Abstract
Autocrine growth hormone (GH) induced cell proliferation, invasion-metastasis and drug resistance in breast cancer cells. Curcumin has an apoptotic effect on colon, melanoma, cervix, and breast cancer cells. Autophagy and endoplasmic reticulum (ER) stress are essential cellular processes activated under nutrient deprivation, pathogen infection and drug exposure. Our aim in this study is to investigate the time-dependent effect of curcumin on ER stress and autophagy and potential increase of curcumin efficiency by bafilomycin treatment. Autocrine GH expression triggered resistant profile against curcumin-induced cell viability loss in MCF-7 cells. However, this effect was prevented by the time-dependent manner in MCF-7 cells. In GH+ breast cancer cells bafilomycin increase curcumin-induced cell viability loss by MTT cell viability assay. In conclusion, autocrine GH-triggered curcumin resistance was overcome by autophagy inhibition condition by bafilomycin treatment in a dose-dependent manner in MCF-7 GH+ breast cancer cells.
1. Introduction
Breast cancer is the most common cancer type among women worldwide and is the fifth most common cause of death from cancer in women [1]. Prolactin (PRL) and Growth hormone (GH) are essential hormones acting on mammary gland development and their expressions have been determined in breast cancer tissue samples [2]. Curcumin, a natural plant-derived compound, isolated from Curcuma Longa has anti-proliferative, anti-oxidant, anti-inflammatory, anti-carcinogenic properties. Beside apoptotic cell death, curcumin induced autophagy and endoplasmic reticulum stress in cancer cells [3]. Autophagy is a cellular self-degradative process that is essential in energy source balancing under nutrient stress conditions. Misfolded or aggregated proteins, intracellular pathogens, damaged organelles (mitochondria, endoplasmic reticulum, etc.) can be eliminated through autophagy activation in order to maintain cell survival [4].
Our aim in this study is to demonstrate the role of endoplasmic reticulum stress/autophagy in autocrine GH-mediated curcumin resistant in MCF-7 breast cancer cells and potential incline on the curcumin triggered apoptotic cell death through autophagy suppression condition by bafilomycin treatment.
2. Material and Methods
Time-dependent curcumin (Sigma Aldrich, C1386, Munich, Germany) effect on cell viability was determined by mitotracker/DAPI (Thermo fisher Scientific, Mitotracker Red CMXRos, M7512, DAPI, D1306, Waltham, MA, USA) staining. Autophagy and ER stress key molecules (Cell signaling technology, Autophagy antibody sampler kit, 4445, ER stress antibody sampler kit, 9956, Danvers, MA, USA) expression profile was determined by immunoblotting in MCF-7 wt (ATCC, Manassas, VA, USA) and GH+ breast cancer cells. The potential additional effect of bafilomycin (Abcam, ab120497, Cambridge, UK) on curcumin-induced cell viability loss was determined by MTT (Sigma Aldrich, M2128, St. Louis, MO, USA) cell viability assay. Curcumin-mediated autophagy induction was determined by GFP-LC3 plasmid transfection (Promega, psetz-gfplc3, Madison, WI, USA), following drug exposure with/without bafilomycin and analyzed by FACS flow cytometer (BD Bioscences, 556547, San Jose, CA, USA). The activation of CHOP following curcumin exposure and the preventable effect of CHOP activation was observed by bafilomycin treatment in MCF-7 wt and GH+ cells were performed by FACS flow analysis. Bafilomycin and curcumin co-treatment on cell cycle and apoptotic cell death was demonstrated by hanging drop assay.
3. Results
Although time-dependent curcumin (20 μM) treatment decrease cell viability loss in MCF-7 wt and GH+ breast cancer cells, autocrine GH-induced drug resistant was overcome by time dependent manner in MCF-7 cells. Curcumin resistance was observed in early drug (3–9 h) exposure in MCF-7 breast cancer cells by mitotracker/DAPI staining (data not shown). In the Figure 1, when we checked the expression profile of ER stress and autophagy key markers following curcumin exposure in MCF-7 wt and GH+ breast cancer cells, PERK expression was slightly upregulated after 9 h curcumin treatment in MCF-7 wt cells but significant upregulation was observed after 6 h in MCF-7 GH+ breast cancer cells. Although BIP expression was increased following 6 h curcumin exposure in MCF-7 wt cells, this sharp effect was not determined in autocrine GH expressing MCF-7 cells as compared to wt MCF-7 cells. In addition, prolonged curcumin exposure triggered IRE1α expressing MCF-7 wt cells, but the early IRE1α response was determined in MCF-7 GH+ breast cancer cells (Figure 2A). When we investigate the time-dependent curcumin effect on autophagy essential proteins such as Beclin-1, Atg-5, Atg-12 significant upregulation was observed only after 3 h curcumin treatment in MCF-7 wt breast cancer cells. Beclin-1, Atg-5 and Atg-12 expressions were upregulated after 12 h curcumin exposure in MCF-7 GH+ breast cancer cells (Figure 2B).
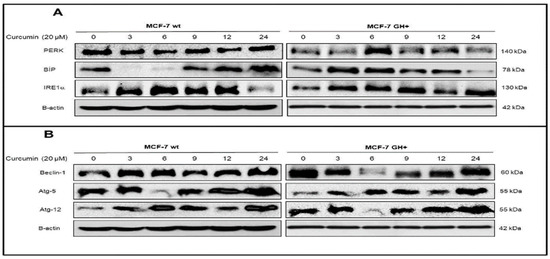
Figure 1.
Curcumin triggers autophagy and ER stress in a time-dependent manner.
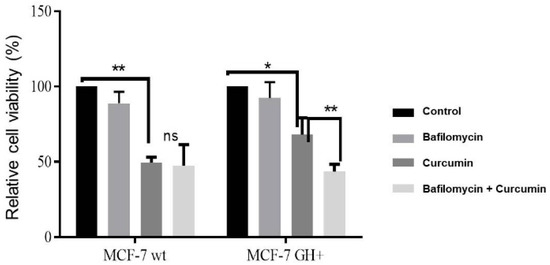
Figure 2.
Bafilomycin increased curcumin-induced cell viability loss in MCF-7 wt and GH+ breast cancer cells.
To determine the potential apoptotic effect of curcumin under autophagy suppression conditions in autocrine GH expressing MCF-7 breast cancer cells, we performed MTT cell viability assay, hanging drop. According to the MTT cell viability assay. Curcumin decreased cell viability by 50% and 60% in MCF-7 wt and GH+ cells, respectively. However, curcumin-mediated cell viability loss was increased to 60% and 53% under autophagy inhibition conditions by bafilomycin in MCF-7 wt and GH+ cells, respectively (Figure 2).
When we checked the LC3-II cleavage formation following curcumin exposure in MCF-7 wt and GH+ cells, bafilomycin prevented curcumin-induced LC3-II cleavage in MCF-7 wt and GH+ cells (data not shown). To demonstrate the curcumin-mediated CHOP activation, we performed mCherry-CHOP plasmid transfection and FACS flow cytometer analysis. When we use bafilomycin to prevent curcumin-induced autophagy, CHOP activation was also decrease in MCF-7 wt and GH+ cells (data not shown). In addition, we observed a significant decline in colony diameters following curcumin and bafilomycin co-treatment in both MCF-7 wt and GH+ breast cancer cells (Figure 3). Concomitantly, curcumin induced apoptotic cell death was increased under autophagy inhibition conditions by bafilomycin in each MCF-7 breast cancer cells.
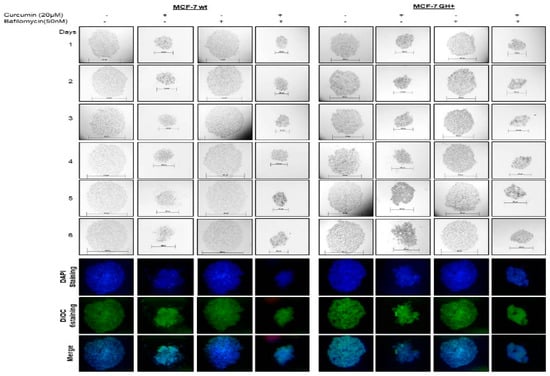
Figure 3.
Bafilomycin increased curcumin-mediated invasion-metastasis prevention in autocrine GH expressing MCF-7 breast cancer cells. (Dioc6: Termo Fisher Scientific, D273, Waltham, MA, USA).
In this study, we showed that autocrine GH triggered curcumin resistant in a time-dependent manner in MCF-7 breast cancer cells by activating. ER stress and autophagy key players expressions. Forced GH expression induced curcumin resistance overcome by autophagic inhibitor and curcumin co-treatment in MCF-7 breast cancer cells.
4. Conclusions
Curcumin resistance due to autocrine GH expression overcome by time-dependent curcumin exposure in MCF-7 breast cancer cells. Curcumin upregulated ER stress key markers were upregulated as an early response in MCF-7 breast cancer cells following curcumin treatment, which led to sharp decrease in cell viability when the autophagy was inhibited.
References
- Sun, Y.S.; Zhao, Z.; Yang, Z.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Berry, P.A.; Jiang, J.; Lobie, P.E.; Langenheim, J.F.; Chen, W.Y.; Frank, S.J. Growth Hormone Signaling in Human T47D Breast Cancer Cells: Potential Role for a Growth Hormone Receptor-Prolactin Receptor Complex. Mol. Endocrinol. 2011, 25, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.O.; Yadav, R.K.; Kim, H.R.; Chae, H.J. ER stress: Autophagy induction, inhibition and selection. Autophagy 2015, 11, 1956–1977. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).