Synthesis of New 2-Oxo-1,2-Dihydropyridine-3-Carboxylic Acid Derivatives †
Abstract
:1. Introduction
2. Experimental
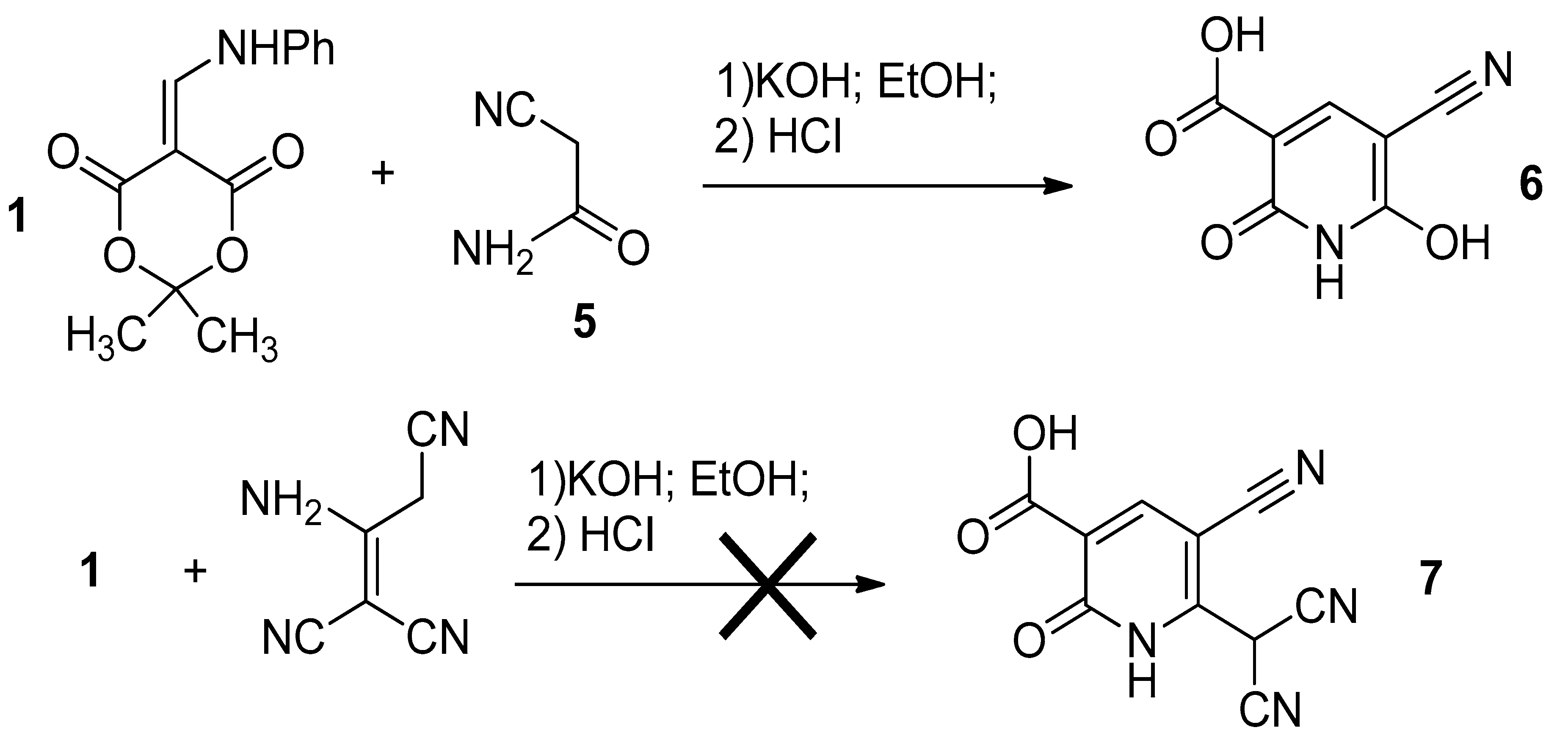
2.1. Anilinomethylidene Derivative of Meldrum’s Acid
2.2. 2,2-Dimethyl-5-(Phenylamino)Methylene-1,3-Dioxane-4,6-Dione (1)
2.3. Compounds 3 and 6 (General Procedure)
Funding
References
- Di Marco, V.B.; Tappro, A.; Dolmella, A.; Bombi, G.G. Complexation of 2-hydroxynicotinic and 3-hydroxypicolinic acids with zinc (II). Solution state study and crystal structure of trans-diaqua-bis-(3-hydroxypicolinato) zinc (II). Inorg. Chim. Acta 2004, 357, 135–142. [Google Scholar] [CrossRef]
- Yoshito, O.; Fumiaki, H.; Yoshihiro, T.; Takayasu, K. Studies on the interaction of pyridone carboxylic acids with metals. Chem. Pharm. Bull. 1992, 40, S692–S696. [Google Scholar]
- Fossa, P.; Menozzi, G.; Dorigo, P.; Floreani, M.; Mosti, L. Synthesis and pharmacological characterization of functionalized 2-pyridones structurally related to the cardiotonic agent milrinone. Bioorg. Med. Chem. 2003, 11, 4749–4759. [Google Scholar] [CrossRef] [PubMed]
- Dotsenko, V.V.; Krivokolysko, S.G.; Chernega, A.N.; Litvinov, V.P. Anilinomethylidene derivatives of cyclic 1,3-dicarbonyl compounds in the synthesis of new sulfur-containing pyridines and quinolines. Russ. Chem. Bull. 2002, 51, 1556–1561. [Google Scholar] [CrossRef]

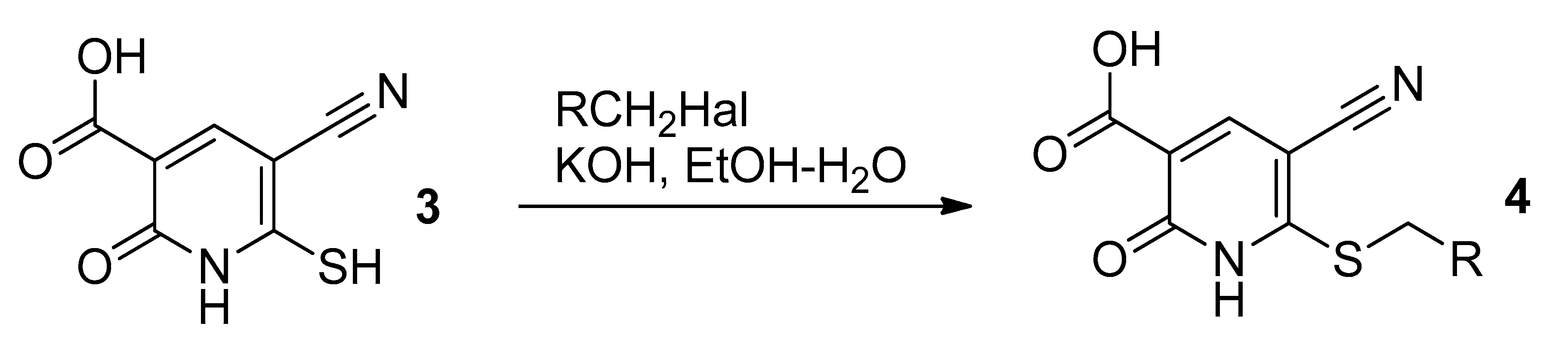
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dotsenko, V.V.; Russkih, A.A.; Aksenov, N.A.; Aksenova, I.V. Synthesis of New 2-Oxo-1,2-Dihydropyridine-3-Carboxylic Acid Derivatives. Proceedings 2019, 41, 24. https://doi.org/10.3390/ecsoc-23-06523
Dotsenko VV, Russkih AA, Aksenov NA, Aksenova IV. Synthesis of New 2-Oxo-1,2-Dihydropyridine-3-Carboxylic Acid Derivatives. Proceedings. 2019; 41(1):24. https://doi.org/10.3390/ecsoc-23-06523
Chicago/Turabian StyleDotsenko, Victor V., Alena A. Russkih, Nikolai A. Aksenov, and Inna V. Aksenova. 2019. "Synthesis of New 2-Oxo-1,2-Dihydropyridine-3-Carboxylic Acid Derivatives" Proceedings 41, no. 1: 24. https://doi.org/10.3390/ecsoc-23-06523
APA StyleDotsenko, V. V., Russkih, A. A., Aksenov, N. A., & Aksenova, I. V. (2019). Synthesis of New 2-Oxo-1,2-Dihydropyridine-3-Carboxylic Acid Derivatives. Proceedings, 41(1), 24. https://doi.org/10.3390/ecsoc-23-06523




