Thermodynamic Phase Control of Poly(TFEMA) Nucleation and Surface Deposition in Supercritical CO2–Toluene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Carbon dioxide (CO2), obtained from Airgas (Philadelphia, PA, USA); certified purity 99.9995%.
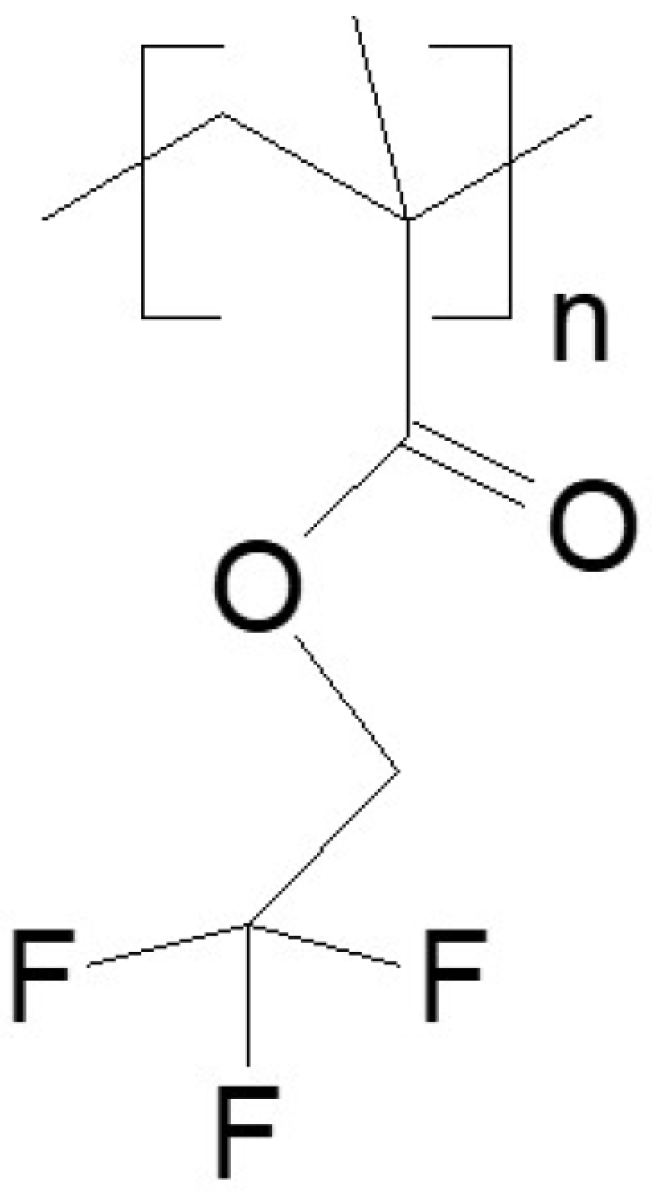
- Poly(2,2,2-trifluoroethyl methacrylate) [poly(TFEMA); trifluoroethyl methacrylate homopolymer], obtained from Specific Polymers (Castries, France). Supplier data: , , and .
- Toluene (C7H8; 99.8%), obtained from Sigma-Aldrich (St. Louis, MO, USA).
- Fluorine-doped tin oxide (FTO) coated glass substrates, 25 mm × 25 mm, obtained from Ossila Ltd. (Solpro Business Park, Windsor Street, Sheffield, S4 7WB, UK).
2.2. Substrate Preparation
- Initial wash: Substrates were washed with a solution of deionized (DI) water and Alconox detergent (Powder Precision Cleaner, Sigma-Aldrich), and they were then rinsed with DI water and placed into a polypropylene substrate cleaning rack (Ossila, Product Code E102).
- Ultrasonic cleaning with Hellmanex: The rack was placed in a Branson Ultrasonic Cleaner (Model 1510; Branson Ultrasonics Corp., Wallingford, CT, USA). A Hellmanex III working solution (prepared by diluting 2 mL Hellmanex III, Ossila, in 100 mL DI water) was added to the rack compartments. The ultrasonic bath reservoir was filled with water to a level just below the top of the rack height to ensure full immersion. Sonication was performed for 15 min at ambient temperature.
- Rinsing: Substrates were removed and rinsed with DI water to remove Hellmanex residues.
- Sequential solvent sonication: Substrates were sonicated first in isopropyl alcohol (IPA, ≥99.7%, Sigma-Aldrich) for 15 min, followed by acetone (≥99.5%, Sigma-Aldrich) for 15 min.
- Final rinses and drying: After solvent sonication, substrates were rinsed with acetone and then with IPA, and they were quickly dried using a stream of high-pressure dry air to prevent residues or liquid spots.
- Plasma treatment: Substrates were plasma-cleaned for 4 min in a Solarus 950 advanced plasma system (Gatan Inc., Pleasanton, CA, USA). The plasma environment consisted of 75% argon and 25% oxygen at 25 psi, with feed gases of 99.995% purity.
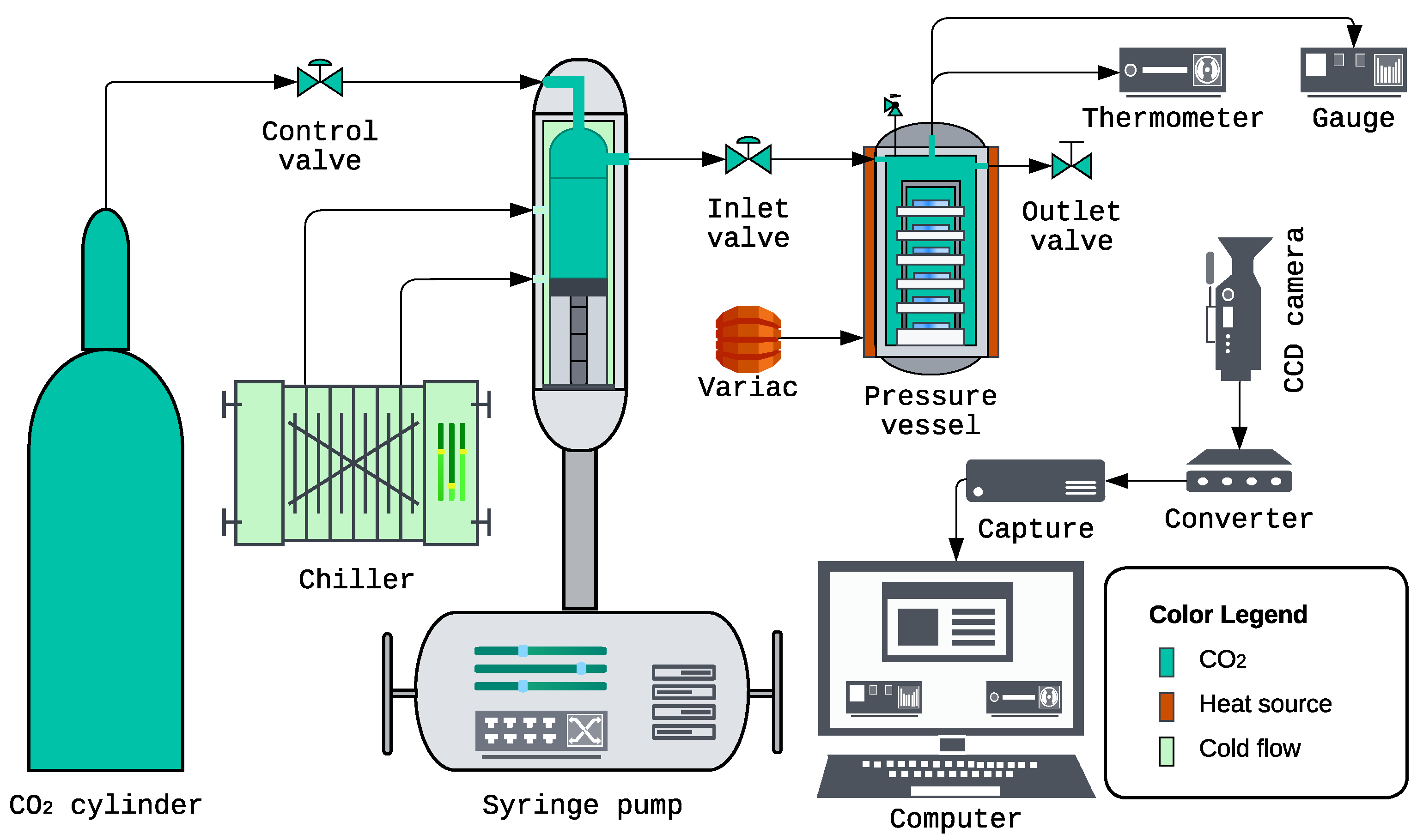
2.3. Supercritical Deposition System and Instrumentation
- CO2 cylinder: High-purity CO2 was supplied from a compressed-gas cylinder via high-pressure stainless-steel tubing and a control valve to the syringe pump, providing a clean and continuous feed.
- Syringe pump: Teledyne ISCO 260D (Teledyne Isco, Lincoln, NE, USA) pressurized and delivered CO2 to the pressure vessel with precise control of delivery rate and final set pressure, enabling reproducible attainment of the one-phase, cloud-point, and two-phase conditions.
- Pump-head chiller: Neslab CFT-25 refrigerated recirculator (Neslab Instruments Inc., Portsmouth, NH, USA) cooled the syringe pump head to increase CO2 density during each loading cycle, thereby increasing the mass delivered per stroke and reducing the number of charging iterations needed to reach the target vessel pressure.
- High-pressure vessel: Parr 4768 reactor (Parr Instrument Company, Moline, IL, USA) housed the substrates, polymer, cosolvent, and scCO2 during deposition (nominal capacity 600 mL). The effective working volume was experimentally determined by an Archimedes displacement procedure to be 424.89 mL, with an estimated uncertainty of ±3 mL. Key specifications: head style VGR; maximum working pressure 3000 psi at 350 °C; and maximum working temperature 350 °C with flat PTFE gasket.
- Heating and temperature monitoring: A Staco Variac autotransformer (Model 3PN1010B; Staco Energy Products Co., Dayton, OH, USA; input 120 V, output 0–140 V, 12 A, 1.4 kVA) powered a heavy-duty heating tape (HTS/Amptek AWD-051-060, Stafford, TX, USA; 120 V, 312 W) wrapped around the vessel to regulate temperature. The internal temperature was measured with a J-type thermocouple inserted through the head of the vessel (the rod extending into the chamber) and read on an OMEGA HH506 digital thermometer (Omega Engineering Inc., Norwalk, CT, USA; 0.1 °C readout resolution). All deposition experiments were carried out at a nominal set point of 40.0 °C, with the vessel temperature stable within approximately during the 30 min hold. This set point was then converted to for use where an absolute temperature is required.
- Pressure monitoring: The vessel pressure was measured using an OMEGA PX309-10KGI pressure transducer with readout on an OMEGA DP400TP high-speed panel meter (Omega Engineering Inc., Norwalk, CT, USA). The combined system precision was of full scale (including linearity, hysteresis, and repeatability), providing reliable real-time control. Pressures were read in psi and converted to MPa for reporting; on the 10,000 psi range, this specification corresponds to an absolute uncertainty of approximately MPa. Throughout this work, tabulated pressures in MPa should therefore be regarded as nominal set points with this uncertainty, unless a different value is stated explicitly.
- Video monitoring: A Vanxse CCTV Mini HD 1/3 CCD 960H Auto Iris Camera (Model BX2812; Shenzhen Kaixing Security Technology Co., Ltd., Shenzhen, China; NTSC) recorded the HH506 (temperature) and DP400TP (pressure) displays. The signal passed through a NOVPEAK BNC CCTV S-Video to VGA converter (Model UPD39 E01A; SQdeal) and an ATCCPYDM VGA-to-USB 2.0 capture card (Model V2U-LO) prior to transfer to the computer.
- Data acquisition: Video files containing synchronized pressure and temperature readings were captured and archived for each run using OBS Studio 30.0.2 (OBS Project, open-source). These time-stamped recordings were used for post hoc frame-by-frame analysis to verify vessel conditions and improve the accuracy of the reported experimental values across all deposition regimes.
2.4. Cloud-Point References and Set-Point Selection
2.5. Deposition Procedures and Compositions
- Common operating protocol (applies to all three conditions).
- Charge and fixturing. Dispense the prescribed toluene volume (Table 3) into the open vessel using a beaker and calibrated pipette. Mount one cleaned substrate on each of the top three rack levels; place the weighed poly(TFEMA) on the fourth level, directly beneath the lowest substrate.
- Sealing and heating. Install the head; tighten bolts in a star pattern in 5 lb-ft increments to a final torque of 25 lb-ft (∼34 N·m). Apply heat with a Variac-driven tape and equilibrate at 40.0 °C (313.2 K).
- CO2 introduction and purge. With the syringe pump set to 20 mL min−1, pressurize to ∼3.45 MPa (∼500 psi), then purge three times by venting to ∼2.07 MPa (∼300 psi) and repressurizing to remove air and moisture while retaining polymer and toluene (outlet valve cracked only minimally).
- Stabilization. Resume CO2 addition and follow the pressure path specific to the targeted regime (as specified in Pressure paths and rationale, below). Start the 30 min deposition clock only when both temperature (313.2 K) and pressure are stable.
- Pressure paths and rationale.
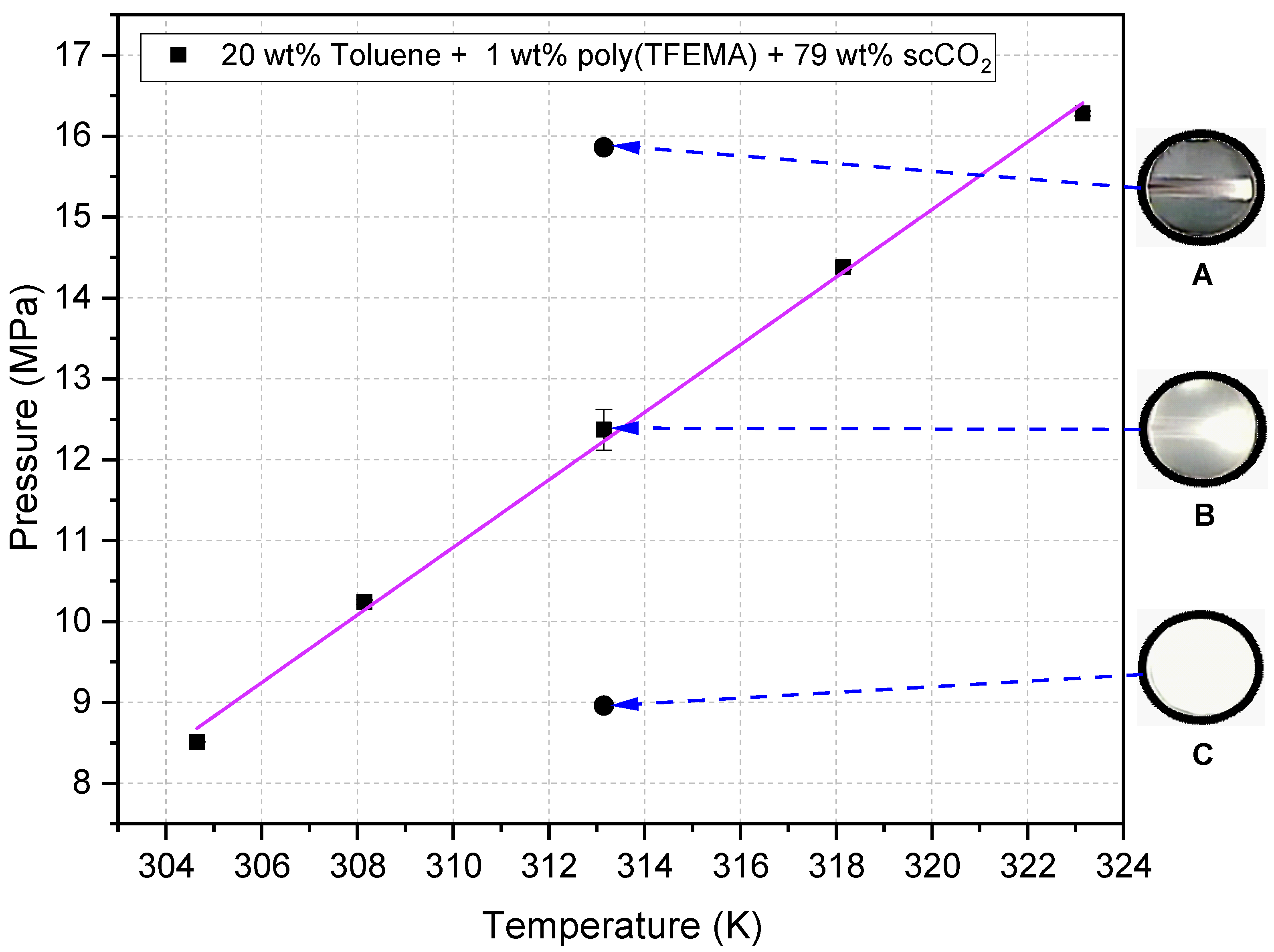
- One-phase, 15.86 MPa. Pressurize directly at 20 mL min−1 to 15.86 MPa at 313.2 K; close the inlet valve to isolate; hold 30 min. This sets a homogeneous one-phase state throughout the treatment, consistent with the clear-field phase-monitor image in Figure 5A.
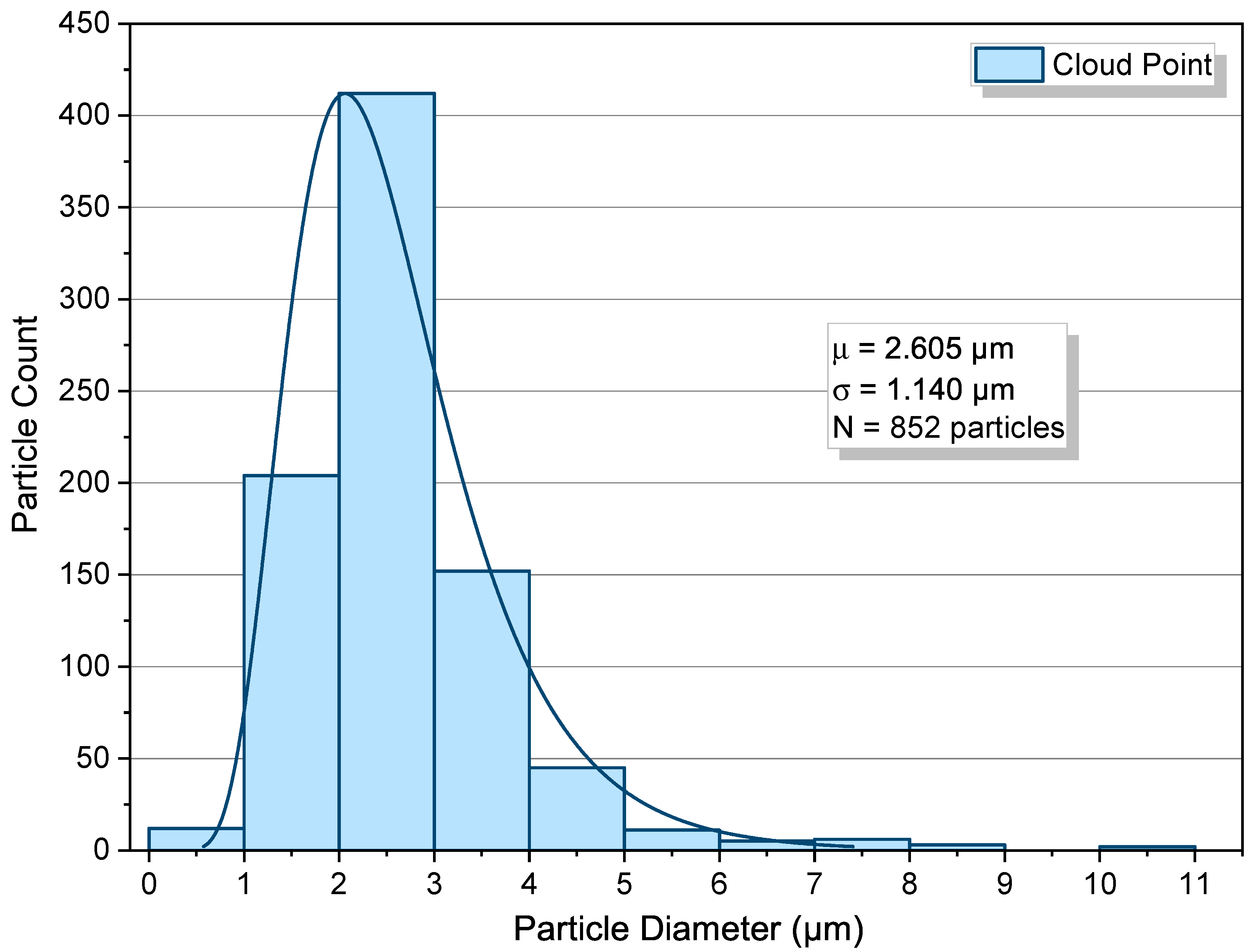
- Cloud point, 12.37 MPa. First pressurize to ≈12.62 MPa at 313.2 K (one-phase region) to ensure complete dissolution; then reduce slowly to 12.37 MPa by cracking the outlet valve to minimize solvent or polymer loss; hold 30 min. The visual endpoint is the first persistent haze captured through the phase monitor (Figure 5B), our reversible-turbidity criterion.
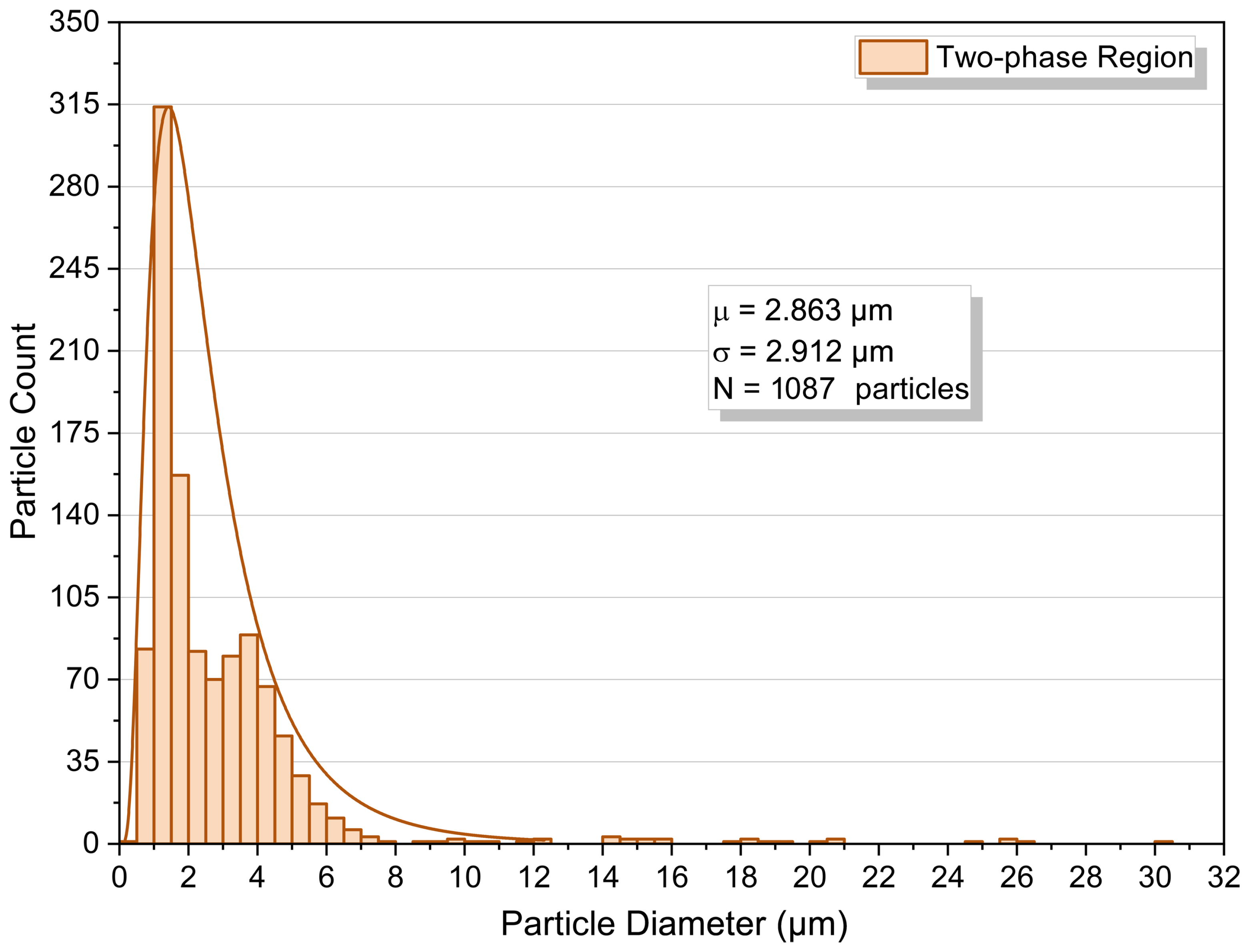
- Two-phase, 8.96 MPa. First pressurize to ≈12.41 MPa at 313.2 K to dissolve polymer; then decompress slowly to 8.96 MPa; hold 30 min. The phase monitor shows sustained turbidity at this isobar (Figure 5C), confirming operation in the polymer-saturated two-phase region.
- Post-treatment handling.
- Immerse the vessel in an ice–water bath (0.0 °C) while sealed to precipitate toluene from the supercritical phase and quench further mass transfer.
- When the external thermometer reads ∼16.0 °C, vent slowly and continuously to ambient pressure, remove the head, and retrieve the substrates for ex situ characterization.
2.6. SEM Preparation and Imaging
3. Results and Discussion
3.1. Phase-State Control of Deposition (Overview)
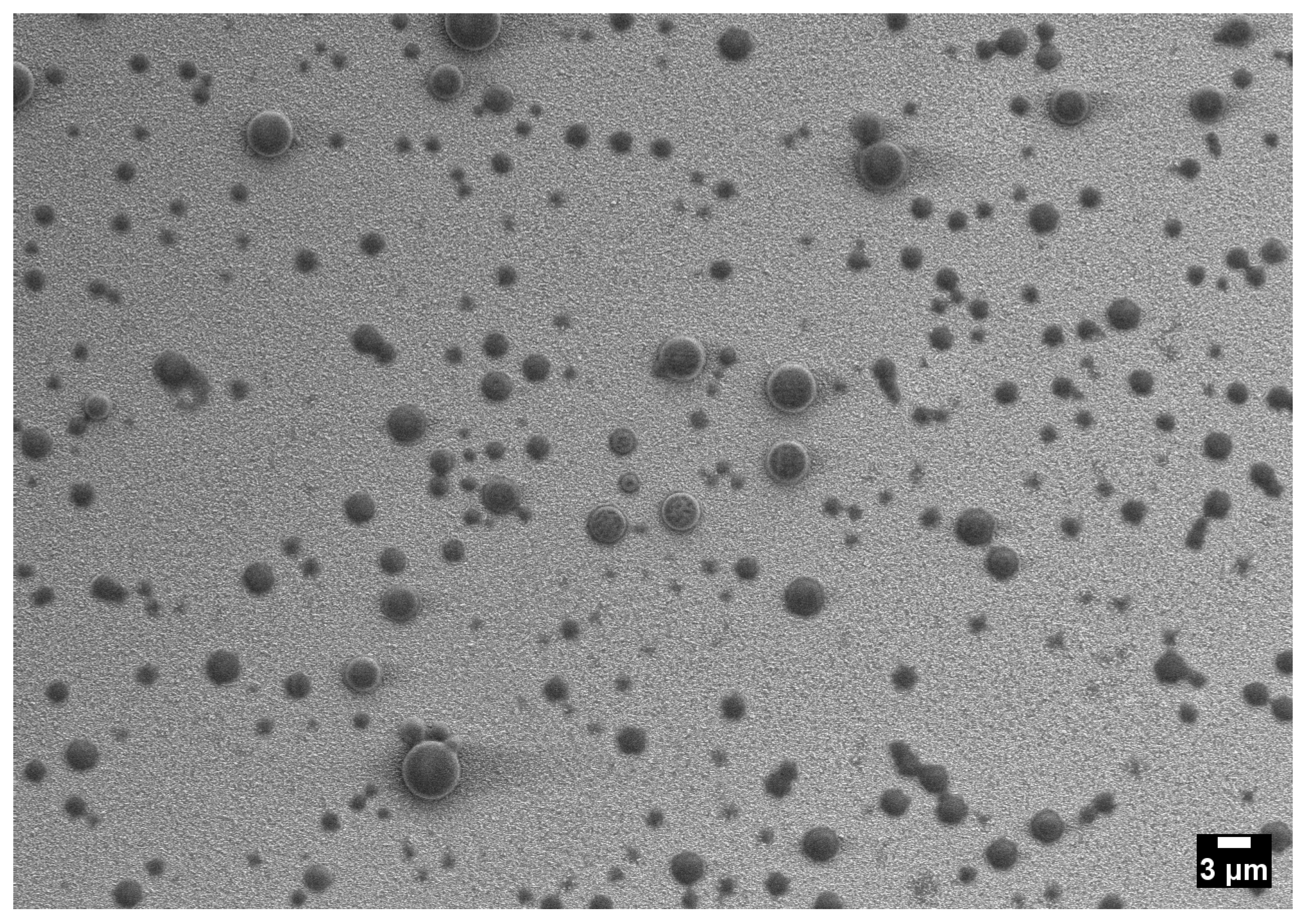
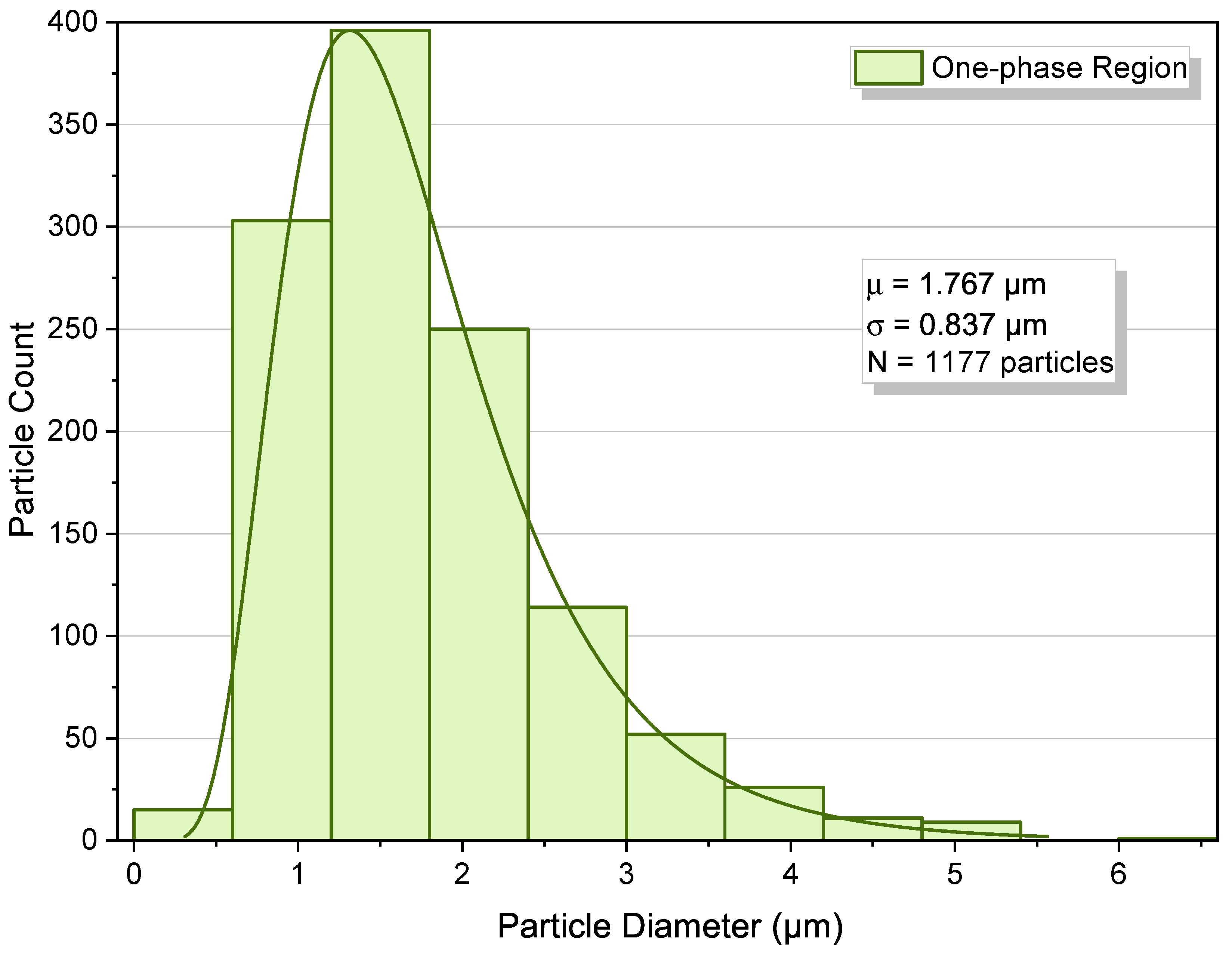
3.2. One-Phase Region
3.3. Cloud-Point Condition
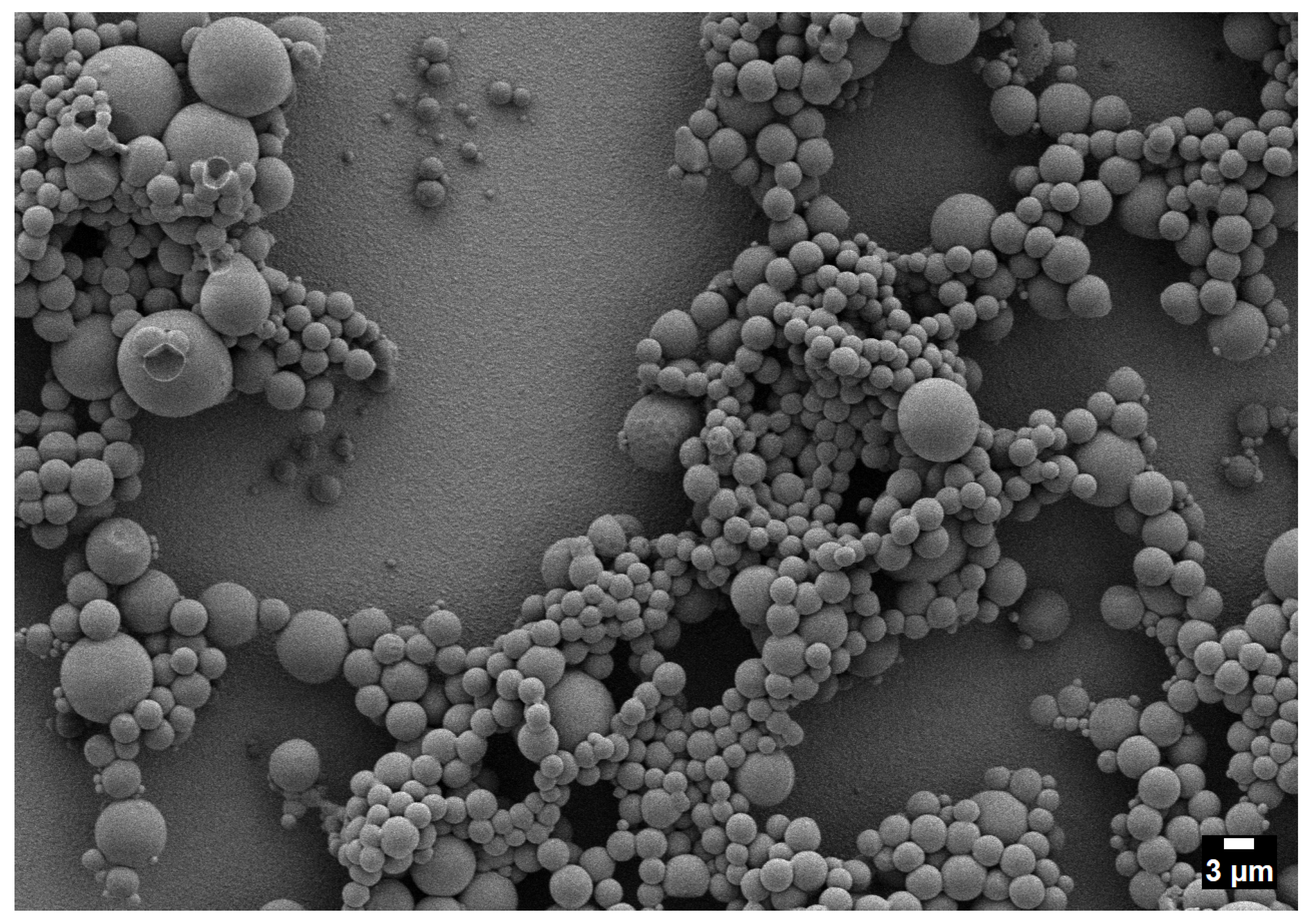
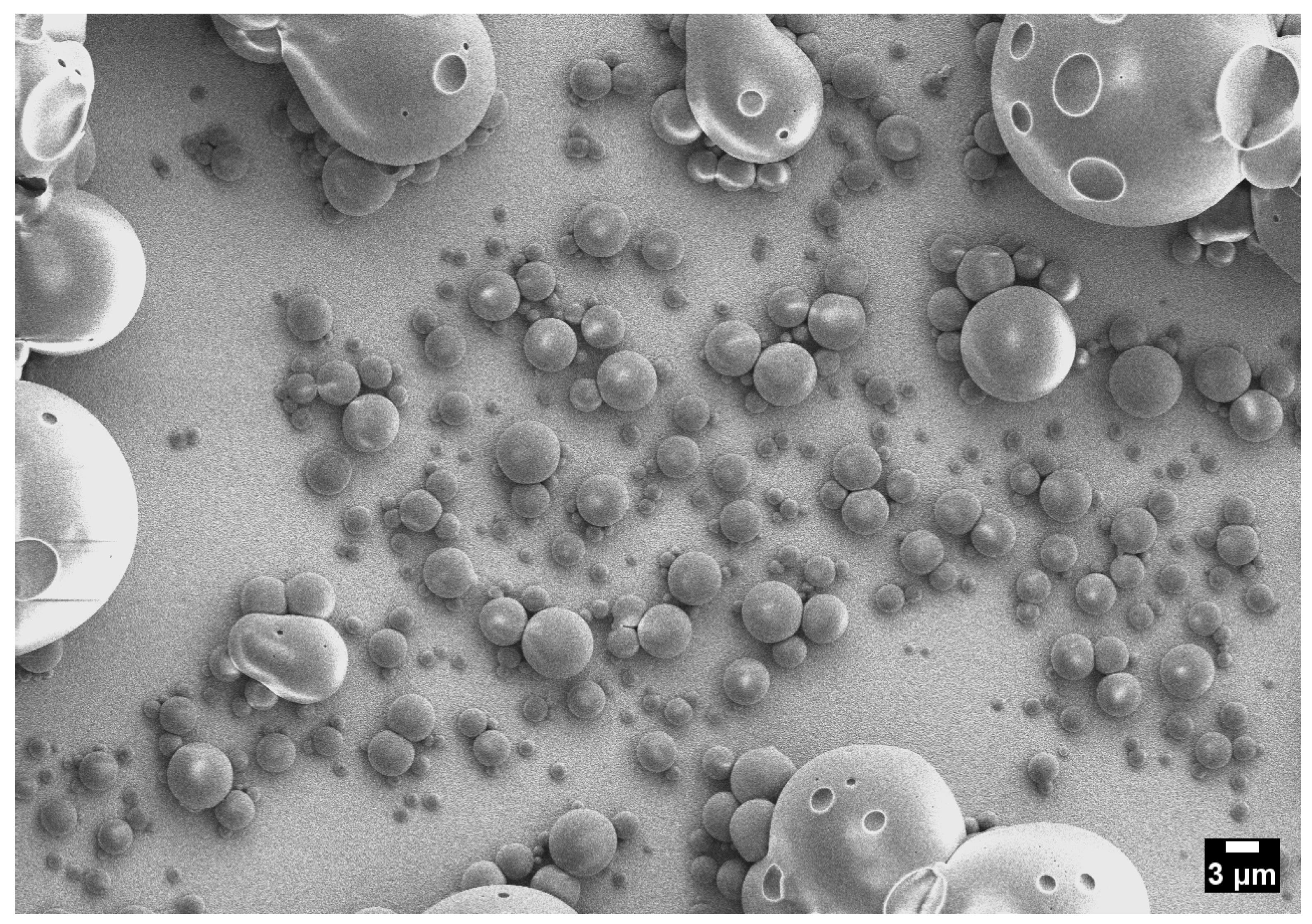
3.4. Two-Phase Region
Origin of Crater-like Pits (Outgassing Mechanism)
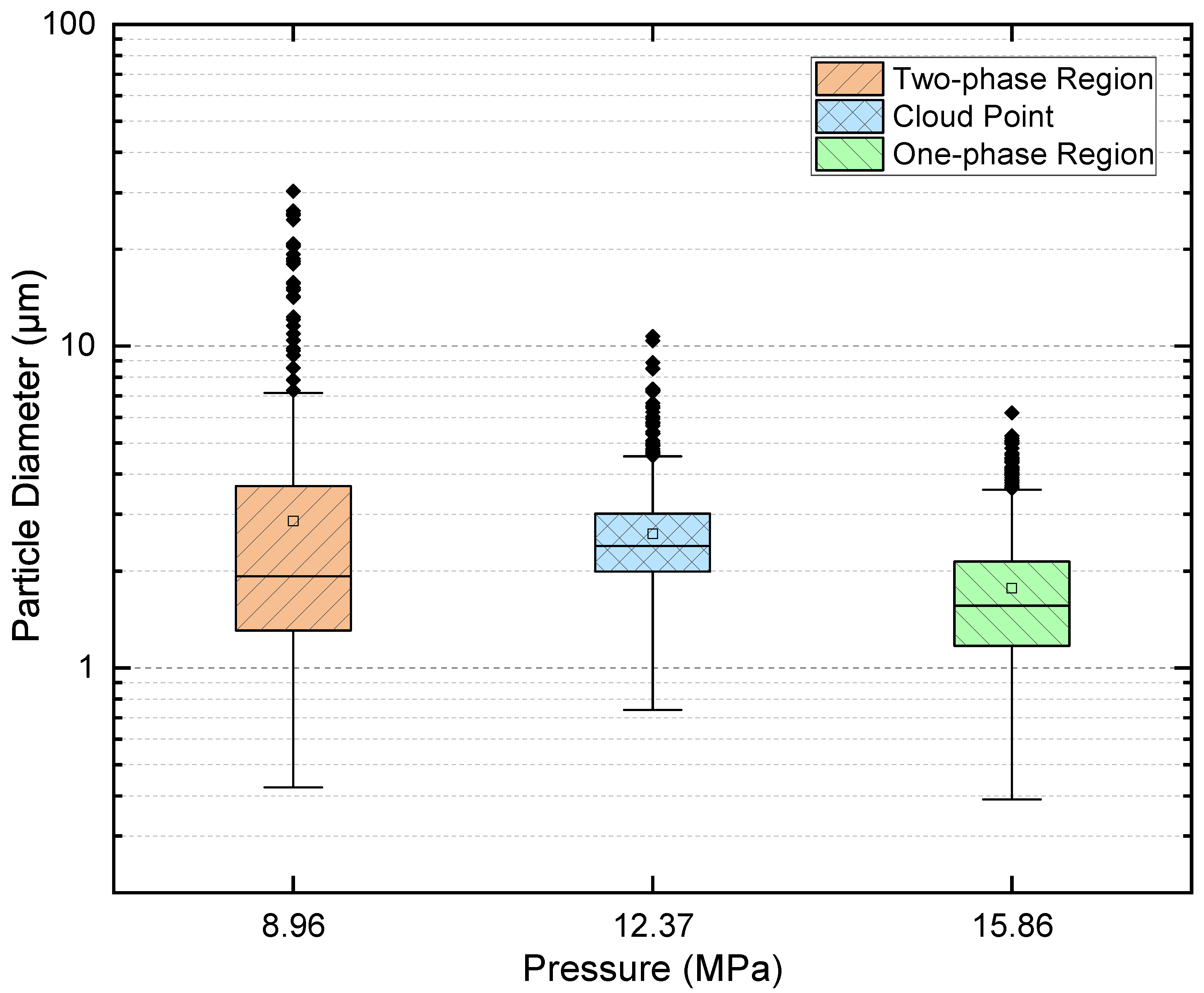
3.5. Quantitative Cross-Condition Comparison and Mechanistic Synthesis
- One-phase operation favors compact, relatively uniform deposits with minimal interparticle interaction.
- Cloud-point operation increases coverage and shifts sizes upward while retaining a single mode and only modest dispersion.
- Two-phase operation yields the broadest dispersions and the largest outliers as a result of coalescence and vent-induced hollowing.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AG–HGK | Altunin–Gadetskii–Haar–Gallagher–Kell (equation of state for CO2) | |
| CCD | Charge-coupled device (camera) | |
| CV | Coefficient of variation () | |
| DI | Deionized (water) | |
| EOS | Equation of state | |
| FTO | Fluorine-doped tin oxide | |
| IPA | Isopropyl alcohol | |
| IQR | Interquartile range | |
| PDI | Polydispersity index | |
| PSD | Particle-size distribution | |
| PTFE | Polytetrafluoroethylene | |
| scCO2 | Supercritical carbon dioxide | |
| SEM | Scanning electron microscopy | |
| TFEMA | 2,2,2-trifluoroethyl methacrylate (poly(TFEMA) denotes the homopolymer) | |
| Glass transition temperature | ||
| Nomenclature | ||
| Symbol | Description | Units |
| P | Pressure | MPa |
| T | Temperature | K (or °C) |
| Supercritical CO2 density (EOS) | kg m−3 | |
| Mass of scCO2 charged | g | |
| Mass of toluene charged | g | |
| Volume of toluene charged | mL | |
| Mass of poly(TFEMA) | g | |
| Polymer mass fraction | wt% | |
| Cloud-point pressure | MPa | |
| N | Sample size (particle count) | – |
| Mean particle diameter | μm | |
| Standard deviation of diameter | μm | |
| Coefficient of variation () | – | |
| Critical nucleus radius | nm (or m) | |
| Nucleation free-energy barrier | J |
References
- Ovaskainen, L.; Rodriguez-Meizoso, I.; Birkin, N.A.; Howdle, S.M.; Gedde, U.; Wågberg, L.; Turner, C. Towards superhydrophobic coatings made by non-fluorinated polymers sprayed from a supercritical solution. J. Supercrit. Fluids 2013, 77, 134–141. [Google Scholar] [CrossRef]
- Vasylyshyn, T.; Patsula, V.; Filipová, M.; Konefal, R.L.; Horák, D. Poly(glycerol monomethacrylate)-encapsulated upconverting nanoparticles prepared by miniemulsion polymerization: Morphology, chemical stability, antifouling properties and toxicity evaluation. Nanoscale Adv. 2023, 5, 6979–6989. [Google Scholar] [CrossRef]
- Lv, C.; Liao, X.; Zou, F.; Tang, W.; Xing, S.; Li, G. Generating porous polymer microspheres with cellular surface via a gas-diffusion confined scCO2 foaming technology to endow the super-hydrophobic coating with hierarchical roughness. Chem. Eng. J. 2022, 442, 136192. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, W.; Wang, Y.; Lin, Z.; Ji, H.; Chen, X.; Li, G.; Ma, Y.; Xie, L. Preparation of PP/PC Light-Diffusing Materials with UV-Shielding Property. Polym. Compos. 2023, 44, 5553–5566. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Bai, H.; Li, L.; Li, J. Polymeric nanoporous materials fabricated with supercritical CO2 and CO2-expanded liquids. Chem. Soc. Rev. 2014, 43, 6938–6953. [Google Scholar] [CrossRef] [PubMed]
- Barroso, T.; Temtem, M.; Casimiro, T.; Aguiar-Ricardo, A. Development of pH-responsive poly(methylmethacrylate-co-methacrylic acid) membranes using scCO2 technology. Application to protein permeation. J. Supercrit. Fluids 2009, 51, 57–66. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kanehashi, S. Polymeric membrane gas separation performance improvements through supercritical CO2 treatment. J. Membr. Sci. 2018, 566, 239–248. [Google Scholar] [CrossRef]
- Ratcharak, O.; Sane, A. Surface coating with poly(trifluoroethyl methacrylate) through rapid expansion of supercritical CO2 solutions. J. Supercrit. Fluids 2014, 89, 106–112. [Google Scholar] [CrossRef]
- Leitner, W. Designed to dissolve. Nature 2000, 405, 129–130. [Google Scholar] [CrossRef]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of supercritical carbon dioxide in materials processing and synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A Review of CO2 Applications in the Processing of Polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Tutek, K.; Masek, A.; Kosmalska, A.; Cichosz, S. Application of Fluids in Supercritical Conditions in the Polymer Industry. Polymers 2021, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Kortsen, K.; Fowler, H.R.; Jacob, P.L.; Krumins, E.; Lentz, J.C.; Souhil, M.R.; Taresco, V.; Howdle, S.M. Exploiting the tuneable density of scCO2 to improve particle size control for dispersion polymerisations in the presence of poly(dimethyl siloxane) stabilisers. Eur. Polym. J. 2022, 168, 111108. [Google Scholar] [CrossRef]
- Hakuta, Y.; Hayashi, H.; Arai, K. Fine particle formation using supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003, 7, 341–351. [Google Scholar] [CrossRef]
- Yan, H.; Sato, T.; Komago, D.; Yamaguchi, A.; Oyaizu, K.; Yuasa, M.; Otake, K. Electrochemical Synthesis of a Polypyrrole Thin Film with Supercritical Carbon Dioxide as a Solvent. Langmuir 2005, 21, 12303–12308. [Google Scholar] [CrossRef]
- Medina-Gonzalez, Y.; Camy, S.; Condoret, J.S. scCO2/Green Solvents: Biphasic Promising Systems for Cleaner Chemicals Manufacturing. ACS Sustain. Chem. Eng. 2014, 2, 2623–2636. [Google Scholar] [CrossRef]
- Wood, C.D.; Tan, B.; Zhang, H.; Cooper, A.I. Chapter 21—Supercritical Carbon Dioxide as a Green Solvent for Polymer Synthesis. In Thermodynamics, Solubility and Environmental Issues; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 383–396. [Google Scholar] [CrossRef]
- Khanyile, A.; Andrew, J.; Paul, V.; Sithole, B. A comparative study of supercritical fluid extraction and accelerated solvent extraction of lipophilic compounds from lignocellulosic biomass. Sustain. Chem. Pharm. 2022, 26, 100608. [Google Scholar] [CrossRef]
- do Espirito Santo, A.T.; Siqueira, L.M.; Almeida, R.N.; Vargas, R.M.F.; do N Franceschini, G.; Kunde, M.A.; Cappellari, A.R.; Morrone, F.B.; Cassel, E. Decaffeination of yerba mate by supercritical fluid extraction: Improvement, mathematical modelling and infusion analysis. J. Supercrit. Fluids 2021, 168, 105096. [Google Scholar] [CrossRef]
- Marco, I.D.; Riemma, S.; Iannone, R. Supercritical Carbon Dioxide Decaffeination Process: A Life Cycle Assessment Study. Chem. Eng. Trans. 2017, 57, 1699–1704. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, T.; Liao, X.; Zhou, Y.; Chen, S.; Chen, J.; Xiong, W. Extraction of Camphor Tree Essential Oil by Steam Distillation and Supercritical CO2 Extraction. Molecules 2022, 27, 5385. [Google Scholar] [CrossRef]
- Yıldırım, M.; Erşatır, M.; Poyraz, S.; Amangeldinova, M.; Kudrina, N.O.; Terletskaya, N.V. Green Extraction of Plant Materials Using Supercritical CO2: Insights into Methods, Analysis, and Bioactivity. Plants 2024, 13, 2295. [Google Scholar] [CrossRef] [PubMed]
- Handy, K.; Tepper, G.C. Incorporation of Poly(TFEMA) in Perovskite Thin Films Using a Supercritical Fluid. Molecules 2023, 28, 5385. [Google Scholar] [CrossRef]
- von Schnitzler, J.; Eggers, R. Mass transfer in polymers in a supercritical CO2-atmosphere. J. Supercrit. Fluids 1999, 16, 81–92. [Google Scholar] [CrossRef]
- Furtado, A.I.; Bonifácio, V.D.B.; Viveiros, R.; Casimiro, T. Design of Molecularly Imprinted Polymers Using Supercritical Carbon Dioxide Technology. Molecules 2024, 29, 926. [Google Scholar] [CrossRef]
- Naguib, H.E.; Park, C.B.; Song, S.W. Effect of Supercritical Gas on Crystallization of Linear and Branched Polypropylene Resins with Foaming Additives. Ind. Eng. Chem. Res. 2005, 44, 6685–6691. [Google Scholar] [CrossRef]
- Luna-Bárcenas, G.; Mawson, S.; Takishima, S.; DeSimone, J.M.; Sanchez, I.C.; Johnston, K.P. Phase behavior of poly(1,1-dihydroperfluorooctylacrylate) in supercritical carbon dioxide. Fluid Phase Equilibria 1998, 146, 325–337. [Google Scholar] [CrossRef]
- Blackburn, J.M.; Long, D.P.; Cabañas, A.; Watkins, J.J. Deposition of Conformal Copper and Nickel Films from Supercritical Carbon Dioxide. Science 2001, 294, 141–145. [Google Scholar] [CrossRef]
- Cabañas, A.; Long, D.P.; Watkins, J.J. Deposition of Gold Films and Nanostructures from Supercritical Carbon Dioxide. Chem. Mater. 2004, 16, 2028–2033. [Google Scholar] [CrossRef]
- Rasadujjaman, M.; Watanabe, M.; Kondoh, E. Codeposition of Cu/Ni thin films from mixed precursors in supercritical carbon dioxide solutions. Jpn. J. Appl. Phys. 2014, 53, 5–7. [Google Scholar] [CrossRef]
- Pandiyarajan, S.; Hsiao, P.J.; Liao, A.H.; Ganesan, M.; Manickaraj, S.S.M.; Lee, C.T.; Huang, S.T.; Chuang, H.C. Influence of ultrasonic combined supercritical-CO2 electrodeposition process on copper film fabrication: Electrochemical evaluation. Ultrason. Sonochem. 2021, 74, 105555. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, M.; Liu, C.C.; Pandiyarajan, S.; Lee, C.T.; Chuang, H.C. Post-supercritical CO2 electrodeposition approach for Ni-Cu alloy fabrication: An innovative eco-friendly strategy for high-performance corrosion resistance with durability. Appl. Surf. Sci. 2022, 577, 151955. [Google Scholar] [CrossRef]
- Hosokawa, S.; Tomita, E.; Kanehashi, S.; Ogino, K. Study on effect of supercritical CO2 on structural ordering and charge transporting property in thiophene-based block copolymer. Jpn. J. Appl. Phys. 2022, 61, 021001. [Google Scholar] [CrossRef]
- Sajfrtová, M.; Cerhová, M.; Jandová, V.; Dřínek, V.; Daniš, S.; Matějová, L. The effect of type and concentration of modifier in supercritical carbon dioxide on crystallization of nanocrystalline titania thin films. J. Supercrit. Fluids 2018, 133, 211–217. [Google Scholar] [CrossRef]
- Wei, M.; Wang, K.; Yanagida, M.; Sugihara, H.; Morris, M.A.; Holmes, J.D.; Zhou, H. Supercritical fluid processing of mesoporous crystalline TiO2 thin films for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2007, 17, 3888–3893. [Google Scholar] [CrossRef]
- Sanli, D.; Bozbag, S.E.; Erkey, C. Synthesis of nanostructured materials using supercritical CO2: Part I. Physical transformations. J. Mater. Sci. 2012, 47, 2995–3025. [Google Scholar] [CrossRef]
- Kaleva, A.; Heinonen, S.; Nikkanen, J.P.; Levänen, E. Synthesis and Crystallization of Titanium Dioxide in Supercritical Carbon Dioxide (scCO2). IOP Conf. Ser. Mater. Sci. Eng. 2017, 175, 012034. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A Cost-Effective Supercapacitor Material of Ultrahigh Specific Capacitances: Spinel Nickel Cobaltite Aerogels from an Epoxide-Driven Sol-Gel Process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Pu, N.W.; Wang, C.A.; Sung, Y.; Liu, Y.M.; Ger, M.D. Production of few-layer graphene by supercritical CO2 exfoliation of graphite. Mater. Lett. 2009, 63, 1987–1989. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Wang, W.; Zhao, Y. Preparation of Two Dimensional Atomic Crystals-BN, WS2 and MoS2 by Supercritical CO2 Assisted with Ultrasonic. Ind. Eng. Chem. Res. 2013, 52, 4379–4382. [Google Scholar] [CrossRef]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Ekart, M.P.; Bennett, K.L.; Ekart, S.M.; Gurdial, G.S.; Liotta, C.L.; Eckert, C.A. Cosolvent Interactions in Supercritical Fluid Solutions. AIChE J. 1993, 39, 235–248. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Ting, Y.S.; Hsieh, C.M. Prediction of solid solute solubility in supercritical carbon dioxide with organic cosolvents from the PR+COSMOSAC equation of state. Fluid Phase Equilibria 2017, 431, 48–57. [Google Scholar] [CrossRef]
- Ren, H.; Song, J.; Xu, Q.; Yin, J. Solubility of the silver nitrate in supercritical carbon dioxide with ethanol and ethylene glycol as double cosolvents: Experimental determination and correlation. Chin. J. Chem. Eng. 2019, 27, 400–404. [Google Scholar] [CrossRef]
- Gurina, D.L.; Antipova, M.L.; Odintsova, E.G.; Petrenko, V.E. The study of peculiarities of parabens solvation in methanol- and acetone-modified supercritical carbon dioxide by computer simulation. J. Supercrit. Fluids 2017, 126, 47–54. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Knutson, B.L.; Pouillot, F.; Liotta, C.L.; Eckert, C.A. Spectroscopic study of structure and interactions in cosolvent-modified supercritical fluids. J. Phys. Chem. 1993, 97, 11823–11834. [Google Scholar] [CrossRef]
- Yang, H.; Zhong, C. Modeling of the solubility of aromatic compounds in supercritical carbon dioxide–cosolvent systems using SAFT equation of state. J. Supercrit. Fluids 2005, 33, 99–106. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, T.; Li, J.; Pang, Q.; Yang, H.; Liu, G.; Huang, H.; Zhu, Y. Dynamics Simulation of the Effect of Cosolvent on the Solubility and Tackifying Behavior of PDMS Tackifier in Supercritical CO2 Fracturing Fluid. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130985. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Han, B. Critical points and phase behavior of toluene-CO2 and toluene-H2-CO2 mixture in CO2-rich region. J. Supercrit. Fluids 2000, 18, 185–192. [Google Scholar] [CrossRef]
- Matsukawa, H.; Tsuji, T.; Otake, K. Measurement of the Density of Carbon Dioxide/Toluene Homogeneous Mixtures and Correlation with Equations of State. J. Chem. Thermodyn. 2022, 164, 106618. [Google Scholar] [CrossRef]
- Wu, J.; Pan, Q.; Rempel, G.L. Pressure-Density-Temperature Behavior of CO2/Acetone, CO2/Toluene, and CO2/Monochlorobenzene Mixtures in the Near-Critical Region. J. Chem. Eng. Data 2004, 49, 976–979. [Google Scholar] [CrossRef]
- Rowane, A.J.; Mallepally, R.R.; Bamgbade, B.A.; Newkirk, M.S.; Baled, H.O.; Burgess, W.A.; Gamwo, I.K.; Tapriyal, D.; Enick, R.M.; McHugh, M.A. High-temperature, high-pressure viscosities and densities of toluene. J. Chem. Thermodyn. 2017, 115, 34–46. [Google Scholar] [CrossRef][Green Version]
- Pitzer, K.S.; Schreiber, D.R. Improving equation-of-state accuracy in the critical region; equations for carbon dioxide and neopentane as examples. Fluid Phase Equilibria 1988, 41, 1–17. [Google Scholar] [CrossRef]
- Ameduri, B. Fluoropolymers: The right material for the right applications. Chem.–Eur. J. 2018, 24, 18830–18841. [Google Scholar] [CrossRef]
- Ciardelli, F.; Rubino, G.; Ranieri, G.; Licciulli, A.; Laviano, R. Fluorinated polymeric materials for the protection of monumental buildings. Macromol. Symp. 2000, 152, 211–222. [Google Scholar] [CrossRef]
- Leivo, E.; Wilenius, T.; Kinos, T.; Vuoristo, P.; Mäntylä, T. Properties of thermally sprayed fluoropolymer PVDF, ECTFE, PFA and FEP coatings. Prog. Org. Coat. 2004, 49, 69–73. [Google Scholar] [CrossRef]
- Kwon, S.; Bae, W.; Lee, K.; Byun, H.S.; Kim, H. High Pressure Phase Behavior of Carbon Dioxide + 2,2,2-Trifluoroethyl Methacrylate and + Poly(2,2,2-trifluoroethyl methacrylate) Systems. J. Chem. Eng. Data 2007, 52, 89–92. [Google Scholar] [CrossRef]
- Kwon, S.; Bae, W.; Kim, H. The Effect of CO2 in Free-radical Polymerization of 2,2,2-Trifluoroethyl Methacrylate. Korean J. Chem. Eng. 2004, 21, 910–914. [Google Scholar] [CrossRef]
- Zelaya, J.R.; Tepper, G.C. Cloud Point Behavior of Poly(trifluoroethyl methacrylate) in Supercritical CO2–Toluene Mixtures. Molecules 2025, 30, 1199. [Google Scholar] [CrossRef]
- Byun, H.S.; Kim, C.R.; Yoon, S.D. Cloud-point measurement of binary and ternary mixtures for the P(MMA-co-PnFPA) in supercritical fluoric solvents. J. Supercrit. Fluids 2017, 120, 226–239. [Google Scholar] [CrossRef]
- Xu, A.; Zhang, L.; Ma, J.; Ma, Y.; Geng, B.; Zhang, S. Preparation and surface properties of poly(2,2,2-trifluoroethyl methacrylate) coatings modified with methyl acrylate. J. Coat. Technol. Res. 2016, 13, 795–804. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G. Moisture Stability of Perovskite Solar Cells Processed in Supercritical Carbon Dioxide. Molecules 2021, 26, 7570. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G.C. Efficient perovskite solar cells processed in supercritical carbon dioxide. J. Supercrit. Fluids 2021, 171, 105203. [Google Scholar] [CrossRef]
- Annohene, G.; Pascucci, J.; Pestov, D.; Tepper, G.C. Supercritical fluid-assisted crystallization of CH3NH3PbI3 perovskite films. J. Supercrit. Fluids 2020, 156, 104684. [Google Scholar] [CrossRef]
- Annohene, G.; Tepper, G.C. Low temperature formation of CH3NH3PbI3 perovskite films in supercritical carbon dioxide. J. Supercrit. Fluids 2019, 154, 104604. [Google Scholar] [CrossRef]
- Schmelzer, J.; Röpke, G.; Priezzhev, V.B. Nucleation Theory and Applications; Wiley Online Library: Hoboken, NJ, USA, 2005; Volume 76, p. 472. [Google Scholar] [CrossRef]
- Oxtoby, D.W. Homogeneous nucleation: Theory and experiment. J. Phys. Condens. Matter 1992, 4, 7627. [Google Scholar] [CrossRef]
- Kalikmanov, V.I. Classical Nucleation Theory. In Nucleation Theory; Springer: Dordrecht, The Netherlands, 2013; pp. 17–41. [Google Scholar] [CrossRef]
- Kashchiev, D. Nucleation; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Kalikmanov, V.I. Heterogeneous Nucleation. In Nucleation Theory; Springer: Dordrecht, The Netherlands, 2013; pp. 253–276. [Google Scholar] [CrossRef]
- Liu, X. Heterogeneous nucleation or homogeneous nucleation? J. Chem. Phys. 2000, 112, 9949–9955. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
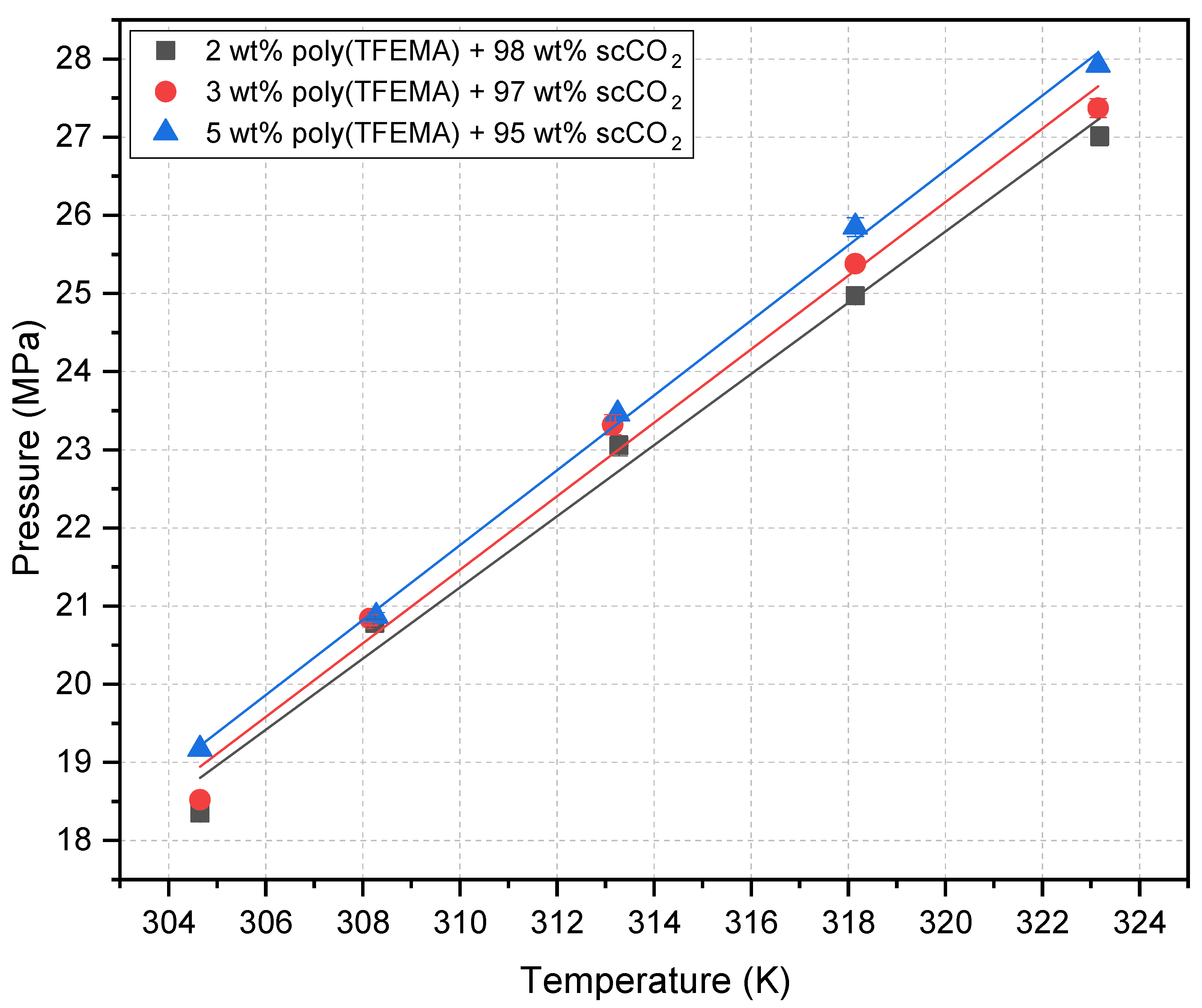
| Temperature (K) | Cloud Point Pressure (MPa) | ||
|---|---|---|---|
| 2 wt% Poly(TFEMA) | 3 wt% Poly(TFEMA) a | 5 wt% Poly(TFEMA) | |
| 304.7 | 18.35 | 18.52 | 19.17 |
| 308.2 | 20.78 | 20.84 | 20.87 |
| 313.2 | 23.05 | 23.32 | 23.46 |
| 318.2 | 24.97 | 25.38 | 25.85 |
| 323.2 | 27.01 | 27.37 | 27.92 |
| Pressure (MPa) | Classification |
|---|---|
| 15.86 ± 0.17 | One-phase |
| 12.37 ± 0.25 | Cloud point |
| 8.96 ± 0.17 | Two-phase |
| Regime | P (MPa) | (kg m−3) | (g) | (g) | (mL) | (g) |
|---|---|---|---|---|---|---|
| One-phase | 15.86 | 793.9 | 337.3 | 85.4 | 99.0 | 4.3 |
| Cloud point | 12.37 | 729.2 | 309.8 | 78.4 | 91.0 | 3.9 |
| Two-phase | 8.96 | 477.8 | 203.0 | 51.4 | 59.6 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelaya, J.R.; Tepper, G.C. Thermodynamic Phase Control of Poly(TFEMA) Nucleation and Surface Deposition in Supercritical CO2–Toluene. Colloids Interfaces 2025, 9, 78. https://doi.org/10.3390/colloids9060078
Zelaya JR, Tepper GC. Thermodynamic Phase Control of Poly(TFEMA) Nucleation and Surface Deposition in Supercritical CO2–Toluene. Colloids and Interfaces. 2025; 9(6):78. https://doi.org/10.3390/colloids9060078
Chicago/Turabian StyleZelaya, James R., and Gary C. Tepper. 2025. "Thermodynamic Phase Control of Poly(TFEMA) Nucleation and Surface Deposition in Supercritical CO2–Toluene" Colloids and Interfaces 9, no. 6: 78. https://doi.org/10.3390/colloids9060078
APA StyleZelaya, J. R., & Tepper, G. C. (2025). Thermodynamic Phase Control of Poly(TFEMA) Nucleation and Surface Deposition in Supercritical CO2–Toluene. Colloids and Interfaces, 9(6), 78. https://doi.org/10.3390/colloids9060078







