Abstract
The importance of electrospinning to produce biomimicking micro- and nano-fibrous matrices is realized by many who work in the area of fibers. Based on the solubility of the materials to be spun, organic solvents are typically utilized. The toxicity of the utilized organic solvent could be extremely important for various applications, including tissue engineering, biomedical, agricultural, etc. In addition, the high viscosities of such polymer solutions limit the use of high polymer concentrations and lower down productivity along with the limitations of obtaining desired fiber morphology. This emphasizes the need for a method that would allay worries about safety, toxicity, and environmental issues along with the limitations of using concentrated polymer solutions. To mitigate these issues, the use of emulsions as precursors for electrospinning has recently gained significant attention. Presence of dispersed and continuous phase in emulsion provides an easy route to incorporate sensitive bioactive functional moieties within the core-sheath fibers which otherwise could only be hardly achieved using cumbersome coaxial electrospinning process in solution or melt based approaches. This review presents a detailed understanding of emulsion behavior during electrospinning along with the role of various constituents and process parameters during fiber formation. Though many polymers have been studied for emulsion electrospinning, poly(ε-caprolactone) (PCL) is one of the most studied polymers for this technique. Therefore, electrospinning of PCL based emulsions is highlighted as unique case-study, to provide a detailed theoretical understanding, discussion of experimental results along with their suitable biomedical applications.
1. Electrospinning: Past and Present
Electrospinning, a top-to-bottom nano-manufacturing technique, is a relatively simple and versatile method that has attracted excessive research interest to fabricate nano-fibrous matrices of high flexibility, porosity, and specific surface area [1]. In a typical electrospinning set-up, an electrostatically charged polymer droplet is ejected through a spinneret in the form of a polymer-jet which ultimately gets spun into micro- or nano-scale fibers/mat [2,3]. Electrospun nanofibrous matrices have been prepared using various polymeric systems such as solution [4], emulsion [5], melt [6], or near-gel resin [7,8,9], and applied in applications including air filtration [10], oil-water separation [7,11], composite reinforcement [1], smart-textile [12], super-capacitor [13], gas sensor [14], catalysis [15], controlled drug delivery [16], wound dressing [17], and as tissue engineering scaffolds [18] to name a few. The surface morphology of electrospun matrices, mimicking the native extracellular matrix (ECM) in terms of topology, chemistry, biology, and mechanical properties, is highly advantageous for tissue engineering application [3,19,20]. The electrospinning technique invented in 19th century has reached far beyond the original use of polymer solution as precursor to prepare nano-fibrous matrices. In the early 1900, John Francis Cooley (UK patent, GB 6385, Title: Improved methods of and apparatus for electrically separating the relatively volatile liquid component from the component of relatively fixed substances of composite fluids) filed two patents (US692631 and US745276) and demonstrated the apparatus for dispersion of fluids, generation of filament mesh using electrostatic force, spinneret systems for coaxial spinning, air-assisted spinning and rotating collectors, but more details of the electrospinning process were described much later by Anton Formhals in 1929–1944, who published 22 patents that covered numerous aspects of the electrospinning process [21]. Between 1964 and 1969, Geoffrey Taylor provided pioneering mathematical models on how a strong electric field affects a droplet’s morphology. Specifically, when the electric field strength increased beyond a threshold value, the spherical droplet of polymer solution evolved into a cone and ensue a liquid jet (now commonly referred to as Taylor cone). Afterward, the limitations of characterization tools during that era slowed advancement of electrospinning technique for 20 years until in the 1990s, mechanistic studies of fiber generation were carried out by Reneker and Chun (1996), then there are fascinating contributions from a variety of groups [22,23]. The primary development history nodes of electrospinning are summarized in Figure 1. Over the years electrospun fiber-based matrices, core-sheath fibers, and nanocomposites have been prepared using synthetic and natural polymers. Perhaps associated with numerous advantages, electrospinning process holds some drawbacks which need to be addressed and resolved in upcoming research, such as excess use of organic/toxic solvents especially for hydrophobic polymers, in processability of low/highly viscous precursors, low productivity in terms of total output, and poor control over fiber alignment and morphology [24]. The use of organic solvents such as dimethylformamide (DMF), tetrahydrofuran (THF), benzene-based solvents restrict the direct incorporation of any biomolecules, cells into the electrospinning precursor to produce electrospun matrices for tissue engineering application. The residual organic solvent in the matrices prohibits several growth factors and causes inflammation, resulting serious health concerns when it is applied as an ECM. The toxic nature of these solvents and their adverse impact on environmental life make to restrict their use in electrospinning dope [25].
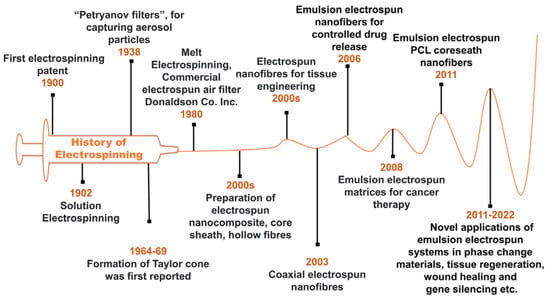
Figure 1.
History of emulsion electrospinning.
In the surge of ecological and sustainable technologies, it necessitates to minimize the usage of toxic solvents in electrospinning systems too. In this regard, use of water is a good alternative for producing electrospun matrices from hydrophilic polymers like poly(vinyl alcohol) (PVA), poly(ethylene oxide) (PEO), poly(ethylene glycol) (PEG) etc., although they can also be solubilized and electrospun from organic solvents and addition of binary solvent is reported to improve the spinnability of such systems [26,27,28]. However, for hydrophobic polymers like polystyrene, poly(ε-caprolactone) (PCL), poly(L-lactide) (PLLA) poly(acrylonitrile) (PAN) etc. use of organic solvents becomes mandatory [29,30,31]. Additionally, for enhancing the productivity of electrospun matrices in the view of commercialization, researchers are continuously improving the electrospinning process. A few studies have described methodical improvement of the characteristics of nanofibers and reviewed the process parameters to narrow the diameter-distribution of electrospun fibers [32].
Initially, the core-shell electrospinning technology was the only viable option for producing stable nanofibers from thermosensitive polymers, rubbery latex etc. For example, morphologically stable composite nanofibers of polybutadiene rubber with uniformly distributed elemental Ag NPs were prepared via co-axial electrospinning combined with in situ chemical crosslinking followed by the removal of PVP component from the corresponding core–sheath nanofibers [33]. Similar kinds of studies with very fascinating fiber morphology like honeycomb, patterned etc. have been widely reported by other research groups [34,35]. But, recently electrospinning of polymer emulsions, simply emulsion electrospinning has emerged as a novel approach to tackle the issues like material incompatibility, overall productivity and excessive use of organic solvents etc. in electrospinning. Fabrication of electrospun matrices of unique core-sheath morphology can be obtained a single nozzle using dilute polymer solutions as dispersed phase of emulsion and from low molecular weight polymers as well are highly fascinating aspects of emulsion electrospinning [36]. The method is highly advantageous for inclusion of water-soluble bioactive agents into electrospun matrices of hydrophobic polymers [37]. The hydrophobicity of polymer provides a barrier from external conditions (solvent exposure, shear stresses) and prevents the burst-release of hydrophilic bioactive agents, thus offers a sustained and controlled release over a long-period [38]. Furthermore, emulsion electrospinning offers the flexibility towards controlling the rheological properties of system by adjusting the emulsion parameters and produces matrices of uniform fiber diameter even at low polymer concentration [39]. On contrary, emulsion electrospinning often requires a template or carrier polymer to enhance the spinnability of the system and emulsion electrospun matrices have poor aqueous dimensional stability due to leaching of template polymer and surfactant [40]. Pickering emulsions stabilized using amphiphilic nanomaterials (Pickering particles) are a viable choice to prepare emulsion electrospun nanocomposite matrices instead from conventional emulsions [41,42]. Additionally, Pickering emulsions are advantageous due to their high stability, insolubility of Pickering particles, and introduction of inorganic functionalities into electrospun matrices which also enhances the mechanical properties of electrospun matrices [43]. A wide range of polymers, including biopolymers (e.g., proteins, polysaccharides, chitosan, gelatin etc.) and biocompatible polymers (e.g., PVA, PEO, PCL, PLLA, poly(L-lactide-co-glycolide) (PLGA) have been successfully emulsion electrospun to develop uniform fibrous matrices [3]. The well-known biodegradability, biocompatibility and low toxicity of these polymers have encouraged the high usage of emulsion electrospun matrices based on these polymers for food, biomedical, pharmaceutical, and other applications [44,45,46]. When the emulsion electrospinning of a polymer system is emphasized, it is observed that PCL and its blends rank the top and being a biocompatible, biodegradable and bioresorbable polymer, majority of the electrospun matrices have been used as a tissue engineering scaffold. The synthesis, physio-chemical properties, degradation behavior, different techniques to fabricate PCL based porous materials and their bio-medical and other applications have been briefly discussed in several literatures [47,48,49,50,51]. The present review is therefore focused on the recent advancements in emulsion electrospinning of PCL and how the scope of this technique can further be expanded to generate porous structures for applications not limiting to tissue engineering. Also, the effect of process parameters onto ultimate fiber morphology and how the structure property correlation is linked with end use will be discussed in detail. Figure 2 depicts the literatures devoted to emulsion electrospinning in last decade, significantly more than 30% of literatures are based on PCL as their major component whereas PLLA, PLGA occupied 20% each and remaining are less than 5% each (silk fibroin, gelatin, polyacrylic acid, polystyrene etc.). Overall, more than 75% of available literature is on PCL, either as their main component or in blended form. Therefore, in detail covering of such PCL based systems alongside a brief covering of other polymer-based systems will portray a brief idea about the potential of this rapidly growing system.
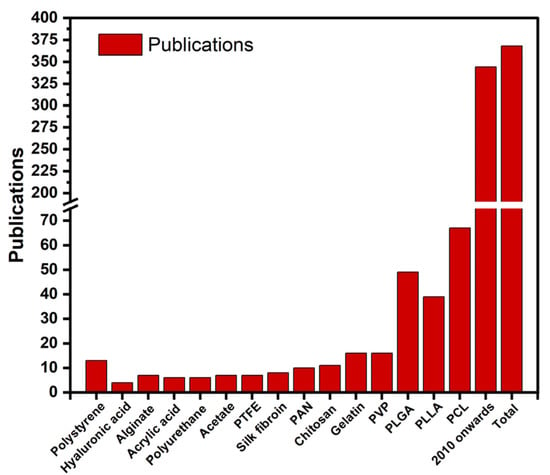
Figure 2.
Publications originating from emulsion electrospinning of different polymers (Source—SciFinder, 2010 onwards and total indicates total number of publications since 2010 and total number of publications to date respectively).
2. Emulsion Electrospinning: Clean and Safe Electrospinning
Emulsion electrospinning is an advanced fascinating technique where with the use of single nozzle/syringe, core-sheath and multi-channel fibers can be produced. It is also termed as ‘green electrospinning’ because of its sustainable nature which promotes low/restricted use of organic solvents. The basic component of this process is an emulsion which are categorized as water-in-oil (w/o) or oil-in-water (o/w) depending on whether the water phase is dispersed or continuous and the solvent rich aquaphobic phase is commonly known as oil phase. These two phases interact with each other through stabilizers viz., emulsifiers/surfactants emulsifier (e.g., Tweens, Spans etc.), Pickering particles (e.g., nano silica, nano clay etc.), biopolymers (e.g., gelatin, alginate etc.), and globular biopolymers (e.g., soy proteins, whey protein etc.) [52,53,54].
The advantages of emulsion electrospinning are as follows. First, the scope of conventional electrospinning is somewhat limited to very low polymer concentration since otherwise it leads to the formation of submicron size fibers. Whereas emulsion provides appropriate rheological properties to a polymer system so that elongation of the polymer phase can take place more smoothly and discards the probability of electro spraying. Further, increasing aqueous fractions also assist in modulation of fiber diameter within nano size range even at higher polymer concentration. [36] Second, hydrophilic bioactive agents that are mostly intolerant and poorly soluble in hydrophobic organic solvents can easily be incorporated within hydrophobic polymer matrix by using a predominantly aqueous electrospinning medium like w/o high internal phase emulsions. Third, electrospinning of w/o emulsions results into core-sheath fibers with hydrophilic bioactive components embedded within core of hydrophobic barrier polymer, shielding the sensitive hydrophilic component from external circumstances (solvent exposure, shear pressures) and thus retains their bioactivity [37]. Fourth, being encapsulated by the core-shell fiber matrix, these bioactive agents need to cross the barrier prior to release process, thus reducing their burst release. Further, being a biphasic system emulsion allows distinct matrixes for the bioactive compound and polymer thus removes the difficulty of finding a common media [38]. Lastly, it offers scalability on both needle and needleless setup which is not possible with co-axial and blend electrospinning setup [28].
2.1. Process Overview
In order to form an emulsion, two immiscible liquids, usually oil and water, are mechanically agitated along with the stabilizer as depicted in Figure 3. Stabilizer stabilizes emulsion by reducing the interfacial tension between the two phases thereby allowing formation of smaller dispersed droplet under mechanical agitation and by avoiding droplet coalescence through formation of a barrier between two phases [55]. The disperse droplets start to split into smaller units as soon as the energy exerted by the shear forces due to turbulent micro-eddies on the droplet’s interphase exceeds the cohesive forces of the liquid in droplets [56]. Therefore, the amount of energy required to break a droplet is there for directly proportional to interfacial tension. Other than process and thermodynamic variables, the emulsification is influenced by various emulsion parameters including dispersed phase volume, viscosity, and mutual solubility of both phases [57]. Electrospinning of such emulsions is termed as emulsion electrospinning.
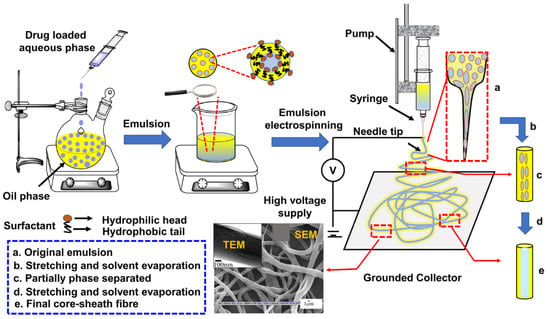
Figure 3.
Process flowchart of w/o emulsion electrospinning. SEM and TEM images are Reprinted with permission from Ref [61]; Copyright 2016, Elsevier.
The most fundamental electrospinning setup is depicted in Figure 3 comprises of three major components, a syringe pump, an electrically conducting spinneret, a conducting grounded collector separated by a predetermined distance and a high voltage power supply. The most common setup in the laboratory is a plastic barrel syringe carries the polymer solution and a metallic needle with blunt tip as the spinneret and the feed rate of polymer solution to be electrospun is controlled by the syringe pump [58]. To charge the polymer solution, one electrode of the power supply is connected to metallic tip of the syringe holding the spinning solution, and the other is connected to the collector of opposite polarity. When a high voltage (0.1–30 kV) is applied to the spinneret, it creates potential difference between the spinneret and collector, and droplet undergoes two types of electrostatic forces, the Coulombic force exerted by the external electric field and mutual electrostatic repulsion between surface charges and as pointed out in Figure 4c. Because of this, there exists some localized charge separation within the liquid. Charges with the same sign as the spinneret’s polarity will move toward the surface of the droplet and generate extra charges. As the voltage slowly goes up, more and more charges will build up, making the droplet’s surface charge density go up. Under these circumstances, surface tension tends to make a droplet round so that its total surface free energy remains as low as possible. However, electrostatic repulsion tends to change the shape of the droplet, so its surface area will grow to reduce the repulsion. Finally, the droplet is presumed to have a shape that makes the sum of electrostatic energy and surface free energy as small as possible [59]. When the intensity of the electric field reaches a specific critical threshold, the liquid drop elongates into a conical shape known as the Taylor cone, indicating that electrostatic repulsions overcome the surface tension of the polymer solution as shown in Figure 4a. As soon as the polymer droplet’s surface charge is overcome, a polymer jet forms right from the apex of the Taylor cone and continues to be ejected and accelerated under electric field in a steady manner until viscoelastic and surface tension forces prevent the jet in moving forward. The solution jet initially follows a straight course, but after a certain distance from the spinneret, the jet begins to whip out in a chaotic form owing to the rapid solvent evaporation and increase in jet surface charge density, which destabilizes the polymer jet as shown in Figure 4b [58]. This phenomenon is referred to the bending instability. As the jet swirls in the direction of the collector, higher order instabilities manifest themselves, resulting in a completely chaotic trajectory and leads to the formation of a randomly oriented, nonwoven polymeric fibrous mat on the collector [60].
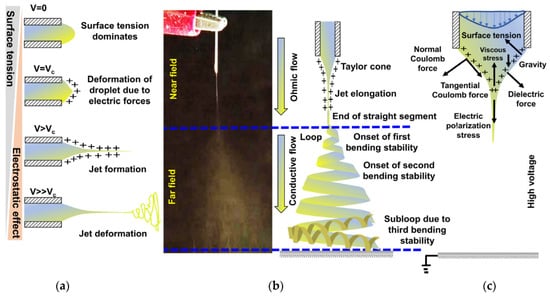
Figure 4.
(a) Effect of applied voltage during electrospinning of a polymer fluid (b) Digital photograph of electrospinning process along with the consequences in each zone, and (c) forces acting on a Taylor cone during its electro-jetting process.
Unlike normal electrospinning, in emulsion electrospinning, the solvent evaporates at a much faster rate from sheath as compared to that of core, resulting rapid increase in the outer layer’s viscosity in comparison to the inner layer. Following that, emulsion droplets are induced to move inward from the surface to the center along with the instantaneous condensation and stretching into elliptical shapes (Figure 3) due to the force exerted by a high-voltage electric field [62,63]. This leads to the segregation of polymer jets, initially into two jets and, eventually, into large number of jets, to counteract the instability [64]. During this process, the emulsion droplets can be drawn out in the nanofibers to form a core-shell structure (TEM image Figure 3, [61]), or they can stay as droplets to form a multi-core structure. The electrostatic force, which affects the constant splitting of polymer droplets causes the patterning/deposition of nanofibers on the collector. Further, the deformation and attenuation of the emulsion droplets are also controlled by opposite directional drag force exhibited by the surrounding atmosphere. Other forces acting on the charged jet include gravity, coulombic repulsion force, surface tension, and viscoelastic forces as demonstrated in Figure 4b [65]. Droplet expansion is caused by coulombic repulsion and electrostatic forces. Surface tension and viscoelastic forces help to contract the charged droplet, reducing the interface area between the air and the jet [66]. With rapid solvent evaporation on the grounded collector, the core-shell nanofibers are finally obtained as schematically shown in Figure 3. Non-spinnable drugs, growth factors, and other active substances can be dispersed in the polymer solution and electrospun using emulsion electrospinning. Furthermore, combination of synthetic polymers (as the backbone material) with natural polymers (on the surface to improve cell adhesion) can fabricate composite fibrous scaffolds with optimal mechanical and compatibility properties for biomedical applications. Pal et al. demonstrated the creation of cotton-wool-like fluffy nanofibrous mats via emulsion electrospinning of PCL and chitosan, resulted in the formation of a chitosan-PCL based core-shell fibrous suitable for skin tissue engineering [67]. Furthermore, the core-shell nanofibers of acrylic acid grafted with PCL and hydroxyapatite be created through the electrospinning of o/w emulsions [68]. Unlike nanomaterials (e.g., nanotubes, nanorods, and nanowires), which are mostly created through synthetic, bottom-up methods, top-down fabrication is used to create electrospun fibers. These results in obtaining continuous fibers, that are relatively simple to align, assemble, and process into applications in industries such as food, cosmetics, pharmaceuticals, and biomedicine [69]. Doshi et al. identified the internal and external parameters that govern the structural morphology of electrospun nanofibers. The major factors are environmental humidity and temperature (as external parameters) as well as applied voltage, tip to collector distance, conductivity, and viscosity of the polymer solution (as internal parameters). The effects of various parameters on fruitfulness of electrospinning are discussed in detail in the following sections.
2.2. Polymers in Emulsion Electrospinning
With the rapid development of new materials derived from renewable resources, a wide range of materials, including biopolymers (e.g., proteins, polysaccharides) and biocompatible polymers (e.g., poly(vinyl alcohol) (PVA) [70,71,72], poly(ε-caprolactone) (PCL) [5], poly(lactic acid) (PLA) [73,74,75,76], poly(lactic-co-glycolic acid) (PLGA) [77,78,79,80], poly(vinylpyrrolidone) (PVP) [81,82,83,84], poly(ethylene oxide) (PEO) [29,39,85], poly(ethylene glycol) (PEG) [86,87] etc., and other synthetic polymers like polystyrene (PS) [88,89] and polyurethane (PU) [90,91] etc. have also been successfully electrospun to uniform fibers from their emulsions. The details of different PCL based emulsion systems used to produce electrospun nano-fibrous matrices are summarized in Table 1. Emulsion parameters (continuous phase, dispersed phase, and emulsifier), emulsion electrospinning parameters (voltage; kV, tip to collector distance; cm, and flow rate; mL/h), and the bio-medical application of matrices produced from electrospinning of water-in-oil (w/o), oil-in-water (o/w), and non-aqueous emulsions are outlined.

Table 1.
Emulsion electrospun PCL nanofibrous matrices for biomedical applications.
3. Parameters Affecting Emulsion Electrospinning
There are several factors that affect the electrospinning process. These factors are classified as emulsion parameters, electrospinning parameters, and environmental parameters. The emulsion parameters include the solvent, polymer concentration, viscosity, disperse phase volume fraction, nature of emulsifier and emulsion conductivity. The electrospinning parameters include the applied voltage, distance between needle tip and collector, type of collector, flow rate, and needle diameter. The environmental parameters include relativity humidity and temperature. All these parameters have a significant influence in the formation of bead free uniform fibers. Therefore, it is crucial to thoroughly compresence the effects of different electrospinning parameters to have better understanding of electrospinning technique and fabrication of uniform polymeric nanofibers.
3.1. Effect of Emulsion Parameters
3.1.1. Polymer Concentration, Viscosity, and Volume Fraction of Dispersed Phase (ϕd)
The polymer concentration is one of the key factors governing the electrospinnability of solution and fiber morphology. Since the process is based on uniaxial stretching of a charged jet which is strongly dependent on the extent of entanglement of polymeric chains, and therefore concentration of polymer solution significantly impacts the stretching of charged jet, and morphology of electrospun fibers. [126] If the concentration of polymer solution is very low like as shown in dilute regime I and semi dilute regime-I (Figure 5), the applied voltage and surface tension cause the entangled polymer chains to break up before reaching the collector which is undesirable and beaded nanofibers produced ultimately. [127] Further in semi dilute regime-II (Figure 5), increasing concentration of polymer solution increases the solution viscosity by raising the chain entanglements and which facilitates to overcome the surface tension of droplets and formation of uniform bead free electrospun nanofibers [128]. Increase in polymer solution concentration above a critical value (the concentration at which bead free uniform nanofibers are produced) impedes the solution flow through the spinneret (concentrated solution regime (Figure 5), the polymer solution dries and blocks the spinneret), which eventually results in the formation of defective or beaded nanofibers. As the viscosity of polymer solution increases above a critical value, so does the bead size and the average distance between beads on the fiber. At the same time, the bead shape transitions from spherical to spindle-like as depicted in Figure 5. Beads and beaded fibers, on the other hand, are less likely to form in more viscous solutions [3].
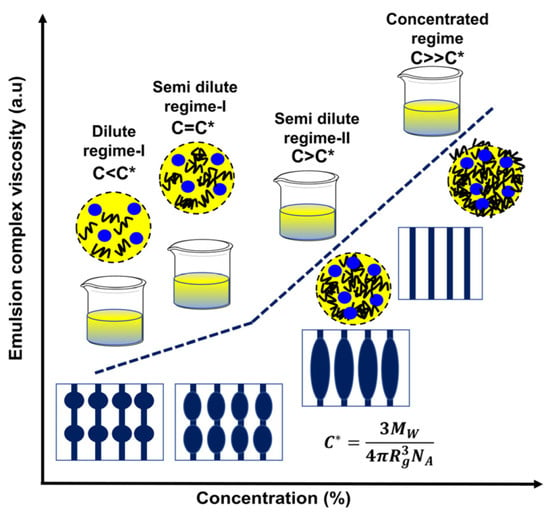
Figure 5.
Effect of polymer concentration and complex viscosity on the morphology of emulsion electrospun nanofibers.
Additionally, the diameter of the fiber may increase with increasing the polymer concentration. [129] Samanta et al. studied the effect of PCL concentration, concentration of template polymer (PVA), and ϕd onto the morphology of w/o emulsion (high internal phase emulsion, HIPE) PCL fibers. [120] A decrease in average fiber diameter (from 479 ± 8 to 345 ± 16 nm, Figure 6b) with increasing ϕd (from 0.75 to 0.90) was observed due to elongation of highly conducting aqueous phase present in large quantity and a decrease in overall solid content of the emulsion. Similar trend was also observed for emulsion electrospun PCL-silk fibroin fibrous mats from w/o emulsions. [108] Whereas, when the PCL concentration was varied (0 to 40 wt.%) at a constant ϕd of 0.75, larger diameter co-continuous fibers were obtained (mean fiber diameter increased from 414 ± 7 to 678 ± 11 nm, Figure 6a) since, an increase in PCL concentration led to the formation of more viscous continuous phase along with higher overall solid content per unit volume of the emulsion [120]. Additionally, the concentration of template polymer also played a significant role in the formation of bead free continuous fibers. No continuous fibers could be obtained from HIPEs below an optimum template polymer concentration (here, ≥8 wt.%). Hence the template polymer not only served as a stabilizer for HIPE, but also served as a template, protecting the droplet coalescence, and ensuring the continuity of the emerging fiber from the Taylor cone. An increase in the mean fiber diameter (from 210 ± 12 to 1180 ± 11 nm) was reported with increase in template polymer concentration (from 8 to 20 wt.%) within the disperse phase, attributing to higher viscosity, emulsion stability and increase in the solid content at higher template polymer concentration [120]. Detailed study on viscoelastic interaction between template polymer and other emulsion components by Pal et al. confirmed the caging of disperse phase droplets by template polymer, which governed the uniform stretching and orientation of both the phases of emulsion [5]. During rheological study an increase in storage and loss modulus was observed due to formation of caged dispersed droplets within continuous phase. Further, at lower template polymer concentration, beaded fibers produced due to slippage of continuous phase over dispersed droplets while very high PVA concentration led to gelling of emulsion and no fibers could be produced as depicted in Figure 6c. At high polymer (PCL) concentration in dispersed phase resulted in formation of bigger droplets which diminished the caging effect; thus, droplets were not effectively stretched, and oriented, and thicker fibers were produced eventually (Figure 6c) [5]. Another study revealed that the amount of required template polymer concentration can be reduced if the PCL surface is grafted by a hydrophilic polymer like poly(acrylic acid) [68]. Similar effects of PCL concentration on the morphology of emulsion electrospun fibers were discussed and further demonstrated that use of template polymer can be restricted if emulsion was stabilized using Pickering particles instead of conventional surfactants, thus leading to a greener approach [121]. These kinds of electrospun nanocomposite matrices have superior mechanical properties as compared to that of previous once. The enhancement in mechanical properties by inclusion of Pickering particles in polymer matrix was attributed to effective load transfer from polymer matrix to Pickering nanoparticles due to strong secondary interactions between the two [121]. Interestingly, pore formation on fiber surface was observed at high PCL concentration. This was possibly due to the occurrence of phase separation in polymer rich and poor zones during electrospinning where polymer precipitation and solvent evaporation occurred simultaneously. Such pore formation was not observed in the neat PCL based electrospun matrices at similar PCL concentration, which was obvious due to absence of aqueous phase [61]. Similar effects of polymer concentration and ϕd onto the core-shell nanofibers was observed in case of other PCL based emulsion systems like PCL-gelatin [130], PCL-poly(methyl methacrylate) (PCL-PMMA) [131], PCL-chitosan [67], PCL-trypsin [112], and PCL-PVA [125]. The average fiber diameter increased, and the number of beaded fibers decreased as the polymer concentration increased, the other solvent properties being constant, likely resulting from the increased viscosity of the dope dispersion associated to higher resistance for the solution to be stretched under the applied electrostatic force [112].
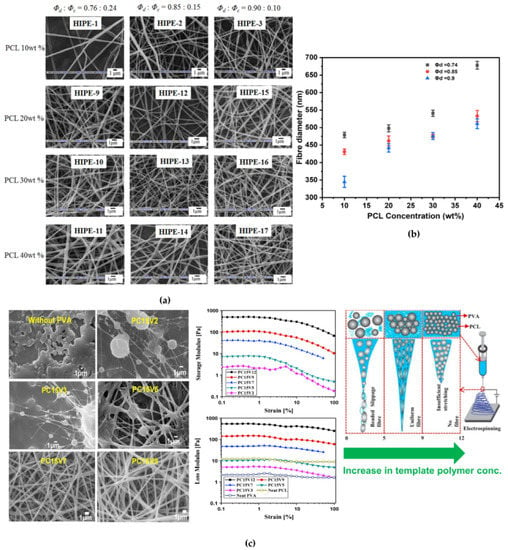
Figure 6.
Effect of (a) polymer concentration, (b) ϕd Reproduced with permission from ref [120]; Copyright 2016, Elsevier, and (c) template polymer concentration on the diameter and morphology of emulsion (HIPE) electrospun PCL nanofibers Reproduced with permission from ref [5]; Copyright 2017, Elsevier.
3.1.2. Emulsifier
Emulsion preparation employs a stabilizer (surfactant and/or Pickering particles) which is one of the essential ingredients which controls emulsion electrospinning process and morphology of electrospun fibers thus produced. Surfactants commonly having a non-polar tail (lipophilic) and polar head (hydrophilic) adsorb at liquid-liquid (oil-water) interface during emulsification. The surfactant causes droplet disruptions by reducing the interfacial tension and prevents aggregation of dispersed droplets by increasing the repulsive forces exhibited by the protective surfactant layer surrounding the dispersed droplets [132]. Commonly used surfactants for stabilization of PCL emulsions include anionic; SDS, cationic; TEBAC, and non-ionic; Sorbian monooleate (span 80). Kinetics of droplet coalescence and disruptions are strongly dependent upon the rate at which surfactant molecules cover the liquid-liquid (droplet) interphase [133]. According to Zhang et al. during emulsion electrospinning, small surfactant molecules are divided into two parts, one part could be in the core and the surfactant’s hydrophobic part (tail) remain as an array in the sheath. The remaining surfactants could move from droplet phase to outer surface due to charge repulsion and evenly distribute on fiber surface with their hydrophilic groups in air [3]. Li et al. studied the effect of emulsifier (stabilized by span 80) during emulsion electrospinning of poly(L-lactide-co-ε-caprolactone) and observed that around 10,000 times elongation of emulsion droplets occurs within tens of milliseconds, along its path to collector (tip to collector distance-15 cm) and band-like electrospun fibers were obtained at distances of 0.2 to 0.5 cm as well as some elliptical-shape drops in electrospun fibers at distances of 2.0–2.5 cm, and 3.0–3.5 cm as shown in Figure 7a–b. This can be attributed as, during emulsion electrospinning emulsion droplets are elongated along with the polymer jet, accompanied by the rapid jet-solidification due to the water and solvent evaporation but the liquid emulsifier (at room temperature) remains as it is and move from the emulsion spheres to the outer region and even to the surfaces of fibers due to the charge repulsion. Thus, emulsifier divided into two parts one with the polymer another with the water-soluble drugs and helps to fabricate the core–shell type fibers [62]. In another study, the effects of different surfactants, non-ionic; span 80 and Pluronic F108, anionic; SDS, and cationic TEABC, at varying concentrations on morphology of emulsion electrospun PCL/bovine serum albumin (PCL/BSA) nanofibers were studied [105]. It was observed that in presence of any of the surfactant electrospinnability of emulsion with low PCL concentration was enhanced and branched or uniform fibers were obtained as depicted in Figure 7c. Electrospinning of an emulsion prepared at 0.4 wt.% SDS produced the thinnest and the most uniform nanofibers (167 ± 39 nm) attributing to high conductivity of PCL emulsions. Electrospun PCL/BSA nanofibers produced from emulsion containing different surfactants at varying concentration differed in fiber morphology (Figure 7d) and mechanical properties. Results suggest that surfactants have the ability to modulate the fiber morphology via electrostatic and hydrogen bonding, depending on their chemical structure and surfactant-polymer interactions. For example, PCL-BSA electrospun mats containing TEABC surfactant with benzyl group presented maximum tensile strength but least elongation at break due to inherent structural rigidity of TEABC, whereas electrospun fiber with surfactants containing long alkyl chains (span 80, SDS and F108) showed moderate (for span 80 and SDS) to poor (for F108) tensile strength as shown in graph 7f. However, high elongation at break was observed for surfactant F108 owing to the presence of ductile PEO chains as shown in graph 7e [105]. Interestingly, electrospun PCL fibers produced from emulsion systems containing surfactant brij-58 depicted the co-continuous morphology. Further, increase in fiber diameter with increasing brij-58 concentration referred to increase in solid content and formation of relatively bigger droplets [120]. Emulsion electrospun P(LLA-CL) nanofibers containing span 80 demonstrated high strength and modulus because of the reason that majority of span 80 molecules aggregated into nanorods and acted as reinforcing agents [118]. However, surfactants used in emulsion electrospinning are usually difficult to remove and might introduce concerns related to biocompatibility of electrospun fibers [134]. Furthermore, in some instances surfactant molecules migrate from liquid-liquid interphase to the surface and result in bulging and rough fiber morphology [39].
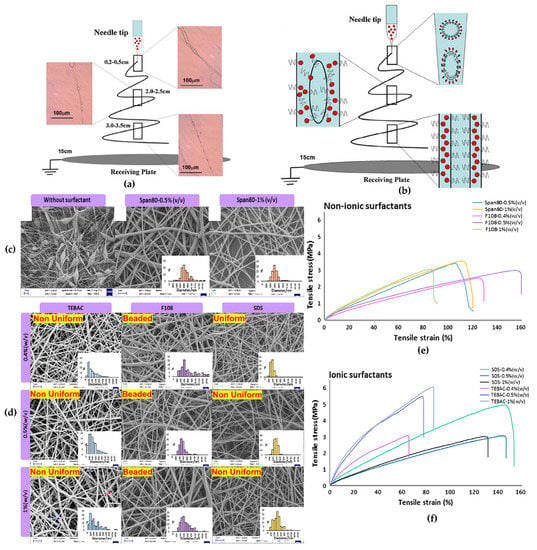
Figure 7.
(a,b) Role of emulsifier and its distribution during core–shell structure formation at each stage of electrospinning, Reprinted with permission from ref [62]; Copyright 2010, Elsevier; (c) Effect of surfactant concentration on fiber formation and morphology (d) Effect of various surfactants on fiber morphology, and (e,f) effect of nature of emulsifier on mechanical properties of fibers obtained during emulsion electrospinning, Reprinted with permission from ref [105]; Copyright 2015, Taylor & Francis.
Recently, instead of conventional surfactants colloidal amphiphilic nanoparticles known as Pickering particles has gained an increasing interest among the researchers owing to inherent biocompatibility and other fascinating properties of Pickering particles leading to a greener approach [47]. Pickering particles forming a densely packed morphology at liquid-liquid interphase protect the droplets from flocculation and coalescence through steric stabilization mechanism [135]. Compared to conventional surfactants, the thickness of particle-stabilized liquid-liquid interface is at least equal to the size of particle-monolayer, which provides stronger protecting barrier against droplet flocculation and coalescence [136]. Additionally, particle stabilized interfacial layer has good mechanical stability due to the presence of lateral attractive capillary forces caused by the deformation of fluid interface around the particles [137]. Hence, electrospun fibrous mats with reduced toxicity are expected to be produced from emulsion electrospinning which is attributed to favoring polymer-particle and particle-particle interactions. During electrospinning of PCL-grafted-poly(acrylic acid) based o/w emulsions enhanced storage modulus was observed for emulsions containing nano-hydroxyapatite (HAp) in comparison to those prepared without HAp, thus suggesting the increased oil-water phase interactions [68]. Similarly, PCL based electrospun nanocomposite matrices of higher tensile strength and modulus were obtained when fabricated from Pickering emulsions stabilized with modified montmorillonite nano clay (MMT) and no toxic effects of MMT inclusion on cell-proliferation efficacy of PCL matrices were observed [61].
In another study, PCL based Pickering emulsions stabilized with hydrophobically modified silica nanoparticles (mSiO2) depicted reduction in emulsion droplet size with increasing mSiO2 and generation of thicker diameter PCL fibers upon electrospinning [121]. The mSiO2 acted as an external nucleating agent and increased the crystallinity of emulsion electrospun nanocomposite PCL/mSiO2 matrices. Furthermore, the reinforcing effect of mSiO2 enhanced the tensile properties of PCL/mSiO2 nanocomposite matrices and increased growth profile of fibroblast L929 cells was observed. Interestingly, the PCL based nanocomposite fibrous matrices prepared from emulsion electrospinning of PCL Pickering emulsions also demonstrated good biomineralization efficacy as well and hence proving their high suitability for bone tissue engineering application [68,138].
3.1.3. Conductivity and Surface Tension of Phases
During emulsion electrospinning, Taylor cone formation and diameter of nanofibers produced are strongly governed by the emulsion conductivity and surface tension which is mainly dependent upon properties of polymer, solvent, and surfactant, and availability of ionizable salts [105,139]. In case of emulsions with low conductivity dispersed droplets, the poor surface charge and high surface tension hinders the Taylor cone formation and production of uniform electrospun fibers. Increasing emulsion conductivity up to a critical value enhances the surface charges on emulsion droplets to form a highly stable Taylor cone and decreases the fiber diameter as well [140]. However, increasing emulsion conductivity at par (or above a critical value) have deteriorating effects on Taylor cone formation due to the fact, Taylor cone formation is primarily controlled by the electrostatic force of the surface charges generated by the applied external electric field (electric field component, tangential to surface of the fluid induces the electrostatic force). An ideal dielectric polymer solution won’t have enough charges in it to move to the surface of the fluid. This means that the electrostatic force generated by an electric field will be enough to form a Taylor cone and start the electrospinning process [139]. A conductive polymer solution, on the other hand, will have enough free charges to travel onto the fluid’s surface, form a Taylor cone, and start the electrospinning process. But when the conductivity of the solution goes up significantly, the tangential electric field goes down a lot. This decreases the electrostatic force along the surface of the fluid, which detrimentally affects the formation of the Taylor cone [139]. The straight jet section is elongated and thinned by a combination of Coulomb and electrostatic forces.
Indeed, electrospinnable emulsions are mainly consist of non-miscible polymers, not the immiscible solvents and these immiscible polymers leads to the formation of a highly viscous homogeneous solution that upon electrospinning produces an ordered polymeric blend nanofiber [34,35,141,142]. For example, immiscible blend of silk fibroin and PCL was emulsified and investigated by assessing the morphology of emulsion electrospun PCL-silk fibroin fibers [108]. A reduction in silk fibroin concentration from 10 to 5 wt.% reduced the emulsion viscosity while increased the conductivity. It was proposed that for emulsions with high viscosity, cohesion among the polymer chains hindered the conductivity. Emulsions with low conductivity showed appreciable spinnability but at very high silk fibroin content emulsion was not electrospun. Though, viscosity and conductivity of the system to be electrospun are not inter-related at ambient temperature, it was predicted that superior emulsion electrospinning was enabled by the synergistic effects of optimal high viscosity and low conductivity. Another study on PCL-chitosan core-sheath nanofibers generation via emulsion electrospinning discussed the effect of electrical conductivity on fiber diameter and morphology [143]. With increasing PCL content, the relative contribution of protonation of amine groups of chitosan decreased, so does the conductivity of the emulsion while viscosity was increased (Figure 8j), and surface tension not changed significantly. With increasing PCL content at a constant chitosan content, the average diameter of the nanofibers was increased from 214 ± 90 to 715 ± 330 nm as depicted in Figure 8a–i. The reduction in chitosan content w.r.t PCL also decreased the electric forces required to electrospun PCL-chitosan emulsion system and the PCL sheath of the fibers became thicker with thus leading to decreased core to sheath diameter ratio as shown through TEM images in Figure 8k, suggesting the effect of electrical conductivity of core component. Similarly, inclusion of cefazolin (as Na+ salt) in electrospinning PCL based systems resulted in an increased charge density which imposed the thinning forces on the stretching jet -and resulted in smaller diameter fibers [144] as observed for systems like PCL-PMMA [131], PCL-chitosan [67], and PCL-silk fibroin [106]. The effect of ionic nature of surfactant and its concentration on emulsion conductivity, spinnability and properties of produced PCL/BSA nanofibers was focused [105]. Incorporation of low number of surfactants, TEBAC (cationic) or SDS (anionic) had nominal and much higher increase in conductivity of the system, respectively, whereas non-ionic surfactants, span80 and F108 had no influence on emulsion conductivity. It is due to the fact, in case of non-ionic surfactants, the surfactant molecules do not bear any surface ionic charge and the association of surfactant molecules is likely governed by hydrogen bonding and/or hydrophobic interactions [145]. Broad diameter distribution was observed for PCL-BSA fibers that electrospun from emulsions loaded with non-ionic surfactants. Also, the tensile strength of PCL/BSA nanofibers was not influenced much by the ionic nature of surfactant whereas elongation at break and hydrophilicity (water contact angle) were highly correlated with surfactant type. Similar results also depicted when quality by design approach based on design of experiments was used to achieve predictable critical quality attributes for PCL emulsions containing ionizable antimicrobial agent (doxycycline HCl) formulated with a surfactant blend [111].
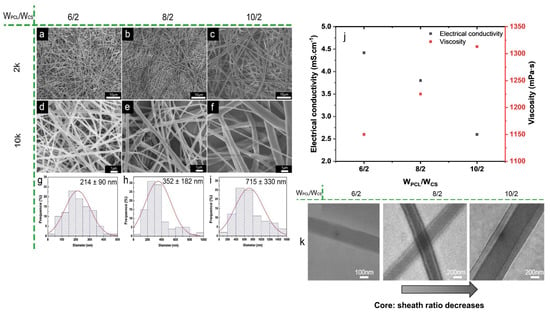
Figure 8.
(a) FE-SEM images (a–f) showing the effect of conductivity on fiber diameter and diameter distributions (g–i) of emulsion electrospun PCL-chitosan fibers. (j) Effect of blend ratio on emulsion conductivity and viscosity, and (k) TEM images of core-shell structured nanofibers with different PCL/CS weight ratios, Reprinted with permission from ref [143]; Copyright 2019, Elsevier.
3.1.4. Solvent for the Polymer
The solvent for polymer dissolution is another crucial parameter governing the formation of smooth and bead free electrospun nanofibers depending upon the polymer-solvent interactions. The solvent selection is primarily based on two parameters, firstly, excellent solubility of the polymer or polymer blend need to be electrospun and second, the solvent should have considerable/moderate volatility (moderate boiling temperature) [139]. In addition to volatility of the solvent, its conductivity and dipole moment are also important. Solvents with low to moderate boiling temperature are generally preferred because their high evaporation rates ease the polymer precipitation during transfer of polymer solution from spinneret tip to collector and extraction of residual solvent from the obtained electrospun nanofibrous mats. Solvents with high volatility (low boiling temperature), on the other hand, are mostly avoided because the drying/coagulation of polymer jet at the spinneret tip hinders the electrospinning process. Solvents with extremely high boiling temperature are also avoided due to their poor extraction during and post-electrospinning, thus preventing complete drying of nanofibers and causing fusion of fibers [139].
Effect of different solvent systems at different blend ratios (chloroform: DMF; 60:40, 75:25, and 85:15) onto the porosity of trypsin entrapped emulsion electrospun PCL nanofibers suggests that for solvent-blends having higher fractions of chloroform (lower boiling temperature, better solubility, and conductivity) coarser PCL nanofibers with reduced porosity are obtained (Mean fiber diameter- 141 ± 44 nm, porosity- 32 ± 3%). In contrast, increasing the quantity of DMF in the solvent combination induces an increase in solution conductivity and a greater elongation of the polymer jet during the electrospinning process, resulting in fibers with smaller diameters and higher porosity (Mean fiber diameter- 118 ± 52 nm, porosity- 56 ± 1%). Thus, indicating the effects of solvent volatility and conductivity [112]. Another study on emulsion electrospun PCL/PVA-gelatin nanofibrous matrices demonstrated the morphology of fibers are also strongly dependent on disperse phase solvent compositions. As depicted in Figure 9 blend ratio 7:3 (deionized water: acetic acid, DW:AA) results in uniform bead-free fiber as compared to 8:2 (irrespective of the nature of surfactant), which may be attributed to the high-water surface tension in the aqueous solutions with the higher volume ratio of water as compared to acetic acid [146]. In general, the effect of solvents onto the morphology of electrospun matrices is similar for both solution as well as emulsion-based systems. Effect of different solvent systems, (a) acetone, (b) acetone: chloroform (30:70), (c) DCM: methanol (90:10), and (d) chloroform: DMF (70:30) in terms of their ability to form uniform electrospun PCL nanofibers was investigated [147]. When acetone was solely used as the solvent for PCL, poor electrospinnability with formation of beaded fibers was observed, whereas acetone: chloroform blend significantly increased the electrospinnability of PCL, indicating higher solubility of PCL in chloroform than acetone [148]. The electrospinnability of PCL from DCM: methanol blend was also very poor pertaining to low dielectric constant and conductivity values of the main solvent, DCM [147]. The chloroform: DMF composition found to be the best solvent system for PCL electrospinning which produced smooth electrospun fibers of thinnest diameter with small pores along the fiber surface. Although DMF is not a good solvent for PCL, its high dielectric constant value and electrolyte nature contributed to electrospinning of bead free PCL nanofibers [149]. Furthermore, a solvent-blend system plays crucial role in fabrication of porous electrospun nanofibers this is due to the reason that in a solvent-blend, one of the solvents is generally a non-solvent for the polymer, and the different evaporation rates of the solvent and non-solvent lead to phase separation (polymer rich and poor zones) and thus generation of electrospun porous nanofibers [150].
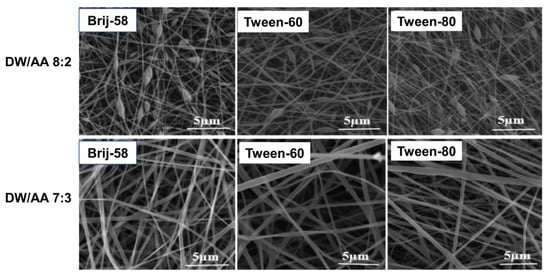
Figure 9.
Effect of employed solvent on morphology emulsion electrospun fibers, Reprinted with permission from ref [146]; Copyright 2019, Wiley.
3.2. Effect of Process Parameters
3.2.1. Applied Voltage
Electrospinning is a highly intriguing process which encompasses nonlinear electro-hydrodynamics at extremely high speeds, complex rheology, and charge, mass, and heat transfer within the polymer jet migrating from spinneret tip to collector. The process includes three stages: jet initiation, jet elongation (with/out branching and/or splitting), and jet solidification into nanofibers [58]. In each stage mentioned above, applied voltage plays a significant role in fiber formation and it takes place once the applied voltage crosses the threshold limit and induces necessary charges on electrospinning fluid along with the electric field. At a critical voltage, spherical droplet of polymer fluid deforms into a Taylor cone and transforms into ultrafine nanofibers on the collector. This critical applied voltage (VC, kV) varies for different polymers and given by the Equation (1), where H is tip to collector distance), L is length of the spinneret, R is radius of the tube (H, L, and R in cm), and γ is surface tension of the fluid (dyncm-1). The factor 1.3 comes from 2 cos 49.3° by considering cone has a semi vertical angle close to a possible equilibrium value of 49.3° [22,151].
Once the electrically charged polymer jet starts to eject from the apex of Taylors cone, it follows a nearly straight path for a certain distance known as near-field region as depicted in Figure 4b. The length of this straight segment can be estimated by the Equation (2), where , σ is the surface charge, Q is the flow rate, k is the electrical conductivity, ρ is the density, E is the electrical field strength, I is the current passing through the jet, r0 is the internal radius of the jet [152,153].
During this span of time the viscoelastic and surface tension forces prevent the jet from moving forward, as a result forward acceleration is gradually attenuated [126]. As the jet travels towards the collector its diameter reduces and at some distance away from the collector acceleration becomes zero or constant. At this point, any perturbation due to mutual electrostatic repulsion destroys the straight jet trajectory, introduces instability and the polymer jet enters to the far-field regime where it undergoes through axisymmetric (Rayleigh instability) and non-axisymmetric instabilities (whipping or bending instability) and finally reaches to collector as depicted in Figure 4b [154,155,156,157,158].
It has been experimentally already proven that the shape, diameter, porosity, and mechanical properties of the electrospun fiber is highly dependent on applied voltage. Above the critical voltages, an increase in applied voltage causes more ejection of polymer fluid and higher acceleration rate and reduced flight time towards the collector, thus reduced jet stretching and formation of large dimeter electrospun fibers as depicted in Figure 10a. At much higher voltage, branching of the individual fibers takes place which is mainly associated with the induction of multiple jets during electrospinning thus reducing the electrostatic forces and overall fiber stretching and eventually smaller dimeter electrospun fibers [159,160]. Effect of the applied voltage on the morphology and mechanical properties of emulsion electrospun PLGA-chitosan nanofibrous scaffolds reported the formation of beaded fibers at voltage 8kV, increasing the voltage to 12 kV causes the beads to vanish but results in the creation of non-uniform nanofibers. On the other hand, voltage of 16 kV produces uniform nanofibers (Figure 10b). It may be inferred that a stronger electrical field caused by higher voltage causes a greater pull in the emulsion jet trajectory, resulting in the development of bead-free, more uniform, and finer PLGA/chitosan/PVA nanofibers [161]. An increase in average fiber diameter with a much wider distribution curve were obtained when the applied voltage was increased from 10 to 25 kV. Whereas morphological analysis of electrospun PCL nanofibers suggested significant change without affectation of their physicochemical properties. Furthermore, at higher voltages (20 to 25 kV) the polymer jet expelled from spinneret tip was randomly welded (coalesced) across its length before their deposition on collector, hence, electrospun nanofibers with interlaced morphology were produced and had higher mechanical strength [162]. Similar type of conglutinations among the fibers produced at higher electrospinning voltages have also been reported [163,164,165]. It was hypothesized that the fusion among the fibers produced at higher voltages improved the load transfer capacity between the fibers and hence increased tensile strength and the load transfer between fibers occurred via van der Waals interactions and mechanical interlocking [165]. The dimeter of electrospun fibers also dictates the rate of cell growth and proliferation [166,167]. Badami et al. reported that electrospun fibers with small mean diameters (≤140 nm) could inhibit cell infiltration and proliferation, owing to the smaller pore size of the scaffolds [166]. Whereas fiber diameter range between 700–1400 nm promotes cell migration, on contrary, electrospun PCL scaffolds with mean fiber diameter ≥1600 nm presented decreased attachment and proliferation of fibroblasts [167].
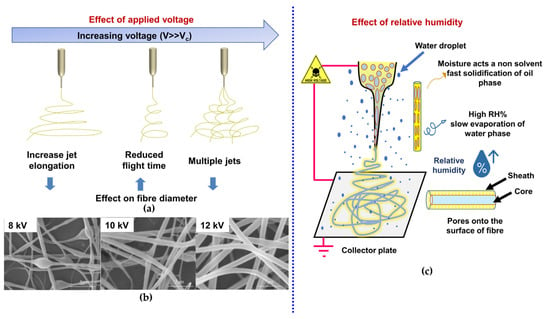
Figure 10.
(a) Effect of applied voltage on electrospinning of a polymer fluid (b) Effect of applied voltage on emulsion electrospun fiber morphology, Reprinted with permission from ref [161]; Copyright 2014, Hindawi, and (c) Effect of humidity onto the morphology of emulsion electrospun fiber.
3.2.2. Flow Rate
Flow rate of polymeric fluid through the spinneret tip during electrospinning process is another important parameter which controls morphology of the electrospun nanofibers and to obtain a bed free continuous electrospun fibers the critical flow rate varies with polymer systems to be electrospun [139]. Increased flow rate above the critical value may result in electrospun beaded fibers, while an optimum flow rate is essential to exceed the thermodynamic instability observed at minimum flow rate. Computational and experimental studies on electrospun PCL nanofibers reported that high flow rate favored the pore formation on fiber surface [168].
It was proposed that with increasing flow rate polymer droplet suspended at the spinneret tip grew larger, which in turn increased the diameter of polymer jet and electrospun fibers. At low flow rates, the solvent may have sufficient time to evaporate before deposition of nanofibers on to the collector, hence thinner and uniform fiber was produced. Conversely, high flow rates probably above the quasi-stable point result in unstable jets thus the polymer fluid is not completely carried away to the collector and production of large dimeter beaded fibers [139]. To summarize, increasing the flow rates above a critical value cause an increase in fiber diameter, pore diameter, and formation of beaded fibers, attributing primarily to incomplete solvent evaporation and relatively low stretching of polymer jet between spinneret tip and collector plate [169].
3.2.3. Tip to Collector Distance
The distance between the spinneret tip and the collector plate in the electrospinning set-up significantly influences fiber diameter and morphology, since it determines the rate of solvent evaporation, fiber deposition time, and whipping or jet instability. It has been proven that a based upon the electrospinning dope characteristics at an applied voltage, a minimum (optimum) distance between spinneret tip and collector plate is essential to obtain uniform electrospun fibers, otherwise beaded fibers are produced if the distance is too less or too far [170]. In general, increasing distance up to certain limit decrease the diameter of electrospun fibers and similar trend was observed for electrospun PCL-HA composite nanofibers where the increasing tip to collector distance (from 5 to 25 cm) decreased the average fiber diameter from 597 to 306 nm [171]. It is explained that increasing distance has a direct influence on the jet flight time and electric field strength. Longer spinning distance provides more time for stretching of polymer jet before fiber deposition on collector and the solvent also have more time to evaporate, hence, fiber diameter is prone to decrease. Numerous studied reports the effect of distance between spinneret tip and collector on fiber morphology and conclude that defective and large diameter nanofibers are obtained with less distance, whereas increasing distance narrow down the nanofiber’s diameter [139].
3.3. Effect of Ambient Parameters
Humidity and Temperature
Apart from emulsion parameters and electrospinning process parameters, ambient (environmental) factors such as relative humidity and temperature have been reported to tune the diameter and morphology of electrospun nanofibers. Humidity modifies the diameter of nanofibers by controlling the precipitation/solidification of charged polymer jet during its migration from spinneret tip to collector plate under the influence of external electric filed as depicted in Figure 10c [170]. This phenomenon, however, is also associated with chemical composition of the polymer or polymer blend to be electrospun. The variation in relative humidity during electrospinning of PCL showed that increase in relative humidity up to a critical level resulted in pore formation on fiber surface and above the critical level pores were diminished, however, fiber diameter did not change significantly [172].
Increasing temperature from 25 to 45 °C during electrospinning of PCL caused an overall decrease in fiber yield as the fiber diameter decreased owing reduction in viscosity of PCL leading to more splitting and stretching of polymer jet [173]. Orientation of PCL chains within the electrospun matrices also altered with the electrospinning temperature, an increased and decreased chain orientation was observed for PCL nanofibers electrospun in temperature range of 25 to 35 °C and 35 to 40 °C, respectively. Interestingly, except for higher PCL concentration (20 wt.%) the degree of crystallinity showed a temperature dependence rather than a concentration dependence, where 12% enhancement in crystallinity of electrospun fibers was observed form matrices produced in the temperature range of 35 to 55 °C. Further, the PCL solution concentration and electrospinning temperature were also interrelated since dilute PCL solutions (12 and 16 wt.%) showed higher electrospinning temperature window compared to concentrated PCL solution (20 wt.%). It is proposed that temperature and solution concentration are the regulating factors for solution viscosity, conductivity, and surface tension which all together contribute to variation in dimeter and morphology of electrospun fibers [174,175,176]. On the other hand, electrospinning temperature close to melting temperature of PCL lead to rapid solvent evaporation thus restrict the jet stretching as the intermolecular forces between the polymer chains dominate and higher dimeter fibers are produced.
The effect of all parameters discussed so far on emulsion electrospun fibre morphology is now summarized in Figure 11 and Table 2.

Figure 11.
Various parameters that affect emulsion electrospinning, nanofiber morphology and how they are interconnected.

Table 2.
Parameters affecting emulsion electrospun fiber morphology.
4. Application of Emulsion Electrospun Nanofibrous PCL Matrices
The application of emulsion electrospun nanofibrous PCL matrices applied in biomedical and other fields is summarized in Figure 12. The biomedical applications include wound healing, bone, vascular and nervous tissue regeneration, encapsulation of functional bioactive components, and controlled drug release. Enzyme immobilization, oil-water separation, air filtration, fuel purification, catalysis, and buffer material for phase change materials are a few other applications of emulsion electrospun PCL matrices.
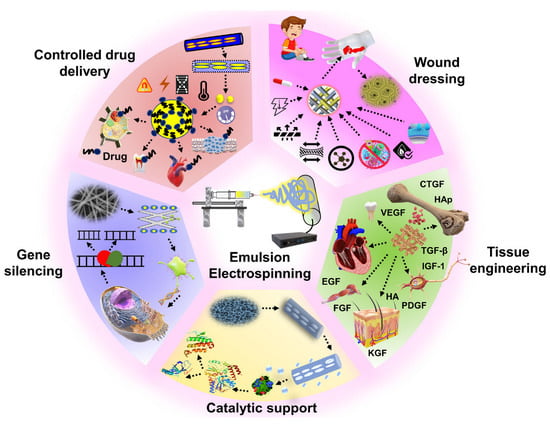
Figure 12.
Potential applications of emulsion electrospun PCL matrices.
4.1. Encapsulation and Controlled Drug Release
Many bioactive molecules like collagen, trypsin, BSA, silk-fibroin, and essential drugs (like ciprofloxacin, ampicillin, captopril, cefazolin, gentamicin, doxorubicin etc.) that are needed during tissue repair, bone regeneration, etc. are prone to degradation upon burst release into active cell media [177]. These chemicals may therefore have a limited bioavailability due to the instantaneous breakdown of their structure and low absorption during intake [3]. Since electrospinning is a straightforward and adaptable encapsulation technique and does not require extreme temperatures or pressures, can be potentially used for effective entrapping and delivery of bioactive compounds. [38] Mainly, coaxial and emulsion electrospinning are used for efficient encapsulation of active compounds. Coaxial electrospinning, however, necessitates a special assembly, coaxial needles with at least two syringe pumps and is more complicated than monoaxial electrospinning. Also, in comparison to other approaches, choosing the appropriate polymers and setting-up the optimum process parameters takes longer time [178]. Moreover, emulsion-based electrospun nanofibers matrices have offered improved stability, bioavailability, and adaptable release profiles of bioactive agents during processing and delivery as compared to other electrospinning-based encapsulation techniques as depicted in Figure 13a [3]. Studies suggest release of bioactive compounds from surface of electrospun fibers/polymer matrix is mainly governed by factors such as, matrix property (composition, structure, swelling, and biodegradability), characteristics of release medium (diffusion coefficient, medium-driven flow, pH, and temperature), and drug properties (hydrophilic/hydrophobic, loading method, drug interactions with polymer matrix, and degradation kinetics of drug carrier) [179,180]. Among these factors, diffusion coefficient, swelling of polymer matrix, and degradation kinetics of drug carrier are the major ones [97]. The diffusion coefficient of drug depends on fibers’ morphology, fiber-diameter distribution, crystallinity, and other physicochemical properties (like core-sheath ratio) partition coefficient of drug, and the initial concentration of bioactive compounds [3,181].
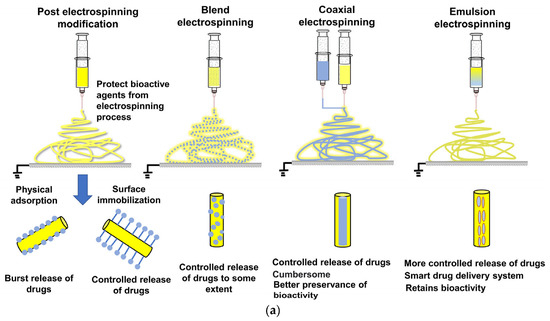
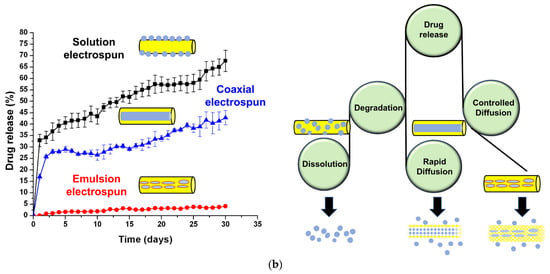
Figure 13.
(a) Various modes of drug encapsulation through electrospinning (b) Schematic of cefazolin drug release from different types of electrospun PCL matrix, Reprinted with permission from ref [144]; Copyright 2018, Elsevier.
Experimental and computational studies (finite element and composite smeared finite elements method) suggested that three-layered scaffolds of PLGA and PCL produced by sequential emulsion electrospinning had delayed release kinetics and made it highly suitable for post-operative therapy [180]. The cumulative drug release amount was inversely related to thickness of PCL layer which controlled the initial burst release of hydrophilic drugs. The prominent overlapping of experimental results with values predicted by numerical models suggested the accuracy of numerical models in predicting the drug release profile to surrounding medium. Controlled and sustained release behavior of antibiotic drug, Eugenol (extract of cloves, known for therapeutic properties) loaded on the emulsion electrospun PCL-PVA-CS composite fibrous matrices was evaluated for 5 days [182]. The emulsion electrospun PCL composite matrices showed initial burst release followed by sustained release of eugenol continuing over two weeks. Further, cell viability analysis of eugenol loaded PCL matrices did not cause any cell death, and thus, can be effectively used for wound dressing and tissue regeneration applications. In another study, antibiotic agent, cefazolin incorporated nanofibrous PCL mats were fabricated by blend, emulsion and co-axial electrospinning methods and compared for their drug release profile and antimicrobial activity [144]. The drug release amounts from electrospun PCL matrices were higher (~68% for blend and ~43% for co-axial) and significantly low (~5% for emulsion electrospun) system as depicted in Figure 13b. The slower drug release profile for emulsion electrospun PCL matrices was mainly due to the efficient encapsulation of bioactive molecules within hydrophobic shell as compared to others. The drug release profile data fitted best with the first order kinetic model and suggested Pseudo-Fickian diffusion behavior. Based on the drug release profile, the suggested potential applications include antibacterial gauzes and coatings for short and long terms urinary catheters.
Emulsion electrospun PVA-PCL nanofibers with core-sheath morphology having different core (PVA) swelling ratio in PBS, controlled the in-vitro release profile of model drugs, Rhodamine B and BSA [183]. The hydrolysis degree of PVA varied the swelling ratio of core, hence, varying diffusion pathways of PBS/drug and controlled release of encapsulated BSA. A novel needleless emulsion electrospinning technique with massive production rate was used to fabricate core-sheath nanofibers of PCL and Pluronic F-68 (F68, triblock-co-polymer of PEO and PPO) to encapsulate diverse range of bioactive compounds [184]. Biocompatible polymer F68 forming the core of nanofibers (above its critical micelle concentration) effectively stabilized and controlled the release of proteineous bioactive compounds through non-covalent interactions, and protein unfolding. Further, excellent viability and proliferation of mesenchymal stem cells and controlled drug delivery rate governed by diffusion and degradation mechanisms confirmed the potential of developed PCL-F68 core-sheath nanofibers as drug delivery carrier.
Several studies have been demonstrated, for PCL based scaffolds, eradication of inherent hydrophobicity of PCL through blending with hydrophilic polymers which promoted the rate of cell adhesion and proliferation [106,113]. Hence, PCL and PEO based composite nanofibrous matrices loaded with bioactive platelet-derived growth factor were prepared through emulsion electrospinning [113]. The emulsion formulation considerably regulated the adsorption and release profile of bioactive compounds. The electrostatic interactions between incorporated ceramic phase and bioactive compounds slowed down the release rate of such compounds. Whereas a cationic surfactant enhanced the release rate of bioactive compounds by reducing the electrostatic interactions. The incorporation of platelet-derived growth factor into nanofibrous matrices increased the osteogenic differentiation of human mesenchymal stem cells. The increased alkaline phosphatase activity contributed to improved cell attachment and reorganized cytoskeletal filaments. Unlike Pickering stabilizers, emulsion preparation conditions, specially, extent of sonication and surfactant concentration (above their critical micelle concentration) modify the bioactivity of proteins loaded on electrospun scaffolds and their release profile as well [111,113]. Polyhedral oligomeric silsesquioxane and methyl methacrylate (POSS-MMA) based copolymer was used to stabilize the liquid-liquid interface of emulsions [114]. Such emulsions were electrospun to obtain bi-continuous core-shell electrospun nanofibers with enhanced encapsulation capability having PCL and POSS-MAA in core and sheath, respectively. This study suggested that POSS based copolymers can effectively be used for stabilization and preservation of functional materials through encapsulation and to develop coating formulations for advanced medical devices.
4.2. Tissue Engineering
Tissue engineering, also called regenerative medicine, combines the approaches of medicine, biology, and engineering to develop biological substitutes that restore, maintain, or improve the damaged tissue functions [170,185]. It focuses on reconstructing and regenerate damaged tissues by implanting a scaffold seeded with cells at desired sites [186]. Where the scaffold’s morphology analogues to natural ECM and having multi-functionality, provides support to seeded cells during cell differentiation, proliferation, migration, growth, and ECM deposition. It also assists in controlled delivery of biochemical factors and nutrients and offers pathways for excretion of metabolic wastes. Therefore, significant efforts have been put into developing polymeric nanofibrous scaffolds with large specific surface area through electrospinning with morphology mimicking the natural ECM at sub-micron and nanometer scale [106,187,188,189,190]. Use of 3D scaffold is necessary for most of the tissue regeneration because their 3D biomimetic environment helps cell differentiation, genetic material, expression, ECM secretion, and cellular metabolisms. Therefore, 3D electrospun nanofibrous scaffolds with a certain thickness and cell infiltration capability are critical for tissue engineering. For this electrospinning have been combined with other fabrication techniques like 3D printing, emulsion templating etc. [50]. The designing strategies to convert 2D electrospun matrices into 3D architectures are summarized in the reference [191].
Recently, emulsion electrospinning of biocompatible polymers has gained increasing attention as emulsion electrospun core-sheath nanofibers offer sustained delivery of cell laden bioactive molecules, growth factors, and drugs [192]. Moreover, among different biocompatible polymers, PCL based emulsion electrospun matrices have a major contribution as tissue engineering scaffolds [61,68,124,125]. Attempts have also been made to prepare PCL based nanocomposite electrospun matrices from Pickering emulsions stabilized using nanomaterials (Pickering stabilizers) such as clay, silica, hydroxyapatite, silver etc. [61,121,123]. The nanocomposite fibrous PCL scaffolds prepared from emulsions stabilized with hydrophobically modified nano clay presented enhanced crystallinity, tensile strength, and tensile modulus in comparison to solution electrospun neat PCL matrices. [61] Further, presence of nano clay did not hamper the cell proliferation efficiency of nanocomposite matrices assessed against human breast cancer cells (MCF7) and Osteoblast cells (MG63). Hence, suggesting the potential of emulsion electrospun matrices as tissue engineering scaffold. Interestingly, emulsion electrospun PCL nanocomposite matrices on post-electrospinning modification achieved rough surface which facilitated the enhanced cell growth and proliferation [124,125]. Therefore, the following sections discuss the applications of emulsion electrospun PCL nanofibrous matrices in various tissue engineering applications.
4.2.1. Bone Tissue Engineering
Bones primarily made-up of hydroxyapatite and collagen, have multi-functionality in the body. Bones provide structural framework and strength, regulate blood pH, and level of calcium and phosphate for metabolic activities [193,194]. Recently, orthopedics treatments involving bone grafts have gained high clinical demand due to increased number of bone defects caused by bone infections, bone tumors, and bone loss through trauma [37]. However, use of both autologous and allogeneic bones is limited clinically since the previous one may need 100% gene modification, likely require combination therapies and later one is associated with problem in finding donors and greater chance of morbidity. Therefore, periodontal regenerative procedure based on tissue engineering approach is applied for bone regeneration to overcome such limitations [195]. The main principle is to restrict the migration of rapidly proliferating epithelial cells into the defect location by establishing a barrier membrane between the epithelial tissue and bone or bone transplant as depicted in Figure 14a [196,197]. Thus, a bone tissue engineering scaffold should have biocompatibility, tunable biodegradability, and interconnected porous architecture for superior migration of growth factors. Further, bioactive sterile environment for cell growth, and sufficient mechanical properties to withstand the physiological pressure excreted by the body movements of patient are also desired. Electrospun nanofibrous scaffolds from various natural and synthetic polymers such as alginate, chitosan, collagen, PCL, polyglycolic acid (PGA), PLA, and PLGA have been highly appreciated for bone regeneration as they satisfy the above-mentioned criteria [198,199,200,201,202]. Therefore, use of electrospun nanofibrous organic-inorganic composite scaffolds loaded with bioactive components such as bone morphogenetic proteins, growth factors, and periosteum etc. is vital for efficient bone-regeneration [37,147].
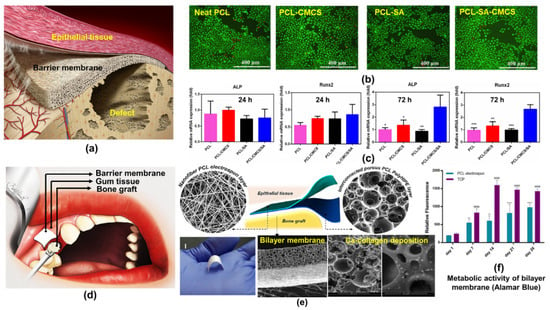
Figure 14.
(a) Schematic showing guided bone regeneration, Reprinted with permission from ref [208]; Copyright 2017, Wiley, (b) Live/dead assay showing osteoblasts (MC3T3-E1) viability (c) Osteogenic gene expression on the fibrous PCL-CMCS-SA scaffolds, (One-way analysis of variance was used to analyze the significance of differences between groups where, * p < 0.05; ** p < 0.01; *** p < 0.001), Reprinted with permission from ref [96]; Copyright 2020, Elsevier (d) PCL based bilayer membrane as a bone graft (e) macro and SEM images of bone graft (f) Metabolic activity of bilayer bone graft, (analysis of variance was used to analyze the significance of differences between groups where **** and ΦΦΦΦ for p ≤ 0.001, ** p ≤ 0.01, while comparing means of 3 samples) Reprinted with permission from ref [147]; Copyright 2019, MDPI.
Biopolymer, collagen-based membranes are commonly used for bone regeneration, however, their potential for antigenicity, poor mechanical properties, and rapid degradation are serious concerns [203,204]. Alternatively, synthetic polymers such as PGA, PLA and PCL based membranes have been commonly investigated for bone tissue engineering. The PCL-based membranes which possess prolong degradation kinetics, hence, not producing an overly acidic environment during their degradation are highly attractive [205,206]. Recently, plasma functionalized resilient PCL bilayer membranes were developed by sequential electrospinning and emulsion templating and examined for their efficacy as bone tissue engineering scaffolds [147]. The top electrospun layer of bilayer membrane showed good barrier properties for prevention of soft tissue invasion. Whereas, the emulsion templated PCL base promoted the cellular infiltration, deposition of collagen and minerals, and blood vessel ingrowth as illustrated in Figure 14 (d, e, and f). In another study, periosteum loaded emulsion electrospun composite fibrous scaffolds of PCL/carboxymethyl chitosan (CMCS) /sodium alginate (SA) were developed and investigated for the periosteum repair to facilitate bone defect regeneration [96]. PCL component strengthened the fibrous scaffolds while carboxymethyl chitosan and sodium alginate components stimulated the ECM of natural periosteum. The emulsion electrospun composite fibrous scaffolds presented good mechanical properties, excellent biocompatibility, and osteoblasts (MC3T3-E1) viability as demonstrated through live-dead assay in Figure 14b. In addition to cell adhesion and growth, it also promoted the differentiation of osteogenic cells. qPCR assay (Figure 14c) suggested no significant difference was observed in the expression of early osteoblastic genes Runx2 and ALP for 72h when only PCL and sodium alginate were used. Interestingly, the addition of CMCS significantly promoted osteogenesis as depicted in Figure 14c. In-vitro and in-vivo studies on w/o emulsion electrospun PVA-strontium ranelate PCL core-sheath nanofibrous scaffolds suggested that encapsulated strontium ranelate was capable of simulating osteogenic differentiation of human mesenchymal stem cells [207]. Further, the boosted Osteocalcin (OCN), runt-related transcription factor 2 (Runx2), alkaline phosphatase (ALP), and collagen I (Col-I) gene expression, and calcium deposition enhanced the bone formation and reduced the bone resorption.
4.2.2. Skin Tissue Engineering and Wound Dressing
Skin, the largest organ of the body protects us from pathogen invasion by providing an external barrier. Burns, diabetes, trauma, surgery, bedsores, ageing, and congenital giant nevi are the main causes of skin damage, and depending on healing-duration, these injuries are acute or chronic. High socio-economic expenses are associated with the skin abnormalities, which are one of the leading causes of morbidity and mortality worldwide [209]. The two primary methods for skin regeneration are autograft and allograft, both of which possess limitations including unsatisfactory donor sites, health problems associated with transplants, delayed rate of healing, and scar formation [210,211,212,213]. Alternatively, biopolymer based flexible porous fibrous scaffolds with good moisture vapor permeability, gas transmissibility, biodegradability, high exudates absorbing capability, biocompatibility, and mechanical properties analogues to native skin tissues are potential choice for guided skin regeneration [214]. These scaffolds essentially keep the wound and surrounding area moist, absorb secretions, and inhibit bacterial-growth and support ECM regeneration through cell attachment, proliferation, and migration. In this regard, the well-established features of electrospun fibrous matrices are appealing as a potential scaffold for skin tissue regeneration [37].
Synergistic effects on structural, mechanical, and biochemical properties have been observed for solution electrospun matrices produced from a blend of natural and synthetic polymers. However, large specific surface area and inherent small pores demonstrated by solution electrospun PCL matrices are undesirable and causes curled fibroblast morphology, high adsorption of non-specific proteins, and poor cell infiltration as shown in Figure 15a,e [215]. On other hand, emulsion electrospun PCL-silk fibroin (SF) matrices loaded with hyaluronan (HA) eradicated the above-mentioned concerns. The PCL-silk fibroin-hyaluronan matrices with increased hydrophilicity suppressed the adsorption of non-specific proteins, exhibited a more spread-out cell morphology, reduced the fibrosis-tissue thickness, and enhanced the macrophages adhesion as depicted in Figure 15b,f [215]. Further, hyaluronan incorporated PCL-silk fibroin nanofibrous matrices showed well developed cytoskeleton, enhanced proliferation of human primary skin fibroblasts with filopodia protrusions (Figure 15c,d). Which in turn promoted the cell-cell interaction, increased the penetration of cellular strands into the scaffolds along the oriented long pores (Figure 15g,h), but decreased the collagen I production. Furthermore, hyaluronan interacted well with cell surface receptors (CD44) to activate TGF-β1/MMPs signaling pathways that were conducive to cell migration, hence, suggested the importance of bioactive factors like hyaluronan in skin tissue engineering. In another study, biodegradable core-sheath nanofibrous scaffolds of PCL encapsulating the hyaluronan and epidermal growth factor were developed by emulsion electrospinning [216]. The BSA release rate from PCL based fibrous scaffolds was enhanced in presence of hyaluronan due to increased hydrophilicity of the fibers. Synergistic effect of hyaluronan and epidermal growth factor on accelerated cell-infiltration (in-vitro, human skin keratinocytes and fibroblasts) and epidermis regeneration (in-vivo) was observed. Up-regulated collagen and TGF-β1 gene expression, increased collagen III to collagen I ratios, a thicker epidermis and organized dermis layers proved the potential of developed matrices as effective wound dressing material.
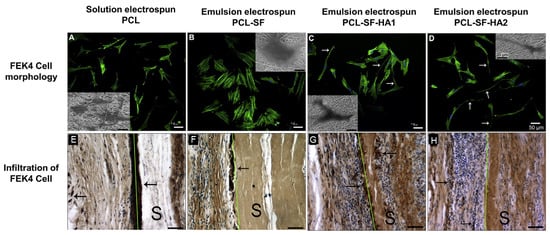
Figure 15.
(A–D) Effect of various electrospinning methods on FEK4 cell morphology (E–H) In vivo assessment of cellular infiltration via staining of CD68+ macrophages, Reprinted with permission from ref [215]; Copyright 2012, Elsevier.
Ketoprofen loaded binary matrices of PCL and gelatin, developed by o/w emulsion electrospinning and crosslinked with glutaraldehyde on post-electrospinning demonstrated potential suitability as wound healing materials [217]. The crosslinked binary matrices possessing moderate hydrophilicity (water contact angle, ~76°) are beneficial for biomedical application as enhanced dimensional stability and cell adhesion and proliferation have been demonstrated than too hydrophilic matrices [218,219]. In-vitro release experiments, for binary PCL-gelatin matrices, showed sustained release of ketoprofen up to 4 days. The ketoprofen-release followed a non-Fickian diffusion mechanism where the crosslinked gelatin barrier encapsulating PCL and ketoprofen suppressed the burst release. In addition, drug loaded PCL-gelatin binary matrices having high porosity and moderate hydrophilicity presented enhanced in-vitro attachment and proliferation of L929 mouse fibroblasts up to 7 days than solution electrospun PCL matrices. Similar observations for cell-attachment and proliferation have also been observed for emulsion electrospun PCL matrices loaded with antibiotic drug gentamicin [97] and emulsion electrospun core-sheath PCL-PVA-gelatin nanofibrous matrices [146], hence, confirming their suitability as wound dressing materials.
PCL-PVA-chitosan nanofibrous matrices loaded with eugenol were prepared by electrospinning of w/o or o/w emulsions and examined for their antimicrobial activity [182]. Eugenol loading within electrospun matrices reduced the porosity and hydrophilicity of matrices, as expected and in-vitro release experiments demonstrated burst release of eugenol during initial 8 h followed by sustained release up to 120 h. Higher retention of eugenol for matrices electrospun from o/w emulsions ascribed to its higher affinity for PCL and protective PVA-chitosan barrier surrounding the PCL phase. The antimicrobial activity of matrices electrospun from w/o emulsions was higher for both the bacteria, S. aureus and P. aeruginosa than those produced from o/w emulsions due to higher release percentage of eugenol. Moreover, the eugenol loading in emulsion electrospun matrices did not induce any cytotoxicity on normal human dermal fibroblast cells during in-vitro incubation of 7 days, hence, appealing their potential as wound dressing materials.
Variation in stirring period during emulsion preparation produced the emulsion electrospun PCL-chitosan fluffy 3D scaffolds and assessed for skin tissue engineering [67]. The developed nano/micro-fibrous 3D scaffolds encapsulating chitosan in the core had excellent stability in water (swelling of 80%), low hydrolytic degradation rate, thus promoting the elimination of crosslinking with toxic reagents. The 3D fibrous morphology promoted in-vitro cell adhesion and proliferation. Further, deposition of ECM proteins, reduced scarring property during culture period, healing of full thickness wound (in-vivo, rat model) within three weeks proved the effectiveness of developed fibrous scaffolds for skin tissue engineering.
4.3. Other Applications
Besides potential biomedical applications of emulsion electrospun neat/blend PCL nanofibrous matrices, these have also been applied as catalytic support [112], encapsulation for phase change materials (PCM), and in micro-RNA therapeutics [220,221].
The prevailing biocatalytic enzyme immobilization strategies like covalent multipoint linking, sol–gel encapsulation, and layer-by-layer (LbL) assembly are efficient enough. However, their major drawbacks include exorbitant, labor intensive and sluggish and, hence, they are still inadequate for large-scale and continuous process application. Emulsion electrospun sub-micron fibrous PCL membranes encapsulated with trypsin (in-situ) were assessed for their catalytic property. The hydrolytic degradation ability of PCL-trypsin matrices on synthetic low molecular weight substrate, Nα-benzoyl-DL-arginine-p-nitroanilide and milk protein casein was investigated [112]. It was observed that the non-homogeneous distribution of the active compounds within emulsion electrospun matrices was governed by the electrostatic forces between charged and uncharged components and their localization near the fibers’ surface allowed the high remembrance of enzyme activity. Further, improved heat and storage stability, and satisfactory retention of catalytic activity (~60%, for 4th repeat experiment) confirmed the efficient encapsulation of hydrophilic trypsin within a hydrophobic polymer, PCL, which otherwise was a challenge. Thus, emulsion electrospinning is highly suitable for creating efficient catalytic fibrous matrices for conversion of low-cost materials into high value products at large scale. Further, for the applications like simultaneous bio-catalysis and filtration, since it allows the production of interconnected fibrous matrices with high specific surface area, good mechanical property that can sustain high enzyme load, and possess adaptability, adequate substrate accessibility, and significantly control on the mass transfer rate.
In the last decade, PCM has gained significant research attention in both academia and industry and found application in diversified fields. On the other hand, proper encapsulation of hydrophobic phase change material remains a challenge since ideally it requires hydrophilic encapsulating material that favors phase separation and prevents migration via permeation through the pore wall. Further, such hybrid structures often absorb moisture and swells at high relative humidity and that makes it unsuitable for end application [222]. So, introduction of proper buffering capability is essential. Temperature buffering hybrid structures by encapsulation of a PCM into a hydrophilic polymer, PVA using emulsion electrospinning was demonstrated [222]. Addition of low amount of non-ionic surfactant, tween 20 (0.32 wt.% of total emulsion) resulted in increased heat storage capacity of the developed structures. However, hydrophilicity of the developed matrices in moderate to high relative humidity made the handling extremely difficult. Co-axial electrospinning was therefore applied to improve the stability of hydrophilic segment by developing hybrid core-shell matrices, where a hydrophobic PCL encapsulated the PVA/PCM emulsion. The increased loading of PCM within hybrid core-shell fibrous structure enhanced the heat storage capacity per gram of the fiber. While the PCL core supported the retention of fiber morphology even in high relative humidity conditions, thus suggesting their prospective food packaging applications. A similar study reported the fabrication of core-shell nanofibrous matrices for thermal energy storage using co-axial electrospinning of o/w emulsions of PVA and PCM (organic paraffin, phase change temperature of 18 °C) and PCL solution [223]. The encapsulation efficiency of PCM within the fibrous matrices was as high as 67%.
Recently, manipulation of gene expression by using micro and short interfering-RNA (miRNA and siRNA) based therapeutics has received significant research attention for treating various inherited and acquired human diseases like cancer, viral infections, cardiovascular disease etc. Nevertheless, the efficient delivery of these functional materials into the targeted cell/tissue is still a challenge since any discrepancy may lead to the instability and degradation of mi/siRNAs. A bilayer vascular scaffold was prepared using emulsion and dual-power electrospinning of poly (ethylene glycol)-b-poly(L-lactide-co-ε-caprolactone) (PELCL) and dual-power electrospinning of PCL and gelatin, respectively, for controlled delivery of micro-RNAs [221]. The inner layer of bilayer scaffold loaded with polyplex/micro-RNA-126 (miRNA-126) complexes was prepared by emulsion electrospinning which regulated the response of endothelial cells, whereas PCL and gelatin based outer layers enhanced the mechanical stability of scaffolds. The encapsulation efficiency of miRNA-126 complexes in bilayer fibrous scaffolds was ~68% and its sustained release was observed for 56 days. Further, bilayer scaffolds loaded with miRNA-126 improved the endothelialisation in-vivo. Another study also reported the enhanced vascular endothelial cell adhesion and proliferation for emulsion electrospun dual-functional fibrous membranes of PELCL and functionalized PCL encapsulating miRNA-126 in comparison to neat PELCL membranes [220]. These studies suggested that well encapsulated miRNA-based therapeutics made via emulsion electrospinning route can be successfully utilized for the remedy of complex inherited and acquired human diseases (like cancer, cardiovascular disease remedy etc.) in near future.
5. Conclusions and Future Perspective
The techniques to fabricate polymeric nanofibers using green and sustainable approaches have always been sought by material scientists. Several advances in processing methods have been carried out over the past decades out of which electrospinning of polymer solutions has emerged as one of the most versatile techniques. But this technique has some grails like productivity and toxicity. Electrospinning of polymer solutions results in formation of micro- to nanofibers at substantial ease where the stability of Taylor cone and fiber spinning is significantly impacted by the polymer’s molecular weight, concentration, chain entanglements, and solution viscosity. Increasing these parameters also leads to the generation of submicron size fibers, which are easily produced by standard textile fiber processing procedures, therefore negating out the benefits of the electrospinning process. Thus, the production of ultrafine nanofibers using electrospinning is particularly difficult since this can only be accomplished at compromised polymer concentration or molecular weight in solution. Here comes the beauty of emulsion electrospinning. This review demonstrated emulsions can be used to impart appropriate rheological properties to a polymer system so that elongation of the polymer phase can take place more smoothly while increasing aqueous fractions and also aid in modulation of fiber diameter even at higher polymer concentration. Further, conventional electrospinning consumes large number of organic solvents which cannot be reused or recycled, and worldwide restrictions and legislations have led to limit or avoid use of toxic organic solvents. Here also emulsion electrospinning justifies it’s need as an approach which would alleviate concerns regarding safety, toxicology, and environmental problems since it deals with a high fraction of green solvent like water. Further, this technique combines the co-axial and single-nozzle electrospinning approaches to produce fibers with diversified morphologies ranging from core-sheath to multichannel, and so on. Use of water based polymeric emulsions (w/o, o/w, or double emulsions) for electrospinning act as sensitive hydrophilic drug reservoirs within hydrophobic/amphiphilic polymers which was almost impossible to obtain with conventional electrospinning and thus overcome the challenge of incorporation and sustained release of the hydrophilic bioactive molecules, such as drugs, proteins and DNA.
An exhaustive list of PCLs based emulsion electrospun systems, emulsion electrospinning parameters (emulsion type, applied voltage, tip to collector distance, and flow rate) and end-use of obtained nano-fibrous matrices is presented and discussed in this review. The mechanism of fiber formation within emulsion electrospinning and the effects of emulsion type, template polymer, rheological properties, electrospinning parameters, and ambient conditions on fiber-morphology are deliberated in detail. Precise control over the emulsion properties and electrospinning parameters is required to achieve desired outcome from the nanofibrous scaffolds. It should be noted that the present studies which have been performed on emulsion electrospinning primarily generate 2D nano-fibrous matrices. On the other hand, in tissue engineering 3D resilient scaffolds mimicking the native ECM are highly appreciated. Hence, efforts should be directed to develop robust and flexible 3D scaffolds within emulsion electrospinning. The majority of emulsion electrospun matrices employed as tissue engineering scaffolds are based on PCL which are uncrosslinked, hence reflect poor mechanical strength. Therefore, efforts can be dedicated to fabricating emulsion electrospun crosslinked matrices of PCL and other polymers which will be mechanically, chemically, and thermally resilient. The developed crosslinked emulsion electrospun polymeric matrices will definitely be suitable for widespread applications. Different statistical models can also be developed to understand and correlate the mechanism of emulsion electrospinning, fiber-morphology, and fiber-strength, and hence, their suitability for a particular application can be scrutinized. Moreover, advances should also be focused on increasing the production rate of emulsion electrospinning without compromising the fiber-strength.
Author Contributions
S.G.: Conceptualization, Methodology, Investigation, Data curation, Writing—original draft. A.Y.: Conceptualization, Investigation, Data curation, Writing—original draft, P.M.G.: Methodology, Investigation, Data curation, Writing—original draft, R.K.S.: Conceptualization, Supervision, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support provided by the Indian Institute of Technology, Delhi, and the Department of Science and Technology, Ministry of Science and Technology, India (Dy. no. C/5142/IFD) to perform this research work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Wang, G.; Yu, D.; Kelkar, A.D.; Zhang, L. Electrospun Nanofiber: Emerging Reinforcing Filler in Polymer Matrix Composite Materials. Prog. Polym. Sci. 2017, 75, 73–107. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of Food Proteins and Polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion Electrospinning: Fundamentals, Food Applications and Prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Salas, C. Solution Electrospinning of Nanofibers. In Electrospun Nanofibers; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–108. ISBN 978-0-08-100907-9. [Google Scholar]
- Pal, J.; Wu, D.; Hakkarainen, M.; Srivastava, R.K. The Viscoelastic Interaction between Dispersed and Continuous Phase of PCL/HA-PVA Oil-in-Water Emulsion Uncovers the Theoretical and Experimental Basis for Fiber Formation during Emulsion Electrospinning. Eur. Polym. J. 2017, 96, 44–54. [Google Scholar] [CrossRef]
- Bachs-Herrera, A.; Yousefzade, O.; del Valle, L.J.; Puiggali, J. Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications. Appl. Sci. 2021, 11, 1808. [Google Scholar] [CrossRef]
- Gurave, P.M.; Nandan, B.; Srivastava, R.K. Emulsion Templated Dual Crosslinked Core-Sheath Fibrous Matrices for Efficient Oil/Water Separation. Colloids Surf. Physicochem. Eng. Asp. 2022, 635, 128037. [Google Scholar] [CrossRef]
- Gurave, P.M.; Singh, S.; Yadav, A.; Nandan, B.; Srivastava, R.K. Electrospinning of a Near Gel Resin To Produce Cross-Linked Fibrous Matrices. Langmuir 2020, 36, 2419–2426. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Gurave, P.M.; Singh, S.; Pal, J.; Samanta, A.; Yadav, A.L.; Nandan, B. A Process for Producing Cross-Linked Polymeric Matrices. Indian Patent No. 399845, 23 June 2022. [Google Scholar]
- Lu, T.; Cui, J.; Qu, Q.; Wang, Y.; Zhang, J.; Xiong, R.; Ma, W.; Huang, C. Multistructured Electrospun Nanofibers for Air Filtration: A Review. ACS Appl. Mater. Interfaces 2021, 13, 23293–23313. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Samal, S.K.; Wang, F.; Gao, B.; Pan, H.; Xu, H.; Yao, J.; Zhan, X.; De Smedt, S.C.; et al. Core–Sheath Structured Electrospun Nanofibrous Membranes for Oil–Water Separation. RSC Adv. 2016, 6, 41861–41870. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Gupta, A.; Srivastava, R.; Nandan, B. Bio-Inspired Design of Electrospun Poly(Acrylonitrile) and Novel Ionene Based Nanofibrous Mats as Highly Flexible Solid State Polymer Electrolyte for Lithium Batteries. Chem. Eng. J. 2022, 440, 135926. [Google Scholar] [CrossRef]
- Barik, R.; Raulo, A.; Jha, S.; Nandan, B.; Ingole, P.P. Polymer-Derived Electrospun Co3O4 @C Porous Nanofiber Network for Flexible, High-Performance, and Stable Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11002–11014. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S.; Tuller, H.L.; Kim, I.-D. Heterogeneous Sensitization of Metal–Organic Framework Driven Metal@Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef]
- Raulo, A.; Singh, S.; Gupta, A.; Srivastava, R.; Nandan, B. Metal Oxide Heterostructure Decorated Carbon Nanofiber as a Novel Redox Catalyst for High Performance Lithium-Sulfur Batteries. Appl. Surf. Sci. 2021, 569, 151054. [Google Scholar] [CrossRef]
- Kajdič, S.; Planinšek, O.; Gašperlin, M.; Kocbek, P. Electrospun Nanofibers for Customized Drug-Delivery Systems. J. Drug Deliv. Sci. Technol. 2019, 51, 672–681. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Wang, Y.; Cui, W. Advanced Electrospun Hydrogel Fibers for Wound Healing. Compos. Part B Eng. 2021, 223, 109101. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.; Shin, Y.M.; Shin, H. Current Progress in Application of Polymeric Nanofibers to Tissue Engineering. Nano Converg. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Gurave, P.M.; Mishra, S.; Nandan, B.; Srivastava, R.K. Tuned Interactions within Inclusion Complex to Generate Electrospun Matrices of Superior Strength. Mater. Today Commun. 2021, 29, 102794. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Tucker, N.; Kny, E.; Thomas, S. Electrical Spinning to Electrospinning: A Brief History. In Soft Matter Series; Kny, E., Ghosal, K., Thomas, S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; Chapter 1; pp. 1–23. ISBN 978-1-78801-100-6. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Dzenis, Y.A.; Reneker, D.H. Delamination Resistant Composites Prepared by Small Diameter Fiber Reinforcement at Ply Interfaces. U.S. Patent 6,265,333, 24 July 2001. [Google Scholar]
- Hong, J.; Yeo, M.; Yang, G.H.; Kim, G. Cell-Electrospinning and Its Application for Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 6208. [Google Scholar] [CrossRef]
- Jamalzadeh, L.; Ghafoori, H.; Sariri, R.; Rabuti, H.; Nasirzade, J.; Hasani, H.; Aghamaali, M.R. Cytotoxic Effects of Some Common Organic Solvents on MCF-7, RAW-264.7 and Human Umbilical Vein Endothelial Cells. Avicenna J. Med. Biochem. 2016, 4, e33453. [Google Scholar] [CrossRef]
- Li, F.; Chang, X.; Yang, H.; Xu, Z. Study on the Electrospinnability of Polyvinyl Alcohol Solutions by Using Water/N, N-Dimethylacetamide or Water/N, N-Dimethylformamide as Solvents. J. Macromol. Sci. Part B 2017, 56, 682–696. [Google Scholar] [CrossRef]
- Maccaferri, E.; Ortolani, J.; Mazzocchetti, L.; Benelli, T.; Brugo, T.M.; Zucchelli, A.; Giorgini, L. New Application Field of Polyethylene Oxide: PEO Nanofibers as Epoxy Toughener for Effective CFRP Delamination Resistance Improvement. ACS Omega 2022, 7, 23189–23200. [Google Scholar] [CrossRef] [PubMed]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning Based on Benign Solvents: Current Definitions, Implications and Strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; EI-Shanshory, A.; El-Newehy, M.; EI-Hamshary, H.A.; Al-Deyab, S.S.; He, C.; Mo, X. Dexamethasone Loaded Core–Shell SF/PEO Nanofibers via Green Electrospinning Reduced Endothelial Cells Inflammatory Damage. Colloids Surf. B Biointerfaces 2015, 126, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-H.; Wu, D.-Q.; Chu, C.-C. Dual Functions of Polyvinyl Alcohol (PVA): Fabricating Particles and Electrospinning Nanofibers Applied in Controlled Drug Release. J. Nanoparticle Res. 2013, 15, 1395. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Wang, Z.; Jing, X. Ultrafine PEG–PLA Fibers Loaded with Both Paclitaxel and Doxorubicin Hydrochloride and Their in Vitro Cytotoxicity. Eur. J. Pharm. Biopharm. 2009, 72, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Chatterton, N.P.; Yang, J.-H.; Wang, X.; Liao, Y.-Z. Coaxial Electrospinning with Triton X-100 Solutions as Sheath Fluids for Preparing PAN Nanofibers. Macromol. Mater. Eng. 2012, 297, 395–401. [Google Scholar] [CrossRef]
- Wu, H.; Hu, Q.; Zhang, L.; Fong, H.; Tian, M. Electrospun Composite Nanofibers of Polybutadiene Rubber Containing Uniformly Distributed Ag Nanoparticles. Mater. Lett. 2012, 84, 5–8. [Google Scholar] [CrossRef]
- Povolo, M.; Maccaferri, E.; Cocchi, D.; Brugo, T.M.; Mazzocchetti, L.; Giorgini, L.; Zucchelli, A. Damping and Mechanical Behaviour of Composite Laminates Interleaved with Rubbery Nanofibers. Compos. Struct. 2021, 272, 114228. [Google Scholar] [CrossRef]
- Yan, G.; Yu, J.; Qiu, Y.; Yi, X.; Lu, J.; Zhou, X.; Bai, X. Self-Assembly of Electrospun Polymer Nanofibers: A General Phenomenon Generating Honeycomb-Patterned Nanofibrous Structures. Langmuir 2011, 27, 4285–4289. [Google Scholar] [CrossRef]
- Sy, J.C.; Klemm, A.S.; Shastri, V.P. Emulsion as a Means of Controlling Electrospinning of Polymers. Adv. Mater. 2009, 21, 1814–1819. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.u.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning Nanofiber Scaffolds for Soft and Hard Tissue Regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Wen, P.; Wen, Y.; Zong, M.-H.; Linhardt, R.J.; Wu, H. Encapsulation of Bioactive Compound in Electrospun Fibers and Its Potential Application. J. Agric. Food Chem. 2017, 65, 9161–9179. [Google Scholar] [CrossRef]
- Spano, F.; Quarta, A.; Martelli, C.; Ottobrini, L.; Rossi, R.M.; Gigli, G.; Blasi, L. Fibrous Scaffolds Fabricated by Emulsion Electrospinning: From Hosting Capacity to in Vivo Biocompatibility. Nanoscale 2016, 8, 9293–9303. [Google Scholar] [CrossRef]
- Drummond, J.L.; Andronova, K.; Al-Turki, L.I.; Slaughter, L.D. Leaching and Mechanical Properties Characterization of Dental Composites. J. Biomed. Mater. Res. 2004, 71B, 172–180. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions Stabilized with Solid Nanoparticles: Pickering Emulsions. Colloids Surf. Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- French, D.J.; Brown, A.T.; Schofield, A.B.; Fowler, J.; Taylor, P.; Clegg, P.S. The Secret Life of Pickering Emulsions: Particle Exchange Revealed Using Two Colours of Particle. Sci. Rep. 2016, 6, 31401. [Google Scholar] [CrossRef]
- Ridel, L.; Bolzinger, M.-A.; Gilon-Delepine, N.; Dugas, P.-Y.; Chevalier, Y. Pickering Emulsions Stabilized by Charged Nanoparticles. Soft Matter 2016, 12, 7564–7576. [Google Scholar] [CrossRef] [PubMed]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of Bicomponent PCL/Gelatin and PCL/Collagen Nanofibers Electrospun from Alternative Solvent System. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Biocompatibility Evaluation of Emulsion Electrospun Nanofibers Using Osteoblasts for Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 1952–1968. [Google Scholar] [CrossRef]
- Agrawal, M.; Yadav, A.; Nandan, B.; Srivastava, R.K. Facile Synthesis of Templated Macrocellular Nanocomposite Scaffold via Emulsifier-Free HIPE-ROP. Chem. Commun. 2020, 56, 12604–12607. [Google Scholar] [CrossRef]
- Labet, M.; Thielemans, W. Synthesis of Polycaprolactone: A Review. Chem. Soc. Rev. 2009, 38, 3484. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Yadav, A.; Ghosh, S.; Samanta, A.; Pal, J.; Srivastava, R.K. Emulsion Templated Scaffolds of Poly(ε-Caprolactone)—A Review. Chem. Commun. 2022, 58, 1468–1480. [Google Scholar] [CrossRef]
- Yadav, A.; Pal, J.; Nandan, B.; Srivastava, R.K. Macroporous Scaffolds of Cross-Linked Poly(ε-Caprolactone) via High Internal Phase Emulsion Templating. Polymer 2019, 176, 66–73. [Google Scholar] [CrossRef]
- Agrawal, M.; Yadav, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Dual-Functionalized Pickering HIPE Templated Poly(ε-Caprolactone) Scaffold for Maxillofacial Implants. Int. J. Pharm. 2023, 633, 122611. [Google Scholar] [CrossRef]
- Ghosh, S.; Yadav, A.; Rani, S.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. 3D Printed Hierarchical Porous Poly(ε-Caprolactone) Scaffolds from Pickering High Internal Phase Emulsion Templating. Langmuir 2023, 39, 1927–1946. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, D.; Nandan, B.; Srivastava, R.K. Emulsion Templated Porous Funnel from Polypropylene Waste for Efficient Oil Separation and Spillage Management. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129563. [Google Scholar] [CrossRef]
- Gurave, P.M.; Dubey, S.; Nandan, B.; Srivastava, R.K. Pickering Emulsion-Templated Nanocomposite Membranes for Excellent Demulsification and Oil–Water Separation. ACS Appl. Mater. Interfaces 2022, 14, 54233–54244. [Google Scholar] [CrossRef]
- Welch, C.F.; Rose, G.D.; Malotky, D.; Eckersley, S.T. Rheology of High Internal Phase Emulsions. Langmuir 2006, 22, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Aldemir Dikici, B.; Claeyssens, F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Bowlin, G.L. Electrospinning Jets and Nanofibrous Structures. Biomicrofluidics 2011, 5, 013403. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Electrospun Composite Matrices of Poly(ε-Caprolactone)-Montmorillonite Made Using Tenside Free Pickering Emulsions. Mater. Sci. Eng. C 2016, 69, 685–691. [Google Scholar] [CrossRef]
- Li, X.; Su, Y.; Liu, S.; Tan, L.; Mo, X.; Ramakrishna, S. Encapsulation of Proteins in Poly(l-Lactide-Co-Caprolactone) Fibers by Emulsion Electrospinning. Colloids Surf. B Biointerfaces 2010, 75, 418–424. [Google Scholar] [CrossRef]
- Xu, X.; Zhuang, X.; Chen, X.; Wang, X.; Yang, L.; Jing, X. Preparation of Core-Sheath Composite Nanofibers by Emulsion Electrospinning. Macromol. Rapid Commun. 2006, 27, 1637–1642. [Google Scholar] [CrossRef]
- Advanced Nanofibrous Materials Manufacture Technology Based on Electrospinning, 1st ed.; Liu, Y., Wang, C., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group, LLC: Abingdon, UK, 2019; ISBN 978-0-429-08576-5. [Google Scholar]
- Ding, B.; Li, C.; Miyauchi, Y.; Kuwaki, O.; Shiratori, S. Formation of Novel 2D Polymer Nanowebs via Electrospinning. Nanotechnology 2006, 17, 3685–3691. [Google Scholar] [CrossRef]
- Almería, B.; Deng, W.; Fahmy, T.M.; Gomez, A. Controlling the Morphology of Electrospray-Generated PLGA Microparticles for Drug Delivery. J. Colloid Interface Sci. 2010, 343, 125–133. [Google Scholar] [CrossRef]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maulik, D.; Dhara, S. Nano-/Microfibrous Cotton-Wool-Like 3D Scaffold with Core–Shell Architecture by Emulsion Electrospinning for Skin Tissue Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 3563–3575. [Google Scholar] [CrossRef]
- Pal, J.; Skrifvars, M.; Nandan, B.; Srivastava, R.K. Electrospun Composite Matrices from Tenside-Free Poly(ε-Caprolactone)-Grafted Acrylic Acid/Hydroxyapatite Oil-in-Water Emulsions. J. Mater. Sci. 2017, 52, 2254–2262. [Google Scholar] [CrossRef]
- Anu Bhushani, J.; Anandharamakrishnan, C. Electrospinning and Electrospraying Techniques: Potential Food Based Applications. Trends Food Sci. Technol. 2014, 38, 21–33. [Google Scholar] [CrossRef]
- Shibata, T.; Yoshimura, N.; Kobayashi, A.; Ito, T.; Hara, K.; Tahara, K. Emulsion-Electrospun Polyvinyl Alcohol Nanofibers as a Solid Dispersion System to Improve Solubility and Control the Release of Probucol, a Poorly Water-Soluble Drug. J. Drug Deliv. Sci. Technol. 2022, 67, 102953. [Google Scholar] [CrossRef]
- Ngoensawat, U.; Pisuchpen, T.; Sritana-anant, Y.; Rodthongkum, N.; Hoven, V.P. Conductive Electrospun Composite Fibers Based on Solid-State Polymerized Poly(3,4-Ethylenedioxythiophene) for Simultaneous Electrochemical Detection of Metal Ions. Talanta 2022, 241, 123253. [Google Scholar] [CrossRef]
- Nageeb El-Helaly, S.; Abd-Elrasheed, E.; Salim, S.A.; Fahmy, R.H.; Salah, S.; EL-Ashmoony, M.M. Green Nanotechnology in the Formulation of a Novel Solid Dispersed Multilayered Core-Sheath Raloxifene-Loaded Nanofibrous Buccal Film; In Vitro and In Vivo Characterization. Pharmaceutics 2021, 13, 474. [Google Scholar] [CrossRef] [PubMed]
- Hemmatian, T.; Seo, K.H.; Yanilmaz, M.; Kim, J. The Bacterial Control of Poly (Lactic Acid) Nanofibers Loaded with Plant-Derived Monoterpenoids via Emulsion Electrospinning. Polymers 2021, 13, 3405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ko, F.K.; Hamad, W.Y. Effects of Emulsion Droplet Size on the Structure of Electrospun Ultrafine Biocomposite Fibers with Cellulose Nanocrystals. Biomacromolecules 2013, 14, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Maretschek, S.; Greiner, A.; Kissel, T. Electrospun Biodegradable Nanofiber Nonwovens for Controlled Release of Proteins. J. Control. Release 2008, 127, 180–187. [Google Scholar] [CrossRef]
- Qi, H.; Hu, P.; Xu, J.; Wang, A. Encapsulation of Drug Reservoirs in Fibers by Emulsion Electrospinning: Morphology Characterization and Preliminary Release Assessment. Biomacromolecules 2006, 7, 2327–2330. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Shabani, I.; Ahvaz, H.H.; Soleimani, M. PLGA/Gelatin Hybrid Nanofibrous Scaffolds Encapsulating EGF for Skin Regeneration: Plga/Gelatin Hybrid Nanofibrous Scaffolds. J. Biomed. Mater. Res. A 2015, 103, 2225–2235. [Google Scholar] [CrossRef]
- Peh, P.; Lim, N.S.J.; Blocki, A.; Chee, S.M.L.; Park, H.C.; Liao, S.; Chan, C.; Raghunath, M. Simultaneous Delivery of Highly Diverse Bioactive Compounds from Blend Electrospun Fibers for Skin Wound Healing. Bioconjug. Chem. 2015, 26, 1348–1358. [Google Scholar] [CrossRef]
- Niu, J.; Dai, Y.; Guo, H.; Xu, J.; Shen, Z. Adsorption and Transformation of PAHs from Water by a Laccase-Loading Spider-Type Reactor. J. Hazard. Mater. 2013, 248–249, 254–260. [Google Scholar] [CrossRef]
- Norouzi, M.; Soleimani, M.; Shabani, I.; Atyabi, F.; Ahvaz, H.H.; Rashidi, A. Protein Encapsulated in Electrospun Nanofibrous Scaffolds for Tissue Engineering Applications: Protein Encapsulated in Nanofibers. Polym. Int. 2013, 62, 1250–1256. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, B.; Xu, J.; Xie, E.; Wang, T.; Xu, Z. Electrospinning–Thermal Treatment Synthesis: A General Strategy to Decorate Highly Porous Nanotubes on Both Internal and External Side-Walls with Metal Oxide/Noble Metal Nanoparticles. Nanoscale 2013, 5, 2835. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Herves, A.; Wedepohl, S.; Calderón, M. One-Pot Synthesis of Doxorubicin-Loaded Multiresponsive Nanogels Based on Hyperbranched Polyglycerol. Chem. Commun. 2015, 51, 5264–5267. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Youn, D.-Y.; Jo, S.M.; Oh, S.-G.; Kim, I.-D. Micelle-Mediated Synthesis of Single-Crystalline β(3C)-SiC Fibers via Emulsion Electrospinning. ACS Appl. Mater. Interfaces 2011, 3, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, B.; Chen, J.; Tian, L.; Huang, L.; Tu, M.; Tan, S. Structure Design and Photocatalytic Properties of One-Dimensional SnO2-TiO2 Composites. Nanoscale Res. Lett. 2015, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiong, T.; Jiang, S.; Liu, S.; Hou, H. Mechanical Properties and Chemical Resistance of Electrospun Polyterafluoroethylene Fibres. RSC Adv. 2016, 6, 24250–24256. [Google Scholar] [CrossRef]
- Hosseini, A.; Ramezani, S.; Tabibiazar, M.; Mohammadi, M.; Golchinfar, Z.; Mahmoudzadeh, M.; Jahanban-Esfahlan, A. Immobilization of α-Amylase in Ethylcellulose Electrospun Fibers Using Emulsion-Electrospinning Method. Carbohydr. Polym. 2022, 278, 118919. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, T.; Chen, F.; Wei, W.; Liu, C.; He, S.; Li, X. Electrospun Fibers with Plasmid BFGF Polyplex Loadings Promote Skin Wound Healing in Diabetic Rats. Mol. Pharm. 2012, 9, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, G.; Popa, A.M.; Rossi, R.M.; Maniura-Weber, K.; Puigmartí-Luis, J.; Crespy, D.; Fortunato, G. Tunable Release of Hydrophilic Compounds from Hydrophobic Nanostructured Fibers Prepared by Emulsion Electrospinning. Polymer 2015, 66, 268–276. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Y.; Huang, X.; Yue, T. Controlled Release of Protein from Core-Shell Nanofibers Prepared by Emulsion Electrospinning Based on Green Chemical. J. Appl. Polym. Sci. 2015, 132, 41811. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Xie, Y.; Wang, L.; Yang, L.; Yu, J.; Miyamoto, A.; Sun, F. Development of FGF-2-Loaded Electrospun Waterborne Polyurethane Fibrous Membranes for Bone Regeneration. Regen. Biomater. 2021, 8, rbaa046. [Google Scholar] [CrossRef]
- Zhou, W.; Gong, X.; Li, Y.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Environmentally Friendly Waterborne Polyurethane Nanofibrous Membranes by Emulsion Electrospinning for Waterproof and Breathable Textiles. Chem. Eng. J. 2022, 427, 130925. [Google Scholar] [CrossRef]
- Wang, Z.; Song, X.; Cui, Y.; Cheng, K.; Tian, X.; Dong, M.; Liu, L. Silk Fibroin H-Fibroin/Poly(ε-Caprolactone) Core-Shell Nanofibers with Enhanced Mechanical Property and Long-Term Drug Release. J. Colloid Interface Sci. 2021, 593, 142–151. [Google Scholar] [CrossRef]
- Su, S.; Bedir, T.; Kalkandelen, C.; Ozan Başar, A.; Turkoğlu Şaşmazel, H.; Bulent Ustundag, C.; Sengor, M.; Gunduz, O. Coaxial and Emulsion Electrospinning of Extracted Hyaluronic Acid and Keratin Based Nanofibers for Wound Healing Applications. Eur. Polym. J. 2021, 142, 110158. [Google Scholar] [CrossRef]
- Johnson, P.M.; Lehtinen, J.M.; Robinson, J.L. Surfactant Interactions and Solvent Phase Solubility Modulate Small Molecule Release from Emulsion Electrospun Fibers. AIChE J. 2021, 67, e17470. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.P.; Ahonen, M.; Fangueiro, R.; Gouveia, I.C. Chelidoniummajus L. Incorporated Emulsion Electrospun PCL/PVA_PEC Nanofibrous Meshes for Antibacterial Wound Dressing Applications. Nanomaterials 2021, 11, 1785. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Tao, H.; Jin, L.; Wan, Z.; Dai, F.; Xiang, W.; Deng, H. Carboxymethyl Chitosan/Sodium Alginate-Based Micron-Fibers Fabricated by Emulsion Electrospinning for Periosteal Tissue Engineering. Mater. Des. 2020, 194, 108849. [Google Scholar] [CrossRef]
- Tariq, S.; Rahim, A.; Muhammad, N.; Rahman, S.U.; Azhar, U.; Sultana, K.; Sharif, F.; Siddiqi, S.A.; Zaman, M.; Rehman, F. Controllable Delivery from Gentamicin Loaded Polycaprolactone/Grafted Silica Nanoparticles Composite Mats. J. Mol. Liq. 2019, 290, 111205. [Google Scholar] [CrossRef]
- Giannetti, R.; Abraham, G.A.; Rivero, G. The Role of Emulsion Parameters in Tramadol Sustained-Release from Electrospun Mats. Mater. Sci. Eng. C 2019, 99, 1493–1501. [Google Scholar] [CrossRef]
- Cui, C.; Wen, M.; Zhou, F.; Zhao, Y.; Yuan, X. Target Regulation of Both VECs and VSMCs by Dual-Loading MiRNA-126 and MiRNA-145 in the Bilayered Electrospun Membrane for Small-Diameter Vascular Regeneration: Target Regulation of VECs and VSMCs by MiRNAs. J. Biomed. Mater. Res. A 2019, 107, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, S.K.; Shamloo, A. Bilayered Heparinized Vascular Graft Fabricated by Combining Electrospinning and Freeze Drying Methods. Mater. Sci. Eng. C 2019, 94, 1067–1076. [Google Scholar] [CrossRef]
- Hu, J.; Prabhakaran, M.P.; Tian, L.; Ding, X.; Ramakrishna, S. Drug-Loaded Emulsion Electrospun Nanofibers: Characterization, Drug Release and in Vitro Biocompatibility. RSC Adv. 2015, 5, 100256–100267. [Google Scholar] [CrossRef]
- Dong, L.; Li, L.; Song, Y.; Fang, Y.; Liu, J.; Chen, P.; Wang, S.; Wang, C.; Xia, T.; Liu, W.; et al. MSC-Derived Immunomodulatory Extracellular Matrix Functionalized Electrospun Fibers for Mitigating Foreign-Body Reaction and Tendon Adhesion. Acta Biomater. 2021, 133, 280–296. [Google Scholar] [CrossRef]
- Li, L.; Qian, Y.; Lin, C.; Li, H.; Jiang, C.; Lv, Y.; Liu, W.; Cai, K.; Germershaus, O.; Yang, L. The Effect of Silk Gland Sericin Protein Incorporation into Electrospun Polycaprolactone Nanofibers on in Vitro and in Vivo Characteristics. J. Mater. Chem. B 2015, 3, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shan, H.; Wang, J.; Wang, X.; Yang, X.; Ding, J. Laden Nanofiber Capsules for Local Malignancy Chemotherapy. J. Biomed. Nanotechnol. 2019, 15, 939–950. [Google Scholar] [CrossRef]
- Hu, J.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Emulsion Electrospinning of Polycaprolactone: Influence of Surfactant Type towards the Scaffold Properties. J. Biomater. Sci. Polym. Ed. 2015, 26, 57–75. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Qian, Y.; Li, X.; Singh, G.K.; Zhong, L.; Liu, W.; Lv, Y.; Cai, K.; Yang, L. Electrospun Poly (ε-Caprolactone)/Silk Fibroin Core-Sheath Nanofibers and Their Potential Applications in Tissue Engineering and Drug Release. Int. J. Biol. Macromol. 2011, 49, 223–232. [Google Scholar] [CrossRef]
- Antonova, L.V.; Krivkina, E.O.; Rezvova, M.A.; Sevostyanova, V.V.; Tkachenko, V.O.; Glushkova, T.V.; Akentyeva, T.N.; Kudryavtseva, Y.A.; Barbarash, L.S. A Technology for Anti-Thrombogenic Drug Coating of Small-Diameter Biodegradable Vascular Prostheses. Sovrem. Tehnol. V Med. 2020, 12, 6. [Google Scholar] [CrossRef]
- Roy, T.; Maity, P.; Rameshbabu, A.; Das, B.; John, A.; Dutta, A.; Ghorai, S.; Chattopadhyay, S.; Dhara, S. Core-Shell Nanofibrous Scaffold Based on Polycaprolactone-Silk Fibroin Emulsion Electrospinning for Tissue Engineering Applications. Bioengineering 2018, 5, 68. [Google Scholar] [CrossRef]
- Sevostianova, V.V.; Mironov, A.V.; Krivkina, E.O.; Khanova, M.V.; Velikanova, E.A.; Matveeva, V.G.; Glushkova, T.V.; Antonova, L.V.; Barbarash, L.S. Biodegradable Poly(ε-Caprolactone) VEGF-Containing Vascular Patches for Angioplasty. In Proceedings of the AIP Conference Proceedings, Tomsk, Russia, 1–5 October 2019; p. 020321. [Google Scholar]
- Liu, J.; Nie, H.; Xu, Z.; Guo, F.; Guo, S.; Yin, J.; Wang, Y.; Zhang, C. Construction of PRP-Containing Nanofibrous Scaffolds for Controlled Release and Their Application to Cartilage Regeneration. J. Mater. Chem. B 2015, 3, 581–591. [Google Scholar] [CrossRef]
- Badawi, M.A.; El-Khordagui, L.K. A Quality by Design Approach to Optimization of Emulsions for Electrospinning Using Factorial and D-Optimal Designs. Eur. J. Pharm. Sci. 2014, 58, 44–54. [Google Scholar] [CrossRef]
- Pinto, S.C.; Rodrigues, A.R.; Saraiva, J.A.; Lopes-da-Silva, J.A. Catalytic Activity of Trypsin Entrapped in Electrospun Poly(ϵ-Caprolactone) Nanofibers. Enzym. Microb. Technol. 2015, 79, 8–18. [Google Scholar] [CrossRef]
- Briggs, T.; Arinzeh, T.L. Examining the Formulation of Emulsion Electrospinning for Improving the Release of Bioactive Proteins from Electrospun Fibers: Formulation of Emulsion Electrospinning. J. Biomed. Mater. Res. A 2014, 102, 674–684. [Google Scholar] [CrossRef]
- Bauer, A.J.P.; Zeng, T.; Liu, J.; Uthaisar, C.; Li, B. The Enhanced Encapsulation Capacity of Polyhedral Oligomeric Silsesquioxane-Based Copolymers for the Fabrication of Electrospun Core/Shell Fibers. Macromol. Rapid Commun. 2014, 35, 715–720. [Google Scholar] [CrossRef]
- Li, X.; Su, Y.; Zhou, X.; Mo, X. Distribution of Sorbitan Monooleate in Poly(l-Lactide-Co-ε-Caprolactone) Nanofibers from Emulsion Electrospinning. Colloids Surf. B Biointerfaces 2009, 69, 221–224. [Google Scholar] [CrossRef]
- Wu, C.; An, Q.; Li, D.; Wang, J.; He, L.; Huang, C.; Li, Y.; Zhu, W.; Mo, X. A Novel Heparin Loaded Poly(l-Lactide-Co-Caprolactone) Covered Stent for Aneurysm Therapy. Mater. Lett. 2014, 116, 39–42. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Ding, X.; Kai, D.; Ramakrishna, S. Emulsion Electrospun Nanofibers as Substrates for Cardiomyogenic Differentiation of Mesenchymal Stem Cells. J. Mater. Sci. Mater. Med. 2013, 24, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, Y.; He, C.; Wang, H.; Fong, H.; Mo, X. Sorbitan Monooleate and Poly(L-Lactide-Co-ε-Caprolactone) Electrospun Nanofibers for Endothelial Cell Interactions. J. Biomed. Mater. Res. A 2009, 91, 878–885. [Google Scholar] [CrossRef]
- Yan, S.; Xiaoqiang, L.; Shuiping, L.; Xiumei, M.; Ramakrishna, S. Controlled Release of Dual Drugs from Emulsion Electrospun Nanofibrous Mats. Colloids Surf. B Biointerfaces 2009, 73, 376–381. [Google Scholar] [CrossRef]
- Samanta, A.; Nandan, B.; Srivastava, R.K. Morphology of Electrospun Fibers Derived from High Internal Phase Emulsions. J. Colloid Interface Sci. 2016, 471, 29–36. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Facile Fabrication of Composite Electrospun Nanofibrous Matrices of Poly(ε-Caprolactone)–Silica Based Pickering Emulsion. Langmuir 2017, 33, 8062–8069. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Nano-Silver Stabilized Pickering Emulsions and Their Antimicrobial Electrospun Fibrous Matrices. Biomed. Phys. Eng. Express 2017, 3, 035011. [Google Scholar] [CrossRef]
- Samanta, A.; Takkar, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Hydroxyapatite Stabilized Pickering Emulsions of Poly(ε-Caprolactone) and Their Composite Electrospun Scaffolds. Colloids Surf. Physicochem. Eng. Asp. 2017, 533, 224–230. [Google Scholar] [CrossRef]
- Pal, J.; Singh, S.; Sharma, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Emulsion Electrospun Composite Matrices of Poly(ε-Caprolactone)-Hydroxyapatite: Strategy for Hydroxyapatite Confinement and Retention on Fiber Surface. Mater. Lett. 2016, 167, 288–296. [Google Scholar] [CrossRef]
- Pal, J.; Sharma, S.; Sanwaria, S.; Kulshreshtha, R.; Nandan, B.; Srivastava, R.K. Conducive 3D Porous Mesh of Poly(ε-Caprolactone) Made via Emulsion Electrospinning. Polymer 2014, 55, 3970–3979. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Haider, S.; Al-Zeghayer, Y.; Ahmed Ali, F.A.; Haider, A.; Mahmood, A.; Al-Masry, W.A.; Imran, M.; Aijaz, M.O. Highly Aligned Narrow Diameter Chitosan Electrospun Nanofibers. J. Polym. Res. 2013, 20, 105. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M.K. A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Hong, Y.; Zhao, Y.; Qiu, S.; Wang, C.; Wei, Y. Influence of Solvents on the Formation of Ultrathin Uniform Poly(Vinyl Pyrrolidone) Nanofibers with Electrospinning. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 3721–3726. [Google Scholar] [CrossRef]
- Daelemans, L.; Steyaert, I.; Schoolaert, E.; Goudenhooft, C.; Rahier, H.; De Clerck, K. Nanostructured Hydrogels by Blend Electrospinning of Polycaprolactone/Gelatin Nanofibers. Nanomaterials 2018, 8, 551. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Pinto, J.; Nanni, G.; Fragouli, D.; Athanassiou, A. Enhanced Oil Removal from Water in Oil Stable Emulsions Using Electrospun Nanocomposite Fiber Mats. RSC Adv. 2018, 8, 7641–7650. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural Emulsifiers—Biosurfactants, Phospholipids, Biopolymers, and Colloidal Particles: Molecular and Physicochemical Basis of Functional Performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Calvo, E.; de Malmazet, E.; Risso, F.; Masbernat, O. Coalescence of Water Drops at an Oil–Water Interface Loaded with Microparticles and Surfactants. Ind. Eng. Chem. Res. 2019, 58, 15573–15587. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Shen, M.; Cao, X.; Zhang, L.; Xu, J.; Yu, J.; Shi, X. Electrospun Poly(Lactic-Co-Glycolic Acid)/Halloysite Nanotube Composite Nanofibers for Drug Encapsulation and Sustained Release. J. Mater. Chem. 2010, 20, 10622. [Google Scholar] [CrossRef]
- Dickinson, E. Use of Nanoparticles and Microparticles in the Formation and Stabilization of Food Emulsions. Trends Food Sci. Technol. 2012, 24, 4–12. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Lips, A. Comparison of Solid Particles, Globular Proteins and Surfactants as Emulsifiers. Phys. Chem. Chem. Phys. 2008, 10, 1608. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Schmitt, V. Solid-Stabilized Emulsions. Curr. Opin. Colloid Interface Sci. 2008, 13, 217–227. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Li, X.; Hu, X.; Zhou, W.; Dong, X.; Wang, C.; Yang, Z.; Binks, B.P. Facile Preparation of Bioactive Nanoparticle/Poly(ε-Caprolactone) Hierarchical Porous Scaffolds via 3D Printing of High Internal Phase Pickering Emulsions. J. Colloid Interface Sci. 2019, 545, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.Z.; Zhang, H.D.; Li, M.M.; Duvail, J.L.; Jiang, X.Y.; Yin, H.L. Advances in Three-Dimensional Nanofibrous Macrostructures via Electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Pal, S.; Srivastava, R.K.; Nandan, B. Crystallization and Polymorphic Behaviour of Melt Miscible Blends of Crystalline Homopolymers with Close Melting Temperatures under Confinement. Polymer 2022, 256, 125249. [Google Scholar] [CrossRef]
- Pal, S.; Srivastava, R.K.; Nandan, B. Effect of Spinning Solvent on Crystallization Behavior of Confined Polymers in Electrospun Nanofibers. Polym. Cryst. 2021, 4, e10209. [Google Scholar] [CrossRef]
- Ma, L.; Shi, X.; Zhang, X.; Li, L. Electrospinning of Polycaprolacton/Chitosan Core-Shell Nanofibers by a Stable Emulsion System. Colloids Surf. Physicochem. Eng. Asp. 2019, 583, 123956. [Google Scholar] [CrossRef]
- Radisavljevic, A.; Stojanovic, D.B.; Perisic, S.; Djokic, V.; Radojevic, V.; Rajilic-Stojanovic, M.; Uskokovic, P.S. Cefazolin-Loaded Polycaprolactone Fibers Produced via Different Electrospinning Methods: Characterization, Drug Release and Antibacterial Effect. Eur. J. Pharm. Sci. 2018, 124, 26–36. [Google Scholar] [CrossRef]
- Kriegel, C.; Kit, K.M.; McClements, D.J.; Weiss, J. Electrospinning of Chitosan–Poly(Ethylene Oxide) Blend Nanofibers in the Presence of Micellar Surfactant Solutions. Polymer 2009, 50, 189–200. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Pezeshki-Modaress, M.; Zandi, M. Biphasic, Tough Composite Core/Shell PCL/PVA-GEL Nanofibers for Biomedical Application. J. Appl. Polym. Sci. 2020, 137, 48713. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.J.; Stride, E.; Edirisinghe, M. Mapping the Influence of Solubility and Dielectric Constant on Electrospinning Polycaprolactone Solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, Y.; Pan, J.; Liu, Y. Macro-Alignment of Electrospun Fibers for Vascular Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 92B, 508–516. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jia, Z.; Liu, J.; Li, Q.; Hou, L.; Wang, L.; Guan, Z. Effect of Electric Field Distribution Uniformity on Electrospinning. J. Appl. Phys. 2008, 103, 104307. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M. Cone-Jet Analytical Extension of Taylor’s Electrostatic Solution and the Asymptotic Universal Scaling Laws in Electrospraying. Phys. Rev. Lett. 1997, 79, 217–220. [Google Scholar] [CrossRef]
- He, J.-H.; Wu, Y.; Zuo, W.-W. Critical Length of Straight Jet in Electrospinning. Polymer 2005, 46, 12637–12640. [Google Scholar] [CrossRef]
- Hohman, M.M.; Shin, M.; Rutledge, G.; Brenner, M.P. Electrospinning and Electrically Forced Jets. II. Applications. Phys. Fluids 2001, 13, 2221–2236. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending Instability of Electrically Charged Liquid Jets of Polymer Solutions in Electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Experimental Characterization of Electrospinning: The Electrically Forced Jet and Instabilities. Polymer 2001, 42, 09955–09967. [Google Scholar] [CrossRef]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Electrospinning: A Whipping Fluid Jet Generates Submicron Polymer Fibers. Appl. Phys. Lett. 2001, 78, 1149–1151. [Google Scholar] [CrossRef]
- Yarin, A.L.; Koombhongse, S.; Reneker, D.H. Bending Instability in Electrospinning of Nanofibers. J. Appl. Phys. 2001, 89, 3018–3026. [Google Scholar] [CrossRef]
- Bhattarai, R.; Bachu, R.; Boddu, S.; Bhaduri, S. Biomedical Applications of Electrospun Nanofibers: Drug and Nanoparticle Delivery. Pharmaceutics 2018, 11, 5. [Google Scholar] [CrossRef]
- Peijs, T. 6.7 Electrospun Polymer Nanofibers and Their Composites. In Comprehensive Composite Materials II; Elsevier: Amsterdam, The Netherlands, 2018; pp. 162–200. ISBN 978-0-08-100534-7. [Google Scholar]
- Ajalloueian, F.; Tavanai, H.; Hilborn, J.; Donzel-Gargand, O.; Leifer, K.; Wickham, A.; Arpanaei, A. Emulsion Electrospinning as an Approach to Fabricate PLGA/Chitosan Nanofibers for Biomedical Applications. BioMed Res. Int. 2014, 2014, 475280. [Google Scholar] [CrossRef]
- Can-Herrera, L.A.; Oliva, A.I.; Dzul-Cervantes, M.A.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers 2021, 13, 662. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Lee, B.-T. Fabrication and Characterization of Cross-Linked Gelatin Electro-Spun Nano-Fibers. J. Biomed. Sci. Eng. 2010, 03, 1117–1124. [Google Scholar] [CrossRef]
- Wei, X.; Xia, Z.; Wong, S.C.; Baji, A. Modelling of Mechanical Properties of Electrospun Nanofibre Network. Int. J. Exp. Comput. Biomech. 2009, 1, 45. [Google Scholar] [CrossRef]
- You, Y.; Won Lee, S.; Jin Lee, S.; Park, W.H. Thermal Interfiber Bonding of Electrospun Poly(l-Lactic Acid) Nanofibers. Mater. Lett. 2006, 60, 1331–1333. [Google Scholar] [CrossRef]
- Badami, A.S.; Kreke, M.R.; Thompson, M.S.; Riffle, J.S.; Goldstein, A.S. Effect of Fiber Diameter on Spreading, Proliferation, and Differentiation of Osteoblastic Cells on Electrospun Poly(Lactic Acid) Substrates. Biomaterials 2006, 27, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(ε-Caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous Electrospun Polycaprolactone Fibers: Effect of Process Parameters. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1878–1888. [Google Scholar] [CrossRef]
- Zhang, S.; Shim, W.S.; Kim, J. Design of Ultra-Fine Nonwovens via Electrospinning of Nylon 6: Spinning Parameters and Filtration Efficiency. Mater. Des. 2009, 30, 3659–3666. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Doustgani, A. Effect of Electrospinning Process Parameters of Polycaprolactone and Nanohydroxyapatite Nanocomposite Nanofibers. Text. Res. J. 2015, 85, 1445–1454. [Google Scholar] [CrossRef]
- Putti, M.; Simonet, M.; Solberg, R.; Peters, G.W.M. Electrospinning Poly(ε-Caprolactone) under Controlled Environmental Conditions: Influence on Fiber Morphology and Orientation. Polymer 2015, 63, 189–195. [Google Scholar] [CrossRef]
- Ramazani, S.; Karimi, M. Investigating the Influence of Temperature on Electrospinning of Polycaprolactone Solutions. e-Polymers 2014, 14, 323–333. [Google Scholar] [CrossRef]
- Nangrejo, M.; Bragman, F.; Ahmad, Z.; Stride, E.; Edirisinghe, M. Hot Electrospinning of Polyurethane Fibres. Mater. Lett. 2012, 68, 482–485. [Google Scholar] [CrossRef]
- Wang, C.; Chien, H.-S.; Yan, K.-W.; Hung, C.-L.; Hung, K.-L.; Tsai, S.-J.; Jhang, H.-J. Correlation between Processing Parameters and Microstructure of Electrospun Poly(D,l-Lactic Acid) Nanofibers. Polymer 2009, 50, 6100–6110. [Google Scholar] [CrossRef]
- Wang, C.; Chien, H.-S.; Hsu, C.-H.; Wang, Y.-C.; Wang, C.-T.; Lu, H.-A. Electrospinning of Polyacrylonitrile Solutions at Elevated Temperatures. Macromolecules 2007, 40, 7973–7983. [Google Scholar] [CrossRef]
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable Electrospun Fibers for Drug Delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Milosevic, M.; Stojanovic, D.B.; Simic, V.; Grkovic, M.; Bjelovic, M.; Uskokovic, P.S.; Kojic, M. Preparation and Modeling of Three-layered PCL/PLGA/PCL Fibrous Scaffolds for Prolonged Drug Release. Sci. Rep. 2020, 10, 11126. [Google Scholar] [CrossRef] [PubMed]
- Petlin, D.G.; Amarah, A.A.; Tverdokhlebov, S.I.; Anissimov, Y.G. A Fiber Distribution Model for Predicting Drug Release Rates. J. Control. Release 2017, 258, 218–225. [Google Scholar] [CrossRef]
- Mouro, C.; Simões, M.; Gouveia, I.C. Emulsion Electrospun Fiber Mats of PCL/PVA/Chitosan and Eugenol for Wound Dressing Applications. Adv. Polym. Technol. 2019, 2019, 9859506. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Shao, P.; Hao, S.; Wang, W.; Yang, Q.; Wang, B. A Novel Multiple Drug Release System in Vitro Based on Adjusting Swelling Core of Emulsion Electrospun Nanofibers with Core–Sheath Structure. Mater. Sci. Eng. C 2014, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Buzgo, M.; Filova, E.; Staffa, A.M.; Rampichova, M.; Doupnik, M.; Vocetkova, K.; Lukasova, V.; Kolcun, R.; Lukas, D.; Necas, A.; et al. Needleless Emulsion Electrospinning for the Regulated Delivery of Susceptible Proteins. J. Tissue Eng. Regen. Med. 2018, 12, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Wobma, H.; Vunjak-Novakovic, G. Tissue Engineering and Regenerative Medicine 2015: A Year in Review. Tissue Eng. Part B Rev. 2016, 22, 101–113. [Google Scholar] [CrossRef]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and Fabrication of Porous Biodegradable Scaffolds: A Strategy for Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, Z.; Zheng, L.; Song, R.; Zhao, Y. Fabrication and Characterization of Electrospun Polycaprolactone Blended with Chitosan-Gelatin Complex Nanofibrous Mats. J. Nanomater. 2014, 2014, 964621. [Google Scholar] [CrossRef]
- Sobreiro-Almeida, R.; Fonseca, D.R.; Neves, N.M. Extracellular Matrix Electrospun Membranes for Mimicking Natural Renal Filtration Barriers. Mater. Sci. Eng. C 2019, 103, 109866. [Google Scholar] [CrossRef] [PubMed]
- Unal, S.; Arslan, S.; Yilmaz, B.K.; Oktar, F.N.; Ficai, D.; Ficai, A.; Gunduz, O. Polycaprolactone/Gelatin/Hyaluronic Acid Electrospun Scaffolds to Mimic Glioblastoma Extracellular Matrix. Materials 2020, 13, 2661. [Google Scholar] [CrossRef] [PubMed]
- Watcharajittanont, N.; Putson, C.; Pripatnanont, P.; Meesane, J. Electrospun Polyurethane Fibrous Membranes of Mimicked Extracellular Matrix for Periodontal Ligament: Molecular Behavior, Mechanical Properties, Morphology, and Osseointegration. J. Biomater. Appl. 2020, 34, 753–762. [Google Scholar] [CrossRef]
- Han, S.; Nie, K.; Li, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. 3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications. Stem Cells Int. 2021, 2021, 8790143. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-Based Systems for Fabrication of Electrospun Nanofibers: Food, Pharmaceutical and Biomedical Applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Sowjanya, J.A.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite Scaffolds Containing Chitosan/Alginate/Nano-Silica for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2013, 109, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Vuong, J.; Hellmich, C. Bone Fibrillogenesis and Mineralization: Quantitative Analysis and Implications for Tissue Elasticity. J. Theor. Biol. 2011, 287, 115–130. [Google Scholar] [CrossRef]
- Shao, S.; Zhou, S.; Li, L.; Li, J.; Luo, C.; Wang, J.; Li, X.; Weng, J. Osteoblast Function on Electrically Conductive Electrospun PLA/MWCNTs Nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef]
- Buser, D.; Hoffmann, B.; Bernard, J.; Lussi, A.; Mettler, D.; Schenk, R.K. Evaluation of Filling Materials in Membrane-Protected Bone Defects. A Comparative Histomorphometric Study in the Mandible of Miniature Pigs.: Filling Materials in Membrane-Protected Bone Defects. Clin. Oral Implant. Res. 1998, 9, 137–150. [Google Scholar] [CrossRef]
- Saghiri, M.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The Role of Angiogenesis in Implant Dentistry Part II: The Effect of Bone-Grafting and Barrier Membrane Materials on Angiogenesis. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e526. [Google Scholar] [CrossRef]
- Dalgic, A.D.; Atila, D.; Karatas, A.; Tezcaner, A.; Keskin, D. Diatom Shell Incorporated PHBV/PCL-Pullulan Co-Electrospun Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 100, 735–746. [Google Scholar] [CrossRef]
- Lin, W.; Chen, M.; Qu, T.; Li, J.; Man, Y. Three-dimensional Electrospun Nanofibrous Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1311–1321. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and Gelatin-Based Electrospun Fibers for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Toloue, E.B.; Karbasi, S.; Salehi, H.; Rafienia, M. Potential of an Electrospun Composite Scaffold of Poly (3-Hydroxybutyrate)-Chitosan/Alumina Nanowires in Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 99, 1075–1091. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration—A Materials Perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-W.; Kim, S.-G. Membranes for the Guided Bone Regeneration. Maxillofac. Plast. Reconstr. Surg. 2014, 36, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of Guided Bone Regeneration: A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Abdollahi Boraei, S.B.; Nourmohammadi, J.; Bakhshandeh, B.; Dehghan, M.M.; Gholami, H.; Gonzalez, Z.; Sanchez-Herencia, A.J.; Ferrari, B. Capability of Core-Sheath Polyvinyl Alcohol–Polycaprolactone Emulsion Electrospun Nanofibrous Scaffolds in Releasing Strontium Ranelate for Bone Regeneration. Biomed. Mater. 2021, 16, 025009. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Rahmati, M.; Blaker, J.J.; Lyngstadaas, S.P.; Mano, J.F.; Haugen, H.J. Designing Multigradient Biomaterials for Skin Regeneration. Mater. Today Adv. 2020, 5, 100051. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M. Cultured Epithelial Autograft (CEA) in Burn Treatment: Three Decades Later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef]
- Clark, R.A.F.; Ghosh, K.; Tonnesen, M.G. Tissue Engineering for Cutaneous Wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S. Progress and Opportunities for Tissue-Engineered Skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Paggiaro, A.O.; Bastianelli, R.; Carvalho, V.F.; Isaac, C.; Gemperli, R. Is Allograft Skin, the Gold-Standard for Burn Skin Substitute? A Systematic Literature Review and Meta-Analysis. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and Synthetic Polymers for Wounds and Burns Dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qian, Y.; Jiang, C.; Lv, Y.; Liu, W.; Zhong, L.; Cai, K.; Li, S.; Yang, L. The Use of Hyaluronan to Regulate Protein Adsorption and Cell Infiltration in Nanofibrous Scaffolds. Biomaterials 2012, 33, 3428–3445. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, Y.; Li, L.; Pan, L.; Njunge, L.W.; Dong, L.; Yang, L. Evaluation of Emulsion Electrospun Polycaprolactone/Hyaluronan/Epidermal Growth Factor Nanofibrous Scaffolds for Wound Healing. J. Biomater. Appl. 2016, 30, 686–698. [Google Scholar] [CrossRef]
- Basar, A.O.; Castro, S.; Torres-Giner, S.; Lagaron, J.M.; Turkoglu Sasmazel, H. Novel Poly(ε-Caprolactone)/Gelatin Wound Dressings Prepared by Emulsion Electrospinning with Controlled Release Capacity of Ketoprofen Anti-Inflammatory Drug. Mater. Sci. Eng. C 2017, 81, 459–468. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Loh, X.J. Controlling Cell Adhesion Using Layer-by-Layer Approaches for Biomedical Applications. Mater. Sci. Eng. C 2017, 70, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Surucu, S.; Turkoglu Sasmazel, H. Development of Core-Shell Coaxially Electrospun Composite PCL/Chitosan Scaffolds. Int. J. Biol. Macromol. 2016, 92, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wen, M.; Zhou, P.; Zhao, Y.; Jia, X.; Fan, Y.; Yuan, X. Electrospun Membranes of PELCL/PCL-REDV Loading with MiRNA-126 for Enhancement of Vascular Endothelial Cell Adhesion and Proliferation. Mater. Sci. Eng. C 2018, 85, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jia, X.; Yang, Y.; Yang, Q.; Gao, C.; Hu, S.; Zhao, Y.; Fan, Y.; Yuan, X. Nanofiber-Mediated MicroRNA-126 Delivery to Vascular Endothelial Cells for Blood Vessel Regeneration. Acta Biomater. 2016, 43, 303–313. [Google Scholar] [CrossRef]
- Chalco-Sandoval, W.; Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Development of an Encapsulated Phase Change Material via Emulsion and Coaxial Electrospinning. J. Appl. Polym. Sci. 2016, 133, 43903. [Google Scholar] [CrossRef]
- Paroutoglou, E.; Fojan, P.; Gurevich, L.; Hultmark, G.; Afshari, A. Thermal Analysis of Organic and Nanoencapsulated Electrospun Phase Change Materials. Energies 2021, 14, 995. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).