Abstract
2,2-Dimethyl-5-((phenylamino)methylene)-1,3-dioxane-4,6-dione, prepared by the reaction of Meldrum’s acid with triethyl orthoformate and aniline, reacts with active methylene nitriles to afford 2-oxo-1,2-dihydropyridine-3-carboxylic acid derivatives, which are useful as drug precursors or perspective ligands.
1. Introduction
It is known that nicotinic acid (niacin, vitamin PP) and its derivatives have a wide spectrum of biological activity. Thus, nicotinic acid and nicotinates have showed hypolipidemic, hypocholesterolemic, neuroprotective and other effects. 2-Oxo-1,2-dihydropyridine-3-carboxylic acid is less studied; however, it is of interest as a complexating agent [1,2] and in pharmaceuticals [3].
Previously, we developed a method the for synthesis of 6-mercapto-2-oxonicotinic acid 3 based on the heterocyclization of aminomethylidene derivative of Meldrum’s acid 1 with cyanothioacetamide 3 (Scheme 1) [4].
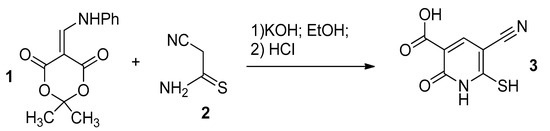
Scheme 1.
The reaction of cyanothioacetamide with anilinomethylidene Meldrum’s acid.
We decided to study the reactions of 5-cyano-2-oxo-1,2-dihydropyridine-3-carboxylic acid 3. Compound 3 easily reacts with alkyl halides regioselectively at the S atom to give sulfides (Scheme 2) 4.
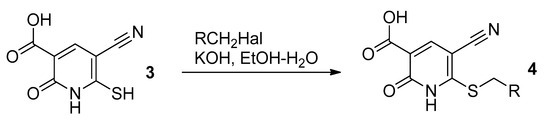
Scheme 2.
S-alkylation of compound 3.
Other active methylene nitriles were also introduced in the reaction. Thus, cyanoacetamide 5 in the presence of KOH reacts with enamino-1,3-diketone 1 followed by acidification to give pyridine 6 in a good yield (Scheme 3). However, we failed to prepare compound 7 starting from a malononitrile dimer.
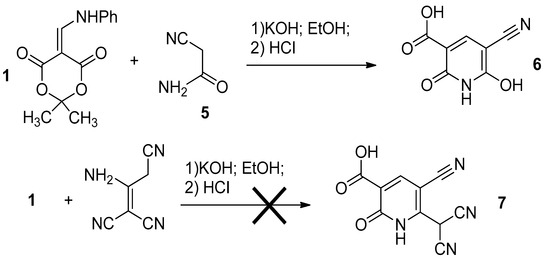
Scheme 3.
The reaction of anilinomethylidene Meldrum’s acid with active methylene nitriles.
2. Experimental
2.1. Anilinomethylidene Derivative of Meldrum’s Acid
A mixture of the powdered Meldrum’s acid (0.1 mol), triethyl orthoformate (21.6 mL, 0.13 mol), and freshly distilled aniline (9.1 mL, 0.1 mol) was refluxed with vigorous stirring for 5 min to afford a syrupy reaction mass. It was diluted with 30 mL of EtOH and refluxed for an additional 3 min. Then, it was cooled with stirring to ∼20 °C and diluted with water to 100 mL. After 2 h, the product was filtered off and washed with water, twice with 60% EtOH, and with hexane.
2.2. 2,2-Dimethyl-5-(Phenylamino)Methylene-1,3-Dioxane-4,6-Dione (1)
Yield 92%, m.p. 156–157 °C. Found (%): C, 63.19; H, 5.32; N, 5.66. C13H13NO4. Calculated (%): C, 63.15; H, 5.30; N, 5.67. 1H NMR, δ: 1.70 (s, 6 H, 2 Me); 7.19-7.51 (m, 5 H, Ph); 8.58 (d, 2 H, –CH =, 3J = 14.7 Hz); 11.27 (d, 1 H, NH, 3J = 14.7 Hz).
2.3. Compounds 3 and 6 (General Procedure)
Potassium hydroxide (1.12 g, 0.02 mol) was added to a vigorously stirred suspension of compound 1 (0.01 mol) and cyano(thio)acetamide (0.01 mol) in 10 mL of EtOH. After 24 h, the reaction mixture was acidified with concentrated HCl to pH 5 and maintained for 3 h. The precipitate that formed was filtered off and washed successively with water and EtOH. The yield of pyridine 3 was 68% and that of pyridine 6 was 74%.
Funding
Authors are grateful for financial support by the Russian Ministry of Education and Science (Project 0795-2020-0010).
References
- Di Marco, V.B.; Tappro, A.; Dolmella, A.; Bombi, G.G. Complexation of 2-hydroxynicotinic and 3-hydroxypicolinic acids with zinc (II). Solution state study and crystal structure of trans-diaqua-bis-(3-hydroxypicolinato) zinc (II). Inorg. Chim. Acta 2004, 357, 135–142. [Google Scholar] [CrossRef]
- Yoshito, O.; Fumiaki, H.; Yoshihiro, T.; Takayasu, K. Studies on the interaction of pyridone carboxylic acids with metals. Chem. Pharm. Bull. 1992, 40, S692–S696. [Google Scholar]
- Fossa, P.; Menozzi, G.; Dorigo, P.; Floreani, M.; Mosti, L. Synthesis and pharmacological characterization of functionalized 2-pyridones structurally related to the cardiotonic agent milrinone. Bioorg. Med. Chem. 2003, 11, 4749–4759. [Google Scholar] [CrossRef] [PubMed]
- Dotsenko, V.V.; Krivokolysko, S.G.; Chernega, A.N.; Litvinov, V.P. Anilinomethylidene derivatives of cyclic 1,3-dicarbonyl compounds in the synthesis of new sulfur-containing pyridines and quinolines. Russ. Chem. Bull. 2002, 51, 1556–1561. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).