Aluminum Triflate—Catalyzed Adamantylation of N-Nucleophiles †
Abstract
:1. Introduction
2. Materials and Methods
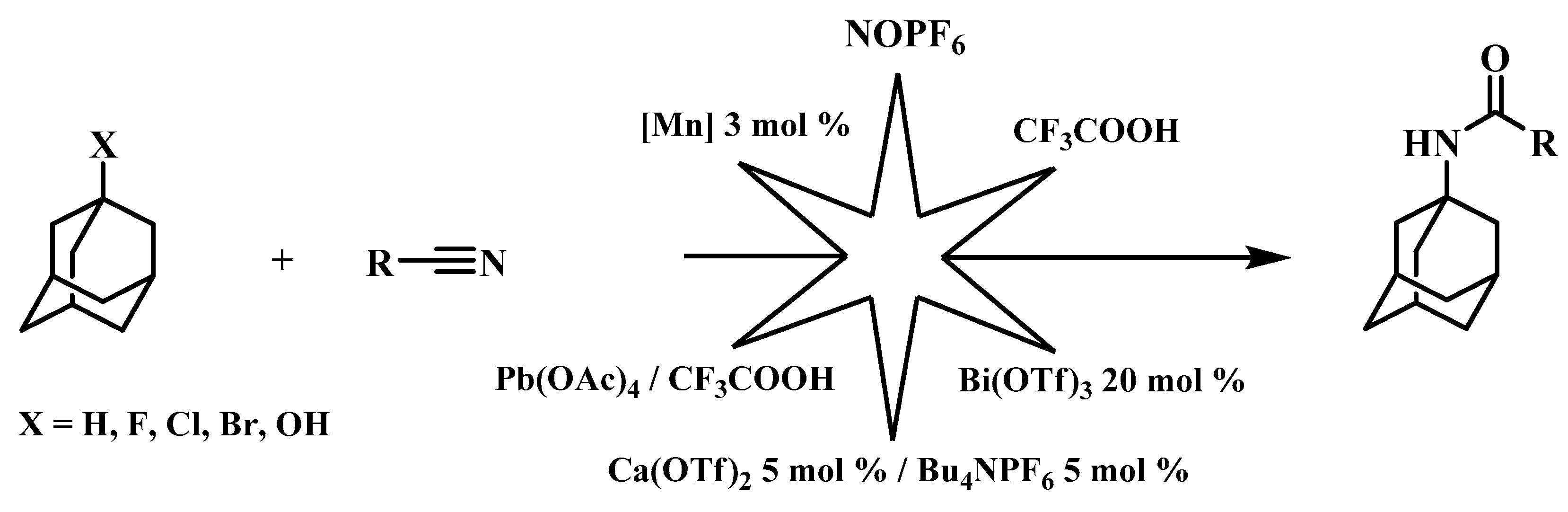
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Klimochkin, Y.N.; Shiryaev, V.A.; Leonova, M.V. Antiviral properties of cage compoundsnew prospects. Russ. Chem. Bull. 2015, 64, 1473–1496. [Google Scholar] [CrossRef]
- Khusnutdinov, R.I.; Shchadneva, N.A.; Khisamova, L.F. Synthesis of N-(adamantan-1-yl)amides by reaction of carboxylic acid amides with 1-bromo(chloro)adamantane catalyzed by manganese compounds. Russ. J. Org. Chem. 2015, 51, 476–479. [Google Scholar] [CrossRef]
- Shokova, E.; Mousoulou, T.; Luzikov, Y.; Kovalev, V. Adamantylation and Adamantylalkylation of amides, nitriles and ureas in trifluoroacetic acid. Synthesis 1997, 9, 1034–1040. [Google Scholar] [CrossRef]
- Shokova, E.A.; Musulu, T.; Luzikov, Y.N.; Kovalev, V.V. Adamantylation and adamantylalkylation of amides, nitriles, and ureas in trifluoroacetic acid. Russ. J. Org. Chem. 1999, 35, 844–856. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J.; Wang, N.; Zhang, Q.; Shi, D.; Sun, K. Solvent-free and direct C(sp3)-H amination of adamantanes by grinding. Tetrahedron 2014, 70, 1395–1400. [Google Scholar] [CrossRef]
- Pat. RU 2455280 C1, Sposobpolucheniya N-(1-adamantyl)acetamida/U.M. Dzhemilev (RU), R.I. Khusutdinov (RU), N.A. Shchadneva (RU), J.J. Majakova (RU), T.M. Oshnjakova (RU). Available online: http://www.freepatent.ru/images/patents/4/2455280/patent-2455280.pdf (accessed on 1 November 2018).
- Shokova, E.; Mousoulou, T.; Luzikov, Y.; Kovalev, V. Adamantylation and Adamantylalkylation of Amides, Nitriles and Ureas in Trifluoroacetic Acid. Synthesis 1997, 1997, 1034–1040. [Google Scholar] [CrossRef]
- Jones, S.R.; Mellor, J.M. Introduction of bridgehed functionality via lead (IV) oxidation of hydrocarbons. Synthesis 1976, 1976, 32–33. [Google Scholar] [CrossRef]
- Callens, E.; Burton, A.J.; Barrett, A.G.M. Synthesis of amides using the Ritter reaction with bismuth triflate catalysis. Tetrahedron Lett. 2006, 47, 8699–8701. [Google Scholar] [CrossRef]
- Yaragorla, S.; Singh, G.; Saini, P.L.; Reddy, M.K. Microwave assisted, Ca(II)-catalyzed Ritter reaction for the green synthesis of amides. Tetrahedron Lett. 2014, 55, 4657–4660. [Google Scholar] [CrossRef]
- Olah, G.A.; Gupta, B.G.B.; Narang, S.C. Synthetic methods and reactions; 66.Nitrosonium ion induced preparation of amides from alkyl (Arylalkyl) halides with nitriles, a mild and selective Ritter-type reaction. Synthesis 1979, 1979, 274–276. [Google Scholar] [CrossRef]
- Turmasova, A.A.; Spesivaya, E.S.; Konshina, D.N.; Konshin, V.V. Adamantylation of β-dicarbonyl compounds. Russ. Chem. Bull. 2012, 61, 1733–1735. [Google Scholar] [CrossRef]
- Turmasova, A.A.; Konshin, V.V.; Konshina, D.N. Synthesis of (1,3-adamantylene)bis-1,3-dicarbonyl compounds. Russ. J. Gen. Chem. 2014, 84, 1273–1276. [Google Scholar] [CrossRef]
- Gohain, M.; Marais, C.; Bezuidenhoudt, B.C.B. Al(OTf)3: An efficient recyclable catalyst for direct nucleophilic substitution of the hydroxyl group of propargylic alcohols with carbon- and heteroatom-centered nucleophiles to construct C-C, C-O, C-N and C-S bonds. Tetrahedron Lett. 2012, 53, 1048–1050. [Google Scholar] [CrossRef]
- Tsypin, V.G.; Pevzner, M.S.; Golod, E.L. Oxidative alkylation of azoles: VII. adamantylation of azoles via oxidative generation of 1-adamantyl cations. Russ. J. Org. Chem. 2001, 37, 1762–1766. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konshin, V.V.; Shcherbinin, V.A.; Lupanova, I.A.; Konshina, D.N. Aluminum Triflate—Catalyzed Adamantylation of N-Nucleophiles. Proceedings 2019, 9, 21. https://doi.org/10.3390/ecsoc-22-05661
Konshin VV, Shcherbinin VA, Lupanova IA, Konshina DN. Aluminum Triflate—Catalyzed Adamantylation of N-Nucleophiles. Proceedings. 2019; 9(1):21. https://doi.org/10.3390/ecsoc-22-05661
Chicago/Turabian StyleKonshin, Valery V., Vitaly A. Shcherbinin, Ida A. Lupanova, and Dzhamilya N. Konshina. 2019. "Aluminum Triflate—Catalyzed Adamantylation of N-Nucleophiles" Proceedings 9, no. 1: 21. https://doi.org/10.3390/ecsoc-22-05661
APA StyleKonshin, V. V., Shcherbinin, V. A., Lupanova, I. A., & Konshina, D. N. (2019). Aluminum Triflate—Catalyzed Adamantylation of N-Nucleophiles. Proceedings, 9(1), 21. https://doi.org/10.3390/ecsoc-22-05661



