An In Situ Forming Bleomycin-Polidocanol Composite Foam for Optimizing Sclerotherapy of High-Risk Airway Venous Malformations
Abstract
1. Introduction
- The “Empty Vein” Effect: The foam completely displaces blood from the malformation’s lumen, which prevents dilution of the sclerosant [21].
- Maximized Endothelial Contact: The large surface area of the foam maximizes the contact between the sclerosing agent and the vascular endothelium [11].
- Prolonged Dwell Time: The foam is retained within the vascular channels four to six times longer than its liquid counterpart, prolonging the therapeutic effect [6].
- Mechanical Displacement: By physically replacing the blood volume, the foam ensures direct and uniform contact between the sclerosant and the vessel wall [8].
- Reduced Systemic Absorption: The lower liquid volume required for foam reduces the risk of systemic toxicity, a critical advantage when using agents like bleomycin [11].
2. Materials and Methods
2.1. Research Design
2.2. Inclusion Criteria
- A diagnosis of a venous malformation confirmed by radiological imaging (magnetic resonance imaging (MRI), CT, or ultrasound) and/or direct endoscopic visualization.
- Location of the venous malformation (VM) within the airway, including the soft palate, tongue, oropharynx, or subglottic space.
- The presence of clinical symptoms attributable to the VM, such as respiratory distress, nocturnal apnea, dysphagia, or dysphonia.
- Age between 4 and 67 years.
- Provision of signed informed consent by the patient or their legal guardian.
2.3. Patient Characteristics
2.4. Treatment Protocol
- Bleomycin (Bleocin®) at a final concentration of 1.5 to 3 mg/mL
- 3% Polidocanol (Aethoxysklerol®)
- Room air
2.5. Efficacy and Safety Assessment
- Complete response: 100% reduction in lesion volume.
- Significant reduction: volume reduction of more than 50% but less than 100%.
- Limited reduction: volume reduction of less than 50%.
2.6. Statistical Analysis
3. Results
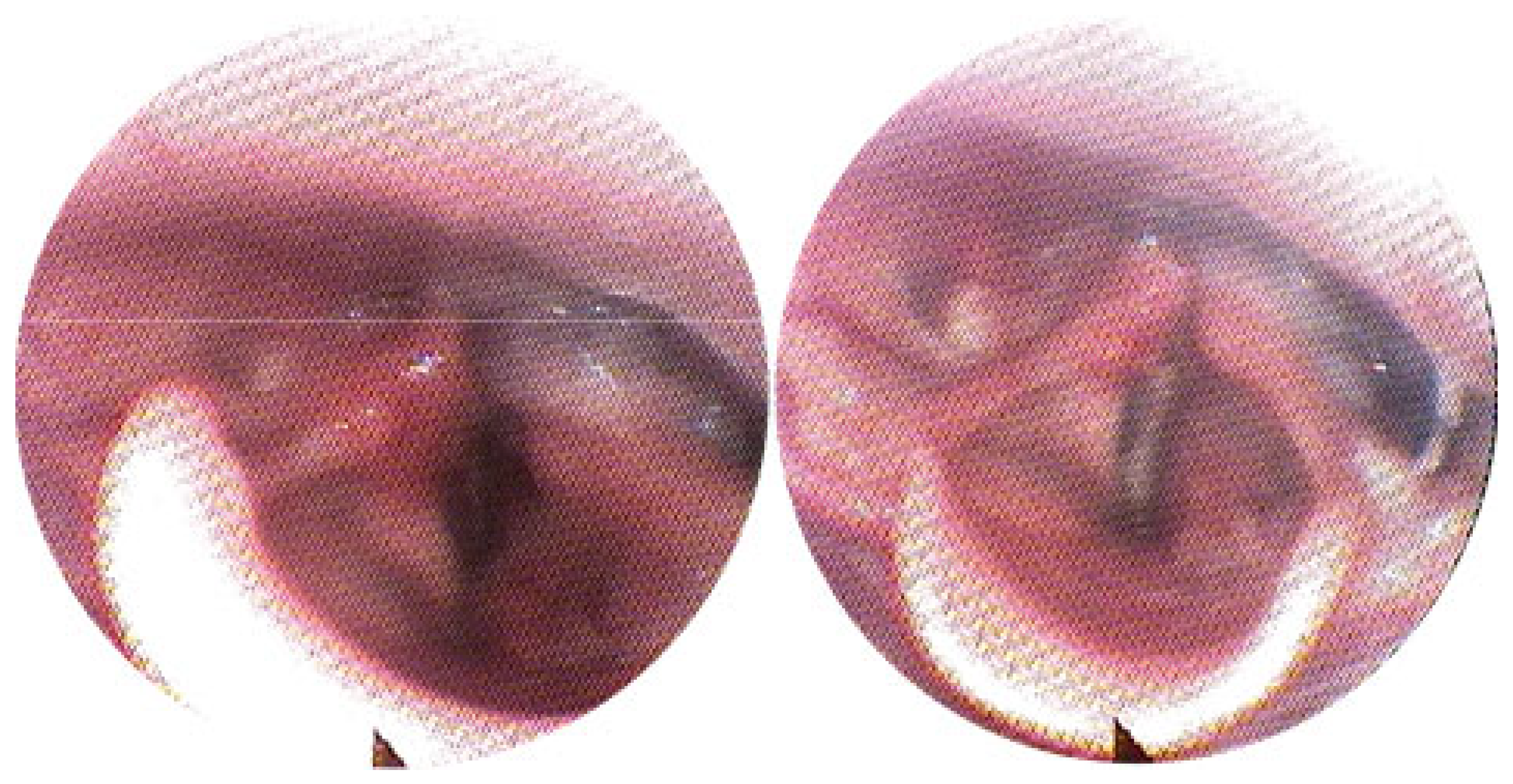
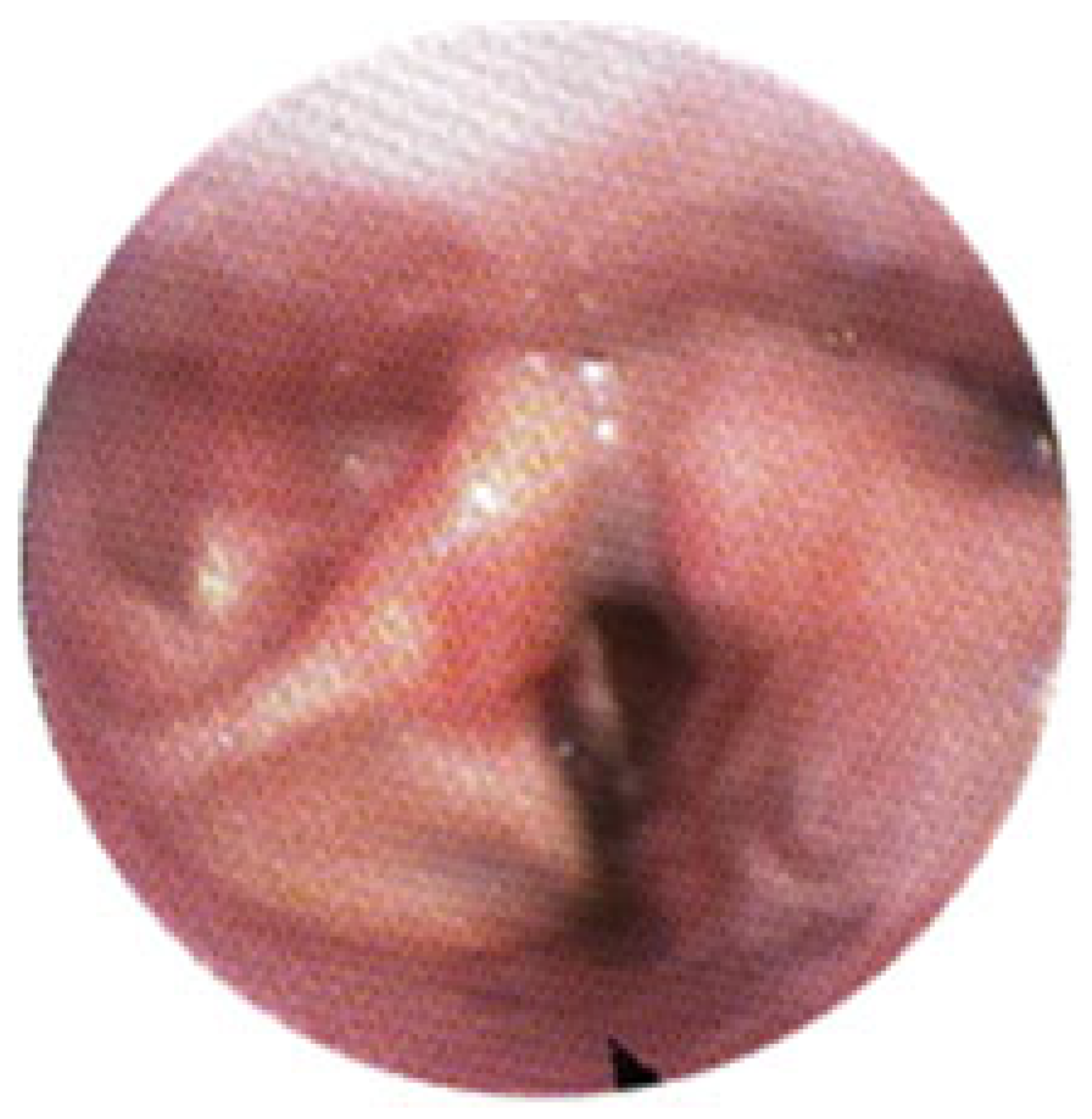
3.1. Magnetic Resonance Imaging (MRI) Findings
3.2. Clinical Outcomes
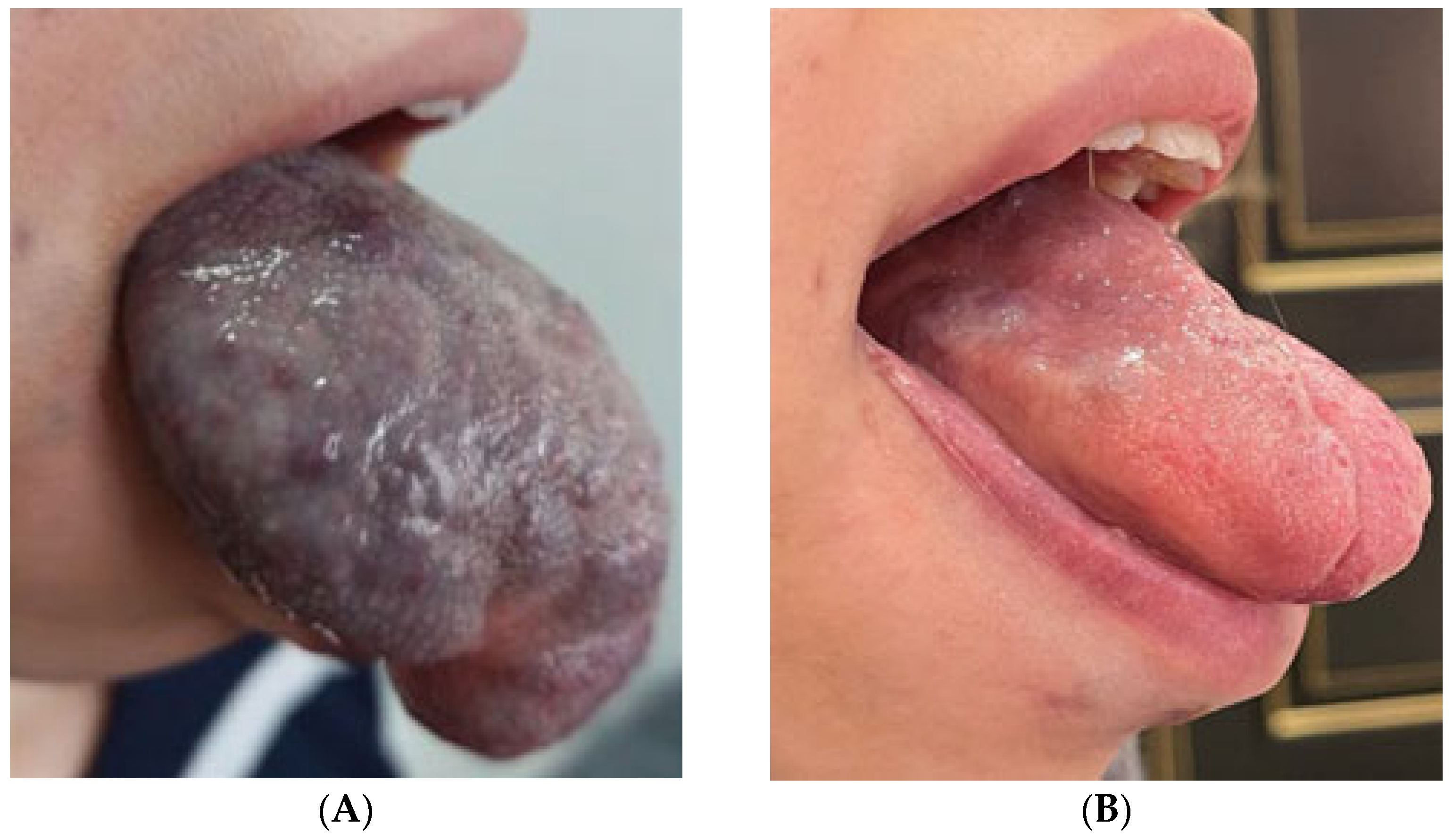
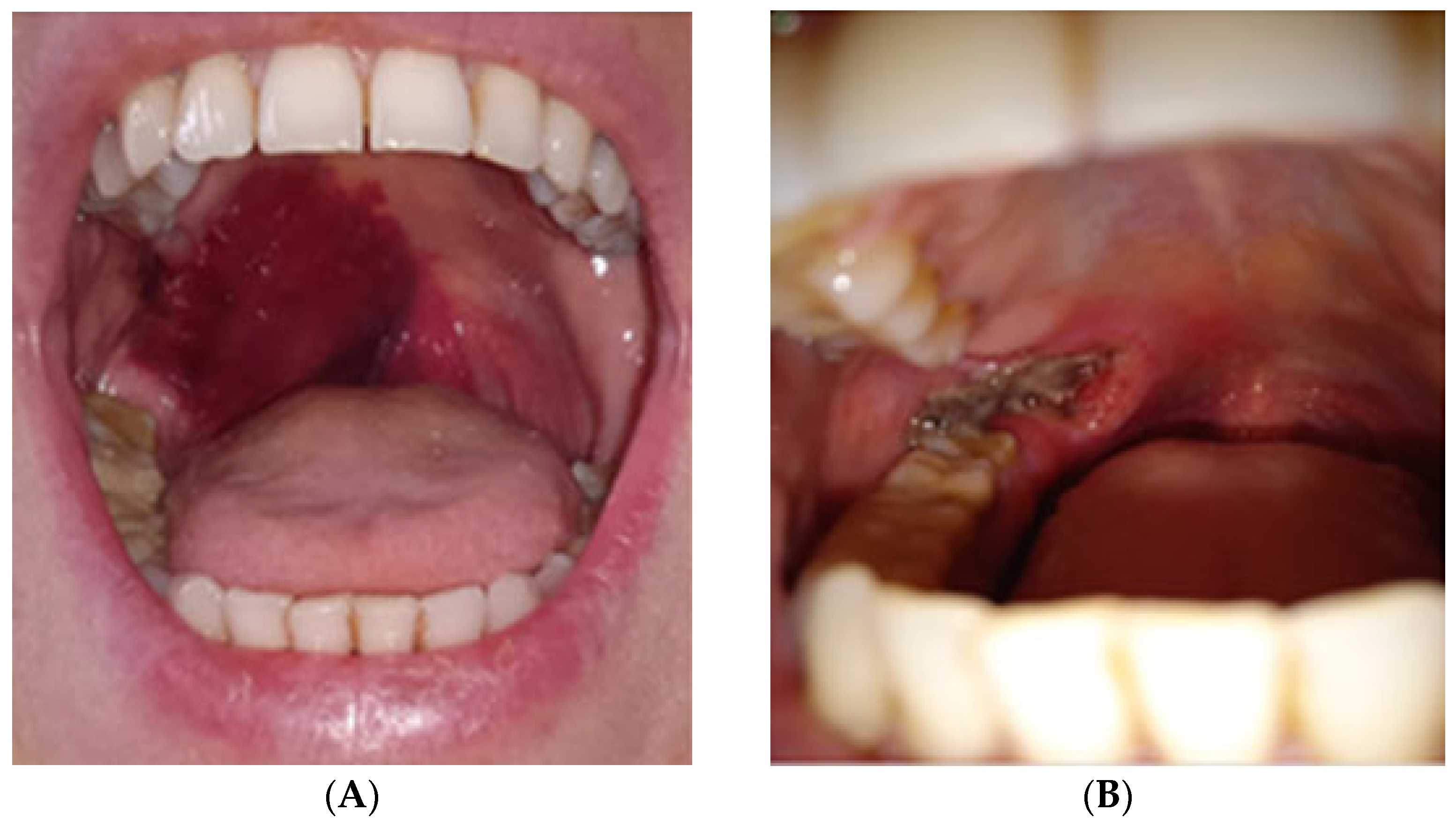
3.3. Complications
- In two patients, the necrosis was subclinical, asymptomatic, and resolved spontaneously without intervention.
- The third case involved a 47-year-old female who developed localized mucosal necrosis secondary to a hematoma, which was associated with significant tenderness. The patient managed the condition with local wound care, and the lesion healed completely within 11 days without the need for systemic therapy (Figure 10).
4. Discussion
- Complete response (based on both clinical and MRI criteria) was achieved in 8 of 14 patients (57.1%).
- ○
- Of these, 6 patients (42.9% of the total cohort) achieved a complete response after a single sclerotherapy session—two with tongue base venous malformations (VMs) and two with soft palate VMs.
- A partial response, characterized by significant lesion regression, was observed in the remaining 4 patients (28.6%).
- ○
- Significant reduction, with >50% volume reduction, in 4 patients (28.6%).
- ○
- Limited reduction, with <50% volume reduction, in 2 patients (14.3%).
- No patients failed to respond to treatment, resulting in a 0% non-response rate.
- It is a minimally invasive procedure that can often be performed under local anesthesia.
- The treatment is scarless, resulting in superior cosmetic outcomes.
- The foam effectively fills complex, multilocular cavities, ensuring uniform distribution of the sclerosant.
- The injection and distribution of the foam can be monitored in real-time under image guidance (ultrasound, endoscopy, or fluoroscopy).
- Venospasm: The injection of foam triggers a reflex contraction of the vein’s smooth muscle layer, promoting luminal collapse, reducing blood outflow, and extending the sclerosant’s exposure time [24].
- Thrombogenic Effect: The close apposition of foam to the endothelium accelerates both the formation and its subsequent organization into an occlusive thrombus [10].
- Prolonged Retention: Due to its high viscosity, microfoam (bubbles < 250 μm) remains at the injection site longer than liquid, ensuring sustained contact [25].
- Uniform Endothelial Destruction: The finely dispersed nature of the foam results in more uniform and profound endothelial damage at the molecular level compared to liquid sclerosants [26].
- Detergent Action of Polidocanol: As a detergent, polidocanol disrupts the cell membranes of the endothelium, initiating the localized inflammatory response necessary for fibrosis [27].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVM | Airway Venous Malformation |
| CR | Complete Response |
| CT | Computed Tomography |
| IRB | Institutional Review Board |
| MRI | Magnetic Resonance Imaging |
| NR | No Response |
| STIR | Short-Tau Inversion Recovery |
| T1WI | T1-Weighted Image |
| T2WI | T2-Weighted Image |
| VM | Venous Malformation |
References
- Garbuzov, R.V.; Polyaev, Y.A.; Stepanov, A.E.; Mylnikov, A.A. Abernathy malformations in children. Experience in endovascular and surgical treatment. Russ. J. Pediatr. Surg. 2020, 24, 71–77. [Google Scholar] [CrossRef]
- Castel, P.; Carmona, F.J.; Grego-Bessa, J.; Berger, M.F.; Viale, A.; Anderson, K.V.; Bague, S.; Scaltriti, M.; Antonescu, C.R.; Baselga, E.; et al. Somatic PIK3CA mutations as a driver of sporadic venous malformations. Sci. Transl. Med. 2016, 8, 332ra42. [Google Scholar] [CrossRef]
- Uebelhoer, M.; Nätynki, M.; Kangas, J.; Mendola, A.; Nguyen, H.L.; Soblet, J.; Godfraind, C.; Boon, L.M.; Eklund, L.; Limaye, N.; et al. Venous malformation-causative TIE2 mutations mediate an AKT-dependent decrease in PDGFB. Hum. Mol. Genet. 2013, 22, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Queisser, A.; Seront, E.; Boon, L.M.; Vikkula, M. Genetic Basis and Therapies for Vascular Anomalies. Circ. Res. 2021, 129, 155–173. [Google Scholar] [CrossRef]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.C.; et al. Vascular Anomalies Classification: Recommendations From the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef]
- Azene, E.; Mitchell, S.; Radvany, M.; Agrawal, N.; Eisele, D.; Weiss, C. Foamed bleomycin sclerosis of airway venous malformations: The role of interspecialty collaboration. Laryngoscope 2016, 126, 2726–2732. [Google Scholar] [CrossRef]
- Kamijo, A.; Hatsushika, K.; Kanemaru, S.; Moriyama, M.; Kase, Y.; Masuyama, K. Five adult laryngeal venous malformation cases treated effectively with sclerotherapy. Laryngoscope 2013, 123, 2766–2769. [Google Scholar] [CrossRef]
- Oomen, K.P.; Paramasivam, S.; Waner, M.; Niimi, Y.; Fifi, J.T.; Berenstein, A.; O, T.M. Endoscopic transmucosal direct puncture sclerotherapy for management of airway vascular malformations. Laryngoscope 2016, 126, 205–211. [Google Scholar] [CrossRef]
- Gurgacz, S.; Zamora, L.; Scott, N.A. Percutaneous sclerotherapy for vascular malformations: A systematic review. Ann. Vasc. Surg. 2014, 28, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, G.; Ren, J.G.; Zhao, Y.F. Bleomycin induces endothelial mesenchymal transition through activation of mTOR pathway: A possible mechanism contributing to the sclerotherapy of venous malformations. Br. J. Pharmacol. 2013, 170, 1210–1220. [Google Scholar] [CrossRef]
- Cabrera, J.; Cabrera García-Olmedo, J.R. Nuevo método de esclerosis en las varices tronculares. Patol. Vasc. 1995, 4, 55–73. [Google Scholar]
- Tessari, L.; Cavezzi, A.; Frullini, A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol. Surg. 2001, 27, 58–60. [Google Scholar] [CrossRef]
- Cavezzi, A.; Mosti, G.; Campana, F.; Tessari, L.; Bastiani, L.; Urso, S.U. Catheter Foam Sclerotherapy of the Great Saphenous Vein, with Perisaphenous Tumescence Infiltration and Saphenous Irrigation. Eur. J. Vasc. Endovasc. Surg. 2017, 54, 629–635. [Google Scholar] [CrossRef]
- Azmoun, S.; Liu, Y.; Bian, K.; Chen, A.; Liu, S. The Effect of Pushing Rate on Foam Stability in the Tessari Method. Dermatol. Surg. 2024, 50, 542–545. [Google Scholar] [CrossRef]
- Shi, X.; Liu, Y.; Li, D.; Tursun, M.; Azmoun, S.; Liu, S. The Stability of Physician-Compounded Foam is Influenced by the Angle of Connector. Ann. Vasc. Surg. 2024, 99, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Breu, F.X.; Cavezzi, A.; Coleridge Smith, P.; Frullini, A.; Gillet, J.L.; Guex, J.J.; Hamel-Desnos, C.; Kern, P.; Partsch, B.; et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology 2014, 29, 338354. [Google Scholar] [CrossRef] [PubMed]
- Chavla, K.K.; Gladysh, G. Composite Foams. In Encyclopedia of Polymer Science and Technology, 3rd ed.; Mark, H.F., Ed.; Wiley-Interscience: Hoboken, NJ, USA, 2003; Volume 9, pp. 267–281. [Google Scholar]
- Zhang, H.; Liu, S.; Chen, A. A Novel Compound Sclerosant: Polidocanol-Bleomycin Foam. Dermatol. Surg. 2020, 46, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gu, H.; Yang, X.; Cai, R.; Shang, Y.; Hu, L.; Wang, Y.; Chen, H.; Lin, X. Bleomycin Polidocanol Foam (BPF) Stability—In Vitro Evidence for the Effectiveness of a Novel Sclerosant for Venous Malformations. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 1011–1018. [Google Scholar] [CrossRef]
- Yang, X.; Chen, H.; Gu, H.; Jin, Y.; Hu, L.; Hua, C.; Wang, Y.; Sun, Y.; Yu, W.; Lin, X. Interim results of bleomycin-polidocanol foam sclerotherapy as a highly efficient technique for venous malformations. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 1066–1073. [Google Scholar] [CrossRef]
- Frullini, A.; Cavezzi, A. Sclerosing foam in the treatment of varicose veins and telangiectases: History and analysis of safety and complications. Dermatol. Surg. 2002, 28, 11–15. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Y.; Yue, H.J.; Guo, W.B.; Chen, L.; Lyu, K.X.; Huang, D.Y.; Lei, W.B. Comparison of pingyangmycin fibrin glue composite and pingyangmycin dexamethasone composite in the treatment of pharyngolaryngeal venous malformation. Chin. J. Otorhinolaryngol. Head Neck Surg. 2023, 58, 552–557. (In Chinese) [Google Scholar] [CrossRef]
- Jia, X.; Mowatt, G.; Burr, J.M.; Cassar, K.; Cook, J.; Fraser, C. Systematic review of foam sclerotherapy for varicose veins. Br. J. Surg. 2007, 94, 925–936. [Google Scholar] [CrossRef]
- Cavezzi, A.; Parsi, K. Complications of foam sclerotherapy. Phlebology 2012, 27 (Suppl. S1), 46–51. [Google Scholar] [CrossRef] [PubMed]
- Cavezzi, A.; Frullini, A.; Ricci, S.; Tessari, L. Treatment of Varicose Veins by Foam Sclerotherapy: Two Clinical Series. Phlebology 2002, 17, 13–18. [Google Scholar] [CrossRef]
- Bai, T.; Liu, Y.; Jiang, W.; Li, Y.; Liu, J.; Yu, C.; Fan, Y. A Review of Sclerosing Foam Stability in the Treatment of Varicose Veins. Dermatol. Surg. 2020, 46, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Parsi, K. Interaction of detergent sclerosants with cell membranes. Phlebology 2015, 30, 306–315. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, W.; Du, C.; Chen, Z.; Du, J.; Zhou, M. Bleomycin induces senescence and repression of DNA repair via downregulation of Rad51. Mol. Med. 2024, 30, 54. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.; Boateng, S.T.; Uddin, M.B.; Banang-Mbeumi, S.; Yadav, R.K.; Bock, C.R.; Folahan, J.T.; Siwe-Noundou, X.; Walker, A.L.; King, J.A.; et al. The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds. Cells 2023, 12, 1671. [Google Scholar] [CrossRef]
- Huang, T.T.; Lampert, E.J.; Coots, C.; Lee, J.M. Targeting the PI3K pathway and DNA damage response as a therapeutic strategy in ovarian cancer. Cancer Treat. Rev. 2020, 86, 102021. [Google Scholar] [CrossRef]
- Sun, B.; Sun, X.; Gao, Q.; Wang, Q. Advances in Sclerosants for Low-Flow Vascular Malformations. Ann. Vasc. Surg. 2025, 122, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Fraissenon, A.; Fortin, F.; Durous, V.; Chauvel-Picard, J.; Gleizal, A.; Viremouneix, L.; Cabet, S.; Guibaud, L. Percutaneous Sclerotherapy of Large Venous Malformations Using Consecutive Polidocanol and Bleomycin Foam: MR Imaging Volumetric and Quality-of-Life Assessment. J. Vasc. Interv. Radiol. 2024, 35, 127–136.e1. [Google Scholar] [CrossRef] [PubMed]
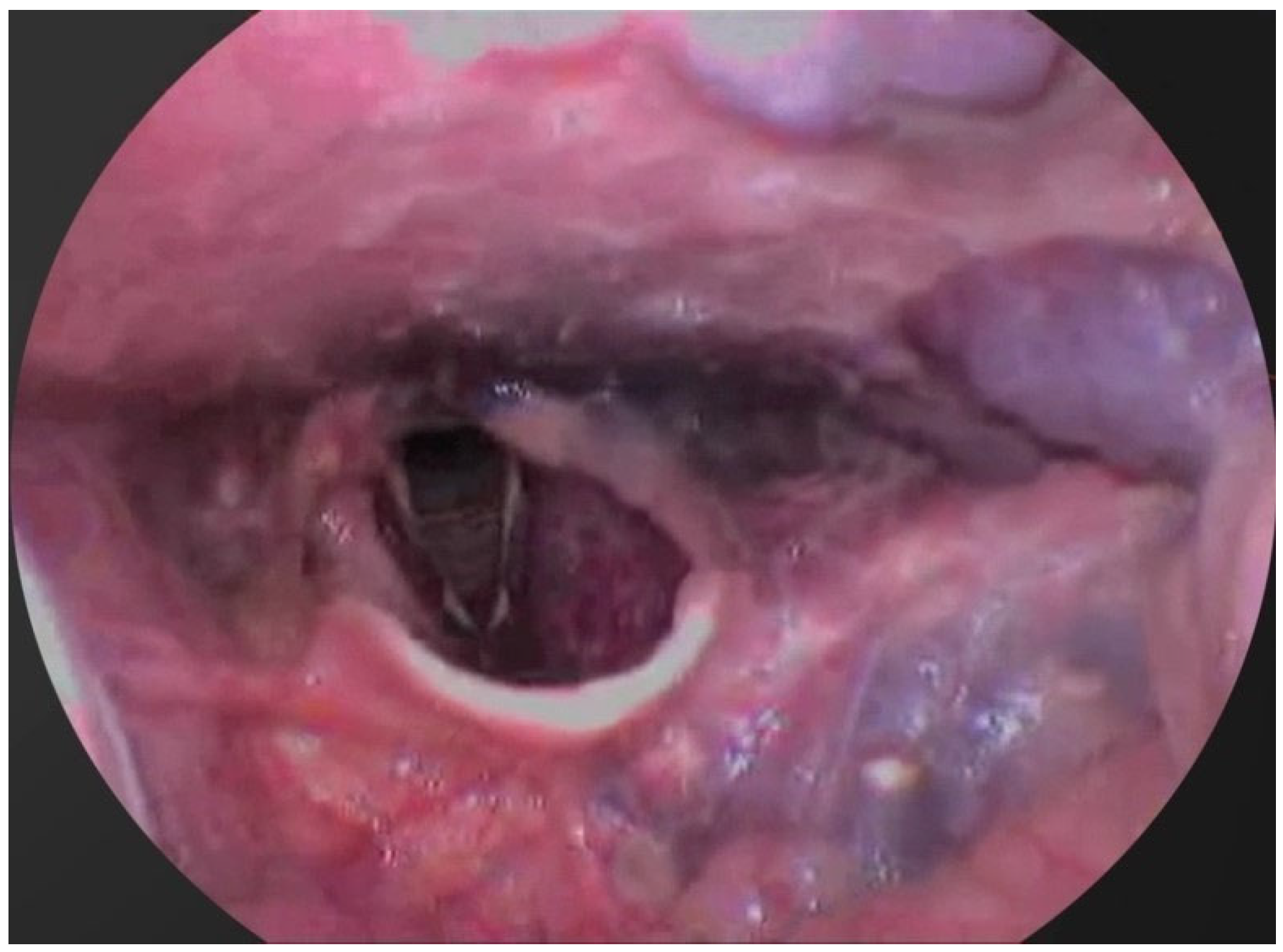

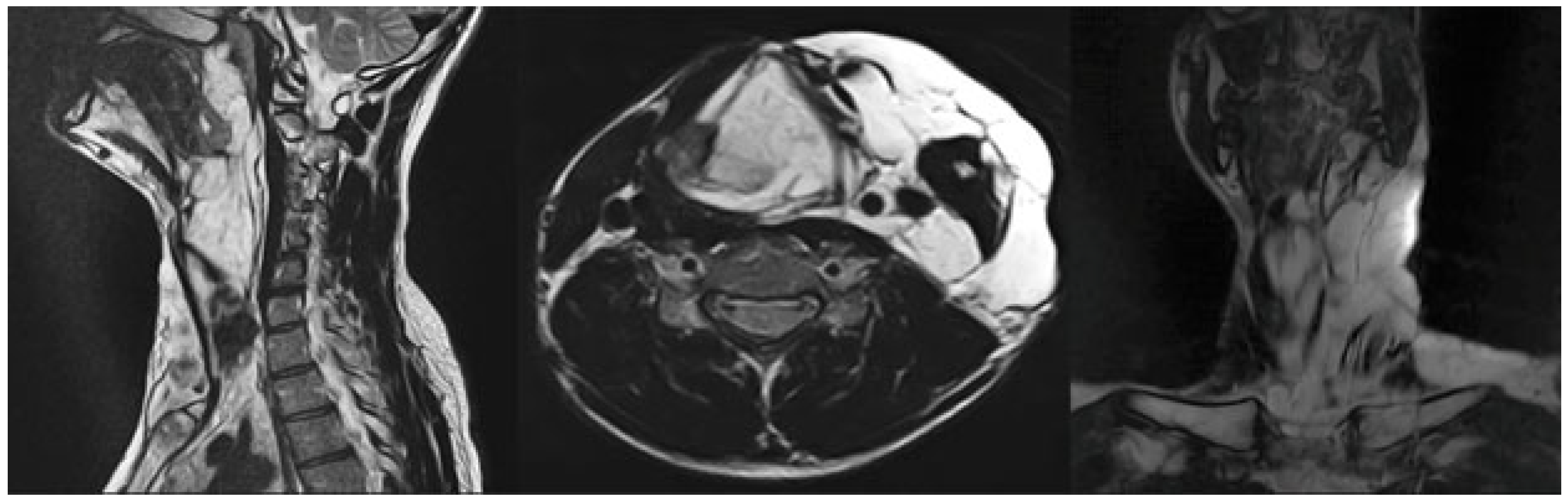
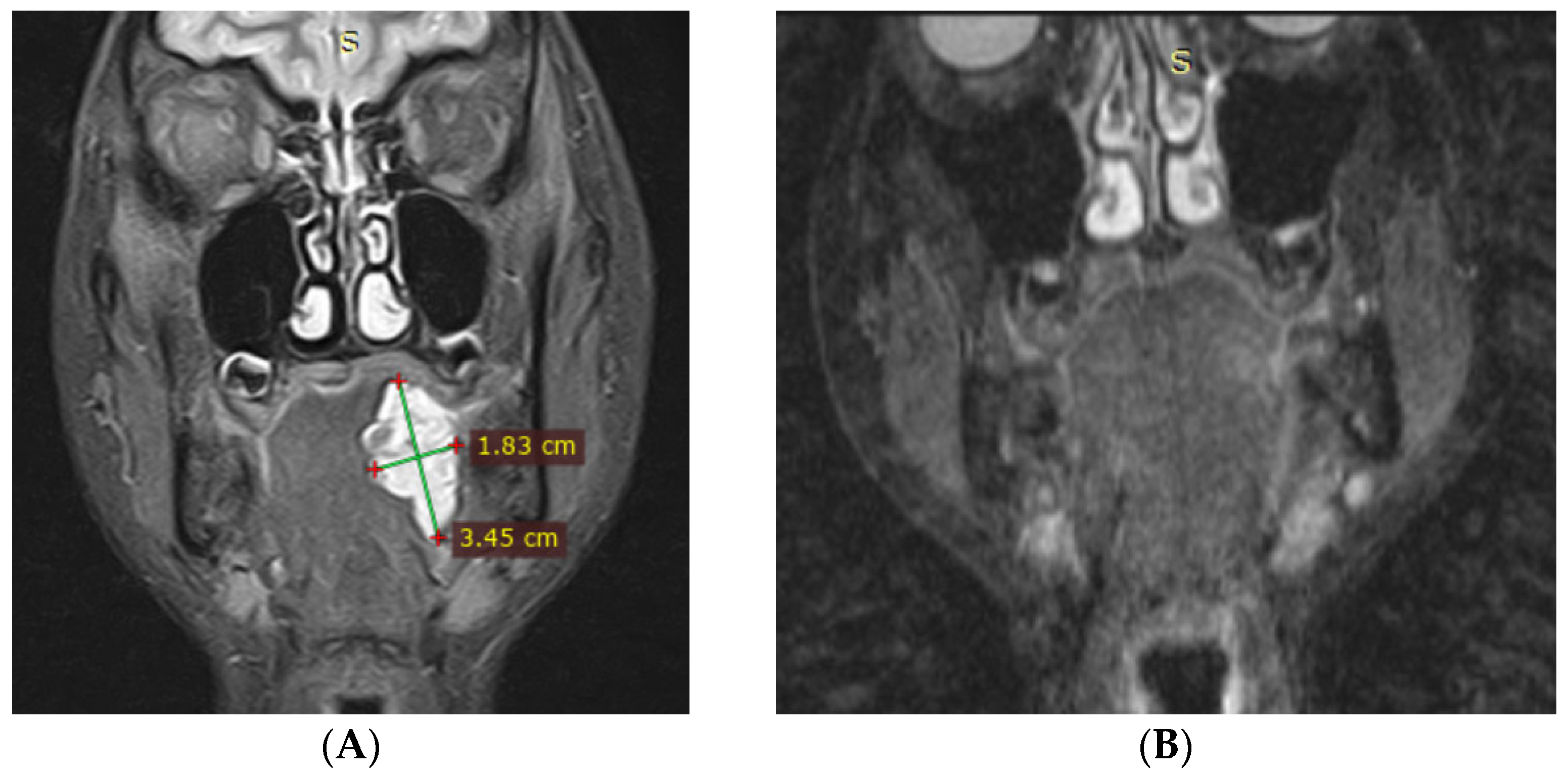

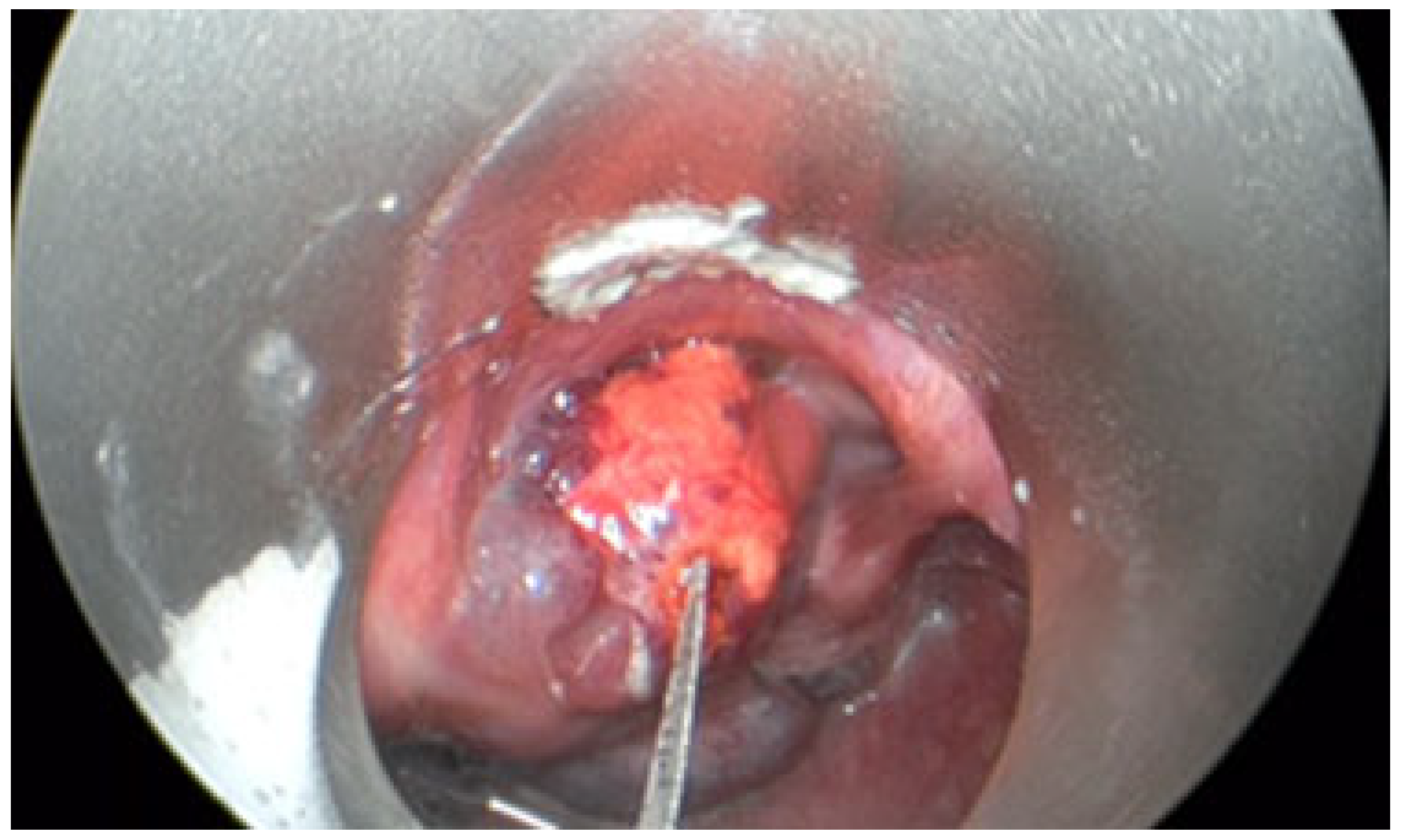
| No. | Sex | Age | Lesion Location | No. of Sessions | Volume Before (mm3) | Volume After (mm3) | Treatment Outcomes | Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 14 | Right tongue root, right floor of mouth | 1 | 6853 | 0 | Complete response (after 1st session) | None |
| 2 | M | 12 | Right tongue root | 2 | 6417 | 0 | Complete response | None |
| 3 | F | 13 | Left tongue root | 1 | 5129 | 0 | Complete response (after 1st session) | None |
| 4 | F | 37 | Oropharynx, posterior wall | 2 | 6938 | 0 | Complete response | None |
| 5 | M | 4 | Right soft palate | 1 | 3287 | 0 | Complete response (after 1st session) | None |
| 6 | F | 47 | Right soft palate | 1 | 2954 | 0 | Complete response (after 1st session) | Yes (painful necrosis) |
| 7 | F | 26 | Soft palate and uvula | 1 | 6711 | 0 | Complete response (after 1st session) | Yes (subclinical necrosis) |
| 8 | M | 4 | Right soft palate | 1 | 4386 | 0 | Complete response (after 1st session) | None |
| 9 | M | 9 | Oropharynx | 6 | 7334 | 3091 | Significant reduction (>50%) | None |
| 10 | M | 23 | Subglottic space, neck | 3 | 8217 | 3248 | Significant reduction (>50%) | None |
| 11 | M | 56 | Tongue and floor of mouth | 3 | 9476 | 3815 | Significant reduction (>50%) | None |
| 12 | F | 14 | Total tongue involvement | 12 | 10,192 | 4090 | Significant reduction (>50%) | None |
| 13 | F | 67 | Soft palate and uvula | 3 | 8743 | 5668 | Limited reduction (<50%) | Yes (subclinical necrosis) |
| 14 | M | 5 | Right soft palate and palatopharyngeal arch | 3 | 9159 | 5340 | Limited reduction (<50%) | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medzhidov, A.; Voznitsyn, L.; Askerov, E.; Vetcher, A.A.; Venkatesan, R.; Telyshev, D. An In Situ Forming Bleomycin-Polidocanol Composite Foam for Optimizing Sclerotherapy of High-Risk Airway Venous Malformations. J. Compos. Sci. 2025, 9, 635. https://doi.org/10.3390/jcs9110635
Medzhidov A, Voznitsyn L, Askerov E, Vetcher AA, Venkatesan R, Telyshev D. An In Situ Forming Bleomycin-Polidocanol Composite Foam for Optimizing Sclerotherapy of High-Risk Airway Venous Malformations. Journal of Composites Science. 2025; 9(11):635. https://doi.org/10.3390/jcs9110635
Chicago/Turabian StyleMedzhidov, Artur, Lev Voznitsyn, Emil Askerov, Alexandre A. Vetcher, Raja Venkatesan, and Dmitry Telyshev. 2025. "An In Situ Forming Bleomycin-Polidocanol Composite Foam for Optimizing Sclerotherapy of High-Risk Airway Venous Malformations" Journal of Composites Science 9, no. 11: 635. https://doi.org/10.3390/jcs9110635
APA StyleMedzhidov, A., Voznitsyn, L., Askerov, E., Vetcher, A. A., Venkatesan, R., & Telyshev, D. (2025). An In Situ Forming Bleomycin-Polidocanol Composite Foam for Optimizing Sclerotherapy of High-Risk Airway Venous Malformations. Journal of Composites Science, 9(11), 635. https://doi.org/10.3390/jcs9110635








