ppm. These chemical shifts were similar enough to those obtained for chemically bonded ceramic/cementitious materials of alkali-activated metakaolin, revealing a wide signal with several overlapped peaks, and their de-convolution indicated the presence of Q4(4Al), Q4(3Al) and Q4(2Al) units at −84, −89 and −92 ppm, respectively [44,45]. Note further that the relatively high intensity of the −89.2 ppm signal observed in Figure 4 was explained by the fact that zeolites NaA and NaP contributed strongly to this signal. The −89.2 ppm resonance, which was associated with Q4(4Al) units in zeolites NaA and NaP, was assigned to the resonance of 27Al nuclei in double four-membered rings (with sodalite cages) of brick zeolites [38,40]. It was also interesting to note that, after the adsorption of NH4+ ions onto alkali brick, the 29Si sites were somewhat better distinguished and, hence, the different MAS NMR lines could be more easily quantified (Figure 4 and Table 1).

Table 1.
De-convolution data was obtained for the 29Si MAS NMR spectra of alkali-activated brick before and after slight acidification (pH 5) and ammonium treatment.
In addition, it was found that by using X-ray diffraction, brick quartz was apparently not (or barely) attacked by alkaline treatment [46]. A resonance at −107.7 ppm in the 29Si MAS NMR spectrum of alkali brick (Figure 4) revealed Q4(0Al) units in quartz [47,48]. The low intensity of this signal in our 29Si MAS-NMR experiments was explained by the relatively short recycle delay. This delay did not allow for a full relaxation of the quartz signal, leading to an under-estimated intensity compared to the other 29Si resonances. In addition to the Q4(0Al) signal corresponding to the quartz mineral, other Q4(0Al) signals were observed at −113.3 and −119.5 ppm (see Figure 4 and Table 1). According to the literature, these peaks could be attributed to highly polymerized amorphous silica structures generated through condensation of silicate tetrahedrons during metakaolin geo-polymerization, thus leading to an increasing fraction of Q4(0Al) [45,49].
From 29Si NMR data (see Table 1), it was possible to estimate the silica contribution of un-reacted aluminosilicate gel, Si(gel)%, to the overall 29Si MAS NMR peak of “zeolitized” brick-metakaolinite from the expression (in atomic %):
where I%(gel) and I%(zeolites) represent the intensity percentages of 29Si resonances ascribed respectively to aluminosilicate gel and zeolites in the 29Si MAS NMR spectrum of alkali-activated brick. Note that in this calculation: (i) we considered only the areas of the signals at −84.5, −94.0 and −100.7 ppm, which were assigned to Q4(4Al), Q4(2Al), Q4(2-1Al) units in aluminosilicate gel(s) [41,43]; and (ii) we did not take into account polymerized amorphous silica structures with Q4(0Al) (i.e., the 29Si NMR peaks at −113.3 ppm and −119.5 ppm, see Table 1). We found at least 70 atomic % of 29Si nuclei present in the “aluminosilicate” gel(s) of the partially zeolitized brick.
From the quantification of the different Q4(mAl) species of modified bricks (see Table 1), Engelhard’s formula (Equation (2)) was used here to estimate the Si/Al molar ratio in aluminosilicates at composite surfaces [50].
We found an atomic Si/Al ratio ranging approximately from 1.35 to 1.53. These Si/Al values result from macroscopic chemical (NMR) analysis of silicon elements in the material. Conversely, the ESEM/EDS results obtained corresponded rather to microscopic chemical analysis of brick surfaces (see Section 3.2.1 and Table 2) and cannot be directly compared with NMR data. For instance, by using the ESEM/EDS technique, we instead found the following atomic Si/Al ratios: (i) Si/Al = 1.58 ± 0.16 for large, targeted zones of surface alkali-brick recovered with both quartz and zeolites; (ii) 1.22 ± 0.08 for targeted zeolites-rich zones; and (iii) 1.11 ± 0.07 for specific zeolitic specimens. As a consequence, it could be stated from ESEM/EDS investigations that alkali-brick grains were mostly coated with zeolitic frameworks (NaA and NaP) (as shown in Figure 1 and Figure 2) and would barely be coated with alumino-silicate gel(s) (as detected macroscopically by NMR). To our mind, composite gels ought to be more present in layer(s) beneath zeolitic coatings.

Table 2.
Elemental (ESEM/EDS) analyses of alkali-activated brick aggregates were performed at different magnifications.
3.3.2. 1H and 23Na MAS NMR
Figure 5 displays solid-state MAS NMR data for 1H nuclei in fine alkali-brick grains in which brick metakaolinite was thoroughly transformed into zeolites. The 1H MAS NMR spectrum of zeolitized brick exhibits an intense signal spreading between ~2 ppm and ~9 ppm (Figure 5A). In this chemical shift range, it can be seen a principal resonance at 4.6 ppm accompanied by weak and sharp peaks at 4.2 ppm and 3.7 ppm. All these 1H NMR resonances were ascribed to a wide distribution of various water molecules and proton species taking place in the zeolitic frameworks (α and β cages) of zeolitized brick [22]. As for the badly resolved resonances in the δ1H range of ~1–3 ppm, they were either non-acidic or weakly acidic hydroxyl groups. These were assigned to isolated silanol/aluminol protons in brick minerals.
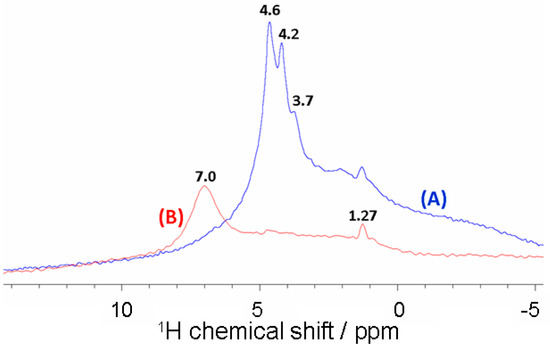
Figure 5.
1H MAS NMR spectra of alkali-activated brick aggregates before (A) and after ammonium treatment (B).
After the addition of an NH4Cl solution ([NH4Cl] = 0.5 mol·L−1) into a treated-brick suspension, the 1H MAS NMR spectrum of the recovered material revealed that zeolitic protons shifted significantly to a lower field (Figure 5B). A single resonance appeared at δ = 7.0 ± 0.1 and was assigned to NH4+ ions which were adsorbed onto the Brønsted sites of the treated brick, leading to NH4+-zeolite complexes [51,52,53]. As for Na+ ions adsorbed onto treated brick, 23Na MAS NMR analysis indicated their near disappearance after NH4Cl treatment, confirming the involvement of an ion-exchange reaction between sodium(I) and ammonium(I) at the brick-water interface according to an equimolar heterogeneous process (Figure 6):
Note that the residual 23Na signals observed in Figure 6B might be due to structural sodium bound to other brick minerals.
>SO−Na+ + NH4+→ >SO−NH4+ + Na+
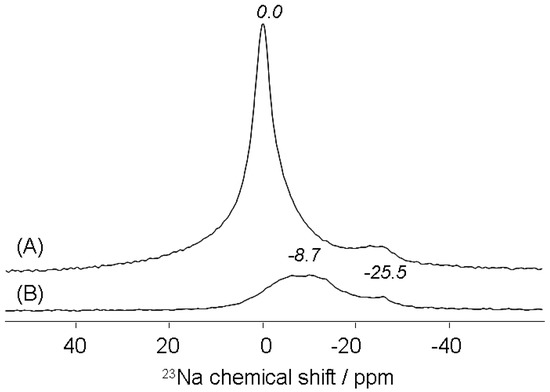
Figure 6.
23Na MAS NMR spectra of zeolitized-brick samples before (A) and after ammonium treatment (B).
On the other hand, in order to generate the H-form of zeolites, alkali-activated brick was acidified progressively at a pH value close to 5 with an HCl solution ([HCl] = 10−3 mol·L−1). This pH value was chosen lower than the pH at the point of zero charge, PZC (pHPZC = 5.85) or the pH at the iso-electric point (pHIEP = 5.9), see Figure S1, indicating the predominant occurrence of positively charged brick surfaces associated with hydroxonium ions. Acidified brick samples were afterward analyzed by 1H and 23Na MAS NMR spectroscopy. The 1H MAS NMR spectrum of the acidified brick revealed the presence of a sharp peak at δ = 4.7 ppm ascribed to H3O+ ions bound to brick zeolites, see Figure 7A. This signal was indeed due to hydrogen nuclei in bridging (structural) hydroxyl groups that underwent a rapid exchange between water molecules and hydroxonium ions in zeolitic cages. Such an observation agreed noticeably well with previous studies about the formation of hydroxyl groups in the low-silica zeolite with nSi/nAl = 1 (during the exchange of Na+ cations by ammonium ions), particularly showing close signals in the chemical shift range of 3.6–4.8 ppm due to Si(OH)Al groups in the α and β-cages [54]. The resonance at δ1H = 4.70 ppm was also found to be similar enough to that observed in the spectra of X and Y zeolites and ascribed to Si(OH)Al groups in sodalite cages [52,55].
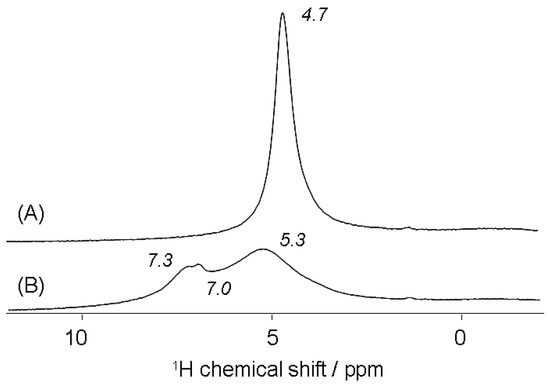
Figure 7.
1H MAS NMR spectra of the acidified alkali-brick at pH 5 before (A) and after ammonium treatment (B).
Acidified alkali-brick was subsequently treated with an NH4Cl solution ([NH4Cl] = 0.5 mol·L−1). The 1H MAS NMR spectrum of the recovered sample displayed three new proton resonances (Figure 7B). The peaks at δ1 ≅ 7.3 ppm and δ1’ = 7.0 ppm were assigned both to NH4+ ions adsorbed onto Brønsted sites of framework zeolites: LTA and NaP (note that the δ1 and δ1’ peaks were not yet assigned to specific zeolites). Whereas the peak at δ2 = 5.3 ppm was attributed to ammonium species involved in fast exchanges with NH4+ (δ1 and δ1’) and hydroxonium ions in excess. A comparable magnetic phenomenon was already evidenced for other zeolites [56,57]. Moreover, by rotating the rotor at a higher speed (from 20 kHz to 60 kHz), the temperature of the rotor increased noticeably, which contributed to affecting the magnetic characteristics of the sample. The obtained 1H MAS NMR spectrum then exhibited a line broadening and 1H NMR signals merging, see Figure 8. This could be explained by hydrogen nuclei that underwent a rapid exchange between ammonium and hydroxonium ions. This suggested the possibility of a transfer of all four hydrogen nuclei from one ammonium ion to bridging hydroxyl groups. The small number of water molecules in the treated brick should also contribute to an increase in the mobility of 1H nuclei along bridging Si−O−−Al chains. The resulting merging signal was centered at δ3 = 5.07 ppm. Note that the weak resonance detected at the lower field in the 1H MAS NMR spectrum (δ1″ = 6.75 ppm) was still ascribed to the own protons of ammonium ions directly bound to bridging Si−O−−Al sites (as Si−O−NH4+−Al).
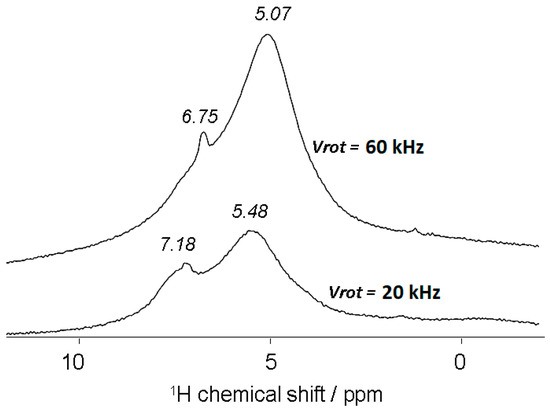
Figure 8.
Magnetic field effects of the rotor speed on the 1H MAS NMR spectrum of alkali-brick grains recovered after their acidification (pH 5) and ammonium treatment.
It could then be contended that upon H3O+/NH4+ exchange, the resonance of zeolitic 1H nuclei were low-field-shifted by Δδ1 = 2.45 ppm (from 4.70 to ~7.15) and Δδ2 = 0.60 ppm (from 4.70 to 5.30), which globally reflected an increasing acid strength of brick protons. Such low-field-shifts induced by ammonium ions were previously observed in 1H Solid-state NMR studies of Brønsted acid sites of other zeolites [52,53,56,57] and flame-derived silica/alumina [51] in which silanols with neighboring aluminum atoms generated bridging AlOHSi groups. However, it is worth noting that 1H, 23Na and 27Al MAS NMR analyses permitted to reveal a progressive decomposition of brick zeolites as the medium pH decreased below 5 (see Figure S2).
3.3.3. 27Al MAS NMR
Figure 9 displays solid-state MAS NMR data for 27Al nuclei in alkali brick before and after NH4Cl treatment. Globally, dominating 27Al resonances observed for studied brick samples fall in the range δ = 59.0–63.6 ppm. Such chemical-shift variations in the local environment of 27Al(IV) within alkali-brick might suggest that Al(IV) sites would exist in distinct aluminosilicate frameworks and would be differentially affected by structural factors, i.e., bond angles/lengths in T–O–T, with T = Si or Al [14,58].
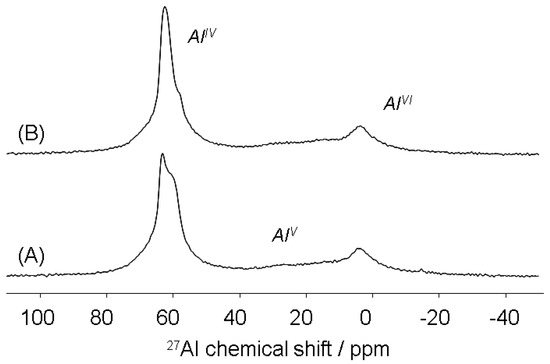
Figure 9.
27Al MAS NMR spectra of alkali-brick samples before (A) and after ammonium treatment (B).
In the 27Al MAS NMR spectrum of an alkali-brick sample, most aluminum atoms are tetrahedrally coordinated (AlIV), which results in three 27Al MAS NMR signals, see Figure 10A. The particular NMR resonances detected in the chemical shift range 57–61 ppm (see Figure 10A) were partially attributed to tetrahedrally coordinated aluminum (bound via oxygen to four 29Si atoms to give Al(OSi)4 units) of both NaA and NaP zeolites [34,59,60,61,62,63,64,65].
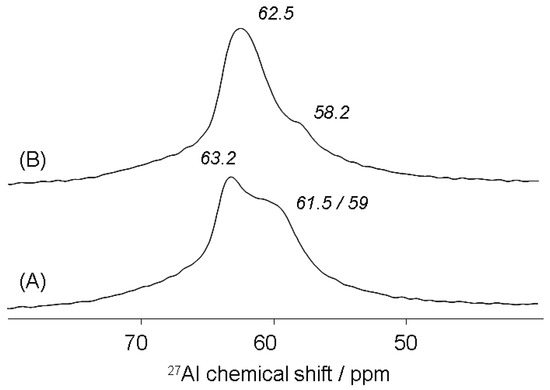
Figure 10.
Detailed MAS NMR spectra of 27AlIV nuclei of alkali brick before (A) and after ammonium treatment (B).
According to the literature [34,63,64,65], a slightly lower chemical shift (about 1 ppm) in the δ27Al range 57.6–59.3 ppm was more expected for NaP than for NaA. As for the NMR resonance at 63.2 ppm (see Figure 10A), it was ascribed to tetrahedrally coordinated aluminum in geo-polymeric gels, which were not transformed into zeolites [66]. Note that, for a series of sodalites (Si/Al = I), Jacobsen et al. (1989) [67] observed a linear dependence of 29Si and 27Al chemical shifts on mean “Si–O–Al” bond angles (θ):
δ(29Si) = −0.702·(θ) + 11.626
δ(27Al) = −0.724·(θ) + 163.982
From Equation (4), we attempted to calculate the mean Si–O–Al angle for brick hydroxyl-sodalite(s) (BS) with a 29Si chemical shift δ(29Si) = −84.5 ppm (see Figure 4). We found: θBS = 136.93°. This angle value permitted us to subsequently estimate the corresponding chemical shift of 27Al nuclei in BS by using Equation (5). We found δ(27Al) = 64.83 ppm. This value was nevertheless higher than that observed experimentally: δ(27Al)exp. = 63.2 ppm. In contrast, by using Lippmaa et al.’s formulae (Equation (6); [68]) indicating the linear dependence of the 27A1 chemical shift on the mean [Al−O−Si] bond angle in framework alumino-silicates, we found a δ(27Al) value: δ(27Al) = 63.53 ppm, which was much closer to the experimental one.
δ(27Al) = −0.50·(θ) + 132 (ppm)
As for 29Si nuclei of alumino-silicate gel(s) detected at −89.2 ppm (which superimposed on those of NaA and NaP zeolites), −94 ppm and −100.7 ppm, these chemical shifts would correspond to Q4(3Al), Q4(2Al) and Q4(1Al) units, respectively; According to Ramdas-Klinowski’s works [69], the 29Si chemical shifts of brick gels could be correlated linearly with the structural parameter, ∑dTT, according to:
in which ∑dTT is defined as:
where m is the number of aluminum atoms in the Si(mAl) unit (in this study, m = 1, 2, 3). Using Equations (7) and (8), one could evaluate the mean Si–O–Al angle (T = Si or AI) in the framework alumino-silicate. We found θ = 141.2°, 140.4° and 142.0° respectively for Q4(3Al), Q4(2Al) and Q4(1Al) units of brick gel(s), see Table 3.
δ(29Si) = 143.03 − 20.34·(∑dTT/Å)
∑dTT = [3.37 × m + 3.24 × (4 − m)] sin(θ/2)

Table 3.
Chemical shifts, δ(29Si) of 29Si nuclei of the different Q4(mAl) units present in modified-brick frameworks.
From these angle values, it was possible to assess the corresponding chemical shifts of 27Al nuclei present in the different Q4(mAl) units of brick gel(s) by employing Equation (6). We found δ(27Al) = 61.4 ppm, 61.8 ppm and 61.0 ppm for Q4(3Al), Q4(2Al) and Q4(1Al) units of brick gel(s), respectively. These δ(27Al) values were consistent with the broad NMR resonances ranging from ~59 to ~61.5 ppm, which were observed in the 27Al MAS NMR spectrum of alkali-brick (see Figure 10A). Likewise, the mean Si–O–Al angle (T = Al) in framework zeolites of alkali-brick was evaluated from Equations (7) and (8): we found: θ = 148.8° for zeolitic Q4(4Al) units (see Table 3); the corresponding chemical shift of 27Al nuclei present in these Q4(4Al) units was determined from Lippmaa et al.’s expression (Equation (6) [68]): δ(27Al) = 57.6 ppm. Also, from Engelhardt et al.’s expression (Equation (9) [43]), the mean Si–O–Al angle (T = AI) in framework zeolites of alkali brick could be estimated (θ = 147.3°): in this case, the corresponding chemical shift value of 27Al nuclei (δ(27Al) = 58.3 ppm) was found to be slightly higher than that determined above.
δ(29Si) = −0.570·(θ) − 5.230
A weak signal observed at 3.7 ppm was attributed to the presence of a small amount of octahedrally coordinated aluminum (AlVI). 27Al nuclei corresponding to penta-coordinated aluminum (AlV) were also present inside alkali-brick networks and gave rise to a signal at approximately δ ≅ 20–30 ppm.
After NH4Cl treatment, the 27Al MAS NMR spectrum of the recovered material changed noticeably in comparison with that of the alkali-brick (Figure 9 and Figure 10). The incorporation of NH4+ ions at high concentration into the alkali-brick suspension strongly displaced alkali cations (Na+) from the extra-framework sites and fulfilled the charge balancing role (as shown in Figure 6 relative to the Na+/NH4+ exchange at the brick surface). As observed by 27Al MAS NMR (Figure 10B), the addition of ammonium to alkali-brick frameworks led to a broader signal at 62.5 ppm as a consequence of an overlapping of the 27AlO4 resonances in the range ~59–63 ppm with an increase in the full width at half height (FWHH). This might be explained by the fact that the interactions of NH4+ ions with Q4 AlO4 sites of aluminosilicate gel(s) would contribute to a decreasing relaxation of the distortional bonding strain around AlIV atoms. Such strains would lead to a shortening of Al–O bonds with improved tetrahedral symmetry when compared to Al bonding strains caused by H-bondings between Al sites and a water-sodium(I) mixture [70]. To support this, we reported here some relevant findings regarding protons’ effects on the environment of silicon and aluminum framework atoms of alumino-silicates [71]. The authors in reference [71] concluded that the removal of protons from double four-membered rings (D4R: being present in sodalite structures) led to a strengthening of Si–O and Al–O bonds (and thereby a decrease of their lengths). In the resulting anionic framework, the Si–O–Al angle (138.4°) became larger (by 7.3°) than that at the bridging hydroxyl site (131.1°). In addition, in Section 3.3.2, it was shown that the adsorption of NH4+ ions onto alkali-brick resulted in acidification of brick particles, as evidenced by the significant shifting towards lower field observed by 1H MAS NMR (see Figure 7B). This finding implied that similar decreases of Si–O–Al angles should exist in framework brick aluminosilicates from the anionic material (>SO−Na+) to the (pseudo)-protonated form, >SO−H3O+NH3, as previously suggested [51,52,53,56,57]. In this assumption, the mean Si–O–Al angles of NH4+-loaded alkali-brick were evaluated by using Lippmaa et al.’s formulae, Equation (6). “Si–O–Si(Al)” angles data showed that: (i) the mean Si–O–Al angle in alkali-brick (148.8°) would be slightly higher (by 1.1°) than that at bridging hydroxyl sites of “protonated” alkali-brick (147.6°). As for aluminosilicate gels, the decrease of the mean Si–O–Al angle should range from ~1° to ~3°.
3.3.4. 2D MAS NMR (27Al-{1H} D-HMQC)
In order to observe 27Al–1H dipolar-mediated correlations, 2D 27Al(1H) D-HMQC experiments were performed on ammonium-loaded H-form of alkali-brick. As shown in Figure 11, two correlations were observed in the two-dimensional 27Al(1H) D-HMQC spectrum: one between 1H nuclei of NH4+ (δ1H = 7.0 ppm) and AlIV (δ27Al ≅ 64 ppm); and the other between 1H nuclei of H3O+ (δ1H = 3.7 ppm) and AlIV (δ27Al ≅ 62 ppm).
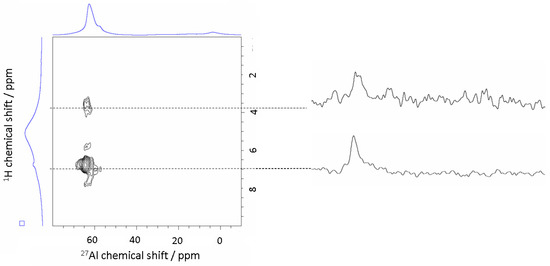
Figure 11.
Observation of 27Al–1H dipolar-mediated correlations from 2D 27Al(1H) D-HMQC experiments performed on the ammonium-loaded H-form of alkali brick.
The observation of an AlIV−NH4+ cross-peak in Figure 11 at (64, 7.0) ppm suggested that Brønsted acid sites Si−OH−Al, with AlIV atoms were able to transfer protons of bridging hydroxyl groups to ammonia molecules. This chemical surface transfer led to a significant low-field shift of the 1H NMR signal, as already observed previously for different types of zeolites [52,53,56,57]. Moreover, the 1H MAS NMR signal reached a maximal intensity at about 5 ppm. At this resonance maximum, some of the NH4+ ions were involved directly in fast exchanges with ammonia adsorbed onto Brønsted sites (δ = 7.0 ppm) and hydroxonium partially present in the H-form of brick zeolites (δ = 3.7 ppm). Such an NH4+/H+ exchange on brick surfaces indicated that not all bridging hydroxyl groups were involved in the signal at low field (i.e.: δ1H = 7.0 ppm). This could be explained by the fact that the hydrogen-exchange rate between 1H nuclei of bridging hydroxyl sites and the four hydrogen nuclei of the ammonium ions became close to that relative to the chemical exchange of bridging hydroxyl groups between the sites. Such mixing exchanges then gave rise to a merging signal which occurred at a higher field shift (and therefore at a lower acidity strength) than that observed for ammonia interacting directly with negatively-charged bridging hydroxyl sites (Si−O−−Al groups), as suggested by Wang and his co-workers [53]. Note that by using 1H MAS NMR and by rotating the rotor at different speeds, the transfer of hydrogen nuclei from ammonium ions to bridging hydroxyl groups was evidenced above (in Section 3.3.2 and Figure 8).
3.4. Aqueous Structural Stability and Cationic Immobilization Characteristics
In order to assess acidity effects on the aqueous structural stability of the brick composite at room temperature, a series of leaching tests were carried out at the following medium pH values: 0.39, 0.63, 0.80, 1.09, 1.27, 1.40, 1.67, 2.15, 2.32, 2.51, 3.70, 4.35 and 4.83. After a leaching time of 4 hours, the amounts of brick elements (Si, Al and Na) leached from the brick composite were determined and represented in the histogram of Figure 12. As seen in this figure, there are some noticeable differences between the leaching patterns of framework elements (Al and Si) and that of the extra-framework element (Na) with medium acidification. The concentrations of Al and Si present in the leachates remained low at reaction pH values above 2.5, suggesting a relative acid stability of the aluminosilicate matrix. Conversely, sodium was released at high concentrations with increasing acidification, revealing a facile exchange of Na+ ions by H3O+ ions during leaching. This was in agreement with recent thermodynamic data revealing weak acid characteristics of Brønsted acid sites in alumino-silicate frameworks of alkali-activated brick [29]. At pH values below 2.5, the acid degradation of the brick composite occurred strongly, and hence the concentrations of Al, Si and Na in the leachates became important (Figure 12).
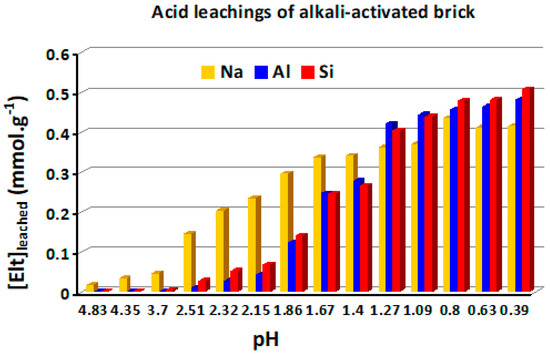
Figure 12.
Histogram representing the evolution of the amounts of Na, Al and Si released in the leachates during the acid leaching of the brick-based composite at room temperature and reaction pH values ranging from 0.39 to 4.83.
On the other hand, the equilibrium reactions between the Na-exchanged forms of the brick composite and aqueous solutions containing one, two or three cationic metals, such as Cd2+, Fe2+, Ni2+ and Pb2+ (Me2+), were previously studied thermodynamically on the basis of both the general properties of divalent metals in water and the global (zeolitic and geo-polymeric) surface characteristics of alkali-brick [46]. These investigations clearly revealed the importance of first hydrated radius, ionic potential and hydration-free energy, and second kinetics and mass-transfer/diffusion on the course of the global ion-exchange process at the brick−water interface [46]. Moreover, thermodynamic calculations concerning interfacial 2Na+/Me2+ ion-exchange reactions revealed that the replacement of the exchangeable cation (Na+) on the composite by Me2+ ions in the solution occurred favorably [46]. This proved that the immobilization efficiency of the composite for cationic metals is better than for Na+ ions. To support this, acid leaching of alkali-brick samples doped with radio-active cations (Cs+ and Rb+) was carried out in a 0.01 M HCl solution (pH~2) at room temperature and under limited acid conditions close to material decomposition (as shown in Figure 13).
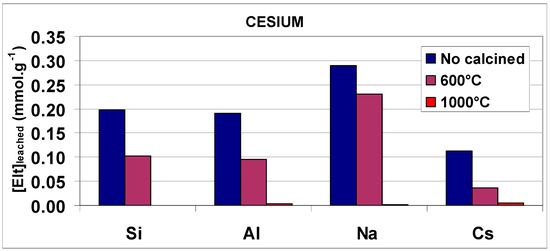
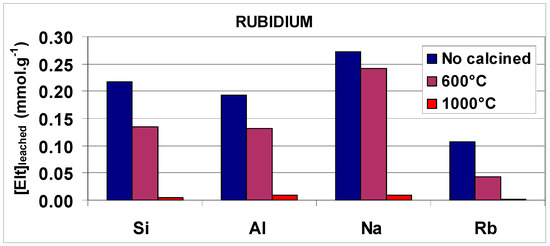
Figure 13.
Histograms representing the evolution of the amounts of Si, Al, Na and Cs (or Rb) released in the leachates during the acid leaching (at pH 2 and room temperature) of the composite no-calcined and calcined at 600 °C and 1000 °C for 4 h.
As expected, the concentrations of Al, Si, Na and radioactive elements in the leachates were found to be relatively high as a result of the acid degradation of the brick composite. By using the same leaching procedure, we afterward leached brick-composite samples which were previously calcined at 600 °C and 1000 °C for 4 h. Leaching results obtained at 600 °C indicated two features: (i) the leach fractions of Al and Si decreased noticeably, showing a slightly increased stability of calcined material; and (ii) the amounts of Na+, Cs+ and Rb+ (as extra-framework monovalent cations, M+) in the leachates diminished as well, showing a positive effect on M+ immobilization. This relative (weak) immobilization could be explained by the loss of water molecules in hydrated cationic shells during calcination, enabling the transformation of outer-sphere “M(H2O)x+-brick” complexes into inner-sphere “M+-brick” complexes (as evidenced by MAS NMR, Boughriet et al., unpublished works). As for leaching results obtained at 1000 °C, they revealed that the concentrations of Al, Si, Na, Cs and Rb present in the leachate became very low, evidencing both a greater stabilization of the brick matrix and a strong immobilization of Na+, Cs+ and Rb+ ions, as already observed for alkali-activated (geo-polymerized) blast furnace slag/fly ash [3,13,72]. This latter point suggested that Na+, Cs+ and Rb+ ions were presumed to be encapsulated inside a material that was structurally modified by heating, as previously mentioned for fly ash-silica fume-based geo-polymers after their calcination [13]. However, more studies on the brick-based composite must be undertaken to gain relevant information about structural/crystalline modifications induced by high temperatures.
4. Conclusions
The brick material was treated with sodium hydroxide using careful temperature control, giving rise to a geo-polymerization followed by a progressive zeolitization of geo-polymeric gels. X-ray diffraction analysis revealed the formation of crystalline structures identified as low-silica zeolites (NaA and NaP). ESEM/EDS microscopic analysis permitted observation of the cubic and spherical specimens at the brick surface and the evaluation of their averaged elemental composition, thus confirming the occurrence of low-silica zeolites with atomic ratios Si/Al close to 1. Solid MAS NMR spectroscopy permitted us to obtain a comprehensive insight into the different 29Si, 27Al and 1H nuclei environments existing in brick-composite networks. 29Si and 27Al MAS RMN studies revealed the still existence of geo-polymerized gels in combination with low-silica zeolites and allowed an estimation of the “geo-polymeric silicon” contribution inside the studied material. 1H MAS NMR studies showed the existence of bridging Si−O−H3O+−Al chains and their implication in rapid exchanges with hydrogen nuclei of water molecules and ammonium ions. Protons transfer from ammonium to bridging Si−O−−Al sites was confirmed by using two-dimensional 27Al(1H) D-HMQC NMR. Detailed spectral analyses of 1H, 29Si and 27Al NMR resonances allowed the assessment of “Si–O–Si(Al)” angles. Structural angle calculation permitted us to evidence the effects of extra-framework cations (Na+, H3O+ and NH4+) on negatively-charged bridging hydroxyl sites (Si−O−−Al groups).
Acid-leaching tests revealed that heating treatment was an effective method to stabilize the significant composite structure, as well as to improve the immobilization of radio-active elements (Cs and Rb).
5. Future Prospects
Alkali-activated brick represents a novel alkali-activated binder on which negative surface charges represent potential immobilization sites for radio-active cationic species. Geo-polymers and low-silica zeolites would be considered interesting binding phases for radio-elements uptake. The low Si/Al ratio in these compounds contributed to high adsorption density that would facilitate ionic exchanges between Na+ ions at the solid surface and radioactive ions (Cs+, Rb+, Sr2+ and Co2+) in the solution. However, a comprehensive insight into interfacial phenomena must still be clarified on the basis of kinetics and thermodynamics investigations. The knowledge obtained here on the compositional, structural and interfacial (ionic) properties of brick-based composite would help: (i) to interpret the interfacial ion exchange; (ii) to understand the uptake and leaching mechanism of radioactive ions; and (iii) to ensure better nuclear waste immobilization and nuclear radiation shielding after calcination/vitrification.
Pyrolysis−Fourier transform infrared (Py-FTIR) spectrometric studies of alkali-brick at elevated temperatures are in prospect for understanding the pyrolysates composition and their evolution over time and analyzing the difference between Na and H form geo-polymer. In addition, studies are underway to evaluate the state of the immobilized radio-elements and their degree of encapsulation inside composite frameworks after calcination at different temperatures. For that, X-ray photoelectron spectroscopy (XPS) should be useful for etching matrix surfaces by ionic Ar+ bombardment at different durations and analyzing surface elements versus formed-crater depth.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ceramics6030108/s1, Figure S1: determination of the point of zero charge and isoelectric point of alkali brick; Figure S2 (a) (1H): 1H MAS NMR spectra of alkali-brick grains vs medium pH; (b) (27Al): 27Al MAS NMR spectra of alkali-brick grains vs medium pH; (c) (23Na): 23Na MAS NMR spectra of alkali-brick grains vs medium pH; Figure S3: ESEM images of alkali-brick grains. Quantitative ESEM/EDS analyses performed on: large zones of surface aggregates (~300–500 µm) —(A); zeolites-rich zones(50–60µm)—(B); different targeted ‘zeolitic’ particles—(C).
Author Contributions
Conceptualization: A.B., O.A., G.T., B.R. and M.W.; methodology: A.B., O.A., N.P., G.D. and M.W.; formal analysis: A.B., O.A., N.P., G.D., G.T., B.R., B.O. and M.W.; investigation: A.B., O.A., G.T., B.R. and M.W.; resources: A.B., O.A., B.O. and M.W.; data curation: A.B., O.A., G.T., B.R. and M.W.; writing—original draft preparation: A.B.; writing—review and editing: A.B.; supervision: A.B., O.A., B.O. and M.W.; project administration: B.O. and M.W.; funding acquisition: M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
Scientific works were undertaken successfully owing to the cooperation between the University of Lille (France) and the University of Bangui (Central African Republic). This collaboration (being still underway) and the Grant-in-Aid to Gildas Doyemet for his Doctoral-Thesis preparation is financially supported by the Embassy of France to Bangui. Financial support from the IR INFRANALYTICS FR2054 for conducting the research is gratefully acknowledged. The Region “Hauts de France” and the French government are warmly acknowledged for the co-funding of these apparatus. Scanning electron microscopy studies were undertaken in the laboratory UMR LOG 8187 at the Department of Earth Sciences (Villeneuve d’Ascq 59655, France). The authors gratefully thank Sandra Vantalon (Chemical Engineer) for carrying out careful ESEM/EDS analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pierrehumbert, R. There is no Plan B for dealing with the climate crisis. Bulletion At. Sci. 2019, 75, 215–221. [Google Scholar] [CrossRef]
- Glasser, F. Application of inorganic cements to the conditioning and immobilisation of radioactive wastes. In Handbook of Advanced Radioactive Waste Conditioning Technologies; Ojovan, M.I., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 67–135. [Google Scholar]
- Komljenovića, M.; Tanasijevića, G.; Džunuzovića, N.; Provis, J.L. Immobilization of cesium with alkali-activated blast furnace slag. J. Hazard. Mater. 2020, 388, 121765. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Tao, D.; Xu, Y.; Li, P.; Cui, H.; Zhai, J. Immobilization of simulated radionuclide 133Cs+ by fly ash-based geopolymer. J. Hazard. Mater. 2013, 262, 325–331. [Google Scholar] [CrossRef]
- Deng, N.; An, H.; Cui, H.; Pan, Y.; Wang, B.; Mao, L.; Zhai, J. Effects of gamma-ray irradiation on leaching of simulated 133Cs+ radionuclides from geopolymer wasteforms. J. Nucl. Mater. 2015, 459, 270–275. [Google Scholar] [CrossRef]
- Kuenzel, C.; Cisneros, J.F.; Neville, T.P.; Vandeperre, L.; Simons, S.J.R.; Bensted, J.; Cheeseman, C.R. Encapsulation of Cs/Sr contaminated clinoptilolite geopolymers produced from metakaolin. J. Nucl. Mater. 2015, 466, 94–99. [Google Scholar] [CrossRef]
- Jang, J.G.; Park, S.M.; Lee, H.K. Physical barrier effect of geopolymeric waste form on diffusivity of cesium and strontium. J. Hazard. Mater. 2016, 318, 339–346. [Google Scholar]
- Wagh, A.S.; Sayenko, S.Y.; Shkuropatenko, V.A.; Tarasov, R.V.; Dykiy, M.P.; Svitlychniy, Y.O.; Virych, V.D.; Ulybkina, E.A. Experimental study on cesium immobilization in struvite structures. J. Hazard. Mater. 2016, 302, 241–249. [Google Scholar] [CrossRef]
- Al-Jubouri, S.M.; Curry, N.A.; Holmes, S.M. Hierarchical porous structured zeolite composite for removal of ionic contaminants from waste streams and effective encapsulation of hazardous waste. J. Hazard. Mater. 2016, 320, 241–251. [Google Scholar] [CrossRef]
- Arbel Haddad, M.; Ofer-Rozovsky, E.; Bar-Nes, G.; Borojovich, E.J.C.; Nikolski, A.; Mogiliansky, D.; Katz, A. Formation of zeolites in metakaolin-based geopolymers and their potential application for Cs immobilization. J. Nucl. Mater. 2017, 493, 168–179. [Google Scholar] [CrossRef]
- El-Naggar, M.R.; Amin, M. Impact of alkali cations on properties of metakaolin and metakaolin/slag geopolymers: Microstructures in relation to sorption of 134Cs radionuclide. J. Hazard. Mater. 2018, 344, 913–924. [Google Scholar] [CrossRef]
- Vandevenne, N.; Iacobescu, R.I.; Pontikes, Y.; Carleer, R.; Thijssen, E.; Gijbels, K.; Schreurs, S.; Schroeyers, W. Incorporating Cs and Sr into blast furnace slag inorganic polymers and their effect on matrix properties. J. Nucl. Mater. 2018, 503, 1–12. [Google Scholar] [CrossRef]
- Tian, Q.; Nakama, S.; Sasaki, K. Immobilization of cesium in fly ash-silica fume based geopolymers with different Si/Al molar ratios. Sci. Total Environ. 2019, 687, 1127–1137. [Google Scholar] [CrossRef]
- Walkley, B.; Ke, X.; Hussein, O.H.; Bernal, S.A.; Provis, J.L. Incorporation of strontium and calcium in geopolymer gels. J. Hazard. Mater. 2020, 382, 121015. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Immobilisation of Radioactive Waste in Cement in: An Introduction to Nuclear Waste Immobilisation, 2nd ed.; Elsevier: Oxford, UK, 2014; Chapter 15; pp. 205–232. [Google Scholar]
- Park, B.; Kim, J.; Ghoreishian, S.M.; Rethinasabapathy, M.; Suk Huh, Y.; Kang, S.-M. Generation of multi-functional core-shell adsorbents: Simultaneous adsorption of cesium, strontium and rhodamine B in aqueous solution. J. Ind. Eng. Chem. 2022, 112, 201–209. [Google Scholar] [CrossRef]
- Jiang, C.; Ni, J.; Jin, G.P. Magnetic potassium cobalt hexacyanoferrate nanocomposites for efficient adsorption of rubidium in solution. Sep. Purif. Technol. 2022, 296, 121383. [Google Scholar] [CrossRef]
- Almas Dutt, M.; Asif Hanif, M.; Nadeem, F.; Bhatti, H.N. A review of advances in engineered composite materials popular for wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 104073. [Google Scholar] [CrossRef]
- Roshanfekr Rad, L.; Anbia, M. Zeolite-based composites for the adsorption of toxic matters from water: A review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Li, L.; Li, H.; Hu, D.; Xiang, Y.; Han, L.; Yu, Y.; Ning, L.; Peng, X. Short communication on ‘Incorporating radionuclide Sr into geopolymer-zeolite A composites: Geopolymerisation characteristics. J. Nucl. Mater. 2022, 563, 153625. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Gao, M.; Bai, H.; Nagasaka, T. Synthesis of zeolite-geopolymer composites with high zeolite content for Pb (II) removal by a simple two-step method using fly ash and metakaolin. J. Clean. Prod. 2022, 378, 134528. [Google Scholar] [CrossRef]
- Dehou, S.C.; Wartel, M.; Recourt, P.; Revel, B.; Mabingui, J.; Montiel, A.; Boughriet, A. Physicochemical, crystalline and morphological characteristics of bricks used for ground waters purification in Bangui region (Central African Republic). Appl. Clay Sci. 2012, 59, 69–75. [Google Scholar] [CrossRef]
- Poumaye, N.; Allahdin, O.; Tricot, G.; Revel, B.; Billon, G.; Recourt, P.; Wartel, M.; Boughriet, A. MAS NMR investigations on a metakaolinite-rich brick after zeolitization by alkaline treatments. Microporous Mesoporous Mater. 2019, 277, 1–9. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Gan, Z. 13C/14N heteronuclear multiple-quantum correlation with rotary resonance and REDOR dipolar recoupling. J. Magn. Reson. 2007, 184, 39–43. [Google Scholar] [CrossRef]
- Tricot, G.; Trébosc, J.; Pourpoint, F.; Gauvin, R.; Delevoye, L. The D-HMQC MAS-NMR Technique. In Annual Reports on NMR Spectroscopy; Webb, G.A., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 81, pp. 145–184. [Google Scholar] [CrossRef]
- Poumaye, N.; Allahdin, O.; Wartel, M.; Boughriet, A. Insights into characterization and adsorptive behaviour of zeolitized brick in water toward cadmium (A very toxic heavy metal to humans). Int. J. Pharm. Pharmaceut. Res. 2018, 13, 1–29. [Google Scholar]
- Poumaye, N.; Allahdin, O.; Lesven, L.; Wartel, M.; Boughriet, A. Adsorption of Iron (II) on Sodic Zeolites─Bearing Brick (In Batch): Insights into Interfacial Chemical Processes and Thermodynamic Equilibria. Int. J. Sci. Res. Methodol. 2019, 11, 88–119. [Google Scholar]
- Boughriet, A.; Allahdin, O.; Poumaye, N.; Tricot, G.; Revel, B.; Lesven, L.; Wartel, M. Micro-Analytical Study of a Zeolites/Geo-Polymers/Quartz Composite, Dielectric Behaviour and Contribution to Brønsted Sites Affinity. Ceramics 2022, 5, 908–927. [Google Scholar] [CrossRef]
- Allahdin, O.; Wartel, M.; Recourt, P.; Revel, B.; Ouddane, B.; Billon, G.; Mabingui, J.; Boughriet, A. Adsorption capacity of iron oxyhydroxide-coated brick for cationic metals and nature of ion surface interactions. Appl. Clay Sci. 2014, 90, 141–149. [Google Scholar] [CrossRef]
- Seliem, M.K.; Komarneni, S. Equilibrium and kinetic studies for dissociation of iron from aqueous solution by synthetic Na-A zeolites: Statistical modelling and optimization. Microporous Mesoporous Mater. 2016, 228, 266–274. [Google Scholar] [CrossRef]
- Tontisirin, S. Synthesis and characterization of co-crystalline zeolite composite of LSX/A. Microporous Mesoporous Mater. 2017, 239, 123–129. [Google Scholar] [CrossRef]
- Sathupunya, M.; Glari, E.; Wongkasemjit, S. ANA and GIS zeolite synthesis directly from alumatrane and silatrane by sol-gel process and microwave technique. J. Eur. Ceram. Soc. 2002, 22, 2305–2314. [Google Scholar] [CrossRef]
- Zubowa, H.-L.; Kosslick, H.; Müller, D.; Richter, M.; Wilde, L.; Fricke, R. Crystallization of phase-pure zeolite NaP from MCM-22-type gel compositions under microwave radiation. Microporous Mesoporous Mater. 2008, 109, 542–548. [Google Scholar] [CrossRef]
- Behin, J.; Kazemian, H.; Rohani, S. Sonochemical synthesis of zeolite NaP from clinoptilolite. Ultrason. Sonochem. 2016, 28, 400–408. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th ed.; Elsevier: New York, NY, USA, 2007; pp. 1–762. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Apply 29Si, 27Al MAS NMR and selective dissolution in identifying the reaction degree of alkali activated slag-fly ash composites. Ceram. Int. 2017, 43, 12408–12419. [Google Scholar] [CrossRef]
- Lippmaa, E.; Mági, M.; Samoson, A.; Tarmak, M.; Engelhardtl, G. Investigation of the structure of zeolites by solid-state high-resolution 29Si NMR spectroscopy. J. Am. Chem. Soc. 1981, 103, 4992–4996. [Google Scholar] [CrossRef]
- Hunger, M.; Anderson, M.W.; Ojo, A.; Pfelfer, H. Study of the geometry and location of the bridging OH groups in aluminosilicate and silicoaluminophosphate type zeolites using 1H MAS NMR sideband analysis and CP/MAS NMR. Microporous Mater. 1993, 1, 17–32. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Monzo, M.; Vicent, M.; Barba, A.; Palomo, A. Alkaline activation of metakaolin–fly ash mixtures: Obtain of Zeoceramics and Zeocements. Microporous Mesoporous Mater. 2008, 108, 41–49. [Google Scholar] [CrossRef]
- Criado, M.; Fernandez-Jimenez, A.; Palomo, A.; Sobrados, I.; Sanz, J. Effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Part II: 29Si MAS-NMR Survey. Microporous Mesoporous Mater. 2008, 109, 525–534. [Google Scholar] [CrossRef]
- Souayfan, F.; Rozière, E.; Paris, M.; Deneele, D.; Loukili, A.; Justino, C. 29Si and 27Al MAS NMR spectroscopic studies of activated metakaolin-slag mixtures. Constr. Build. Mater. 2022, 322, 126415. [Google Scholar] [CrossRef]
- Engelhardt, G.; Luger, S.; Buhl, J.C.; Felsche, J. 29Si MAS n.m.r, of aluminosilicate sodalites: Correlations between chemical shifts and structure parameters. Zeolites 1989, 9, 182–186. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. 29Si NMR study of structural ordering in aluminosilicates geopolymer gels. Langmuir 2005, 21, 3028–3036. [Google Scholar] [CrossRef]
- Ortega-Zavala, D.E.; Burciaga-Díaz, O.; Escalante-García, J.I. Chemically bonded ceramic/cementitious materials of alkali activated metakaolin processed by cold pressing. Constr. Build. Mater. 2021, 279, 121365. [Google Scholar] [CrossRef]
- Allahdin, O.; Poumaye, N.; Wartel, M.; Boughriet, A. Correlation analysis between cationic metal characteristics and ion-exchange performance of brick-derived zeolites: A comprehensive mechanistic explanation. Mater. Chem. Phys. 2022, 276, 125353. [Google Scholar] [CrossRef]
- Engelhardt, G.; Michel, D. High-Resolution Solid-State NMR of Silicates and Zeolites; Wiley & Sons: London, UK, 1987; p. 485. ISBN 0471915971. [Google Scholar]
- MacKenzie, K.J.D.; Smith, M.E. Multinuclear Solid-State NMR of Inorganic Materials. Pergamon Mater. Ser. 2002, 6, 727. [Google Scholar]
- Lin, H.; Liu, H.; Li, Y.; Kong, X. Properties and reaction mechanism of phosphoric acid activated metakaolin geopolymer at varied curing temperatures. Cem. Concr. Res. 2021, 144, 106425. [Google Scholar] [CrossRef]
- Engelhardt, G. Multinuclear solid-state NMR in silicate and zeolite chemistry. TrAC Trends Anal. Chem. 1989, 8, 343–347. [Google Scholar] [CrossRef]
- Huang, J.; van Vegten, N.; Jiang, Y.; Hunger, M.; Baiker, A. Increasing the Brønsted acidity of flame-derived silica/alumina up to zeolitic strength. Angew. Chem. 2010, 122, 7942–7947. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Dai, W.; Hunger, M. Solid-state nuclear magnetic resonance investigations of the nature, property, and activity of acid sites on solid catalysts. Solid State Nucl. Magn. Reson. 2011, 39, 116–141. [Google Scholar] [CrossRef]
- Wang, C.; Dai, W.; Wu, G.; Guan, N.; Li, L. Application of ammonia probe-assisted solid-state NMR technique in zeolites and catalysis. Magn. Reson. Lett. 2022, 2, 28–37. [Google Scholar] [CrossRef]
- Dyballa, M.; Obenaus, U.; Lang, S.; Gehring, B.; Traa, Y.; Koller, H.; Hunger, M. Brønsted sites and structural stabilization effect of acidic low-silica zeolite A prepared by partial ammonium exchange. Microporous Mesoporous Mater. 2015, 212, 110–116. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Reddy Marthala, V.R.; Wang, W.; Sulikowski, B.; Hunger, M. In situ 1H MAS NMR investigations of the H/D exchange of alkylaromatic hydrocarbons on zeolites H-Y, La, Na-Y, and H-ZSM-5. Microporous Mesoporous Mater. 2007, 99, 86–90. [Google Scholar] [CrossRef]
- Ma, D.; Han, X.; Xie, S.; Bao, X.; Hu, H.; Au-Yeung, S.C.F. An investigation of the roles of surface aluminum and acid sites in the zeolite MCM-22. Chem. Eur. J. 2002, 8, 162–170. [Google Scholar] [CrossRef]
- Kanellopoulos, J.; Gottert, C.; Schneider, D.; Knorr, B.; Prager, D.; Ernst, H.; Freude, D. NMR investigation of proton mobility in zeolites. J. Catal. 2008, 255, 68–78. [Google Scholar] [CrossRef]
- Ruiz-Santaquiteria, C.; Skibsted, J.; Fernández-Jiménez, A.; Palomo, A. Alkaline solution/binder ratio as a determining factor in the alkaline activation of aluminosilicates. Cem. Concr. Res. 2012, 42, 1242–1251. [Google Scholar] [CrossRef]
- Glid, M.; Sobrados, I.; Ben Rhaiem, H.; Sanz, J.; Ben Haj Amara, A. Alkaline activation of metakaolinite-silica mixtures: Role of dissolved silica concentration on the formation of geopolymers. Ceram. Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- Miladinovic, Z.P.; Zakrzewska, J.; Kovacevic, B.T.; Miladinovic, J.M. In situ 27Al NMR kinetic investigation of zeolite A crystallization. Microporous Mesoporous Mater. 2014, 195, 131–142. [Google Scholar] [CrossRef]
- Kulshreshthaa, S.K.; Vijayalakshmia, R.; Sudarsana, V.; Salunkeb, H.G.; Bhargavac, C. Iron oxide nanoparticles in NaA zeolite cages. Solid State Sci. 2013, 21, 44–50. [Google Scholar] [CrossRef]
- Xiao, Y.; Sheng, N.; Chu, Y.; Wang, Y.; Wu, Q.; Liu, X.; Deng, F.; Meng, X.; Feng, Z. Mechanism on solvent-free crystallization of NaA zeolite. Microporous Mesoporous Mater. 2017, 237, 201–209. [Google Scholar] [CrossRef]
- Albert, B.R.; Cheetham, A.K.; Stuart, J.A.; Adams, C.J. Investigations on P zeolites: Synthesis, characterisation, and structure of highly crystalline low-silica NaP. Microporous Mesoporous Mater. 1998, 21, 133–142. [Google Scholar] [CrossRef]
- Nery, J.G.; Mascarenhas, Y.P.; Cheetham, A.K. A study of the highly crystalline, lowsilica, fully hydrated zeolite P ion exchanged with (Mn2+, Cd2+, Pb2+, Sr2+, Ba2+) cations. Microporous Mesoporous Mater. 2003, 57, 229–248. [Google Scholar] [CrossRef]
- Meftah, M.; Oueslati, W.; Chorfi, N.; Ben Haj Amara, A. Effect of the raw material type and the reaction time on the synthesis of halloysite based Zeolite Na-P1. Result Phys. 2017, 7, 1475–1484. [Google Scholar] [CrossRef]
- Fletcher, R.A.; MacKenzie, K.J.D.; Nicholson, C.L.; Shimada, S. The composition range of aluminosilicate geopolymers. J. Eur. Ceram. Soc. 2005, 25, 1471–1477. [Google Scholar] [CrossRef]
- Jacobsen, H.S.; Norby, P.; Bildsce, H.; Jakobsen, H.J. 1:1 Correlation between 27AI and 29Si chemical shifts and correlations with lattice structures for some aluminosilicate sodalites. Zeolites 1989, 9, 491–495. [Google Scholar] [CrossRef]
- Lippmaa, E.; Samoson, A.; Mägi, M. High-resolution 27Al NMR of aluminosilicates. J. Am. Chem. Soc. 1986, 108, 1730–1735. [Google Scholar] [CrossRef]
- Ramdas, S.; Klinowski, J. A simple correlation between isotropic 29Si-NMR chemical shifts and T -0-T angles in zeolite frameworks. Nature 1984, 308, 521–523. [Google Scholar] [CrossRef]
- O’Neil Parker, W., Jr.; SWegner, S. Aluminum in mesoporous silica–alumina. Microporous Mesoporous Mater. 2012, 158, 235–240. [Google Scholar] [CrossRef]
- Limtrakul, J.; Tantanak, D. Cationic, structural, and compositional effects on the surface structure of zeolitic alu minosilicate catalysts. Chem. Phys. 1996, 208, 331–340. [Google Scholar] [CrossRef]
- Jain, S.; Banthia, N.; Troczynski, T. Leaching of immobilized cesium from NaOH-activated fly ash-based geopolymers. Cem. Concr. Compos. 2022, 133, 104679. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).