Abstract
The present study investigated the occurrence of selected micropollutants including emerging contaminants (mainly pharmaceuticals and personal care products- PPCPs) in selected water samples from swimming pool systems. The study area was selected based on the lack of available information regarding the suspected contamination of swimming pools water by PPCPs. The variety and concentration of chemical compounds in these aquatic systems can be quite diversified, presenting a challenge in terms of both purification and quality control. Determination of PPCPs requires very sensitive analytical methods that make it possible to confirm the presence of tested compounds in a complex organic extract. In this field, GC-MS gas chromatography can be used. This system enables to perform Selected Ion Monitoring, which reduces the detection limits of the investigated analyte. This paper aims at presentation of analytical methods and strategies adapted to obtain information on the composition and characteristics of water in swimming pool systems. The sample preparation methodology including solid phase extraction was developed for swimming pool water.
1. Introduction
Maintaining the microbial water quality in order to inhibit the spread of infections and diseases is the priority for every swimming pool owners and managers. According to sanitary and hygienic guidelines, in public swimming pools, disinfection with chlorine compounds is required [1,2]. There is a number of disinfectants that have been used for swimming pool with the potential to produce a wide range of disinfection by-products (DBPs) through the reaction with organic and inorganic matter as it is well established from studies on disinfection of drinking water. Due to the recirculation technology that is applied, higher chlorination levels, higher organic matter content, much more DBPs are formed in swimming pool systems than in drinking water [3].
There are many studies on chemical contaminants in swimming pools focusing on the occurrence of disinfection by-products (DBPs) [3,4,5,6]. However, some authors have concluded that further researches are needed to evaluate potential health risk not only from DBPs but also from other chemicals occurring in swimming pools [7,8]. Research on PPCPs in swimming pools are still in their infancy and available data are limited.
PPCPs are designed to be biologically active also at low concentrations. Long-term exposure to the PPCPs mixture may potentially cause negative health effects. Moreover, their degradation in swimming pool water treatment systems is possible and PPCPs’ by-products may be more relevance to the health of swimmers than their parent compound [9]. These reasons connected with the fact that swimmers have direct contact with the analyzed compounds and their by-products, make it necessary to investigate the occurrence of PPCPs in swimming pools.
The determination of PPCPs requires very sensitive analytical methods that enables to confirm the presence of tested compounds in a complex organic extract. The variety and concentration of chemical compounds in such complex aquatic systems as swimming pool water, is quite diversified, presenting a challenge in terms of quality control. In this field highly sophisticated equipment, such as gas or liquid chromatography with mass spectrometry (GC-MS or LC-MS) can be used. These detection methods are commonly used as analytical techniques to identify and quantify water contaminants such as PPCPs [10,11,12,13,14,15]. They enable the detection of PPCPs from different matrices at sub-ng/g levels [16]. There are many disadvantages and advantages of both LC–MS and GC–MS. There is high importance of selecting the appropriate analysis techniques to obtain the best results. Sample’s nature and complexity are key factors in choosing the best technique [16]. Pharmaceuticals consist of polar compounds that are soluble both in water and polar solvents, which is a particular advantage of LC-MS analysis. On the other hand, personal care products (PCPs) are relatively non-polar. Furthermore, they are more soluble and better extracted in relatively non-polar organic solvents [16]. GC-MS is a highly efficient tool widely used to analyze PCPs at extremely low levels from environmental samples [16].
Both GC-MS and LC-MS analysis require appropriate sample preparation. The essential preparation step is the extraction. Solid Phase Extraction (SPE) or Liquid-Liquid Extraction are reliable ways to perform it. Liquid–Liquid Extraction has been proven to be an efficient technique [17], however it is a time and reagent consuming procedure and it cannot be easily automated. As a result, an alternative method, Solid Phase Extraction, has been developed. When compared to other sample preparation processes, Solid-Phase Extraction offers lower cost due to lower solvent and reagent consumption and greater recoveries as the sample transfer is minimal [18]. Despite the undoubted advantages, SPE does not always perform its task. It happens due to the physicochemical properties of some compounds that strongly adsorbed on the surface of the laboratory vessel walls. This adsorption may cause high loss of the analyte. In the liquid extraction method, the solvent is added directly to the sample. It allows the analytes adsorbed on the laboratory vessel walls to be rinsed.
Both liquid and gas chromatography can possess different detection limits, recoveries, accuracy and the repeatability of obtained results. These features depend on the type of analyzed compound and the conditions of sample extraction.
The paper presents a selection of procedure for determining the concentration of three compounds from the macro-group of Pharmaceutical and Personal Care Products. The goal of this study is to select the type of SPE Tube, the extraction process conditions and the performance parameters of chromatograph during the determinations of the substances.
2. Materials and Methods
The standards of micropollutants: carbamazepine (CBZ), caffeine (CAF) and benzophenone-3 (BP-3) were supplied by Sigma-Aldrich. The properties of the tested compounds are summarized in Table 1. Organic solvents: methanol and acetonitrile of purity grade >99.8% and >99.5% respectively, by Avantor Performance Materials Poland S.A. were also used. Disposable Supelclean™ Tubes by Supelco were applied to Solid Phase Extraction. Six types of SPE tubes were tested: ENVI™-8, ENVI™-18, LC™-8, LC™-18, LC™-CN, LC™-Ph. They are compared in Table 2. The extract was analyzed using a Gas Chromatograph coupled to Mass Spectrometry (GC-MS) with Electronic Ionization, Model 7890B by Perlan Technologies. The extract was separated in SLBTM- 5 ms Capillary GC Column of Supelco with an internal diameter of 0.25 mm, a length of 30 m and a layer thickness of 0.25 μm.

Table 1.
Characteristics of tested compounds.

Table 2.
Characteristics of Supelclean™ Tubes applied to Solid Phase Extraction.
3. Results and Discussion
In the first part of the research a selection of chromatographic conditions was performed. The linearity of the mass detector response was examined. The following GC-MS (EI) operating parameters have been determined:
- the oven temperature program: 80 °C (6 min), 5 °C/min to 260 °C, 20 °C/min to 300 °C,
- the support phase: helium with a flow of 1.1 mL/min,
- injector: 250 °C,
- ion source: 230 °C,
- ion trap: 150 °C,
- ion recording mode: 50–700 m/s.
In order to calibrate the mass detector, the calibration curves were prepared based on standard solutions prepared in methanol, in a concentration range from 0.5 to 10 ng/µL. Linearity of the detector response was checked by linear regression (Figure 1). Parameters of calibration curves are presented in Table 3.
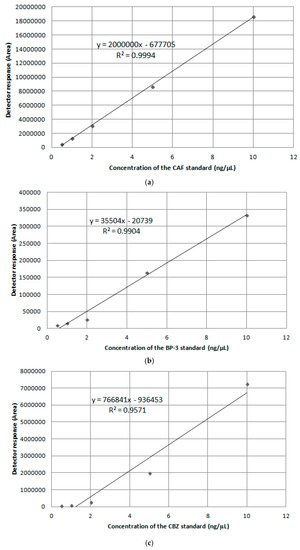
Figure 1.
Calibration curve by GC-MS for (a) CAF; (b) BP-3; (c) CBZ.

Table 3.
Parameters of calibration curves for determining micropollutants by GC-MS.
The obtained values of R2 coefficient show the linearity of the detector’s response. Retention times of compounds allow for proper separation and appropriate identification in complex water matrices. The standard deviations of tR are acceptable.
In the process of identifying and assessing the concentration of micro-organic compounds in swimming pools, the repeatability of the quantitative results is of key importance. Table 4 shows the values of the Coefficient of Variation (CV) that is a measure of the repeatability of the measurements. The Limit of Detection (LOD) was also determined and presented in Table 4. It determines the lowest amount of a substance that can be distinguished from the absence of that substance within a certain confidence interval [19]. The obtained values of CV do not exceed 3% that confirm the high repeatability of the conducted measurements.

Table 4.
Coefficient of Variation (CV) for five concentration levels of tested micropollutants and the Limit of Detection (LOD).
The main step in developing an analytical procedure for the determination of compounds in pool water is the selection of a sample preparation procedure. Because of the complexity of the matrix and the low concentrations of analytes, it is necessary to isolate the analytes from the samples. Solid Phase Extraction was used to separate the compounds from swimming pool water. The optimization of extraction conditions was performed by searching of the appropriate combination of SPE Tube type and the solvents used for both conditioning and elution. It was carried out by inserting the standard at the concentration level of 1 mg/L into the deionized water matrix. Then it was subjected to an SPE process using different type of tubes and different solvents. Recovery and Limit of Quantification (LOQ) were examined for each method of sample preparation. They are listed in Table 5. Based on these parameters, the most optimal methodology was chosen. Conditioning with a mixture of methanol and acetonitrile and extraction in the ENVI-18 Tube was considered the best suited. The worst results were obtained after the conditioning with a mixture of methanol and acetonitrile and extraction in the LC-CN Tube.

Table 5.
Recovery and LOQ for various combinations of SPE Tube types and the solvents.
Table 6 summarizes recoveries obtained in the most optimal Solid Phase Extraction methodology for the various matrices. It was carried out by inserting the standard at the concentration level of 1 mg/L into the different water matrices (the deionized water, the tap water and the swimming pool water). The lowest recovery was obtained for tap water. The recoveries of both deionized water and swimming pool water was 100%. Based on the calculated recovery factors, the accuracy of the results obtained from the chosen analytical method was very good. The repeatability of the results, measured as the standard deviation, was satisfactory. Its value was in the range from 1 to 10%.

Table 6.
Recoveries obtained in the most optimal Solid Phase Extraction methodology (Methanol + Acetonitrile and ENVI-18 Tube) for different matrices.
The Limits of Quantification of tested compounds in different matrices are presented in Table 7. The lowest LOQs were obtained for swimming pool water, while the highest were observed for deionized water. The observed differences show the influence of organic and inorganic substances presence in water matrix on the LOQ value.

Table 7.
Limits of Quantification obtained in the most optimal Solid Phase Extraction methodology (Methanol + Acetonitrile and ENVI-18) for different matrices.
4. Conclusions
- The presented analytical procedure enables the quantification of caffeine, carbamazepine and benzophenone-3 with satisfactory repeatability and accuracy.
- The obtained recovery values ensure the possibility of full quantitative control of the tested micropollutants in samples collected from swimming pool waster systems.
- The developed methodology can be used for analytical control of swimming pool water treatment processes from selected Pharmaceuticals and Personal Care Products.
- The different physicochemical composition of water affect on LOQ. The values of LOQ obtained for swimming pool water were lower than for deionized and tap water.
Author Contributions
Anna Lempart and Edyta Kudlek conceived and designed the experiments, performed the experiments and analyzed the data under the supervision of Mariusz Dudziak; Mariusz Dudziak contributed reagents, materials and analysis tools; Anna Lempart wrote the paper under the supervision and review of Mariusz Dudziak and Edyta Kudlek.
Acknowledgments
This research was supported by research funds for young researchers awarded to the Institute of Water and Wastewater Engineering of the Silesian University of Technology No. BKM/554/RIE-4/2017.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Guidelines for Safe Recreational Water Environments. Volume 2: Swimming Pools and Similar Environments. 2006. Available online: www.who.int/water_sanitation_health/water-quality/recreational/en/ (accessed on 25 September 2017).
- Standards DIN 19643 1-4:2012-11. Aufbereitung von Schwimm und Badebeckenwasser (Water Treatment for Swimming and Bathing Pools); Beuth-Verlag: Berlin, Germany, 2012. [Google Scholar]
- Chowdhury, S.; Alhooshani, K.; Karanfil, T. Disinfection by-products in swimming pool: Occurrences, implications and future needs. Water Res. 2014, 53, 68–109. [Google Scholar] [CrossRef] [PubMed]
- Kanan, A.; Karanfil, T. Formation of disinfection by-products in indoor swimming pool water: The contribution from filling water natural organic matter and swimmer body fluids. Water Res. 2011, 45, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jun, M.J.; Lee, M.H.; Eom, S.W.; Zoh, K.D. Production of various disinfection byproducts in indoor swimming pool waters treated with different disinfection methods. Int. J. Hyg. Environ. Health 2010, 213, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Wyczarska-Kokot, J. Comparison of chloramine concentration in swimming pool water depending on swimming pool intended use. Ecol. Chem. Eng. A 2015, 22, 27–37. [Google Scholar] [CrossRef]
- Terasaki, M.; Makino, M. Determination of chlorinated by-products of parabens in swimming pool water. Int. J. Environ. Anal. Chem. 2008, 88, 911–922. [Google Scholar] [CrossRef]
- Teo, T.L.L.; Coleman, H.M.; Khan, S.J. Chemical contaminants in swimming pools: Occurrence, implications and control. Environ. Int. 2015, 76, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, P.; Bonadonna, L.; Chirico, M.; Caroli, S.; Záray, G. Emerging issues on degradation by-products deriving from personal care products and pharmaceuticals during disinfection processes of water used in swimming pools. Microchem. J. 2014, 112, 13–16. [Google Scholar] [CrossRef]
- Koutsouba, V.; Heberer, T.; Fuhrmann, B.; Schmidt-Baumler, K.; Tsipi, D.; Hiskia, A. Determination of polar pharmaceuticals in sewage water of Greece by gas chromatography-mass spectrometry. Chemosphere 2003, 51, 69–75. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Vanderford, B.J.; Pearson, R.A.; Rexing, D.J.; Snyder, S.A. Analysis of endocrine disruptors, pharmaceuticals, and personal care products in water using liquid chromatography/tandem mass spectrometry. Anal. Chem. 2003, 75, 6265–6274. [Google Scholar] [CrossRef] [PubMed]
- Ollers, S.; Singer, H.P.; Fässler, P.; Müller, S.R. Simultaneous quantification of neutral and acidic pharmaceuticals and pesticides at the low-ng/l level in surface and waste water. J. Chromatogr. A 2001, 911, 225–234. [Google Scholar] [CrossRef]
- Lee, H.B.; Peart, T.E.; Svoboda, M.L. Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2005, 1094, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Zhao, X.; Yang, P. GC-MS and HPLC-MS analysis of bioactive pharmaceuticals and personal care products in environmental matrices. Trends Anal. Chem. 2007, 26, 569–580. [Google Scholar] [CrossRef]
- Mottaleb, M.A.; Bellamy, M.K.; Mottaleb, M.A.; Islam, M.R. Use of LC-MS and GC-MS methods to measure emerging contaminants Pharmaceutical and Personal care Products (PPCPs) in Fish. J. Chromatogr. Sep. Tech. 2015, 6, 267. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadia, Y.; Milani Hosseini, M.R.; Aghaeea, A.; Ahmadia, F.; Berijania, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, D.; Crummett, W.B. Guidelines for data acquisition and data quality evaluation in environmental chemistry. Anal. Chem. 1980, 52, 2242–2249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).