Cross-Validation of Neurodegeneration Biomarkers in Blood and CSF for Dementia Classification
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Taking CSF
2.3. Blood Plasma Collection
2.4. Multiplex Assay
2.5. Determination of AD Signs by CSF Marker Concentration
2.6. Statistical Processing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| CSF | Cerebrospinal fluid |
| ICD-10 | International Classification of Diseases |
| MMSE | Mini-Mental State Examination |
| NCAM-1 | Neural cell adhesion molecule 1 |
| TDP-43 | Transactive response DNA-binding protein 43 |
| MRI | Magnetic resonance imaging |
References
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Badji, A.; Youwakim, J.; Cooper, A.; Westman, E.; Marseglia, A. Vascular Cognitive Impairment—Past, Present, and Future Challenges. Ageing Res. Rev. 2023, 90, 102042. [Google Scholar] [CrossRef]
- Chin, K.S. Pathophysiology of Dementia. Aust. J. Gen. Pract. 2023, 52, 516–521. [Google Scholar] [CrossRef]
- Thangwaritorn, S.; Lee, C.; Metchikoff, E.; Razdan, V.; Ghafary, S.; Rivera, D.; Pinto, A.; Pemminati, S. A Review of Recent Advances in the Management of Alzheimer’s Disease. Cureus 2024, 16, e58416. [Google Scholar] [CrossRef] [PubMed]
- Geldmacher, D.S. Treatment of Alzheimer Disease. Contin. (Minneap Minn.) 2024, 30, 1823–1844. [Google Scholar] [CrossRef] [PubMed]
- Waite, L.M. New and Emerging Drug Therapies for Alzheimer Disease. Aust. Prescr. 2024, 47, 75–79. [Google Scholar] [CrossRef]
- Wu, C.-K.; Fuh, J.-L. A 2025 Update on Treatment Strategies for the Alzheimer’s Disease Spectrum. J. Chin. Med. Assoc. 2025, 88, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Chang, K.-X.; Chen, Y.-F.; Yan, K.; Wang, C.-X.; Hua, Q. Diagnosis of Alzheimer’s Disease: Towards Accuracy and Accessibility. J. Biol. Methods 2024, 11, e99010010. [Google Scholar] [CrossRef]
- Milos, T.; Vuic, B.; Balic, N.; Farkas, V.; Nedic Erjavec, G.; Svob Strac, D.; Nikolac Perkovic, M.; Pivac, N. Cerebrospinal Fluid in the Differential Diagnosis of Alzheimer’s Disease: An Update of the Literature. Expert. Rev. Neurother. 2024, 24, 1063–1079. [Google Scholar] [CrossRef]
- Bridel, C.; Somers, C.; Sieben, A.; Rozemuller, A.; Niemantsverdriet, E.; Struyfs, H.; Vermeiren, Y.; Van Broeckhoven, C.; De Deyn, P.P.; Bjerke, M.; et al. Associating Alzheimer’s Disease Pathology with Its Cerebrospinal Fluid Biomarkers. Brain 2022, 145, 4056–4064. [Google Scholar] [CrossRef]
- Bergström, S.; Remnestål, J.; Yousef, J.; Olofsson, J.; Markaki, I.; Carvalho, S.; Corvol, J.-C.; Kultima, K.; Kilander, L.; Löwenmark, M.; et al. Multi-Cohort Profiling Reveals Elevated CSF Levels of Brain-Enriched Proteins in Alzheimer’s Disease. Ann. Clin. Transl. Neurol. 2021, 8, 1456–1470. [Google Scholar] [CrossRef]
- McGrowder, D.A.; Miller, F.; Vaz, K.; Nwokocha, C.; Wilson-Clarke, C.; Anderson-Cross, M.; Brown, J.; Anderson-Jackson, L.; Williams, L.; Latore, L.; et al. Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease: Current Evidence and Future Perspectives. Brain Sci. 2021, 11, 215. [Google Scholar] [CrossRef]
- Lista, S.; Mapstone, M.; Caraci, F.; Emanuele, E.; López-Ortiz, S.; Martín-Hernández, J.; Triaca, V.; Imbimbo, C.; Gabelle, A.; Mielke, M.M.; et al. A Critical Appraisal of Blood-Based Biomarkers for Alzheimer’s Disease. Ageing Res. Rev. 2024, 96, 102290. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hu, Y.; Cummings, J.; Mattke, S.; Iwatsubo, T.; Nakamura, A.; Vellas, B.; O’Bryant, S.; Shaw, L.M.; Cho, M.; et al. Blood-Based Biomarkers for Alzheimer’s Disease: Current State and Future Use in a Transformed Global Healthcare Landscape. Neuron 2023, 111, 2781–2799. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Blennow, K.; Zetterberg, H.; Dage, J. Blood Biomarkers for Alzheimer’s Disease in Clinical Practice and Trials. Nat. Aging 2023, 3, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Humpel, C.; Hochstrasser, T. Cerebrospinal Fluid and Blood Biomarkers in Alzheimer’s Disease. World J. Psychiatry 2011, 1, 8–18. [Google Scholar] [CrossRef]
- Assfaw, A.D.; Schindler, S.E.; Morris, J.C. Advances in Blood Biomarkers for Alzheimer Disease (AD): A Review. Kaohsiung J. Med. Sci. 2024, 40, 692–698. [Google Scholar] [CrossRef]
- Dhauria, M.; Mondal, R.; Deb, S.; Shome, G.; Chowdhury, D.; Sarkar, S.; Benito-León, J. Blood-Based Biomarkers in Alzheimer’s Disease: Advancing Non-Invasive Diagnostics and Prognostics. Int. J. Mol. Sci. 2024, 25, 10911. [Google Scholar] [CrossRef]
- Arslan, B.; Zetterberg, H.; Ashton, N.J. Blood-Based Biomarkers in Alzheimer’s Disease—Moving towards a New Era of Diagnostics. Clin. Chem. Lab. Med. 2024, 62, 1063–1069. [Google Scholar] [CrossRef]
- Mantellatto Grigoli, M.; Pelegrini, L.N.C.; Whelan, R.; Cominetti, M.R. Present and Future of Blood-Based Biomarkers of Alzheimer’s Disease: Beyond the Classics. Brain Res. 2024, 1830, 148812. [Google Scholar] [CrossRef]
- Zorkina, Y.A.; Morozova, I.O.; Abramova, O.V.; Ochneva, A.G.; Gankina, O.A.; Andryushenko, A.V.; Kurmyshev, M.V.; Kostyuk, G.P.; Morozova, A.Y. Use of modern classification systems for complex diagnostics of Alzheimer’s disease. Zh Nevrol. Psikhiatr Im. S S Korsakova 2024, 124, 121–127. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Ebenau, J.L.; Timmers, T.; Wesselman, L.M.P.; Verberk, I.M.W.; Verfaillie, S.C.J.; Slot, R.E.R.; van Harten, A.C.; Teunissen, C.E.; Barkhof, F.; van den Bosch, K.A.; et al. ATN Classification and Clinical Progression in Subjective Cognitive Decline: The SCIENCe Project. Neurology 2020, 95, e46–e58. [Google Scholar] [CrossRef]
- Hansen, E.O.; Dias, N.S.; Burgos, I.C.B.; Costa, M.V.; Carvalho, A.T.; Teixeira, A.L.; Barbosa, I.G.; Santos, L.A.V.; Rosa, D.V.F.; Ribeiro, A.J.F.; et al. Millipore xMap® Luminex (HATMAG-68K): An Accurate and Cost-Effective Method for Evaluating Alzheimer’s Biomarkers in Cerebrospinal Fluid. Front. Psychiatry 2021, 12, 716686. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.-J.; Guo, J. Biofluid Biomarkers of Alzheimer’s Disease: Progress, Problems, and Perspectives. Neurosci. Bull. 2022, 38, 677–691. [Google Scholar] [CrossRef]
- Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, P.; Kornhuber, J.; Lewczuk, P.; Mroczko, B. Biomarkers for the Diagnosis of Alzheimer’s Disease in Clinical Practice: The Role of CSF Biomarkers during the Evolution of Diagnostic Criteria. Int. J. Mol. Sci. 2022, 23, 8598. [Google Scholar] [CrossRef] [PubMed]
- Barthélemy, N.R.; Salvadó, G.; Schindler, S.E.; He, Y.; Janelidze, S.; Collij, L.E.; Saef, B.; Henson, R.L.; Chen, C.D.; Gordon, B.A.; et al. Highly Accurate Blood Test for Alzheimer’s Disease Is Similar or Superior to Clinical Cerebrospinal Fluid Tests. Nat. Med. 2024, 30, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Slaets, S.; Le Bastard, N.; Martin, J.-J.; Sleegers, K.; Van Broeckhoven, C.; De Deyn, P.P.; Engelborghs, S. Cerebrospinal Fluid Aβ1-40 Improves Differential Dementia Diagnosis in Patients with Intermediate P-tau181P Levels. J. Alzheimers Dis. 2013, 36, 759–767. [Google Scholar] [CrossRef]
- Lewczuk, P.; Esselmann, H.; Otto, M.; Maler, J.M.; Henkel, A.W.; Henkel, M.K.; Eikenberg, O.; Antz, C.; Krause, W.-R.; Reulbach, U.; et al. Neurochemical Diagnosis of Alzheimer’s Dementia by CSF Abeta42, Abeta42/Abeta40 Ratio and Total Tau. Neurobiol. Aging 2004, 25, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Zetterberg, H.; Buchhave, P.; Andreasson, U.; Londos, E.; Minthon, L.; Blennow, K. Prediction of Alzheimer’s Disease Using the CSF Abeta42/Abeta40 Ratio in Patients with Mild Cognitive Impairment. Dement. Geriatr. Cogn. Disord. 2007, 23, 316–320. [Google Scholar] [CrossRef]
- Janelidze, S.; Zetterberg, H.; Mattsson, N.; Palmqvist, S.; Vanderstichele, H.; Lindberg, O.; van Westen, D.; Stomrud, E.; Minthon, L.; Blennow, K.; et al. CSF Aβ42/Aβ40 and Aβ42/Aβ38 Ratios: Better Diagnostic Markers of Alzheimer Disease. Ann. Clin. Transl. Neurol. 2016, 3, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Spies, P.E.; Verbeek, M.M.; van Groen, T.; Claassen, J.A.H.R. Reviewing Reasons for the Decreased CSF Abeta42 Concentration in Alzheimer Disease. Front. Biosci. 2012, 17, 2024–2034. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kim, S.J.; Hong, S.; Kim, Y. Diagnosis of Alzheimer’s Disease Utilizing Amyloid and Tau as Fluid Biomarkers. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Mo, J.-A.; Lim, J.-H.; Sul, A.-R.; Lee, M.; Youn, Y.C.; Kim, H.-J. Cerebrospinal Fluid β-Amyloid1–42 Levels in the Differential Diagnosis of Alzheimer’s Disease—Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0116802. [Google Scholar] [CrossRef]
- Sunderland, T.; Linker, G.; Mirza, N.; Putnam, K.T.; Friedman, D.L.; Kimmel, L.H.; Bergeson, J.; Manetti, G.J.; Zimmermann, M.; Tang, B.; et al. Decreased Beta-Amyloid1-42 and Increased Tau Levels in Cerebrospinal Fluid of Patients with Alzheimer Disease. JAMA 2003, 289, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.; Hesse, C.; Davidsson, P.; Minthon, L.; Wallin, A.; Winblad, B.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Cerebrospinal Fluid Beta-Amyloid(1–42) in Alzheimer Disease: Differences between Early- and Late-Onset Alzheimer Disease and Stability during the Course of Disease. Arch. Neurol. 1999, 56, 673–680. [Google Scholar] [CrossRef]
- Lopez, O.L.; Klunk, W.E.; Mathis, C.A.; Snitz, B.E.; Chang, Y.; Tracy, R.P.; Kuller, L.H. Relationship of Amyloid-Β1–42 in Blood and Brain Amyloid: Ginkgo Evaluation of Memory Study. Brain Commun. 2019, 2, fcz038. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Tan Hehir, C.A.; Baker, D.; et al. Plasma β-Amyloid in Alzheimer’s Disease and Vascular Disease. Sci. Rep. 2016, 6, 26801. [Google Scholar] [CrossRef]
- Lopez, O.L.; Chang, Y.; Ives, D.G.; Snitz, B.E.; Fitzpatrick, A.L.; Carlson, M.C.; Rapp, S.R.; Williamson, J.D.; Tracy, R.P.; DeKosky, S.T.; et al. Blood Amyloid Levels and Risk of Dementia in the Ginkgo Evaluation of Memory Study (GEMS): A Longitudinal Analysis. Alzheimers Dement. 2019, 15, 1029–1038. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Huang, L.-C.; Hsieh, S.-W.; Huang, L.-J. Dynamic Blood Concentrations of Aβ1–40 and Aβ1–42 in Alzheimer’s Disease. Front. Cell Dev. Biol. 2020, 8, 768. [Google Scholar] [CrossRef]
- Shpilyukova, Y.A.; Fedotova, E.Y.; Kuzmina, E.N.; Illarioshkin, S.N. New forms of dementia in neurodegenerative diseases: Molecular basis, phenomenology, and diagnostic capability. Russ. Neurol. J. 2022, 27, 5–13. [Google Scholar] [CrossRef]
- Patra, K.; Soosaipillai, A.; Sando, S.B.; Lauridsen, C.; Berge, G.; Møller, I.; Grøntvedt, G.R.; Bråthen, G.; Begcevic, I.; Moussaud, S.; et al. Assessment of Kallikrein 6 as a Cross-Sectional and Longitudinal Biomarker for Alzheimer’s Disease. Alzheimers Res. Ther. 2018, 10, 9. [Google Scholar] [CrossRef]
- Chen, K.; Gao, T.; Bai, Z.; Yuan, Z. Circulating APP, NCAM and Aβ Serve as Biomarkers for Alzheimer’s Disease. Brain Res. 2018, 1699, 117–120. [Google Scholar] [CrossRef]
- Foulds, P.; McAuley, E.; Gibbons, L.; Davidson, Y.; Pickering-Brown, S.M.; Neary, D.; Snowden, J.S.; Allsop, D.; Mann, D.M.A. TDP-43 Protein in Plasma May Index TDP-43 Brain Pathology in Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Acta Neuropathol. 2008, 116, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.E.; Zachariou, V.; Sudduth, T.L.; Van Eldik, L.J.; Jicha, G.A.; Nelson, P.T.; Wilcock, D.M.; Gold, B.T. Plasma TDP-43 Levels Are Associated with Neuroimaging Measures of Brain Structure in Limbic Regions. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12437. [Google Scholar] [CrossRef] [PubMed]

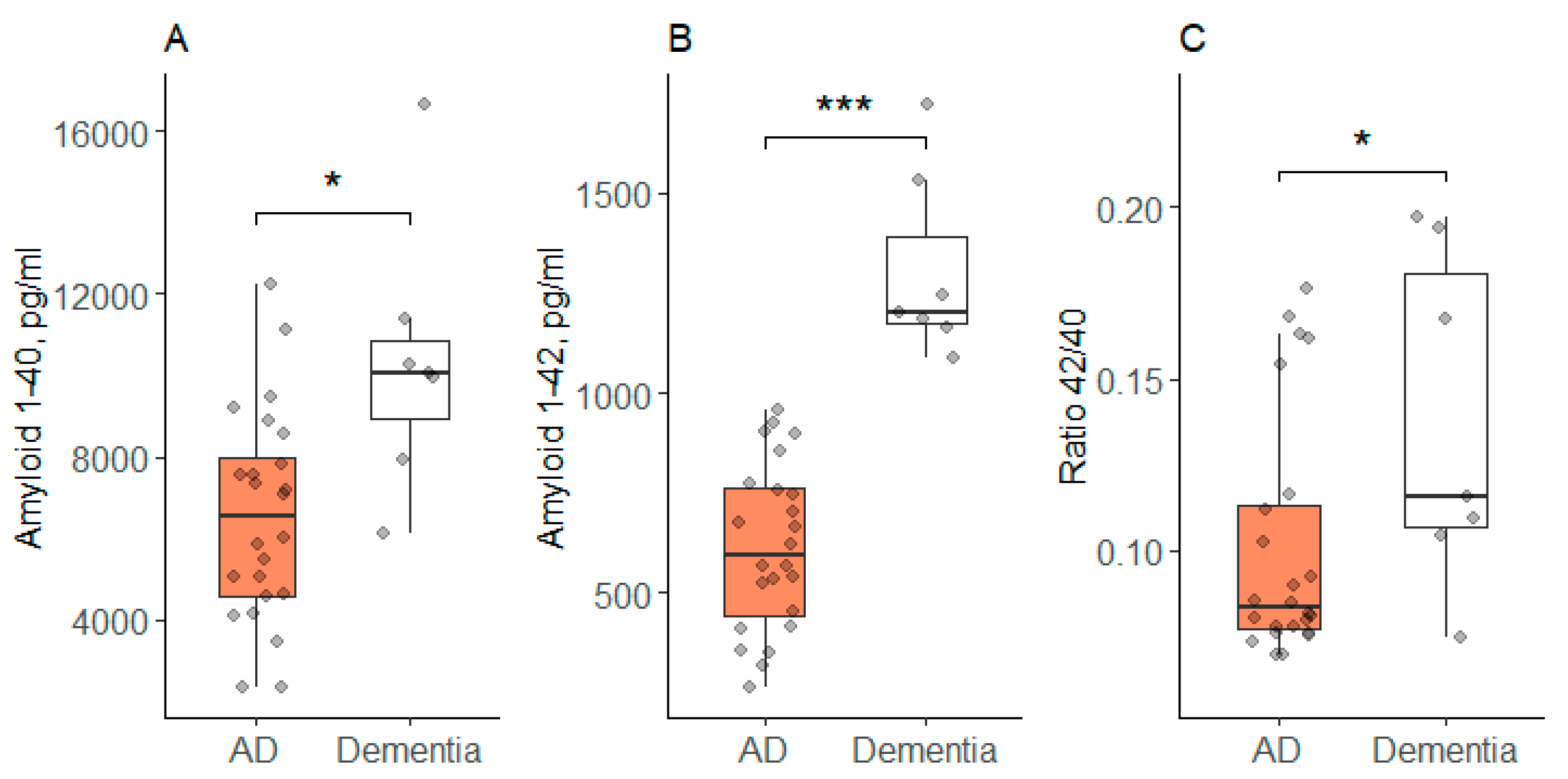
| Parameter | AD (n = 24) | Dementia (n = 7) | F/χ2 | p | ||
|---|---|---|---|---|---|---|
| Mean/Median | SE/Q1; Q3 | Mean/Median | SE/Q1; Q3 | |||
| Age, years | 72 | 2.1 | 75 | 5.3 | 0.29 | 0.60 |
| MMSE, score | 11.8 | 1.3 | 14.7 | 2.7 | 0.91 | 0.37 |
| Concentration of biomarkers in CSF | ||||||
| Aβ40, pg/mL # | 6545 | 527 | 10,337 | 1247 | 7.85 | 0.02 |
| Aβ42, pg/mL | 615 | 42 | 1305 | 88 | 50.30 | <0.001 |
| Ratio 42/40 * | 0.083 | 0.077; 0.113 | 0.116 | 0.107; 0.181 | 3.75 | 0.048 |
| tTau, pg/mL | 2330 | 1656; 3573 | 2759 | 2005; 3370 | 0.22 | 0.64 |
| pTau181, pg/mL * | 150 | 87; 238 | 122 | 88; 244 | 0.009 | 0.92 |
| Concentration of biomarkers in blood plasma | ||||||
| KLK6, pg/mL * | 877 | 692; 1113 | 734 | 578; 904 | 1.88 | 0.17 |
| Aβ40, pg/mL | 598 | 417; 1040 | 912 | 609; 1305 | 1.13 | 0.29 |
| Aβ42, pg/mL | 0.71 | 0.14 | 0.16 | 0.08 | 12.49 | 0.001 |
| tTau, pg/mL | 1.94 | 1.68; 2.38 | 1.94 | 1.52; 1.94 | 1.66 | 0.20 |
| NCAM-1, pg/mL # | 16,957 | 874 | 15233 | 1261 | 1.26 | 0.28 |
| pTau181, pg/mL * | 0.00 | 0.00; 0.01 | 0.00 | 0.00; 0.02 | 0.01 | 0.91 |
| TDP-43, pg/mL * | 26.4 | 0.0; 73.7 | 0.0 | 0.0; 9.8 | 2.65 | 0.10 |
| Neurogranin, pg/mL * | 7464 | 6270; 10,081 | 7144 | 6469; 9048 | 0.06 | 0.81 |
| Parameter 1 | Parameter 2 | r | p |
|---|---|---|---|
| AD Group | |||
| KLK6 | tTau | 0.41 | 0.047 |
| KLK6 | NCAM-1 | 0.47 | 0.02 |
| KLK6 | Tau181 | 0.60 | 0.002 |
| KLK6 | TDP-43 | 0.55 | 0.005 |
| KLK6 | neurogranin | 0.63 | <0.001 |
| NCAM-1 | Aβ42 | 0.44 | 0.03 |
| NCAM-1 | neurogranin | 0.46 | 0.02 |
| TDP-43 | Tau181 | 0.53 | 0.007 |
| TDP-43 | neurogranin | 0.845 | <0.001 |
| Dementia Group | |||
| NCAM-1 | Aβ40 | 0.87 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Published by MDPI on behalf of the Swiss Federation of Clinical Neuro-Societies. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ochneva, A.; Abramova, O.; Zorkina, Y.; Morozova, I.; Ushakova, V.; Pavlov, K.; Andreyuk, D.; Zubkov, E.; Andryushchenko, A.; Tsurina, A.; et al. Cross-Validation of Neurodegeneration Biomarkers in Blood and CSF for Dementia Classification. Clin. Transl. Neurosci. 2026, 10, 2. https://doi.org/10.3390/ctn10010002
Ochneva A, Abramova O, Zorkina Y, Morozova I, Ushakova V, Pavlov K, Andreyuk D, Zubkov E, Andryushchenko A, Tsurina A, et al. Cross-Validation of Neurodegeneration Biomarkers in Blood and CSF for Dementia Classification. Clinical and Translational Neuroscience. 2026; 10(1):2. https://doi.org/10.3390/ctn10010002
Chicago/Turabian StyleOchneva, Aleksandra, Olga Abramova, Yana Zorkina, Irina Morozova, Valeriya Ushakova, Konstantin Pavlov, Denis Andreyuk, Eugene Zubkov, Alisa Andryushchenko, Anna Tsurina, and et al. 2026. "Cross-Validation of Neurodegeneration Biomarkers in Blood and CSF for Dementia Classification" Clinical and Translational Neuroscience 10, no. 1: 2. https://doi.org/10.3390/ctn10010002
APA StyleOchneva, A., Abramova, O., Zorkina, Y., Morozova, I., Ushakova, V., Pavlov, K., Andreyuk, D., Zubkov, E., Andryushchenko, A., Tsurina, A., Kalinina, K., Gurina, O., Chekhonin, V., Kostyuk, G., & Morozova, A. (2026). Cross-Validation of Neurodegeneration Biomarkers in Blood and CSF for Dementia Classification. Clinical and Translational Neuroscience, 10(1), 2. https://doi.org/10.3390/ctn10010002






