Crude Glycerol as an Innovative Corrosion Inhibitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Steel Specimens
2.2. Experimental Procedure
3. Results and Discussion
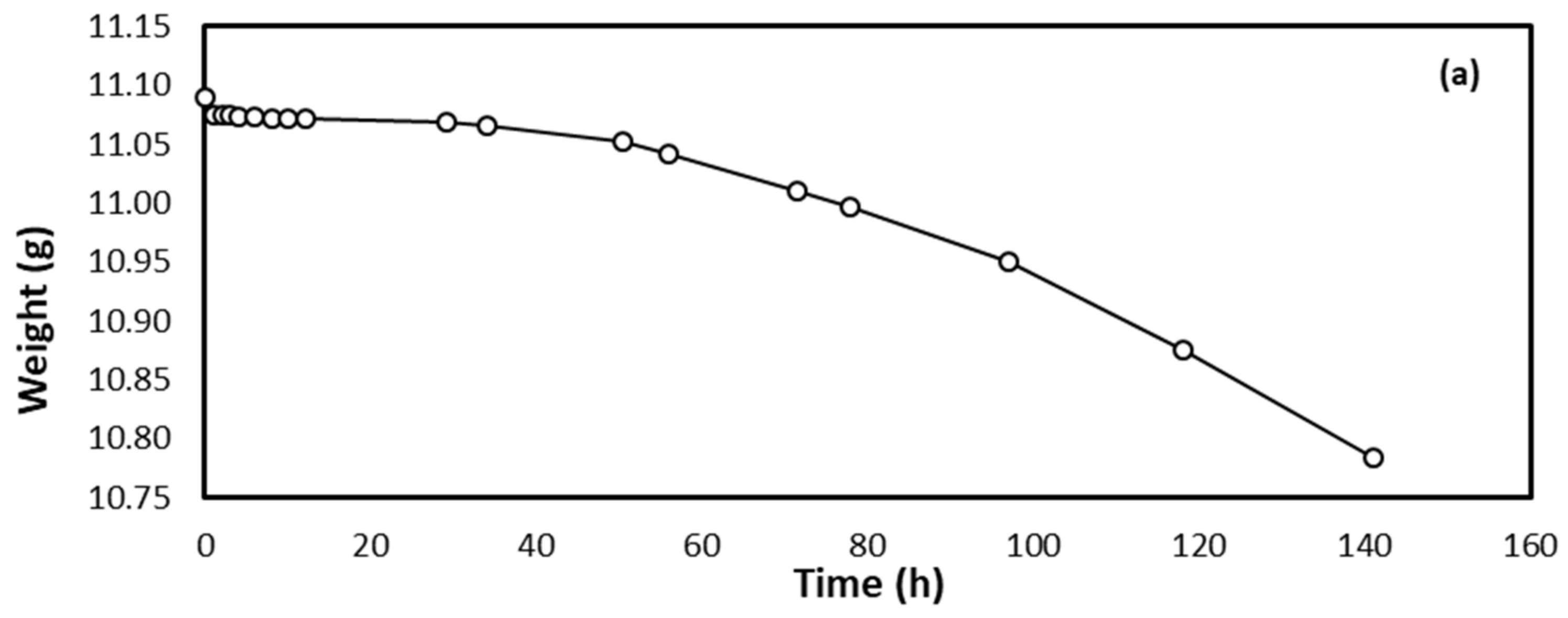
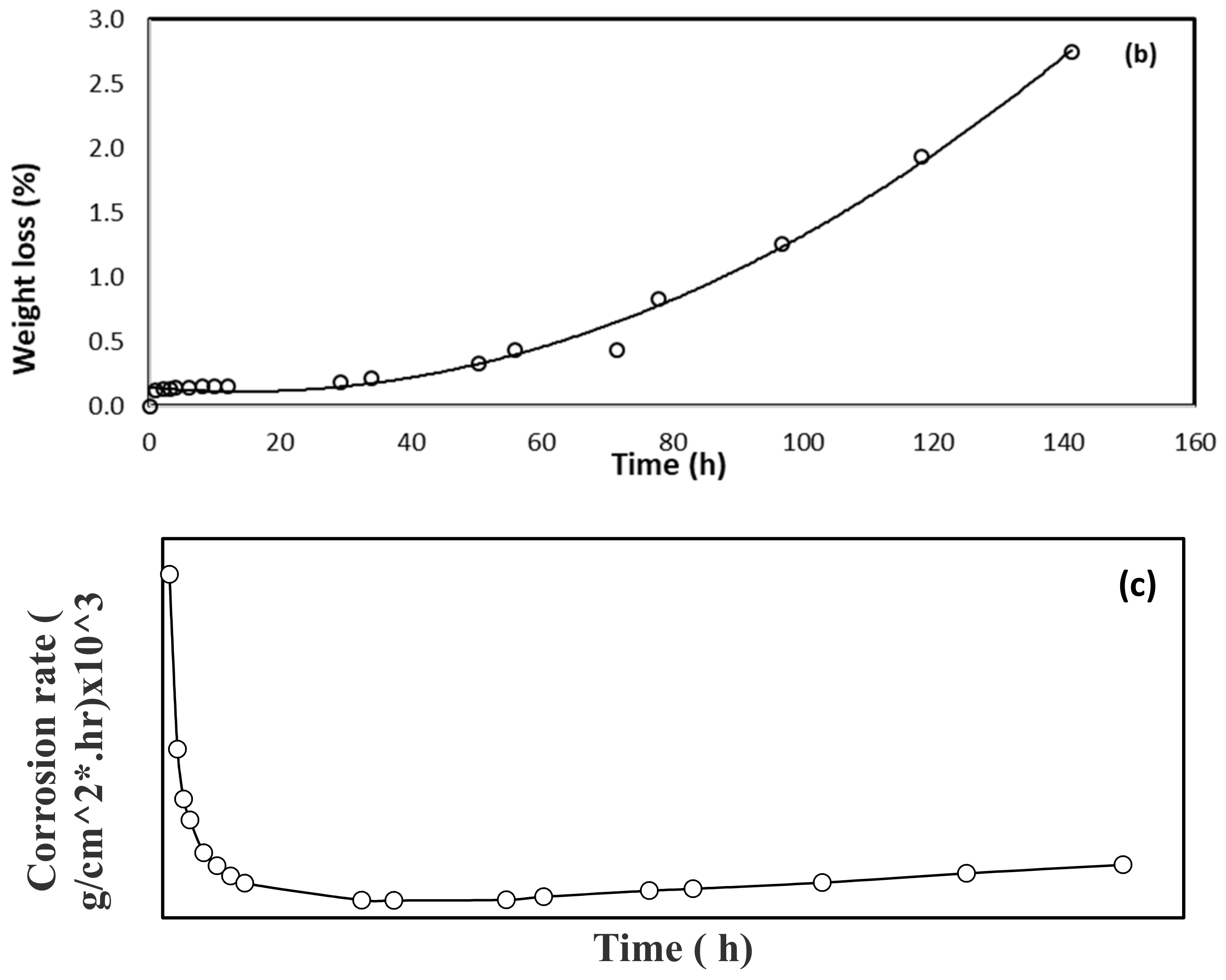
3.1. Corrosion Study
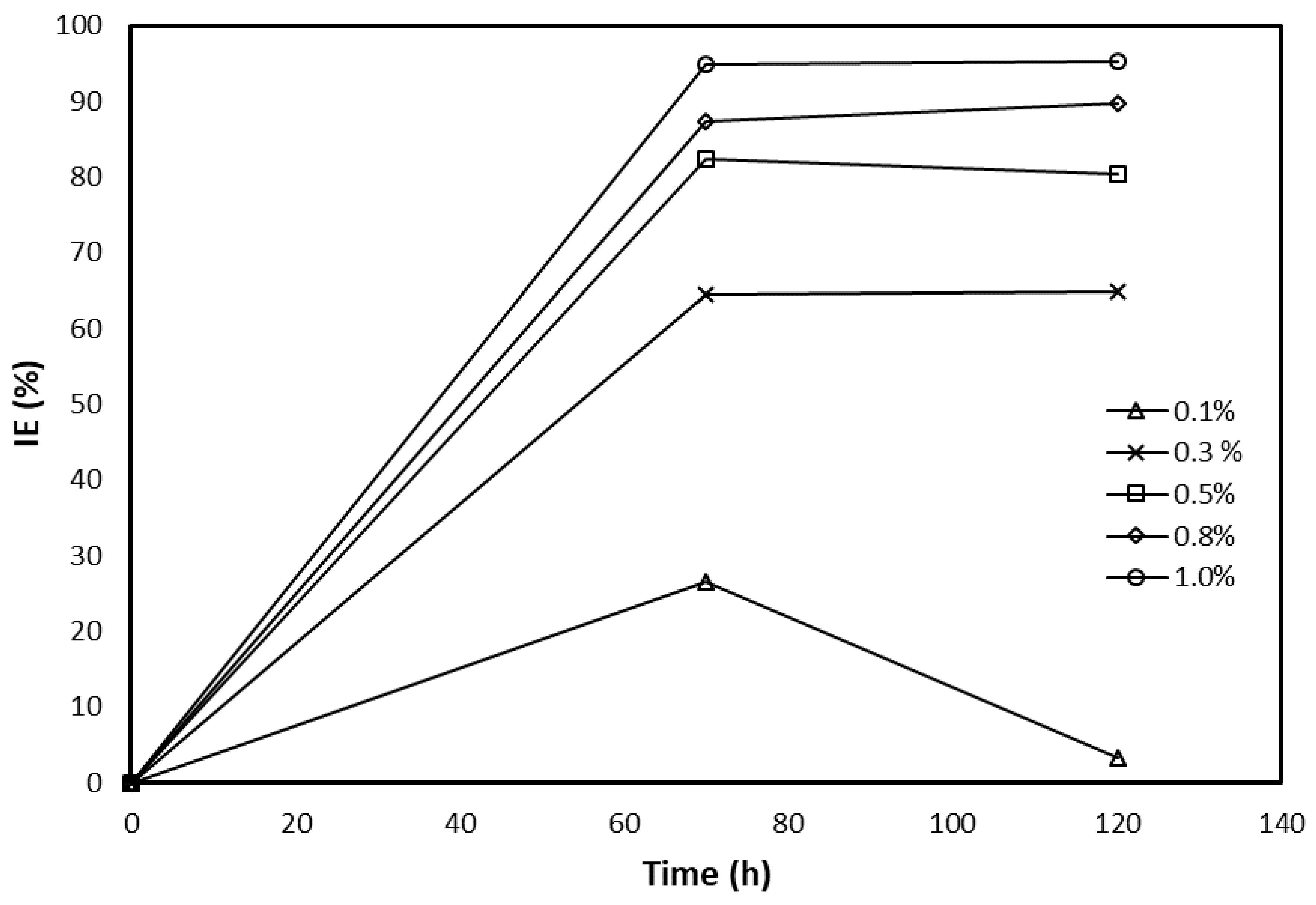
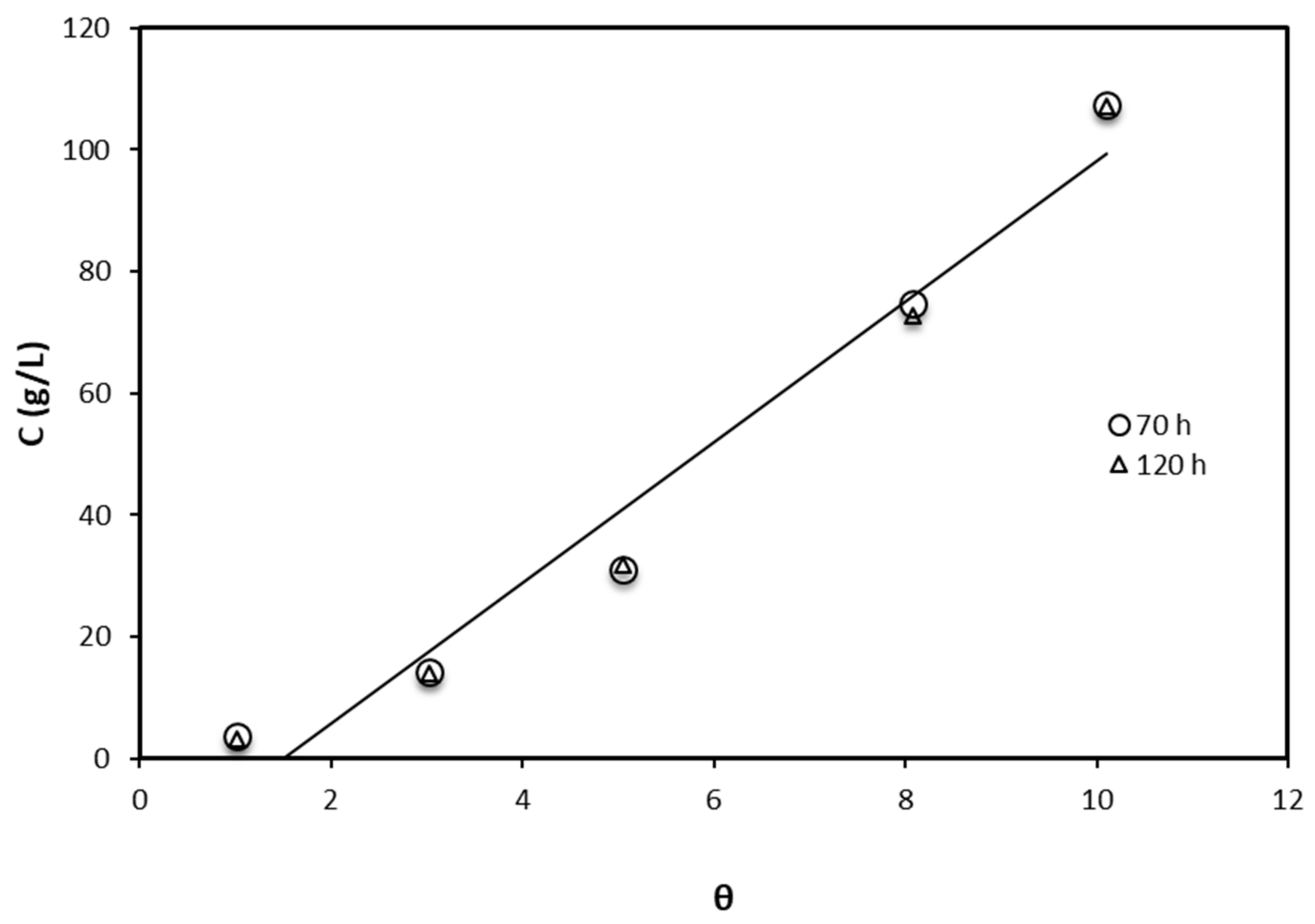
3.2. Crude Glycerol Inhibition Ability
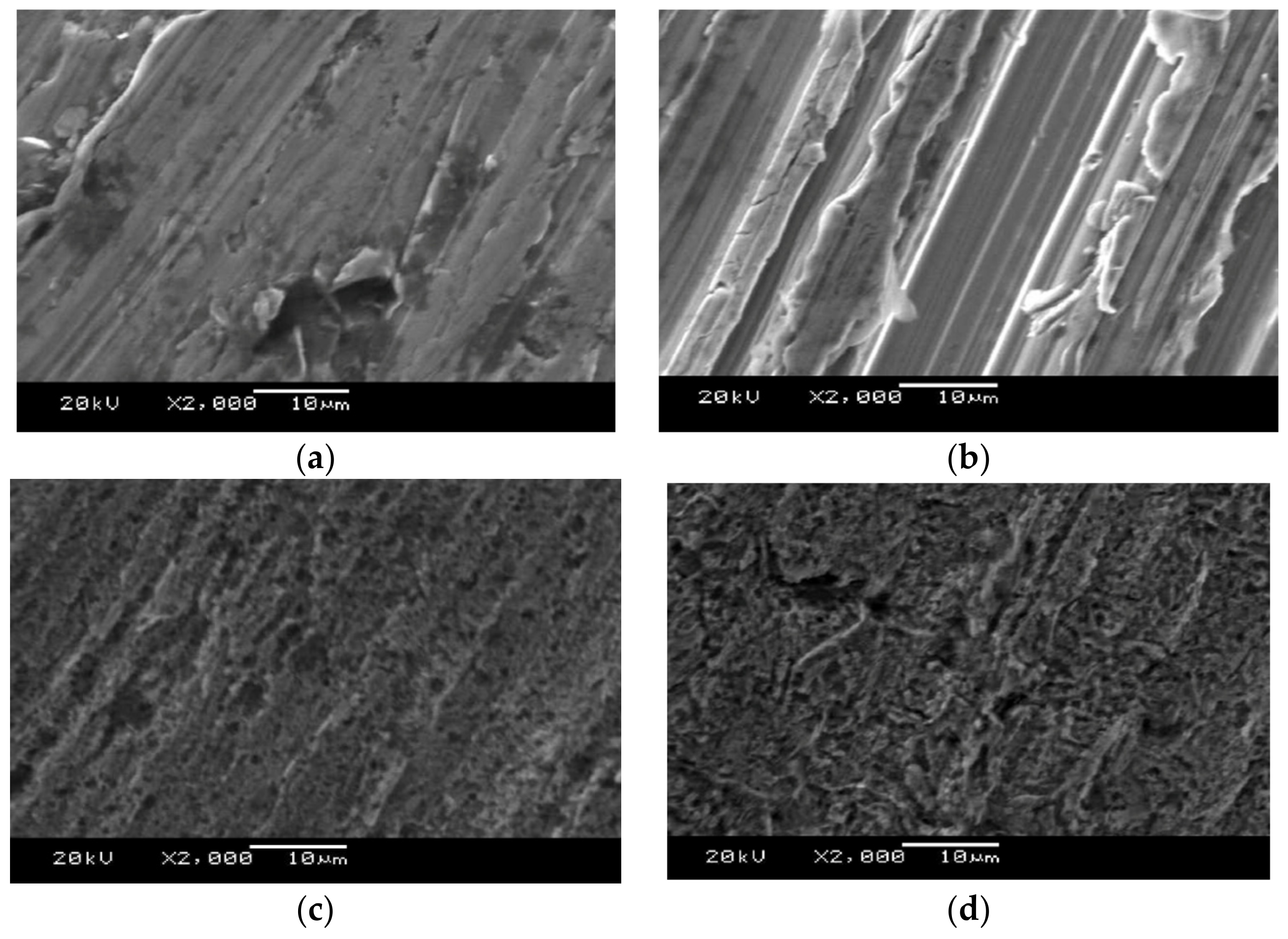
3.3. Scanning Electron Microscopy (SEM)
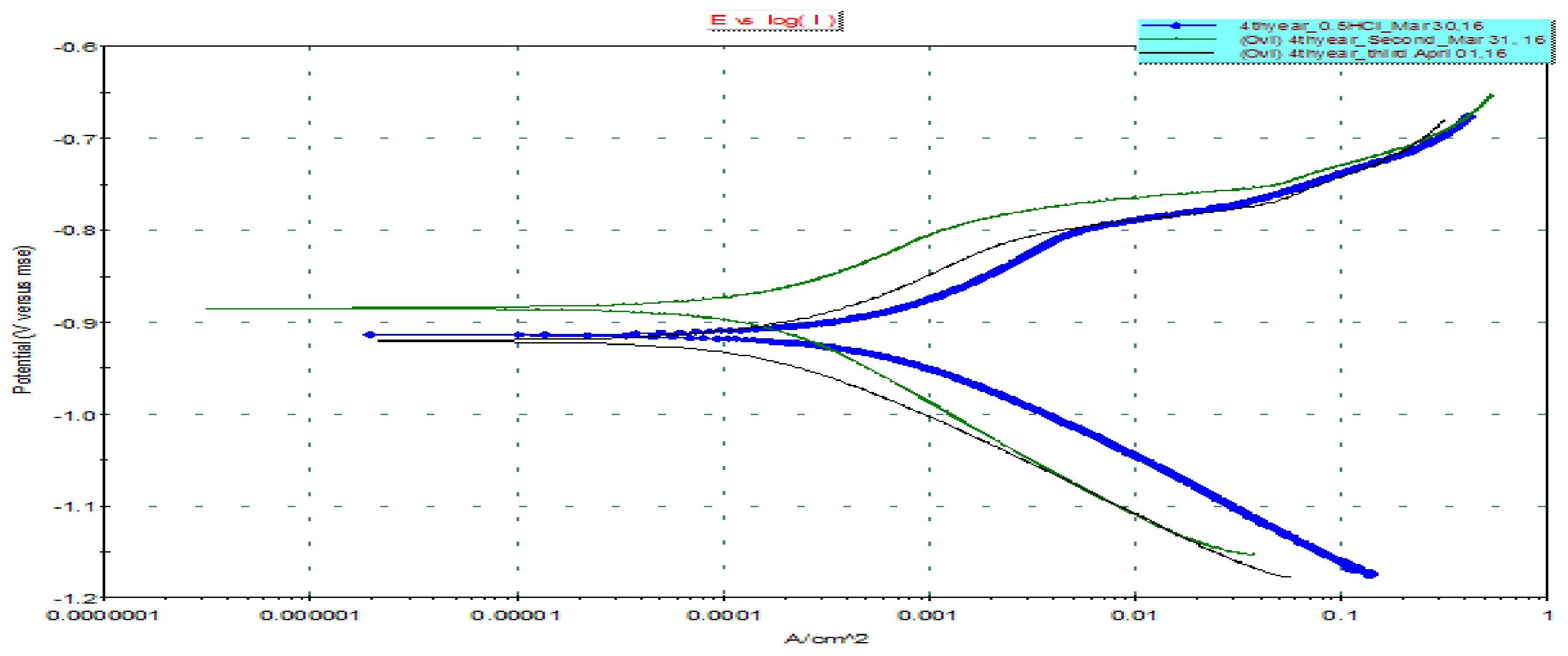
3.4. Potentiodynaminc Polarization Measurements
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Khan, G.; Newaz, K.S.; Basirun, W.J.; Ali, H.B.M.; Faraj, F.L.; Khan, G.M. Application of natural product extracts as green corrosion inhibitors for metals and alloys in acid pickling processes—A review. Int. J. Electrochem. Sci. 2015, 10, 6120–6134. [Google Scholar]
- Cardarelli, F. Materials Handbooks—A Concise Desktop Reference; Springer: London, UK, 2000. [Google Scholar]
- Gerhardus, H.K.; Michiel, P.H.; Bronger, N.G.; Thompson, Y.; Paul, V.; Payer, J.H. Corrosion Cost and Preventive Strategies in the United States. Supplement to Materials Performance; Report number FHWA. RD-01-156; Federal Highway Administration: Mclean, VA, USA, 2002. [Google Scholar]
- Bouklah, M.; Hammouti, B.; Lagrenée, M.; Bentis, F.M. Thermodynamic properties of 2,5-bis (4-methoxyphenyl)-1,3,4-oxadiazole as a corrosion inhibitor for mild steel in normal sulfuric acid medium. Corros. Sci. 2006, 48, 2831–2842. [Google Scholar] [CrossRef]
- Raja, P.B.; Sethuraman, M.G. Natural products as corrosion inhibitor for metals in corrosive media—A review. Mater. Lett. 2008, 62, 113–116. [Google Scholar] [CrossRef]
- Wang, L.; Pu, J.X.; Luo, H.C. Corrosion inhibition of zinc in phosphoric acid solution by 2-mercaptobenzimidazole. Corros. Sci. 2003, 45, 677–683. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Natural honey as corrosion inhibitor for metals and alloys, Copper in neutral aqueous solution. Corros. Sci. 1998, 40, 1845–1850. [Google Scholar] [CrossRef]
- Radojcic, I.; Berkovic, K.; Kovac, S.; Vorkapic-Furac, J. Natural honey and black radish juice as tin corrosion inhibitors. Corros. Sci. 2008, 50, 1498–1504. [Google Scholar] [CrossRef]
- Ahmad, Z. Principles of Corrosion Engineering and Corrosion Control; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Ali, S.A.; Al-Muallem, H.A.; Rahman, S.U.; Saeed, M.T. Bis-isoxazolidines: A new class of corrosion inhibitors of mild steel in acidic media. Corros. Sci. 2008, 50, 3070–3377. [Google Scholar] [CrossRef]
- Mernari, B.; El Attari, H.; Traisnel, M.; Bentiss, F.; Lagrenee, M. Inhibiting effects of 3,5-bis (n-pyridyl)-4-amino-1,2,4-triazoles on the corrosion for mild steel in 1 M HCl medium. Corros. Sci. 1998, 40, 391–399. [Google Scholar] [CrossRef]
- El Achouri, M.; Kertit, S.; Gouttaya, H.M.; Nciri, B.; Bensouda, Y.; Perez, L.; Infante, M.R.; Elkacemi, K. Corrosion inhibition of iron in 1 M HCl by some gemini surfactants in the series of alkanediyl-α,ω-bis-(dimethyl tetradecyl ammonium bromide). Prog. Org. Coat. 2001, 43, 267–273. [Google Scholar] [CrossRef]
- Zeng, D.; Yan, H. Experimental study on a new corrosion and scale inhibitor. J. Environ. Protect. 2013, 4, 671–675. [Google Scholar] [CrossRef]
- Mokhtari, O.; Hamdani, I.; Chetouani, A.; Lahrach, A.; El Halouani, H.; Aouniti, A.; Berrabah, M. Inhibition of steel corrosion in 1M HCl by Jatropha curcas oil. J. Mater. Environ. Sci. 2014, 5, 310–319. [Google Scholar]
- Khaled, K.F. Experimental and theoretical study for corrosion inhibition of mild steel in hydrochloric acid solution by some new hydrazine carbodithioic acid derivatives. Appl. Surf. Sci. 2016, 252, 4120–4128. [Google Scholar] [CrossRef]
- Fouda, A.S.; Elewady, G.Y.; Shalabi, K.; Habbouba, S. Gibberellic acid as green corrosion inhibitor for carbon steel in hydrochloric acid solutions. J. Mater. Environ. Sci. 2014, 5, 767–778. [Google Scholar]
- Sangeetha, M.; Rajendran, S.; Muthumegala, T.S.; Krishnaveni, A. Green corrosion inhibitors-An overview. Zaštita Materijala 2011, 52, 3–19. [Google Scholar]
- Fouda, A.S.; Elewady, G.Y.; Shalabi, K.; Habouba, S. Anise extract as green corrosion inhibitor for carbon steel in hydrochloric acid solutions. Int. J. Innov. Res. Sci. 2014, 3, 11210–11228. [Google Scholar]
- Obot, I.B.; Obi-Ebbed, N.O. 2,3-Diphenylbenzoquinoxaline: A new corrosion inhibitor for mild steel in sulphuric acid. Corros. Sci. 2010, 52, 282–285. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O.; Odozi, N.W. Acenaphtho [1,2-b] quinoxaline as a novel corrosion inhibitor for mild steel in 0.5 M H2SO4. Corros. Sci. 2010, 52, 923–926. [Google Scholar] [CrossRef]
- Obot, I.B.; Obi-Egbedi, N.O. Indeno-1-one [2, 3-b] quinoxaline as an effective inhibitor for the corrosion of mild steel in 0.5 M H2SO4 solution. Mater. Chem. Phys. 2010, 122, 325–328. [Google Scholar] [CrossRef]
- Kesavan, D.; Gopiraman, M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Neha, P.; Shruti, A.; Pallav, S. Greener Approach towards Corrosion Inhibition. Chin. J. Eng. 2013, 2013. [Google Scholar] [CrossRef]
- Singh, W.P.; Bockris, J.O. NACE-96225, Corrosion 96; NACE International: Denver, CO, USA, 24–29 March 1996. [Google Scholar]
- Bewley, B.R.; Berkaliev, A.; Henriksen, H.; Ball, D.B.; Ott, L.S. Waste glycerol from biodiesel synthesis as a component in deep eutectic solvents. Fuel Process. Technol. 2015, 138, 419–423. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The Glycerin Glut: Options for the Value-Added Conversion of Crude Glycerol Resulting from Biodiesel Production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Thompson, J.C.; He, B.B. Characterization of Crude Glycerol from Biodiesel Production from Multiple Feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- Hansen, C.F.; Hernandez, A.; Mullan, B.P.; Moore, K.; Trezona-Murray, M.; King, R.H.; Pluske, J.R. A chemical analysis of samples of crude glycerol from the production of biodiesel in Australia, and the effects of feeding crude glycerol to growing-finishing pigs on performance, plasma metabolites and meat quality at slaughter. Anim. Prod. Sci. 2009, 49, 154–161. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Praveen Kumar, R.; Elavazhagan, S.; Barathiraja, B.; Jayakumar Sunita, M.; Varjani, J. Utilization of Crude Glycerol from Biodiesel Industry for the Production of Value-Added Bioproducts. In Waste to Wealth; Springer Publisher: New York, NY, USA, 2017; pp. 65–82. [Google Scholar]
- Darlene, D. Biofuels Annual 2016; Gain Report No. CA16038; USD Foreign Agricultural Service: Washington, DC, USA, 2016.
- Hu, S.; Luo, X.; Wan, C.; Li, Y. Characterization of Crude Glycerol from Biodiesel Plants. J. Agric. Food Chem. 2012, 60, 5915–5921. [Google Scholar] [CrossRef] [PubMed]
- Al Zubaidi, I.; Ibrahim, H.; Jones, R.; Al zughaibi, M.; Albayyadhi, M.; Darzi, F. Waste glycerol as new green inhibition for metal corrosion in acid medium. In Proceedings of the 3rd International Conference on Fluid Flow, Heat and Mass Transfer (FFHMT’16), Ottawa, ON, Canada, 2–3 May 2016. [Google Scholar]
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alex. Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef]
- Umoren, S.A.; Ebenso, E.E. Studies of the anti-corrosive effect of Raphia hookeri exudate gum-halide mixtures for aluminum corrosion in acidic medium. Pigment Resin Technol. 2008, 37, 17–23. [Google Scholar] [CrossRef]
- Pujar, M.G.; Anita, T.; Shaikh, H.; Dayal, R.K.; Khatak, H.S. Analysis of electrochemical noise data using MEM for pitting corrosion of 316 SS in Chloride Solution. Int. J. Electrochem. Sci. 2007, 2, 301–310. [Google Scholar]
- Oguzie, E.E. Corrosion inhibition of mild steel in hydrochloric acid solution by methylene blue dye. Mater. Lett. 2005, 59, 1076–1079. [Google Scholar] [CrossRef]
- Shreir, L.L.; Jarman, R.A.; Burstein, G.T. Corrosion, 3rd ed.; Newnes-Butterworths: London, UK, 1994; Volume 2. [Google Scholar]
- Umoren, S.A.; Obot, I.B.; Obi-Egbedi, N.O. Raphia hookeri gum as a potential eco-friendly inhibitor for mild steel in sulfuric acid. J. Mater. Sci. 2009, 44, 274–279. [Google Scholar] [CrossRef]
- Nwosu, O.F.; Osarolube, E.; Nnanna, L.A.; Akoma, C.S.; Chigbu, T. Acidic Corrosion Inhibition of Piper guineense Seed Extract on Al Alloy. Am. J. Mater. Sci. 2014, 4, 178–183. [Google Scholar]
- Sivaraju, P.K.; Arulanantham, A. Inhibitive properties of plant extract (Acalyphaindica L.) on mild steel corrosion in 1N phosphoric acid. Int. J. Chem. Technol. Res. 2010, 2, 256–265. [Google Scholar]
- de Souza, F.S.; Spinelli, A. Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci. 2009, 51, 642–649. [Google Scholar] [CrossRef]
- Ansari, A.; Znini, M.; Hamdani, I.; Majidi, L.; Bouyanzer, A.; Hammouti, B. Experimental and theoretical investigations anti-corrosive properties of Menthone on mild steel corrosion in hydrochloric acid. J. Mater. Environ. Sci. 2014, 5, 81–94. [Google Scholar]
- El-Awady, A.A.; Abd-El-Nabey, B.A.; Aziz, S.G. Kinetic thermodynamic and adsorption isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open chain amines. J. Electrochem. Soc. 1992, 139, 2149–2154. [Google Scholar] [CrossRef]
| Ultimate Tensile (kpsi) | Yield Strength (kpsi) | Elongation (%) |
|---|---|---|
| 72.105 | 49.870 | 21.00 |
| C | Mn | P | S | Si | Cu | Ni | Cr | Mo | N | Pb | Sn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.17 | 0.67 | 0.012 | 0.029 | 0.18 | 0.24 | 0.11 | 0.04 | 0.030 | 0.009 | 0.004 | 0.012 |
| Property | |
|---|---|
| Color | Homogeneous dark brownish color |
| Density, g/mL at 20 °C | 1.046 |
| Glycerin content, wt % by mass [33] | 80–85 |
| Matter Organic Non Glycerin, % by mass [33] | <2 |
| Salt, % by mass | <7 |
| pH value | 11.0 |
| Viscosity, centipoise | 286.32 |
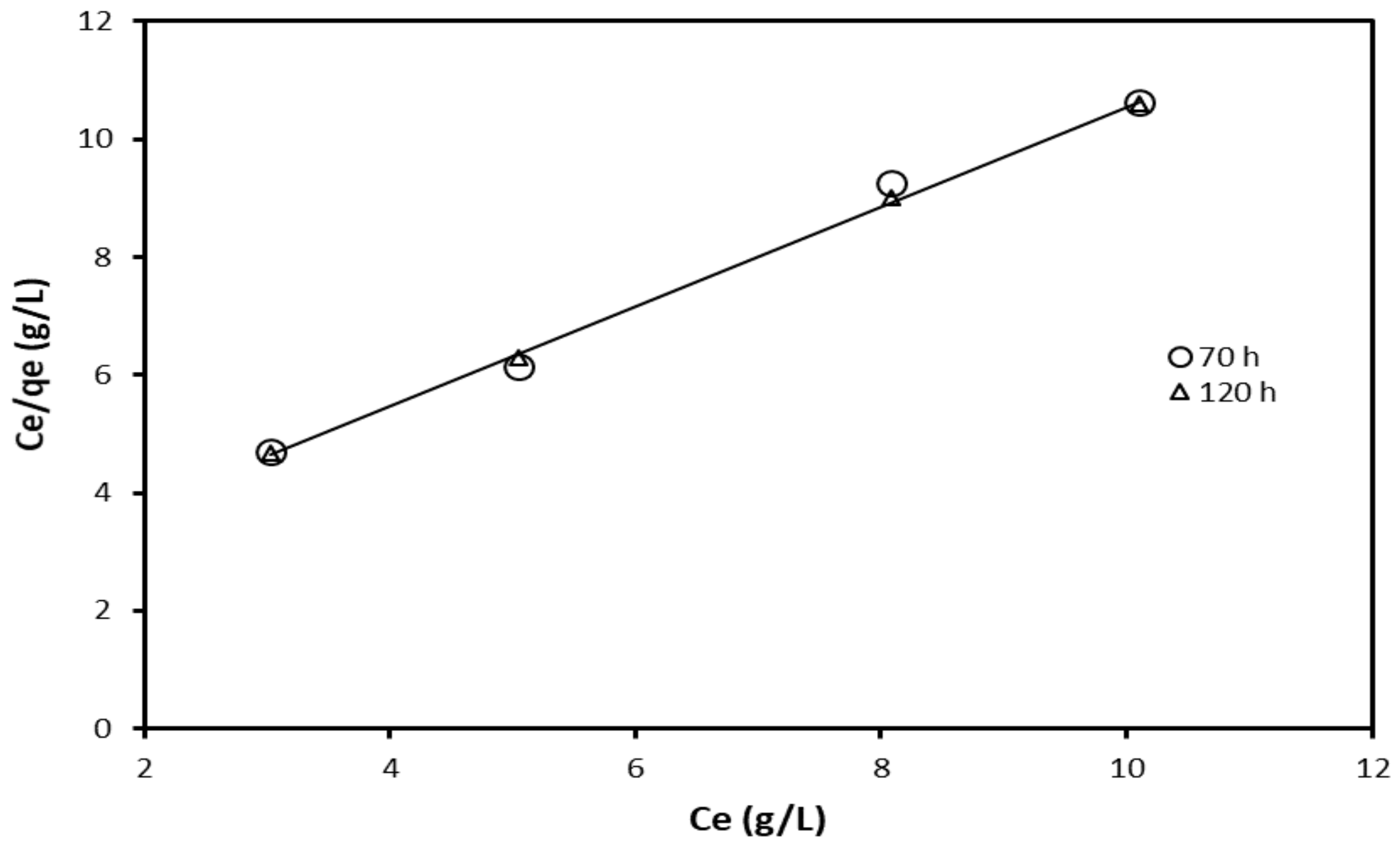
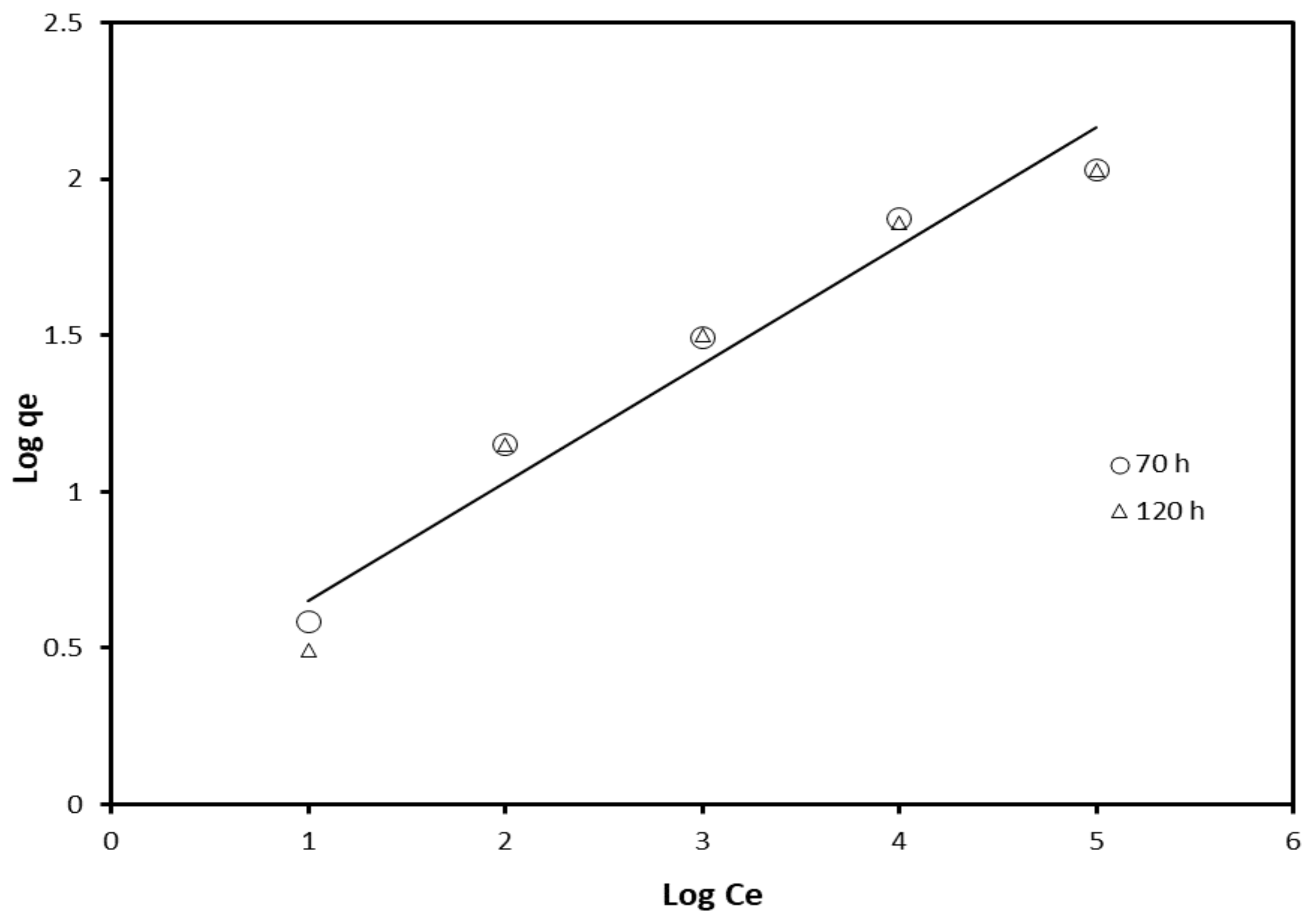
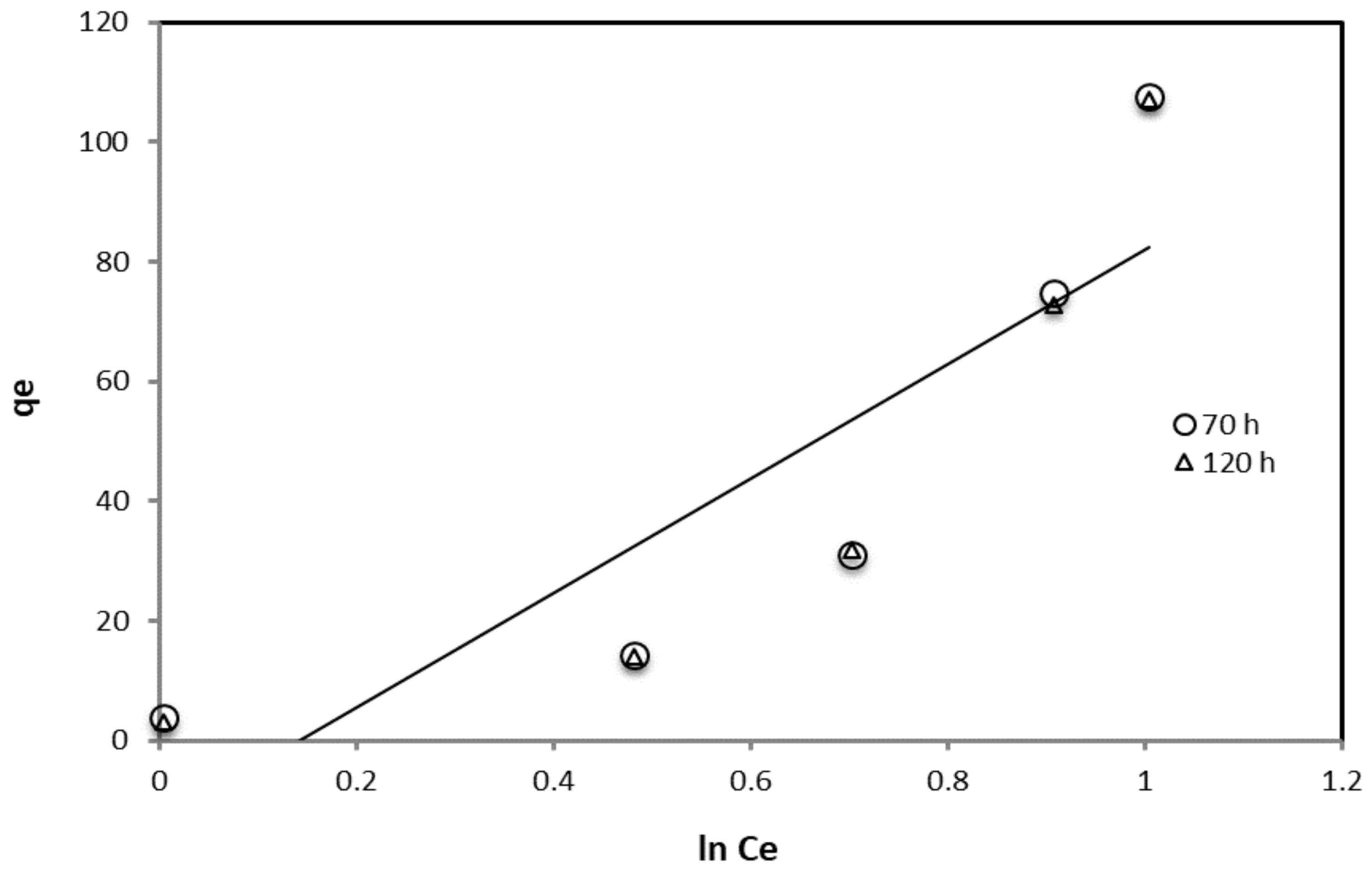
| Adsorption Isotherm Model | Contact Time (h) | R2 | Kad, g/L, Slope | Intercept |
|---|---|---|---|---|
| Langmuir | 70 | 0.999 | 0.848 | 2.069 |
| 120 | 0.994 | 0.869 | 1.970 | |
| Freundlich | 70 | 0.965 | 0.362 | 0.342 |
| 120 | 0.952 | 0.378 | 0.273 | |
| Temkin | 70 | 0.853 | 0.0085 | 0.1848 |
| 120 | 0.8532 | 0.0085 | 0.1859 |
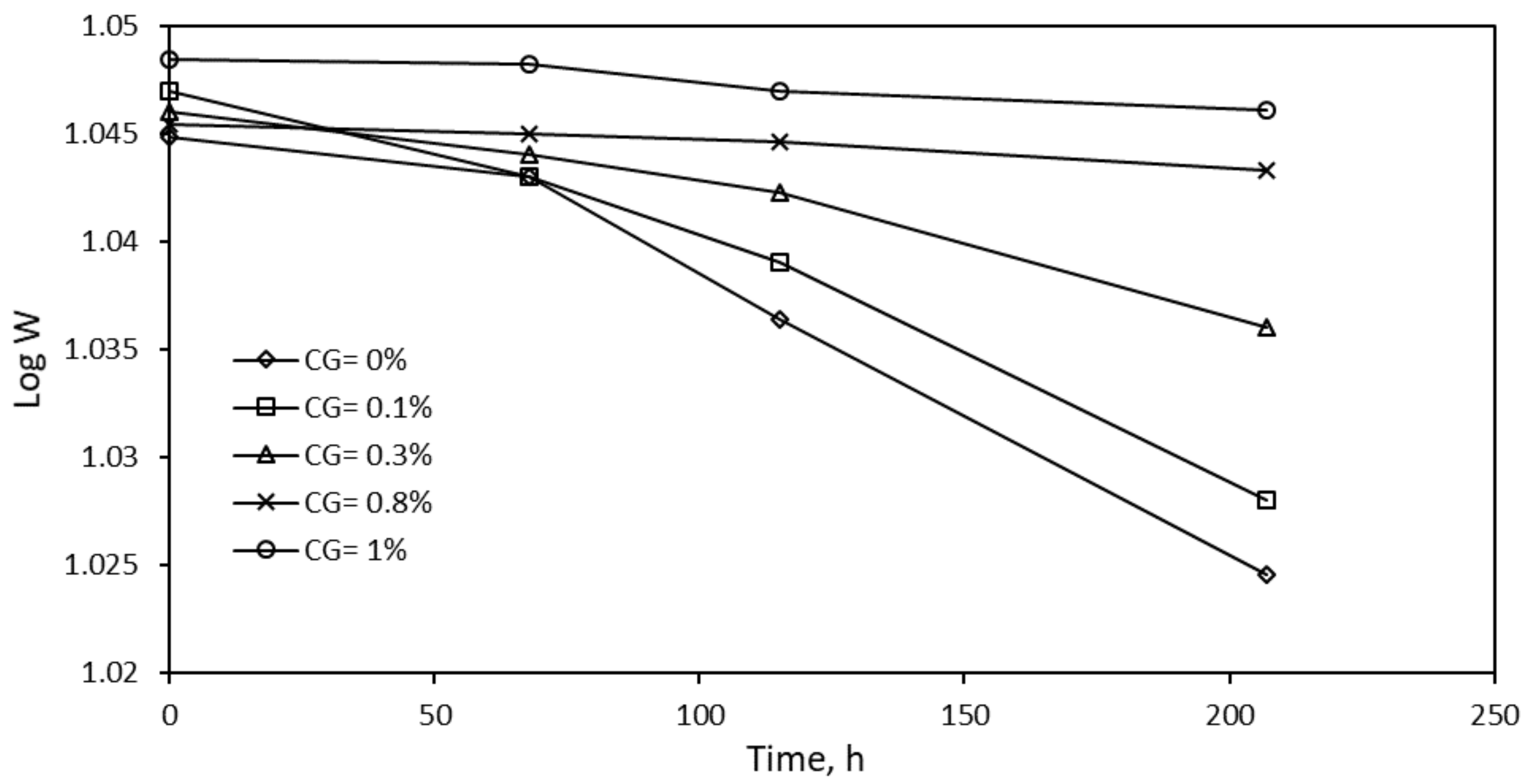
| R2 | K, ×105 | t1/2 (×10−4), s | ∆Gads, kJ/mol | |
|---|---|---|---|---|
| 0% CG | 0.925 | 1 | 6.93 | −12.741 |
| 0.1% CG | 0.956 | 1 | 6.93 | −12.741 |
| 0.3% CG | 0.953 | 5 | 1.386 | −14.441 |
| 0.8%CG | 0.971 | 9 | 0.77 | −13.000 |
| 1.0% CG | 0.935 | 10 | 0.693 | −24.037 |
| Without Inhibition | With CC Inhibition | |
|---|---|---|
| E (I = 0) (mV): | −914.082 | −884.460 |
| Icorr (μA): | 602.7 | 212.5 |
| Ca. Beta (mV): | 108.714 | 149.183 |
| An. Beta (mV): | 120.478 | 108.848 |
| Co. Rate (mpy): | 56.66 | 19.97 |
| Chi-Square: | 0.58 | 5.96 |
| Fit Range (mV): | (−1005), (−811) | (−1021), (−785) |
| Fit Mode: | Auto | Auto |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubaidi, I.A.; Jones, R.; Alzughaibi, M.; Albayyadhi, M.; Darzi, F.; Ibrahim, H. Crude Glycerol as an Innovative Corrosion Inhibitor. Appl. Syst. Innov. 2018, 1, 12. https://doi.org/10.3390/asi1020012
Zubaidi IA, Jones R, Alzughaibi M, Albayyadhi M, Darzi F, Ibrahim H. Crude Glycerol as an Innovative Corrosion Inhibitor. Applied System Innovation. 2018; 1(2):12. https://doi.org/10.3390/asi1020012
Chicago/Turabian StyleZubaidi, Isam Al, Robert Jones, Mohammed Alzughaibi, Moayed Albayyadhi, Farzad Darzi, and Hussameldin Ibrahim. 2018. "Crude Glycerol as an Innovative Corrosion Inhibitor" Applied System Innovation 1, no. 2: 12. https://doi.org/10.3390/asi1020012
APA StyleZubaidi, I. A., Jones, R., Alzughaibi, M., Albayyadhi, M., Darzi, F., & Ibrahim, H. (2018). Crude Glycerol as an Innovative Corrosion Inhibitor. Applied System Innovation, 1(2), 12. https://doi.org/10.3390/asi1020012






