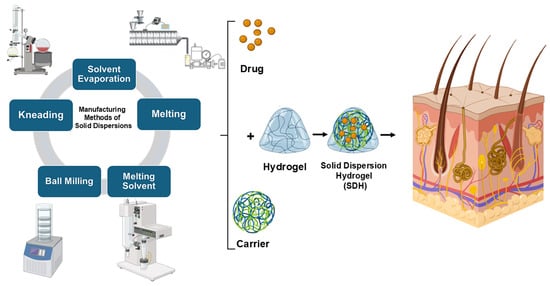
A Mini-Review on Enhancing Solubility in Topical Hydrogel Formulations Using Solid Dispersion Technology for Poorly Water-Soluble Drugs
Abstract
1. Introduction
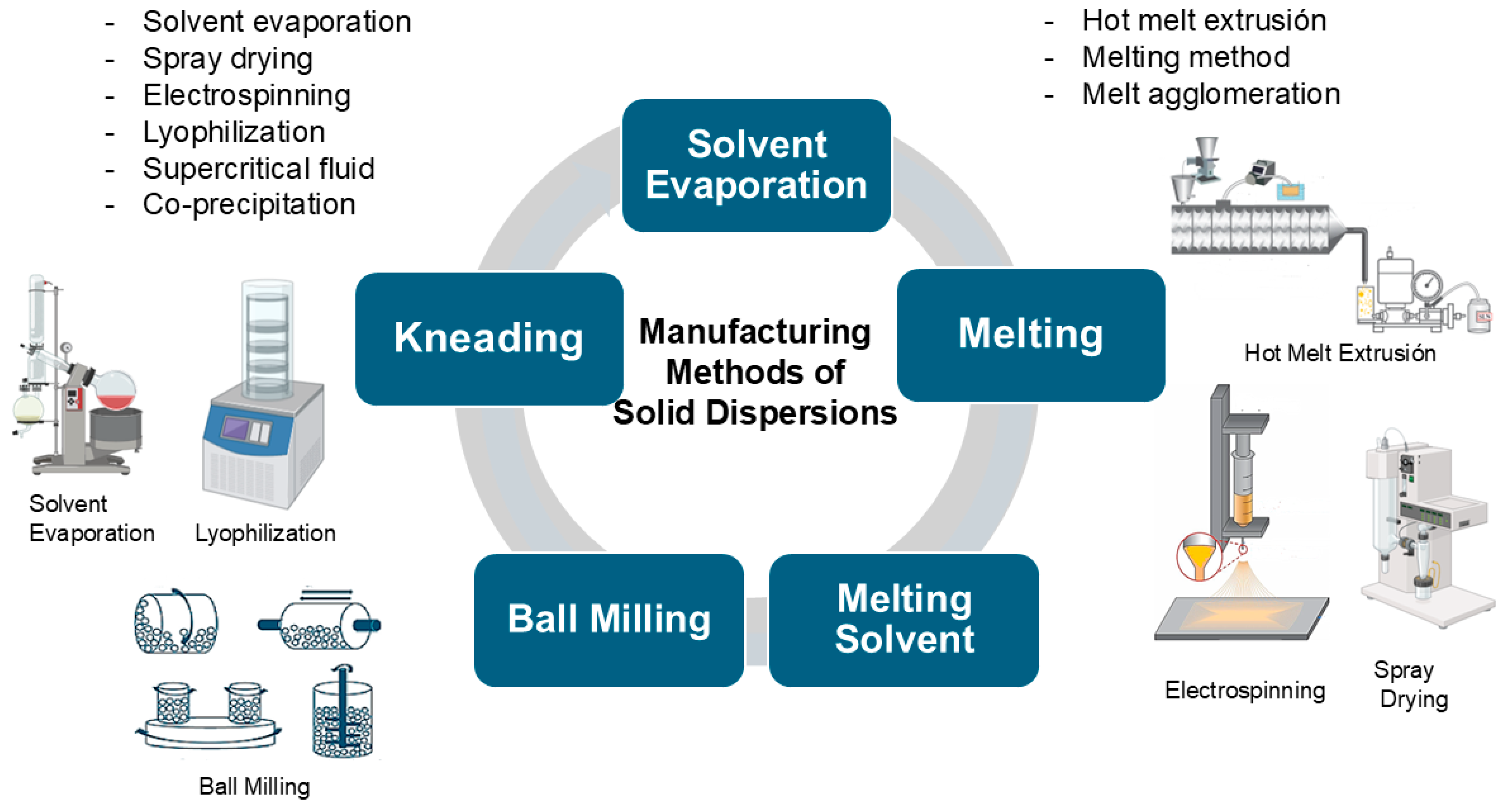
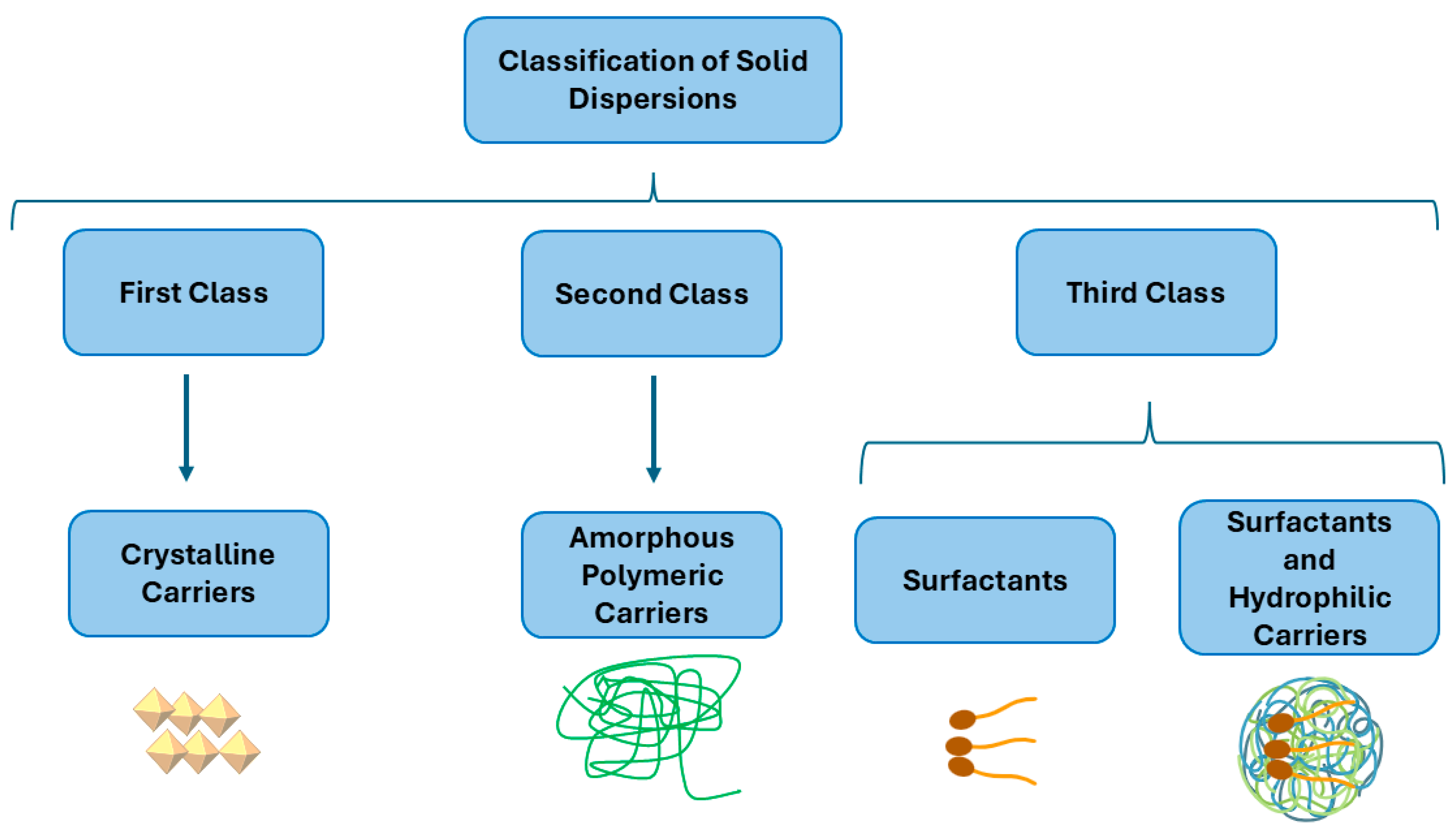
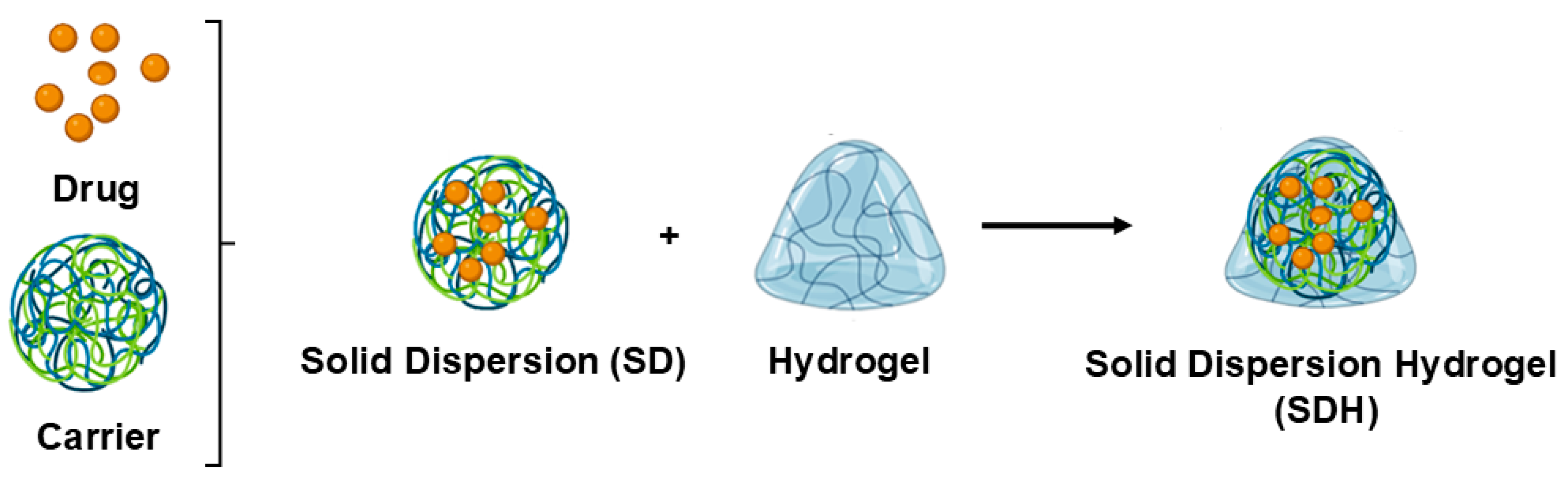
2. Solid Dispersions (SDs)
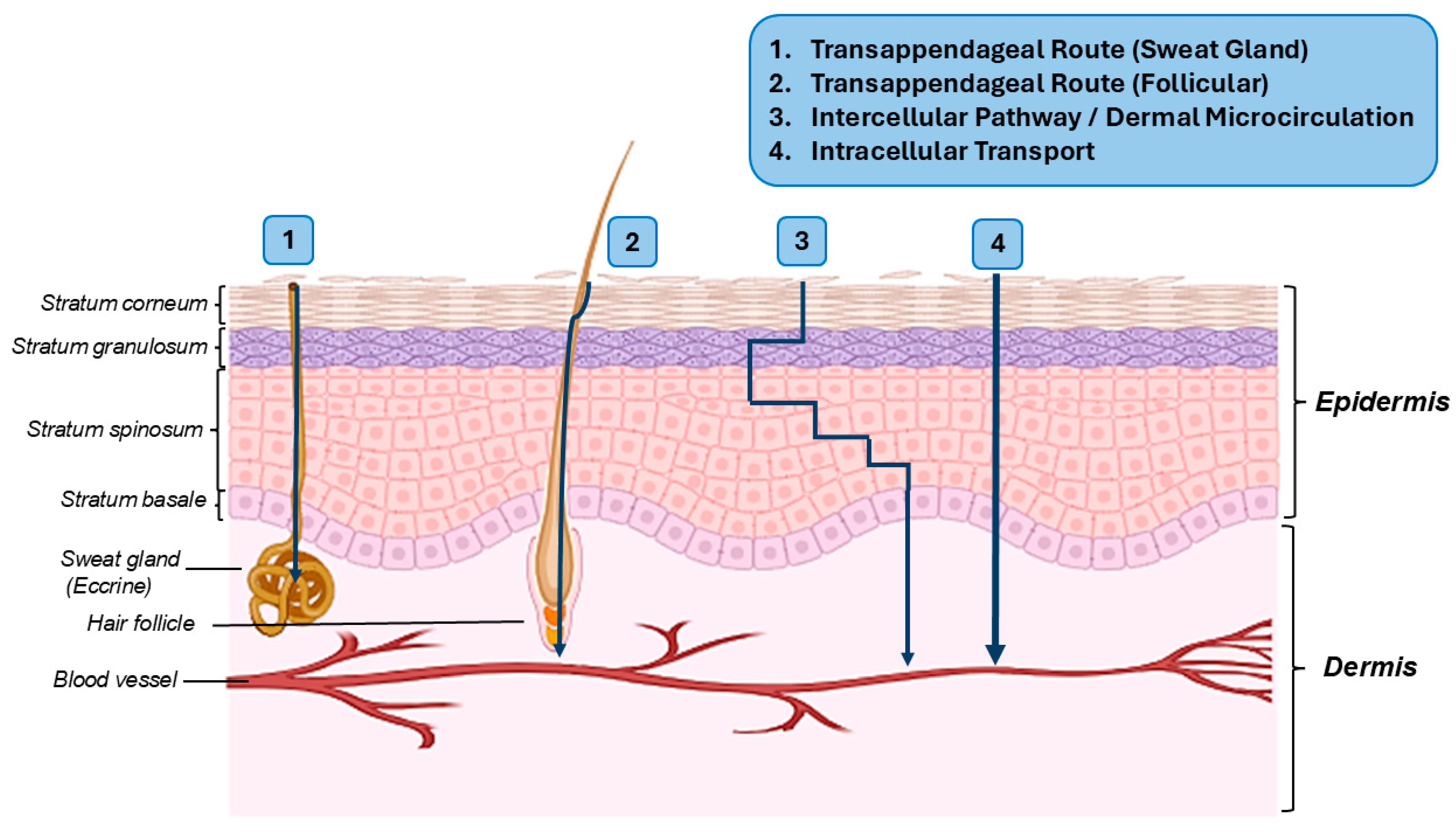
3. Topical Drug Delivery Systems and Their Pathways to the Target Tissues
4. The Current State of SDHs
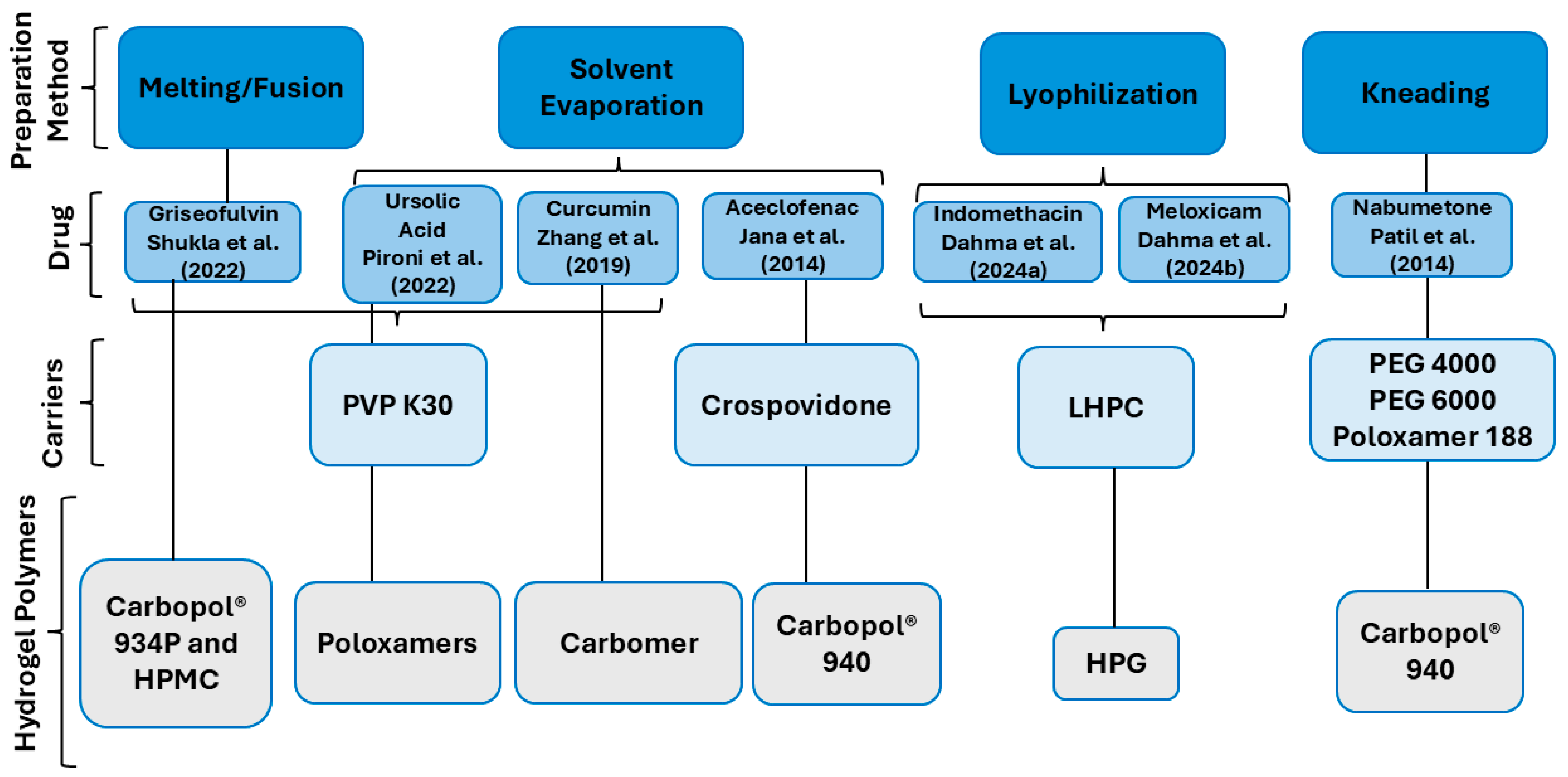
4.1. The Preparation of SDHs
4.2. Characterization of SDHs
4.2.1. SEM
4.2.2. The Degree of Crystallinity
4.2.3. FTIR
4.2.4. Solubility
4.2.5. In Vitro Drug Release
4.2.6. Rheological Studies
4.2.7. Cytotoxicity
4.2.8. Permeation Studies
4.2.9. In Vivo Study
4.3. SDHs as a Promising Carrier System for the Topical Delivery of Poorly Water-Soluble Drugs
4.4. Future Perspectives and Challenges of SDHs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| BCS | Biopharmaceutical Classification System |
| BHA | Butylated Hydroxyanisole |
| BHT | Butylated Hydroxytoluene |
| CD | Cyclodextrin |
| DMSO | Dimethyl Sulfoxide |
| DSC | Differential Scanning Calorimetry |
| FDA | Food and Drug Administration |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| FDM | Fused Deposition Modeling |
| HeLa | Human Ovarian Cells |
| HPG | Hydroxypropyl Guar Gum |
| HPLC | High-Performance Liquid Chromatography |
| HPMC | Hydroxypropylmethylcellulose |
| IND | Indomethacin |
| INDNa-PVPVA | Sodium IND–Copovidone |
| IND-PVPVA | IND–Copovidone |
| J774 | Murine Macrophages |
| L929 | Murine Fibroblasts |
| LHPC | Low-Substituted Hydroxypropyl Cellulose |
| MS | Mass Spectrometry |
| N | Nabumetone |
| NaCl | Sodium Chloride |
| NaOH | Sodium Hydroxide |
| NLC | Nanostructured Lipid Carrier |
| PEG | Polyethylene Glycol |
| PHEMA | Poly(2-hydroxyethyl methacrylate) |
| PLGA-PEG-PLGA | Poly(lactic-co-glycolic acid)-poly(ethylene glycol)-poly(lactic-co-glycolic acid) |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinylpyrrolidone |
| QbD | Quality by Design |
| SD | Solid Dispersion |
| SDH | Solid Dispersion Hydrogel |
| SDS | Sodium Dodecyl Sulfate |
| SEDDS | Self-Emulsifying Drug Delivery System |
| SEM | Scanning Electron Microscopy |
| SLN | Solid Lipid Nanoparticle |
| Tg | Glass Transition Temperature |
| UA | Ursolic Acid |
| Vero CCL-81 | Monkey Epithelial Cells |
| WHO | The World Health Organization |
| XRD | X-Ray Diffraction |
References
- Choi, J.-S.; Lee, S.-E.; Jang, W.S.; Byeon, J.C.; Park, J.-S. Solid dispersion of dutasteride using the solvent evaporation method: Approaches to improve dissolution rate and oral bioavailability in rats. Mater. Sci. Eng. C 2018, 90, 387–396. [Google Scholar] [CrossRef]
- Xu, W.; Sun, Y.; Du, L.; Chistyachenko, Y.S.; Dushkin, A.V.; Su, W. Investigations on solid dispersions of valsartan with alkalizing agents: Preparation, characterization and physicochemical properties. J. Drug Deliv. Sci. Technol. 2018, 44, 399–405. [Google Scholar] [CrossRef]
- Choi, J.S.; Kwon, S.H.; Lee, S.E.; Jang, W.S.; Byeon, J.C.; Jeong, H.M.; Park, J.S. Use of acidifier and solubilizer in tadalafil solid dispersion to enhance the in vitro dissolution and oral bioavailability in rats. Int. J. Pharm. 2017, 526, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Karashima, M.; Sano, N.; Yamamoto, S.; Arai, Y.; Yamamoto, K.; Amano, N.; Ikeda, Y. Enhanced pulmonary absorption of poorly soluble itraconazole by micronized cocrystal dry powder formulations. Eur. J. Pharm. Biopharm. 2017, 115, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.; Kim, T.; Park, H.J.; Kim, J.-Y.; Lee, K.D.; Lee, J.M.; Lee, Y.-W. Extension of the hansen solubility parameter concept to the micronization of cyclotrimethylenetrinitramine crystals by supercritical anti-solvent process. J. Supercrit. Fluids 2016, 111, 112–120. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Zhu, Y.; Chen, Y.; Zou, L.; Liu, W.; Cheng, C.; Fu, D.; Liu, C. Enhancement of the solubility, stability and bioaccessibility of quercetin using protein-based excipient emulsions. Food. Res. Int. 2018, 114, 30–37. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227. [Google Scholar] [CrossRef]
- Reggane, M.; Wiest, J.; Saedtler, M.; Harlacher, C.; Gutmann, M.; Zottnick, S.H.; Piechon, P.; Dix, I.; Müller-Buschbaum, K.; Holzgrabe, U.; et al. Bioinspired co-crystals of imatinib providing enhanced kinetic solubility. Eur. J. Pharm. Biopharm. 2018, 128, 290–299. [Google Scholar] [CrossRef]
- Wong, J.J.L.; Yu, H.; Lim, L.M.; Hadinoto, K. A trade-off between solubility enhancement and physical stability upon simultaneous amorphization and nanonization of curcumin in comparison to amorphization alone. Eur. J. Pharm. Sci. 2018, 114, 356–363. [Google Scholar] [CrossRef]
- Park, J.B.; Park, C.; Piao, Z.Z.; Amin, H.H.; Meghani, N.M.; Tran, P.H.L.; Tran, T.T.D.; Cui, J.-H.; Cao, Q.-R.; Oh, E. pH-independent controlled release tablets containing nanonizing valsartan solid dispersions for less variable bioavailability in humans. J. Drug Deliv. Sci. Technol. 2018, 46, 365–377. [Google Scholar] [CrossRef]
- Chen, B.Q.; Kankala, R.K.; Wang, S.-B.; Chen, A.Z. Continuous nanonization of lonidamine by modified-rapid expansion of supercritical solution process. J. Supercrit. Fluids 2018, 133, 486–493. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured Lipid Carriers (NLCs) as Drug Delivery Platform: Advances in Formulation and Delivery Strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef]
- Mfoafo, K.; Mittal, R.; Eshraghi, A.A.; Omidian, H. Thiolated Polymers: An Overview of Mucoadhesive Properties and Their Potential in Drug Delivery via Mucosal Tissues. J. Drug Deliv. Sci. Technol. 2023, 85, 104596. [Google Scholar] [CrossRef]
- Schittny, A.; Huwyler, J.; Puchkov, M. Mechanisms of increased bioavailability through amorphous solid dispersions: A review. Drug. Deliv. 2020, 27, 110–127. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Multisource (Generic) Pharmaceutical Products: Guidelines on Registration Requirements to Establish Interchangeability. 2018. Available online: https://www.who.int/publications/m/item/annex-6-trs-1003 (accessed on 7 March 2025).
- U.S. Food and Drug Administration (FDA). Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System Guidance for Industry. 2017. Available online: https://www.fda.gov/files/drugs/published/Bioavailability-and-Bioequivalence-Studies-Submitted-in-NDAs-or-INDs-%E2%80%94-General-Considerations.pdf (accessed on 7 March 2025).
- Bashiardes, S.; Christodoulou, C. Orally Administered Drugs and Their Complicated Relationship with Our Gastrointestinal Tract. Microorganisms 2024, 12, 242. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, C.; Jing, L.; Zhou, M.; Gao, J.; Shi, Y.; Zhang, S. Multifunctional nanocomposite hydrogel dressing with low temperature photothermal and controlled antibiotics release for combating bacterial infection. Mater. Des. 2024, 239, 112812. [Google Scholar] [CrossRef]
- Karimkhani, C.; Dellavalle, R.P.; Coffeng, L.E.; Flohr, C.; Hay, R.J.; Langan, S.M.; Nsoesie, E.O.; Ferrari, A.J.; Erskine, H.E.; Silverberg, J.I.; et al. Global skin disease morbidity and mortality an update from the global burden of disease study 2013. JAMA Dermatol. 2017, 153, 406–412. [Google Scholar] [CrossRef]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-Based Drug Delivery Systems for Poorly Water-Soluble Drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q.T. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. J. Pharm. Sci B 2021, 11, 2505–2536. [Google Scholar] [CrossRef]
- Khan, K.U.; Minhas, M.U.; Badshah, S.F.; Suhail, M.; Ahmad, A.; Ijaz, S. Overview of nanoparticulate strategies for solubility enhancement of poorly soluble drugs. Life Sci. 2022, 15, 120301. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Pezzini, B.R.; Stulzer, H.K. Eutectic mixtures as an approach to enhance solubility, dissolution rate and oral bioavailability of poorly water-soluble drugs. Int. J. Pharm. 2020, 588, 119741. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, J. Solid dispersion techniques: A review. Int. J. Res. Eng. Sci. Manag. 2021, 4, 104–111. [Google Scholar]
- Jiménez-Santos, C.J.; Pérez-Martínez, J.I.; Gómez-Pantoja, M.E.; Moyano, J.R. Enhancement of albendazole dissolution properties using solid dispersions with Gelucire 50/13 and PEG 15000. J. Drug. Deliv. Sci. Technol. 2017, 42, 261–272. [Google Scholar] [CrossRef]
- Haser, A.; Cao, T.; Lubach, J.; Listro, T.; Acquarulo, L.; Zhang, F. Melt extrusion vs. spray drying: The effect of processing methods on crystalline content of naproxen-povidone formulations. Eur. J. Pharm. Sci. 2017, 102, 115–1252. [Google Scholar] [CrossRef]
- Motallae, S.; Taheri, A.; Homayouni, A. Preparation and characterization of solid dispersions of celecoxib obtained by spray-drying ethanolic suspensions containing PVP-K30 or isomalt. J. Drug Deliv. Sci. Technol. 2018, 46, 188–196. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.E.; Jang, W.S.; Byeon, J.C.; Park, J.S. Tadalafil solid dispersion formulations based on PVP/VA S-630: Improving oral bioavailability in rats. Eur. J. Pharm. Sci. 2017, 106, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Pyo, Y.C.; Kim, D.H.; Lee, S.E.; Kim, J.K.; Park, J.S. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, H.; Zhao, Y.; Ma, Z. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 10, 74. [Google Scholar] [CrossRef]
- Fan, N.; He, Z.; Ma, P.; Wang, X.; Li, C.; Sun, J.; Sun, Y.; Li, J. Impact of HPMC on inhibiting crystallization and improving permeability of curcumin amorphous solid dispersions. Carbohydr. Polym. 2018, 181, 543–550. [Google Scholar] [CrossRef]
- Fan, N.; Ma, P.; Wang, X.; Li, C.; Zhang, X.; Zhang, K.; Li, J.; He, Z. Storage stability and solubilization ability of HPMC in curcumin amorphous solid dispersions formulated by Eudragit E100. Carbohydr. Polym. 2018, 199, 492–498. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Chen, Y.; Zhang, Z.; Zhu, A.; Qian, F. A high-sensitivity HPLC-ELSD method for HPMC-AS quantification and its application in elucidating the release mechanism of HPMC-AS based amorphous solid dispersions. Eur. J. Pharm. Sci. 2018, 122, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kashani, E.; Maghsoudi, S.; Rezania, H.; Yarazavi, M.; Hajiabbas, M.; Benkovics, G.; Bilensoy, E.; Lacík, I.; Heydari, A. Cyclodextrin polymers containing ionizable and ionic groups: A comprehensive review from classifications and synthesis methods to applications. Mater. Today Chem. 2024, 39, 102186. [Google Scholar] [CrossRef]
- Almeida, H.; Ferreira, B.; Fernandes-Lopes, C.; Araújo, F.; Bonifácio, M.J.; Vasconcelos, T.; Sarmento, B. Third-Generation Solid Dispersion Through Lyophilization Enhanced Oral Bioavailability of Resveratrol. ACS Pharmacol. Transl. Sci. 2024, 7, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycka, K.; Marcinkowska, A. The use of hot melt extrusion to prepare a solid dispersion of ibuprofen in a polymer matrix. Polymers 2023, 15, 2912. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Prezotti, F.; Araújo, F.; Lopes, C.; Loureiro, A.; Marques, S.; Sarmento, B. Third-generation solid dispersion combining Soluplus and poloxamer 407 enhances the oral bioavailability of resveratrol. Int. J. Pharm. 2021, 595, 120245. [Google Scholar] [CrossRef]
- Dahma, Z.; Ibáñez-Escribano, A.; Fonseca-Berzal, C.; García-Rodríguez, J.J.; Álvarez-Álvarez, C.; Torrado-Salmerón, C.; Torrado-Santiago, S.; Torre-Iglesias, P.M. Development, Characterization, and Cellular Toxicity Evaluation of Solid Dispersion-Loaded Hydrogel Based on Indomethacin. Polymers 2024, 16, 2174. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, J.Y.; Chang, J.H.; Liu, X.G.; Xue, H.F.; Wang, R.X.; Liu, C.Z. Soluplus-Mediated Diosgenin Amorphous Solid Dispersion with High Solubility and High Stability: Development, Characterization and Oral Bioavailability. Drug Des. Dev. Ther. 2020, 14, 2959–2975. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Obi, N. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar]
- Ugwu, F.E.; Hyma, P. Enhancement of solubility of poorly water soluble drugs (Amlodipine Besylate) by using Solid Dispersion Technique. Int. J. Res. Anal. Rev. 2024, 11, 562–574. [Google Scholar]
- Bertoni, S.; Albertini, B.; Ferraro, L.; Beggiato, S.; Dalpiaz, A.; Passerini, N. Exploring the use of spray congealing to produce solid dispersions with enhanced indomethacin bioavailability: In vitro characterization and in vivo study. Eur. J. Pharm. BioPharm. 2019, 139, 132–141. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Roh, S.; Nguyen, M.T.N.; Lee, J.S. Structural Control of Nanofibers According to Electrospinning Process Conditions and Their Applications. Micromachines 2023, 14, 2022. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Nakamura, A. A method for preparing an aqueous colloidal dispersion of organic materials by using water-soluble polymers: Dispersion of B-carotene by polyvinylpyrrolidone Kolloid-Z. Z. Für Polym. 1965, 203, 130–133. [Google Scholar] [CrossRef]
- Visan, A.I.; Negut, I. Development and Applications of PLGA Hydrogels for Sustained Delivery of Therapeutic Agents. Gels 2024, 10, 497. [Google Scholar] [CrossRef]
- Kalyani, K.; Sravya, D. A Study on Enhancement of Solubility and Dissolution Properties of Voriconazole by Solid Dispersion Technique. J. Pharm. Insights Res. 2024, 2, 089–096. [Google Scholar] [CrossRef]
- Goldberg, A.H.; Gibaldi, M.; Kanig, J.L. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures III. J. Pharm. Sci. 1966, 55, 487–492. [Google Scholar] [CrossRef]
- Sitotaw, Y.W.; Habtu, N.G.; Gebreyohannes, A.Y.; Nunes, S.P.; Van Gerven, T. Ball Milling as an Important Pretreatment Technique in Lignocellulose Biorefineries: A Review. Biomass Convers. Biorefinery 2023, 13, 15593–15616. [Google Scholar] [CrossRef]
- Bejaoui, M.; Kalfat, R.; Galai, H. The effect of adding PVP to the binary solid dispersion (indomethacin: Kaolin) on the formation of physically stable amorphous drug. J. Pharm. Innov. 2021, 17, 736–746. [Google Scholar] [CrossRef]
- Rojas-Oviedo, I.; Retchkiman-Corona, B.; Quiri-no-Barreda, C.T.; Cárdenas, J.; Schabes-Retchkiman, P. Solubility enhancement of a poorly water soluble drug by forming solid dispersions using mechanochemical activation. Ind. J. Pharm. Sci. 2012, 74, 505. [Google Scholar] [CrossRef]
- Khalid, G.M.; Billa, N. Solid Dispersion Formulations by FDM 3D Printing—A Review. Pharmaceutics 2022, 14, 690. [Google Scholar] [CrossRef]
- El-Badry, M.; Fetih, G.; Fathy, M. Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharm. J. 2009, 17, 217–225. [Google Scholar] [CrossRef]
- Ji, Y.; Paus, R.; Prudic, A.; Lübbert, C.; Sadowski, G. A novel approach for analyzing the dissolution mechanism of solid dispersions. Pharm. Res. 2015, 32, 2559–2578. [Google Scholar] [CrossRef] [PubMed]
- Daravath, B.; Tadikonda, R.R.; Vemula, S.K. Formulation and pharmacokinetics of Gelucire solid dispersions of flurbiprofen. Drug Dev. Ind. Pharm. 2015, 41, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Gao, W.; Taylor, L.S. Impact of Eudragit EPO and hydroxypropyl methylcellulose on drug release rate, supersaturation, precipitation outcome and redissolution rate of indomethacin amorphous solid dispersions. Int. J. Pharm. 2017, 531, 313–323. [Google Scholar] [CrossRef]
- Chiang, C.W.; Tang, S.; Mao, C.; Chen, Y. Effect of Buffer pH and Concentration on the Dissolution Rates of Sodium Indomethacin–Copovidone and Indo-methacin–Copovidone Amorphous Solid Dispersions. Mol. Pharm. 2023, 12, 6451–6462. [Google Scholar] [CrossRef]
- Dahma, Z.; Torrado-Salmerón, C.; Álvarez-Álvarez, C.; Guarnizo-Herrero, V.; Martínez-Alonso, B.; Torrado, G.; Torrado-Santiago, S.; Torre-Iglesias, P.M. Topical Meloxicam Hydroxypropyl Guar Hydrogels Based on Low-Substituted Hydroxypropyl Cellulose Solid Dispersions. Gels 2024, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Benavent, C.; Torrado-Salmerón, C.; Torrado-Santiago, S. Development of a solid dispersion of nystatin with maltodextrin as a carrier agent: Improvements in antifungal efficacy against Candida spp. biofilm infections. Pharmaceuticals 2021, 14, 397. [Google Scholar] [CrossRef]
- Colombo, M.; Lima-Melchiades, G.; Michels, L.R.; Figueiró, F.; Bassani, V.L.; Teixeira, H.F.; Koester, L.S. Solid dispersion of kaempferol: Formulation development, characterization, and oral bioavailability assessment. AAPS PharmSciTech 2019, 20, 106. [Google Scholar] [CrossRef]
- Rusdin, A.; Mohd -Gazzali, A.; Thomas, N.; Megantara, S.; Aulifa, D.L.; Budiman, A.; Muchtaridi, M. Advancing drug delivery paradigms: Polyvinyl pyrolidone (PVP)-based amorphous solid dispersion for enhanced physicochemical properties and therapeutic efficacy. Polymers 2024, 16, 286. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, L.; Wu, B.; Chen, F. Improving solubility of poorly water-soluble drugs by protein-based strategy: A review. Int. J. Pharm. 2023, 634, 122704. [Google Scholar] [CrossRef]
- Ting, J.M.; Porter, I.W.; Mecca, J.M.; Bates, F.S.; Reineke, T.M. Advances in polymer design for enhancing oral drug solubility and delivery. Bioconjugate Chem. 2018, 29, 939–952. [Google Scholar] [CrossRef]
- Tian, Y.; Jacobs, E.; Jones, D.S.; Mc-Coy, C.P.; Wu, H.; Andrews, G.P. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef]
- Patil, S.S.; Mohite, S.K. Development and evaluation of solid dispersion incorporated topical gel of Nabumetone. Res. J. Pharm. Tech. 2014, 7, 1413–1419. [Google Scholar]
- Harrison, I.P.; Spada, F. Hydrogels for atopic dermatitis and wound management: A superior drug delivery vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Cascone, S.; Lamberti, G. Hydrogel-Based Commercial Products for Biomedical Applications: A Review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Croisfelt, F.M.; Tundisi, L.L.; Ataide, J.A.; Silveira, E.; Tambourgi, E.B.; Jozala, A.F.; Souto, E.M.; Mazzola, P.G. Modified-release topical hydrogels: A ten-year review. J. Mater. Sci. 2019, 54, 10963–10983. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Chatterjee, S.; Mohanta, A.; De, A.; Mukherjee, A.; Hazra, A.; Niloy, P.P.; Tudu, M.; Chattopadhyay, K.; Samanta, A. Evaluation of Gum Odina/Carbopol Composite Mucoadhesive Hydrogel on Pharmaceutical Performance: Focusing on Potential Periodontal Treatment. Int. J. Biol. Macromol. 2025, 288, 138708. [Google Scholar] [CrossRef]
- Choi, H.; Choi, W.S.; Jeong, J.O. A Review of Advanced Hydrogel Applications for Tissue Engineering and Drug Delivery Systems as Biomaterials. Gels 2024, 10, 693. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-based biomaterials for pharmaceutical and biomedical applications: A focus on topical drug administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Lai, W.-F.; He, Z.-D. Design and Fabrication of Hydrogel-Based Nanoparticulate Systems for In Vivo Drug Delivery. J. Control. Release 2016, 243, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Clijsen, R.; Baeyens, J.P.; Barel, A.O. In vivo determination of the diclofenac skin reservoir: Comparison between passive, occlusive, and iontophoretic application. Drug Des. Dev. Ther. 2015, 9, 835–840. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, X.; Jia, Y.; Ho, C.T.; Huang, Q. Applications and delivery mechanisms of hyaluronic acid used for topical/transdermaldelivery–areview. Int. J. Pharm. 2020, 578, 119127. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Draelos, Z.D.; Gold, L.F.; Cha, A.; Vlahos, B.; Aikman, L.; Sanders, P.; Wu-Linhares, D.; Cork, M.J. Vehicles for atopic dermatitis therapies: More than just a placebo. J. Dermatol. Treat. 2022, 2022 3, 685–698. [Google Scholar] [CrossRef]
- Anantrao, J.H.; Nath, P.A.; Nivrutti, P.R. Drug penetration enhancement techniques in transdermal drug delivery system: A review. J. Pharm. Res. Int. 2021, 33, 46–61. [Google Scholar] [CrossRef]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A re-view. Ski. Res. Technol. 2021, 27, 299–308. [Google Scholar] [CrossRef]
- Elmowafy, M. Skin penetration/permeation success determinants of nanocarriers: Pursuit of a perfect formulation. Colloids Surf. B Biointerfaces 2021, 203, 111748. [Google Scholar] [CrossRef]
- Ng, K.W. Penetration enhancement of topical formulations. Pharmaceutics 2018, 10, 51. [Google Scholar] [CrossRef]
- Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.D.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Kurmi, B.D. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.; Mo-hammed, Y.; Holmes, A.; Hoek, J.; Pastore, M.; Grice, J.E. Topical drug delivery: History, percutaneous absorption, and product development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef] [PubMed]
- Praça, F.S.; Medina, W.S.; Eloy, J.O.; Petrilli, R.; Campos, P.M.; Ascenso, A.; Bentley, M.V. Evaluation of critical parameters for in vitro skin permeation and penetration studies using animal skin models. Eur. J. Pharm. Sci. 2018, 111, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Raghav, R.S.; Verma, S.; Monika, A. Comprehensive Review on Potential Chemical and Herbal Permeation Enhancers Used in Transdermal Drug Delivery Systems. Recent Adv. Drug Deliv. Formul. Former. Recent Pat. Drug Deliv. Formul. 2024, 18, 21–34. [Google Scholar] [CrossRef]
- Suvarna, V.; Sawant, N.; Jadhav, P.; Desai, N. Bile Acid Nanoparticles-An Emerging Approach for Site Specific Drug Targeting. Curr. Nanomed. 2024, 14, 212–226. [Google Scholar] [CrossRef]
- Derry, S.; Conaghan, P.; Da- Silva, J.A.P. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst. Rev. 2016, 4, 7400. [Google Scholar] [CrossRef]
- Martina, H.; Baker, M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr. Med. Res. Opin. 2017, 33, 1623–1634. [Google Scholar] [CrossRef]
- Bolla, P.K.; Clark, B.A.; Juluri, A.; Cheruvu, H.S.; Renukuntla, J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics 2020, 12, 151. [Google Scholar] [CrossRef]
- Sun, D.D.; Lee, P.I. Crosslinked Hydrogels—A Promising Class of Insoluble Solid Molecular Dispersion Carriers for Enhancing the Delivery of Poorly Soluble Drugs. Acta Pharm. Sin. B 2014, 4, 26–36. [Google Scholar] [CrossRef]
- Pironi, A.M.; Melero, A.; Eloy, J.O.; Guillot, A.J.; Santos, K.P.; Chorilli, M. Solid dipersions included in poloxamer hydrogels have favorable rheological properties for topical application and enhance the in vivo antiinflammatory effect of ursolic acid. J. Drug Deliv. Sci. Technol. 2022, 74, 103602. [Google Scholar] [CrossRef]
- Jana, S.; Ali, S.A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Development of topical gel containing aceclofenac-crospovidone solid dispersion by “Quality by Design (QbD) approach. Chem. Eng. Res. Des. 2014, 92, 2095–2105. [Google Scholar] [CrossRef]
- Shukla, S.; Upadhyay, S.; Gupta, R.K. Formulation and Evaluation of Griseofulvin Solid Dispersion incorporated gel for topical application. Res. J. Pharm. Technol. 2022, 15, 4389–4394. [Google Scholar] [CrossRef]
- Zhang, M.; Zhuang, B.; Du, G.; Han, G.; Jin, Y. Curcumin solid dispersion-loaded in situ hydrogels for local treatment of injured vaginal bacterial infection and improvement of vaginal wound healing. J. Pharm. Pharmacol 2019, 71, 1044–1054. [Google Scholar] [CrossRef]
- Ahmadi-Khoshooei, M.; Fazlollahi, F.; Maham, Y. A review on the application of differential scanning calorimetry (DSC) to petroleum products: Characterization and kinetic study. J. Therm. Anal. Calorim. 2019, 138, 3455–3484. [Google Scholar] [CrossRef]
- Ameh, E.S. A review of basic crystallography and x-ray diffraction applications. Int. J. Adv. Manuf. Technol. 2019, 105, 3289–3302. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved topical drug delivery: Role of permeation enhancers and advanced approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Jagtap, S.; Magdum, C.; Jadge, D.; Jagtap, R. Solubility enhancement technique: A review. J. Pharm. Sci. Res. 2018, 10, 2205–2211. [Google Scholar]
- Bhuyan, C.; Saha, D.; Rabha, B. A brief review on topical gels as drug delivery system. J. Pharm. Res. Int. 2021, 33, 344–357. [Google Scholar] [CrossRef]
- Ibáñez-Escribano, A.; Fonseca-Berzal, C.; Martínez-Montiel, M.; Álvarez-Márquez, M.; Gómez-Núñez, M.; Lacueva-Arnedo, M.; Espinosa-Buitrago, T.; Martín-Pérez, T.; Escario, J.A.; Merino-Montiel, P. Thio-and selenosemicarbazones as antiprotozoal agents against Trypanosoma cruzi and Trichomonas vaginalis. J. Enzym. Inhib. Med. 2022, 37, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Escribano, A.; Meneses-Marcel, A.; Marrero-Ponce, Y.; Nogal-Ruiz, J.J.; Arán, V.J.; Gómez-Barrio, A.; Escario, J.A. A sequential procedure for rapid and accurate identification of putative trichomonacidal agents. J. Microbiol. Methods 2014, 105, 162–167. [Google Scholar] [CrossRef]
- Marena, G.D.; Fonseca-Santos, B.; Matheus Aparecido dos Santos, R.; dos-Santos, K.C.; Bauab, T.M.; Corilli, M. Incorporation of ursolic acid in liquid crystalline systems improves the antifungal activity against Candida sp. J. Pharm. Innov. 2021, 16, 576–586. [Google Scholar] [CrossRef]
- Patel, M.; Patel, A.; Desai, J.; Patel, S. Cutaneous pharmacokinetics of topically applied novel dermatological formulations. AAPS Pharm. Sci. Tech. 2024, 25, 46. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, V.K.; Mahmood, A.; Waghule, T.; Gorantla, S.; Kumar-Dubey, S.; Alexander, A.; Singhvi, G. Revisiting techniques to evaluate drug permeation through skin. Expert Opin. Drug Deliv. 2021, 18, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, C.; Linville, R.M.; Galea, I.; Lambden, E.; Vögele, M.; Chen, C.; Troendle, E.P.; Ruggiu, F.; Ulmschneider, M.B.; Schiøtt, B.; et al. Permeability Benchmarking: Guidelines for Comparing in Silico, in Vitro, and in Vivo Measurements. J. Chem. Inf. Model. 2025, 65, 1234–1249. [Google Scholar] [CrossRef] [PubMed]
| Preparation Methods | Drug: Carriers | Solubility (µg/mL or mg/mL) (Increase in Drug Solubility) | Ref. | ||
|---|---|---|---|---|---|
| Melting | Indomethacin (IND):PEG 4000 IND:Gelucire® 50/13 | Phosphate buffer, pH of 7.4 | El-Badry et al. [52] | ||
| 1:4 (4-fold) | |||||
| 1:4 (3.5-fold) | |||||
| Spray-drying | IND:PVP K25 | Phosphate buffer | Ji et al. [53] | ||
| pH of 4.98: 0.47 mg/mL | |||||
| pH of 6.05: 4.27 mg/mL | |||||
| pH of 7.25: 65.96 mg/mL | |||||
| Solvent evaporation | Flurbiprofen: Gelucire® 44/14 | Phosphate buffer solution, pH of 7.2, 0.618 ± 0.26 mg/mL (3.5-fold) | Daravath et al. [54] | ||
| Solvent evaporation | IND:Eudragit® EPO (3:7) IND:HPMC (5:5 and 1:9) | pH 2.2 Mcllvaine buffer > 80 µg/mL 36 µg/mL 35 µg/mL >80 µg/mL | Xie et al. [55] | ||
| Spray congealing | IND:Gelucire® 50/13: Gelucire® 48/16 (1:9:0) (1:4.5:4.5) (1:2.7:6.3) | Phosphate buffer, pH of 5.8 0.194 ± 0.044 mg/mL (4-fold) 0.466 ± 0.045 mg/mL (19-fold) 0.775 ± 0.025 mg/mL (31-fold) | Bertoni et al. [42] | ||
| Solvent evaporation | IND-Copovidone (IND-PVPVA) sodium IND–Copovidone (INDNa-PVPVA) | Phosphate buffer, pH of 4.7–7.2 INDNa-PVPVA: 250 mg/mL | Chiang et al. [56] | ||
| Lyophilization | IND:LHPC | Acetate buffer, pH of 5.8 656.09 ± 6.28 µg/mL (4.9-fold) | Dahma et al. [38] | ||
| Lyophilization | Meloxicam:LHPC | Acetate buffer, pH of 5.8 709.17 ± 10.15 µg/mL (5.6-fold) | Dahma et al. [57] | ||
| Lyophilization | Nystatin:Maltodextrin | Phosphate buffer, pH of 4.5 (1.3-fold) | Benavent et al. [58] | ||
| Ball milling | IND:Kaolin + 10% PVP + 25% PVP + 50% PVP + 75% PVP | Water 16.66 ± 0.1 µg/mL (1.8-fold) 29.05 ± 0.1 µg/mL (3.1-fold) 43.44 ± 0.1 µg/mL (4.5-fold) 44.44 ± 0.1 µg/mL (4.8-fold) | Bejaoui et al. [49] | ||
| Ball milling | IND:lactose (1:1) | Water 39.18 ± 0.004 µg/mL (2.7-fold) | Rojas-Oviedo et al. [50] | ||
| Solvent and melting | Kaempferol: Poloxamer 407 (1:5) | Water 670.16 ± 90.53 µg/mL (4000 fold) | Colombo et al. [59] | ||
| Factors | Molecular Size | Acidity | Water Solubility | Penetration Enhancer | Protein Binding | Site and Method of Application Considerations |
|---|---|---|---|---|---|---|
| Description | Active ingredients with a molecular size < 500 g/mol can easily pass through the stratum corneum [81]. | Hydrogels with high acidity can penetrate the cell membrane and reach the neutral intracellular space at higher concentrations [88,89]. Acidic formulations with low pKa values ionize easily and cross barrier membranes more effectively [84]. | To pass through the stratum corneum and lipid matrix, the compound must be both water-soluble and lipophilic [84,87]. | Increases penetration through the stratum corneum [84,85]. | The drug concentration is higher in joints and tissues with high albumin concentrations (e.g., inflamed joints) [86]. | Topical hydrogels more easily reach the superficial joints (e.g., knee, fingers) compared to deeper ones (e.g., hip joints) [79]. The bioavailability of the drug can significantly increase with repetitive administration [80]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahma, Z.; Álvarez-Álvarez, C.; de la Torre-Iglesias, P.M. A Mini-Review on Enhancing Solubility in Topical Hydrogel Formulations Using Solid Dispersion Technology for Poorly Water-Soluble Drugs. Colloids Interfaces 2025, 9, 17. https://doi.org/10.3390/colloids9020017
Dahma Z, Álvarez-Álvarez C, de la Torre-Iglesias PM. A Mini-Review on Enhancing Solubility in Topical Hydrogel Formulations Using Solid Dispersion Technology for Poorly Water-Soluble Drugs. Colloids and Interfaces. 2025; 9(2):17. https://doi.org/10.3390/colloids9020017
Chicago/Turabian StyleDahma, Zaid, Covadonga Álvarez-Álvarez, and Paloma Marina de la Torre-Iglesias. 2025. "A Mini-Review on Enhancing Solubility in Topical Hydrogel Formulations Using Solid Dispersion Technology for Poorly Water-Soluble Drugs" Colloids and Interfaces 9, no. 2: 17. https://doi.org/10.3390/colloids9020017
APA StyleDahma, Z., Álvarez-Álvarez, C., & de la Torre-Iglesias, P. M. (2025). A Mini-Review on Enhancing Solubility in Topical Hydrogel Formulations Using Solid Dispersion Technology for Poorly Water-Soluble Drugs. Colloids and Interfaces, 9(2), 17. https://doi.org/10.3390/colloids9020017