Hydroxyapatite is mainly used in biomedical applications because of its very important property—bioactivity. Due to its bioactivity, the stimulation of bone regeneration at the contact between HAp and the biological environment becomes possible [1]. A huge amount of eggshell is produced every day and is considered waste. The eggshell consists of 91–94% calcium carbonate. This fact makes it favorable to use them as a source of calcium oxide in the synthesis of Hap [2].
In this paper, we used the microwave-assisted method to obtain HAp nanopowders. The powders were lately sintered by the Spark Plasma Sintering method to maintain its nanostructure. As reaction precursors, orthophosphoric acid solution, calcium hydroxide (obtained from calcinated and hydrated eggshells), and ammonium hydroxide were used for pH adjustment. In parallel, for comparison, HAp synthesis used commercial calcium hydroxide.
The obtained precipitate was then placed in a microwave oven for 1 h, dried in the oven, and calcinated at 700 degrees Celsius, then characterized.
As seen in Figure 1, there is represented the XRD spectrum of the HAp sample (obtained from eggshells). It can be observed that all the diffraction interferences belong to the HAp phase.
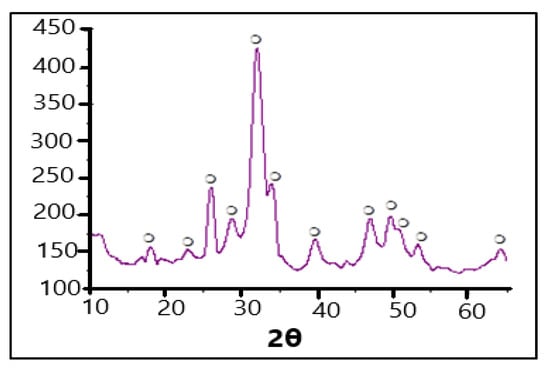
Figure 1.
XRD spectrum of HAp obtained from the eggshells.
The SEM image (Figure 2) presents the microstructure of the HAp powder. From a microstructural point of view, there was obtained a monophasic polycrystalline powder, were the crystalline grains present a needle-like morphology, with nanometric dimensions (ca. 10 nm) and random orientation. The grain boundaries are well defined and the porosity degree is high, the pores being intergranular and interconnected.
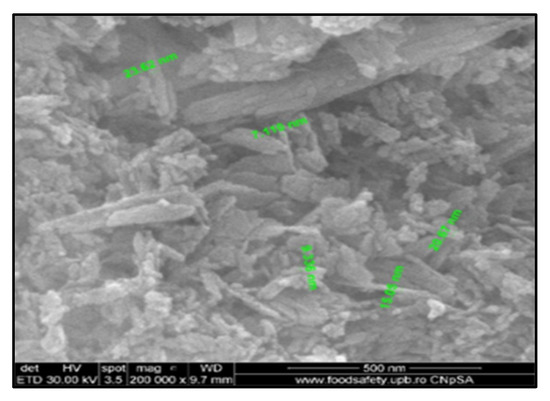
Figure 2.
SEM image of HAp obtained from eggshells.
The synthesis of HAp using the microwave-assisted method leads to the obtaining of a high purity HAp powder, with needle-like crystals that present nanometric dimensions. The use of biogenic waste (eggshells) did not affect the final result, so it can be easily used in HAp synthesis.
Acknowledgments
This paper was supported by the Romanian Ministry of Research and Innovation, Project 51PCCDI/2018 within PNIII and PN 19.23.03.01 within the NUCLEU program.
References
- Agbeboh, N.; Oladele, I.; Daramola, O.; Adediran, A.; Olasukanmi, O.; Tanimola, M. Environmentally sustainable processes for the synthesis of hydroxyapatite. Heliyon 2020, 6, e03765. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, H.; Prakash, S. Synthesis and characterization of hydroxyapatite powder by eggshell. J. Min. Mater. Charact. Eng. 2016, 4, 119–126. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).