Abstract
Tuberculosis (TB) is still one of the world’s deadliest infections. Fast detection of the pathogen M. tuberculosis (MTB) and its genetic resistance markers substantially improves treatment success and outcome. A key element for rapid genetic diagnostics is the efficient extraction of DNA from sputum for qPCR detection at the point of care. We present the fully automated sample preparation of MTB DNA from 3 mL of liquefied sputum and qPCR detection of MTB on a centrifugal microfluidic cartridge. Our method achieves a limit of detection (LoD) between 17 and 57 CFU/mL and provides a purified DNA solution for molecular downstream testing, such as targeted NGS.
1. Introduction
Only a few fully automated point-of-care solutions are available for TB diagnostics [1,2], and none of them provide extracted DNA for subsequent comprehensive antibiotic resistance testing from the same sample. Here, we present a new sample preparation of MTB DNA using centrifugal microfluidics, a technology dedicated to implementing complex workflows on compact cartridges [3]. However, to date, fully automated centrifugal microfluidic sample-to-answer approaches have only been demonstrated for small sample volumes [4] or nasal swabs [5]. The combination of a liquefaction and inactivation (LI) reagent with a filter and lysis (FL) module allows us to increase sample volumes 15-fold from 200 µL to 3000 µL. It enables the detection of MTB on the cartridge and the provision of the DNA solution in a detachable DNA tube for subsequent resistance profiling.
2. Materials and Methods
The cavity for the FL module is integrated into an injection-molded cartridge (Figure 1A,B). A polyethylene frit (PE3510, SPC Technologies, diameter of 9 mm) and a glass microfiber filter (GMF150, Whatman, diameter of 10 mm) are fixed with a press-fit O-ring (8 × 1.25 mm, FKM, IR-Dichtungstechnik). The custom-developed LI reagent consists of 45% isopropanol, 3% NaOH, 0.5% N-lauroyl-sarcosine, and 0.2% Tween. 1 mL of sputum is mixed with 2 mL of LI reagent and incubated for 20 min.
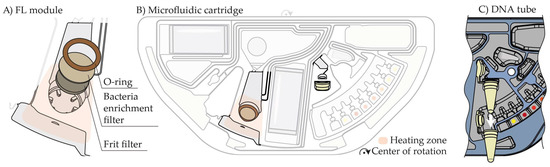
Figure 1.
(A) Exploded view of the FL module. (B) Microfluidic cartridge. (C) Top-side view of the DNA tube.
3. Results and Discussion
For the automation of sample preparation and qPCR detection of MTB from sputum on a centrifugal microfluidic cartridge, the FL module is the key element. It enables the efficient provision of MTB DNA from sputum and consists of a filter frit and a bacteria enrichment filter (Figure 1A). The externally inactivated, liquefied sputum sample is added to the cartridge and filtered, retaining MTB in the bacteria enrichment filter (Figure 2A). A washing step is performed to reduce interfering sputum and LI reagent residues. This is followed by thermal lysis to release the MTB DNA. With this method, large sample volumes of up to 3 mL can be automatically processed and analyzed. Spiking dilutions of H37Rv in sputum identified a LoD between 17 and 57 CFU/mL (Figure 2B). One important achievement is the inactivation and structural weakening of the hard-to-lyse MTB during the LI step while not losing the DNA. MTB DNA can be efficiently released by thermal lysis and subsequently used for qPCR-based MTB detection on the cartridge. Resistance profiles can be generated externally downstream using the DNA solution obtained via the DNA tube interface, as depicted in Figure 1C.
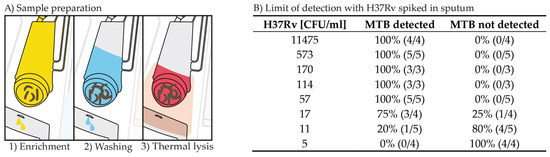
Figure 2.
(A) Sample preparation steps automated by the cartridge. (B) LoD achieved with the automated sample preparation and qPCR detection of the microfluidic cartridge with H37Rv spiked in sputum.
Author Contributions
Conceptualization, J.S.; methodology, J.S.; formal analysis, J.S.; investigation, J.S. and M.B.; writing—original draft, J.S.; visualization, J.S.; project administration, J.S. and M.B.; writing—review and editing, M.B., J.L., H.H. and N.P.; supervision, J.L., H.H. and N.P.; funding acquisition, H.H. and N.P. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support by the Federal Ministry of Education and Research, Germany, within the project BT-SeqDisK (No. 16GWO154) is gratefully acknowledged.
Institutional Review Board Statement
The study was approved by the Nepal Health Research Council and the Ethical committee of the medical faculty of the University of Munic (project ID 17-761).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are partially contained within the original research article Schlanderer et al. (DOI: 10.1039/d3lc00783a).
Conflicts of Interest
We declare that the three unit operation of the integrated workflow is partially protected by patents. The rehydration of the pre-stored reagents is protected by DE102013220257 and family. The switching after the rehydration is patented by DE102016207845 and family. The aliquoting is protected by DE102008003979 and family. All patents are owned by Hahn-Schickard. On the first two, Nils Paust is a co-inventor.
References
- Helb, D.; Jones, M.; Story, E.; Boehme, C.; Wallace, E.; Ho, K.; Kop, J.; Owens, M.R.; Rodgers, R.; Banada, P.; et al. Rapid Detection of Mycobacterium tuberculosis and Rifampin Resistance by Use of On-Demand, Near-Patient Technology. J. Clin. Microbiol. 2010, 48, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-W.; Sakamuri, R.; Kumar, P.; Ferguson, T.M.; Doebler, R.W.; Herrington, K.D.; Talbot, R.P.; Weigel, K.M.; Nguyen, F.K.; Cangelosi, G.A.; et al. Integrated nucleic acid testing system to enable TB diagnosis in peripheral settings. Lab Chip 2020, 20, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef] [PubMed]
- Czilwik, G.; Messinger, T.; Strohmeier, O.; Wadle, S.; Von Stetten, F.; Paust, N.; Roth, G.; Zengerle, R.; Saarinen, P.; Niittymäki, J.; et al. Rapid and fully automated bacterial pathogen detection on a centrifugal-microfluidic LabDisk using highly sensitive nested PCR with integrated sample preparation. Lab Chip 2015, 15, 3749–3759. [Google Scholar] [CrossRef] [PubMed]
- Spindiag. Rhonda SARS-CoV-2 Disk—Spindiag. 2023. Available online: https://www.spindiag.de/rhonda-sars-cov-2-disk/ (accessed on 18 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).