Abstract
In-liquid biosensing is the new frontier of cells real time monitoring and biomarkers detection. In order to improve the stability and electrical properties of an Electrolyte Gated Organic Field Effect Transistor (EGOFET) biosensor, in this study we investigate the effect of the solvent and of the substrate modification on thin films of organic semiconductor Poly(3-hexylthiophene) (P3HT). The studied surface is the relevant interface between the P3HT and the electrolyte acting as gate dielectric for in-liquid detection of an analyte. AFM and XPS characterizations were employed to study the effect of two solvents (toluene and 1,2-dichlorobenzene) and of the adhesion promoter (Ti prime) on the morphological structure and electronic properties of P3HT film. Combining the results from the surface characterizations with electrical measurements, we investigate the changes on the EGOFET performances and stability in DI water with an Ag/AgCl gate electrode.
1. Introduction
Electrolyte Gated Organic Field Effect Transistors (EGOFETs) are emerging as a promising technology for biosensing applications due to their high sensibility, low-voltage operation, biocompatibility and low-cost fabrication [1].
Poly(3-hexylthiophene) (P3HT) is one of the most used materials for this kind of devices due to its relatively high carrier mobility and easy processability [2]. Recent works have exploited P3HT as organic semiconductor for EGOFET biosensors [3], but the main drawback still affecting these devices is their fast degradation when working in ambient conditions [4], also with respect to similar devices as Organic Electro Chemical Transistors (OECTs), which exhibit a better stability in-liquid [5] and a tailorable conductive behavior [6].
In this study we investigate the effect of solvents and adhesion promoter on the morphology and electronic structure of a P3HT film surface. Being this surface the interface between the organic semiconductor and the liquid dielectric in an EGOFET, by optimizing the surface properties we can optimize device performances and stability.
2. Materials and Methods
2.1. Materials and Reagents
Poly(3-hexylthiophene-2,5-diyl) (P3HT) was purchased from Rieke Metals (Mw= 37 kDa, regioregularity > 96%, RMI001-EE), Ti prime adhesion promoter was purchased from MicroChemicals, all other chemicals were purchased from Sigma Aldrich.
2.2. Device Fabrication
Ti adhesion layer (10 nm) and Au layer (100 nm) were e-beam evaporated on Si/SiO2 substrates. Afterwards, source and drain electrodes were photolithographically patterned (channel length = 20 µm, channel width = 2000 µm). After photolithography, half of the samples were treated with the adhesion promoter: Ti prime was spin coated at 4000 rpm for 30 s and dried at 120 °C for 2 minutes on a hot plate. P3HT solutions were prepared with concentration of 2.5 mg/ml in toluene or in 1,2-dichlorobenzene (oDCB) and spin coated at 2000 rpm for 30 s. Finally, the devices were dried overnight at 120 °C under vacuum to completely remove any solvent residues.
Samples for XPS and AFM analyses were fabricated following the same procedures but on clean Si/SiO2 substrates without patterned source and drain electrodes.
2.3. Characterizations
AFM characterization have been performed in tapping mode with a Bruker Atomic Force Microscope to monitor the quality of P3HT film surface.
XPS analysis has been performed using the X-ray source Al Kα 1486.6 eV, pass energy 187.85 eV for survey analysis and 23.50 eV for peaks and valence band analysis.
Electrical characterizations have been performed with a Keysight B2912A Source/Measure unit. Sheet resistances of P3HT films (thickness 30 nm) were measured first. Then the devices were characterized in EGOFET configuration using DI water as gate electrolyte and an Ag/AgCl leak free reference electrode as gate electrode.
3. Results and Discussion
3.1. Tapping Mode AFM
AFM analysis has been carried out on each of the 4 different samples (toluene or oDCB based P3HT solutions, with or without Ti prime). Figure 1a and Figure 1b show P3HT deposited using toluene solution on clean substrate and on SiO2 treated with adhesion promoter. The presence of Ti prime results in a slightly smoother surface topography, with an RMS roughness of 2.41 nm compared to 2.85nm of the film without adhesion promoter. In both cases the surface is strongly disordered. In contrast, films obtained using oDCB as solvent (Figure 1c,d) present a flatter surface topography with RMS roughness around 0.65 nm for both samples and organization in nano-crystalline domains.

Figure 1.
AFM images acquired in tapping mode of P3HT deposited on Si/SiO2 substrate: (a) from toluene solution without adhesion promoter; (b) from toluene solution with Ti prime; (c) from oDCB solution without adhesion promoter; (d) from oDCB solution with Ti prime.
3.2. XPS Characterization
The density of states in the valence band region has been investigated via XPS spectroscopy. Figure 2 shows the spectra obtained for the different samples. Contributions at high binding energy (B.E. > 5 eV) are due to binding σ orbitals from the alkyl chains, while between 0 and 5 eV there are only contributions coming from π orbitals of the conjugated backbones. The peak at 3.4 eV in the spectra of P3HT deposited from the toluene solution can be attributed to localized states, and its broadening in the spectra of P3HT deposited from oDCB solution may be related to a higher degree of delocalization of the molecular orbital, possibly indicating a higher degree of π-π conjugation of different polymer backbones, which could improve the electrical properties of the material [7].
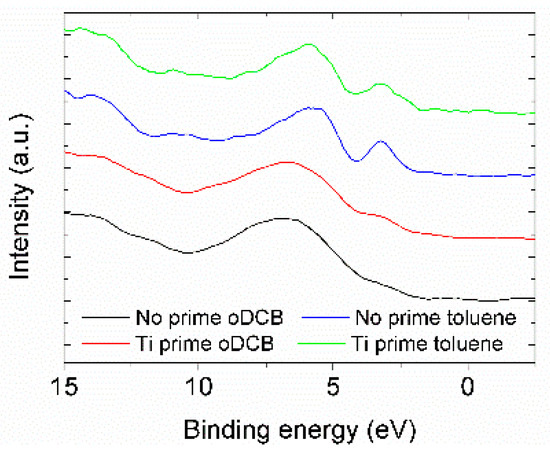
Figure 2.
Density of states in the valence band region measured for the four different films.
3.3. Electrical Characterization
Sheet resistance measurements (Figure 3) show that films obtained using oDCB as solvent instead of toluene are three orders of magnitude more conductive. This result supports the hypothesis of a better delocalization of states coming from the XPS spectra analysis. Moreover, samples treated with Ti prime show lower standard deviation independently on the solvent, suggesting that the presence of the adhesion promoter can improve the device-to-device reproducibility.
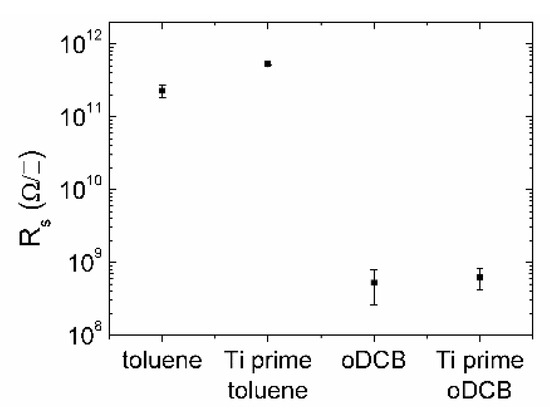
Figure 3.
Sheet resistances measured on a set of minimum three devices for each kind of sample. Devices obtained from oDCB solution generally show a three-orders-of-magnitude improvement in conductivity, while samples treated with Ti prime show higher reproducibility for both solvents.
Transfer characteristics (Ids vs. Vgs) measured using DI water as gate electrolyte are shown in Figure 4, the same measurement has been repeated several times on the same device. The device fabricated from toluene solution without Ti prime (Figure 4a) shows lower transconductance and faster degradation with respect to the one fabricated from oDCB solution with Ti prime (Figure 4b) which remains stable throughout multiple measurements and is able to work in a smaller voltage window.
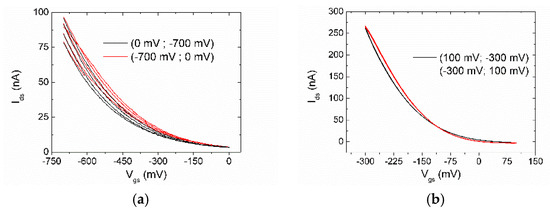
Figure 4.
Transfer characteristics measured multiple times of EGOFET fabricated using P3HT dissolved in: (a) toluene without adhesion promoter; (b) oDCB with Ti prime adhesion promoter.
4. Conclusions
In conclusion, AFM and XPS results suggest that the use of oDCB improves the polymer crystallization, resulting in a film with smoother surface and a higher degree of delocalization of electronic states at the HOMO level (valence band). Sheet resistance measurements quantitatively assess the improvement in conductivity due to the choice of the solvent and suggest that an adhesion promoter treatment may reduce device-to-device variations. Finally, EGOFETs characterization demonstrates that a device fabricated using oDCB as solvent and Ti prime as adhesion promoter is stable during multiple measurements and can work in a smaller voltage window, avoiding the fast degradation obtained for the EGOFET fabricated with toluene solution and no adhesion promoter.
Author Contributions
Design of the devices, M.P., S.L.M. and A.V.; Methodology of the experiments, M.C. and M.P.; Device fabrication, M.P. and A.B.; AFM and XPS Analysis, E.P., M.P. and V.F.; Electrical analysis M.P.; Writing—Review and Editing, M.P., S.L.M. and M.C.; Supervision, C.F.P. and S.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was performed in the framework and financed by POLITO BIOMed LAB, financed by Politecnico di Torino, and DEFLeCT (“Advanced platform for the early detection of not small cells lung cancer”) project, financed by Piedmont Region in the framework of “Health &Well Being” Platform project.
Acknowledgments
The authors want to acknowledge Salvatore Guastella for his work on XPS characterization.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kergoat, L.; Piro, B.; Berggren, M. Advances in organic transistor-based biosensors: From organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 2012, 402, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Ludwigs, S. P3HT Revisited—From Molecular Scale to Solar Cell Devices. Adv. Polym. Sci. 2014, 265, 39–82. [Google Scholar]
- Seshadri, P.; Manoli, K.; Schneiderhan-marra, N.; Anthes, U.; Wierzchowiec, P.; Bonrad, K.; Di, C.; Torsi, L. Label-free procalcitonin analytical detection with an electrolyte-gated organic field-effect transistor based electronic immunosensor. Biosens. Bioelectron. 2018, 104, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sirringhaus, B.H. Device Physics of Solution-Processed Organic Field-Effect Transistors. Adv. Mat. 2005, 17, 2411–2425. [Google Scholar] [CrossRef]
- D’Angelo, P.; Tarabella, G.; Romeo, A.; Giodice, A.; Marasso, S.; Cocuzza, M.; Ravanetti, F.; Cacchioli, A.; Petronini, P.G.; Iannotta, S. Monitoring the adaptive cell response to hyperosmotic stress by organic devices. Mrs Commun. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Angelo, P.D.; Tarabella, G.; Romeo, A.; Marasso, S.L.; Verna, A.; Cocuzza, M.; Peruzzi, C.; Vurro, D.; Iannotta, S.; D’Angelo, P.; et al. PEDOT:PSS Morphostructure and Ion-To-Electron Transduction and Amplification Mechanisms in Organic Electrochemical Transistors. Materials 2018, 12, 9. [Google Scholar] [PubMed]
- Dannetun, P.; Boman, M.; Stafström, S.; Salaneck, W.R.; Lazzaroni, R.; Fredriksson, C.; Brédas, J.L.; Taliani, C.; Dannetun, P.; Boman, M.; et al. The chemical and electronic structure of the interface between aluminum and polythiophene semiconductors. J. Chem. Phys. 1993, 99, 664–671. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).