Abstract
For the past 10 years, disease-modifying therapy (DMT) options for multiple sclerosis (MS) have grown remarkably where DMTs have been shown to reduce the risk of MS relapses. MS patients are advised to begin treatment with a DMT shortly after diagnosis to limit the possibility of disease progression over time. While patients with radiologically isolated syndrome do not require pharmacologic treatment, high-risk patients with clinically isolated syndrome are advised to start DMTs. This article provides evidence-based recommendations for DMT use in MS management, helping healthcare practitioners advise patients on treatment decisions. We aim to provide recommendations for the management of acute MS relapses. The recommendations herein were developed following the gathering of a panel of experts after evaluating international guidelines, and the latest evidence was collected through a comprehensive literature review.
1. Introduction
Recently, the availability of disease-modifying therapies (DMTs) for managing multiple sclerosis has dramatically increased. These treatments are diverse in their therapeutic profiles: they differ in the extent to which they suppress MS relapses (clinical efficacy), as well as their underlying therapeutic mechanisms, duration of action, modes of administration, tolerability, and safety profiles [1]. DMTs are not classified for the purpose of these consensus recommendations. However, it is important to note that DMTs are sometimes classified based on the method of administration, the approach to treatment, and the efficacy profile. They are often divided into “first-line” or “second-line” treatment options, with the latter being possibly restricted in the treatment-naïve patient [2,3,4]. Additionally, some drugs are used without a prescription for the management of MS, such as rituximab and ocrelizumab which are anti-CD20 monoclonal antibodies, and are approved for use in MS [5,6].
Designing a therapeutic regimen for patients with MS has never been as complex and challenging as it is today. Conversely, the diverse characteristics of today’s DMTs provide an unparalleled opportunity to individualize care, taking into account the needs and desires of the patient, while seeking to suppress the activity of the disease [7,8].
The prevalence of MS in Saudia Arabia is rising and the clinical course was found to be primary progressive in 19.1% of cases, relapsing-remitting in 60.7%, and progressive relapsing in 20.2% [9]. Therefore, the Ministry of Health (MOH) has established a working group formed of a multidisciplinary expert panel of neurologists, clinical pharmacists, neuroradiologists, and nurses with clinical experience in MS management, to discuss and agree on evidence-based recommendations. Workshop facilitators compiled all the relevant international guidelines and peer-reviewed articles. During the workshop, the nominal group technique was adopted and consensus was defined as an agreement of at least 75% of the attendees [10]. Following the session, a document describing the agreed-upon recommendations was submitted for review by an expert committee from the Ministry of Health as well as work group leaders.
The overall aim of these recommendations is to support all healthcare professionals who encounter patients with MS and related disorders in their diagnosis and management. This article summarizes recommendations of the major guidelines on DMT use in MS long-term management, acute-MS-relapse treatment, as well as the use of radiology/imaging for the diagnosis and monitoring of patients.
2. Use of Disease-Modifying Therapies for the Management of Multiple Sclerosis
2.1. Need for Early Use of DMTs
The proper use of DMTs has been proven to reduce the possibility of MS relapse. Therefore, all patients must be counselled to begin treatment with a DMT shortly after diagnosis to minimize the disability progression over time and to enhance long-term clinical outcomes [3,11]. The following sections guide the selection of a DMT appropriate for a patient with MS. The prevailing level of MS-disease activity, the presence or absence of concomitant conditions, and the patient’s personal preferences and circumstances are key issues to consider when choosing a DMT.
Fitting MS treatment around a patient’s needs to plan a family is a particular challenge for many MS patients and will be considered in detail in a separate article.
2.2. Classification of DMTs
For the past 10 years, the treatment options for MS have grown significantly, and several DMTs have been used “off-label”, while others have been accepted by international and local regulatory agencies [11]. These DMTs vary significantly in terms of their mechanism of action, route of administration, cost, and side effects [11,12]. The present consensus recommendations will better define when to use DMTs, which DMTs to use, and in which order.
DMTs have not been classified for the purposes of these recommendations. However, it is important to note that DMTs are sometimes classified based on the method of administration, the approach to treatment initiation, or their efficacy profiles [3]. They are also still often referred to as “first-line” or “second-line” treatment options, with the possibility of restriction of the use of a “second-line” DMT in a treatment-naïve patient [4]. Accordingly, formulary restrictions may need to be considered when selecting a DMT.
2.3. Currently Available DMTs for MS in Saudi Arabia
MS management is growing quickly with the introduction of new treatments. It is challenging for any guideline to remain up to date with the evolving market for MS treatments. This is particularly true for new medications, where their limited use thus has far prevented an informed expert opinion. Thus, although we list all currently available DMTs, the focus will be placed on the SFDA-licensed options at the time-point of the workshops, as well as therapies that can be assessed by expert opinion and used in regular clinical practice. Table 1 summarizes the details of the currently available DMTs, their USFDA-approval status, and SFDA-registration status.

Table 1.
Disease-modifying therapies for MS currently available in Saudi Arabia.
2.4. Prescribers of DMTs
The decision between therapies should be made based on a benefit–risk assessment and individualized to serve every patient’s needs after discussions between the patient and his/her neurologist. Certain DMTs (namely, ocrelizumab, rituximab, natalizumab, alemtuzumab, and cladribine) should be prescribed and initiated by a neurologist with extensive clinical experience in the treatment of MS, considering that the use of these therapies requires appropriate patient-selection, comprehensive assessment, and proper monitoring including a pre-treatment work-up and DMT laboratory-monitoring.
General Pre-Treatment Work-Up
The following table provides the minimum recommended baseline-investigations before the initiation of any DMT (Table 2). In addition to the baseline investigations, patients should be updated with all immunizations before initiating therapy with DMTs. A baseline MRI of the brain should also be performed.

Table 2.
Baseline investigations before initiation of a disease-modifying treatment (DMT).
2.5. Assessment of Disease Activity in Treated Patients
In patients treated with DMTs, it is advisable to complete a standardized reference brain-MRI within 6 months of the start of the medication and 12 months after starting treatment, as a form of monitoring [11]. It is advisable to follow specific monitoring recommendations, depending on the DMT used (Table 3).

Table 3.
Disease-modifying therapy (DMT)-specific routine-monitoring recommendations.
2.6. Use of Disease-Modifying Therapies in Specific Subgroups of Patients with MS
2.6.1. Radiologically Isolated Syndrome (RIS)
Available data does not suggest the initiation of DMT in patients with RIS [1]. More answers may be provided once the two ongoing randomized, double-blind, placebo-controlled phase III studies evaluating the ability of dimethyl fumarate (ARISE) or teriflunomide (TERIS) have been published [13]. However, follow-up of patients with RIS by an MS specialist is recommended [14].
2.6.2. Clinically Isolated Syndrome (CIS)
For patients with inactive CIS (no relapses in the previous 2 years and no new MRI lesion-activity imaging), serial imaging may be recommended at least once a year for the first 5 years, with a close follow-up at least every 6 months, rather than initiating DMT [3].
After discussing the pros and cons of treatment, DMTs should be offered to patients with a single clinical demyelinating event and at least two brain lesions characteristic of MS [3]. The decision to select a preferred DMT should be reached after discussions with the patient. The following DMTs may be considered for CIS: interferons [15,16,17], glatiramer acetate, teriflunomide [18,19], and dimethyl fumarate [11].
2.6.3. Inactive Relapsing–Remitting Multiple Sclerosis (RRMS)
Inactive RRMS is MS with no clinical attack (relapses) within the previous 2 years, and no evidence of active, new MRI-lesions [3]. For managing the treatment of naïve-inactive RRMS, a shared decision-making approach between the patient and treating neurologist should be used. Serial imaging may be recommended for a minimum of once a year for the first 5 years, with a close follow-up at least every 6 months, rather than initiating DMT (Figure 1) [3].
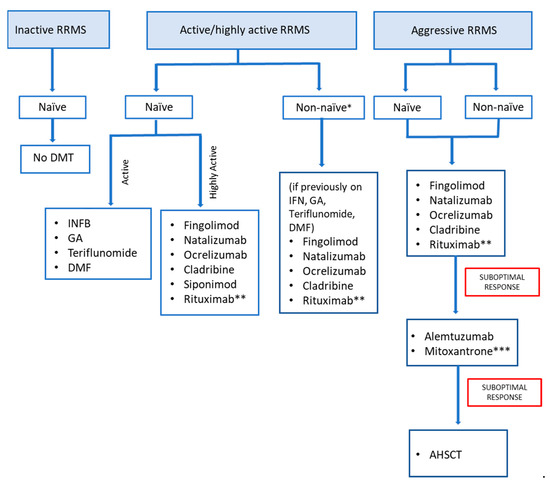
Figure 1.
Management algorithm for using disease-modifying therapies (DMT) for relapsing–remitting multiple sclerosis (RRMS). Figure was created by authors. No permission needed.* Escalate or switch DMT according to the extent of disease activity, safety, tolerability, and adherence. ** Off-label use. *** Only if benefits greatly outweigh risks.
2.6.4. Active or Highly-Active RRMS
Active/highly active RRMS is characterized by relapses and/or MRI activity in the past 12 months [11]. For treating naïve-active/highly active RRMS, early treatment initiation with any of the following DMTs may be considered [11] (Figure 1):
- Interferon beta [20]
- Glatiramer Acetate
- Teriflunomide [21]
- Dimethyl fumarate [22]
- Fingolimod [1,2]
- Natalizumab [3]
- Ocrelizumab [4]
- Cladribine [5]
- Siponimod [6,7]
- Rituximab (off-label)
The choice of therapy is guided by [1,11]:
- Characteristics and medical issues of patients
- Disease severity
- Adverse effects of drugs
- Drug accessibility
2.6.5. Escalation/Switching/Stopping Treatment in a Patient with RRMS
The decision to escalate or switch DMTs may be driven by disease severity and drug safety, tolerability, and adherence. In general, people with MS take the same DMT unless suboptimal response, the appearance of adverse events, or adherence issues are recorded [23].
Washout periods differ for individual DMTs (Table 4), and this must be factored into a strategy for switching treatment [1].

Table 4.
Washout period for switching between disease-modifying therapies.
Switch due to suboptimal response: When considering switching DMTs, there is no consensus on a definition for treatment “failure” or “non-responders”. There is no universally accepted standard time for switching DMTs. In general, when evaluating disease evolution in treated patients, combining MRI with clinical measures should be considered [11,24]. Monitoring disease activity from clinical onset and using MRI for new lesion detection guides treatment decisions [3].
When monitoring response to treatment with a DMT, a reference brain MRI usually during 6 months of starting treatment should be compared with a later brain MRI completed within 12 months after treatment onset [11,24]. Measurement of new T2 lesions is the recommended MRI method, supplemented with GAD-enhancing lesions [11]. Attacks or newly detected lesions on the MRI might develop after starting a DMT and before the treatment becomes effective [3]. Changing from one DMT to another should be discussed with patients who have been taking DMT for a prolonged period of time which is enough for the treatment to have a full response. These patients should be compliant with their therapy when they experience attacks (one or more), unequivocally new MRI-detected lesions (two or more), or worsening disability on examination after more than one year of using a DMT [3].
For non-naïve active/highly active RRMS patients who have previously taken interferons, glatiramer acetate, teriflunomide, dimethyl fumarate, or any of the other treatments, escalation options may be considered: fingolimod, natalizumab, ocrelizumab cladribine, and rituximab (off-label) [25,26].
Switch due to poor adherence: Adherence to treatment for MS patients is affected by tolerability, adverse effects, cost, and route of administration. MS patients on injectable DMTs could suffer from discomfort at the injection site which requires a switch to DMT with a different administration route with less-frequent dosing [3]. Selection of another DMT is also needed when adverse events negatively influence adherence [3]. Due to the different adverse events associated with DMTs (Table 5), engaging patients in the decision-making process regarding treatment options and assessing their needs may also enhance compliance with DMT [3].

Table 5.
Disease-modifying therapy (DMT)-specific and adverse events.
Switching due to laboratory abnormalities: persistent laboratory abnormalities (for example, decreased WBC counts or elevated liver enzymes) may prompt switching of treatment or reduced dosage (where data are available at different doses, for example, for interferons, teriflunomide) or reduced frequency of administration [3].
Switching from natalizumab due to high PML risk: While natalizumab has a good tolerability profile, it is linked to a rare possible adverse event whereby immune system depression allows for JCV activation and progressive multifocal leukoencephalopathy (PML) (see above). Once a patient receiving natalizumab becomes JCV-antibody positive, with an index higher than 0.9, it is necessary to switch therapies [3,41].
Clinicians must inform patients that the discontinuation of natalizumab may be followed by a return to previous MS disease-activity within 6 months of discontinuation [3]. To maintain adequate disease control and reduce the risk of disease activity resuming, the initiation of an alternative treatment option within 6 to 8 weeks of natalizumab discontinuation should be considered. The choice of therapy guide should be based on every patient’s past medical history and available safe treatment options. It is recommended that clinicians consider CSF analysis to exclude asymptomatic progressive multifocal leukoencephalopathy (PML) before starting cell-depleting therapies (cladribine, alemtuzumab, mitoxantrone or hematopoietic stem-cell transplant).
2.6.6. Aggressive MS (Treatment Non-Naïve and Treatment-Naïve)
Aggressive RRMS has one or more of the following characteristics, irrespective of the prior-treatment history [42]:
- EDSS score ≥ 4 within 5 years of starting treatment.
- ≥two relapses with partial resolution over the last 12 months.
- >two MRI scans with new or growing T2-lesions or enhancing lesions, in spite of treatment.
- Lack of improvement while on therapy with one or more DMTs for up to one year.
Treatment for worsening MS should be prescribed and initiated by a neurologist with extensive clinical experience in the treatment of MS [1,43]. The following treatment options can be initiated in aggressive MS, whether or not the patient has received a DMT previously: fingolimod [25], cladribine, natalizumab, ocrelizumab, rituximab (off-label) (Figure 1). Treatment escalation to alemtuzumab should be considered for a patient with aggressive RRMS who demonstrated a partial response to an initial high-efficacy DMT [37,44,45]. Mitoxantrone should not be prescribed to patients due to the large number of severe side effects side-effects, except if the possible benefits greatly trump the risks [3,46,47].
Autologous hematopoietic stem-cell transplantation (AHSCT) after immunoablation has been used for the past two decades for the management of treatment-refractory autoimmune illnesses [48]. A recent study showed that MS activity-free-survival was 69·6% 3 years after transplantation [49]. The recent results of the MIST study suggest that AHSCT is significantly more effective than DMT and can be administered safely [50]. Despite encouraging results for efficacy and safety, AHSCT should be reserved as a final attempt to manage MS refractory to DMT due to its serious immunosuppression and potential side effects [51]. It should also be restricted to centers with transplant hematology expertise [52].
2.6.7. Progressive MS
DMTs for progressive-MS patients should be prescribed and initiated by a neurologist with extensive clinical experience in the treatment of MS.
Ocrelizumab should be considered for patients with PPMS who are ambulatory, with MRI features showing evidence of inflammatory activity, except with risks that exceed benefits [3,11,53,54].
SPMS patients who have superimposed attacks or active lesions on an MRI may benefit from DMT. For active SPMS, any of the following DMTs may be considered (individualized therapy in accordance with expected benefits and risks) [3,11,55]:
Fingolimod.
Natalizumab.
Ocrelizumab.
Cladribine.
Rituximab (off-label).
Siponimod [56].
Randomized controlled trials have not yet directly assessed the discontinuation of DMTs in patients with non-active SPMS. Moreover, clinical trials have not addressed the safety and efficacy of DMT in patients with SPMS who are non-ambulatory. Therefore, we recommend continuing DMT in patients with non-active SPMS who are able to walk without assistance. The use of DMT in non-ambulatory SPMS is advised if the advantages trump the risks [57]. Discontinuation of DMT is recommended in non-ambulatory SPMS patients without evidence of disease activity (no relapses or new MRI lesions) for a minimum of two years.
2.6.8. Use of Biosimilar Agents and Generic Medications
Generic or biosimilar preparations are becoming available as alternatives to some costly, branded products, which will significantly improve the accessibility of many MS treatments and reduce the overall costs of healthcare. In the USA, the use of generic medicines increased from 80% to 90% between 2016 and 2019 [2,3] and most available MS therapies are biological medications. However, biosimilar alternatives to these have yet to be approved for therapeutic use in Saudi Arabia (although biosimilar-interferon is available in Latin America and Iran). Therefore, we recommend using approved generic and biosimilar medications to treat and manage patients with MS, after their approval by the regulatory authorities, internationally (FDA or EMA) and locally for biosimilars, and locally only for generics.
2.7. COVID-19 and MS DMTs (Recommendations as of July 2020)
There is no evidence that shows that MS patients are at a higher risk of contracting COVID-19. However, certain factors could lead to a possible increase in the risk of complications in patients with MS who develop COVID-19 [58]:
- Chronic medical comorbidities, such as bronchial asthma, cardiac disease, diabetes, and malignancy.
- Age older than 65.
- Obesity.
- Restricted mobility.
The effect of DMT on COVID-19 disease severity disease-severity remains unclear. It is necessary that healthcare practitioners discuss with their patients the effect of MS therapies on the possibility of contracting COVID-19. Delaying the start of DMT might reduce the immune system’s ability to respond to infections (alemtuzumab, cladribine, fingolimod, siponimod, ocrelizumab, and rituximab), and is not recommended.
We provide the following specific recommendations regarding COVID-19 and MS:
Patients should reduce COVID-19 infection-risk by following WHO and local-health-authority recommendations [59]:
Practice social distancing by staying home and maintaining 2 m distance from others when needing to go outside.
Frequent handwashing with soap and water.
Frequent use of hand sanitizer.
Respiratory hygiene—covering coughs or sneezes.
Avoiding touching the face.
Disinfect surfaces that are frequently touched.
Follow the Ministry of Health recommendations for mask use in the community.
Healthcare practitioners managing asymptomatic MS-patients with a positive test for COVID-19 should recommend [60]:
Continue the following DMTs: interferon-beta, glatiramer acetate, teriflunomide, fingolimod, siponimod, dimethyl fumarate, or natalizumab.
Healthcare practitioners treating COVID-19 infection in MS patients should recommend [59,61]:
- Continue interferon-β, glatiramer acetate, dimethyl fumarate, fingolimod, teriflunomide, siponimod, or natalizumab if COVID-19 is present but symptoms are mild.
- Otherwise, in patients with symptomatic, severe COVID-19 (e.g., patients with pneumonia, septic shock, or on mechanical ventilation), temporarily stop all DMTs (injectables, oral, and infusion therapies) until the patient is asymptomatic—especially for patients with an increased risk of complications (older age, greater disability, anti-CD 20 B cell therapy) (be aware of the risk of disease reactivation following the discontinuing of fingolimod and natalizumab) [62].
- Delay additional doses of alemtuzumab, cladribine, ocrelizumab, and rituximab in patients with mild symptoms of COVID-19.
KEY POINTS ON THE USE OF DMTS FOR THE MANAGEMENT OF MS
The decision between therapies should depend on a benefit–risk evaluation, and should be customized to serve the individual requirements of the patient after dis-cussions with him/her. Considering specific MS-subtypes:
- RIS: Evidence does not support the initiation of DMT in RIS patients.
- CIS: The following DMTs may be considered for CIS: interferons, glatiramer acetate, teriflunomide, and dimethyl fumarate.
- RRMS: For treatment of naïve-inactive RRMS, serial imaging may be recommended for a minimum of once a year for the initial 5 years, and an extended follow-up at least every 6 months, rather than initiating DMT. For treatment of naïve-active/highly active RRMS, early treatment-initiation with any of the DMTs may be considered, with the choice of therapy guided by the severity of the disease, the patient’s comorbidities and the availability and safety of the drug for that patient. For treatment of non-naïve active/highly active RRMS, the decision to escalate/switch DMTs may be driven by the extent of disease activity and tolerability and compliance with medication. The following treatment options can be initiated in both naïve and non-naïve aggressive MS: fingolimod, cladribine, natalizumab, ocrelizumab, rituximab. In addition, treatment escalation to alemtuzumab can be considered if the response to the initial high-efficacy DMT is suboptimal.
- Progressive MS: PPMS: for PPMS patients who are ambulatory, with MRI features showing evidence of inflammatory activity, treatment with ocrelizumab may be considered, except if there are risks outweighing the benefits. SPMS: For active SPMS, any of the following DMTs may be considered: (1) fingolimod, (2) natalizumab, (3) ocrelizumab, (4) cladribine, (5) rituximab (off-label), and (6) siponimod [40].
- The use of DMTs will need to be individualized with consideration of each medication’s expected benefitThe use of approved generic and biosimilar medications to treat and manage patients with MS should be considered. s and risks.
- MS and COVID-19: Counsel patients to avoid infection with COVID-19 (hygiene, social distancing) Do not delay DMT per se in the absence of a positive COVID-19 test or symptoms. Continue interferon-β, glatiramer acetate, dimethyl fumarate, fingolimod, teriflunomide, siponimod, or natalizumab if COVID-19 is present but symptoms are mild. Delay doses of alemtuzumab, cladribine, ocrelizumab, or rituximab for 2 weeks or until a negative COVID-19 test before initiation and follow-up.
3. Management of Acute Relapse
3.1. Diagnosis of Acute Relapse
Relapses are the most significant feature of RRMS and are usually linked to significant functional impairment and worsening quality of life [63]. Relapses in MS are new or deteriorating neurological losses lasting for 24 h or more without fever or infection [61]. MS relapse typically evolves over hours or days (though sometimes considerably longer), reaching a nadir in several days, and followed by a slow recovery course over a period of weeks to months [63]. Recovery may be complete or partial, with residual long-term disability [63].
Furthermore, clinicians must rule out pseudo relapse, which is a temporary flare-up in MS symptoms unrelated to the long-term disease course of MS. Pseudo relapse is typically caused by an infection, although individual patient factors such as stress may be involved [63]. Treating radiological activities alone as relapses with regard to acute therapy is not recommended.
3.2. Interventions for Acute Relapse
Figure 2 summarizes intervention strategies for managing acute MS relapses.
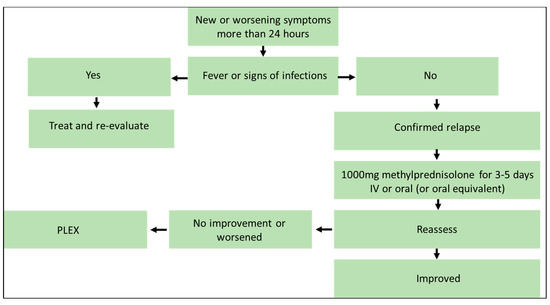
Figure 2.
Algorithm for management of acute relapse. Figure was created by authors. No permission needed.
In patients with mild exacerbations, there is a consensus that immediate treatment may not be required. Treatment should be started as soon as possible for patients with moderate-to-severe confirmed MS attacks. A high dose (1 g/day) of IV methylprednisolone for 3 to 5 days is recommended as first-line treatment [63]. It is also an option to administer high-dose methylprednisolone instead of IV formulation [63]. If oral methylprednisolone is not available, prednisone (1250 mg prednisone = 1000 mg methylprednisolone) is considered an acceptable alternative that provides similar clinical efficacy, bioavailability, and gastric tolerance [64,65,66]. There are few differences in efficacy between the oral and intravenous modes of administration, according to the results of randomized comparisons, and these treatments can be considered equivalent [33,67,68]. Oral prednisone tapering after IV methylprednisolone administration could be considered, taking into consideration the patient’s situation. However, data suggest no benefit for oral tapering [63].
Plasmapheresis (PLEX) has been shown to be effective in the setting of acute MS relapses in subgroup analyses of randomized trials in patients with the relapsing form of the disease, including in patients who have not responded to IV methylprednisolone [69,70]. Thus, PLEX may be considered as a second-line treatment in patients with severe, steroid-resistant relapse [71]. However, the use of intravenous immunoglobulin (IVIG) as a monotherapy for MS relapses is not supported by sufficient data [72].
KEY POINTS ON DIAGNOSIS AND MANAGEMENT OF ACUTE RELAPSE
- A relapse is a new or worsening neurological loss or weakness that lasts a minimum of 24 h, in the absence of fever or infectious disease.
- Clinicians should rule out pseudo-relapse, which is a temporary flare-up in MS symptoms unrelated to the long-term disease course of MS.
- In patients with mild exacerbations, there is a consensus that immediate treatment may not be required.
- In patients with moderate-to-severe confirmed MS relapses, high-dose intravenous/oral methylprednisolone (or the oral equivalent) is recommended as first-line treatment.
- Plasmapheresis may be considered a second-line treatment option.
4. Conclusions
Selecting the appropriate disease-modifying therapy for an MS patient is crucial to reduce the risk of relapse, and should be considered shortly after diagnosis of MS. DMT initiation may be especially beneficial for patients belonging to multiple sclerosis subgroups with aggressive clinical courses, high disease activity, and high risk of relapse.
Author Contributions
Conceptualization, Y.M.A.M. and M.A.A.J.; methodology, Y.M.A.M. and M.A.A.J.; writing—original draft preparation, Y.M.A.M., M.A.A.J. and I.A.A.T.; writing—review and editing. Validation and review, M.A.A. (Maha A. AlAmmari), N.A.F., E.N.A., D.A.A., S.A.A., A.H.A.-J., M.A.A. (Maeed A. AlKathiri), M.M.A., M.A.A. (Mousa A. Almejally), H.Y.A.-M., H.S.A.O., G.H.A., R.H.A.Y., M.A.B., S.A.B., R.F.B., E.J.C., M.H., A.M.K. (Abid M. Kareem), A.M.K. (Amr M. Khardaly), S.M., L.H.S., J.A.S., E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Ministry of Health, Kingdom of Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Yaser Al Malik received a speaker honorarium from Merck, and Roche, consultancy fees from Merck, Genzyme, Novartis, and Roche, and travel support from Roche, Biogen, and Serono. Ibtisam Al Thubaiti received a speaker honorarium from Novartis and Merck Serono, and received consultancy fees from Merck Serono. Maha Abdulaziz AlAmmari received research grants from the KAIMRC. Norah AlFugham received travel support from Sanofi Genzyme. Eman Nassim Ali received a speaker honorarium from Biogen and received travel support from Novartis, Sanofi, and Merck. Mona AlKhawajah received a speaker honorarium, consultancy fees, and travel support from Roche, Merck, Sanofi, Biogen, Novartis, Saja, Hikma, and Actelion. Mousa Almejally received a speaker honorarium from Sanofi and Biogen, and travel support from Saja, Novartis, Biogen, and Merck. Ghadah AlTowaijri received research grants from King Fahad Medical City, a speaker honorarium from Novartis, and travel support from Genzyme and Novartis. Rumaiza Hussein Al Yafeai received a speaker honorarium from Novartis and Roche. Mohammed Ahmed Babakkor received travel support from Bayer. Saeed A. Bohlega received research grants from King Abdulaziz City for Science and Technology, and the King Salman Center for Disability Research, a speaker honorarium from Allergan Biologix, Sanofi, Novartis, and Merck, consultancy fees from Allergan Biologix, Sanofi, Novartis, Merck, and Abbvie, and travel support from Allergan Biologix, Sanofi, Novartis, Merck, and Abbvie. Reem F. Bunyan received a speaker honorarium and travel support from Merck, Novartis, and Roche. Edward J. Cupler received a speaker honorarium from Novartis, Biogen, Sanofi, and Merck and received travel support from Novartis, Biogen, Sanofi, and Merck. Seraj Makkawi received a speaker honorarium from Merck and a speaker honorarium and travel support from Roche. Jameelah Saeedi received speaker honorarium and consultancy fees or travel support from Roche, Novartis, Merck, Hikma, Biologix, Sanofi, Bayer. Eslam Shosha received a speaker honorarium from Biologix, Hikma, and Merck, consultancy fees from Merck and Sanofi, and travel support from Biologix, Merck, Sanofi, and Roche. Mohammad Al Jumah received consultancy fees and speaker honoraria from Merck, Biogen, Biologix, Novartis, Sanofi, Bayer, and Roche and received research grants from Merck. The following authors declared no conflicts of interest regarding the publication of these guidelines: Dema A. Alissa, Maeed ALKathiri, Hessa Sharar AlOtaibi, Mohammed Hakami, Abid Mohammad Kareem, Amr M. Khardaly, Leena H. Saeed, Hajer Almudaiheem, Ahmed Al-Jedai. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Yamout, B.; Sahraian, M.; Bohlega, S.; Al-Jumah, M.; Goueider, R.; Dahdaleh, M.; Inshasi, J.; Hashem, S.; Alsharoqi, I.; Khoury, S.; et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to the MENACTRIMS guidelines. Mult. Scler. Relat. Disord. 2020, 37, 101459. [Google Scholar] [CrossRef] [PubMed]
- Alroughani, R.; Inshasi, J.S.; Deleu, D.; Al-Hashel, J.; Shakra, M.; Elalamy, O.R.; Shatila, A.O.; Al-Asmi, A.; Al Sharoqi, I.; Canibano, B.G.; et al. An overview of high-efficacy drugs for multiple sclerosis: Gulf region expert opinion. Neurol. Ther. 2019, 8, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rae-Grant, A.; Day, G.S.; Marrie, R.A.; Rabinstein, A.; Cree, B.A.; Gronseth, G.S.; Haboubi, M.; Halper, J.; Hosey, J.P.; Jones, D.E.; et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 777–788. [Google Scholar] [CrossRef]
- Metz, L.M. Clinically isolated syndrome and early relapsing multiple sclerosis. Neurology 2019, 25, 670–688. [Google Scholar] [CrossRef] [PubMed]
- Berntsson, S.A.-O.; Kristoffersson, A.; Boström, I.; Feresiadou, A.; Burman, J.; Landtblom, A.M. Rapidly increasing off-label use of rituximab in multiple sclerosis in Sweden—Outlier or predecessor? Acta Neurol. Scand. 2018, 138, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sellebjerg, F.; Blinkenberg, M.; Sorensen, P.A.-O. Anti-CD20 Monoclonal Antibodies for Relapsing and Progressive Multiple Sclerosis. CNS Drugs 2020, 34, 269–280. [Google Scholar] [CrossRef]
- Vermersch, P.; Berger, T.; Gold, R.; Lukas, C.; Rovira, A.; Meesen, B.; Chard, D.; Comabella, M.; Palace, J.; Trojano, M. The clinical perspective: How to personalise treatment in MS and how may biomarkers including imaging contribute to this? Mult. Scler. J. 2016, 22, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Supplements and Featured Publications. Individualized Care and Formulary Management in Multiple Sclerosis; Supplements and Featured Publications: Cranbury, NJ, USA, 2020. [Google Scholar]
- Daif, A.K.; Al-Rajeh, S.; Awada, A.; Al Bunyan, M.; Ogunniyi, A.; AbdulJabar, M.; Al Tahan, A. Pattern of presentation of multiple sclerosis in Saudi Arabia: Analysis based on clinical and paraclinical features. Eur. Neurol. 1998, 39, 182–186. [Google Scholar] [CrossRef]
- McMillan, S.S.; King, M.; Tully, M.P. How to use the nominal group and Delphi techniques. Int. J. Clin. Pharm. 2016, 38, 655–662. [Google Scholar] [CrossRef]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. J. 2018, 24, 96–120. [Google Scholar] [CrossRef]
- Shao, H.; Stoecker, C.; Monnette, A.M.; Shi, L. Cost sharing of disease-modifying treatments (DMTs) as policy lever to improve DMTs’ access in multiple sclerosis. Value Health 2018, 21, 1083–1089. [Google Scholar] [CrossRef]
- Lebrun, C. Radiologically isolated syndrome should be treated with disease-modifying therapy—Commentary. Mult. Scler. J. 2017, 23, 1821–1823. [Google Scholar] [CrossRef] [PubMed]
- Okuda, D.T.; Mowry, E.M.; Beheshtian, A.; Waubant, E.; Baranzini, S.E.; Goodin, D.S.; Hauser, S.L.; Pelletier, D. Incidental MRI anomalies suggestive of multiple sclerosis: The radiologically isolated syndrome. Neurology 2009, 72, 800–805. [Google Scholar] [CrossRef]
- Comi, G.; Filippi, M.; Barkhof, F.; Durelli, L.; Edan, G.; Fernández, O.; Hartung, H.P.; Seeldrayers, P.; Sørensen, P.S.; Rovaris, M.; et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: A randomised study. Lancet 2001, 357, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.A.; Kieseier, B.C.; Arnold, D.L.; Balcer, L.J.; Boyko, A.; Pelletier, J.; Liu, S.; Zhu, Y.; Seddighzadeh, A.; Hung, S.; et al. Pegylated interferon β-1a for relapsing-remitting multiple sclerosis (ADVANCE): A randomised, phase 3, double-blind study. Lancet Neurol. 2014, 13, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Comi, G.; De Stefano, N.; Freedman, M.S.; Barkhof, F.; Polman, C.H.; Uitdehaag, B.M.; Casset-Semanaz, F.; Hennessy, B.; Moraga, M.S.; Rocak, S.; et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): A phase 3 randomised controlled trial. Lancet Neurol. 2012, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Confavreux, C.; O’Connor, P.; Comi, G.; Freedman, M.S.; Miller, A.E.; Olsson, T.P.; Wolinsky, J.S.; Bagulho, T.; Delhay, J.L.; Dukovic, D.; et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 247–256. [Google Scholar] [CrossRef]
- O’Connor, P.; Wolinsky, J.S.; Confavreux, C.; Comi, G.; Kappos, L.; Olsson, T.P.; Benzerdjeb, H.; Truffinet, P.; Wang, L.; Miller, A.; et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N. Engl. J. Med. 2011, 365, 1293–1303. [Google Scholar] [CrossRef]
- Jacobs, L.D.; Beck, R.W.; Simon, J.H.; Kinkel, R.P.; Brownscheidle, C.M.; Murray, T.J.; Simonian, N.A.; Slasor, P.J.; Sandrock, A.W.; CHAMPS Study Group. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N. Engl. J. Med. 2000, 343, 898–904. [Google Scholar] [CrossRef]
- Vermersch, P.; Czlonkowska, A.; Grimaldi, L.M.; Confavreux, C.; Comi, G.; Kappos, L.; Olsson, T.P.; Benamor, M.; Bauer, D.; Truffinet, P.; et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: A randomised, controlled phase 3 trial. Mult. Scler. J. 2014, 20, 705–716. [Google Scholar] [CrossRef]
- Viglietta, V.; Miller, D.; Bar-Or, A.; Phillips, J.T.; Arnold, D.L.; Selmaj, K.; Kita, M.; Hutchinson, M.; Yang, M.; Zhang, R.; et al. Efficacy of delayed-release dimethyl fumarate in relapsing-remitting multiple sclerosis: Integrated analysis of the phase 3 trials. Ann. Clin. Transl. Neurol. 2015, 2, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.S.; Selchen, D.; Arnold, D.L.; Prat, A.; Banwell, B.; Yeung, M.; Morgenthau, D.; Lapierre, Y. Treatment optimization in MS: Canadian MS Working Group updated recommendations. Can. J. Neurol. Sci. 2013, 40, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Jalkh, G.A.-O.; Abi Nahed, R.; Macaron, G.; Rensel, M. Safety of newer disease modifying therapies in multiple sclerosis. Vaccines 2021, 9, 12. [Google Scholar] [CrossRef]
- He, A.; Spelman, T.; Jokubaitis, V.; Havrdova, E.; Horakova, D.; Trojano, M.; Lugaresi, A.; Izquierdo, G.; Grammond, P.; Duquette, P.; et al. Comparison of switch to fingolimod or interferon beta/glatiramer acetate in active multiple sclerosis. AMA Neurol. 2015, 72, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, P.; Lucisano, G.; Pozzilli, C.; Brescia Morra, V.; Ghezzi, A.; Millefiorini, E.; Patti, F.; Lugaresi, A.; Zimatore, G.B.; Marrosu, M.G.; et al. Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain 2015, 138, 3275–3286. [Google Scholar] [CrossRef] [PubMed]
- Copaxone (Glatiramer Acetate) Prescribing Information. Available online: https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf (accessed on 29 July 2020).
- O’Connor, P.W.; Li, D.; Freedman, M.S.; Bar-Or, A.; Rice, G.P.; Confavreux, C.; Paty, D.W.; Stewart, J.A.; Scheyer, R. A Phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 2006, 66, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Tecfidera (Dimethyl Fumarate) Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204063s023lbl.pdf (accessed on 3 August 2020).
- Roman, C.; Menning, K. Treatment and disease management of multiple sclerosis patients: A review for nurse practitioners. J. Am. Assoc. Nurse Pract. 2017, 29, 629–638. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Martinelli, V.; Rocca, M.A.; Annovazzi, P.; Pulizzi, A.; Rodegher, M.; Boneschi, F.M.; Scotti, R.; Falini, A.; Sormani, M.P.; Comi, G.; et al. A short-term randomized MRI study of high-dose oral vs intravenous methylprednisolone in MS. Neurology 2009, 73, 1842–1848. [Google Scholar] [CrossRef]
- Butzkueven, H.; Kappos, L.; Pellegrini, F.; Trojano, M.; Wiendl, H.; Patel, R.N.; Zhang, A.; Hotermans, C.; Belachew, S. Efficacy and safety of natalizumab in multiple sclerosis: Interim observational programme results. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, A.; Calatroni, M.; D’Alessandro, M.; Signa, S.; Bertelli, E.; Cioni, M.; Di Marco, E.; Biassoni, R.; Caridi, G.; Ingrasciotta, G.; et al. Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome. Br. J. Clin. Pharmacol. 2018, 84, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- McAtee, C.L.; Lubega, J.; Underbrink, K.; Curry, K.; Msaouel, P.; Barrow, M.; Muscal, E.; Lotze, T.; Srivaths, P.; Forbes, L.R.; et al. Association of rituximab use with adverse events in children, adolescents, and young adults. JAMA Netw. Open 2021, 4, e2036321. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Lubega, J.; Underbrink, K.; Curry, K.; Msaouel, P.; Barrow, M.; Muscal, E.; Lotze, T.; Srivaths, P.; Forbes, L.R.; et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012, 380, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Coles, A.J.; Fox, E.; Vladic, A.; Gazda, S.K.; Brinar, V.; Selmaj, K.W.; Skoromets, A.; Stolyarov, I.; Bass, A.; Sullivan, H.; et al. Alemtuzumab more effective than interferon β-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 2012, 78, 1069–1078. [Google Scholar] [CrossRef]
- Prescribing Information Mavenclad (Cladribine) Tablets. Available online: https://www.emdserono.com/us-en/pi/mavenclad-pi.pdf (accessed on 29 July 2020).
- Prescribing Information Mayzent (Siponimod) Tablets. Available online: https://www.novartis.com/us-en/sites/novartis_us/files/mayzent.pdf (accessed on 29 July 2020).
- Ho, P.R.; Koendgen, H.; Campbell, N.; Haddock, B.; Richman, S.; Chang, I.H. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: A retrospective analysis of data from four clinical studies. Lancet Neurol. 2017, 16, 925–933. [Google Scholar] [CrossRef]
- Rush, C.A.; MacLean, H.J.; Freedman, M.S. Aggressive multiple sclerosis: Proposed definition and treatment algorithm. Nat. Rev. Neurol. 2015, 11, 379–389. [Google Scholar] [CrossRef]
- Menon, S.; Shirani, A.; Zhao, Y.; Oger, J.; Traboulsee, A.; Freedman, M.S.; Tremlett, H. Characterising aggressive multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1192–1198. [Google Scholar] [CrossRef]
- Havrdova, E.; Arnold, D.L.; Cohen, J.A.; Hartung, H.P.; Fox, E.J.; Giovannoni, G.; Schippling, S.; Selmaj, K.W.; Traboulsee, A.; Compston, D.A.; et al. Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy. Neurology 2017, 89, 1107–1116. [Google Scholar] [CrossRef]
- Coles, A.J.; Twyman, C.L.; Arnold, D.L.; Cohen, J.A.; Confavreux, C.; Fox, E.J.; Hartung, H.P.; Havrdova, E.; Selmaj, K.W.; Weiner, H.L.; et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012, 380, 1829–1839. [Google Scholar] [CrossRef]
- Hartung, H.P.; Gonsette, R.; Konig, N.; Kwiecinski, H.; Guseo, A.; Morrissey, S.P.; Krapf, H.; Zwingers, T. Mitoxantrone in progressive multiple sclerosis: A placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002, 360, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Martinelli Boneschi, F.; Vacchi, L.; Rovaris, M.; Capra, R.; Comi, G. Mitoxantrone for multiple sclerosis. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, M.; Sakellari, I.; Gavriilaki, E.; Kimiskidis, V.K.; Anagnostopoulos, A. Autologous Hematopoietic Cell Transplantation in Multiple Sclerosis: Changing Paradigms in the Era of Novel Agents. Stem Cells Int. 2019, 2019, 5840286. [Google Scholar] [CrossRef] [PubMed]
- Atkins, H.L.; Bowman, M.; Allan, D.; Anstee, G.; Arnold, D.L.; Bar-Or, A.; Bence-Bruckler, I.; Birch, P.; Bredeson, C.; Chen, J.; et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: A multicentre single-group phase 2 trial. Lancet 2016, 388, 576–585. [Google Scholar] [CrossRef]
- Burt, R.K.; Balabanov, R.; Snowden, J.A.; Sharrack, B.; Oliveira, M.C.; Burman, J. Non-myeloablative hematopoietic stem cell transplantation (HSCT) is superior to disease modifying drug (DMD) treatment in highly active Relapsing Remitting Multiple Sclerosis (RRMS): Interim results of the Multiple Sclerosis International Stem cell Transplant (MIST) Randomized Trial (S36.004). Neurology 2018, 90 (Suppl. S15), S36.004. [Google Scholar]
- Grand’Maison, F.; Yeung, M.; Morrow, S.A.; Lee, L.; Emond, F.; Ward, B.J.; Laneuville, P.; Schecter, R. Sequencing of disease-modifying therapies for relapsing-remitting multiple sclerosis: A theoretical approach to optimizing treatment. Curr. Med. Res. Opin. 2018, 34, 1419–1430. [Google Scholar] [CrossRef]
- Gross, R.H.; Corboy, J.R. Monitoring, switching, and stopping multiple sclerosis disease-modifying therapies. Contin. Lifelong Learn. Neurol. 2019, 25, 715–735. [Google Scholar] [CrossRef]
- Wolinsky, J.S.; Montalban, X.; Hauser, S.L.; Giovannoni, G.; Vermersch, P.; Bernasconi, C.; Deol-Bhullar, G.; Garren, H.; Chin, P.; Belachew, S.; et al. Evaluation of no evidence of progression or active disease (NEPAD) in patients with primary progressive multiple sclerosis in the ORATORIO trial. Ann. Neurol. 2018, 84, 527–536. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; De Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Ontaneda, D. Progressive multiple sclerosis. Continuum 2019, 25, 736–752. [Google Scholar]
- Kappos, L.; Bar-Or, A.; Cree, B.; Fox, R.; Giovannoni, G.; Gold, R.; Vermersch, P.; Arnould, S.; Sidorenko, T.; Wolf, C.; et al. Efficacy and safety of siponimod in secondary progressive multiple sclerosis—Results of the placebo controlled, double-blind, Phase III EXPAND study. In Proceedings of the 2016 meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), London, UK, 14 September 2016. [Google Scholar]
- Lizak, N.; Malpas, C.B.; Sharmin, S.; Havrdova, E.K.; Horakova, D.; Izquierdo, G.; Eichau, S.; Lugaresi, A.; Duquette, P.; Girard, M.; et al. Association of sustained immunotherapy with disability outcomes in patients with active secondary progressive multiple sclerosis. JAMA Neurol. 2020, 77, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Marrie, R.A.; Elliott, L.; Marriott, J.; Cossoy, M.; Blanchard, J.; Tennakoon, A.; Yu, N. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology 2014, 83, 929–937. [Google Scholar] [CrossRef]
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. 2020. Available online: http://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 5 August 2020).
- Brownlee, W.; Bourdette, D.; Broadley, S.; Killestein, J.; Ciccarelli, O. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology 2020, 94, 949–952. [Google Scholar] [CrossRef]
- Coles, A.; Anderson, G.G. Covidms ABN Guidance on DMT in the Times Of COVID-19. 2020. Available online: https://multiple-sclerosis-research.org/2020/03/abn-guidance-on-dmt-in-the-times-of-covid-19 (accessed on 5 August 2020).
- Pistor, M.; Hoepner, R.; Hoepner, A.G.; Lin, Y.; Jung, S.; Bassetti, C.L.; Chan, A.; Salmen, A. Multiple Sclerosis immunotherapies and COVID-19 mortality: An analysis of the FDA Adverse Event Reporting System. Ther. Adv. Neurol. Disord. 2022, 15, 17562864221129383. [Google Scholar] [CrossRef] [PubMed]
- Berkovich, R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics 2013, 10, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Metz, L.M.; Sabuda, D.; Hilsden, R.J.; Enns, R.; Meddings, J.B. Gastric tolerance of high-dose pulse oral prednisone in multiple sclerosis. Neurology 1999, 53, 2093. [Google Scholar] [CrossRef] [PubMed]
- Strupp, M. The bioavailability of IV methylprednisolone and oral prednisone in multiple sclerosis. Neurology 2005, 64, 1100. [Google Scholar] [CrossRef]
- Morrow, S.A.; Stoian, C.A.; Dmitrovic, J.; Chan, S.C.; Metz, L.M. MS patients report excellent compliance with oral prednisone for acute relapses. Neurology 2004, 63, 1079–1080. [Google Scholar] [CrossRef]
- Ramo-Tello, C.; Grau-López, L.; Tintoré, M.; Rovira, A.; Ramió i Torrenta, L.; Brieva, L.; Cano, A.; Carmona, O.; Saiz, A.; Torres, F.; et al. A randomized clinical trial of oral versus intravenous methylprednisolone for relapse of MS. Mult. Scler. J. 2014, 20, 717–725. [Google Scholar] [CrossRef]
- Le Page, E.; Veillard, D.; Laplaud, D.A.; Hamonic, S.; Wardi, R.; Lebrun, C.; Zagnoli, F.; Wiertlewski, S.; Deburghgraeve, V.; Coustans, M.; et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): A randomised, controlled, double-blind, non-inferiority trial. Lancet 2015, 386, 974–981. [Google Scholar] [CrossRef]
- Weiner, H.L.; Dau, P.C.; Khatri, B.O.; Petajan, J.H.; Birnbaum, G.; McQuillen, M.P.; Fosburg, M.T.; Feldstein, M.; Orav, E.J. Double-blind study of true vs. sham plasma exchange in patients treated with immunosuppression for acute attacks of multiple sclerosis. Neurology 1989, 39, 1143. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; O’Brien, P.C.; Petterson, T.M.; Noseworthy, J.H.; Lucchinetti, C.F.; Dodick, D.W.; Pineda, A.A.; Stevens, L.N.; Rodriguez, M. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann. Neurol. 1999, 46, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Cortese, I.; Chaudhry, V.; So, Y.T.; Cantor, F.; Cornblath, D.R.; Rae-Grant, A. Evidence-based guideline update: Plasmapheresis in neurologic disorders: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2011, 76, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.; Njue, A.; Lyall, M.; Heyes, A.; Mahler, N.; Philbin, M.; Nazareth, T. Efficacy, safety, and quality-of-life of treatments for acute relapses of multiple sclerosis: Results from a literature review of randomized controlled trials. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 55–78. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).