The aim of this article was the gas chromatography–mass spectrometry (GC-MS) analysis of organic matter from a residual liquor sample (S.C. Alum S.A., Tulcea), extracted by the solid-phase microextraction method (SPMA) and derivatized with N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA) as the silylating agent. The trace organic matter from Bayer liquor (known as poisons) has the ability to inhibit Al(OH)3 (gibbsite) crystallization. The structure of the poisons is essential for understanding how inhibition is induced and consequently, how they can be removed [1,2]. The first step is the extraction of organic poisons from Bayer liquor by solid-phase microextraction (SPME) [3]. Due to the polar functionalized polyhydroxy compounds, and aliphatic and aromatic acids, the derivatization is required to produce more volatile compounds [4]. The most suitable technique of extracted and derivatized organic poisons from Bayer liquor has been shown to be GC-MS [1,5].
The experimental data for this paper were obtained on a gas chromatograph coupled with a mass spectrometer (GC-MS) produced by PerkinElmer, USA. A 50 mL aliquot of the residual liquor sample was acidified to precipitate Al(OH)3 which was removed by centrifugation. From the supernatant acidified with HCl at pH 2, a 20 mL aliquot was transferred to a test flask. The organic matter (poisons) in the sample vial was extracted by the SPME technique on a polyacrylate fiber (PA) of 85 µm. The polyacrylate fiber was immediately exposed in a heated ampoule (700 °C, 10 min), in nitrogen, to the derivatizing agent MTBSTFA/pyridine (7:3). This was followed by GC injection at 270 °C by desorption from the PA fiber for 5 min. The derivatized components of the residual liquor sample were separated chromatographically on a 60 m capillary column with stationary phase Elite-5 MS (phenyl-methyl silicone). The mass chromatogram (16–40 min) of the ions (m/z 73 + 75 + 147) with derivatized organic components is presented in Figure 1.
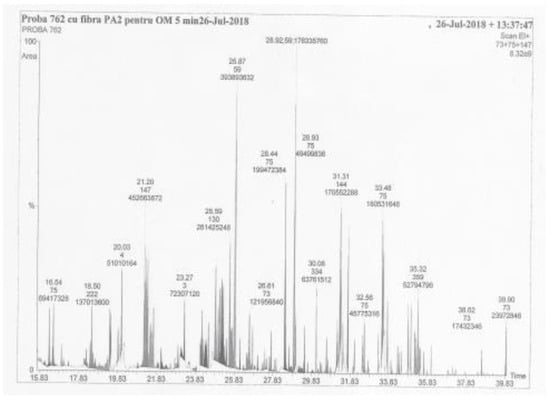
Figure 1.
The mass chromatogram of organic compounds in the residual liquor sample.
In Table 1, non-derivatized organic compounds with their molecular masses were obtained by subtracting the masses of the tert-butyldimethylsilyl (115-1) and trimethylsilyl (73-1) groups from the molecular masses of the compounds in Table 2.

Table 1.
The organic compounds from the residual liquor sample.

Table 2.
The derivatized organic compounds from the residual liquor sample.
The qualitative analysis of the derivatized organic components as tert-butyldimethylsilyl (TBDMS) or trimethylsilyl (TMS) derivatives (esters) of the residual liquor sample, separated by gas chromatography, was performed by comparing their mass spectra with the mass spectra in the NIST (the National Institute of Standards and Technology, U.S.) and NBS (the National Bureau of Standards, U.S.) mass spectrum libraries; 19 compounds were identified (Table 2). For example, see glycerin identification in Figure 2.
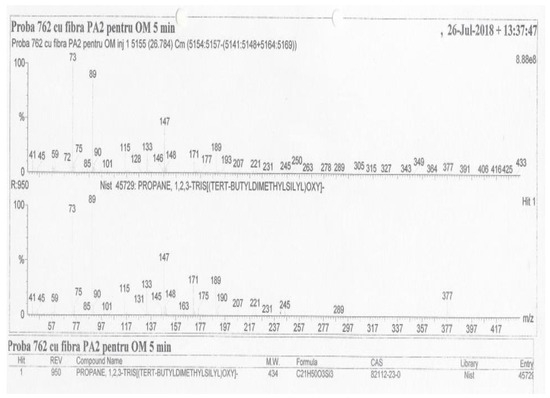
Figure 2.
The identification of glycerin as tert-butyldimethylsilyl (TBDMS) ester (No. 10 in Table 2).
Conclusions
The 19 organic compounds were identified in a residual liquor sample by comparing their mass spectra with the mass spectra in the NIST and NBS mass spectrum libraries.
The calculation of the concentration of the 19 identified components was performed from the area of their peaks in the mass chromatogram of the ions with m/z 73 + 75 + 147 and from the TOC analysis (854 mg/L) for the residual liquor sample.
The concentrations (in mg/L) of the 19 components identified in the residual liquor sample are given in Table 1.
References
- Maher, Q.E. Structural Determination, Identification and Removal of Bayer Liquor Organic Poisons. Master’s Thesis, Macquarie University, Sydney, Australia, 2015. [Google Scholar]
- Power, G.; Loh, J. Organic compounds in the processing of lateritic bauxites to alumina part 1: Origins and chemistry of organics in the Bayer process. Hydrometallurgy 2010, 105, 1–29. [Google Scholar] [CrossRef]
- Sigma-Aldrich, Co. Supelco, Solid Phase Microextraction: Theory and Optimization of Conditions. Bulletin 1998, 923, 1–8. [Google Scholar]
- Schummer, C.; Delhomme, O.; Appenzeller, B.M.R.; Wennig, R.; Millet, M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 2009, 77, 1473. [Google Scholar] [CrossRef] [PubMed]
- Power, G.; Loh, J.S.C.; Wajon, J.E.; Busetti, F.; Joll, C. A review of the determination of organic compounds in Bayer process liquors. Anal. Chim. Acta 2011, 689, 8–21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).