Abstract
Psoriasis is a chronic and relapsing skin disease that affects approximately 2–3% of the world population and is considered as an inflammatory disease. Defective keratinocyte proliferation and differentiation programs, aberrant immune responses are the major factors that have been implicated in its pathogenesis. Thick scaly plaques, which are psoriasis characteristic formed by the abnormal maturation of keratinocytes and epidermal hyperplasia. The aim of this study is to establish the initial stage of psoriasis model by LPS (lipopolysaccharide) treatment to HaCaT (transformed keratinocyte) cells. MTT assay of LPS was performed to hallmark the initial stage of psoriasis. ELISA assay was performed to determine the cytokine release as response to LPS for psoriasis initial stage. According to the MTT assay results 200 ng/mL used as the test dose and 8µg/mL LPS determined as the toxic dose at 72th hour. LL-37 was the first cytokine whose expression increased in the formation of the immune response. Besides, TNF-α release occurs soon after. The presence of immune system cells is needed to maintain IL-17 and IL-1β release. The cytokine release at the 72th hour decreased to control level due to the lack of immune cells in the culture medium.
1. Introduction
Psoriasis is a chronic, multifactorial, inflammatory skin disease characterized by T cell-mediated keratinocyte hyperproliferation and shiny white scales. The etiology of the disease relies on both genetic and environmental factors. Psoriasis can be triggered by many different causes such as damage, trauma, infection, environmental factors, drugs, metabolic factors, alcohol and smoking [1]. Keratinocytes are the basic and dense cell type of the epidermis. Keratinocytes are arranged in five layers in the epidermis; stratum basale, stratum spinozum, sratum granulose, stratum lusidum and stratum corneum. In the process of epidermal terminal differentiation, the prismatic cells in the basal layer become more and more flat as they move upwards and lose their nuclei and cornify. Programmed cell death is the fundamental step of cornification [2]. While this process is normally 28–30 days, it decreases to 3–4 days in psoriatic skin. Psoriasis characteristic of thick scaly plaques formed by abnormal maturation and hyperproliferation of keratinocytes. This hyperproliferation is driven by cytokines secreted by activated resident immune cells, infiltrating T cells, dendritic cells and cells of the innate immune system, as well as the keratinocytes themselves [3]. The aim of this study is to establish a model of the psoriasis’ initial stage by LPS (lipopolysaccharide) treatment of keratinocytes, the main cells affected in the pathogenesis of psoriasis, using transformed cell line HaCaT.
2. Materials and Methods
DMEM-high glucose (Gibco) medium was mixed with 10% FBS (Gibco), 1% penicillin-streptomycin (Gibco) and 1x amphotericin B (Capricorn) for HaCaT cell culture. Cells were grown in 5% CO2, 95% air humidified incubator at 37 °C.
To construct the cell culture psoriasis model, it was planned to use LPS to initiate the keratinocyte response by inducing expression of proinflammatory cytokines. For LPS test dose and toxic dose determination, the MTT assay was performed according to the manufacturer’s instructions (Vybrant MTT Cell Proliferation Assay Kit). After 24 h incubation, cells were treated at a concentration of 0, 10, 50, 100, 200, 500, 1000, 1500 ng/mL LPS (Sigma-L2630) diluted with PBS, as triplicated. Readings were then taken at 12, 24, 48 and 72 h. No toxicity was seen after the first trial and the MTT test was repeated with LPS treatment at higher concentrations to determine the LD50 dose. Cells were treated at a concentration of 0, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5, 6, 6.5, 7, 7.5 and 8 mg/mL LPS at 24, 48 and 72 h.
The release of IL-1β, IL-17, TNF-α and LL-37, which are pro-inflammatory and inflammatory mediators, in the media of the cultured cells were determined by ELISA assay (YLBiont).100.000 cells were seeded per well of 6-well plate. After 24 h, cells were treated with 200 ng/mL LPS as the test dose. The culture medium was collected at 1, 2, 4, 8, 12, 24, 48, and 72 h, and the amount of cytokine release was determined with ELISA. The collected media at the specified times were kept at +4 °C until ELISA measurement was performed. ELISA assay was performed according to the manufacturer’s instructions
3. Results
According to the results of cell survival rate, cells treated with 200 ng/mL LPS were showed the most increase in proliferation (Figure 1). The MTT test was repeated with LPS treatment at higher concentrations since no toxicity was observed after the first trial. According to the cell survival rates, LD50 dose of LPS was determined 8 mg/mL at 72th hour (Figure 2).
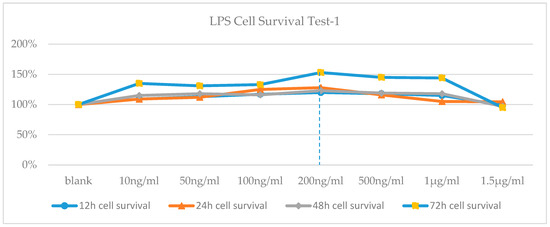
Figure 1.
Cell Viability-time chart of LPS-applied cells.
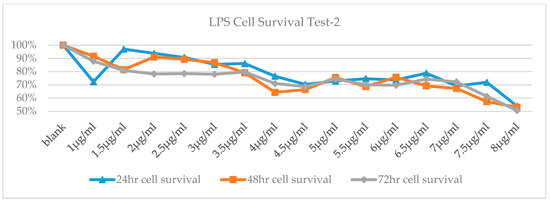
Figure 2.
Cell Viability-time chart of high-dose LPS.
It was observed that the release of the antimicrobial peptide, LL-37, started at 1.5th hour and reached its peak at the 8th hour, and it started to decrease after 24th hour. Likewise, TNF-α release started to increase at 3th hour, between 4th–8th hours had a partial plateau (which showed an increase of 25% at the 8th hour) and then decrease quickly (Figure 3). It was observed that there was no significant change in IL-17 release until 24th hour, there was an increase in 24th hour (48%) and immediately decreased. It was observed that IL-1β release started to increase in 12th hour, increased by 50% at 24th hour and returned to baseline at 72th hour. (Figure 4).
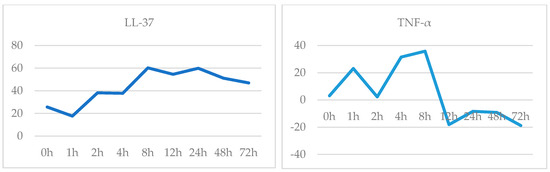
Figure 3.
LL-37 (ng/mL) and TNF-α (ng/L) release-time graph.
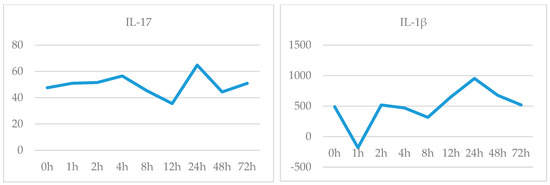
Figure 4.
IL-17 (ng/L) and IL-1β (pg/L) release-time graph.
4. Discussion
The interrelations of cytokines expressed in psoriatic skin determine the hyperproliferation of keratinocytes, increased neovascularization and inflammation [4]. Immune system activation, which is the first step of psoriasis pathogenesis, was evaluated by cytokine release in this study. The cytokines stimulate the expression of key growth factors and immune genes, including proinflammatory cytokines, chemokines, and antimicrobial peptides, which amplify immune circuits, accelerate keratinocyte turnover, and impact epidermal differentiation [1,5]. Expression of the TNF-α, Type I cytokine, increased at 3th hour and LL-37 (an antimicrobial peptide) increased at 1.5th hour and both reached its peak level at the 8th hour, suggesting that the activation of the immune system is too early. The antimicrobial peptide LL-37, which plays an important role in the development of the immune response, begins to increase at the second hour and indicates that response has begun, TNF-α release starts soon after. TNF-α is secreted by many cells involved in the pathogenesis of psoriasis, such as keratinocytes, dendritic cells, NK cells, Th1, Th17 and Th22. TNF-α is a multifunctional cytokine that regulates inflammation, immune response, cell motility, cell cycle, tissue regeneration and apoptosis. It plays roles in both the onset and chronic phase of psoriasis [6]. Previous studies have also shown increased TNF-α expression in active plaques. TNF-α expression describes the relationship between keratinocyte proliferation and immune activation and tissue inflammation [7]. In our study, the release of TNF-α reached up to the highest level between 4–8 h and it contribute to support this information. Cytokines such as IL-17, TNF-α and IL-1β play a role in the formation of keratinocyte response in psoriasis by themselves or in combination. IL-17, TNF-α and IL-1β cause keratinocyte proliferation, but also increase the production of other chemokines and cytokines [1]. The presence of immune system cells is needed to maintain IL-17 and IL-1β release as well as TNF-α. Our results showed that the cytokine release at 72th hour is same as control level, we think that this decrease is due to the lack of immune cells in the culture medium.
Acknowledgments
This study was supported by Pamukkale University Scientific Research Projects Coordination Unit with grant number 2016SABE007.
References
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Tschachler, E. Control of cell death-associated danger signals during cornification prevents autoinflammation of the skin. Exp. Dermatol. 2018, 27, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Krueger, J.G. The Immunopathogenesis of Psoriasis. Dermatol. Clin. 2015, 33, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Baliwag, J.; Barnes, D.H.; Johnston, A. Cytokines in Psoriasis. Cytokine 2015, 73, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Schonthaler, H.B.; Guinea-Viniegra, J.; Tschachler, E. Psoriasis: What we have learned from mouse models. Nat. Rev. Rheumatol. 2010, 6, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Grine, L.; Dejager, L.; Libert, C.; Vandenbroucke, R.E. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015, 26, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Shobaili, H.A.; Qureshi, M.G. Pathophysiology of Psoriasis: Current Concepts. In Psoriasis, Hermenio Lima; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).