Abstract
Vampire bats likely first appeared in South America in the early Miocene (~20 Ma) and evolved to feed upon the blood of native South American mammals of medium to large body size, in particular, xenarthrans–ground sloths, armadillos, pampatheres, and glyptodonts, and native ungulates–notoungulates and litopterns. Following the formation of the Panamanian Isthmus (~5 Ma), vampire bats immigrated into North America as participants in the Great American Biotic Interchange, following their preferred prey species, probably xenarthrans. The earliest records of vampire bats are the extinct species Desmodus archaeodaptes from three early Pleistocene faunas in Florida. The large extinct vampire D. stocki occurs in 18 late Pleistocene faunas in the southern US and Mexico. The giant extinct vampire D. draculae is known from eight late Pleistocene faunas from Mexico and Belize south to Brazil and Argentina. The late Pleistocene extinction of D. draculae and D. stocki coincided with the extinction of their primary source of blood, the mammalian megafauna. The common vampire bat D. rotundus survived and now occurs throughout tropical America because it had a broader prey base, feeding on the blood of a variety of medium- to large-sized mammals, and currently preying preferentially on non-native domestic livestock.
Keywords:
vampire bats; Desmodus; xenarthrans; Cingulata; Pilosa; Quaternary; evolution; paleoecology; extinction 1. Introduction
Three living species of vampire bats (Chiroptera: Phyllostomidae: Desmodontinae), the common vampire bat Desmodus rotundus, the white-winged vampire bat Diaemus youngi, and the hairy-legged vampire bat Diphylla ecaudata, occur throughout tropical America, from Mexico, Central America, and South America as far south as Uruguay, Argentina, and Chile [1]. Desmodus rotundus has the broadest geographic distribution among the three species, occurring the farthest north, within several hundred kilometers of the US border in northeastern Mexico, and the farthest south in central Chile [1,2]. Desmodus also has the most extensive fossil record of the three vampire bat genera, known from 45 sites ranging in age from latest Pliocene or early Pleistocene to late Holocene and occurring over nearly 80° of latitude from northern California to the northeastern coast of Argentina (Appendix A). Almost half of these fossil records are in Pleistocene faunas from the southern United States, north of the current geographic range of the genus. There are four late Pleistocene records of Diphylla, all from within the modern Neotropical range of D. ecaudata. Diaemus lacks a fossil record.
The feeding preferences of the three living vampire bats are well known [2,3]. Desmodus rotundus prefers mammal blood, with domestic livestock as the favored “prey” or “blood donor” species, whereas Diphylla ecaudata and Diaemus youngi mostly prefer avian blood but will feed on the blood of livestock as well. Given its preference for feeding on large mammals and good fossil record, our study focuses on the current and past feeding behavior of Desmodus, including the extant species D. rotundus and three extinct Quaternary species, D. archaeodaptes, D. draculae, and D. stocki. Since domestic livestock have only been present in the New World for the past 500 years with the advent of European colonization, we focus on Quaternary fossil sites containing Desmodus and evaluate the associated native large mammals to observe trends that might reflect the preferred prey species of vampires prior to the extinction of the mammalian megafauna at the end of the Pleistocene and before the European introduction of cattle and horses.
We review all fossil vampire bat records in North America and South America and associated ground sloths and other large xenarthrans from those same sites to determine if there is enough evidence to suggest a “special relationship” (i.e., coevolution or parasite/host association) between these two groups of mammals. Since Desmodus evolved in South America and subsequently dispersed northward as part of the Great American Biotic Interchange (GABI or the Interchange), we focus on possible prey species of vampire bats that also evolved in South America and were part of the GABI, in particular the various groups of xenarthrans. The fossil record indicates that extinct species of Desmodus were present in the southern US and/or Mexico from the early Pleistocene (late Blancan) to the late Pleistocene (Rancholabrean). Most of the North American sites containing extinct vampire bats also have associated faunas of large mammals, allowing us to evaluate possible prey species.
The following three hypotheses will be evaluated in this study: 1. Vampire bats evolved in South America in the Miocene and their original preferred “prey” or “blood donor” species are to be found among endemic groups of South American mammals, primarily species of large body size, including xenarthrans (ground sloths and several cingulate groups–pampatheres, glyptodonts, armadillos), South American native ungulates (SANU, e.g., litopterns, notoungulates), and large rodents such as capybaras; 2. Desmodus participated in the Great American Biotic Interchange and followed its favored prey northward into Central America, Mexico, and the southern US sometime after the connection of North America and South America at the Panamanian isthmus in the early Pliocene at about 5 Ma; 3. Two species of vampire bats present in late Pleistocene faunas, Desmodus draculae and D. stocki, both larger than the extant D. rotundus, became extinct in the late Rancholabrean, presumably because their preferred prey species among the Pleistocene megafauna also became extinct.
2. Methods and Materials
We have each studied Late Cenozoic mammals from North America and South America throughout our careers, although concentrating on different taxonomic groups, in particular bats (GSM, NJC) and ground sloths (HGM). For GSM and HGM, our shared interest in the Great American Biotic Interchange (GABI or Interchange) is a legacy of our major professor at the University of Florida, the late S. David Webb, who contributed perhaps more than any other North American paleontologist to the study of the Interchange [4,5,6,7,8,9]. In previous papers [10,11,12,13,14,15,16,17], we have studied various aspects of the GABI and the mammals that participated in the Interchange, and also the association and possible interactions or coevolution in the Pleistocene between vampire bats of the genus Desmodus and various groups of large mammals of South American origin that reached North America during the GABI. We also review the South American large mammal fauna that participated in the Interchange and evaluate which of those species were most likely to have been the primary “prey” or more accurately “blood donors” of Pleistocene vampires.
Appendix A provides a standard set of data for each of the published fossil records of vampire bats (Phyllostomidae: Desmodontinae), including: fossil sites with vampire bats; latitude and longitude; elevation; site type (e.g., cave, karst deposit, open site); age, including radiocarbon dates if available; vampire bat species present; associated genera or species of large mammals (>45 kg) of South American origin, including ground sloths, other large xenarthrans, South American native ungulates (SANU), and capybaras; and pertinent references. Each of the vampire bat localities is assigned a number in Appendix A. These numbered localities are indicated on a map of the Western Hemisphere in Figure 1. The modern distribution of D. rotundus is also indicated on this map by the shaded area.
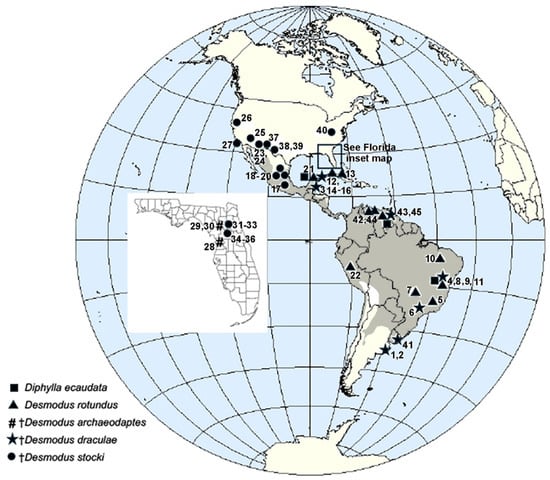
Figure 1.
Map of the Western Hemisphere showing the location of the 45 Quaternary fossil sites known to contain fossils of vampire bats (Phyllostomidae: Desmodontinae), including fossils of two extant species Desmodus rotundus and Diphylla ecaudata and three extinct species Desmodus archaeodaptes, Desmodus draculae, and Desmodus stocki. Data for the numbered localities are provided in Appendix A. The gray shading indicates the modern distribution of Desmodus rotundus. The inset map shows the Quaternary records of Desmodus from Florida.
Abbreviations used in the text: CAS (Central American Seaway); GABI (Great American Biotic Interchange); ka (kilo annum or thousands of years before present); LF (Local Fauna); Ma (Mega annum or millions of years before present); NALMA (North American Land Mammal Age); SALMA (South American Land Mammal Age); SANU (South American native ungulates); yrBP (radiocarbon years before present).
3. Fossil Record of Vampire Bats
There are four Late Quaternary records of the living species Diphylla ecaudata, all from caves occurring within its modern geographic range (Appendix A; Figure 1): Gruta de Loltún in the Yucatán peninsula of Mexico (site 21), Cueva del Guácharo in Venezuela (site 43), and two caves in Brazil, Toca da Barriguda and Toca da Boa Vista (sites 8, 9). Among the three genera of vampire bats, Desmodus has the most extensive fossil record, known from 45 Quaternary and Holocene sites in both North America and South America (Appendix A; Figure 1). There are three extinct species of Desmodus: D. archaeodaptes, D. draculae, and D. stocki, as well as a fourth named extinct species, D. puntajudensis from Cuba, now synonymized with D. rotundus (see discussion below). The living species D. rotundus has a fairly extensive Late Quaternary fossil record. Ray et al. [18] last reviewed the fossil record of vampire bats, including a total of 25 sites, 15 from the United States, four from Mexico, two from Cuba, three from Venezuela, and one from Brazil. All 25 of these sites contained fossil remains of Desmodus and two sites mentioned above from Mexico and Venezuela also had fossils of Diphylla. In the following 37 years there have been two additional fossil discoveries of Diphylla, both from Brazil, and the number of fossil records of Desmodus has nearly doubled. Most of these are from South America, including seven additional records from Brazil and one more from Venezuela, as well as the first known fossils of Desmodus from Argentina (two sites), Peru, and Uruguay. Four additional fossil records of D. stocki from the US have been published since 1988, two from Arizona and one each from Texas and West Virginia. More than half of the fossil records of Desmodus (28 sites) are extralimital to the modern range of the genus, including 18 from the United States, five from Cuba, three from Mexico, and two from Argentina. For a genus now confined to the New World tropics, Desmodus had a remarkable latitudinal range of nearly 80° in the Late Quaternary, from 40°47′ Nlatitude in Potter Creek Cave in northern California to 38°26′ S latitude at Centinela del Mar along the Atlantic coast of northeastern Argentina.
Biogeographic and genomic evidence indicates that vampire bats of the subfamily Desmodontinae (family Phyllostomidae) almost certainly evolved in South America [19,20,21,22], probably in the early Miocene. Although the oldest well-dated fossil site containing Desmodus is from the early Pleistocene of Florida (Appendix A, Figure 1), the record from Kiyú in Uruguay is probably late Pliocene, and thus slightly older. The Florida record consists of a complete humerus referred to the extinct species D. archaeodaptes from the early Pleistocene (late Blancan NALMA; ~2 Ma) Inglis 1A LF (site 28) [10,23]. Two other records of D. archaeodaptes, Haile 16A and Haile 21A, are also from early Pleistocene karst deposits in northern peninsular Florida, but are somewhat younger than Inglis 1A and are referred to the early Irvingtonian NALMA (~1.0–1.6 Ma). Haile 21A (site 30) is the type locality of D. archaeodaptes, represented by a complete braincase (type specimen) and a proximal humerus, and Haile 16A (site 29) has several partial limb bones referred to this species [10,23]. D. archaeodaptes is similar in size to D. rotundus but differs from the living species in several characters of the braincase [23]. All three of the Florida early Pleistocene faunas with D. archaeodaptes also contain species of cave-dwelling vespertilionid bats, indicating these sites originally formed in caves, even though they were collected as karst fissure/sinkhole deposits.
The two earliest records of Desmodus from South America are both from open sites that could be as old as late Pliocene or early Pleistocene but are not precisely dated, one site each in Uruguay and Venezuela. Ubilla et al. [24] identified a complete humerus of a large species of vampire bat, referred to Desmodus aff. draculae, from the Kiyú fauna in Uruguay (site 41) that is no younger than middle Pleistocene and possibly as old as late Pliocene. Czaplewski and Rincón [17] reported the distal humerus of a large vampire bat, cf. Desmodus, from El Breal de Orocual, an asphalt seep deposit in Venezuela (site 45) of either late Pliocene or early Pleistocene age based on the associated mammalian fauna. Even though the Desmodus humeri from Uruguay and Venezuela are similar in age to the early Pleistocene Desmodus from three sites in Florida, these records represent different species. The South American records are from a large species similar in size to the giant extinct vampire D. draculae from the Late Quaternary (see below), whereas the Florida early Pleistocene records are from a considerably smaller species, D. archaeodaptes. The record of a vampire bat from El Breal de Orocual in Venezuela is particularly significant because this site is located in northern South America and contains a diverse large mammal fauna containing species of both North American and South American origin, documenting an early Interchange age [17,25]. All three Florida early Pleistocene faunas with Desmodus, Inglis 1A, Haile 16A, and Haile 21A, are also early Interchange sites containing large mammal faunas with species of both North and South American origin.
After the early Pleistocene (early Irvingtonian) records of Desmodus archaeodaptes from Florida, all other fossil records of Desmodus from North America are late Pleistocene in age (Rancholabrean), with the exception of one possible Holocene occurrence. The apparent absence of Desmodus in the medial to late Irvingtonian (~0.25–1.0 Ma) of Florida and elsewhere in North America is probably an artifact of the fossil record and does not reflect a real absence of vampire bats. Cave faunas of this age are not common in temperate North America, and most of these are in more northerly latitudes where Desmodus would be less likely to have occurred [26,27]. No medial to late Irvingtonian cave faunas and only a few karst deposits of this age are known from Florida, including the McLeod Limerock Mine and Coleman 2A. The medial Irvingtonian McLeod Limerock Mine was collected before the advent of screenwashing, so very few small mammals are known from that fauna. The late Irvingtonian Coleman 2A LF was screenwashed and produced three species of cave-dwelling vespertilionid bats, but no Desmodus [28]. Excavations in Slaughter Canyon Cave in southern New Mexico had a primary goal of procuring a sample of Desmodus. Although this late Irvingtonian fauna produced thousands of fossils of the extinct free-tailed bat, Tadarida constantinei, and small samples of four other bat species, no fossils of vampire bats were recovered [29,30].
Jones [31] described the first extinct vampire bat, the large species Desmodus stocki, from the late Pleistocene (Rancholabrean) San Josecito Cave in northern Mexico. The next year, Gut [32] described a second extinct species of large vampire, D. magnus, from the late Pleistocene Reddick 1 site in Florida. In reporting seven humeri that he referred to D. stocki from Potter Creek Cave in California, Hutchison [33] established that Jones and Gut had recognized the presence of large, extinct vampire bats at about the same time from different late Pleistocene sites but were not aware of each other’s work and described them as separate species. Hutchison [33] synonymized D. magnus with D. stocki, the latter species having priority. His taxonomic conclusion was confirmed by Morgan [10], who studied a large sample of D. magnus (=D. stocki) from the type locality Reddick 1 and several other Florida Rancholabrean faunas.
Fourteen late Pleistocene records and one Holocene report of Desmodus stocki are distributed across the southern tier of US states from Florida to California, with an outlying record from West Virginia, and there are also four records of this large extinct vampire bat from Mexico (Appendix A; Figure 1). In the following discussion, the citations for previously published records of D. stocki are listed in Appendix A. Citations are provided only for records published since the review of fossil vampire bats by Ray et al. [18]. Fifteen records of D. stocki, 11 from the US and four from Mexico, were reported by Ray et al. [18], whereas four additional records of this species from the US have been reported since. The largest concentration of late Pleistocene sites with D. stocki is in northern peninsular Florida, with six records from karst deposits that represent former caves, Arredondo 2A, Haile 1A, 11B, and Reddick 1A, 1B, 1C (sites 31–36). The single US record of D. stocki that is not from the southern tier of states is from New Trout Cave in West Virginia (site 40) [34], about 1200 km north of the Florida records and the northernmost record (38°36′ N of this species of vampire bat in eastern North America. Only the record of D. stocki from Potter Creek Cave in California is farther north. About 2500 km west of the Florida sites, there are two records of D. stocki from caves in the Trans-Pecos region of southwestern Texas, Sierra Diablo Cave and Terlingua (sites 38, 39). The specimens of D. stocki from Sierra Diablo Cave were collected within the past decade or so [35]. A single record of Stock’s vampire bat from New Mexico is from U-Bar Cave in the southwestern part of the state (site 37). There are three records of D. stocki from Arizona, Rampart Cave in the Grand Canyon in the northwestern part of the state (site 25; Figure 2) and two records reported in the past 20 years, both from the southeastern part of the state near Tucson, Arkenstone Cave (site 23) and La Tetera Cave (site 24) [36,37]. The westernmost records of D. stocki are from California, a cave on San Miguel Island, one of the Channel Islands off the coast of southern California (site 27), and Potter Creek Cave in northern California (site 26), which is the northernmost record of a vampire bat (40°47′ N. There are also four records of D. stocki from Mexico, including three records from the northern part of the country, San Josecito Cave (=Cueva de San Josecito) in the state of Nuevo Leon, and Cueva de La Presita from San Luis Potosí (Site 19), and one record from central Mexico, a cave at Cerro de Tlapacoya in the state of Mexico (site 17).

Figure 2.
National Fossil Day poster for 2019 showing the Shasta ground sloth Nothrotheriops shastensis with the extinct vampire bat Desmodus stocki hanging overhead, Rampart Cave, Grand Canyon National Park, Arizona. Vince Santucci and the National Park Service Paleontology Program gave us permission to reproduce this poster.
With the exception of a late Holocene (~3–5000 yrBP) cave deposit on San Miguel Island off the coast of southern California [38,39], all other records of Desmodus stocki are late Pleistocene in age. All US records of D. stocki are north of the current geographic range of D. rotundus. The modern range of D. rotundus approaches to within several hundred kilometers of the US/Mexican border in southern Tamaulipas (~25° N) in northeastern Mexico and southern Sonora (~28° N) in northwestern Mexico [1,2,26,40,41]. See current distribution of D. rotundus on the map in Figure 1. Three of the four Mexican records of D. stocki, including the type locality of San Josecito Cave, are located above 1000 m in elevation on the Mexican plateau in the northern part of the country. This is outside the current range of D. rotundus, which occurs at similar latitudes in northern Mexico but at lower elevations nearer the coasts of the Gulf of California and Gulf of Mexico.
The largest of all Desmodus species, the extinct giant vampire D. draculae, occurs in eight Late Quaternary faunas in the Neotropical region, including single sites in Mexico, Belize, and Venezuela, two sites in Argentina, and three sites in Brazil (Appendix A; Figure 1). The two late Pliocene or early Pleistocene records of a large Desmodus from Uruguay and Venezuela mentioned above [17,24] were compared with D. draculae but were not definitively identified as that species. The two records from the northeast coast of Argentina are somewhat south of the geographic range of D. rotundus [42,43], while all other late Pleistocene fossil records of D. draculae are within the modern range of D. rotundus. Only a single record of D. draculae was listed in Ray et al. [18], the type locality of the species from Cueva del Guácharo in Venezuela (site 43) [23]. Seven additional records of D. draculae have been reported since 1988. The northernmost record of this species is from Loltún Cave (Gruta de Loltún) on the Yucatán peninsula of Mexico (site 21) [18,37,44,45]. A second record of D. draculae from the Yucatán peninsula was reported from Cebada Cave in Belize (site 3) [14]. The largest concentration of fossil localities containing D. draculae is in Brazil with three sites, all located south of the equator, including two caves in the state of Bahia, Toca dos Ossos (site 11) [46] and Toca da Boa Vista (site 9) [13] and Santana Cave in the state of São Paulo (site 6) [47]. The southernmost records of D. draculae are from two sites on the Atlantic Coast of Argentina in Buenos Aires province (~38°S), Centinela de Mar and La Ballenera, several hundred kilometers south of the southernmost current range of D. rotundus. The late Pleistocene La Ballenera site is a former cave (site 2) [43], while the late Holocene Centinela de Mar locality is an open site (site 1) [42].
The living vampire bat Desmodus rotundus has a rather widespread Late Quaternary fossil record (Appendix A; Figure 1), identified from ten cave sites within the modern range of the species, six from Brazil, three from Venezuela, and one from Mexico, as well as five cave sites from Cuba that represent the only locally extinct or extirpated population of D. rotundus. Some of these specimens of D. rotundus are almost certainly Holocene in age, including several of the Cuban records (Appendix A). A confirmed late Pleistocene record of D. rotundus consists of a braincase from Gruta dos Brejões in Brazil adhered to the underside of a ground sloth coprolite with a 14C age of 12,200 radiocarbon yr (Appendix A) [13]. Late Quaternary records of D. rotundus published since Ray et al. [18] consist of five records from Brazil (Appendix A): Gruta dos Brejões (site 4) [13,48], Serra da Mesa (site 7) [49], Toca da Barriguda (site 8) [13], Toca da Boa Vista (site 9) [13,46,50,51,52,53], and Toca do Gordo do Garrincho (site 11) [54]. A late Pleistocene record of Desmodus from Jatun Uchco, Peru (site 22) was not identified to species [55].
The extinct/extirpated Cuban vampire bat was first reported as the extant species Desmodus rotundus by Koopman [56] based on a fossil skull from a Late Quaternary deposit in Cueva Lamas west of Havana on Cuba’s northwestern coast (site 14). The Cuban vampire was later identified from Cueva Centenario de Lenin, a cave at Punta Judas about midway along the northern coast of Cuba (site 13), and described as an extinct subspecies, D. rotundus puntajudensis [57]. In reporting a third record of Desmodus from Cueva de Paredones near Havana (site 16), Suarez [58] considered the Cuban vampire to be an endemic species, D. puntajudensis. Following detailed comparisons of a complete skull of Desmodus from the late Holocene Cueva de Los Nesophontes in northwestern Cuba (site 15) and other vampire bat fossils from Cuba, including a fifth cave site, Cuevas Blancas in western Cuba (site 12) [59], with a large sample of modern specimens of D. rotundus from throughout its geographic range, Orihuela [60,61] concluded that the Cuban vampire was conspecific with D. rotundus, as originally reported by Koopman [56]. We concur with the taxonomic conclusions of Koopman and Orihuela and consider the Cuban vampire to be the same species as the common Neotropical vampire D. rotundus. A vampire bat probably dispersed to Cuba from Mexico in the late Pleistocene across the narrow Yucatán Channel that separates westernmost Cuba from the Yucatán peninsula. There is a late Pleistocene record of D. rotundus from a cave in the Yucatán peninsula, Gruta de Loltún [18,45,62].
Fossils of Desmodus rotundus are associated with those of D. draculae in three cave deposits in widely separated sites in the Neotropics (Appendix A; Figure 1), Gruta de Loltún in the Yucatán peninsula of Mexico [45,62], Cueva del Guácharo in Venezuela, the type locality of D. draculae [18], and Toca da Boa Vista in Brazil [12]. These associations document that D. draculae was sympatric with the much smaller D. rotundus throughout the extensive range of both species in the Neotropical region during the Late Quaternary. The fact that D. rotundus survived and D. draculae became extinct was almost certainly a factor of their differing prey bases, probably related to body size, with D. draculae specializing on the blood of members of the now-extinct Pleistocene megafauna, whereas D. rotundus fed on a wider variety of medium-sized mammals that did not become extinct. Vampire bat extinctions are covered in more detail in Section 6 at the end of the paper.
4. Evolutionary History of Vampire Bats
As discussed above, the oldest record of the vampire bat subfamily Desmodontinae (family Phyllostomidae) is probably from the late Pliocene Kiyú Fauna in Uruguay, followed by the extinct species Desmodus archaeodaptes from the early Pleistocene (about 2 Ma) Inglis 1A LF in Florida [10,11,23]. Fossils of an extinct species of large Desmodus from Venezuela [17] and Uruguay [24] may be as old as, or even somewhat older (late Pliocene?) than the Inglis 1A record, but these two South American records are not precisely dated. However, the remarkable cranial, dental, and postcranial modifications of vampire bats for a diet of feeding on the blood of vertebrates, not to mention the numerous unique features of their soft anatomy, physiology, genetics, and behavior [63,64] compared to more typical insectivorous or omnivorous phyllostomid bats, suggest a much longer evolutionary history. In a review of the molecular time scale to evaluate the origin of various feeding strategies of bats in the Phyllostomidae, Baker et al. [21] used genomic data to predict the lineage that gave rise to vampire bats diverged from the remainder of the family sometime between the early Oligocene and early Miocene, an age range of about 10 million years (~31–21 Ma). In our experience, the molecular clock tends to overestimate the timing of the origin or divergence of various groups of Neotropical bats when compared to the actual fossil record [21,65,66]. The fossil record of the Phyllostomidae is rather meager [67,68], with the oldest members of this family consisting of two insectivorous or possibly carnivorous phyllostomines from the early Miocene (~21 Ma), including two lower jaws of a recently described extinct genus and species Americanycteris cyrtodon from Panama [69] and an isolated lower molar of an indeterminate genus from Argentina [70]. The molecular phylogeny of Rojas et al. [22] places the branching of Diphylla from the other vampire bats at about 20 Ma and the split between Desmodus and Diaemus at about 13–12 Ma. Thus, the desmodontine crown group must have begun blood-feeding at least between 20 and 12 Ma. The date of 21 Ma for the oldest phyllostomid fossils agrees with the younger end of the age range for the divergence of vampire bats from other phyllostomids based on the molecular time scale [21], suggesting that a Miocene age of about 20–12 Ma is a reasonable estimate for the origin of vampire bats. This age range is also supported by the occurrence of the Desmodontinae near the base of the phyllostomid phylogenetic tree [22,71].
We have identified five time intervals during the evolutionary history of vampire bats, when they encountered significant changes in the mammalian fauna that likely necessitated shifts in their feeding habits, as well as changes in their geographic distribution. These intervals and approximate time ranges are: 1. South American (~20–9 Ma); 2. GABI–late Miocene (9–5.3 Ma); 3. GABI–Pliocene-Pleistocene (5.3 Ma–10 ka); 4. Holocene (10 ka–1500 AD/CE; 5. Post-Columbian (1500 AD/CE to present).
4.1. South American
The first and longest of the time intervals in the evolutionary history of vampire bats was the “South American,” beginning with the origin of vampire bats in the Miocene by about 20–12 Ma and lasting until the onset of the Great American Biotic Interchange at about 9 Ma. Vampire bats almost certainly originated in South America when it was an island continent during the Miocene [21,22,66] and thus would have evolved to feed upon the blood of endemic groups of South American mammals, specifically mammals of medium to large body size based on their current feeding preferences. Among the groups of mammals present in South America in the Miocene, other bats and most marsupials, primates, and caviomorph rodents would have been too small to serve as potential prey species, leaving only two major groups of large mammals, xenarthrans and South American native ungulates (SANU), as the most likely prey or hosts. Among xenarthrans, four families of ground sloths (Pilosa: Phyllophaga): Megalonychidae, Megatheriidae, Mylodontidae, Nothrotheriidae) had already appeared and diversified in the South American fossil record by the early to middle Miocene [72]. The second group within the Pilosa, the anteaters (Vermilingua: Myrmecophagidae), has a rather sparse fossil record, first appearing in the early Miocene [72,73] and are much smaller than the majority of ground sloths, so are less likely as prey species. Several groups of armored xenarthrans (Cingulata) appeared in the Eocene and had become fairly diverse by the Miocene, including armadillos (Chlamyphoridae, Dasypodidae), glyptodonts (Glyptodontidae or Chlamyphoridae: Glyptodontinae), and pampatheres (Pampatheriidae). Although it has no effect on our discussion of vampire bat evolution, recent studies of the ancient DNA of the South American Pleistocene glyptodont Doedicurus [74,75] suggest that glyptodonts, long placed in the separate cingulate family Glyptodontidae [72], should be classified as the subfamily Glyptodontinae within the armadillo family Chlamyphoridae that includes all of the living genera of armadillos except Dasypus, which is now the only extant genus in a second armadillo family, the Dasypodidae. This change in taxonomy does not alter the fact that glyptodonts (Glyptodontidae or Glyptodontinae) were a unique group of heavily armored cingulates lacking movable bands in the carapace, that first appeared in the Eocene, attained large size by the Miocene, and reached megafaunal status in the Pleistocene when many species exceeded 1000 kg in body mass [76]. The other major group of large mammals present in South America in the early Miocene consisted of a diverse assemblage of native ungulates, primarily the orders Notoungulata and Litopterna [72,77]. South American native ungulates (SANU) first appeared in the Paleocene and became extinct at the end of the Pleistocene, although the group underwent a significant loss of diversity after the Miocene. Unlike the xenarthrans, in which multiple taxa dispersed into North America at various times during the GABI, only one representative of the SANU, the toxodont Mixotoxodon, reached North America. Mixotoxodon is known from numerous sites in Central America and several sites in southern Mexico and also occurred as far north as Texas [78]. It is not possible to determine which of the various groups of native South American mammals, in particular xenarthrans and SANU, might have been the favored prey of early vampire bats during the Miocene, because the Desmodontinae have no fossil record during this time interval.
4.2. GABI–Late Miocene
The second time interval in the evolutionary history of vampire bats, “GABI–late Miocene”, began in the late Miocene (~9 Ma) with the onset of the Great American Biotic Interchange. It ended with the connection of South America with North America at the Panamanian Isthmus in the early Pliocene (~5 Ma) [79], marking the beginning of the Pliocene-Pleistocene phase of the GABI (see below). The mammals in South America available for vampire bats to feed upon in the late Miocene would have been similar to the fauna available during the preceding South American period, consisting of xenarthrans and SANU. The late Miocene in South America was also characterized by several lineages of very large caviomorph rodents that may have been prey of vampire bats, including species in the Dinomyidae, Neoepiblemidae, and capybaras (Caviidae: Hydrochoerinae) [72]. The beginning of the GABI in temperate North America is defined by the first appearance of two unrelated genera of ground sloths of South American origin, Pliometanastes (Megalonychidae) and Thinobadistes (Mylodontidae). These two sloths crossed the narrow Central American Seaway (CAS) in the late Miocene and eventually arrived in temperate North America, in part characterizing the early Hemphillian NALMA between 9 and 7 Ma [4,12,80]. Pliometanastes has been identified in a number of early Hemphillian sites from Florida to California [11,12,81,82,83] as well as Mexico [84]. Thinobadistes has a more limited fossil record with several early Hemphillian occurrences from Florida and Texas [85]. While Pliometanastes and Thinobadistes must have passed through Mesoamerica on their way northward in the late Miocene, the earliest well-dated records of both genera are from temperate North America. Although Pliometanastes was reported from the late Miocene (late Hemphillian) San Gerardo de Limoncito fauna in easternmost Costa Rica near the border with Panama [86], Valerio et al. [87] now consider this record as a species of Zacatzontli, Z. cotobrusensis. There is also a late Miocene record of the pampathere Scirrotherium from the San Gerardo de Limoncito fauna [88]. The occurrence of the horse Dinohippus mexicanus in the San Gerardo de Limoncito fauna [88], as well as the presence of the megalonychid sloth Zacatzontli, originally described from the late Hemphillian of Mexico [89], both suggest the age of the Costa Rican fauna is more likely late Hemphillian (~7–5 Ma).
Cyonasua is a large member of the raccoon family Procyonidae and sister taxon of the middle and late Miocene genus Arctonasua of North American origin that dispersed to South America across the CAS in the late Miocene (Huayquerian SALMA; ~7 Ma) [90]. New genera in three other families of North American mammals, Amahuacatherium in the Gomphotheriidae [91], Sylvochoerus and Waldochoerus in the Tayassuidae [92], and Surameryx in the Dromomerycidae [93], have been described from presumed late Miocene deposits in the western Amazon Basin in Brazil and Peru, and were considered to be members of the late Miocene Interchange fauna. However, these records are controversial, and other authors argue that the supposed late Miocene gomphothere, peccaries, and dromomerycid (cervid?) from the western Amazon are actually Pleistocene in age and were not participants in the late Miocene phase of the GABI [94,95,96]. Ground sloths and procyonids appear to have crossed the CAS by overwater dispersal, as most authors agree there is no evidence for a direct land connection between the two continents in the late Miocene [8,80] but see Campbell et al. [97] for a contrasting opinion.
Although the possibility exists that Desmodus or a predecessor flew across the CAS in the late Miocene prior to the connection of the two continents in the early Pliocene, there is no fossil evidence of the pre-early Pleistocene existence of vampire bats in North America, suggesting an overland route during the Pliocene or early Pleistocene was more probable. This hypothesis is supported by the observation that Desmodus is not a particularly strong flier [98,99] and would have been unlikely to fly across the CAS. However, we know that Desmodus flew across oceanic water gaps to offshore islands several times in its history, including Cuba, Trinidad, and one of the California Channel Islands. The extant species D. rotundus has been identified in five Late Quaternary cave deposits in Cuba, as discussed in more detail above [56,57,58,59,60,61]. Desmodus presumably flew from the Yucatán Peninsula, across the narrow Yucatán Channel, and arrived in western Cuba where four of the five records of the now-extirpated Cuban vampire are located. The Yucatán Channel is currently only about 200 km wide and would have been somewhat narrower during the Last Glacial Maximum in the late Pleistocene (~25–20 ka) when sea level was at its lowest.
Desmodus also has been recorded on two other islands located very close to continental areas that would have been the source for vampire bats. Desmodus rotundus and Diaemus youngi occur on the island of Trinidad in the southeastern Caribbean Sea [100], located about 10 km from Venezuela off the northern coast of South America. Trinidad is on the South American continental shelf and would have been directly connected to South America during late Pleistocene low sea level stands [101]. Guthrie [38,39] reported fossils of D. stocki from San Miguel Island about 40 km off the California coast. The northern Channel Islands of San Miguel, Santa Rosa, and Santa Cruz would have been joined into one larger island during late Pleistocene low sea level stand and would have extended to within 20 km of the coast of California, although separated from the mainland by a narrow channel with depths exceeding 100 m.
While not synchronous with the late Miocene phase of the GABI, megalonychid sloths were also able to cross the Caribbean Sea by overwater dispersal and become established on islands in the Greater Antilles. The oldest sloth from the West Indies is an indeterminate genus and species from the early Oligocene of Puerto Rico [102]. The oldest named sloth from the West Indies is Imagocnus zazae, described from the early Miocene Domo de Zaza locality in south-central Cuba [103]. The oldest known ground sloth record from Hispaniola is represented by an unassociated partial tibia and scapula that are recognized as a single taxon from the late Miocene-early Pliocene of the Dominican Republic [104]. While not yet formally named, characters of the Dominican tibia are suggestive of a close relationship with Megalocnus from Cuba. All three of these Antillean sloth records from the mid to late Cenozoic precede the known Late Quaternary record of vampire bats in Cuba. There are no fossils of vampire bats from Hispaniola or Puerto Rico [105,106], although sloths were present on both islands. The fossil record documents that sloths were an established part of the mammalian fauna of the Greater Antilles by the Miocene, exclusive of Jamaica; as the largest terrestrial mammals, they were a potential food source for vampire bats.
There are at least two species of large vertebrates of South American origin that are first recorded in North America in the earliest Pliocene (latest Hemphillian; ~5 Ma) and could have arrived either in the latest Miocene or shortly after the land connection of the two continents in the earliest Pliocene. The giant, flightless, phorusrhacid bird, Titanis walleri, of South American origin is best known from several early Pleistocene (late Blancan) faunas in Florida and was long thought to be a member of the Pliocene-Pleistocene phase of the GABI [4]. One of the largest samples of Titanis is from the latest Blancan Inglis 1A LF that also contains Desmodus archaeodaptes [107,108]. However, a single toe bone of Titanis from the Nueces River Fauna on the Gulf of Mexico coastal plain in Texas occurs in a mixed fauna of latest Hemphillian and Rancholabrean vertebrates [109]. The age of the Titanis toe bone from the Nueces River Fauna was later confirmed to be early Pliocene (latest Hemphillian) in age (~5 Ma) based on an analysis of rare earth elements [110]. It is highly unlikely that a huge flightless bird could have crossed the CAS by overwater dispersal, thus it is most likely that Titanis entered North America in the early Pliocene shortly after the Panamanian isthmus formed. A megalonychid ground sloth from Tecolotlán, Jalisco, Mexico, Zacatzontli tecolotlanensis, is also early Pliocene in age (latest Hemphillian) based on a radioisotopic date of 4.85 Ma on an associated ash deposit and correlation with the well-known latest Hemphillian Rancho El Ocote Fauna from Guanajuato, Mexico [89]. The fossil record has established that two ground sloths, Pliometanastes and Thinobadistes, had previously crossed the CAS by overwater dispersal in the late Miocene about 9 Ma, and therefore overwater dispersal by Zacatzontli in the latest Miocene, especially across a very narrow water gap, was possible. We also suggest the possibility that the two xenarthrans reported from the late Hemphillian San Gerardo de Limoncito fauna in Costa Rica, the ground sloth Zacatzontli cotobrusensis and the pampathere, Scirrotherium, may also have entered North America across a dry land connection in the early Pliocene rather than by overwater dispersal across the CAS in the late Miocene [86,87,88]. A large armored xenarthran such as a pampathere seems an unlikely candidate for overwater dispersal.
4.3. GABI–Pliocene-Pleistocene
A third time period in our proposed history of vampire bats “GABI–Pliocene-Pleistocene” phase began in the early Pliocene at about 5 Ma and ended with the extinction of the Pleistocene megafauna at about 10 ka. The onset of the Plio-Pleistocene phase of the GABI was initiated by the formation of the Panamanian isthmus at about 5 Ma [79], which resulted in the widespread northward dispersal of the South American biota and the southward dispersal of the North American biota across a direct land connection. The fossil record documents that during this Plio-Pleistocene phase of the GABI, the dispersal of large mammals in both directions was most active between about 5 and 1 Ma [4,5,12,80], although it should be noted that the interchange of plants and animals between the two continents is still ongoing. This phase of the Interchange is particularly significant to our discussion because it documents the first fossil evidence of Desmodus in the early Pleistocene. Vampire bats apparently took advantage of the land connection between the two continents to migrate into North America following their favored prey species northward.
The mammals with South American affinities in South America available for vampire bats to feed upon in the Pliocene and Pleistocene would have been similar to the previous GABI–late Miocene phase, including a wide variety of large xenarthans, native South American ungulates, and several species of large caviomorph rodents. As noted above [72], the diversity of SANU dropped rather dramatically after the Miocene, and only one SANU, Mixotoxodon larensis, extended its range into North America. Following the connection of the two continents in the early Pliocene, a diverse group of large mammals of North American origin reached South America in the late Pliocene and early Pleistocene (Uquian and Ensenadan SALMAs) and would have first encountered vampire bats, including: cats (Felidae), dogs/wolves (Canidae), bears (Ursidae), horses (Equidae), tapirs (Tapiridae), peccaries (Tayassuidae), camels (Camelidae), deer (Cervidae), and several genera of gomphotheres (Gomphotheriidae). Several large carnivores and tapirs, peccaries, lamine camelids, and deer still form a conspicuous component of the modern fauna of large mammals in South America within the distribution of vampire bats.
At this same time, in the late Pliocene and early Pleistocene (Blancan and early Irvingtonian NALMAs), a cohort of large mammals from South America dispersed northward, becoming widespread in Mesoamerica and the southern US [4,5,11,12,15,80]. A diverse Plio-Pleistocene contingent of large Interchange mammals in North America primarily consisted of xenarthrans, including: three families and six genera of cingulates: Dasypus (Dasypodidae); Holmesina, Pampatherium, and Plaina (Pampatheriidae); Glyptotherium and Pachyarmatherium (Glyptodontidae or Chlamyphoridae: Glyptodontinae, see above); four families and seven genera of ground sloths: Megalonyx, Meizonyx, Nohochichak, and Xibalbaonyx (Megalonychidae) in the Neotropical portion of North America, as well as Megalonyx in the more northern temperate region of the continent; Eremotherium (Megatheriidae); Paramylodon (Mylodontidae); and Nothrotheriops (Nothrotheriidae); and the giant anteater Myrmecophaga (Myrmecophagidae). Three other genera of large mammals from South America reached North America in the Blancan or Irvingtonian: a SANU, the toxodont Mixotoxodon (Notoungulata: Toxodontidae); and two genera of caviomorph rodents, the capybaras Neochoerus and Phugatherium (Rodentia: Caviidae: Hydrochoerinae). It is not clear if the capybaras represent two separate immigration events or if Neochoerus was descended from Phugatherium, possibly in North America.
Myrmecophaga is known from a single fossil site in North America, the early Pleistocene (early Irvingtonian) El Golfo de Santa Clara Fauna in Sonora in northwestern Mexico [111]. Considering its rarity, it is doubtful that the giant anteater was an important prey species of Desmodus in temperate North America. The oldest records of Mixotoxodon in Mesoamerica are from two early/middle Pleistocene (Irvingtonian) faunas in El Salvador, Barranca del Sisimico [112] and Río Tomayate [113,114]. Mixotoxodon larensis was widespread in late Pleistocene faunas in Mesoamerica, including all Central American countries except Belize, namely Guatemala [115], El Salvador, Honduras [112], Nicaragua [116], Costa Rica [117], and Panama [118], as well as Michoacán and Veracruz in southern Mexico [119]. There is also a single record of Mixotoxodon from the southern US, a tooth from a late Pleistocene site on the Gulf Coastal Plain of Texas [78]. The absence of Mixotoxodon from late Blancan and early Irvingtonian faunas in Florida that contain Desmodus archaeodaptes, as well as the absence of this toxodont from all Rancholabrean faunas in the southern US containing D. stocki, rules out this genus as an important prey species of vampire bats in temperate North America. However, it is possible that the large, extinct vampire D. draculae fed on Mixotoxodon larensis in the tropical region of Mesoamerica where both species are known to occur. Although there are no late Pleistocene localities in Mesoamerica where Desmodus and Mixotoxodon are found together, both species occur in sites on the Yucatán peninsula or in the general vicinity. D. draculae has been identified from two caves in the Yucatán peninsula, Gruta de Loltún in Mexico and Cebada Cave in Belize [14,45]. The closest sites with Mixotoxodon are Río la Pasion/Santa Amelia in the Yucatán peninsula in Guatemala [115] and La Estribera in Veracruz, Mexico [119].
The large capybara Neochoerus aesopi was widespread throughout North America in the late Pleistocene from Nicaragua [116] to northern Mexico [120,121] and in the southeastern US from Texas to Florida and as far north as North Carolina [122,123,124]. In the late Blancan and early Irvingtonian, Neochoerus occidentalis occurred in several sites in Mexico [125]) and an unidentified species of Neochoerus is known from numerous late Blancan and Irvingtonian localities in Florida [122,126]. Two older species of Neochoerus, N. cordobai from the early Blancan of central Mexico [127] and N. dichroplax from the late Blancan of Arizona and Florida [126,128] were transferred to its presumed ancestral/sister genus Phugatherium by Vucetich et al. [129], with both species now referred to Phugatherium dichroplax. Neochoerus and Desmodus co-occur in two sites, the late Blancan Inglis 1A LF in Florida and the Rancholabrean Cerro de Tlapacoya LF in Mexico (Appendix A).
McDonald [15] documented that the family-level diversity of large xenarthrans in North America was similar to that in South America during the Plio-Pleistocene, but the generic and species-level diversity within xenarthran families was much lower in North America where only one or at most two genera and species representing each of these families were present during any given time interval (i.e., Blancan, Irvingtonian, or Rancholabrean). The one exception to this statement is the ground sloth family Megalonychidae, which had considerably more genera and species in North America than did the other xenarthran families, especially in Mexico and Central America. The diversity of megalonychids in Mesoamerica during the Pliocene and Pleistocene indicates a more complicated biogeographic and evolutionary history in North America compared to the other three ground sloth families [89,130,131,132]. This appears to be, in part, a result of endemic evolution of the Megalonychidae in both temperate North America and tropical Mesoamerica. The early Hemphillian Pliometanastes evolved into Megalonyx in the late Miocene (late Hemphillian), followed by the evolution of a series of four successive species of Megalonyx from the late Miocene through the late Pleistocene in temperate North America, together with a fifth species, M. obtusidens, from the early/middle Pleistocene (Irvingtonian) of El Salvador [130], which occurred at the same time as the larger M. wheatleyi in temperate North America. Contemporary with the various species of Megalonyx during the early Pliocene to the late Pleistocene was a diverse group of more distantly related megalonychids in southern Mexico and Central America that apparently were derived from successive dispersal events from South America and/or endemic radiation in Mesoamerica. These included: Zacatzontli tecolotlanensis from the early Pliocene (latest Hemphillian) of Jalisco, southern Mexico [89]; Z. cotobrusensis from the latest Hemphillian San Gerardo de Limoncito fauna in Costa Rica [86,87]; Meizonyx salvadorensis from the early to middle Pleistocene of El Salvador and late Pleistocene of Oaxaca, southern Mexico [130,132]); Nohochichak xibalbahkah from the late Pleistocene of the Yucatán peninsula, Mexico [131]; and three species of Xibalbaonyx from the Yucatán peninsula and Jalisco, Mexico [133]. This greater diversity of sloths in the northern Neotropics, within the range of vampire bats (three extant vampire species and the extinct Desmodus draculae), compared to the lesser sloth diversity in temperate North America at the northern geographic limit of vampire bats where a single species, now extinct (D. stocki), occurred in northern Mexico and the southern US, may have made these sloth taxa a more likely food resource in a tropical region with less climatic stress than the seasonal cold at more northern latitudes.
As previously noted, this radiation of Plio-Pleistocene megalonychids in Mesoamerica is unrelated to the diverse assemblage of small megalonychid ground sloths from the Greater Antilles that were not participants in the GABI but arrived in the West Indies from South America much earlier, in the Oligocene to Miocene. Megalonychids reached the Greater Antilles either by dispersing overwater across the Caribbean Sea or using the hypothesized GAARlandia route along the now-submerged Aves Ridge that consisted of either a peninsula or series of closely spaced islands extending northward from South America to the Greater Antilles in the late Eocene and early Oligocene [134,135,136].
Prior to the early Pleistocene (~2 Ma), our hypothesis on the evolutionary history of vampire bats is speculative owing to the lack of a fossil record of this group before that time. The documented history of vampire bats began in the early Pleistocene when Desmodus first appeared in the fossil record. As noted above, two records of Desmodus, one from Uruguay [24] and another from Venezuela [17], could be as old as late Pliocene (~2.6–3.0 Ma) but may be as young as early Pleistocene (~1.0–2.6 Ma), whereas the earliest record from North America is from the early Pleistocene (~2 Ma) of Florida [10,11,25]. Because of the well-documented fossil record of Desmodus in the Pleistocene, beginning about 2 Ma and extending through the end of this epoch to about 10 ka, we focus our analysis on large mammals that were present in North America and South America during this time period. The extinction of the large vampire bats D. draculae and D. stocki, that occurred at the end of this third period in the evolutionary history of vampire bats, was almost certainly related to the late Pleistocene extinction of the megafauna in the New World because species of now-extinct large mammals in both North and South America would have been the primary blood sources for these two large vampire bats (See discussion below under Section 6).
We hypothesize that Desmodus first reached North America in the early Pleistocene as a participant in the GABI, and fed upon the blood of large, extinct xenarthrans of South American origin that were also participants in the GABI. We base this in part on the presence of the extinct D. archaeodaptes in one of the most diverse North American Interchange faunas, the early Pleistocene (latest Blancan; ~2 Ma) Inglis 1A LF from peninsular Florida [10,25]. Eight species of large vertebrates of South American origin that participated in the GABI are known from Inglis 1A [4,11]: the giant flightless bird Titanis; three genera of ground sloths, Eremotherium, Megalonyx, and Paramylodon; the armadillo Dasypus, the pampathere Holmesina; the glyptodont Glyptotherium; and the capybara Neochoerus (=Phugatherium?). Two somewhat younger late early Pleistocene (early Irvingtonian; ~1.6–1.0 Ma) faunas from peninsular Florida, Haile 16A and Haile 21A, also document the presence of D. archaeodaptes in association with large Interchange mammals. In Haile 16A, D. archaeodaptes occurs with a diverse South American Interchange fauna similar to that of Inglis 1A, including the ground sloths Eremotherium, Megalonyx, and Paramylodon and the cingulates, Dasypus, Holmesina, and the small glyptodont Pachyarmatherium. The type locality of D. archaeodaptes, Haile 21A, has only two genera of large Interchange mammals, the giant sloth Eremotherium and Dasypus, both of which occur in all three Florida early Pleistocene faunas in association with Desmodus.
The first appearance of a nothrothere sloth in North America, Nothrotheriops texanus, was in the early Irvingtonian, somewhat later than the arrival of most other genera of large Interchange mammals in the temperate part of the continent discussed above. The earliest records for this sloth are in the early Irvingtonian Leisey 1A and Pool Branch faunas in Florida [137,138], but the species was distributed from coast to coast in the southern United States, including early Irvingtonian records from Texas, El Golfo de Santa Clara in Sonora, northwestern Mexico, and the Anza-Borrego Desert in southern California [16,139,140]. Although several late Blancan records of Nothrotheriops were reported from the Anza-Borrego Desert [139], the generic identity of these specimens is questionable (HGM). After the Irvingtonian, the range of Nothrotheriops retracted to the west, and during the Rancholabrean, the succeeding species, N. shastensis is known primarily from the western United States south through Mexico to Belize but is not known from Florida [16,141].
The late Pliocene or early Pleistocene El Breal de Orocual Fauna from Venezuela in northern South America is similar in age to Inglis 1A and has produced a fossil record of a large species tentatively referred to Desmodus, in association with a diverse fauna of large mammals of both South American and North American origin that participated in the Interchange [17,25]. Large mammals of South American origin from El Breal de Orocual include 11 genera: two genera of ground sloths, six genera of cingulates, an anteater, a large caviomorph rodent, and a SANU, a toxodont [25]. The large mammals of North American origin from El Breal de Orocual, consist of genera that arrived in South America during the Interchange sometime after the early Pliocene, including: four genera of large carnivorans, a horse, a tapir, two genera of peccaries, a camel, and a gomphotheriid proboscidean [25].
This third interval in the history of vampire bats continued throughout the remainder of the Pleistocene, with the fauna of large mammals of both North American and South American origin available as prey remaining fairly stable on both continents. Large xenarthrans associated with Desmodus in late Pleistocene sites in both North America and South America are listed in Appendix A. The Pliocene-Pleistocene interval ended in the late Pleistocene with the extinction of the majority of large mammals in both North and South America, presumably including the large mammals that were important prey species for the two large vampire bat species that also became extinct at this same time, D. draculae and D. stocki.
4.4. Holocene
The fourth interval in the history of vampire bats “Holocene” was much shorter than the previous three time periods, including most of the Holocene, beginning about 10 ka and ending with the arrival of Europeans in the New World about 500 years ago. There are supposed Holocene records of both large, extinct vampire bats, D. draculae from Argentina [42] and D. stocki from San Miguel Island in southern California [38,39], but these large vampires did not survive to the present time. Moreover, the Holocene ages of these two faunas are not based on radiocarbon dates taken directly on the vampire bat bones but are from the associated mammalian fauna in Argentina [42] or radiocarbon dates on associated organic material from San Miguel Island [38,39]. The extant vampire species Desmodus rotundus and Diphylla ecaudata are known from late Pleistocene fossil sites (Appendix A) and have survived to the present time. The third living species of vampire bat, Diaemus youngi, has no fossil record. As mentioned above, the three living vampire bats have similar geographic distributions at the present time, limited to the Neotropical region in southern Mexico, Central America, and the tropical portion of South America.
Only three mammals of South American origin survived in temperate North America after the Pleistocene, all of fairly small body size (˂10 kg), the Virginia opossum Didelphis virginiana, the nine-banded armadillo Dasypus novemcinctus (a much smaller species than the late Pleistocene D. bellus), and the North American porcupine Erethizon dorsatum. Compared to the late Pleistocene, a much-reduced fauna of large mammals (>45 kg), all of North American origin, survived the end-Pleistocene extinction event in temperate North America, including gray wolf, puma, jaguar, and three species of bears (black bear, brown bear, and polar bear) among carnivorans and 12 species of artiodactyls, including collared peccary, pronghorn, five species of cervids (caribou, elk, moose, mule deer, and white-tailed deer), mountain goat, bighorn sheep, Dall sheep, American bison, and muskox. Only four of these large mammals (puma, jaguar, collared peccary, and white-tailed deer) occur in the tropical region of North America in southern Mexico and Central America, where they would have been (and still are) sympatric with vampire bats, including Desmodus rotundus. Three additional species of large mammals of North American origin co-occur with vampire bats in Mesoamerica, Baird’s tapir, white-lipped peccary, and Central American red brocket deer. Two species of large mammals of South American origin survive in Central America, the giant anteater Myrmecophaga tridactyla and the lesser capybara Hydrochoerus isthmius.
In South America, only four large mammals of South American origin survived the end-Pleistocene extinction: giant armadillo Priodontes maximus; Myrmecophaga tridactyla; and two capybaras Hydrochoerus hydrochaeris and H. isthmius. All SANU became extinct at the end of the Pleistocene. Large mammals of North American origin that survived the Pleistocene extinction in South America include: jaguar, puma, maned wolf, and spectacled bear, among carnivorans; three species of tapirs (Baird’s tapir, South American tapir, mountain tapir); three species of peccaries (collared peccary, white-lipped peccary, Chacoan peccary); two species of lamine camels (guanaco and vicuña, not including the domesticated llama and alpaca); and six species of deer (white-tailed deer, marsh deer, pampas deer, red brocket deer, and two species of Andean deer–huemul and taruca; this list does not include several species of smaller cervids [<45 kg], such as smaller species of brocket deer and pudu). A few of these surviving large mammals are primarily restricted to higher elevations in the Andean region (spectacled bear, guanaco, vicuña, and two species of Andean deer), and probably rarely encounter vampire bats.
Desmodus rotundus was the only species among the three species of Desmodus known from the late Pleistocene that survived to the present, presumably because they were generalists, feeding on a wider variety of mammalian prey than their extinct late Pleistocene counterparts. Several of the surviving large mammals in both North and South America are known to be fed upon by D. rotundus, as are a variety of medium-sized mammals and even non-mammals. A discussion of the living species of mammals known to be prey species of D. rotundus is presented below under Section 5. A local population of D. rotundus in Cuba did become extinct, or more accurately extirpated, during the Holocene [60,61]. The Cuban population of D. rotundus apparently survived until the late Holocene, but then disappeared from the island, probably following the late Holocene extinction of small ground sloths in Cuba that likely constituted its primary food source [56,142,143,144].
4.5. Post-Columbian
The fifth and still ongoing time period in the evolutionary history of vampire bats, “Post-Columbian”, began about 500 years ago in the late Holocene, with the arrival of Europeans in the New World. The post-Pleistocene fauna of native large mammals in the tropical regions of both North America and South America that comprised the primary food source of Desmodus rotundus remained the same as in the previous Holocene period, with the difference being the introduction of domesticated livestock by Europeans, including cows, horses, pigs, goats, and sheep, as well as chickens, turkeys, geese, and ducks. At present, domestic livestock are the primary source of blood for D. rotundus in the Neotropics [2,3,145] (see more detailed discussion under Section 5). This demonstrates not only a dramatic dietary change in D. rotundus over a period of less than 500 years, but also the ecological flexibility of this species in rapidly shifting its primary prey base, a character that almost certainly contributed to its survival when two other species of Desmodus became extinct at the end of the Pleistocene. The reintroduction of horses into South America is of interest as extinct species of horse were present in faunas from North America and dispersed into South America as part of the GABI. Thus, they would have been a potential food source for vampire bats up to the Pleistocene extinction event when they became extinct in the New World. After their extinction there was a hiatus of about 10 ka until the reintroduction of horses by Europeans. The current inclusion of horses in the diet of vampire bats is a return to a previous potential food source.
5. Paleoecology of Vampire Bats
5.1. Diet of Living Vampire Bats
Among the three living species of vampire bats, Diaemus youngi and Diphylla ecaudata are primarily avian vampires, feeding on the blood of birds and rarely feeding on large mammals, including domestic livestock [3]. Desmodus rotundus preys on a wide variety of vertebrates for its blood meals [146], but at the present time feeds primarily on introduced livestock (cows, horses, pigs, goats, and sheep), poultry (chickens and turkeys), and occasionally humans [2,3,145]. Our interest in the feeding behavior of vampire bats specifically involves Desmodus because it prefers to feed on the blood of mammals and, as we have documented above, has an excellent Quaternary fossil record, including three extinct Pleistocene species. Our focus is on the diet of Desmodus prior to the appearance of domesticated animals in the New World in the late 15th century. Paleoindian people arrived in the Western Hemisphere much earlier, certainly by 13–15 ka and possibly during the Last Glacial Maximum about 21–23 ka [147], although it is unlikely humans have ever been an important source of blood for vampire bats. Domesticated dogs were the only mammals Paleoindian people brought with them from the Old World.
There are numerous field observations of the common vampire bat Desmodus rotundus in tropical America interacting with, and in many cases feeding upon, the blood of native Neotropical mammals of medium to large size. The following are the species and general localities of mammals known to be “prey” of D. rotundus: nine-banded armadillo Dasypus novemcinctus in Mexico [148]; giant armadillo Priodontes maximus in Brazil [149]; southern tamandua (=collared anteater) Tamandua tetradactyla [150]; capybara Hydrochoerus hydrochaeris in Venezuela [151,152] and Brazil [153]; southern sea lion Otaria flavescens on the coast of Chile [154,155]; South American fur seal Arctocephalus australis on the coast of Argentina [156]; puma or mountain lion Puma concolor [150]; South American tapir Tapirus terrestris in Brazil [157,158,159] and Ecuador [160]; collared peccary Dicotyles tajacu in Brazil [159,161]; white-tailed deer Odocoileus virginianus in Mexico [162,163]; and red brocket deer Mazama americana in Brazil [157,159]. Four of these mammals are South American in origin, nine-banded armadillo, giant armadillo, southern tamandua, and capybara, whereas five of these species are North American in origin, puma, tapir, peccary, and two species of deer, and would not have been present in South America prior to the connection of the two continents in the early Pliocene.
One of the most interesting records of vampire bat feeding behavior consists of camera trap recordings of Desmodus rotundus feeding on the giant armadillo Priodontes maximus in the Cerrado region in Matto Grosso state in western Brazil [149]. The authors observed a vampire bat on the back or carapace of a giant armadillo, presumably trying to feed on the blood in the soft tissue between the 11 to 13 movable bands composed of bony plates (osteoderms) that separate the anterior and posterior portions of the carapace (bucklers) that form solid shields of osteoderms. Another vampire was observed on camera trying to feed on the tail of a giant armadillo, probably on the soft tissue between the bony caudal rings. Priodontes maximus is the largest living species of armadillo, with adults reaching 1.5 m in length and 45 kg in weight [164]. This is somewhat greater than the weight of the extinct “beautiful” armadillo Dasypus bellus that co-occurs with extinct species of Desmodus in seven Florida Pleistocene sites (Appendix A). D. bellus was a larger version of the living D. novemcinctus, both of which possess nine movable bands of osteoderms in the carapace. These observations on the current feeding behavior of D. rotundus on the giant armadillo provide important insights into how the extinct vampires D. draculae and D. stocki may have fed upon large extinct cingulates, such as the beautiful armadillo, pamapatheres, and glyptodonts.
In laboratory studies of the feeding behavior of Desmodus rotundus, Greenhall [3] documented a vampire bat feeding on two medium-sized Neotropical mammals, Dasypus novemcinctus and the prehensile-tailed porcupine Coendou sp. Greenhall [3] (p. 118) observed “An armadillo, Dasypus, although apparently protected by its armor, was bitten on the tail between the scutes as well as on the foot, with no defensive reaction.” On the same page, Greenhall noted “A prehensile-tailed porcupine, Coendou, was quickly bitten on the part of the tail not covered with spines. One bat tried in vain to attack the naked feet which were tucked under its body. Both the armadillo and porcupine could be natural hosts for Desmodus, since the bats quickly located vulnerable biting sites despite protective scutes or spines.” Greenhall [3] (pp. 118–119) also provided photos showing a vampire feeding on the armored tail of an armadillo and the naked tail of a porcupine, both in the laboratory.
5.2. Diet of Extinct Vampire Bats
In a chapter on “Feeding Behavior” in a book on the “Natural History of Vampire Bats” [165], Arthur Greenhall [3] (p. 112) asked the question “What wildlife did vampire bats feed upon before the introduction of domestic animals? Some unknown factors have allowed Desmodus rotundus, Diaemus youngi, and Diphylla ecaudata to survive, while those vampires of the Pleistocene, such as Desmodus draculae, became extinct even though similar blood donors as capybaras and man were available then and now.”
Several previous authors have discussed the potential prey species of extinct vampire bats. Koopman [56] identified a skull of Desmodus rotundus in association with two species of extinct megalonychid ground sloths Megalocnus rodens and Mesocnus torrei (=Parocnus brownii) from a Late Quaternary fossil deposit in Cueva Lamas in western Cuba. Koopman [56] (p. 3–4) was the first author to suggest that vampire bats may have fed upon the blood of extinct ground sloths in his statement “We may assume that Desmodus reached Cuba after prey was available in the form of ground sloths and large ground birds, and that these species became extinct and thus the ecological niche for vampires disappeared, the latter also died out in Cuba.” The only surviving non-volant mammals in Cuba are eight species of capromyid rodents and a single species of the primitive lipotyphlan “insectivore” Solenodon cubanus [166], all of which were apparently unsuitable prey species for the now-extirpated Cuban population of D. rotundus. Small ground sloths also occurred on two other islands in the Greater Antilles, Hispaniola and Puerto Rico [134,135] but vampire bat fossils are not known from either of those islands [105,106].
Morgan [10] (p. 194) proposed that the earliest described species of vampire bat “Desmodus archaeodaptes or its progenitor probably entered North America in the late Pliocene as a participant in the Great American Interchange [=GABI], perhaps following the northward dispersal of its principal food source…The large, slow-moving ground sloths or another of the groups of South American immigrant mammals must have originally provided the major source of blood for Desmodus.” His hypothesis was based, in part, on the first appearance of Desmodus in temperate North America in three early Pleistocene faunas in Florida that also contained diverse assemblages of large Interchange vertebrates of South American origin (see more detailed discussion below).
Trajano and de Vivo [47] were the first authors to comment on the possible feeding behavior of the giant vampire Desmodus draculae, reporting a skull of this species from a late Pleistocene deposit in Santana Cave in the Ribeira River Valley from the state of São Paulo in southern Brazil. Trajano and de Vivo [47] (p. 458) stated “Considering its large size, we suggest that D. draculae was a vampire species which preyed preferentially on large mammals, presently extinct. Remains of such a megafauna have been found in caves of the Ribeira Valley, including the ungulate Toxodon platensis and giant ground sloths like Scelidotherium sp. and Eremotherium sp. …With the extinction of the mammalian megafauna, D. draculae would not have been able to move successfully to smaller prey, to which the other vampire species were better suited.” Cartelle and Abuhid [46] reported a nearly complete skull of D. draculae from a cave in the state of Bahia in Brazil, Toca dos Ossos, that was associated with a cranium of the extinct horse Equus (Amerhippus) neogeus. Ubilla et al. [24] discussed the associated fauna of large mammals from the Plio-Pleistocene Kiyú Fauna in Uruguay that also produced a humerus of a large vampire bat similar to D. draculae, mostly consisting of native South American taxa including ground sloths, glyptodonts, litopterns, notoungulates, and dinomyid rodents, as well as large, flightless phorusrhacid birds (Appendix A).
Brizuela and Tassara [43] reported Desmodus draculae from the late Pleistocene La Ballenera site in Argentina, along the Atlantic coast near Miramar in Buenos Aires province (38° S), about 400 km south of the closest modern record of D. rotundus in northern Argentina (35° S). According to Brizuela and Tassara [43] (p. 174), “…this record suggests that they [D. draculae] could have exploited the large caves of giant extinct sloths (Mylodontidae), some of which have been suggested as possible prey of these vampires.” Previous authors [167] have suggested that loess caves in Argentina may have been excavated by ground sloths. Many more such caves/burrows in southeastern Brazil have also been attributed to the digging activities of ground sloths [168,169,170] and occur within the former distribution of D. draculae. An on-line story based on the record of D. draculae from the La Ballenera site [171] was accompanied by an illustration showing a ground sloth resting in one of these excavated caves, with vampire bats roosting on the cave ceiling above the sloth. In this story, one of the coauthors of the original study, Daniel Tassara, was quoted, “The jaw of Desmodus draculae was found inside a cave or burrow 1.2 m (3.9 feet) in diameter attributed to a giant sloth of the family Mylodontidae, such as Scelidotherium…”.
Pardiñas and Tonni [42] noted that the Centinela del Mar site in northeastern Argentina of late Holocene age (~300 yrBP) is the southernmost record of Desmodus draculae and the youngest dated record of this species. This site is located slightly farther south than the late Pleistocene record of D. draculae from La Ballenera. There are no large extinct mammals at Centinela del Mar as they disappeared at the end of the Pleistocene. Pardiñas and Tonni [42] hypothesized that the late Holocene population of D. draculae may have fed upon medium-sized caviomorph rodents, including the vizcacha (Lagostomus maximus), as well as extant species of cervids and camelids. Considering that Centinela del Mar is near the Atlantic coast, it is also possible that D. draculae from this site fed on pinnipeds, as D. rotundus has been observed feeding on the southern sea lion on the coast of Chile [154,155] and on the South American fur seal on the coast of Argentina [156].
The only other post-Pleistocene record of an extinct vampire bat is Desmodus stocki identified from a late Holocene (~3–5 ka) archeological site in a cave on San Miguel Island, one of the Channel Islands off the coast of southern California [38,39]. No large mammals were reported from this cave deposit, but Ray et al. [18] suggested the vampires may have fed upon pinnipeds that occur on this island. The Channel Islands pygmy mammoth, Mammuthus exilis, also occurred on San Miguel Island, and may have been a prey species of D. stocki [172].
In their review of the fossil bats of Mesoamerica, Arroyo-Cabrales and Polaco [173] (p. 160) noted that “The large size of the extinct species, D. [Desmodus] draculae and D. stocki, may be due to the large-sized animals, like ground sloths and many other megafaunal mammals, on which they fed. These large ‘mega-vampires’ then were depleted of their food supply at the time of megafaunal extinction.”
McDonald and Jefferson [16] documented the association of the extinct vampire bat Desmodus stocki and the Shasta ground sloth Nothrotheriops shastensis in five late Pleistocene cave sites in western North America. Three of these caves are in the southwestern US: Potter Creek Cave in California; Rampart Cave in Arizona (Figure 2); and U-Bar Cave in New Mexico, and two of the caves are in Mexico, Cueva de San Josecito (San Josecito Cave in most literature citations) in the state of Nuevo Leon and Cueva de La Presita in the state of San Luis Potosí, both located in north-central Mexico within one degree latitude of the Tropic of Cancer (23°26′ N). Harris [35] has since added a sixth site that documents the co-occurrence of D. stocki and N. shastensis, Sierra Diablo Cave in the Trans-Pecos region of southwestern Texas. McDonald and Jefferson [16] (p. 321) noted that “The association of N. shastensis and D. stocki in a number of assemblages suggests a similar latitudinal and altitudinal restriction of both species by minimal winter temperatures. Given the sloth’s low metabolic rate and the thermal restrictions exhibited by modern vampire bats, this lower limiting temperature falls in the range of 10 to 20 °C.” McDonald and Jefferson [16] focused on the geographic distribution of Stock’s vampire bat and Shasta ground sloth, in particular latitudinal and altitudinal limitations, as well as their co-occurrence in cave faunas with the possible moderating influence of temperatures in caves during the winter months, but they did not address the potential feeding preferences of D. stocki, possibly including N. shastensis.
Here we examine the association of the three extinct species of Desmodus with members of the extinct Pleistocene megafauna, specifically mammals of South American origin that participated in the GABI, to see if we can establish a pattern between these faunal associations and possible feeding behavior by the extinct vampire bats. Appendix A provides a list of the associated genera or species of large mammals of South American origin for most of the 45 Quaternary sites that contains vampire bats. Some of these fossil localities with Desmodus lack associated large mammals or lack data on the associated mammalian fauna. This focus on large mammals associated with the GABI is based on our hypothesis that vampire bats evolved in South America and their original prey consisted of mammals of South American origin of large body size. The oldest evidence of fossil vampire bats in South America are late Pliocene or early Pleistocene records of a large species of Desmodus from Uruguay and Venezuela. There are also late Pleistocene records of the giant extinct vampire, D. draculae, from Argentina, Brazil, and Venezuela and the living species Diphylla ecaudata and Desmodus rotundus from Brazil and Venezuela (Appendix A). Even though vampire bats are South American in origin, two of the three extinct vampire species, D. archaeodaptes and D. stocki, are known only from Pleistocene sites in North America, including the southern US (both species) and Mexico (D. stocki). D. draculae is the only extinct vampire bat species known from both continents, with late Pleistocene records from Mexico and Belize, together with the South American records mentioned above (Appendix A). The following discussion focuses on the North American record of the three extinct vampire species, followed by a brief analysis of the South American late Pleistocene record of D. draculae and several records of large vampire bats, consisting of fragmentary fossils of indeterminate species, from earlier sites (late Pliocene or early Pleistocene) having affinities with this species.
The three sites with the oldest extinct vampire bat species, Desmodus archaeodaptes, share the following characters: (1) located in peninsular Florida; (2) date to the early Pleistocene (latest Blancan or early Irvingtonian, ~1–2 Ma); (3) consist of karst deposits that represent former cave systems; and (4) preserve Interchange faunas with from two to eight species of mammals of South American origin that participated in the GABI [11] (Appendix A). Two of these sites, Inglis 1A (late Blancan) and Haile 16A (early Irvingtonian), are among the most diverse Interchange faunas known from temperate North America [4,11]. Inglis 1A has ten genera of GABI vertebrates, eight of which are of large body size (>45 kg), three ground sloths (Eremotherium, Megalonyx, Paramylodon), glyptodont (Glyptotherium), pampathere (Holmesina), armadillo (Dasypus), capybara (Neochoerus), and a giant flightless bird (Titanis). Haile 16A has eight Interchange mammal genera, including six of large body size, five of which are shared with Inglis 1A (Eremotherium, Megalonyx, Paramylodon, Holmesina, Dasypus), as well as a smaller glyptodont (Pachyarmatherium) that does not occur at Inglis 1A. Two of the Interchange genera from Inglis 1A and Haile 16A, the giant ground sloth Eremotherium and Dasypus, are also shared with a third Florida early Irvingtonian fauna, Haile 21A, the type locality of D. archaeodaptes. A fourth genus of ground sloth, Nothrotheriops, occurs in two Florida early Irvingtonian open sites, Leisey Shell Pit and Pool Branch [137,138], but Desmodus is not known from either of those sites. Since Nothrotheriops texanus and D. archaeodaptes occur in correlative early Irvingtonian faunas in Florida, although not the same sites, it is reasonable to assume these two species will eventually be found together in a Florida early Irvingtonian karst deposit. The larger, late Pleistocene species of Nothrotheriops, N. shastensis, is not known from Florida. But as previously noted by McDonald and Jefferson [16] and discussed above, during the Rancholabrean N. shastensis and D. stocki do co-occur in multiple cave sites in western North America.
Six Rancholabrean sites containing Desmodus stocki occur within about 60 km of one another in the karst terrain of northern peninsular Florida, distributed in two clusters of three sites each. All of these sites are less than 100 m in elevation, are karst fissure deposits that were former caves, and were discovered in commercial limestone mines. Arredondo 2A, Haile 1A, and Haile 11B occur in Alachua County, and the three Reddick sites (Reddick 1A, 1B, 1C) are in Marion County, the next county to the south. The three Reddick sites are located within several hundred meters of one another in the same abandoned limestone quarry and probably represent different parts of the same now-collapsed cave system. The Reddick sites have the largest known sample of D. stocki, or of any species of fossil vampire bat, with more than 500 specimens representing a minimum of 54 individuals [10,32]. Although the Reddick 1 sites are treated as three separate localities in Appendix Awe discuss their associated mammalian faunas together. Four genera of large mammals of South American origin occur in the Reddick 1 faunas, the ground sloths Megalonyx and Paramylodon and the cingulates Holmesina and Dasypus. Paramylodon and Dasypus also occur in the Arredondo 2A LF and Dasypus is present in the Haile 11B LF (Appendix A).
The following is a summation of the number of Pleistocene (late Blancan, early Irvingtonian, and Rancholabrean) sites in Florida (total of nine sites) in which large Interchange mammals and one giant bird (total of nine genera) occur in association with Desmodus (D. archaeodaptes or D. stocki): Dasypus–7 sites; Paramylodon–5 sites; Megalonyx–4 sites; Eremotherium–3 sites; Holmesina–3 sites; Glyptotherium–1 site; Pachyarmatherium–1 site; Neochoerus–1 site; Titanis–1 site. The four genera of large Interchange vertebrates that occur with Desmodus in only one Florida site are either late Blancan (Glyptotherium, Neochoerus, Titanis) or early Irvingtonian (Pachyarmatherium). Eremotherium is associated with Desmodus in three Florida early Pleistocene sites but does not occur with Desmodus in any Florida late Pleistocene faunas. This may be a taphonomic bias, because the late Blancan and early Irvingtonian species Eremotherium eomigrans is known primarily from karst deposits in Florida that represent former caves or sinkhole ponds, including the type locality Haile 7C [174], whereas the late Pleistocene species E. laurillardi occurs in open sites near the Florida coast. Four genera of large Interchange mammals, Megalonyx, Paramylodon, Holmesina, and Dasypus, are associated with Desmodus in both early Pleistocene (late Blancan and early Irvingtonian) and late Pleistocene (Rancholabrean) sites in Florida. A list of Florida Pleistocene sites with Desmodus and associated large GABI mammals is provided in Appendix A.
Excluding Florida, all other US fossil records of vampire bats are late Pleistocene cave sites with Desmodus stocki. These localities lack the diverse associated fauna of large Interchange mammals of South American origin found in Florida Pleistocene sites with fossil vampire bats. They have at most two species of large Interchange mammals, both ground sloths, the Shasta ground sloth, Nothrotheriops shastensis and/or Jefferson’s ground sloth, Megalonyx jeffersonii (Appendix A). In the following discussion, for the sites in western North America and Mexico with D. stocki that have only one sloth or no associated Interchange mammals of South American origin, we also list several large, extinct mammals of North American affinity from those sites that could have been prey for D. stocki.
The only fossil record of D. stocki from the eastern US outside of Florida is from the late Pleistocene (Rancholabrean) New Trout Cave fauna in West Virginia. The vampire bat was recovered from level C in New Trout Cave that was radiocarbon dated at 29,400 ± 1700 yrBP [34]. New Trout Cave documents the co-occurrence of D. stocki with several large, extinct mammals of North American origin, including dire wolf Canis (=Aenocyon) dirus, horse Equus sp., peccary Platygonus compressus, and woodland muskox Bootherium bombifrons [175,176]. Two mammals of South American origin are known from the Pleistocene of West Virginia, the ground sloth Megalonyx jeffersonii and the extinct armadillo Dasypus bellus, although neither of these species has been identified from New Trout Cave [177].
There are eight records of D. stocki from the western US, seven are late Pleistocene cave deposits, three from Arizona, one from California, one from New Mexico, and two from Texas, and one is a late Holocene cave deposit in California (Appendix A). Potter Creek Cave in northern California is one of four sites in the US where this vampire bat occurs in association with the ground sloth Nothrotheriops shastensis [16]. A second species of ground sloth, Megalonyx jeffersonii, has also been identified from Potter Creek Cave (Appendix A), together with a fairly diverse fauna of large, extinct mammals of North American origin, including: giant short-faced bear Arctodus simus, Aenocyon dirus, Equus sp., Platygonus sp., shrubox Euceratherium collinum, mastodon Mammut, and mammoth Mammuthus [27].
Arizona has three late Pleistocene cave sites with Desmodus, the largest concentration of US fossil sites containing vampire bats outside of Florida. Rampart Cave in Grand Canyon National Park in northwestern Arizona also records the co-occurrence of D. stocki and Nothrotheriops shastensis [16]. The association of these two species was enshrined on a poster of Rampart Cave for the National Fossil Day in October 2019, sponsored by the National Park Service (Figure 2). Rampart Cave is located in the side of a cliff above the Colorado River, so it does not have a particularly diverse large mammal fauna. In addition to N. shastensis, other large extinct mammals are the cheetah-like cat Miracinonyx trumani, Equus sp., and Harrington’s extinct mountain goat Oreamnos harringtoni [178,179,180,181,182]. The two other late Pleistocene cave deposits in Arizona with D. stocki, Arkenstone Cave and La Tetera Cave, are located within 1 km of one another in Colossal Cave County Park, Pima County, in the Sonoran Desert in the southern part of the state [36,37]. The mammalian fauna from Arkenstone Cave consists of D. stocki, two species of small insectivorous bats (Myotis), and a cricetid rodent, but no larger mammals that could have been prey for vampire bats [36]. La Tetera Cave has two large mammals of North American affinity in association with D. stocki, the horse Equus conversidens and a large camel Camelops hesternus [37]. La Tetera Cave also contains a stratified paleoguano deposit, apparently produced by D. stocki, that has a calibrated radiocarbon age of 23,745 yrBP [37]. Analysis of the La Tetera guano deposit for ancient DNA from both the vampire bats and their prey species should be attempted.
U-Bar Cave is a late Pleistocene cave deposit in southern New Mexico that has produced several postcranial specimens of Desmodus stocki [35,183,184], associated with Nothrotheriops shastensis [16]. U-Bar Cave has an exceptionally diverse vertebrate fauna numbering over 100 species [35,183], with 64 species of mammals, including N. shastensis and ten members of the extinct Pleistocene megafauna with North American affinities: Aenocyon dirus, Arctodus simus, two species of Equus, Camelops hesternus, mountain deer Navahoceros fricki, two pronghorns Capromeryx furcifer and Stockoceros conklingi, Oreamnos harringtoni, and Euceratherium collinum. Although the vampire bat fossils were collected from spoil resulting from guano mining operations in the cave, Harris [184] attributed the Desmodus fossils to the mid Wisconsin, with associated radiocarbon dates from 26,150 to 35,890 yrBP.
Two late Pleistocene cave sites in the Trans-Pecos region of southwestern Texas have fossils of Desmodus stocki, Sierra Diablo Cave and Terlingua (Appendix A). Sierra Diablo Cave is located in the Diablo Plateau, Rio Grande drainage in Hudspeth County [35] and documents the association of D. stocki and Nothrotheriops shastensis. This cave was not mentioned by McDonald and Jefferson [16] because it was not excavated until 2011 and 2012 [35]. The vertebrate fauna from Sierra Diablo Cave has not been extensively studied or published but has more than 60 species of mammals [35], including N. shastensis and eight members of the extinct Pleistocene megafauna with North American affinities: American lion Panthera atrox, three species of Equus, the llama Hemiauchenia macrocephala, the antilocaprids Capromeryx furcifer and Stockoceros conklingi, and Oreamnos harringtoni. Postcranial elements of D. rotundus were derived from clay sediments in a limestone crevice about 100 m below the surface at the Little Thirty-eight Mine near Terlingua in the Big Bend region, Brewster County, Texas [18,85,185]. In a paper describing a fossil land snail from the Terlingua deposit, Cockerell [186] (p. 52) was one of the first authors to report a fossil vampire bat, where in a footnote he stated “A quantity of bat remains was also recovered, in which Dr. Gerrit S. Miller recognizes…a race somewhat larger than Desmodus rotundus murinus” [extant Mexican subspecies of the common vampire bat]. Three genera of large mammals of North American affinity, Equus, Bison, and Ovis, were identified from the Terlingua site, in association with D. stocki [185,186].
Four records of Desmodus stocki have been reported from Mexico: San Josecito Cave (=Cueva de San Josecito) and Cueva de La Boca, both in the state of Nuevo León, Cueva de La Presita (=cave near Matehuala) in the state of San Luís Potosí, and a cave at Cerro de Tlapacoya in the state of Mexico [18,31,62,187,188,189,190]. One of the best-known fossil vampire bat localities is San Josecito Cave in northern Mexico. Stock [191] was the first paleontologist to mention the presence of vampire bat fossils from San Josecito Cave. However, it was not until 15 years later that Jones [31] recognized this bat as a new species, distinctly larger than the extant D. rotundus from Mexico, and named it D. stocki in honor of Chester Stock. San Josecito Cave was one of two late Pleistocene cave sites from Mexico in which McDonald and Jefferson [16] documented the co-occurrence of D. stocki and Nothrotheriops shastensis. San Josecito Cave and Potter Creek Cave are the only two sites where D. stocki is associated with both N. shastensis and Megalonyx jeffersonii. San Josecito Cave has a diverse fauna of 12 species of large, extinct mammals of North American affinity [190]: Aenocyon dirus, Florida cave bear Tremarctos floridanus, sabertooth cat Smilodon fatalis, Panthera atrox, Equus conversidens, large tapir Tapirus haysii (=T. merriami?), Platygonus compressus, Camelops hesternus, Navahoceros fricki, Stockoceros conklingi, Oreamnos harringtoni, and Euceratherium collinum. A second record of D. stocki from Nuevo León, Cueva de La Boca, was reported by Arroyo-Cabrales and Polaco [188], who also identified Euceratherium collinum from this cave.
Cueva de La Presita in northern San Luis Potosí is only about 150 km southwest of San Josecito Cave and, together with the latter cave, documents the association of Desmodus stocki and Nothrotheriops shastensis [16]. This locality is the same as “Matehuala (cave near)” listed by Ray et al., [18]. Five species of large, extinct ungulates of North American affinity also occur in Cueva de La Presita [188,192]: Equus sp., the antilocaprids Capromeryx furcifer and Stockoceros conklingi, and the camelids Camelops hesternus and Hemiauchenia macrocephala. Alvarez [189] identified a mandible of D. stocki from a locality listed as Cerro de Tlapacoya by Ray et al. [18] and Tlapacoya by Arroyo-Cabrales and Polaco [173], located in the state of Mexico, about 25 km southeast of Mexico City. According to Ray et al. [18] (p. 23) “This specimen [D. stocki mandible] came from a small cave in a layer immediately overlying another dated at 14,000 years. This deposit is separate from and considerably younger than those nearby from which Alvarez [193] had earlier reported Pleistocene faunas”. The latter fauna from Tlapacoya also produced a tooth of the large capybara Neochoerus pinckneyi (=N. aesopi) [193].
There are two records of Desmodus draculae in North America, both of which are located on the Yucatán peninsula in the tropical region of Mesoamerica, Gruta de Loltún (=Loltún Cave) in the state of Yucatán, Mexico and Cebada Cave in Belize (Appendix A, Figure 1). Loltún Cave is one of the best known late Pleistocene cave deposits in Mexico [44,45]. The first vampire bat fossil recorded from Loltún Cave was a humerus of the living species Diphylla ecaudata [194]. Subsequent publications reported additional vampire bat fossils from Gruta de Loltún, including D. rotundus and the first North American record of D. draculae [18,44,62]. Loltún Cave is one of only three cave sites in the Neotropical region that documents the late Pleistocene co-occurrence of three species of vampire bats, D. draculae, D. rotundus, and D. ecaudata; the other two caves are Cueva del Guácharo in Venezuela [23] and Toca da Boa Vista in Brazil [13]. All three of these sites are within the current geographic ranges of D. rotundus and D. ecaudata. Loltún Cave also has a large sample of fossils representing a diverse fauna of 23 species of extant Neotropical bats, all of which still occur on the Yucatán peninsula in the general vicinity of the cave [45]. No ground sloths or other Interchange mammals of South American origin have been identified from Loltún Cave, but there are four species of large mammals with North American affinities, Aenocyon dirus, Equus conversidens, Hemiauchenia, sp., and the gomphothere Cuvieronius hyodon [45]. Four genera and five species of ground sloths have been identified from late Pleistocene cave sites in the Yucatán peninsula: a mylodontid identified as Paramylodon harlani from Cueva de Spukil (=Actun Spukil) in the state of Yucatán [188]; two genera and three species of megalonychids from underwater caves or cenotes in the state of Quintana Roo, Mexico, Nohochichak xibalbahkah [131], Xibalbaonyx oviceps, and X. exinferis [195,196]; and Nothrotheriops shastensis from Actun Lak, a cave in Belize [141]. However, to date, no fossils of vampire bats, including D. draculae, have been found in direct association with ground sloths in Pleistocene cave sites in the Yucatán peninsula.
Czaplewski et al. [14] reported a skull of D. draculae from Cebada Cave in Belize that they considered to be late Pleistocene in age. They also identified eight other species of Neotropical bats from Cebada Cave, six phyllostomids and two vespertilionids, all of which are extant species that still occur in the general vicinity of the cave and were considered probable Holocene occurrences. The only extinct large mammal from Cebada Cave is the Florida cave bear Tremarctos floridanus. Several other large mammals are known from caves in Belize but not in direct association with D. draculae: Nothrotheriops shastensis from Actun Lak cave, mentioned above [141]; and the large armadillo Dasypus bellus, and three large mammals with North American affinities, Panthera atrox, Tremarctos floridanus, and Equus conversidens from Extinction Cave [197]. The sloth Eremotherum laurillardi was reported from a late Pleistocene deposit in a cenote at Cara Blanca in Belize [198] but it is the only mammal so far recovered from the site.
The associated species of ground sloths and other large mammals of South American origin associated with fossil records of Desmodus from South America are listed in Appendix A. Because our focus is on the better-known North American record, we provide only a brief synopsis of the South American fossil record of Desmodus and associated large mammals. We concentrate on the seven late Pleistocene sites from South America with the large, extinct species D. draculae, as well as two older late Pliocene or early Pleistocene sites with a large species of Desmodus that has affinities with D. draculae. The type locality of D. draculae, Cueva de Guácharo in Venezuela, lacks an associated fauna of large mammals [23] (Appendix A). Trajano and de Vivo [47] reported the second known occurrence of D. draculae in South America from Santana Cave in the state of São Paolo in southern Brazil, and also noted two genera of ground sloths, Eremotherium and Scelidotherium, that were found in caves in the same general vicinity in the Ribeira River Valley (Appendix A). Brizuela and Tassara [43] mentioned the presence of mylodontid sloths and the glyptodont Glyptodon reticulatus associated with D. draculae from the late Pleistocene La Ballenera site in northeastern Argentina. As noted above, they suggested that a mandible of D. draculae from La Ballenera may have been preserved in a sediment-filled burrow excavated by a ground sloth.
Two caves in the state of Bahia in Brazil, Toca dos Ossos and Toca da Boa Vista, contain fossils of Desmodus draculae in association with diverse late Pleistocene (Lujanian) faunas of large mammals of both South American and North American origin [13,46]. Auler et al. [53] citing Cartelle [50,51,52], documented a remarkably diverse fauna of large mammals from Toca dos Ossos, including 13 species of South American origin: six ground sloths, Eremotherium laurillardi, Glossotherium aff. lettsomi, Catonyx cuvieri, Ocnotherium giganteum, Mylodontopsis ibseni, and Nothrotherium maquinense; the glyptodont Glyptodon clavipes; the pampathere Pampatherium humboldtii; the extant giant anteater Myrmecophaga tridactyla; the extant capybara Hydrochoerus hydrochaeris and extinct capybara Neochoerus sulcidens; and two SANU, the toxodonts Toxodon platensis and Trigonodops lopesi. Mammals of North American origin from Toca dos Ossos include seven species: the sabertooth cat Smilodon populator; the horses Equus neogeus and Hippidion principale; the extant peccary Dicotyles tajacu; the extinct llama Palaeolama major; the extant deer Odocoileus virginianus; and the gomphothere Notiomastodon platensis.
Both Desmodus draculae and D. rotundus occur in Toca da Boa Vista [13,46]. The associated Pleistocene fauna from this cave includes five large mammals of South American origin: the ground sloths Catonyx cuvieri and Nothrotherium maquinense, Myrmecophaga tridactyla, and two large extinct primates, Caipora bambuiorum and Cartelles coimbrafilhoi (previously referred to Protopithecus brasiliensis); two medium-sized South American mammals, the six-banded armadillo, Euphractus sexcinctus, and the prehensile-tailed porcupine, Coendou prehensilis; and eight species with North American affinities, four large carnivorans, Smilodon populator, extant puma Puma concolor, canid Protocyon troglodytes, and bear Arctotherium brasiliense, Equus neogeus, and three artiodactyls, Dicotyles tajacu, the extant guanaco Lama guanicoe, and extant brocket deer Mazama gouazoubira [46,53].
The late Pliocene or early Pleistocene Kiyú Fauna from Uruguay documents a humerus of a large vampire bat, Desmodus aff. draculae in association with a diverse fauna of large mammals of South American origin, including medium to large ground sloths, glyptodonts, litopterns, notoungulates, and dinomyid rodents, as well as large phorusrhacid birds [24]. El Breal de Orocual is a late Pliocene or early Pleistocene tar pit site in Venezuela that contains a large Desmodus [17] in association with a diverse fauna of large mammals of both South American and North American origin that participated in the Interchange [25] (Appendix A). The genera of large mammals from El Breal de Orocual with South American affinities include: two ground sloths, Eremotherium sp. and a megalonychid; the armadillo Propraopus sulcatus; three glyptodonts, Glyptodon sp., Hoplophorus sp., and Pachyarmatherium cf. leiseyi; two pampatheres, Holmesina occidentalis and Pampatherium humboldtii; the anteater cf. Myrmecophaga sp.; the capybara cf. Chapalmatherium sp.; and a SANU, the toxodont Mixotoxodon larensis. El Breal de Orocual has an equally diverse fauna of genera of large mammals of North American origin: two sabertooth cats, Smilodon sp. and a homotheriine; the canid cf. Protocyon sp.; the ursid Arctotherium cf. wingei; the horse Hippidion sp.; the tapir Tapirus new sp.; two peccaries, Platygonus sp. and Tayassu sp.; the lamine camel Palaeolama sp.; and an indeterminate gomphotheriid proboscidean. The late Pliocene/early Pleistocene Kiyú and El Breal de Orocual faunas document the presence of a very large species of Desmodus in South America long before the widespread occurrence of D. draculae in the late Pleistocene.
Several additional Brazilian caves document diverse faunas of xenarthans and SANU, in association with fossils of the extant Desmodus rotundus. Czaplewski and Cartelle [13] identified D. rotundus from Gruta dos Brejões (Appendix A). Remarkably, a braincase of D. rotundus from Gruta dos Brejões was found adhering to a coprolite referred to the ground sloth Nothrotherium maquinense that radiocarbon dated at 12,200 yrBP. The diversity of eight species of sloths identified from this cave exceeds that from most other cave sites in South America, including: in addition to N. maquinense, Eremotherium laurillardi, Ocnotherium giganteum, Glossotherium aff. lettsomi, Glossotherium robustum, Catonyx cuvieri, Mylodon darwini, and Mylodontopsis ibseni. Other South American mammals from this cave are Myrmecophaga tridactyla, the pampathere Pampatherium humboldtii, the macraucheniid Xenorhinotherium bahiense, and the porcupine, Coendou. Large mammals of North American affinity include the gomphothere Notiomastodon platensis (=Haplomastodon waringi) and the lamine camelid Palaeolama major [48]. D. rotundus was reported as a fossil from Toca da Barriguda in Bahia, Brazil [13], associated with the sloth, Nothrotherium maquinense, collared anteater Tamandua tetradactyla, and an extinct porcupine, Coendou magnus [199].
5.3. Evolution of Feeding Behavior in Vampire Bats
Among the list of known prey species of Desmodus rotundus (see above), we focus on the species of mammals of South American origin, including two species of armadillos, Dasypus novemcinctus and Priodontes maximus, an anteater Tamandua tetradactyla, and two species of caviomorph rodents, the capybara Hydrochoerus hydrochaeris and the prehensile-tailed porcupine Coendou sp. The observations of D. rotundus feeding on living armadillos, including both D. novemcinctus and P. maximus [3,149], report it feeding on the area of soft tissue between the movable bands of osteoderms on the back and between the bony rings on the tail and an exposed foot, feeding on the feet of a capybara, and feeding or attempting to feed on the naked tail and the feet of a captive prehensile-tailed porcupine. These observations provide important insights into not only the possible prey of the extinct species of Desmodus, but also how they may have fed upon these native Neotropical mammals. We suspect that the feet of ground sloths and various extinct cingulates, including glyptodonts, pampatheres, and armadillos, all of which were large in body size and presumably slow-moving, may have been a particularly vulnerable area for feeding by extinct vampires. We present hypothetical illustrations showing the extinct vampire bat Desmodus stocki feeding on the foot of Harlan’s ground sloth Paramylodon harlani (Figure 3) and on the ear of a sleeping Shasta ground sloth Nothrotheriops shastensis (Figure 4) and the giant extinct vampire Desmodus draculae feeding at the base of the tail of the pampathere Pampatherium (Figure 5). These illustrations are artist’s conceptions for esthetic purposes and do not necessarily reflect co-occurrences of the taxa illustrated at a given locality.

Figure 3.
Hypothetical reconstruction of the extinct vampire bat Desmodus stocki feeding on the foot of Harlan’s ground sloth Paramylodon harlani. Note that this reconstruction does not illustrate a known behavior as documented in the fossil record. Illustration by Nicholas J. Czaplewski.
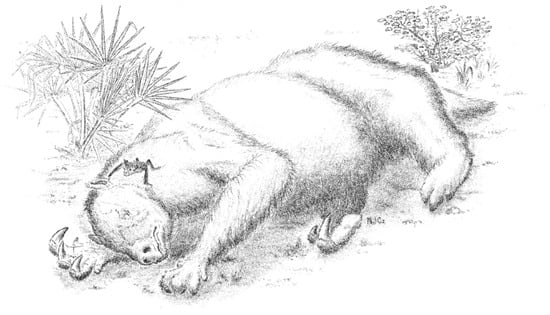
Figure 4.
Hypothetical reconstruction of the extinct vampire bat Desmodus stocki about to feed on the ear of a sleeping Shasta ground sloth Nothrotheriops shastensis. Note that this reconstruction does not illustrate a known behavior as documented in the fossil record. Illustration by Nicholas J. Czaplewski.

Figure 5.
Hypothetical reconstruction of the giant extinct vampire bat Desmodus draculae feeding where the tail meets the posterior portion of the carapace of the pampathere Pampatherium. Note that this reconstruction does not illustrate a known behavior as documented in the fossil record. Illustration by Nicholas J. Czaplewski.
Possibly feeding on the feet of large, extinct xenarthrans may also provide insight into the evolution of the unique (for a bat) terrestrial locomotion of Desmodus. In attempting to understand the reason(s) for the evolution of terrestrial locomotion in vampire bats, a quote from Altenbach [99] (p. 72) about the locomotor abilities of D. rotundus presents a good starting point for our discussion. “The adaptations for specialized locomotion in Desmodus are spectacular. A low-efficiency style of flight reflects an evolutionary trade-off for superb agility in terrestrial locomotion and for jumping into flight with a blood meal comprising a large proportion of body weight. Although other bats exhibit varying degrees of terrestrial agility and some are able to jump from a horizontal surface into flight, no others are known to have such specializations for these locomotor patterns.” Altenbach [98,99] described the locomotor morphology of D. rotundus in exquisite detail, and as a result we have a comprehensive understanding of the functional morphology of terrestrial locomotion and jumping of D. rotundus. In the Forward to his classic work on the locomotor morphology of D. rotundus, Altenbach [98] (p. vi) noted that his descriptions and illustrations of the functional morphology and terrestrial locomotion of D. rotundus focused on the muscular and skeletal systems of the front limb. He specifically stated that “Only description of the anatomical details of the pectoral girdle and limb is included in this work. Admittedly the pelvic girdle and limb are important in locomotion in the vampire bat and show specializations for its unique locomotor mechanisms. However, I feel that the most spectacular of the anatomical specializations for terrestrial locomotion and certainly the most important specializations for flight are those of the pectoral limb and girdle, and I have focused my attention on these structures.”
Further observations on the terrestrial locomotion of extant vampire bats have been presented by Schutt et al. [200], Schutt [201], Schutt and Simmons [202], Riskin and Hermanson [203], and Riskin et al. [204]. Several statements by Schutt and Simmons [202] (p. 148) are particularly relevant to our ideas on the feeding behavior of extinct vampire bats. “Desmodus feeds primarily from the ground…Alighting some distance from their intended prey, the common vampire bat, Desmodus rotundus, makes its approach using a quadrupedal gait that varies between walking, spider-like scrambling, jumps into short flights, and hopping. Desmodus must consume between 50% and 100% of its body mass in blood each night to survive (McNab, 1973) [26]. Jumping functions to get the heavily loaded vampire bat airborne from a horizontal surface, a critical first step in initiating flight back to the roost following a blood meal. Jumping may also be useful to vampire bats in avoiding terrestrial predators and probably prevents them from being trampled by large prey such as cattle.”
Morgan [10] described the morphology of the humerus, radius-ulna, femur, and tibia of Desmodus stocki based on a large fossil sample from the late Pleistocene Reddick 1 LF in Florida and also made comparisons between the limbs of D. stocki and those of extant D. rotundus. Figure 6 illustrates the four major limb bones, humerus, radius-ulna, femur, and tibia, of D. stocki, together with comparative photos of these same four limbs in D. rotundus and the basal phyllostomid bat Macrotus waterhousii. Table 1 provides a series of measurements of the humerus, radius-ulna, femur, and tibia of D. stocki from Reddick 1 and of a sample of those limbs of D. rotundus from the Neotropical region, as well as measurements of the humerus of the two other extinct vampire bat species, D. archaeodaptes and D. draculae. Morgan’s [10] study revealed that the most striking morphological differences between the limbs of Desmodus and those of more typical phyllostomid bats, as well as between D. stocki and D. rotundus, are in the hind limb not the fore limb. The humerus and radius of D. stocki average somewhat longer but overlap in length with those two limb elements of D. rotundus, whereas both elements in D. stocki are more robust, particularly in the width of the proximal and distal ends (Table 1). The length of the humerus of D. stocki averages 10–15% longer than the humerus in a sample of modern D. rotundus, whereas the width of the proximal and distal ends of the humerus of D. stocki average 20–30% broader than in equivalent measurements of D. rotundus. Two complete humeri of a large species of Desmodus, one of which is part of the type specimen of D. draculae from Cueva del Guácharo in Venezuela and one of which is tentatively referred to that species from Kiyú, Uruguay, are 25–30% longer and the proximal and distal ends are 30–40% broader than in the humerus of D. rotundus, and are about 15–20% larger in the four measurements of the humerus than in D. stocki (Table 1). The total length of the femur of D. stocki from Reddick 1 is similar in both the mean and observed range compared to D. rotundus, whereas both the mean and observed range of the length of the tibia in D. stocki are slightly less than in the living species, even though D. stocki is larger in almost all other cranial and postcranial measurements (Table 1) [10]. However, the widths of these two limb elements are much broader and more robust in D. stocki, particularly the shafts of the femur and tibia, which are nearly 40% broader than in D. rotundus (Table 1; see Figure 6). We were unable to locate a femur or tibia of D. draculae or D. archaeodaptes for comparison with D. stocki and D. rotundus. The basal phyllostomid Macrotus is essentially incapable of terrestrial locomotion and has a very slender femur (Figure 6I) and tibia (Figure 6L) that cannot support its weight.
Figure 6.
Comparative photos of limb bones of the extinct vampire bat Desmodus stocki (A,D,G,J), the extant common vampire bat D. rotundus (B,E,H,K), and Waterhouse’s leaf-nosed bat Macrotus waterhousii (C,F,I,L). There are two views of the humerus (A–C), radius-ulna (D–F), femur (G–I), and tibia (J–L) for each of these three species. All photos are reproduced at the same scale.

Table 1.
Measurements (in mm) of the humerus, radius-ulna, femur, and tibia of late Pleistocene Desmodus stocki from Florida and modern specimens of D. rotundus. Measurements of the humerus are also provided for D. stocki from San Josecito Cave, Mexico and Potter Creek Cave, California, D. draculae from Cueva del Guácharo, Venezuela, and D. aff. draculae from Kiyú, Uruguay. Missing measurements are indicated by “–”. Extinct species are indicated by “†”. Mean (M), observed range (OR), and sample size (SS) are provided for most measurements.
The robust hind limbs of Desmodus rotundus are related to its ability to jump straight up from the ground as high as 1 m and then take off in flight, even with a full blood meal still in its digestive system [98,99,205]. The strong limbs may also be related to the unique, modified bounding gait of these bats at higher speeds during terrestrial locomotion [203]. The more robust hind limbs of D. stocki compared to D. rotundus are probably related to the different prey species of these two vampires. Desmodus stocki probably fed on larger species of mammals, acquiring a heavier blood meal during feeding and thus requiring a stronger hind limb, with more robust femur and tibia, to achieve flight after a vertical leap from the ground. The femur and tibia of D. draculae have not been reported but presumably would have been even larger and more robust than those of D. stocki.
Altenbach’s [98,99] studies of the terrestrial locomotion of Desmodus rotundus covered the “how” of walking by vampire bats in remarkable detail, but he did not address the “why” of vampire bat terrestrial locomotion. Why would a bat that was perfectly well-adapted for powered flight essentially “re-evolve” the ability to walk and run on the ground? We offer the following scenario for the feeding of the large vampires D. stocki and D. draculae on extinct xenarthans and other large megafaunal mammals. Rather than landing or jumping on the back of a ground sloth or glyptodont, landing on the ground a short distance away and then employing a slow, stealthy approach using terrestrial locomotion (probably a diagonal sequence walk as in D. rotundus [206]), may have been a more effective strategy for the vampire bat to approach its unwary prey than landing directly on the animal. The ability to run is probably important to vampires that need to quickly move away from large prey animals that move while the bat is feeding. Given the long, coarse fur of sloths, the presence of dermal ossicles in the skin of some mylodont sloths [207], or the extensive bony armor and limited areas of vulnerable skin in cingulates, a vampire bat would need to be more selective in accessing exposed areas of skin. There was almost certainly some exposed skin on the feet of ground sloths and other large xenarthrans such as glyptodonts and pampatheres. The extinct vampires may also have fed on the skin between movable bands on the carapace of pampatheres and the large extinct species of Dasypus or at the posterior edge of the carapace where it meets the tail or between the bony rings on the tail in those cingulates (See Figure 6). Glyptodonts had a solid carapace with no movable bands and thus would have had no exposed soft tissue on the back but did have soft tissue between the separate bony rings on the tail and between the carapace and tail, much like armadillos and pampatheres. As noted above, this type of feeding behavior, extracting blood from the soft tissue between the movable bands in the carapace and between the bony tail rings, has been documented in modern D. rotundus when feeding on two living species of armadillos, Priodontes maximus and Dasypus novemcinctus.
Why vampire bats evolved in the Miocene from a more typical, presumably omnivorous or insectivorous, basal phyllostomid bat is not readily apparent from the fossil record. Most authors [208,209,210,211,212] have proposed that ancestral phyllostomids and noctilionoids were omnivorous, not strictly insectivorous, based on dental and cranial morphology, although reconstructing their phylogeny is fraught with problems of convergent morphological and molecular evolution [213]. An omnivorous ancestry allowed phyllostomids to diversify into a wide variety of feeding roles, some of which retained omnivory and insectivory but also led to the development of carnivory and sanguinivory (vampire bats), as well as frugivory and nectarivory. By the time vampire bats first appeared in the fossil record in the early Pleistocene, most of the highly derived features of their cranial and postcranial anatomy were already present [10,18,23].
Slaughter [214] proposed that vampire bats may have evolved from a frugivorous bat that had teeth adapted for cutting through the hard rind of fruit. Several other authors have suggested that ancestral vampire bats may have fed on ectoparasites (e.g., ticks) of large mammals, which eventually led to an entirely sanguinivorous diet [208,215,216]. Altenbach [98] suggested that vampire bats initially fed on small terrestrial vertebrates and that terrestrial locomotion developed in response to this mode of feeding. Fenton [217] proposed that feeding on insects or their larvae at wounds of large mammals may have led to the evolution of sanguinivory in vampire bats. His “wound theory” of the origin of blood-feeding in bats took into account certain anatomical features, in particular the robust upper incisors of some insectivorous species in the Phyllostomidae that could have evolved into the sharp, curved, cutting blades observed in living vampires. In addition, Fenton [217] supported his hypothesis noting the flexible feeding strategies that characterize many phyllostomids, and the availability of large prey in the Miocene of South America, including native South American ungulates and xenarthrans. Schutt [201] and Schutt and Simmons [202] (p. 151) proposed the “arboreal omnivore hypothesis” of vampire bat origins. According to this theory, “Miocene protovampires exploited arboreal food sources such as insects, small reptiles, birds, and mammals, much as do some extant phyllostomines (e.g., Vampyrum spectrum). During the mid- to late Miocene, protovampires would have encountered larger and increasingly diverse arboreal vertebrates. Under these conditions, protovampires would have undergone dietary and behavioral changes which allowed them to exploit larger animals as a food source—by feeding only on their blood. Derived conditions for terrestrial blood-feeding (e.g., robust hindlimb bones) may have evolved as the Desmodus…lineage came down from the trees to exploit terrestrial vertebrates.”
We have already discussed the large mammalian prey available to evolving vampire bats in the Miocene in South America. We concentrated on the diversity of xenarthrans in South America, whereas Fenton [217] focused on South American ungulates (SANU), probably because Desmodus rotundus prefers to feed on large ungulates, specifically domestic livestock. An examination of the fossil record demonstrates that South American ungulate diversity had greatly decreased by the time the Interchange occurred in late Miocene and Pliocene/Pleistocene, whereas the diversity of large xenarthrans remained fairly high and stable during this time period in both South America, their continent of origin, and also in North America following the Interchange [15]. Only one SANU, the toxodont Mixotoxodon, reached North America during the Interchange, whereas four families of ground sloths and three families of cingulates had reached temperate North America, in particular Florida, by the early Pleistocene [11,12,15].
6. Extinction of Desmodus draculae and D. stocki
Among the four species of vampire bats recorded from late Pleistocene fossil sites in North America and South America (Appendix A), two are extinct, Desmodus draculae and Desmodus stocki, and two are extant, Desmodus rotundus and Diphylla ecaudata. A third extinct species, Desmodus archaeodaptes, became extinct in the early Pleistocene. We evaluate several hypotheses proposed to explain the Late Quaternary extinction of the two largest species, D. draculae and D. stocki, including climate change and the extinction of their presumed prey base of large mammals. To understand how climate change in the late Pleistocene may have led to the extinction of D. draculae and/or D. stocki, we assess the climatic factors that affect the modern distribution of D. rotundus because it is the most widely distributed of the extant vampires and belongs to the same genus as the two larger, extinct species. D. rotundus is restricted to the New World tropics, where its northern and southern distributional limits closely track the boundaries of the Neotropical region [218,219]. According to McNab [26] (p. 140), “The limits of the present-day distribution [of D. rotundus] in Mexico closely parallel the 10° C minimal isotherm in January.” Based on this temperature limitation, D. rotundus is currently found throughout the lowlands of Mexico to about 28° North latitude on the west coast in the state of Sonora and 25° North on the east coast in the state of Tamaulipas. Temperature isotherms in Mexico are also correlated with the limits in elevational distribution of D. rotundus. Higher elevations (>1000 m) on the Mexican Plateau as far south as central Mexico are cooler than the 10° C minimal winter isotherm and are thus unsuitable for D. rotundus. These temperature limits also apply to South America south of the equator where the distribution of D. rotundus closely parallels the 10° C minimal isotherm for July, with the southern limits of the species in Uruguay, northern Argentina, and central Chile [1,2]. McNab [26] suggested that fossil records of Desmodus beyond the current geographic limits of D. rotundus indicated changes in climate, specifically that warmer temperatures during the Pleistocene allowed the extinct D. stocki to survive farther north than the current northern limit of D. rotundus in Mexico. Van de Vuurst et al. [220] predicted that with ongoing human-caused climate change, the range of D. rotundus could increase northward into the southwestern US and possibly Florida. Indeed, with winter temperatures warmer than the 10° C isotherm and a thriving cattle industry, D. rotundus could almost certainly survive in the southern half of the Florida peninsula at the present time. However, an inhospitable temperature regime for this species (colder than the 10° C isotherm in the winter months) currently exists along the northern Gulf of Mexico from Texas to northern Florida, thus serving as a thermal barrier to a northern extension of its range from northeastern Mexico.
McNab [26] thought the occurrence of the extinct vampire Desmodus stocki in several late Pleistocene fossil localities in Florida north of the l0° C isotherm for January indicated that this isotherm was located about 200 km northward of its present position in Florida during the late Pleistocene. The 10° C winter isotherm currently crosses the Florida peninsula at about the level of Tampa Bay (28° N), whereas the late Pleistocene Arrendondo, Haile, and Reddick localities with D. stocki are between 29° and 30° North in northern Florida (Appendix A). However, McNab [26] did not take into account that D. stocki was larger than D. rotundus and thus may have had a somewhat greater tolerance for cooler temperatures than the smaller living species (but also would have had greater nutrient demands).
Morgan [10] (p. 198) hypothesized that “… the occurrence of D. stocki in areas north of the present range of D. rotundus indicates one of two things: (1) the large extinct vampire bat was able to withstand somewhat cooler winter temperatures than Recent vampires or (2) climatic conditions were different, specifically winter temperatures were warmer in the late Pleistocene.” Measurements in Table 1 confirm that D. stocki was as much as 25% larger than living D. rotundus in postcranial dimensions and presumably was considerably heavier as well. We calculated probable body weights for these bats using the equation ln y = a(ln x) + b and their humeral midshaft diameters (“shaft widths” in Table 2) with the parameters set for humeral midshaft widths by Gunnell et al. [221] (Table 1); slope constant a= 2.32 and y-intercept b = 1.94). The predicted weight range for extant D. rotundus (23.8–52.3 g) based on humerus shaft widths (Table 2) fits well with actual live weights of 15–50 g for this species (Animal Diversity Web). Our predicted weight range for D. stocki is 34.5–81.3 g (Table 2), with the maximum predicted weight of the extinct species about 35% greater than that of D. rotundus. The greater body mass of D. stocki may have allowed this species to withstand somewhat cooler winter temperatures than 10° C, especially considering that almost all fossil records of D. stocki are either from caves (western US and Mexico) or karst deposits that were formerly caves (Florida) that would have ameliorated cooler winter temperatures somewhat (See discussion in McDonald and Jefferson [16]). Caves generally have a relatively constant year-round temperature that approximates the mean annual temperature at a given latitude and elevation [222,223].

Table 2.
Predicted body weights for Desmodus species based on humerus midshaft widths in Table 1 and using the relevant equation and parameters of Gunnell et al. [221].
Studies indicate that Pleistocene climates in temperate North America, particularly during the latest Pleistocene Wisconsin glacial interval, were more equable than at present, with warmer winters and cooler summers [224,225]. D. stocki may have been well suited to the late Pleistocene climate in the warm temperate region of the southern United States, which was by no means tropical or even subtropical, but probably lacked the prolonged winter freezes characteristic of the present climate of this region, excluding peninsular Florida. Webb [226] (p. 23) noted that “Vampire bats (Desmodus) in the “Arredondo Clay” at Reddick 1A, Haile XIB [=11B], and Arrendondo IIA [=2A] suggest warmer winters than presently recorded.” Gut [32] and Brodkorb [227] placed the Florida sites with D. stocki in the Sangamonian interglacial or the Illinoian glacial (=penultimate glacial), both of early Rancholabrean age. The Sangamonian interglacial was characterized by somewhat warmer temperatures than present. Gut [32] (p. 537) stated “The two localities [Reddick 1A and Haile 11B] from which the extinct bat [D. magnus = D. stocki] is known both represent the Illinoian stage of the Pleistocene, whereas none of the numerous vertebrate localities from the later stages of the Pleistocene is known to contain Desmodus.” However, Morgan and Emslie [228] considered these same sites with D. stocki in northern peninsular Florida to be late Pleistocene (late Rancholabrean) in age and characterized by a subtropical climate that permitted the dispersal of numerous tropical mammals and birds from Mesoamerica to Florida along the Gulf Coast Savanna Corridor [226] during the low sea level stand of the Wisconsin glacial. Any warming event, even those of relatively short duration, could in effect have allowed Desmodus to be found in faunas with taxa having a more northern distribution and thus be part of a non-analog fauna [225].
Although the larger size of Desmodus stocki may have afforded this species a greater tolerance for cooler winter temperatures at the northern limits of its range in the southern US, large size among vampire bats is not necessarily correlated with their occurrence in regions with cooler temperatures. The largest known vampire bat, the extinct D. draculae, is known only from late Pleistocene sites in the New World tropics, where its geographic range almost entirely overlaps with that of the living D. rotundus, from the Yucatán peninsula in southern Mexico and Belize south to Venezuela, Brazil, and northern Argentina (Appendix A, Figure 1). The two sites in Argentina with D. draculae are about 400 km farther south than the southernmost current occurrence of D. rotundus [43]. The calculated body weight of D. draculae is 110 g (Table 2), based on the midshaft diameter of a complete humerus of this species from Cueva del Guácharo in Venezuela and using the formula proposed by Gunnell et al. [221]). This is more than twice the body weight of the largest D. rotundus (~50 g) and is a little heavier than the second and third largest New World bats, the big-eared woolly bat Chrotopterus auritus and greater spear-nosed bat Phyllostomus hastatus, but much lighter than the spectral bat Vampyrum spectrum, which is the largest living Western Hemisphere bat (maximum weight ~190 g; Animal Diversity Web). Based on the data presented here, it appears that the relative size of the two large, extinct species of vampire bats was not affected by climatic factors but was more likely related to the size of their preferred prey species.
Another argument against the climate model to explain the extinction of Pleistocene vampire bats is the local extinction or extirpation of D. rotundus in the late Holocene of Cuba [61], long after the major climatic changes in the late Pleistocene. Moreover, Cuba lies south of the Tropic of Cancer (23°27′ N) and would have had a tropical climate throughout the Pleistocene and Holocene, so the local extinction of D. rotundus was not a result of cooler winter temperatures. The disappearance of vampire bats from Cuba has been attributed to the extinction of ground sloths on the island in the late Holocene (~4200 yrBP), the vampire bat’s primary food source, and was not related to climate change [56,142,143,144].
We agree with several previous authors [47,173] who hypothesized that the extinction of Desmodus draculae and D. stocki resulted from the extinction of the Pleistocene megafauna in both North America and South America, species of which were the primary source of blood for these two vampire species. We have further suggested that large mammals (>100 kg) of South American origin, particularly xenarthrans, including ground sloths, large armadillos, pampatheres, and glyptodonts, were the favored prey for these two large extinct species of vampire bats. All ground sloths and all but one species of large (>45 kg), armored cingulates became extinct at the end of the Pleistocene, which led to the extinction of D. draculae and D. stocki. There are only two surviving xenarthrans of appreciable size (~45–100 kg in body mass), both restricted to the Neotropical region, the giant armadillo Priodontes maximus and the giant anteater Myrmecophaga tridactyla. Both occur in South America, and the giant anteater is also found as far north as Honduras and Nicaragua in Central America. The geographic ranges of the giant armadillo and giant anteater overlapped with that of D. draculae in the Pleistocene. Priodontes maximus is known to be a prey species of D. rotundus, as is the smaller anteater Tamandua tetradactyla (see discussion above). Both Priodontes and Myrmecophaga currently live far south of the known late Pleistocene range of D. stocki in the southern US and Mexico, although there is a single early Pleistocene record of Myrmecophaga from the El Golfo de Santa Clara Fauna in the state of Sonora, northwestern Mexico [111].
Data presented here support the extinction of the mammalian megafauna in the late Pleistocene in the New World as the primary factor leading to the extinction of D. draculae and D. stocki. The similarity in timing of the extinction of the megafauna and of these two large vampire species that fed on the large mammals is the best evidence for the correlation between these extinctions. In Cuba, the extinction of the indigenous vampire bat does not occur until the late Holocene, after the indigenous sloths became extinct [143,144]. Also, the fact that extant D. rotundus is present in the late Pleistocene and occurs with D. draculae in several faunas but did not become extinct, strongly suggests the common vampire bat fed on a wider variety of medium-sized and large mammals, much as they do at present. D. rotundus was apparently able to change prey species rather quickly, as indicated by their shift to feeding on non-native domestic livestock within the past 500 years.
Based on the evolutionary history of vampire bats and their faunal associations, we argue that large xenarthrans of South American origin were the primary prey of the two large, extinct species of vampire bats, D. draculae and D. stocki. In the western US, there was a limited fauna of mammals of South American affinity in the late Pleistocene, consisting of three ground sloths, Nothrotheriops shastensis (associated with D. stocki in four US sites), Megalonyx jeffersonii (occurs with D. stocki in two sites), and Paramylodon harlani (not known to be associated with D. stocki in the western US but associated with this extinct vampire bat in three Florida late Pleistocene faunas). While an association of vampire bats with the largest of the extinct ground sloths in North America, the Pan American giant sloth Eremotherium laurillardi, has not been documented, this may be primarily a taphonomic bias because all records of D. stocki are from cave and karst sites (Appendix A) and almost all Rancholabrean records of Eremotherium in North America are from open sites. Large mammals of North American origin, particularly ungulates, including horses, tapirs, peccaries, camels, deer, and gomphotheres, were also likely included in the diet of the extinct vampires, especially in the southern US and Mexico. Several extant species of tapirs (Tapiridae), peccaries (Tayassuidae), and deer (Cervidae) still occur within the former geographic range of D. draculae in southern Mexico, Belize, and South America, suggesting that species in these three families of mammals were probably not important prey of this extinct vampire in those regions. Whether the large xenarthrans and other large mammals from South America and North America became extinct in the latest Pleistocene because of climate change [222] or overkill/overhunting by Paleoindian people [229], we are convinced the large mammal extinctions led to the extinction of D. draculae and D. stocki.
7. Future Research
We recommend two areas of research on the available sample of vampire bat fossils, radiocarbon dating and ancient DNA (aDNA) analyses. We are not aware of any radiocarbon dates taken directly on bones of vampire bats. All of the radiocarbon dates listed in Appendix A are derived from various organic materials (e.g., bones of large mammals, coprolites of ground sloths, organic sediments, etc.) associated with vampire bat fossils, mostly from cave deposits. Direct dates on very small bones, such as those of vampire bats, are now possible using the AMS (accelerator mass spectrometry) radiocarbon dating method [225,230]. It would be highly desirable to obtain AMS 14C dates directly from vampire bat fossils to determine the youngest well-dated occurrences of D. draculae and D. stocki. Radiocarbon dates would also help to confirm our premise that the extant D. rotundus and extinct D. draculae, found together as fossils in several late Pleistocene deposits in the Neotropical region, were contemporaneous. The only records of extinct vampire bats considered to be Holocene in age, including D. draculae from Centinela de Mar, Argentina [42] and D. stocki from San Miguel Island, southern California [38,39], were based on the association of vampire bat fossils with radiocarbon dates on other organic materials. Until radiocarbon dates can be obtained directly from the supposed late Holocene vampire bat fossils, we question these records and suggest they actually may be late Pleistocene in age. It would also be desirable to obtain radiocarbon dates from the extirpated population of D. rotundus in Cuba. Although no bones of the Cuban vampire bat have been dated, late Holocene AMS 14C dates were obtained from bones of another bat species (scapulae of Artibeus jamaicensis) found in close association with a skull of D. rotundus from Cueva de los Nesophontes in northwestern Cuba [61].
A second new area of study of vampire bat fossils involves the analysis of ancient DNA (aDNA). If enough collagen can be recovered from Late Quaternary (late Pleistocene or Holocene) bones of Desmodus to obtain AMS radiocarbon dates, then that same collagen sample could also be used to analyze the aDNA of the bones to determine the possible genomic relationships of the two extinct late Pleistocene species, D. draculae and D. stocki. Another possible use of aDNA analysis would be on a sample of paleoguano from La Tetera Cave in southern Arizona. La Tetera Cave has produced a fairly large fossil sample (35 specimens) of D. stocki, as well as an extensive stratified deposit interpreted to be ancient guano of D. stocki [37]. A sample of the guano deposit yielded a late Pleistocene calibrated radiocarbon date of 23,745 yrBP, which could be interpreted as the only radiocarbon date directly associated with an extinct species of Desmodus, provided the guano was produced by D. stocki. Ancient DNA analysis of the guano could potentially provide the identity of the bat that produced the guano, presumably D. stocki, as well as aDNA from possible prey species of this extinct vampire bat.
8. Conclusions
Evidence from the fossil record and genomic data support our hypothesis that vampire bats (Phyllostomidae: Desmodontinae) evolved in South America in the Miocene between about 20 and 14 Ma. We propose that these early vampire bats would have fed on the blood of large mammals native to South America, including xenarthrans such as ground sloths and glyptodonts and native ungulates such as notoungulates and litopterns. Among these mammal groups of South American origin, the ground sloths Pliometanastes and Thinobadistes participated in the late Miocene phase of the Great American Biotic Interchange (GABI), dispersing to North America about 9 Ma. Additional taxa of South American mammals dispersed to North America in the Pliocene and early Pleistocene from about 5 to 1 Ma, including four families of ground sloths, glyptodonts, pampatheres, and a large armadillo. Given their limited foraging range, vampire bats probably followed these xenarthrans, their favored prey/blood donor species, into North America as participants in the Pliocene-Pleistocene phase of GABI, after the formation of a dry land connection with South America via the Isthmus of Panama at about 5 Ma. The extinct vampire bat Desmodus archaeodaptes is first recorded in North America in the early Pleistocene (~2 Ma) of Florida. A second larger species of Desmodus first appeared in South America in the late Pliocene or early Pleistocene in Uruguay and Venezuela. Two large, extinct species of Desmodus were widely distributed in late Pleistocene cave and karst deposits in the New World: D. stocki from the southern US and northern Mexico and the giant Neotropical vampire D. draculae from southern Mexico and Belize south to Venezuela, Brazil, and Argentina. Both large species of Desmodus became extinct at the end of the Pleistocene, coinciding with the extinction of their primary source of blood, ground sloths and other megafaunal mammals in North and South America. The timing of the extinction of the two large vampires has not been precisely documented due to the lack of radiocarbon dates obtained directly from bones of these species–a focal point for future research. The smaller living vampire bat D. rotundus has a rather extensive late Pleistocene fossil record in the Neotropics, co-occurring with the extinct D. draculae in several sites. Desmodus rotundus did not become extinct in the late Pleistocene because it was a generalist, feeding on a wide variety of medium-sized to large species of mammals that also survive to the present, and now supplementing its diet with the blood of livestock introduced by Europeans about 500 years ago.
Author Contributions
Conceptualization, G.M. and H.G.M.; methodology, G.M.; validation, G.M., H.G.M., N.J.C.; formal analysis, G.M., H.G.M., N.J.C.; investigation, G.M., H.G.M., N.J.C.; resources, G.M., H.G.M., N.J.C.; writing—original draft preparation, G.M.; writing, G.M., H.G.M., N.J.C.; visualization, G.M. and N.J.C.; supervision, G.M.; project administration, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Most of our data on fossil vampire bats and their association with extinct Pleistocene mammals from North American and South America are presented here. Any additional data on these topics are freely available and may be obtained directly from the authors.
Acknowledgments
H.G.M. and G.M. are indebted to the late S. David Webb for instilling in us a lifelong interest in the Great American Biotic Interchange. We were both Webb’s graduate students at the University of Florida in the mid-1970s during the time when he was formulating his innovative ideas on the faunal dynamics of the Interchange. G.M. thanks Ann Pratt for helping to collect a large fossil sample of Desmodus stocki from the Reddick 1 site in Florida, and Pierce Brodkorb, Michael Frazier, and H. James Gut, all now deceased, for collecting vampire bat fossils from several Pleistocene sites in Florida. Rachel Narducci of the Florida Museum of Natural History loaned us a sample of limb bones of Desmodus stocki for comparative analysis and photography. Omar Linares and Clayton Ray were instrumental in helping G.M. further his interest in the fossil record of vampire bats, and in providing him the opportunity to work with them in the description of Desmodus draculae, collected by Linares in Venezuela. N.J.C. is indebted to William D. Peachey for mentoring, training in caving skills, and opportunities to investigate vampire fossils in Arizona. We are very grateful to Lucius Toll for taking the photos of bat limb bones in Figure 6. Joe Cook and Jonathan Dunnum of the Museum of Southwestern Biology, University of New Mexico, loaned us several bat skeletons for photography.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Localities of vampire bats from Quaternary fossil sites in North America and South America. The fossil vampire bat site records are assigned a number, and those numbered sites are located on the map in Figure 1. The fossil sites are listed alphabetically by country and alphabetically by site name within a country, except for the U.S. where the sites are alphabetical by state and then alphabetical by sites within a state, except Florida where the sites are listed chronologically from oldest to youngest. A standard set of data, where available, are provided for each record of a fossil vampire bat, including: latitude, longitude, and elevation; site type (e.g., cave, karst deposit, open site); age; associated genera or species of large mammals (>45 kg) of South American origin; and pertinent references. Extinct species are indicated by a dagger (†). Abbreviations: Co. (County); PBDB (Paleobiology Database); E (early); L (late); N (north); S (south); W (west); yrBP (years before present for radiocarbon dates); 14C (radiocarbon).
Table A1.
Localities of vampire bats from Quaternary fossil sites in North America and South America. The fossil vampire bat site records are assigned a number, and those numbered sites are located on the map in Figure 1. The fossil sites are listed alphabetically by country and alphabetically by site name within a country, except for the U.S. where the sites are alphabetical by state and then alphabetical by sites within a state, except Florida where the sites are listed chronologically from oldest to youngest. A standard set of data, where available, are provided for each record of a fossil vampire bat, including: latitude, longitude, and elevation; site type (e.g., cave, karst deposit, open site); age; associated genera or species of large mammals (>45 kg) of South American origin; and pertinent references. Extinct species are indicated by a dagger (†). Abbreviations: Co. (County); PBDB (Paleobiology Database); E (early); L (late); N (north); S (south); W (west); yrBP (years before present for radiocarbon dates); 14C (radiocarbon).
| Locality | Latitude, Longitude, & Elevation | Site Type | Age | Vampire Bat Species | Associated Large Mammals of South American Origin (Genus/Species) 1 | References |
| Argentina | ||||||
| 1. Centinela del Mar Buenos Aires Province | 38°26′ S 58°13′ W | open | L. Holocene | † Desmodus cf. draculae | None | [42] |
| 2. La Ballenera Buenos Aires Province | 38° 19′ S 57° 56′ W | cave | L. Pleistocene Lujanian | † Desmodus draculae | Mylodontidae Glyptodon reticulatus | [43] |
| Belize | ||||||
| 3. Cebada Cave Chiquibul Cave System Cayo District | cave | L Pleistocene Rancholabrean | † Desmodus draculae | None | [16] | |
| Brazil | ||||||
| 4. Gruta dos Brejões Bahia State | cave | L. Pleistocene; 14C date of 12,200 yrBP 2 | Desmodus rotundus | Catonyx cuvieri Eremotherium laurillardi, Glossotherium aff. lettsomi, Glossotherium robustum, Mylodon darwini, Mylodon ebseni, Ocnotherium giganteum Nothrotherium maquinense Myrmecophaga tridactyla Pampatherium humboldtii Coendou sp. Xenorhinotherium bahiense | [13,48] | |
| 5. Lapa da Lagoa do Sumidouro, near Lagoa Santa Minas Gerais State | 19°38′ S 43°53′ W | cave | L. Pleistocene or Holocene | Desmodus rotundus | [18,231] | |
| 6. Santana Cave Ribeira River Valley São Paulo State | 24°32′ S 48°42′ W | cave | L. Pleistocene | † Desmodus draculae | Eremotherium Scelidotherium (from other nearby caves) | [47] |
| 7. Serra da Mesa Goiás State | cave | L. Pleistocene or Holocene | Desmodus rotundus | none | [49] | |
| 8. Toca da Barriguda Bahia State | cave | Desmodus rotundus Diphylla ecaudata | Nothrotherium maquinense, Tamandua tetradactyla, Coendou magnus | [13] | ||
| 9. Toca da Boa Vista Bahia State | 600 m | cave | L. Pleistocene | †Desmodus draculae Desmodus rotundus Diphylla ecaudata | Catonyx cuvieri Nothrotherium maquinense Myrmecophaga tridactyla Euphractus sexcinctus Coendou prehensilis Cartelles coimbrafilhoi Caipora bambuiorum | [13,46,50,51,52,53] |
| 10. Toca do Gordo do Garrincho, Serra da Capivara Piauí State | cave | L. Pleistocene or Holocene | Desmodus rotundus | [54] | ||
| 11. Toca dos Ossos Bahia State | cave | L. Pleistocerne | † Desmodus draculae | Catonyx cuvieri, Eremotherium laurillardi, Glossotherium aff. lettsomi, Mylodonopsis ibseni, Nothrotherium maquinense, Ocnotherium giganteum, Glyptodon clavipes, Pampatherium humboldtii, Myrmecophaga tridactyla, Hydrochoerus hydrochaeris, Neochoerus sulcidens, Toxodon platensis, Trigonodops lopesi | [46,50,51,52,53] | |
| Cuba | ||||||
| 12. Cuevas Blancas Habana Province | 22°53′ N 82°19′ W | cave | Holocene 7864 ± 96 yrBP | Desmodus rotundus | None | [59,60] |
| 13. Cueva Centenario de Lenin Villa Clara Province | 22°24′ N 79°01′ W | cave | Holocene | Desmodus rotundus (=D. puntajudensis type locality) | [57,58,60] | |
| 14. Cueva Lamas Habana Province | 23°45′ N 82°32′ W | cave | L. Pleistocene or Holocene | Desmodus rotundus | Megalocnus rodens, Mesocnus torrei | [56,60] |
| 15. Cueva de los Nesophontes Habana-Matanzas Provinces | cave | L. Holocene | Desmodus rotundus | None | [61] | |
| 16. Cueva de Paredones Habana Province | cave | L. Pleistocene or Holocene | Desmodus rotundus | [58,60] | ||
| Mexico | ||||||
| 17. Cerro de Tlapacoya Estado de Mexico | 19°18′ N 98°55′ W 2240 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Neochoerus aesopi | [18,173,189,193] |
| 18. Cueva de la Boca Nuevo Leon | 25°25′ N 100°09′ W 540 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | None | [40,175] |
| 19. Cueva de la Presita 3 San Luis Potosí | 23°30′ N 100°37′ W 1540 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Nothrotheriops shastensis | [16,18,173,188] |
| 20. Cueva de San Josecito (=San Josecito Cave) Nuevo León | 24°06′ N 99°49′ W 2250 m | cave | L. Pleistocene Rancholabrean | † Desmodus stocki (type locality) | Megalonyx jeffersonii, Nothrotheriops shastensis | [16,18,31,33,173,191] |
| 21. Gruta de Loltún (=Loltún Cave) Yucatán | 20°15′ N 89°28′ W 40 m | cave | L. Pleistocene | †Desmodus draculae Desmodus rotundus Diphylla ecaudata | None | [18,45,62,194] |
| Peru | ||||||
| 22. Jatun Uchco Departamento de Huánuco | cave | Pleistocene | Desmodus sp. | Diablotherium, Megatherium, Scelidodon | [55] | |
| United States | ||||||
| Arizona | ||||||
| 23. Arkenstone Cave Pima Co. | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | None | [36] | |
| 24. La Tetera Cave Pima Co. | cave | L. Pleistocene Rancholabrean 23,745 yrBP | † Desmodus stocki | None | [37] | |
| 25. Rampart Cave Mohave Co. | 36°06′ N 113°56′ W | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Nothrotheriops shastensis | [16,18,181] |
| California | ||||||
| 26 Potter Creek Cave Shasta Co. | 40°47′ N 122°17′ W | cave | L. Pleistocene Rancholabrean | † Desmodus stocki | Nothrotheriops shastensis Megalonyx jeffersonii | [16,33,232] |
| 27. San Miguel Island, Santa Barbara Co | 34° N 120°20′ W | cave arch. site 3 | Holocene ~3–5000 yr BP | † Desmodus stocki | none | [38,39] |
| Florida | ||||||
| 28. Inglis 1A Citrus Co. | 29°01′ N 82°41′ W El.: sea level | karst fissure/ sinkhole | E. Pleistocene L. Blancan | † Desmodus archaeodaptes | Titanis walleri Eremotherium eomigrans Megalonyx leptostomus Paramylodon harlani Dasypus bellus Glyptotherium texanum Holmesina floridanus Neochoerus sp. | [10,11,23] |
| 29. Haile 16A Alachua Co. | 29°41′ N 82°34′ W El.: 30 m | karst fissure/ sinkhole | E. Pleistocene E. Irvingtonian | † Desmodus archaeodaptes | Eremotherium eomigrans Megalonyx wheatleyi Paramylodon harlani Dasypus bellus Holmesina floridanus Pachyarmatherium leiseyi | [10,11,23] |
| 30. Haile 21A Alachua Co. | 29°41′ N 82°35′ W El.: 30 m | karst fissure/ sinkhole | E. Pleistocene E. Irvingtonian | † Desmodus archaeodaptes (type locality) | Eremotherium eomigrans Dasypus bellus | [10,11,23] |
| 31. Arredondo 2A Alachua Co. | 29°37′ N 82°24′ W El.: 30 m | karst fissure/ sinkhole | L. Pleistocene Rancholabrean | † Desmodus stocki | Paramylodon harlani Dasypus bellus | [10,11,18,227] |
| 32. Haile 1A Alachua Co. | 29°41′ N 82°34′ W El.: 30 m | karst fissure/ sinkhole | L. Pleistocene Rancholabrean | † Desmodus stocki | none | [10,11,32] |
| 33. Haile 11B Alachua Co. | 29°41′N’ 82°34′W El.: 30 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki | Dasypus bellus | [18,33] |
| 34. Reddick 1A Marion Co. | 29°22′ N 82°11′ W El.: 22 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki (type locality of † D. magnus) | Megalonyx jeffersonii Paramylodon harlani Dasypus bellus Holmesina septentrionalis | [10,11,32,233] |
| 35. Reddick 1B Marion Co. | 29°22′ N 82°11′ W El.: 22 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki | Megalonyx jeffersonii Paramylodon harlani Dasypus bellus | [10] |
| 36 Reddick 1C Marion Co. | 29°22′ N 82°11′ W El.: 22 m | karst fissure/ sinkhole | L Pleistocene Rancholabrean | † Desmodus stocki | none | [10] |
| New Mexico | ||||||
| 37. U-Bar Cave Hidalgo Co. | 31°29′ N 108°26′ W El.: 1570 m | cave | L. Pleistocene Rancholabrean 26,150–35,890 yrBP (14C dates) | † Desmodus stocki | Nothrotheriops shastensis | [16,18,35,183,184] |
| Texas | ||||||
| 38. Sierra Diablo Cave Hudspeth Co. | El.: 1660 m | cave | L. Pleistocene Rancholabrean ~35,000 yrBP | † Desmodus stocki | Nothrotheriops shastensis | [35] |
| 39 Terlingua Brewster Co. | 29°19′ N 103°31′ W | mine/ fissure | L. Pleistocene | † Desmodus stocki | none | [185,186] |
| West Virginia | ||||||
| 40 New Trout Cave Pendleton Co. | 38°39’ N 79°23′ W El.: 570 m | cave | L. Pleistocene Rancholabrean >29,400 yrBP | † Desmodus stocki | Megalonyx jeffersonii | [18,34,175,176,232] |
| Uruguay | ||||||
| 41. Kiyú locality Raigón Formation San José Department | open site | L. Pliocene or E. to M. Pleistocene | † Desmodus aff. draculae | medium to large ground sloths, glyptodonts, litopterns, notoungulates, dinomyid rodents, and large phorusrhacid birds | [24] | |
| Venezuela | ||||||
| 42. Cueva de la Brújula Miranda State | 10.45° N 66.77° W | cave | Holocene | Desmodus rotundus | none | [18,234] |
| 43. Cueva del Guácharo Monagas State | 10°10’ N’ 62°33′ W | cave | L. Pleistocene/Holocene | †Desmodus draculae (type locality) Desmodus rotundus Diphylla ecaudata | none | [18,23] |
| 44 Cueva de Quebrada Honda Aragua State | 09°56′ N 67°15′ W | cave | Holocene | Desmodus rotundus | none | [18,235] |
| 45. El Breal de Orocual Monagas State | open site tar pit | L. Pliocene to E. Pleistocene | cf. Desmodus sp. | Eremotherium sp., Megalonychidae, Propraopus sulcatus, Glyptodon sp., Hoplophorus sp., Pachyarmatherium cf. leiseyi, Holmesina occidentalis, Pampatherium humboldtii, cf. Myrmecophaga, cf. Chapalmatherium, Mixotoxodon larensis | [17,25] |
1 Associated genera and species of large mammals of North American origin are discussed in the text. 2 A braincase of Desmodus rotundus from Gruta dos Brejões was adhered to the underside of ground sloth coprolite with a 14C age of 12,200 radiocarbon yr [13]. 3 The record of Desmodus stocki from Cueva de La Presita was listed by Ray et al. [18] from “Matehuala (cave near)”, now known as Cueva de La Presita [188,192].
References
- Koopman, K.F. Systematics and Distribution. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 7–17. [Google Scholar]
- Greenhall, A.M.; Joermann, G.; Schmidt, U. Desmodus rotundus. Mammal. Spec. 1983, 202, 1–6. [Google Scholar] [CrossRef]
- Greenhall, A.M. Feeding behavior. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 111–131. [Google Scholar]
- Webb, S.D. Mammalian faunal dynamics of the Great American Interchange. Paleobiology 1976, 2, 220–234. [Google Scholar] [CrossRef]
- Webb, S.D. A history of savanna vertebrates in the New World. Part II: South America and the Great Interchange. Ann. Rev. Ecol. Syst. 1978, 9, 393–426. [Google Scholar] [CrossRef]
- Webb, S.D. Late Cenozoic mammal dispersals between the Americas. In The Great American Biotic Interchange; Stehli, F.G., Webb, S.D., Eds.; Plenum Press: New York, NY, USA, 1985; pp. 357–386. [Google Scholar]
- Webb, S.D. Ecogeography and the Great American Interchange. Paleobiology 1991, 17, 266–280. [Google Scholar] [CrossRef]
- Webb, S.D. The Great American Biotic Interchange: Patterns and processes. Ann. Missouri Bot. Gard. 2006, 93, 245–257. [Google Scholar] [CrossRef]
- Stehli, F.G.; Webb, S.D. (Eds.) The Great American Biotic Interchange; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Morgan, G.S. Neotropical Chiroptera from the Pliocene and Pleistocene of Florida. Bull. Amer. Mus. Natl. Hist. 1991, 206, 176–213. [Google Scholar]
- Morgan, G.S. The Great American Biotic Interchange in Florida. Bull. Florida Mus. Nat. Hist. 2005, 45, 271–311. [Google Scholar] [CrossRef]
- Morgan, G.S. Vertebrate fauna and geochronology of the Great American Biotic Interchange in North America. New Mexico Mus. Nat. Hist. Sci. Bull. 2008, 44, 93–140. [Google Scholar]
- Czaplewski, N.J.; Cartelle, C. Pleistocene bats from cave deposits in Bahia, Brazil. J. Mammal. 1998, 79, 784–803. [Google Scholar] [CrossRef]
- Czaplewski, N.J.; Krejca, J.; Miller, T.E. Late Quaternary Bats from Cebada Cave, Chiquibul Cave System, Belize. Carib. J. Sci. 2003, 39, 23–33. [Google Scholar]
- McDonald, H.G. Paleoecology of extinct xenarthrans and the Great American Biotic Interchange. Bull. Florida Mus. Nat. Hist. 2005, 45, 313–333. [Google Scholar]
- McDonald, H.G.; Jefferson, G.T. Distribution of Pleistocene Nothrotheriops (Xenarthra, Nothrotheriidae) in North America. Nat. Hist. Mus. LA Co. Sci. Ser. 2008, 41, 313–331. [Google Scholar]
- Czaplewski, N.J.; Rincón, A.D. A giant vampire bat (Phyllostomidae, Desmodontinae) from the Pliocene-Pleistocene El Breal de Orocual asphaltic deposits (tar pits), Venezuela. Hist. Biol. 2020, 33, 2438–2443. [Google Scholar] [CrossRef]
- Ray, C.E.; Linares, O.J.; Morgan, G.S. Paleontology. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 19–30. [Google Scholar]
- Koopman, K.F. Zoogeography. In Biology of Bats of the New World Family Phyllostomatidae, Part 1; Baker, R.J., Jones, J.K., Jr., Carter, D.C., Eds.; Texas Tech Press: Lubbock, TX, USA, 1976; pp. 39–47. [Google Scholar]
- Koopman, K.F. Biogeography of the bats of South America. In Mammalian Biology in South America; Mares, M.A., Genoways, H.H., Eds.; Pymatuning Laboratory of Ecology at the University of Pittsburgh: Linesville, PA, USA, 1982; Volume 6, pp. 273–302. [Google Scholar]
- Baker, R.J.; Bininda-Emonds, O.R.P.; Mantilla-Meluk, H.; Porter, C.A.; van den Bussche, R.A. Molecular time scale of diversification of feeding strategy morphology in New World leaf-nosed bats (Phyllostomidae): A phylogenetic perspective. In Evolutionary History of Bats: Fossils, Molecules and Morphology; Gunnell, G.F., Simmons, N.B., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 385–409. [Google Scholar]
- Rojas, D.; Warsi, O.M.; Dávalos, L.M. Bats (Chiroptera: Noctilionoidea) challenge a recent origin of extant Neotropical diversity. Syst. Biol. 2016, 65, 432–448. [Google Scholar] [CrossRef]
- Morgan, G.S.; Linares, O.J.; Ray, C.E. New species of fossil vampire bats (Mammalia, Chiroptera, Desmodontidae) from Florida and Venezuela. Proc. Biol. Soc. Washington 1988, 101, 912–928. [Google Scholar]
- Ubilla, M.; Gaudioso, P.; Perea, D. First fossil record of a bat (Chiroptera, Phyllostomidae) from Uruguay (Plio-Pleistocene, South America): A giant desmodontine. Hist. Biol. 2021, 33, 137–145. [Google Scholar] [CrossRef]
- Rincón, A.D.; Parra, G.E.; Prevosti, F.J.; Alberdi, M.T.; Bell, C.J. A preliminary assessment of the mammalian fauna from the Pliocene-Pleistocene El Breal de Orocual locality, Monagas state, Venezuela. Mus. North. Arizona Bull. 2009, 65, 593–620. [Google Scholar]
- McNab, B.K. Energetics and the distribution of vampires. J. Mammal. 1973, 54, 131–144. [Google Scholar] [CrossRef]
- Kurtén, B.; Anderson, E. Pleistocene Mammals of North America; Columbia University Press: New York, NY, USA, 1980; 442p. [Google Scholar]
- Martin, R.A. Fossil mammals from the Coleman IIA Fauna, Sumter County. In Pleistocene Mammals of Florida; Webb, S.D., Ed.; University Presses of Florida: Gainesville, FL, USA, 1974; pp. 35–99. [Google Scholar]
- Morgan, G.S. The extinct free-tailed bat Tadarida constantinei and associated vertebrates from Pleistocene deposits in Slaughter Canyon Cave, Carlsbad Caverns National Park, southeastern New Mexico. New Mexico Geol. 2003, 25, 43. [Google Scholar]
- Morgan, G.S.; Lucas, S.G. Pleistocene vertebrates from southeastern New Mexico. New Mex. Geol. Surv. Guideb. 2006, 57, 317–335. [Google Scholar]
- Jones, J.K. Pleistocene bats from San Josecito Cave, Nuevo Leon, Mexico. Univ. Kansas Publ. Mus. Nat. Hist. 1958, 9, 389–396. [Google Scholar]
- Gut, H.J. A Pleistocene vampire bat from Florida. J. Mammal. 1959, 40, 534–538. [Google Scholar] [CrossRef]
- Hutchison, J.H. A Pleistocene vampire bat (Desmodus stocki) from Potter Creek Cave, Shasta County, California. PaleoBios 1967, 3, 1–6. [Google Scholar]
- Grady, F.; Arroyo-Cabrales, J.; Garton, E.R. The northernmost occurrence of the Pleistocene vampire bat Desmodus stocki Jones (Chiroptera: Phyllostomatidae: Desmodontinae) in Eastern North America. Smithson. Contrib. Paleobiol. 2002, 93, 73–75. [Google Scholar]
- Harris, A.H. Pleistocene vertebrates of Southwestern USA and Northwestern Mexico. Available online: www.utep.edu/leb/PleistNM/ (accessed on 1 July 2025).
- Czaplewski, N.J.; Peachey, W.D. Late Pleistocene bats from Arkenstone Cave, Arizona. Southwest. Natur. 2003, 48, 597–609. [Google Scholar] [CrossRef]
- Czaplewski, N.J.; Mead, J.I.; Peachey, W.D. Late Pleistocene vertebrate fauna and bat guano deposit of La Tetera Cave, Arizona, USA. J. Cave Karst Stud. 2025. [Google Scholar]
- Guthrie, D.A. Analysis of avifaunal and bat remains from midden sites on San Miguel Island. In The California Islands: Proceedings of a Multidisciplinary Symposium; Power, D.M., Ed.; Santa Barbara Museum of Natural History: Santa Barbara, CA, USA, 1980; pp. 689–702. [Google Scholar]
- Guthrie, D.A. Fossil Vertebrates from Pleistocene Terrestrial Deposits on the Northern Channel Islands, Southern California. In Contributions to the Geology of the Northern Channel Islands, Southern California; Pacific Section, American Association of Petroleum Geologists (AAPG): Bakersfield, CA, USA, 1998; pp. 187–192. [Google Scholar]
- Hall, E.R. The Mammals of North America, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1981; Volume 1, 1181p. [Google Scholar]
- Suzan, A.G. Common Vampire Bat, Desmodus rotundus. In Mammals of Mexico; Ceballos, G., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2014; pp. 688–689. [Google Scholar]
- Pardiñas, U.F.J.; Tonni, E.P. A giant vampire (Mammalia, Chiroptera) in the Late Holocene from the Argentinean pampas: Paleoenvironmental significance. Palaeogeo. Palaeoclimat. Palaeoecol. 2000, 160, 213–221. [Google Scholar] [CrossRef]
- Brizuela, S.; Tassara, D.A. New record of the vampire Desmodus draculae (Chiroptera) from the late Pleistocene of Argentina. Ameghiniana 2021, 58, 169–176. [Google Scholar] [CrossRef]
- Arroyo-Cabrales, J.; Alvarez, T. Restos Óseos de Murciélagos (Orden Chiroptera) Procedentes de las Excavaciones Arqueológicas en las Grutas de Loltún, Yucatán, México; Instituto Nacional de Antropología e Historia: Mexico City, Mexico, 1990; Volume 194, pp. 1–103. [Google Scholar]
- Arroyo-Cabrales, J.; Alvarez, T. A Preliminary Report of the Late Quaternary Mammal Fauna from Loltún Cave, Yucatán, Mexico. In Ice Age Cave Faunas of North America; Schubert, B.W., Mead, J.I., Graham, R.W., Eds.; Indiana University Press: Bloomington, IN, USA, 2003; pp. 262–272. [Google Scholar]
- Cartelle, C.; Abuhid, V.S. Chiroptera do Pleistoceno final-Holoceno da Bahia. Acta Geol. Leopold. 1994, 39, 429–440. [Google Scholar]
- Trajano, E.; de Vivo, M. Desmodus draculae Morgan, Linares and Ray, 1988, reported for Southeastern Brazil, with paleoecological comments (Phyllostomidae, Desmodontinae). Mammalia 1991, 55, 456–459. [Google Scholar]
- Barleto, E.A.; de Souza, H.N.; Lessa, A.G. Conservação do patrimônio paleontológico, arqueológico, e cultural na Apa Gruta de Brejões/Vereda do Romão Gramacho–BA. In Proceedings of the 29th Brazilian Congress of Speleology, Ouro Preto, Brazil, 7–10 June 2007; pp. 39–46. [Google Scholar]
- Fracasso, M.P.A.; Salles, L.O. Diversity of Quaternary bats from Serra da Mesa (State of Goiás, Brazil). Zootaxa 2005, 817, 1–19. [Google Scholar] [CrossRef]
- Cartelle, C. Edentata e Megamamíferos Herbívoros Extintos da Toca dos Ossos (Ourolândia, BA, Brasil). Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 1992. [Google Scholar]
- Cartelle, C. A fauna local de mamíferos pleistocênicos da Toca da Boa Vista (Laje dos Negros, BA). Ph.D. Thesis, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 1995. [Google Scholar]
- Cartelle, C. Pleistocene Mammals of the Cerrado and Caatinga of Brazil. In Mammals of the Neotropics: The Central Neotropics; Eisenberg, J.B., Redford, K.H., Eds.; University of Chicago Press: Chicago, IL, USA, 1999; pp. 27–46. [Google Scholar]
- Auler, A.S.; Piló, L.B.; Smart, P.L.; Wang, X.; Hoffmann, D.; Richards, D.A.; Edwards, R.L.; Neves, W.A.; Cheng, H. U-series dating and taphonomy of Quaternary vertebrates from Brazilian caves. Palaeogeog. Palaeoclimat. Palaeoecol. 2006, 240, 508–522. [Google Scholar] [CrossRef]
- Hadler, P.; Mayer, E.L.; Motta, F.; Ribeiro, A.M. Fossil bats from the Quaternary of Serra da Capivara, northeast Brazil. Quat. Internat. 2018, 464, 411–416. [Google Scholar] [CrossRef]
- Shockey, B.; Salas Gismondi, R.; Baby, P.; Guyot, L.P.; Baltazar, M.; Huamán, L.; Clack, A.; Stucchi, M.; Pujos, F.; Emerson, J.; et al. New Pleistocene cave faunas of the Andes of Central Perú: Radiocarbon ages and the survival of low latitude Pleistocene DNA. Palaeontol. Elect. 2009, 12, 15. [Google Scholar]
- Koopman, K.F. A fossil vampire bat from Cuba. Breviora 1958, 90, 1–4. [Google Scholar]
- Wołoszyn, B.W.; Mayo, N.A. Postglacial remains of a vampire bat (Chiroptera: Desmodus) from Cuba. Acta Zool. Cracoviensia 1974, 19, 253–265. [Google Scholar]
- Suárez, W. Taxonomic status of the Cuban vampire bat (Chiroptera: Phyllostomidae: Desmodontinae: Desmodus). Carib. J. Sci. 2005, 41, 761–767. [Google Scholar]
- Jiménez, O.; Condis, M.M.; García, E. Vertebrados post-glaciales en un residuario fósil de Tyto alba scopoli (Aves: Tytonidae) en el occidente de Cuba. Rev. Mexicana Mastozool. 2005, 9, 84–111. [Google Scholar]
- Orihuela, J. Skull variation of the vampire bat Desmodus rotundus (Chiroptera: Phyllostomidae): Taxonomic implications for the Cuban fossil vampire bat Desmodus puntajudensis. Chirop. Neotrop. 2011, 17, 963–976. [Google Scholar]
- Orihuela, J. Late Holocene fauna from a cave deposit in western Cuba: Post-Columbian occurrence of the vampire Desmodus rotundus (Phyllostomidae: Desmodontinae). Carib. J. Sci. 2012, 46, 297–312. [Google Scholar] [CrossRef]
- Arroyo-Cabrales, J.; Ray, C.E. Revisión de los vampiros fósiles (Chiroptera: Phyllostomidae: Desmodontinae) de México. In Homenaje al Profesor Ticul Álvarez; Arroyo-Cabrales, J., Polaco, O.J., Eds.; Instituto Nacional de Antropología e Historia (INAH): Mexico City, Mexico, 1997; Volume 357, pp. 69–86. [Google Scholar]
- Mendoza, Z.; Xiong, Z.; Escalera-Zamudio, M.; Runge, A.K.; Thézé, J.; Streicker, D.; Frank, H.K.; Loza-Rubio, E.; Liu, S.; Ryder, O.A.; et al. Hologenomic adaptations underlying the evolution of sanguivory in the common vampire bat. Nat. Ecol. Evol. 2018, 2, 659–668. [Google Scholar] [CrossRef]
- Blumer, M.; Brown, T.; Freitas, M.B.; Destro, A.L.; Oliveira, J.A.; Morales, A.E.; Schell, T.; Greve, C.; Pippel, M.; Jebb, D.; et al. Gene losses in the common vampire bat illuminate molecular adaptations to blood feeding. Sci. Adv. 2022, 8, eabm6494. [Google Scholar] [CrossRef] [PubMed]
- Teeling, E.C.; Springer, M.S.; Madsen, O.; Bates, P.; O’Brien, S.J.; Murphy, W.J. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 2005, 307, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.K. Review of the origins and biogeography of bats in South America. Chirop. Neotrop. 2009, 15, 391–410. [Google Scholar]
- Morgan, G.S.; Czaplewski, N.J. Evolutionary history of the Neotropical Chiroptera: The fossil record. In Evolutionary History of Bats: Fossils, Molecules, and Morphology; Gunnell, G.F., Simmons, N.B., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 105–161. [Google Scholar] [CrossRef]
- Simmons, N.B.; Gunnell, G.F.; Czaplewski, N.J. Fragments and Gaps: The fossil Record. In Phyllostomid Bats: A Unique Mammalian Radiation; Fleming, T.H., Dávalos, L.M., Mello, M.A.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2020; pp. 63–86. [Google Scholar] [CrossRef]
- Morgan, G.S.; Czaplewski, N.J.; Rincon, A.F.; Bloch, J.I.; Wood, A.R.; MacFadden, B.J. A new early Miocene bat (Chiroptera: Phyllostomidae) from Panama confirms middle Cenozoic chiropteran dispersals between the Americas. J. Mammal. Evol. 2023, 30, 963–993. [Google Scholar] [CrossRef]
- Czaplewski, N.J. Colhuehuapian bats (Mammalia: Chiroptera) from the Gran Barranca, Chubut province, Argentina. In The Paleontology of Gran Barranca: Evolution and Environmental Change Through the Middle Cenozoic of Patagonia; Madden, R.H., Carlini, A.A., Vucetich, M.G., Kay, R.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 240–252. [Google Scholar]
- Baker, R.J.; Solari, S.A.; Cirranello, A.; Simmons, N.B. Higher level classification of phyllostomid bats with a summary of DNA synapomorphies. Acta Chirop. 2016, 18, 1–38. [Google Scholar] [CrossRef]
- McKenna, M.C.; Bell, S.K. Classification of Mammals Above the Species Level; Columbia University Press: New York, NY, USA, 1997; 631p. [Google Scholar]
- McDonald, H.G.; Vizcaíno, S.F.; Bargo, M.S. Fossil Vermilinguas-An Overview. In The Biology of the Xenarthra; Vizcaíno, S.F., Loughry, J., Eds.; University of Florida Press: Gainesville, FL, USA, 2008; pp. 64–78. [Google Scholar]
- Delsuc, F.; Gibb, G.C.; Kuch, M.; Billet, G.; Hautier, L.; JSouthon, J.; Rouillard, J.-M.; Fernicola, J.C.; Vizcaíno, S.F.; MacPhee, R.D.E.; et al. The phylogenetic affinities of the extinct glyptodonts. Cur. Biol. 2016, 26, R141–R156. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Scanferla, A.; Soibelzon, E.; Bonini, R.; Ochoa, J.; Cooper, A. Ancient DNA from the extinct South American giant glyptodont Doedicurus sp. (Xenarthra: Glyptodontidae) reveals that glyptodonts evolved from Eocene armadillos. Molec. Ecol. 2016, 25, 3499–3508. [Google Scholar] [CrossRef]
- Fariña, R.A.; Vizcaíno, S.F.; Bargo, M.S. Body mass estimations in Lujanian (late Pleistocene-early Holocene of South America) mammal megafauna. Mastozool. Neotrop. 1998, 5, 87–108. [Google Scholar]
- Kramarz, A.G.; MacPhee, R.D.E. Did some extinct South American native ungulates arise from an afrothere ancestor? A critical reappraisal of Avilla and Mothé’s (2021) Sudamericingulata-Panameridungulata hypothesis. J. Mammal. Evol. 2022, 30, 67–77. [Google Scholar] [CrossRef]
- Lundelius, E.L.; Bryant, V.M., Jr.; Mandel, R.; Thies, K.J.; Thoms, A. The first occurrence of a toxodont (Mammalia, Notoungulata) in the United States. J. Vert. Paleontol. 2013, 33, 229–232. [Google Scholar] [CrossRef]
- O’Dea, A.; Lessios, H.A.; Coates, A.G.; Eytan, R.I.; Restrepo-Moreno, S.A.; Cione, A.L.; Collins, L.S.; de Queiroz, A.; Farris, D.W.; Norris, R.D.; et al. Formation of the Isthmus of Panama. Sci. Adv. 2016, 2, e1600883. [Google Scholar] [CrossRef] [PubMed]
- Woodburne, M.O. The Great American Biotic Interchange: Dispersals, tectonics, climate, sea level and holding pens. J. Mammal. Evol. 2010, 17, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Hirschfeld, S.E.; Webb, S.D. Plio-Pleistocene megalonychid sloths of North America. Bull. Fla. State Mus. Biol. Sci. 1968, 12, 213–296. [Google Scholar] [CrossRef]
- Hirschfeld, S.E. Pliometanastes protistus (Edentata: Megalonychidae) from Knight’s Ferry, California. PaleoBios 1981, 36, 1–16. [Google Scholar]
- McDonald, H.G.; Morgan, G.S. Ground sloths of New Mexico. New Mexico Mus. Nat. Hist. Sci. Bull. 2011, 53, 652–663. [Google Scholar]
- McDonald, H.G. Fossil Xenarthra of Mexico: A review. In Avances en los Estudios Paleomastozoológicos en México; Montellano Ballesteros, M., Arroyo-Cabrales, J., Eds.; Serie Arqueológica; Instituto Nacional de Antropología e Historia (INAH): Mexico City, Mexico, 2002; pp. 227–248. [Google Scholar]
- Webb, S.D. Osteology and Relationships of Thinobadistes segnis, the First mylodont Sloth in North America. In Advances in Neotropical Mammalogy; Redford, K.H., Eisenberg, J.F., Eds.; Sandhill Crane Press: Gainesville, FL, USA, 1989; pp. 469–532. [Google Scholar]
- Laurito, C.A.; Valerio, A.L. Primer registro fósil de Pliometanastes sp. (Mammalia, Xenarthra, Megalonychidae) para el Mioceno Superior de Costa Rica, América Central. Una nueva pista en la comprensión del Pre-GABI. Rev. Geol. Amér.Cent. 2012, 47, 95–108. [Google Scholar] [CrossRef]
- Valerio, A.L.; Laurito, C.; McDonald, H.G.; Rincón, A.D. Megalonychid sloths from the Early Late Hemphillian (Late Miocene), Curré Formation, San Gerardo de Limoncito, Costa Rica. Rev. Geol. Amér. Cent. 2022, 66, 1–15. [Google Scholar] [CrossRef]
- Laurito, C.A.; Valerio, A.L. Scirrotherium antelucanus, una nueva especie de Pampatheriidae (Mammalia, Xenarthra, Cingulata) del Mioceno Superior de Costa Rica, América Central. Rev. Geol. Amér. Cent. 2013, 49, 45–62. [Google Scholar] [CrossRef]
- McDonald, H.G.; Carranza-Castañeda, O. Increased xenarthran diversity of the Great American Biotic Interchange: A new genus and species of ground sloth (Mammalia, Xenarthra, Megalonychidae) from the Hemphillian (late Miocene) of Jalisco, Mexico. J. Paleontol. 2017, 91, 1069–1082. [Google Scholar] [CrossRef]
- Baskin, J.A.; Valenciano, A. Procyonidae (Mammalia, Carnivora) and the Great American Biotic Interchange. In Windows into Sauropsid and Synapsid Evolution; Essays in Honor of Prof. Louis L. Jacobs; Lee, Y.-N., Ed.; Dinosaur Science Center Press: Republic of Korea, 2023; pp. 341–365. [Google Scholar]
- Campbell, K.E.; Frailey, C.D.; Romero-Pittman, L. The Late Miocene Gomphothere Amahuacatherium peruvium (Proboscidea: Gomphotheriidae) from Amazonian Peru: Implications for the Great American Faunal Interchange; Série D, Estudios Regionales; Instituto de Geología, Minería y Metalurgia: Lima, Peru, 2000; Volume 23, pp. 1–152. [Google Scholar]
- Frailey, C.D.; Campbell, K.E. Two new genera of peccaries (Mammalia, Artiodactyla, Tayassuidae) from Upper Miocene deposits of the Amazon Basin. J. Paleontol. 2012, 86, 852–877. [Google Scholar] [CrossRef]
- Prothero, D.R.; Campbell, K.E.; Beatty, B.L.; Frailey, C.D. New late Miocene dromomerycine artiodactyl from the Amazon Basin: Implications for Interchange dynamics. J. Paleontol. 2014, 88, 423–443. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.G. The palaeobiogeography of South American gomphotheres. J. Palaeogeog. 2013, 2, 19–40. [Google Scholar]
- Mothé, D.; Avilla, L. Mythbusting evolutionary issues on South American Gomphotheriidae (Mammalia: Proboscidea). Quat. Sci. Rev. 2015, 110, 23–35. [Google Scholar] [CrossRef]
- Gasparini, G.M.; Parisi Dutra, R.; Perini, F.A.; Croft, D.A.; Cozzuol, M.A.; Missagia, R.V.; Lucas, S.G. On the supposed presence of Miocene Tayassuidae and Dromomerycinae (Mammalia, Cetartiodactyla) in South America. Amer. Mus. Novitates 2021, 3968, 1–27. [Google Scholar] [CrossRef]
- Campbell, K.E., Jr.; Heizler, M.; Frailey, C.D.; Romero-Pittman, L.; Prothero, D.R. Upper Cenozoic chronostratigraphy of the south-western Amazon Basin. Geology 2001, 29, 595–598. [Google Scholar] [CrossRef]
- Altenbach, J.S. Locomotor Morphology of the Vampire Bat, Desmodus Rotundus; Special publication No. 6; American Society of Mammalogists: Pittsburgh, PA, USA, 1979; pp. 1–137. [Google Scholar]
- Altenbach, J. Locomotion. In Natural History of Vampire Bats; Greenhall, A.M., Schmidt, U., Eds.; CRC Press: Boca Raton, FL, USA, 1988; pp. 71–83. [Google Scholar]
- Goodwin, G.G.; Greenhall, A.M. A review of the bats of Trinidad and Tobago. Bull. Amer. Mus. Nat. Hist. 1961, 122, 187–302. [Google Scholar]
- Koopman, K.F. Land bridges and ecology of bat distribution on islands of the northern coast of South America. Evolution 1958, 12, 429–439. [Google Scholar] [CrossRef]
- MacPhee, R.D.; Iturralde-Vinent, M. Orgin of the Greater Antillean land mammal fauna, 1: New Tertiary fossils from Cuba and Puerto Rico. Amer. Mus. Novitates 1995, 3141, 1–31. [Google Scholar]
- MacPhee, R.D.E.; Iturralde-Vinent, M. First Tertiary land mammal from Greater Antilles: An early Miocene sloth (Xenarthra, Megalonychidae) from Cuba. Amer. Mus. Novitates 1994, 3094, 1–42. [Google Scholar]
- Viñola-Lopez, L.W.; Suárez, E.E.C.; Vélez-Juarbe, J.; Milan, J.N.A.; Bloch, J.I. The oldest known record of a ground sloth (Mammalia, Xenarthra, Folivora) from Hispaniola: Evolutionary and paleobiogeographical implications. J. Paleontol. 2022, 96, 684–691. [Google Scholar] [CrossRef]
- Morgan, G.S. Patterns of Extinction in West Indian Bats. In Biogeography of the West Indies: Patterns and Perspectives, 2nd ed.; Woods, C.A., Sergile, F.E., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 369–407. [Google Scholar]
- Velazco, P.M.; O’Neill, H.; Gunnell, G.F.; Cooke, S.B.; Rimoli, R.; Rosenberger, A.L.; Simmons, N.B. Quaternary bat diversity in the Dominican Republic. Amer. Mus. Novitates 2013, 3779, 1–20. [Google Scholar] [CrossRef]
- Brodkorb, P. A giant flightless bird from the Pleistocene of Florida. Auk 1963, 80, 111–115. [Google Scholar] [CrossRef]
- Gould, G.C.; Quitmyer, I.R. Titanis walleri: Bones of contention. Bull. Florida Mus. Nat. Hist. 2005, 45, 201–229. [Google Scholar] [CrossRef]
- Baskin, J.A. The giant flightless bird Titanis walleri (Aves: Phorusrhacidae) from the Pleistocene coastal plain of south Texas. J. Vert. Paleontol. 1995, 15, 842–844. [Google Scholar] [CrossRef]
- MacFadden, B.J.; Labs-Hochstein, J.; Hulbert, R.C., Jr.; Baskin, J.A. Revised age of the Late Neogene terror bird (Titanis) in North America during the Great American Interchange. Geology 2007, 35, 123–126. [Google Scholar] [CrossRef]
- Shaw, C.A.; McDonald, H.G. First record of giant anteater (Xenarthra, Myrmecophagidae) in North America. Science 1987, 236, 186–188. [Google Scholar] [CrossRef]
- Webb, S.D.; Perrigo, S.C. Late Cenozoic vertebrates from Honduras and El Salvador. J. Vert. Paleontol. 1984, 4, 237–254. [Google Scholar] [CrossRef]
- Cisneros, J.C. New Pleistocene vertebrate fauna from El Salvador. Rev. Brasileira Paleontol. 2005, 8, 239–255. [Google Scholar] [CrossRef]
- Cisneros, J.C. The fossil mammals of El Salvador. New Mexico Mus. Nat. Hist. Sci. Bull. 2008, 44, 375–380. [Google Scholar]
- Dávila, S.L.; Stinnesbeck, S.R.; Gonzalez, S.; Lindauer, S.; Escamilla, J.; Stinnesbeck, W. Guatemala’s Late Pleistocene (Rancholabrean) fauna: Revision and interpretation. Quat. Sci. Rev. 2019, 219, 277–296. [Google Scholar] [CrossRef]
- Lucas, S.G.; Garcia, R.; Espinosa, E.; Alvarado, G.E.; Hurtado de Mendoza, L.; Vega, E. The fossil mammals of Nicaragua. New Mexico Mus. Nat. Hist. Sci. Bull. 2008, 44, 417–429. [Google Scholar]
- Lucas, S.G.; Alvarado, G.E.; Vega, E. The Pleistocene mammals of Costa Rica. J. Vert. Paleontol. 1997, 17, 413–427. [Google Scholar] [CrossRef]
- Lucas, S.G. Late Pleistocene mammals from El Hatillo, Panamá. Rev. Geol. Amér. Cent. 2014, 50, 139–151. [Google Scholar]
- Polaco, O.J.; Guzmán, A.F.; Ramírez, G.T. Occurrence of toxodonts in the Pleistocene of Mexico. Cur. Res. Pleist. 2004, 21, 113–115. [Google Scholar]
- Mead, J.I.; Baez, A.; Swift, S.L.; Carpenter, M.C.; Hollenshead, M.; Czaplewski, N.J.; Steadman, D.W.; Bright, J.; Arroyo-Cabrales, J. Tropical marsh and savanna of the Late Pleistocene in northeastern Sonora, Mexico. Southwest. Nat. 2006, 51, 226–239. [Google Scholar] [CrossRef]
- Carbot-Chanona, G.; Eng-Ponce, J.; Gomez-Perez, L.E. Description of Neochoerus specimens from the late Pleistocene (Rancholabrean) of Chiapas, and comments on the taxonomic identity of the fossil capybaras from other Mexican localities. Bol. Soc. Geol. Mexicana 2020, 72. [Google Scholar] [CrossRef]
- Ahearn, M.E. A Revision of the North American Hydrochoeridae. Master’s Thesis, University of Florida, Gainesville, FL, USA, 1981; 99p. [Google Scholar]
- Sanders, A.E. Additions to the Pleistocene mammal faunas of South Carolina, North Carolina, and Georgia. Trans. Amer. Phil. Soc. 2002, 92, 1–152. [Google Scholar] [CrossRef]
- Baskin, J.A.; Gervais, P.D.; Gervais, C.J. A late Pleistocene capybara (Rodentia, Caviidae, Hydrochoerinae) from near Houston, Texas, USA, with a brief review of North American fossil capybaras. Proc. Acad. Nat. Sci. Phila. 2020, 167, 57–68. [Google Scholar] [CrossRef]
- Carranza-Castañeda, O. Roedores caviomorphos (Rodentia Hydrochoeridae) del Blancano temprano-tardío–Irvingtoniano de los estados de Guanajuato, Jalisco y Sonora, México: Relación con Phugatherium dichroplax. Rev. Mexicana Cienc. Geol. 2016, 33, 297–315. [Google Scholar]
- Ahearn, M.E.; Lance, J.F. A new species of Neochoerus (Rodentia: Hydrochoeridae) from the Blancan (late Pliocene) of North America. Proc. Biol. Soc. Wash. 1980, 93, 435–442. [Google Scholar]
- Carranza-Castañeda, O.; Miller, W.E. Roedores caviomorfos de la Mesa Central de México, Blancano Temprano (Plioceno Tardío) de la Fauna Local Rancho Viejo, Estado de Guanajuato. Univ. Nac. Autó. México Inst. Geol. Rev. 1988, 7, 182–199. [Google Scholar]
- Hulbert, R.C., Jr. A new early Pleistocene tapir (Mammalia: Perissodactyla) from Florida, with a review of Blancan tapirs from the state. Bull. Fla. Mus. Nat. Hist. 2010, 49, 67–126. [Google Scholar] [CrossRef]
- Vucetich, M.G.; Deschamps, C.M.; Pérez, M.E. The first capybaras (Rodentia, Caviidae, Hydrochoerinae) involved in the Great American Biotic Interchange. Ameghiniana 2015, 52, 324–333. [Google Scholar] [CrossRef]
- Webb, S.D.; Perrigo, S.C. New Megalonychid Sloths from El Salvador. In The Evolution and Ecology of Armadillos, Sloths and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1985; pp. 113–120. [Google Scholar]
- McDonald, H.G.; Chatters, J.C.; Gaudin, T.J. A new genus of megalonychid ground sloth (Mammalia, Xenarthra) from the late Pleistocene of Quintana Roo, Mexico. J. Vert. Paleontol. 2017, 37, e1307206. [Google Scholar] [CrossRef]
- McDonald, H.G.; Arroyo-Cabrales, J.; Alarcón-Durán, I.; Espinosa-Martínez, D.V. First record of Meizonyx salvadorensis (Mammalia: Xenarthra: Pilosa) from the late Pleistocene of Mexico and its evolutionary implications. J. Syst. Palaeontol. 2020, 18, 1829–1851. [Google Scholar] [CrossRef]
- Stinnesbeck, S.R.; Frey, E.; Stinnesbeck, W. New insights on the paleogeographic distribution of the Late Pleistocene ground sloth genus Xibalbaonyx along the Mesoamerican Corridor. J. South Amer. Ear. Sci. 2018, 85, 108–120. [Google Scholar] [CrossRef]
- MacPhee, R.D.E.; White, J.L.; Woods, C.A. New megalonychid sloths (Phyllophaga, Xenarthra) from the Quaternary of Hispaniola. Amer. Mus. Novitates 2000, 3303, 1–32. [Google Scholar] [CrossRef]
- White, J.L.; MacPhee, R.D.E. The sloths of the West Indies: A Systematic and Phylogenetic Review. In Biogeography of the West Indies: Patterns and Perspectives; Woods, C.A., Sergile, F.E., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 201–236. [Google Scholar]
- Iturralde-Vinent, M.A.; MacPhee, R.D.E. New evidence for late Eocene-early Oligocene uplift of Aves Ridge and paleogeography of GAARlandia. Geol. Acta 2023, 21, 1–10. [Google Scholar] [CrossRef]
- McDonald, H.G. The Shasta Ground Sloth Nothrotheriops shastensis (Xenarthra, Megatheriidae) in the Middle Pleistocene of Florida. In The Evolution and Ecology of Armadillos, Sloths, and Vermilinguas; Montgomery, G.G., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1985; pp. 95–104. [Google Scholar]
- McDonald, H.G. Gravigrade xenarthrans from the early Pleistocene Leisey Shell Pit 1A, Hillsborough County, Florida. Bull. Fla. Mus. Nat. Hist. 1995, 37, 345–373. [Google Scholar] [CrossRef]
- Cassiliano, M.L. Biostratigraphy of Blancan and Irvingtonian mammals in the Fish Creek-Vallecito Creek section, southern California, and a review of the Blancan-Irvingtonian boundary. J. Vert. Paleontol. 1999, 19, 169–186. [Google Scholar] [CrossRef]
- Croxen, F.W., III; Shaw, C.A.; Sussman, D.R. Pleistocene geology and paleontology of the Colorado River Delta at Golfo de Santa Clara, Sonora, Mexico. In Proceedings of the 2007 Desert Symposium; Reynolds, R.E., Ed.; Studies Consortium and LSA Associates, Inc.: Santa Ana, CA, USA; California State University: Long Beach, CA, USA, 2007; pp. 84–89. [Google Scholar]
- De Iuliis, G.; McDonald, H.G.; Stanchly, N.; Spenard, J.; Powis, T.G. Nothrotheriops shastensis (Sinclair) from Actun Lak: First record of Nothrotheriidae (Mammalia, Xenarthra, Pilosa) from Belize. Ameghiniana 2015, 52, 153–171. [Google Scholar] [CrossRef]
- Morgan, G.S.; Woods, C.A. Extinction and the zoogeography of West Indian land mammals. Biol. J. Linn. Soc. 1986, 28, 167–203. [Google Scholar] [CrossRef]
- Steadman, D.W.; Martin, P.S.; MacPhee, R.D.E.; Jull, A.J.T.; McDonald, H.G.; Woods, C.A.; Iturralde-Vinent, M.; Hodgins, G.W. Asynchronous extinction of late Quaternary sloths on continents and islands. Proc. Natl. Acad. Sci. USA 2005, 102, 11763–11768. [Google Scholar] [CrossRef]
- MacPhee, R.D.E.; Iturralde-Vinent, M.A.; Jiménez Vázquez, O. Prehistoric sloth extinctions in Cuba: Implications of a new “Last” Appearance Date. Carib. J. Sci. 2007, 43, 94–98. [Google Scholar] [CrossRef]
- Bobrowiec, P.E.D.; Lemes, M.R.; Gribel, R. Prey preference of the common vampire bat (Desmodus rotundus, Chiroptera) using molecular analysis. J. Mammal. 2015, 96, 54–63. [Google Scholar]
- Carter, G.; Brown, B.; Razik, I.; Ripperger, S. Penguins, Falcons, and Mountain Lions: The Extraordinary Host Diversity of Vampire Bats. In 50 Years of Bat Research; Lim, B.K., Fenton, M.B., Brigham, R.M., Mistry, S., Kurta, A., Gillam, E.H., Russell, A., Ortega, J., Eds.; Springer: Cham, Switzerland, 2021; pp. 151–170. [Google Scholar] [CrossRef]
- Bennett, M.R.; Bustos, D.; Pigati, J.S.; Springer, K.B.; Urban, T.M.; Holliday, V.T.; Reynolds, S.C.; Budka, M.; Honke, J.S.; Hudson, A.M.; et al. Evidence of humans in North America during the Last Glacial Maximum. Science 2021, 373, 1528–1531. [Google Scholar] [CrossRef]
- Ríos-Solís, J.A.; López-Acosta, J.C.; MacSwiney, M.C. Potential attack of the common vampire bat (Desmodus rotundus) on nine-banded armadillo (Dasypus novemcinctus) in northern Oaxaca, México. Therya Not. 2021, 2, 147–150. [Google Scholar] [CrossRef]
- De Oliveira, M.B.; de Andrade, H.S.F.; Cordeiro, J.L.P.; de Oliveira, L.F.B. Potential feeding event of Priodontes maximus (Cingulata: Dasypodidae) by Desmodus rotundus (Chiroptera: Desmodontinae) in the Cerrado, Western Brazil. Not. Mamífer. Sudamer. 2022, 4, 2–10. [Google Scholar] [CrossRef]
- Kays, R. Candid Creatures: How Camera Traps Reveal the Mysteries of Nature; Johns Hopkins University Press: Baltimore, MD, USA, 2016; 261p. [Google Scholar]
- Carranza, J.; Campo, D.R. Incidencias del murciélago hematófago Desmodus rotundus sobre los indígenas Yanomami de Venezuela. Doñana Acta Vertebr. 1982, 7, 113. [Google Scholar]
- Carranza, J. Murciélago hematófago Desmodus rotundus parasitando a un chiguire Hydrochoerus hydrochaeris. Doñana Acta Vertebr. 1982, 9, 414–415. [Google Scholar]
- Gonçalves, F.M.; Magioli, M.; Bovendorp, R.S.; de Barros Ferraz, K.M.P.M.; Cagnoni, L.B.; Moreira, M.Z.; Galetti, M. Prey choice of the common vampire bat on introduced species in an Atlantic Forest land-bridge island. Acta Chiropt. 2020, 22, 167–174. [Google Scholar] [CrossRef]
- Mann Fischer, G. Biología del vampiro: Biol. Trabajo. Inst. Biol. “Juan Noe”, Santiago de Chile, Univ. Chile 1951, 12–13, 3–24. [Google Scholar]
- Catenazzi, A.; Donnelly, M.A. Sea lion Otaria flavescens as host of the common vampire bat Desmodus rotundus. Marine Ecol. Prog. Ser. 2008, 360, 285–289. [Google Scholar] [CrossRef]
- Barquez, R.M.; Mares, M.A.; Braun, J.K. The Bats of Argentina; Special publications no. 42; Museum of Texas Tech University: Lubbock, TX, USA, 1999; pp. 1–275. [Google Scholar]
- Galetti, M.; Pedrosa, F.; Keuroghlian, A.; Sazima, I. Liquid lunch–vampire bats feed on invasive feral pigs and other ungulates. Front. Ecol. Environ. 2016, 14, 505–506. [Google Scholar] [CrossRef]
- Gnocchi, A.P.; Srbek-Araujo, A.C. Common Vampire Bat (Desmodus rotundus) feeding on Lowland Tapir (Tapirus terrestris) in an Atlantic Forest remnant in southeastern Brazil. Biota Neotrop. 2017, 17. [Google Scholar] [CrossRef]
- Zortéa, M.; Silva, D.A.; Calaça, A.M. Susceptibility of targets to the vampire bat Desmodus rotundus are proportional to their abundance in Atlantic Forest fragments? Iheringia. Sér. Zool. 2018, 108, 2015–2018. [Google Scholar] [CrossRef]
- Castellanos, A.X.; Banegas, G.A. Vampire bats bite lowland tapirs in Yasuni National Park, Ecuador. Tapir Conserv. 2015, 24, 7. [Google Scholar]
- Voigt, C.C.; Kingston, T. Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Sánchez-Cordero, V.; Botello, F.; Magaña-Cota, G.; Iglesias, J. Vampire bats, Desmodus rotundus, feeding on white-tailed deer, Odocoileus virginianus. Mammalia 2011, 75, 91–92. [Google Scholar] [CrossRef]
- Tello-Mera, E.L.; Mandujano, S. Primer registro fotográfico de murciélagos hematófagos Desmodus rotundus (Chiroptera: Phyllostomidae) alimentándose de Odocoileus virginianus (Artiodactyla: Cervidae) en la Reserva de la Biosfera Tehuacán-Cuicatlán, México. Mammal. Notes 2016, 3, 17–19. [Google Scholar] [CrossRef]
- Silveira, L.; de Almeida Jácomo, A.T.; Malzoni Furtado, M.; Mundim Torres, N.; Sollmann, R.; Vynn, G. Ecology of the giant armadillo (Priodontes maximus) in the grasslands of central Brazil. Edentata 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Greenhall, A.M.; Schmidt, U. (Eds.) Natural History of Vampire Bats; CRC Press: Boca Raton, FL, USA, 1988; 246p. [Google Scholar]
- Borroto-Paez, R.; Mancina, C.A. Biodiversity and conservation of Cuban mammals: Past, present, and invasive species. J. Mammal. 2017, 98, 964–985. [Google Scholar] [CrossRef]
- Dondas, A.; Isla, F.I.; Carballido, J.L. Paleocaves exhumed from the Miramar Formation (Ensenadan Stage-age, Pleistocene), Mar del Plata, Argentina. Quat. Internat. 2009, 210, 44–50. [Google Scholar] [CrossRef]
- Frank, H.T.; Althaus, C.E.; Dario, E.M.; Tramontina, F.R.; Adriano, R.M.; de Lima Almeida, M.; Ferreira, G.F.; Nogueira, R.; Breier, R. Underground chamber systems excavated by Cenozoic ground sloths in the state of Rio Grande do Sul, Brazil. Rev. Bras. Paleontol. 2015, 18, 273–284. [Google Scholar] [CrossRef]
- Pereira Lopes, R.; Frank, H.T.; Sekiguchi de Carvalho Buchmann, F.; Caron, F. Megaichnus igen. nov.: Giant paleoburrows attributed to extinct Cenozoic mammals from South America. Ichnos 2016, 24, 133–145. [Google Scholar] [CrossRef]
- Audi, C.; Meyer, D.; Yeuw, T.T.; Barrionuevo Baraldo, K.; Fey, J.D.; Spanghero, N.F.; Schereiber Munhoz, M.; de Oliveira, B.J.; Sekiguchi de Carvalho Buchmann, F. Fotogrametria de um icnofóssil escavado por preguiças-gigantes (Megaichnus major). Rev. Bras. Paleontol. 2022, 25, 208–218. [Google Scholar] [CrossRef]
- de Lazaro, E. 100,000-year-old fossil of giant vampire bat found in Argentina. Sci News, 26 July 2021. Available online: https://www.sci.news./paleontology/desmodus-draculae-fossil-09898.html (accessed on 10 July 2025).
- Agenbroad, L.D. Giants and Pygmies: Mammoths of Santa Rosa Island, California (USA). Quat. Internat. 2012, 255, 2–8. [Google Scholar] [CrossRef]
- Arroyo-Cabrales, J.; Polaco, O.J. Fossil bats from Mesoamerica. Arch. Mus. Nac. Rio J. 2008, 66, 155–160. [Google Scholar]
- De Iuliis, G.; Cartelle, C. A new giant megatheriine ground sloth (Mammalia: Xenarthra: Megatheriidae) from the late Blancan to early lrvingtonian of Florida. Zool. J. Linn. Soc. 1999, 47, 495–515. [Google Scholar] [CrossRef]
- Grady, F.; Garton, E.R. Pleistocene fauna from New Trout Cave. Capital Area Cav. Bull. 1982, 1, 62–69. [Google Scholar]
- Grady, F.; Garton, E.R. Paleontology and historic field trip of the John Guilday Cave Preserve (Trout Rock). West Virginia Speleo. Surv. Bull. 2000, 14, 241–244. [Google Scholar]
- Guilday, J.E.; McCrady, S. Armadillo remains from Tennessee and West Virginia caves. Nat. Speleo. Soc. Bull. 1966, 28, 183–184. [Google Scholar]
- Wilson, R.W. Preliminary study of the fauna of Rampart Cave, Arizona. Contrib. Paleontol. Carnegie Inst. Publ. 1942, 530, 169–185. [Google Scholar]
- Mead, J.I. The last 30,000 years of faunal history within the Grand Canyon, Arizona. Quat. Res. 1981, 15, 311–326. [Google Scholar] [CrossRef]
- Mead, J.I.; Tweet, J.S.; Santucci, V.L.; Tobin, B.; Chambers, C.L.; Thomas, S.C.; Carpenter, M.C. Chapter 11. Pleistocene/Holocene cave fossils from Grand Canyon National Park: Ice Age (Pleistocene) flora, fauna, environments, and climate of the Grand Canyon, Arizona. In Grand Canyon National Park Centennial Paleontological Resource Inventory (Non-Sensitive Version); Santucci, V.L., Tweet, J.S., Eds.; Natural Resource Report. NPS/GRCA/NRR–2020/2103; U.S. Department of the Interior, National Park Service, Natural Resource Stewardship and Science: Fort Collins, CO, USA, 2020; pp. 403–463. [Google Scholar]
- Carpenter, M.C. Late Pleistocene Aves, Chiroptera, Perissodactyla, and Artiodactyla from Rampart Cave, Arizona. Master’s Thesis, Northern Arizona University, Flagstaff, AZ, USA, 2004. [Google Scholar]
- Hodnett, J.P.; White, R.S.; Carpenter, M.; Mead, J.I.; Santucci, V.L. Miracinonyx trumani (Carnivora: Felidae) from the Rancholabrean of the Grand Canyon, Arizona and its implications for the ecology of the “American Cheetah”. New Mexico Mus. Nat. Hist. Sci. Bull. 2022, 88, 157–185. [Google Scholar]
- Harris, A.H. Preliminary report on the vertebrate fauna of U-Bar Cave, Hidalgo County, New Mexico. New Mexico Geol. 1985, 7, 74–77+84. [Google Scholar] [CrossRef]
- Harris, A.H. Reconstruction of Mid-Wisconsin environments in southern New Mexico. Nat. Geog. Res. 1987, 3, 142–151. [Google Scholar]
- Ray, C.E.; Wilson, D.E. Evidence for Macrotus californicus from Terlingua, Texas. Occ. Pap. Mus. Texas Tech Univ. 1979, 57, 1–10. [Google Scholar]
- Cockerell, T.D.A. An apparently extinct Euglandina from Texas. Proc. Colorado Mus. Nat. Hist. 1930, 9, 52–53. [Google Scholar]
- Arroyo-Cabrales, J. Sinopsis de los murciélagos fósiles de Mexico. Rev. Soc. Mexicana Paleontol. 1992, 5, 1–14. [Google Scholar]
- Arroyo-Cabrales, J.; Polaco, O.J. Caves and the Pleistocene vertebrate paleontology of Mexico. In Ice Age Cave Faunas of North America; Schubert, B.W., Mead, J.I., Graham, R.W., Eds.; Indiana University Press: Bloomington, IN, USA, 2003; pp. 273–291. [Google Scholar]
- Alvarez, T. Nuevo registro para el vampiro del Pleistoceno Desmodus stocki de Tlapacoya, Mexico. An. Esc. Nac. Cienc. Biol. Mexico 1972, 19, 163–165. [Google Scholar]
- Arroyo-Cabrales, J.; Johnson, E.; Cruz, J.A. San Josecito Cave and its paleoecological contributions for Quaternary studies in Mexico. Quaternary 2021, 4, 34. [Google Scholar] [CrossRef]
- Stock, C. The Cave of San Josecito, Mexico: New Discoveries of the Vertebrate Life of the Ice Age. 1943. Available online: https://calteches.library.caltech.edu/87/1/Stock.pdf (accessed on 10 July 2025).
- Polaco, O.J.; Butrón, M.L. Mamíferos Pleistocenicos de la Cueva La Presita, San Luís Potosí, Mexico. In Homenaje al Profesor Ticul Alvarez; Arroyo-Cabrales, J., Polaco, O.J., Eds.; Instituto Nacional de Antropología e Historia: Mexico City, Mexico, 1997; pp. 279–296. [Google Scholar]
- Alvarez, T. Restos fósiles de mamíferos de Tlapacoya, Estado de Mexico (Pleistoceno-Reciente). Univ. Kansas Mus. Nat. Hist. Misc. Publ. 1969, 51, 93–112. [Google Scholar]
- Alvarez, T. Restos de mamíferos recientes y pleistocénicos procedentes de la Grutas de Loltún, Yucatán, Mexico. Inst. Nac. Antropol. Hist. Depart. Prehist. Cuad. Trab. 1982, 29, 7–35. [Google Scholar]
- Stinnesbeck, S.R.; Frey, E.; Olguín, J.A.; Stinnesbeck, W.; Zell, P.; Mallison, H.; González, A.G.; Núñez, E.A.; Morlet, A.V.; Mata, A.T.; et al. Xibalbaonyx oviceps, a new megalonychid ground sloth (Folivora, Xenarthra) from the late Pleistocene of the Yucatán Peninsula, Mexico, and its paleobiogeographic significance. PalZ 2017, 91, 245–271. [Google Scholar] [CrossRef]
- Stinnesbeck, S.R.; Stinnesbeck, W.; Frey, E.; Avíles Olguín, J.; González González, A. Xibalbaonyx exinferis n. sp. (Megalonychidae), a new Pleistocene ground sloth from the Yucatán Peninsula, Mexico. Hist. Biol. 2020, 33, 1952–1963. [Google Scholar] [CrossRef]
- Churcher, C.S. Pleistocene mammals from Extinction Cave, Belize. Canadian J. Ear. Sci. 2020, 57, 366–376. [Google Scholar] [CrossRef]
- Larmon, J.T.; McDonald, H.G.; Ambrose, S.; DeSantis, L.R.G.; Lucero, L.J. A year in the life of a giant ground sloth during the Last Glacial Maximum in Belize. Sci. Adv. 2019, 5, eaau1200. [Google Scholar] [CrossRef]
- Alves-Silva, L.; Cherkinsky, A.; Dantas, M.A.T. Late Pleistocene mammals from northeastern Brazil caves: Taxonomy, radiocarbon dating, isotopic paleoecology (δ13C), and paleoenvironment reconstruction (δ13C, δ18O). Quat. Internat. 2023, 668, 7–13. [Google Scholar] [CrossRef]
- Schutt, W.A., Jr.; Hermanson, J.W.; Chang, Y.H.; Cullinane, D.; Altenbach, J.S.; Muradali, F.; Bertram, J.E.A. Functional morphology of the common vampire bat, Desmodus rotundus. J. Exp. Biol. 1997, 200, 3003–3012. [Google Scholar] [CrossRef]
- Schutt, W.A., Jr. The Chiropteran Hindlimb Morphology and the Origin of Blood Feeding in Bats. In Bat Biology and Conservation; Kunz, T.H., Racey, P.A., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 157–168. [Google Scholar]
- Schutt, W.A., Jr.; Simmons, N.B. Quadrupedal Bats: Form, Function, and Evolution. In Functional and Evolutionary Ecology of Bats; Zubaid, A., McCracken, G.F., Kunz, T.H., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 145–159. [Google Scholar]
- Riskin, D.K.; Hermanson, J.W. Biomechanics: Independent evolution of running in vampire bats. Nature 2005, 434, 292. [Google Scholar] [CrossRef]
- Riskin, D.K.; Parsons, S.; Schutt, W.A., Jr.; Carter, G.G.; Hermanson, J.W. Terrestrial locomotion of the New Zealand short-tailed bat Mystacina tuberculata and the common vampire bat Desmodus rotundus. J. Exp. Biol. 2006, 209, 1725–1736. [Google Scholar] [CrossRef]
- Crespo, R.F.; Burns, R.J.; Linhart, S.B. Load-lifting capacity of the vampire bat. J. Mammal. 1970, 51, 627–629. [Google Scholar] [CrossRef]
- Jones, M.F.; Hasiotis, S.T. Terrestrial behavior and trackway morphology of Neotropical bats. Acta Chiropt. 2018, 20, 229–250. [Google Scholar] [CrossRef]
- McDonald, H.G. An overview of the presence of osteoderms in sloths: Implications for osteoderms as a plesiomorphic character of the Xenarthra. J. Mammal. Evol. 2018, 25, 485–493. [Google Scholar] [CrossRef]
- Gillette, D.D. Evolution of feeding strategies in bats. Tebiwa 1975, 18, 39–48. [Google Scholar]
- Ferrarezzi, H.; Gimenez, E.A. Systematic patterns and the evolution of feeding habits in Chiroptera (Archonta: Mammalia). J. Comp. Biol. 1996, 1, 75–94. [Google Scholar]
- Rojas, D.; Vale, A.; Ferrero, V.; Navarro, L. When did plants become important to leaf-nosed bats? Diversification of feeding habits in the family Phyllostomidae. Mol. Ecol. 2011, 20, 2217–2228. [Google Scholar] [CrossRef]
- Dumont, E.R.; Dávalos, L.M.; Goldberg, A.; Voigt, C.C.; Rex, K.; Santana, S.E. Morphological innovation, diversification and the invasion of a new adaptive zone. Proc. Roy. Soc. London B 2012, 279, 1797–1805. [Google Scholar] [CrossRef]
- Yohe, L.R.; Velazco, P.M.; Rojas, D.; Gerstner, B.E.; Simmons, N.B.; Dávalos, L.M. Bayesian hierarchical models suggest oldest known plant-visiting bat was omnivorous. Biol. Let. 2015, 11, 20150501. [Google Scholar] [CrossRef]
- Dávalos, L.M.; Cirranello, A.L.; Geisler, J.H.; Simmons, N.B. Understanding phylogenetic incongruence: Lessons from phyllostomid bats. Biol. Rev. 2012, 87, 991–1024. [Google Scholar] [CrossRef]
- Slaughter, B.H. Evolutionary Trends of Chiropteran Dentitions. In About Bats; Slaughter, B.H., Walton, D.W., Eds.; Southern Methodist University Press: Dallas, TX, USA, 1970; pp. 51–83. [Google Scholar]
- Turner, D.C. The Vampire Bat: A Field Study in Behavior and Ecology; Johns Hopkins University Press: Baltimore, MD, USA, 1975; 145p. [Google Scholar]
- Hill, J.E.; Smith, J.D. Bats: A Natural History; British Museum (Natural History): London, UK, 1984; 243p. [Google Scholar]
- Fenton, M.B. Wounds and the origin of blood-feeding in bats. Biol. J. Linnean Soc. 1992, 47, 161–171. [Google Scholar] [CrossRef]
- Morrone, J.J. Neotropical Biogeography: Regionalization and Evolution; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G.; Carmona, A.; Arana, M.; Mercado-Gómez, J.D. Biogeographic regionalization of the Neotropical region: New map and shapefile. An. Acad. Bras. Cien. 2022, 94, e20211167. [Google Scholar] [CrossRef] [PubMed]
- Van de Vuurst, P.; Qiao, H.; Soler-Tovar, D.; Escobar, L.E. Climate change linked to vampire bat expansion and rabies virus spillover. Ecography 2023, 2024, e06714. [Google Scholar] [CrossRef] [PubMed]
- Gunnell, G.F.; Worsham, S.R.; Seiffert, E.R.; Simons, E.L. Vampyravus orientalis Schlosser (Chiroptera) from the early Oligocene (Rupelian), Fayum, Egypt—Body mass, humeral morphology and affinities. Acta Chirop. 2009, 11, 271–278. [Google Scholar]
- McDonald, H.G. Paleoecology of the extinct Shasta ground sloth, Nothrotheriops shastensis, (Xenarthra, Nothrotheriidae): The physical environment. New Mexico Mus. Nat. Hist. Sci. Bull. 2022, 88, 33–43. [Google Scholar]
- Medina, M.J.; Antic, D.; Borges, P.A.V.; Borko, S.; Fiser, C.; Lauritzen, S.-E.; Martín, J.L.; Oromi, P.; Pavlek, M.; Premate, E.; et al. Temperature variation in caves and its significance for subterranean ecosystems. Sci. Rep. 2023, 13, 20735. [Google Scholar] [CrossRef]
- Graham, R.W.; Lundelius, E.L., Jr. Coevolutionary disequilibrium and Pleistocene extinctions. In Quaternary Extinctions: A Prehistoric Revolution; Martin, P.S., Klein, R.G., Eds.; University of Arizona Press: Tucson, AZ, USA, 1984; pp. 223–249. [Google Scholar]
- Semken, H.A., Jr.; Graham, R.W.; Stafford, T.W., Jr. AMS 14C analysis of Late Pleistocene non-analog faunal components from 21 cave deposits in southeastern North America. Quat. Internat. 2010, 217, 240–255. [Google Scholar] [CrossRef]
- Webb, S.D. Chronology of Florida Pleistocene mammals. In Pleistocene Mammals of Florida; Webb, S.D., Ed.; University Press of Florida: Gainesville, FL, USA, 1974; pp. 5–31. [Google Scholar]
- Brodkorb, P. The Pleistocene avifauna of Arredondo, Florida. Bull. Fla. St. Mus. Biol. Sci. 1959, 4, 269–291. [Google Scholar]
- Morgan, G.S.; Emslie, S.D. Tropical and western influences in vertebrate faunas from the Pliocene and Pleistocene of Florida. Quat. Internat. 2010, 217, 143–158. [Google Scholar]
- Martin, P.S. Twilight of the Mammoths; University of California Press: Berkeley, CA, USA, 2007; 276p. [Google Scholar]
- Stafford, T.W., Jr.; Semken, H.A., Jr.; Graham, R.W.; Klippel, W.F.; Markova, A.; Smirnov, N.G.; Southon, J. First AMS 14C dates documenting contemporaneity of non-analog species in late Pleistocene mammal communities. Geology 1999, 27, 903–906. [Google Scholar] [CrossRef]
- Winge, H. Jordfundne og nulevende F1agermus (Chiroptera) fra Lagoa Santa, Minas Geraes, Brasilien: Med udsigt over F1agermusenes indbyrdes Slaegstkab. E Museo Lundii 1893, 2, 1–92. [Google Scholar]
- Paleobiology Database. Available online: https://paleobiodb.org/ (accessed on 22 February 2024).
- Olsen, S.J. Additional remains of Florida’s Pleistocene vampire. J. Mammal. 1960, 41, 458–462. [Google Scholar] [CrossRef]
- Linares, O.J. Quir6pteros subf6siles encontrados en las cuevas Venezolanas. Parte III. Desmodus rotundus en la Cueva de la Brújula (Mi. 1) Miranda. Bol. Soc. Venez. Espeleo. 1970, 3, 33–36. [Google Scholar]
- Linares, O.J. Quir6pteros subf6siles encontrados en las cuevas Venezolanas. Parte I. Dep6sito de la Cueva de Quebrada Honda (Designaci6n de Catastro Ar-l). Bol. Soc. Venez. Espeleo. 1968, 1, 119–145. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.