Abstract
As the therapeutic landscape for metastatic clear cell renal cell carcinoma (mccRCC) expands to include vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR TKIs) and immunotherapies, new challenges are in place for evaluating and treating refractory disease. Assessing and managing refractory disease has several elements: (1) the mechanism(s) of front-line treatment, (2) timing of progressive disease, (3) rapidity and sites of progressing disease, (4) use of adjuvant therapy, and (5) incorporation of surgical and radiation techniques. These variables all have distinct impact on the biology of refractory or resistant mccRCC. A better understanding of the essential mechanisms of both primary and secondary immunotherapy resistance will inform biomarker development and therapeutic strategies in the refractory setting. This paper addresses the current understanding of treatment sequencing in refractory mccRCC, focusing on treatment options with prospective clinical trial data, considers refractory mccRCC after adjuvant immunotherapy, and incorporates radiation or surgical resection for oligoprogressive disease.
Introduction
Refractory metastatic clear cell renal cell carcinoma (mccRCC) poses unique challenges regarding treatment sequencing, selection, and integration of definitive surgery or stereotactic body radiation therapy (SBRT). Ultimately, one must consider choice of front-line treatment, resistance mechanisms, disease kinetics, and disease relapse after adjuvant immunotherapy to determine subsequent therapies at time of disease progression or relapse. Currently, no validated biomarkers are approved to help guide treatment selection; however, certain strategies exist to help one navigate this ever-changing landscape and will be reviewed in this article.
Tyrosine Kinase Inhibitors in mccRCC
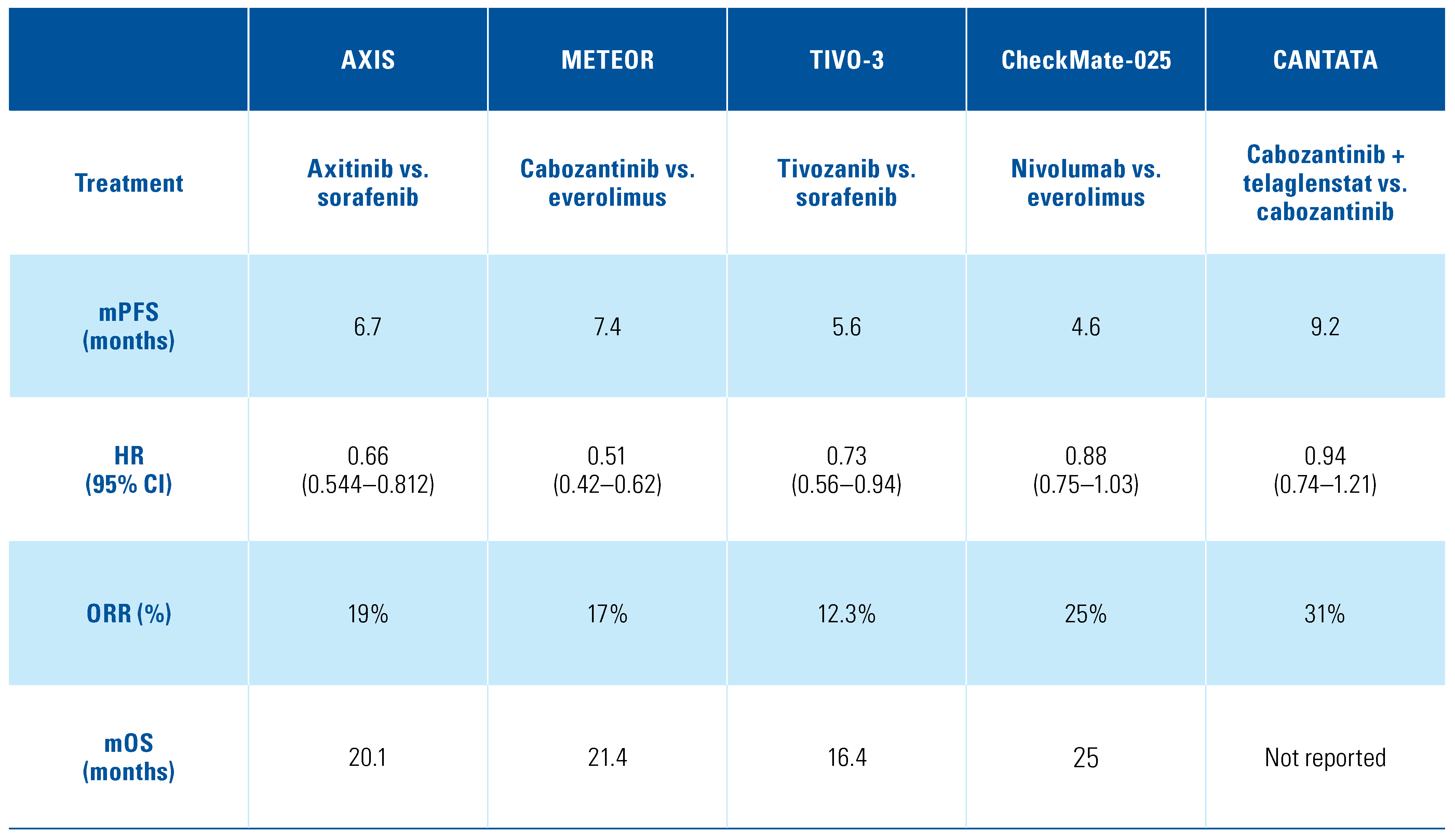
The mainstay for mccRCC treatments relies on targeting angiogenesis and the vascular endothelial growth factor receptor (VEGFR); these become upregulated following loss of the von Hippel-Lindau (VHL) protein and accumulation of hypoxia-inducible factor (HIF), leading to aberrant signaling in angiogenesis, proliferation, and metabolism[,]. Multiple therapies, predominantly antiangiogenic tyrosine kinase inhibitors (TKIs), were developed to target the vascular endothelial growth factor (VEGF) pathway, and seven of these therapies are currently by the United States Food and drug Administration (FDA) and listed on the National Comprehensive Cancer Network (NCCN) guidelines: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib, sunitinib, and tivozanib[]. While these therapies have served as the backbone of treatment, responses are heterogeneous, with some patients exhibiting intrinsic resistance and many patients developing acquired resistance despite initial response or disease stability. Several mechanisms of resistance have been defined including upregulation of other angiogenic drivers and increased tumor invasiveness using alternative pathways (MET or AXL), which led to VEGF resistance and eventual disease progression[,]. Multiple trials in the second line after prior exposure to anti-VEGF therapies have been conducted and demonstrated improvements in progression-free and overall survival. Three TKI therapeutics (axitinib, cabozantinib, and tivozanib) received their FDA approval specifically for patients who have progressed on at least one or more prior systemic therapies[,,]. The first phase 3 trial of axitinib versus sorafenib, entitled AXIS, and including patients who progressed after sunitinib or cytokines, demonstrated a progression-free survival (PFS) for axitinib over sorafenib (hazard ratio [HR], 0.66; 95% CI, 0.544–0.812; P < 0.001)[]. Tolerability of axitinib was similar to sorafenib and also led to higher objective response rates (ORRs), 19% for axitinib and 9% for sorafenib, and did not demonstrate a statistically significant difference in median overall survival between the two arms, but more than half the patients on each arm went on to subsequent therapy. Cabozantinib inhibits the activity of c-MET, VEGFR, AXL, and other tyrosine kinases, thereby leading to reduced tumor angiogenesis, motility, and invasiveness, which make it an ideal choice in the refractory mccRCC setting[]. The phase 3 trial of cabozantinib versus everolimus, entitled METEOR, demonstrated both an improvement in PFS and overall survival (OS) for cabozantinib over everolimus (HR, 0.66; 95% CI, 0.53–0.83; P = 0.00026)[]. Tivozanib, which received FDA approval in 2021, functions by selectively inhibiting the phosphorylation of VEGFR-1, VEGFR-2, and VEGFR-3, and due to this potency and selectively it may confer less toxicity and improved tolerability for patients[]. TIVO-3, a phase 3 clinical trial of tivozanib versus sorafenib, studied patients who had disease progression on at least two prior therapies including anti-VEGF and immune checkpoint inhibitors. The trial met its primary endpoint, and tivozanib as a third- or fourth-line agent improved PFS compared with sorafenib (HR, 0.73; 95% CI, 0.56–0.94; P = 0.016)[]. Finally, combination therapy targeting the mammalian target of rapamycin (mTOR) pathway and VEGFR proved beneficial for patients in the refractory mccRCC setting. In a multicenter, open-label, phase 2 trial of lenvatinib plus everolimus versus lenvatinib or everolimus monotherapy, lenvatinib plus everolimus yielded a strong signal toward improved PFS compared with everolimus (HR, 0.40; 95% CI, 0.24–0.68; P = 0.0005) but only a weak signal toward improved PFS compared with lenvatinib monotherapy (HR, 0.66; 95% CI, 0.39–1.10; P = 0.12)[]. Exploiting these pathways in refractory mccRCC is important and with more specific TKIs approved—for example tivozanib, which has demonstrated improved PFS in heavily pretreated patients with better tolerability and manageable toxicity—should be the cornerstone of future treatment combinations. Key phase 3 trials are listed in Table 1.

Table 1.
Key phase 3 clinical trials in refractory metastatic kidney cancer.
Immune Checkpoint Inhibition in mccRCC
mRCC is an immunogenic tumor, and therapies utilizing humanized monoclonal antibodies to block the negative regulatory signal between programmed death 1 (PD-1) on the T-cell and its ligand programmed death 1 ligand 1 (PD-L1) on the tumor cell, termed immune checkpoint inhibitors (ICIs), have shown remarkable and durable responses. ICI monotherapy has been studied in refractory mccRCC with the PD-1 inhibitor nivolumab. CheckMate-025 was a phase 3 clinical trial comparing nivolumab versus everolimus, where all patients had progressed on at least one prior antiangiogenic therapy. Nivolumab monotherapy improved the ORR over everolimus (25% vs. 5%; odds ratio, 5.98; 95% CI, 3.68–9.72; P < 0.001) and demonstrated a median OS benefit of 25 months compared with 19.6 months (HR, 0.73; 98.5% CI, 0.57–0.93; P = 0.002) []. Another immunogenic pathway in T-cell surface receptor signaling involves the cytotoxic T-lymphocyte associated protein 4 (CTLA-4), which is a coreceptor that controls peripheral tolerance and development of autoimmunity. Ipilimumab is a humanized monoclonal antibody against CTLA-4 and was combined with nivolumab in the first-line setting for mRCC in the pivotal first-line mccRCC trial CheckMate-214. This phase 3 clinical trial compared the combination of nivolumab plus ipilimumab versus sunitinib[]. The OS and ORR were higher with the combination of nivolumab plus ipilimumab compared with sunitinib amid patients with International Metastatic RCC Database Consortium (IMDC) intermediate- and poor-risk disease and remains superior at 4-year follow-up (HR, 0.65; 95% CI, 0.54–0.78)[]. For the refractory mRCC setting, several trials have tested the dual ICI combination ipilimumab plus nivolumab (ipi-nivo). FRACTION-RCC was a phase 2 clinical trial that tested the combination ipi-nivo in heavily pretreated patients with mccRCC who had previously received and progressed on ICIs. The study demonstrated a meager ORR of 15.2% as well as a median PFS of only 16.1 weeks[]. Furthermore, risk-adaptive trials have been conducted with varying responses. Three phase 2 clinical trials (TITAN-RCC, OMNIVORE, HCRN GU16–620) investigated treatment intensification with the addition of ipilimumab after progression or lack of response on PD-1 monotherapy[,,]. All three studies showed modest activity of ipilimumab in the salvage setting after nivolumab with limited responses, and the use of CTLA-4 after PD-1 refractory disease remains uncertain.
Combination ICI Plus VEGFR TKI in Refractory mccRCC
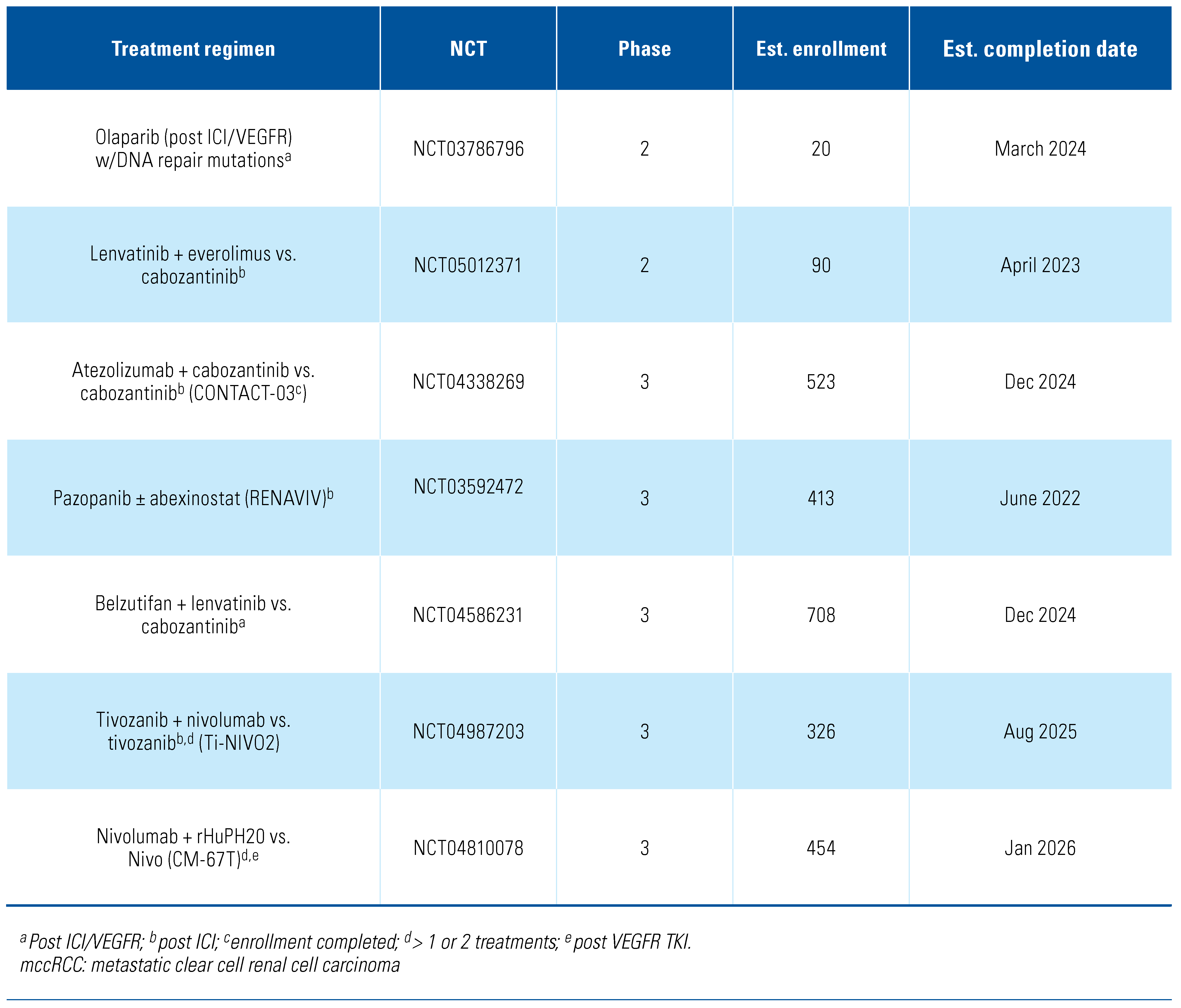
Additionally, front-line combinations of ICIs with or without VEGFR TKI are now standard of care for all IMDC-risk mccRCC patients, but single-center retrospective studies have shown that patients who progress on ICI plus VEGFR TKI or ICI plus ICI have an ORR of 25% and a median PFS of 12 months (95% CI, 8.2–24.5) when initiated on further treatments[]. The CANTATA study was a phase 3 trial of cabozantinib plus telaglenastat, an oral glutaminase (GLS) inhibitor that blocks glutamine utilization and critical downstream pathways, versus cabozantinib plus placebo in previously treated mccRCC patients who received ≥ 1 antiangiogenic therapy or ipi-nivo. In this study, 62% of the enrolled patients had progression on prior ICI. Cabozantinib plus telaglenastat demonstrated a median PFS of 9.2 months and cabozantinib plus placebo demonstrated a median PFS of 9.3 months (HR, 0.94; 95% CI, 0.74–1.21; stratified log-rank P = 0.65), which was not statistically significant. However, in a prespecified subgroup analysis for patients who received prior ICI, cabozantinib plus telaglenastat yielded a numerically longer median PFS than cabozantinib plus placebo (11.1 vs. 9.2 months, respectively; unstratified HR, 0.77; 95% CI, 0.56–1.06)[]. KEYNOTE-146 was a single-arm, phase 1b/2 clinical trial of the combination of lenvatinib plus pembrolizumab in treatment-naïve, previously treated ICI-naïve and ICI-pretreated mccRCC patients[]. The objective response at week 24 in the ICI-pretreated cohort was 55.8% (95% CI, 45.7–65.5), and the median PFS was 12.2 months (95% CI, 9.5–17.7), but due to the single-cohort nature of the trial, it is difficult to ascertain the effectiveness of these individual study drugs in this setting. Current trials are ongoing to address the issue of ICI continuation after progression on ICI combination or monotherapy. CONTACT-03 (NCT04338269) is a randomized phase 3 study of cabozantinib plus atezolizumab versus cabozantinib monotherapy in patients with mRCC (clear cell and non-clear cell, papillary or unclassified) who had radiographic disease progression during or following first- or second-line ICI treatment[]. This trial has dual primary endpoints of PFS and OS that will further elucidate the role of cabozantinib plus atezolizumab in the ICI-refractory setting. CONTACT-03 enrollment was completed in 2022. Another ongoing trial, TiNivo-2 (NCT04987203), is a randomized phase 3 trial of tivozanib plus nivolumab versus tivozanib monotherapy in patients who have previously progressed on 1 or 2 lines of therapy, including an ICI (during or within 6 weeks of treatment discontinuation)[]. Both trials will likely be completed by 2025, and yield insight as to whether combination therapy with VEGF TKI and either PD-1 or PD-L1 inhibition is superior to TKI monotherapy in ICI-refractory mccRCC. Other select ongoing studies in the refractory setting are listed in Table 2.

Table 2.
Selected ongoing clinical trials in refractory mccRCC.
Treatment Selection After Adjuvant Treatment
Recently published data from the large, randomized, phase 3 clinical trial KEYNOTE-564 of pembrolizumab versus placebo in high-risk ccRCC post-nephrectomy established the role of ICI in the adjuvant setting. Pembrolizumab monotherapy significantly prolonged disease-free survival (DFS) compared with placebo (DFS at 24 months, 77.3% vs. 68.1%; HR for recurrence or death, 0.68; 95% CI, 0.53–0.87; P = 0.002 [two-sided])[]. Thus, high-risk ccRCC patients who progress after adjuvant ICI pose a challenging situation. One must factor in timing of relapse as well as incorporation of surgery or SBRT to sites of progression if amenable. When systemic therapy is required to treat relapsed disease, rechallenge with ICI is possible, but no current data exists to provide efficacy of these treatments post-adjuvant ICI therapy. Other tumor types may provide some clues: for example, in malignant melanoma, retrospective studies have shown that patients who progressed during anti–PD-1 monotherapy had no response to subsequent anti–PD-1 systemic therapy to treat metastatic disease. However, they were able to retain sensitivity to future anti–PD-1 after completion of adjuvant therapy[]. Consequently, since adjuvant ICI therapy was only approved in high-risk ccRCC in 2021, no data is available to infer that any rechallenge after completion of adjuvant treatment with ICI would be beneficial. Randomized clinical trials have proven that VEGFR TKI monotherapy or VEGFR plus mTOR inhibitors in mccRCC post-ICI prolongs PFS and OS, and these regimens may be the preferred treatment approach prior to an ICI rechallenge. Rechallenge ICI in ccRCC patients after completion of adjuvant ICI should only be conducted in randomized clinical trials. Lastly, targeting other mechanisms will also depend on timing of disease relapse after completing adjuvant therapy.
Alternative Modalities in Refractory mccRCC
Incorporation of radiotherapy (RT) or surgery should be considered at time of first relapse or during oligometastatic progression. First, SBRT for extracranial sites and stereotactic radiosurgery (SRS) for intracranial sites can provide both local control for patients with oligometastatic progression and palliative relief[,]. Clinical studies have also examined the efficacy of SBRT with systemic VEGFR TKI or ICI. RAPPORT was a phase 1/2 trial that explored the safety and efficacy of total metastatic stereotactic ablative body radiotherapy (SABR) to oligometastatic mccRCC followed by anti– PD-1 treatment. ORR was 63% in 30 evaluable patients and estimated 1- and 2-year OS rates were 90% and 74%, respectively[]. Ongoing clinical trials (CYTOSHRINK [NCT04090710] and SAMURAI [NCT05327686]) are now accruing to study how to effectively combine SBRT or SRS to the primary tumor, with systemic treatments to improve survival outcomes in first-line mccRCC[]. Second, consolidative surgery remains an option in the refractory mccRCC setting. Either at the time of oligoprogression or for palliative purposes, surgical approaches can also be considered after the start of highly effective systemic therapy with ICI combinations. Two completed phase 3 clinical trials, SURTIME and CARMENA, both demonstrated that cytoreductive nephrectomy (CN) may be delayed until a stable or partial response is achieved on VEGFR TKI[,]. However, both trials completed enrollment prior to approval of current combination therapies (ICI plus ICI or ICI plus VEGFR TKI). There are three ongoing studies (NORDIC-SUN [NCT03977571], PROBE [NCT04510597], and CYTO-KIK [NCT04322955]) which will provide prospective evidence regarding optimal timing of CN in the setting of ICI-based combinations in mccRCC. Lastly, surgical resection of metastases at time of progression is undefined. One must consider size and location of the metastatic site as well as tumor biology, along with patient performance status, before taking a surgical approach. Optimal management decisions require a multidisciplinary team approach.
In conclusion, the treatment landscape is continually evolving in first-line mccRCC, and changes with preferred front-line options will dictate second-line therapy and beyond to limit cross-tolerance and overcome resistance mechanisms. Combining other modalities (SBRT and surgery) may have roles in specific refractory settings, and we must also consider patient preferences and tolerability factors in refractory mccRCC.
Acknowledgments
- Stephanie Berg: Advising/Consulting: Exelixis, BMS, Eisai, Pfizer, SeaGen, Sanofi
- Martin Angel: No relevant disclosures.
- Kathryn E. Beckermann: Research funding: Bristol-Myers Squibb-IASLC-LCFA. Advising/consulting: Aravive, Aveo, BMS, Exelexis, Sanofi, Seagen, Astellas.
- Frede Donskov: No relevant disclosures.
- Chung-Han Lee: Research funding: BMS, Calithera, Eisai, Eli Lilly, Exelixis, Merck, Pfizer; Advising/ Consulting: Amgen, Aveo, BMS, Exelixis, Eisai, Merck, Pfizer, EMD Serono, Cardinal Health; Honoraria: AiCME, IDEOlogy Health, Intellisphere, Research to Practice.
- Pavlos Msaouel: Research funding: Takeda, BMS, Mirati Therapeutics, Gateway for Cancer Re-search, and UT MD Anderson Cancer Center; Advising/consulting: Mirati Therapeutics, BMS, Exelixis, Axiom Healthcare, Exelixis, Pfizer Takeda, Bristol Myers Squibb, Mirati Therapeutics, Gateway for Cancer Re-search, and UT MD Anderson Cancer Center.
- Rana R. McKay: Research funding: Bayer, Tempus; Consulting: Aveo, Astra Zeneca, Bayer, BMS, Calithera, Caris, Dendreon, Exelixis, JNJ, Myovant, Merck, Novartis, Pfizer, Sanofi, Sorrento Therapeutics, Tempus.
- Tian Zhang: PI/Research funding: Acerta, Novartis, Merrimack, Abbvie/StemCentrx, Merck, Regeneron, Mirati Therapeutics, Janssen, Astra Zeneca, Pfizer, OmniSeq, Personal Genome Diagnostics, Astellas, Eli Lilly, CPRIT; Advisory Board/Consultant: Merck, Exelixis, Sanofi-Aventis, Janssen, Astra Zeneca, Pfizer, Amgen, BMS, Pharmacyclics, SeaGen, Calithera, QED Therapeutics, Eisai, Aveo, Eli Lilly, Aravive, Astellas, MJH Associates, Vaniam, Aptitude Health, PeerView.
Competing Interests
See "Acknowledgments" for details.
Abbreviations
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| ICI | immune checkpoint inhibitor |
| mccRCC | metastatic clear cell renal cell carcinoma |
| ORR | objective response rate |
| OS | overall survival |
| PD-1 | programmed death 1 |
| PFS | progression-free survival |
| SBRT | stereotactic body radiation therapy |
| TKI | tyrosine kinase inhibitor |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| FDA | United States Food and drug Administration |
References
- Choueiri, T.K.; Kaelin, W.G., Jr. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat. Med. 2020, 26, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Bacigalupa, Z.A.; Rathmell, W.K. Beyond glycolysis: Hypoxia signaling as a master regulator of alternative metabolic pathways and the implications in clear cell renal cell carcinoma. Cancer Lett. 2020, 489, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to systemic therapies in clear cell renal cell carcinoma: Mechanisms and management strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Siska, P.J.; Beckermann, K.E.; Rathmell, W.K.; Haake, S.M. Strategies to overcome therapeutic resistance in renal cell carcinoma. Urol. Oncol. 2017, 35, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczylik, C.; Hutson, T.E.; et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011, 378, 1931–1939. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Mainwaring, P.N.; Rini, B.I.; Donsko, F.; et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1814–1823. [Google Scholar] [CrossRef]
- Salgia, N.J.; Zengin, Z.B.; Pal, S.K. Tivozanib in renal cell carcinoma: A new approach to previously treated disease. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923818. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Pal, S.K.; Escudier, B.J.; Atkins, M.B.; Hutson, T.E.; Porta, C.; et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): A phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. 2020, 21, 95–104. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 2020, 126, 4156–4167. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueri, T.K.; et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020, 5, e001079. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Kluger, H.M.; George, S.; Tykodi, S.S.; Kuzel, T.M.; Perets, R.; et al. FRACTION-RCC: Innovative, high-throughput assessment of nivolumab + ipilimumab for treatment-refractory advanced renal cell carcinoma (aRCC). J. Clin. Oncol. 2020, 38 (Suppl. 15), 5007. [Google Scholar] [CrossRef]
- Grimm, M.-O.; Esteban, E.; Barthélémy, P.; Schmidinger, M.; Busch, J.; Valderrama, B.P.; et al. Efficacy of nivolumab/ipilimumab in patients with initial or late progression with nivolumab: Updated analysis of a tailored approach in advanced renal cell carcinoma (TITAN-RCC). J. Clin. Oncol. 2021, 39 (Suppl. 15), 4576. [Google Scholar] [CrossRef]
- McKay, R.R.; McGregor, B.A.; Xie, W.; Braun, D.A.; Wei, X.; Kyriakopoulos, C.E.; et al. Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: A response-based phase II study (OMNIVORE). J. Clin. Oncol. 2020, 38, 4240–4248. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Jegede, O.; Haas, N.B.; McDermott, D.F.; Bilen, M.A.; Stein, M.; et al. Phase II study of nivolumab and salvage nivolumab + ipilimumab in treatment-naïve patients (pts) with advanced renal cell carcinoma (RCC) (HCRN GU16-260). J. Clin. Oncol. 2020, 38 (Suppl. 15), 5006. [Google Scholar] [CrossRef]
- Ged, Y.; Gupta, R.; Duzgol, C.; Knezevic, A.; Shapnik, N.; Kotecha, R.; et al. Systemic therapy for advanced clear cell renal cell carcinoma after discontinuation of immune-oncology and VEGF targeted therapy combinations. BMC Urol. 2020, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Tannir, N.M.; Agarwal, N.; Porta, C.; Lawrence, N.J.; Motzer, R.J.; Lee, R.J.; et al. CANTATA: Primary analysis of a global, randomized, placebo (Pbo)-controlled, double-blind trial of telaglenastat (CB-839) + cabozantinib versus Pbo + cabozantinib in advanced/metastatic renal cell carcinoma (mRCC) patients (pts) who progressed on immune checkpoint inhibitor (ICI) or anti-angiogenic therapies. J. Clin. Oncol. 2021, 39 (Suppl. 15), 4501. [Google Scholar] [CrossRef]
- Lee, C.H.; Shah, A.Y.; Rasco, D.; Rao, A.; Taylor, M.H.; Di Simone, C.; et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): A phase 1b/2 study. Lancet Oncol. 2021, 22, 946–958. [Google Scholar] [CrossRef]
- Pal, S.K.; Albiges, L.; Rodriguez, C.; Liu, B.; Doss, J.; Khurana, S.; et al. CONTACT-03: Randomized, open-label phase III study of atezolizumab plus cabozantinib versus cabozantinib monotherapy following progression on/after immune checkpoint inhibitor (ICI) treatment in patients with advanced/metastatic renal cell carcinoma. J. Clin. Oncol. 2021, 39 (Suppl. 6), TPS370. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Albiges, L.; Hammers, H.J.; et al. TiNivo-2: A phase 3, randomized, controlled, multicenter, open-label study to compare tivozanib in combination with nivolumab to tivozanib monotherapy in subjects with renal cell carcinoma who have progressed following one or two lines of therapy where one line has an immune checkpoint inhibitor. J. Clin. Oncol. 2022, 40 (Suppl. 6), TPS405. [Google Scholar]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.-H.; et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.N.; Shoushtari, A.N.; Chauhan, D.; et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy. Ann. Oncol. 2020, 31, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Flippot, R.; Dalban, C.; Laguerre, B.; Borchiellini, D.; Gravis, G.; Négrier, S.; et al. Safety and efficacy of nivolumab in brain metastases from renal cell carcinoma: Results of the GETUG-AFU 26 NIVOREN Multicenter Phase II Study. J. Clin. Oncol. 2019, 37, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.; Desai, K.; Wei, W.; Kinsey, E.M.; Kao, C.; George, D.J.; et al. Clinical outcomes in patients with metastatic renal cell carcinoma and brain metastasis treated with ipilimumab and nivolumab. J. Immunother. Cancer 2021, 9, e003281. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Bressel, M.; Wood, S.T.; Shaw, M.G.; Loi, S.; Sandhu, S.K.; et al. Stereotactic radiotherapy and short-course pembrolizumab for oligometastatic renal cell carcinoma-The RAPPORT Trial. Eur. Urol. 2022, 81, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Lalani, A.-K.A.; Swaminath, A.; Pond, G.R.; Kapoor, A.; Chu, W.; Bramson, J.L.; et al. Hotte Phase II trial of cytoreductive stereotactic hypofractionated radiotherapy with combination ipilimumab/nivolumab for metastatic kidney cancer (CYTOSHRINK). J. Clin. Oncol. 2020, 38 (Suppl. 6), TPS761. [Google Scholar] [CrossRef]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.-B.; Bensalah, K.; et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef]
- Bex, A.; Mulders, P.; Jewett, M.; Wagstaff, J.; van Thienen, J.V.; Blank, C.U.; et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019, 5, 164–170, Erratum in JAMA Oncol.2019, 5, 271. [Google Scholar] [CrossRef]
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.