2022 WUOF/SIU International Consultation on Urological Diseases: Management of Toxicity and Side Effects of Systemic Therapy for Renal Cell Carcinoma
Abstract
:Introduction
General Principles of RCC Toxicity Management
Toxicity of VEGFR TKIs
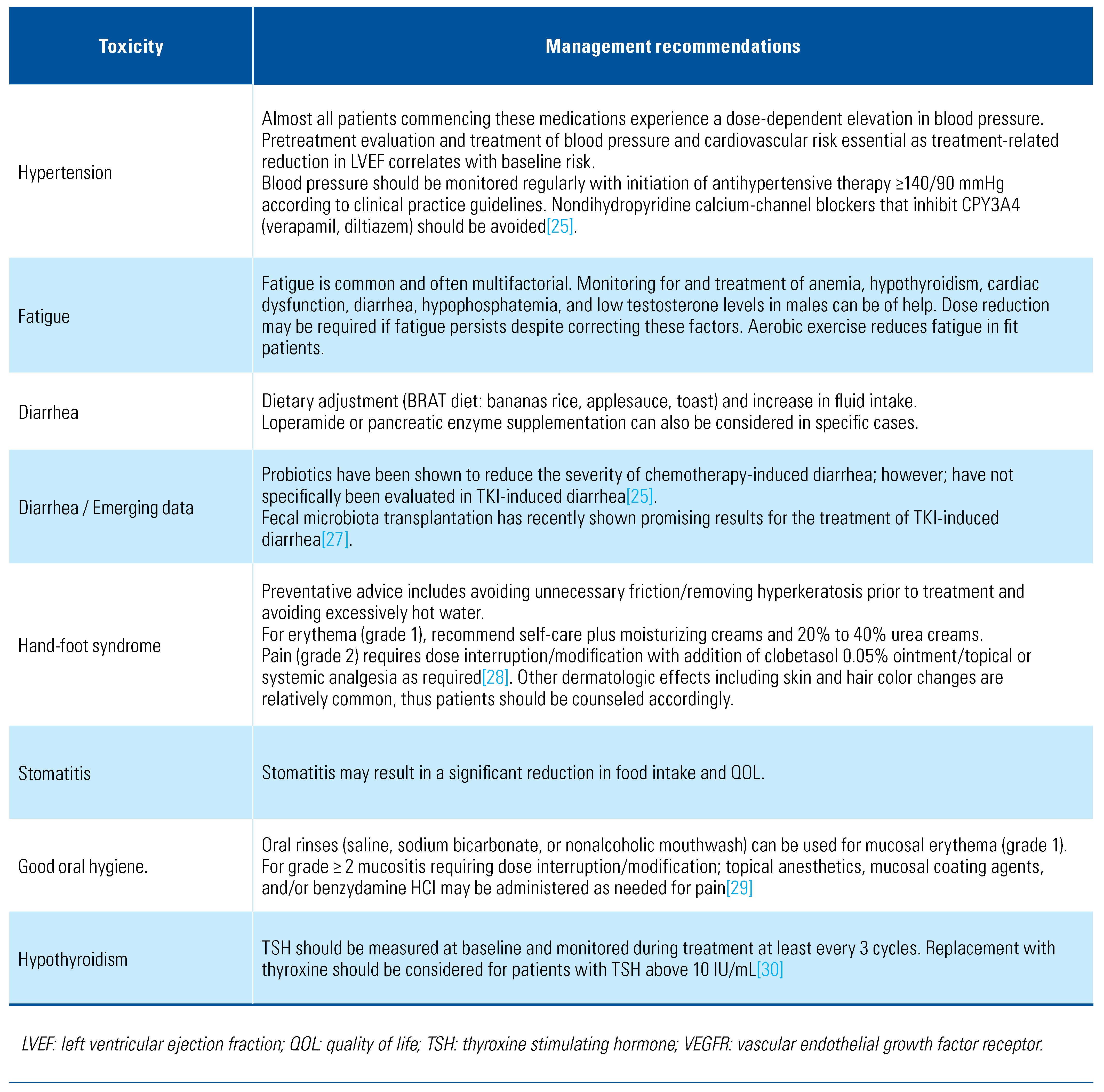
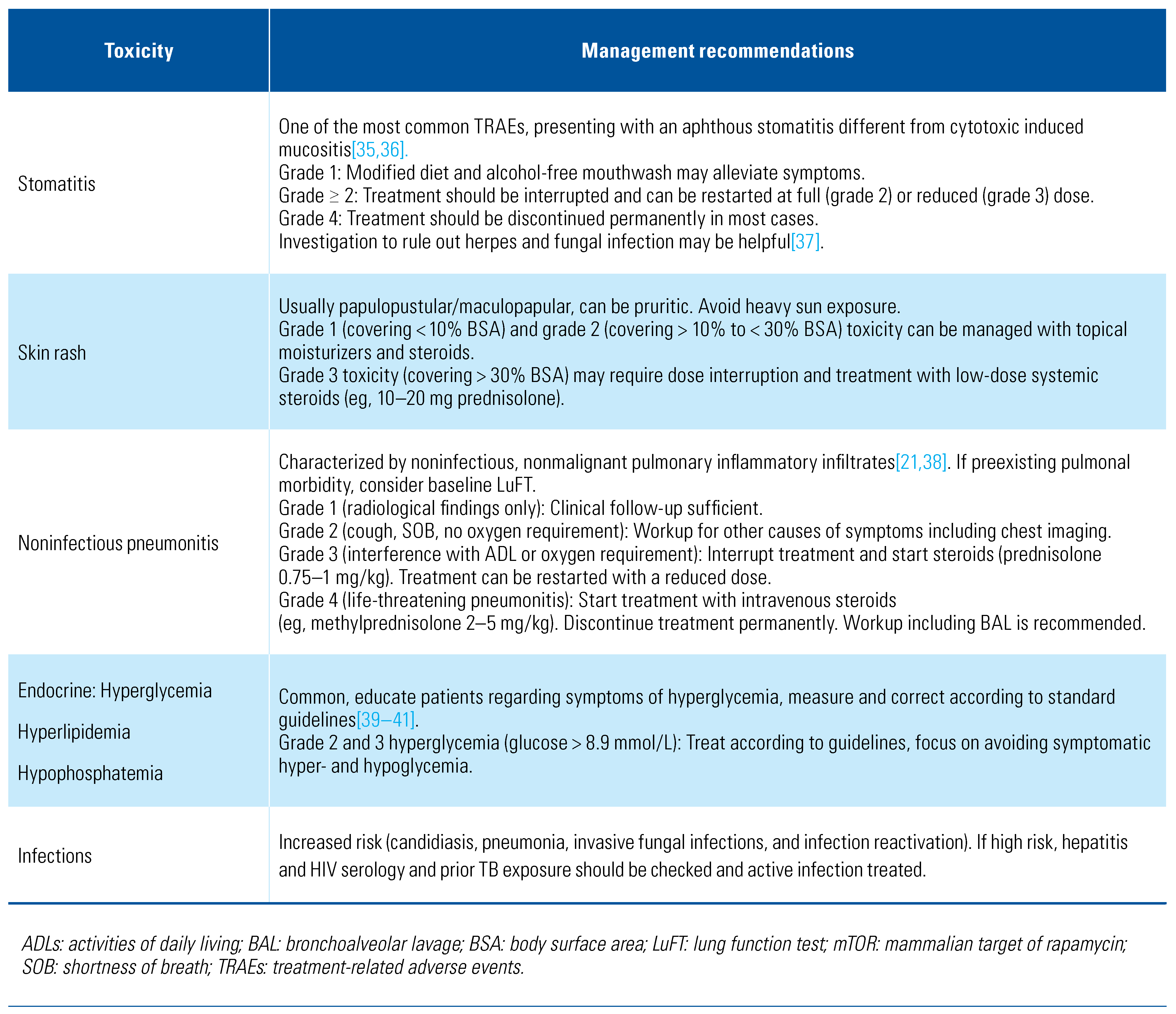
Management of VEGFR TKI–Associated Toxicities
Toxicity of mTOR Inhibitors
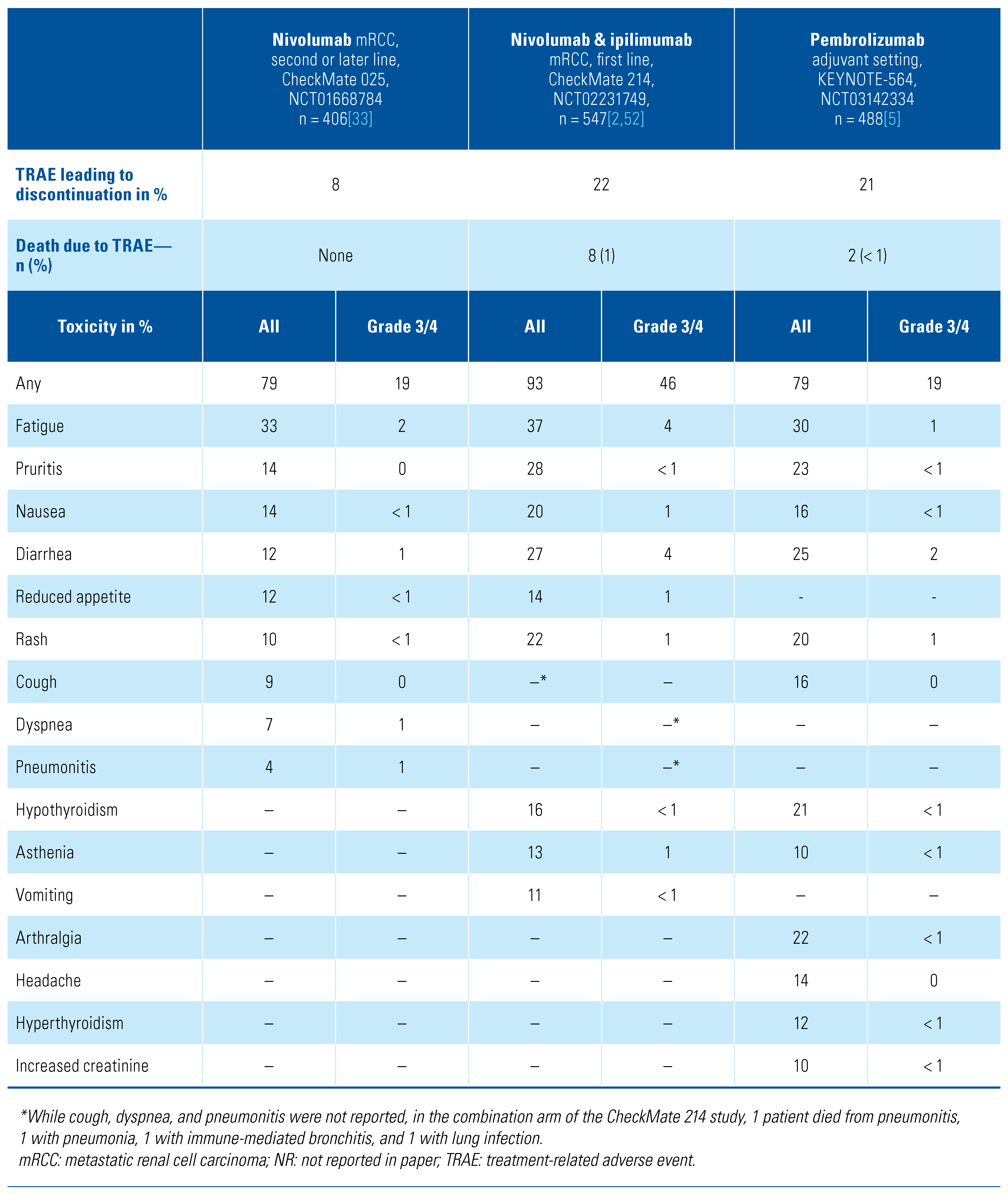
Toxicity of Immune Checkpoint Inhibitors
Mechanism, Spectrum, and Frequency of CPI-Associated Toxicities
Mechanisms of Immune-Related Adverse Events
Range of Immune-Related Adverse Events
Immune-Related Adverse Events in RCC
Management of CPI-Associated Toxicities
General Principles for Management of Immune- Related Adverse Events
Corticosteroids and Corticosteroid-Sparing Agents in Immune-Related Adverse Events
RCC Outcomes in Patients Who Experience Immune-Related Adverse Events
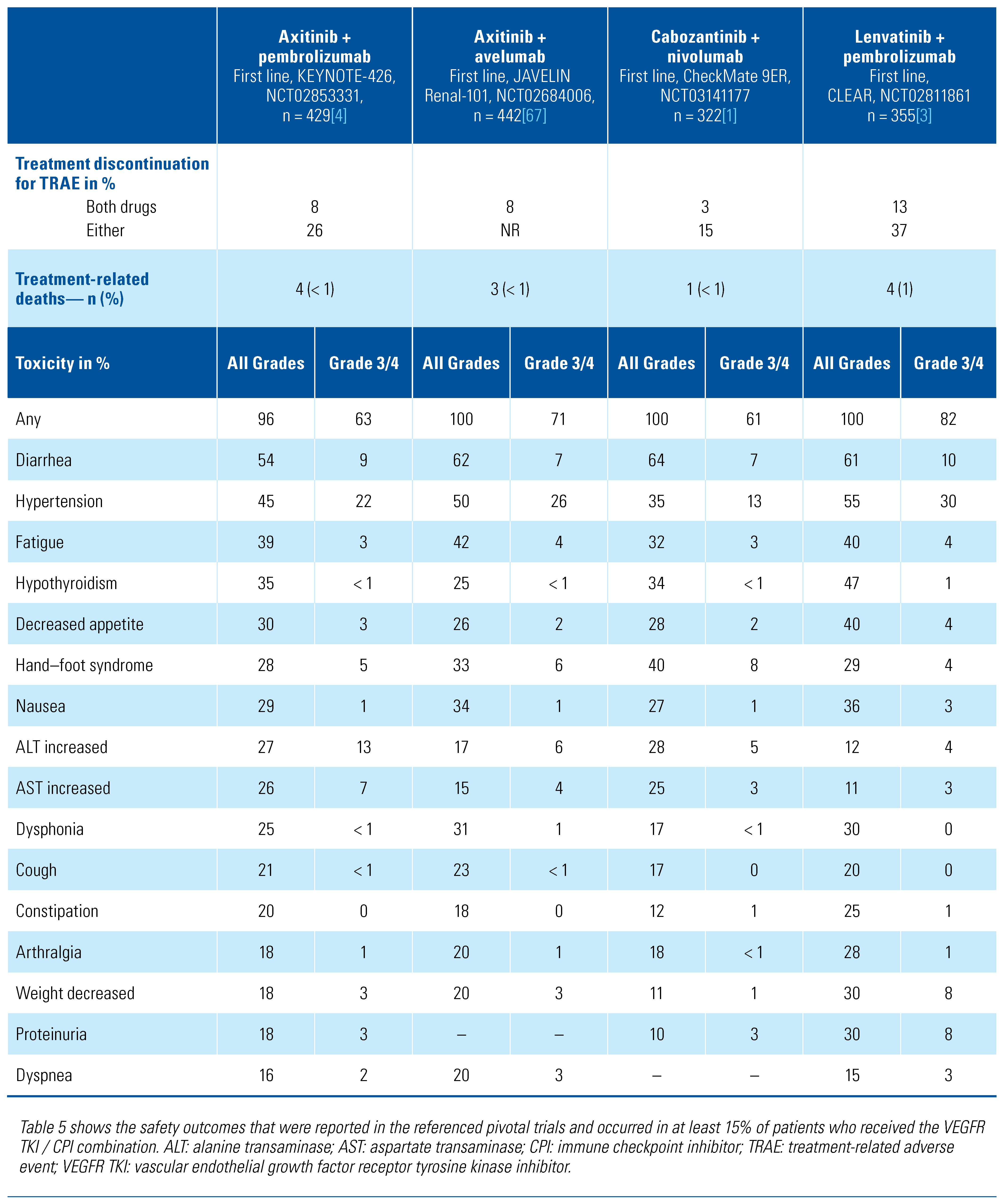
Toxicities of Combination VEGFR TKI/CPI Regimens
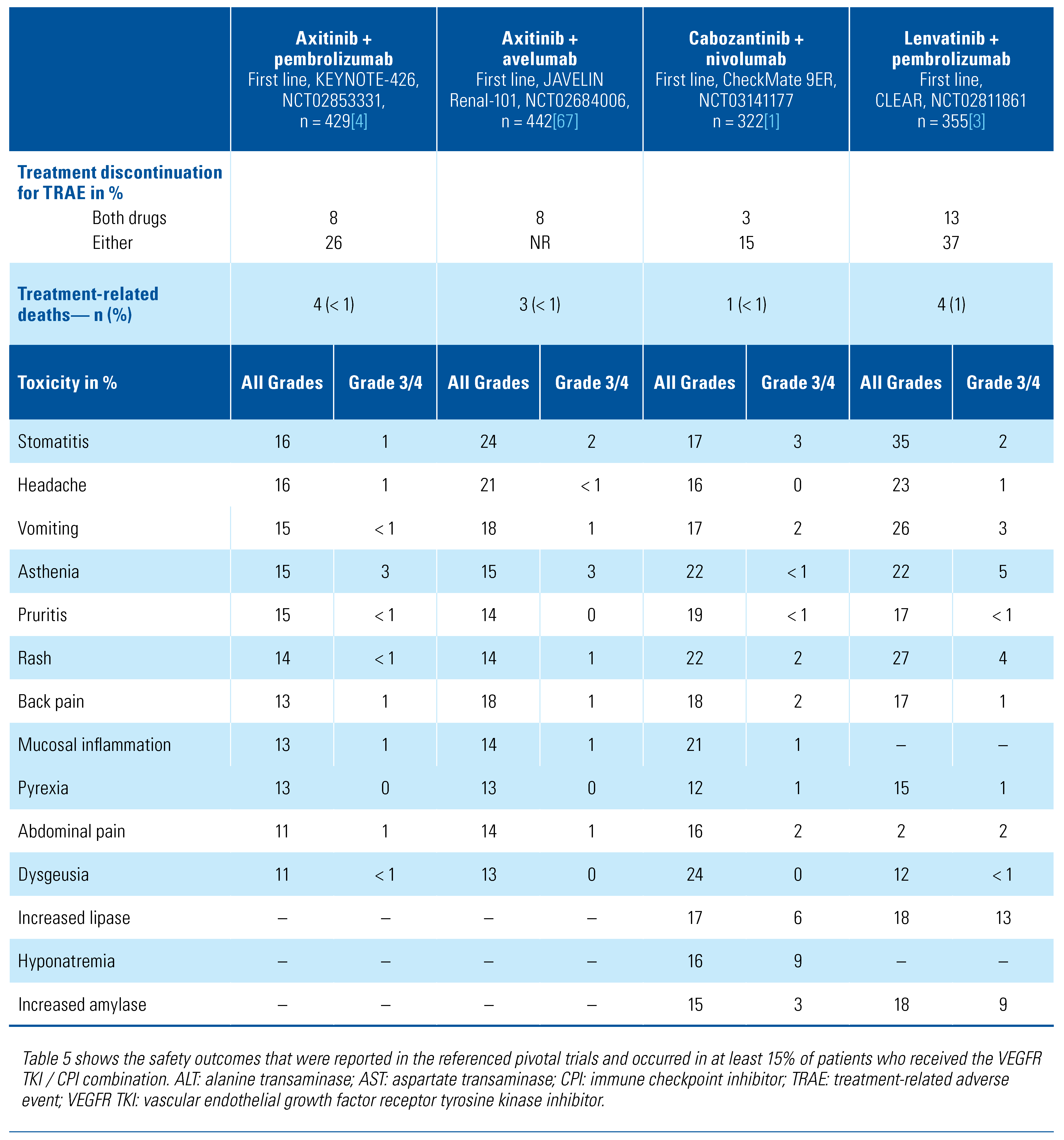
Spectrum and Frequency of Toxicities with TKI/CPI Combination Regimens
Management of Toxicities Associated with TKI/CPI Combination Regimens
- VEGFR TKIs have considerably shorter half-lives than CPIs. Axitinib has the shortest half-life at 2.5–6 hours, those of lenvatinib and cabozantinib are 28 hours and 100–120 hours, respectively. The half-lives of both pembrolizumab and nivolumab are around 26 days. Thus, VEGFR TKI–driven toxicity, especially from axitinib, typically starts to improve within a few days of treatment interruption, including when used in an axitinib plus CPI combination[69].
- In some cases, directed investigation may help to differentiate the cause, assess impact and severity, and guide management such as sigmoidoscopy and biopsy for evaluation of colitis; assessment of the pituitary fossa by magnetic resonance imaging (MRI) for hypophysitis; and cardiac MRI to identify immune-mediated myocarditis.
- First stop the TKI. Improvement in toxicity should be seen within a few days if the toxicity is TKI related.
- If there is no improvement after 5 to 7 days, or less for axitinib, interruption of the CPI and initiation of steroids should be considered following a recognized irAE guideline.
- Consider immediate interruption of both agents for severe, clinically significant toxicities.
- Continue to use appropriate supportive measures according to the toxicity.
- Ongoing regular assessment is required until improvement or resolution with vigilance for reemergence during steroid wean or following further treatment.
Toxicities of Novel Therapeutic Approaches
Patient Selection and Toxicity Prediction
Summary
Competing Interests
Abbreviations
| CPI | immune checkpoint inhibitor |
| CTCAE | Common Terminology Criteria for Adverse Event |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| irAE | immune-related adverse event |
| mRCC | metastatic renal cell carcinoma |
| mTOR | mammalian target of rapamycin |
| mTORI | mammalian target of rapamycin inhibitor |
| PD-1 | programmed cell death 1 receptor |
| RCC | renal cell carcinoma |
| TRAE | treatment-related adverse event |
| VEGFR | vascular endothelial growth factor receptor |
References
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Chang, Y.H.; et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N. Engl. J. Med. 2021, 385, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Markowitz, J.S.; Bei, D.; An, G. Enzyme- and transporter-mediated drug interactions with small molecule tyrosine kinase inhibitors. J. Pharm. Sci. 2014, 103, 3810–3833. [Google Scholar] [CrossRef] [PubMed]
- Cancer Institute, N. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Available online: https://www.meddra.org/ (accessed on 24 February 2022).
- Rini, B.I.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczylik, C.; Hutson, T.E.; et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet 2011, 378, 1931–1939. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The alliance A031203 CABOSUN trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2010, 28, 1061–1068. [Google Scholar] [CrossRef]
- Motzer, R.J.; Nosov, D.; Eisen, T.; Bondarenko, I.; Lesovoy, V.; Lipatov, O.; et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J. Clin. Oncol. 2013, 31, 3791–3799. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef]
- Lee, J.L.; Kim, M.K.; Park, I.; Ahn, J.H.; Lee, D.H.; Ryoo, H.M.; et al. RandomizEd phase II trial of Sunitinib four weeks on and two weeks off versus Two weeks on and One week off in metastatic clear-cell type REnal cell carcinoma: RESTORE trial. Ann. Oncol. 2015, 26, 2300–2305. [Google Scholar] [CrossRef]
- Chen, C.; Fang, H.; Jiao, Y.; Zhou, Y.; Guo, Q.; Lv, Z. Clinical efficacy and complication rate of sunitinib 2/1 versus 4/2 schedule for the treatment of metastatic renal cell cancer: A systematic review and meta-analysis. Clin. Genitourin. Cancer. 2019, 17, 319–331. [Google Scholar] [CrossRef]
- Deng, H.; Li, M.; Wu, Q.; Wang, L.; Hong, Z.; Yi, F.; et al. A 2/1 sunitinib dosing schedule provides superior antitumor effectiveness and less toxicity than a 4/2 schedule for metastatic renal cell carcinoma: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 313. [Google Scholar] [CrossRef]
- Thiery-Vuillemin, A.; Gravis, G.; Schlürmann, F.; Bompas, E.; Rolland, F.; Gross-Goupil, M.; et al. Randomized phase II study to assess the efficacy and tolerability of sunitinib by dose administration regimen in anti-angiogenic naïve patients with metastatic renal cell carcinoma (mRCC): Final analysis of SURF study. J. Clin. Oncol. 2022, 40 (Suppl. 6). [Google Scholar] [CrossRef]
- Rini, B.I.; Melichar, B.; Ueda, T.; Grünwald, V.; Fishman, M.N.; Arranz, J.A.; et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: A randomised double-blind phase 2 trial. Lancet Oncol. 2013, 14, 1233–1242. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Motzer, R.J.; Hutson, T.E.; Choueiri, T.K.; Kollmannsberger, C.; Bjarnason, G.A.; et al. COMPARZ post hoc analysis: Characterizing pazopanib responders with advanced renal cell carcinoma. Clin. Genitourin. Cancer 2019, 17, 425–435. [Google Scholar] [CrossRef]
- Rodriguez-Pascual, J.; Cheng, E.; Maroto, P.; Duran, I. Emergent toxicities associated with the use of mTOR inhibitors in patients with advanced renal carcinoma. Anticancer. Drugs. 2010, 21, 478–486. [Google Scholar] [CrossRef]
- Hudes, G.; Carducci, M.; Tomczak, P.; Dutcher, J.; Figlin, R.; Kapoor, A.; et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 2007, 356, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; Oudard, S.; Hutson, T.E.; Porta, C.; Bracarda, S.; et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet 2008, 372, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Paluri, R.K.; Sonpavde, G.; Morgan, C.; Rojymon, J.; Mar, A.H.; Gangaraju, R. Renal toxicity with mammalian target of rapamycin inhibitors: A meta-analysis of randomized clinical trials. Oncol. Rev. 2019, 13, 455. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Melichar, B.; Fishman, M.N.; Oya, M.; Pithavala, Y.K.; Chen, Y.; et al. Axitinib dose titration: Analyses of exposure, blood pressure and clinical response from a randomized phase II study in metastatic renal cell carcinoma. Ann. Oncol. 2015, 26, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Schmidinger, M. Understanding and managing toxicities of vascular endothelial growth factor (VEGF) inhibitors. EJC Suppl. 2013, 11, 172–191. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Rossi, E.; Thomas, A.M.; Schnizari, G.; Masucci, L.; Quaranta, G.; et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat. Commun. 2020, 11, 4333. [Google Scholar] [CrossRef]
- Anderson, R.; Jatoi, A.; Robert, C.; Wood, L.S.; Keating, K.N.; Lacouture, M.E. Search for evidence-based approaches for the prevention and palliation of hand–foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). Oncologist 2009, 14, 291–302. [Google Scholar] [CrossRef]
- Brown, T.J.; Gupta, A. Management of cancer therapy-associated oral mucositis. JCO Oncol. Pract. 2020, 16, 103–109. [Google Scholar] [CrossRef]
- Wolter, P.; Stefan, C.; Decallonne, B.; Dumez, H.; Bex, M.; Carmeliet, P.; et al. The clinical implications of sunitinib-induced hypothyroidism: A prospective evaluation. Br. J. Cancer 2008, 99, 448–454. [Google Scholar] [CrossRef]
- Soefje, S.A.; Karnad, A.; Brenner, A.J. Common toxicities of mammalian target of rapamycin inhibitors. Target. Oncol. 2011, 6, 125–129. [Google Scholar] [CrossRef]
- Porta, C.; Osanto, S.; Ravaud, A.; Climent, M.A.; Vaishampayan, U.; White, D.A.; et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur. J. Cancer 2011, 47, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Rini, B.I.; Battle, D.; Figlin, R.A.; George, D.J.; Hammers, H.; Hutson, T.; et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J. Immunother. Cancer 2019, 7, 354. [Google Scholar] [CrossRef]
- Grünwald, V.; Weikert, S.; Pavel, M.E.; Hörsch, D.; Lüftner, D.; Janni, W.; et al. Practical management of everolimus-related toxicities in patients with advanced solid tumors. Onkologie 2013, 36, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Boers-Doets, C.B.; Epstein, J.B.; Raber-Durlacher, J.E.; Ouwerkerk, J.; Logan, R.M.; Brakenhoff, J.A.; et al. Oral adverse events associated with tyrosine kinase and mammalian target of rapamycin inhibitors in renal cell carcinoma: A structured literature review. Oncologist 2012, 17, 135–144. [Google Scholar] [CrossRef]
- Peterson, D.E.; Boers-Doets, C.B.; Bensadoun, R.J.; Herrstedt, J. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v139–v151. [Google Scholar] [CrossRef]
- Albiges, L.; Chamming’s, F.; Duclos, B.; Stern, M.; Motzer, R.J.; Ravaud, A.; et al. Incidence and management of motor inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann. Oncol. 2012, 23, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Improving care and promoting health in populations: Standards of medical care in diabetes−2021. Diabetes Care 2021, 44, S7–S14. [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Ipid modification to reduce cardiovascular risk. Eur Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Gaasbeek, A.; Meinders, A.E. Hypophosphatemia: An update on its etiology and treatment. Am. J. Med. 2005, 118, 1094–1101. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer– immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gerber, D.E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: A review. Semin. Cancer Biol. 2020, 64, 1044–1579. [Google Scholar] [CrossRef] [PubMed]
- Teufel, A.; Zhan, T.; Härtel, N.; Bornschein, J.; Ebert, M.P.; Schulte, N. Mini- review management of immune related adverse events induced by immune checkpoint inhibition. Cancer Lett. 2019, 456, 80–87. [Google Scholar] [CrossRef]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Prasad, V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef]
- Cella, D.; Grünwald, V.; Nathan, P.; Doan, J.; Dastani, H.; Taylor, F.; et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: A randomised, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 994–1003. [Google Scholar] [CrossRef]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.; Grünwald, V.; Escudier, B.; Hammers, H.J.; George, S.; Nathan, P.; et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): A randomised, phase 3 trial. Lancet Oncol. 2019, 20, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins Sherry Anadkat, M.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J. Natl. Compr. Canc Netw. 2019, 17, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sykiotis, G.P.; Maillard, M.; Fraga, M.; Ribi, C.; Kuntzer, T.; et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019, 20, e54–e64. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Ernstoff, M.; Wang, Y.; Menzies, A.; Puzanov, I.; Grivas, P.; et al. Rechallenge patients with immune checkpoint inhibitors following severe immune-related adverse events: Review of the literature and suggested prophylactic strategy. J. Immunother. Cancer 2020, 8, e000604. [Google Scholar] [CrossRef]
- Rzeniewicz, K.; Larkin, J.; Menzies, A.M.; Turajlic, S. Immunotherapy use outside clinical trial populations: Never say never? Ann. Oncol. 2021, 32, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Buti, S.; Agostinelli, V.; Bersanelli, M. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin. Oncol. 2019, 46, 362–371. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Yang, J.C.; Hughes, M.; Kammula, U.; Royal, R.; Sherry, R.M.; Topalian, S.L.; et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 2007, 30, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Takagi, T.; Kondo, T.; Homma, C.; Tachibana, H.; Fukuda, H.; et al. Clinical-kidney cancer association between immune-related adverse events and prognosis in patients with metastatic renal cell carcinoma treated with nivolumab. Urol. Oncol. 2019, 37, 355.e21–355.e29. [Google Scholar] [CrossRef]
- Verzoni, E.; Cartenì, G.; Cortesi, E.; Giannarelli, D.; de Giglio, A.; Sabbatini, R.; et al. Real-world efficacy and safety of nivolumab in previously- treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: The Italian expanded access program. J. Immunother. Cancer 2019, 7, 99. [Google Scholar] [CrossRef]
- Martini, D.J.; Hamieh, L.; McKay, R.R.; Harshman, L.C.; Brandao, R.; Norton, C.K.; et al. Durable clinical benefit in metastatic renal cell carcinoma patients who discontinue pd-1/pd-l1 therapy for immune-related adverse events. Cancer Immunol. Res. 2018, 6, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Rini, B.I.; Atkins, M.B.; Choueiri, T.K.; Thomaidou, D.; Rosbrook, B.; Thakur, M.; et al. Time to resolution of axitinib-related adverse events after treatment interruption in patients with advanced renal cell carcinoma. Clin. Genitourin. Cancer 2021, 19, e306–e312. [Google Scholar] [CrossRef]
- Study of Cabozantinib in Combination with Nivolumab and Ipilimumab In Patients with Previously Untreated Advanced or Metastatic Renal Cell Carcinoma—Full Text View—ClinicalTrials.gov [Internet]. Available online: https://clinicaltrials.gov/ct2/show/NCT03937219.
- Apolo, A.B.; Nadal, R.; Girardi, D.M.; Niglio, S.A.; Ley, L.; Cordes, L.M.; et al. Phase I study of cabozantinib and nivolumab alone or with ipilimumab for advanced or metastatic urothelial carcinoma and other genitourinary tumors. J. Clin. Oncol. 2020, 38, 3672–3684. [Google Scholar] [CrossRef]
- Jonasch, E.; Donskov, F.; Iliopoulos, O.; Rathmell, W.K.; Narayan, V.K.; Maughan, B.L.; et al. Belzutifan for Renal Cell Carcinoma in von Hippel– Lindau Disease. N. Engl. J. Med. 2021, 385, 2036–2046. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Bauer, T.M.; Papadopoulos, K.P.; Plimack, E.R.; Merchan, J.R.; McDermott, D.F.; et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: A phase 1 trial and biomarker analysis. Nat. Med. 2021, 27, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. 4), iv96–iv110. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Hessel, C.; Halabi, S.; Sanford, B.; Michaelson, M.D.; Hahn, O.; et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update. Eur. J. Cancer. 2018, 94, 115–125. [Google Scholar] [CrossRef] [PubMed]
- van der Veldt, A.A.M.; Boven, E.; Helgason, H.H.; van Wouwe, M.; Berkhof, J.; de Gast, G.; et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br. J. Cancer 2008, 99, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Diekstra, M.H.M.; Swen, J.J.; Boven, E.; Castellano, D.; Gelderblom, H.; Mathijssen, R.H.J.; et al. CYP3A5 and ABCB1 polymorphisms as predictors for sunitinib outcome in metastatic renal cell carcinoma. Eur. Urol. 2015, 68, 621–629. [Google Scholar] [CrossRef]
- de Velasco, G.; Gray, K.P.; Hamieh, L.; Urun, Y.; Carol, H.A.; Fay, A.P.; et al. Pharmacogenomic markers of targeted therapy toxicity in patients with metastatic renal cell carcinoma. Eur. Urol. Focus. 2016, 2, 633–639. [Google Scholar] [CrossRef]
- Diekstra, M.H.; Belaustegui, A.; Swen, J.J.; Boven, E.; Castellano, D.; Gelderblom, H.; et al. Sunitinib-induced hypertension in CYP3A4 rs4646437 A-allele carriers with metastatic renal cell carcinoma. Pharmacogenomics 2017, 17, 42–46. [Google Scholar] [CrossRef]
- van der Zanden, L.F.M.; Vermeulen, S.H.; Oskarsdottir, A.; Maurits, J.S.F.; Diekstra, M.H.M.; Ambert, V.; et al. Description of the EuroTARGET cohort: A European collaborative project on TArgeted therapy in renal cell cancer—GEnetic- and tumor-related biomarkers for response and toxicity. Urol. Oncol. 2017, 35, 529.e9–529.e16. [Google Scholar] [CrossRef]
- Andrews, M.C.; Duong, C.P.M.; Gopalakrishnan, V.; Iebba, V.; Chen, W.S.; Derosa, L.; et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
This is an open access article under the terms of a license that permits non-commercial use, provided the original work is properly cited. © 2022 The Authors. Société Internationale d'Urologie Journal, published by the Société Internationale d'Urologie, Canada.
Share and Cite
Young, K.; Schmitt, A.M.; Mukherji, D.; Spain, L.; Schmidinger, M.; Pickering, L.M. 2022 WUOF/SIU International Consultation on Urological Diseases: Management of Toxicity and Side Effects of Systemic Therapy for Renal Cell Carcinoma. Soc. Int. Urol. J. 2022, 3, 484-498. https://doi.org/10.48083/SYAB9165
Young K, Schmitt AM, Mukherji D, Spain L, Schmidinger M, Pickering LM. 2022 WUOF/SIU International Consultation on Urological Diseases: Management of Toxicity and Side Effects of Systemic Therapy for Renal Cell Carcinoma. Société Internationale d’Urologie Journal. 2022; 3(6):484-498. https://doi.org/10.48083/SYAB9165
Chicago/Turabian StyleYoung, Kate, Andreas M. Schmitt, Deborah Mukherji, Lavinia Spain, Manuela Schmidinger, and Lisa M. Pickering. 2022. "2022 WUOF/SIU International Consultation on Urological Diseases: Management of Toxicity and Side Effects of Systemic Therapy for Renal Cell Carcinoma" Société Internationale d’Urologie Journal 3, no. 6: 484-498. https://doi.org/10.48083/SYAB9165
APA StyleYoung, K., Schmitt, A. M., Mukherji, D., Spain, L., Schmidinger, M., & Pickering, L. M. (2022). 2022 WUOF/SIU International Consultation on Urological Diseases: Management of Toxicity and Side Effects of Systemic Therapy for Renal Cell Carcinoma. Société Internationale d’Urologie Journal, 3(6), 484-498. https://doi.org/10.48083/SYAB9165





