1. Introduction
Mucous membrane pemphigoid (MMP) is a rare autoimmune blistering disease, affecting one or more mucosae. The oral cavity is generally involved, often in form of desquamative gingivitis (Figure 1).
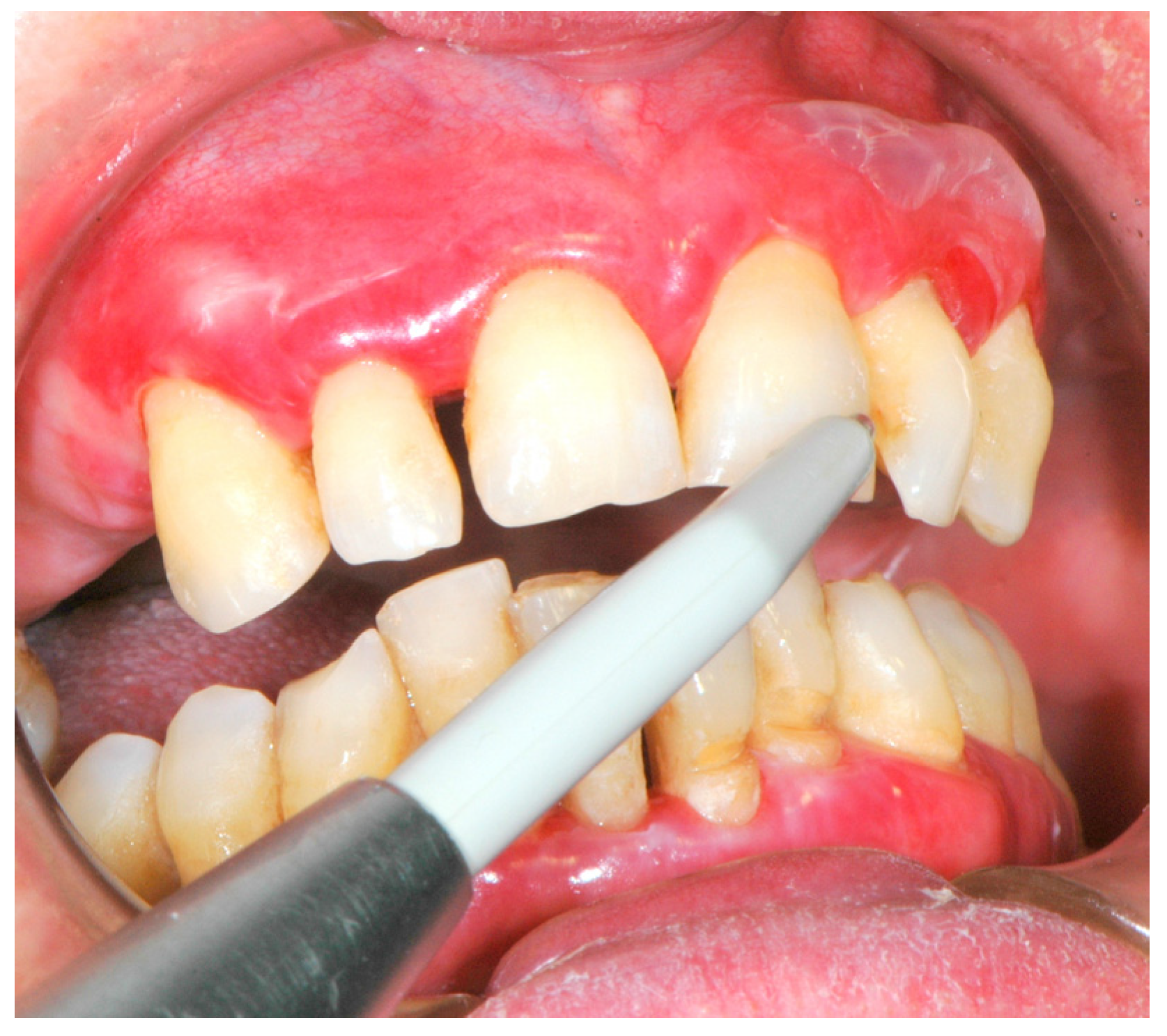
Figure 1.
Desquamative gingivitis: common clinical oral manifestation of mucous membrane pemphigoid.
Immunofluorescence (direct and indirect), histopathology (Figure 2), together with sera autoantibodies dosage by enzyme-linked immunosorbent assay (ELISA) are currently used to diagnose this disease. Saliva is currently employed for diagnostic purposes in multiple fields, providing a wide pool of biomarkers, and representing a not-invasive method suitable for screening and follow-up procedures [1,2].
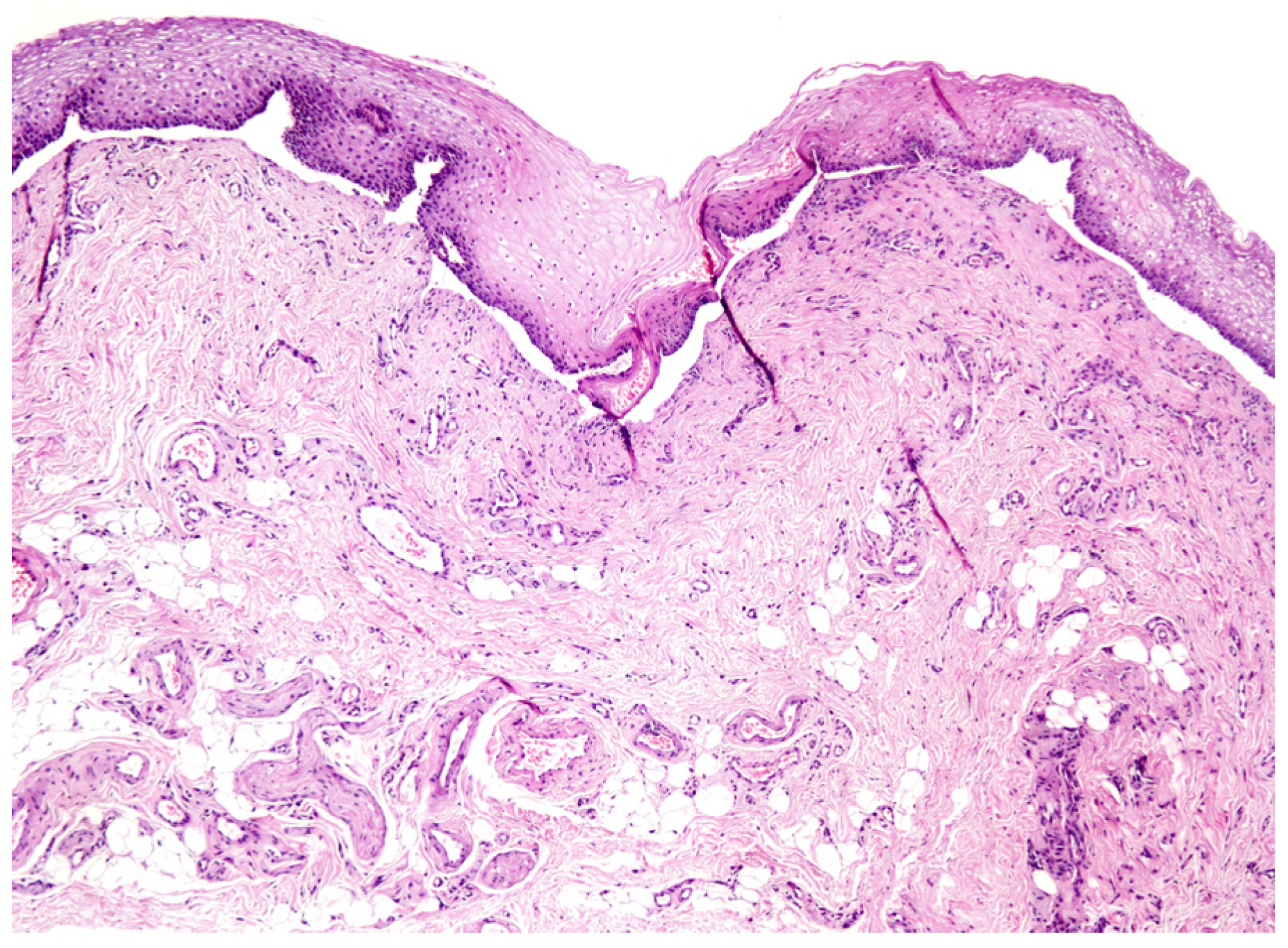
Figure 2.
Histopathological picture of MMP (4× magnification).
2. Aim
The aims of the present study were to analyze the efficacy of ELISA in the detection of anti BP180 NC 16a antibodies, comparing salivary and sera specimens, in patients affected by MMP; and to evaluate the correlation between the severity of the clinical manifestations and the anti BP180 titers detected.
3. Materials & Method
Patients referred to Oral Medicine unit of Policlinico di Bari, from January 2011 to December 2016, with a histopathological diagnosis of MMP confirmed by direct immunofluorescence (DIF) test were enrolled. Every patient underwent a serum and a saliva sample collection at the beginning of the diagnostic process. BP180 titers were assessed using commercially available ELISA kit (cut off: 9 U/mL). Clinical Harman’s score was recorded for every patient. Sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and accuracy of ELISA performed on salivary samples were calculated in comparison to sera specimens. The concordance between the two tests was evaluated using the Cohen’s kappa coefficient (k). The correlation between clinical Harman’s scores and ELISA titers was evaluated using t-Student test (significative values with p < 0.05).
4. Results
Twenty-five patients were enrolled, 19 females and 6 males (F:M = 3.16), mean age 63.5 (SD ± 10.9). Patients data are resumed in Table 1. Sensitivity and specificity values were 83.3% and 53.8% respectively, NPV was 77.8% and PPV was 62.5%, accuracy resulted with a value of 68%. The Cohen’s k value was 0.367 (concordance: “fair”). The association between the clinical scores and both the salivary and sera anti-BP180 titers resulted to be statistically significant (p < 0.05).

Table 1.
Enrolled patients’ demographic data, anti-BP 180 sera and saliva titers detected using ELISA, and Harman’s scores. The positive values (>9 U/mL) are bolded.
5. Discussion
From this study a good sensitivity of ELISA performed on saliva emerged, while specificity resulted lower when compared to sera data. Nowadays, few studies about ELISA effectiveness in patients affected by pemphigoid are available, and even less about muco-membranous forms. The concordance between the two samples used resulted to be fair, suggesting the chance to employ saliva as a not-invasive tool to help the diagnostic process and to track the course of the pathology [1,2,3,4]. In fact, this study showed a statistical correlation between clinical conditions and antibodies titers. A greater reliability of ELISA could be achieved extending the range of dosable antigens of the commercial kits, and using crevicular fluid samples instead of whole saliva, providing higher levels of antibodies.
Conflicts of Interest
The authors have no conflict of interests to declare.
References
- Koopai, M.; Mortazavi, H.; Khatami, A.; Khodashenas, Z. Salivary and Serum Anti-Desmoglein 1 and 3 ELISA and Indirect Immunofluorescence in Pemphigus Vulgaris: Correlations with Serum ELISA, Indirect Immunofluorescence and Disease Severity. Acta Dermatovenerol. Croat. 2018, 26, 91–99. [Google Scholar] [PubMed]
- Esmaili, N.; Mortazavi, H.; Kamyab-Hesari, K.; Aghazadeh, N.; Daneshpazhooh, M.; Khani, S.; Chams-Davatchi, C. Diagnostic accuracy of BP180 NC16a and BP230-C3 ELISA in serum and saliva of patients with bullous pemphigoid. Clin. Exp. Dermatol. 2015, 40, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, D.; Lorenzini, G.; Drakoulakos, D.; Belazi, M.; Mihailidou, E.; Velkos, G.; Mourellou-Tsatsou, O.; Antoniades, D. Detection of pemphigus desmoglein 1 and desmoglein 3 autoantibodies and pemphigoid BP180 autoantibodies in saliva and comparison with serum values. Eur. J. Oral Sci. 2006, 114, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Kelly, C.; Challacombe, S.J.; Donaldson, A.N.; Dart, J.K.; Gleeson, M.; MMP Study Group 2009–14; Setterfield, J.F. Salivary IgA and IgG antibodies to bullous pemphigoid 180 noncollagenous domain 16a as diagnostic biomarkers in mucous membrane pemphigoid. Br. J. Dermatol. 2016, 174, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).