Abstract
This study presents a computational tool for analyzing gas mixtures resulting from the integration of biomethane and hydrogen into natural gas networks. It calculates key properties, such as relative density, higher heating value and Wobbe index, based on composition, temperature and pressure, using the van der Waals equation to model real gas behavior. The tool also offers interactive 3D visualizations to explore how these properties vary under different conditions. With prediction errors below 0.5% at 20 atm, it provides a reliable basis for assessing technical feasibility, regulatory compliance and energy efficiency in the integration of alternative gases into existing networks.
1. Introduction
The increasing incorporation of renewable gases, such as biomethane (bio-CH4) and hydrogen (H2), into natural gas (NG) networks has intensified the need for thorough evaluation of the resulting mixtures, as their physicochemical characteristics differ significantly from those of conventional NG and may affect combustion behavior, energy efficiency and compliance with quality standards [1].
This study adopts a twofold methodological approach to characterize such mixtures. The first method consists of a comparative analysis between two pure gases, typically NG and either bio-CH4 or H2, by calculating and comparing key thermodynamic and combustion-related properties, including relative density, higher heating value (HHV) and Wobbe index. This provides a reference framework that highlights the inherent performance differences between the gases.
The second method simulates a wide range of gas compositions, focusing on blends with varying bio-CH4 concentrations mixed into NG. By systematically varying these proportions and analyzing the resulting properties under different operational conditions, such as temperature and pressure, the method offers a broader understanding of mixture behavior and its suitability for injection into existing infrastructure.
Combined, these approaches provide a comprehensive basis for evaluating the impact of renewable gas integration on gas quality, energy transmission and regulatory compliance.
2. Methods
The computational tool, developed in C#, features a user-friendly interface that allows input gas compositions, definition of operating conditions, and automatic calculation of key thermodynamic properties. All graphical outputs—including 3D surface plots and comparison charts—are generated natively to ensure consistency between simulations and visual representations. Results are updated in real time based on user-defined parameters.
The first methodological approach evaluates the impact of bio-CH4 injection into the NG pipeline on gas quality, relative to technical and regulatory thresholds defined by Portgás (Portugal) specifications and in compliance with European Standard EN 16726:2015 and national regulations [1,2,3]. The NG composition was sourced from Portgás [1] and contains approximately 88% CH4, along with notable fractions of ethane, propane, other hydrocarbons, CO2, and nitrogen (Figure 1). In contrast, the bio-CH4 stream contains 94% CH4, with small amounts of oxygen, nitrogen, CO2, and traces of H2S [2].
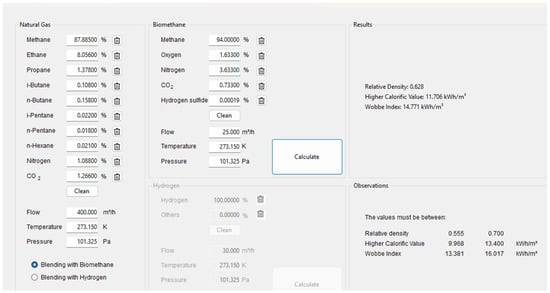
Figure 1.
Interface of the developed C# application used to simulate gas blending. The user defines the composition, flow rate, temperature, and pressure of both NG and bio-CH4. Upon calculation, the tool outputs thermodynamic properties.
A blended stream was then defined using flow rates of 400 m3/h for NG and 25 m3/h for bio-CH4, under identical thermodynamic conditions (273.15 K and 101.325 kPa). The tool was subsequently used to calculate relative density, HHV, and Wobbe index for the pure gases and the resulting mixture.
The second methodological approach consists of a parametric study in which CH4 content is systematically varied within both the bio-CH4 and NG components. This approach enables the simulation of a wide range of blending scenarios, accounting for supply variability, purification levels, and differences in gas origin. It is particularly useful for analyzing how changes in CH4 concentration affect key thermodynamic and combustion-related properties. This analysis simplifies the CH4 variation as proportional. However, in practice, the remaining composition influences the gas behavior in a complex, non-linear way.
Both approaches rely on the van der Waals equation of state, which offers a more accurate representation of real gas behavior by incorporating intermolecular forces and finite molecular volume factors neglected in ideal gas models [4]. This explicit accounting for molecular interactions and volume allows the model to significantly reduce the error associated with the ideal gas law, particularly under the elevated pressures. Specifically, the parameters a (molecular attraction) and b (molecular volume) are used in the modeling framework.
3. Results
In this simulation, Figure 1, the C# application was used to configure a gas blending scenario combining standard NG and bio-CH4, as defined in Methods. After selecting Blending with Bio-CH4 option the model computed the blended stream composition and the corresponding quality indicators, including relative density, higher calorific value, and Wobbe index.
In the second method, Figure 2, a parametric analysis was carried out by systematically varying the CH4 concentration in both NG and bio-CH4 from 80% to 99% on a molar basis. For each simulated composition, the remaining fraction was proportionally distributed among typical diluent or inert components such as carbon dioxide, nitrogen, or light hydrocarbons, depending on the characteristics of each stream. The flow rates were fixed at 300 m3/h for NG and 30 m3/h for bio-CH4, with operating conditions maintained at 273.15 K and 101.325 kPa, representing standard reference conditions. This approach enabled the evaluation of how variations in CH4 purity influence key thermodynamic properties, including relative density, higher calorific value, and Wobbe index, across a wide range of potential blending scenarios. Although this method offers valuable insights into the trends associated with CH4 variation, it also underscores a limitation: the exclusion of the specific calorific contributions and densities of other gas components, which can significantly impact on the overall quality of the final mixture.
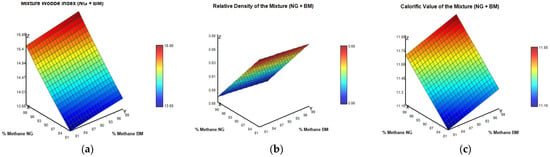
Figure 2.
Thermodynamic properties of NG–BM mixtures as functions of CH4 concentration in both gas streams under standard conditions (273.15 K and 1 atm): (a) Mixture Wobbe Index; (b) Relative Density of the Mixture; (c) Calorific Value of the Mixture.
4. Discussion
The results shown in Figure 1 confirm the effectiveness of the tool in predicting essential thermodynamic and combustion properties of gas mixtures under defined conditions. For the simulated blend of NG and bio-CH4, the tool calculated a relative density of 0.628, a higher calorific value of 11.706 kWh/m3, and a Wobbe index of 14.771 kWh/m3. All values remain within the regulatory limits displayed in the interface, reinforcing the technical viability of injecting this mixture into existing gas distribution networks [2,3].
The three surface plots presented illustrate how the Wobbe index, relative density, and higher calorific value of the NG and bio-CH4 mixture respond to simultaneous variations in CH4 content, ranging from 80% to 99% in each gas stream. As expected, all properties exhibit a positive correlation with increasing CH4 concentration. The Wobbe index (Figure 2a) varies from approximately 13.57 to 15.88 kWh/m3, the relative density (Figure 2b) increases from 0.56 to 0.68, and the calorific value (Figure 2c) rises from about 11.16 to 11.88 kWh/m3.
However, this analysis presents an important limitation. The variation in CH4 concentration was applied proportionally, assuming a uniform adjustment of the remaining composition. In practice, minor components such as ethane, propane, carbon dioxide, and nitrogen possess distinct thermophysical properties that influence gas behavior in complex, non-linear ways. Propane and ethane, for example, significantly enhance the calorific value and Wobbe index, while nitrogen and CO2 act as diluents, reducing both parameters [5]. As a result, proportionally decreasing these components while increasing CH4 may lead to non-representative or distorted outcomes, particularly when assessing regulatory thresholds or injection constraints.
These observations highlight the necessity of employing compositionally realistic gas profiles in sensitivity analyses aimed at evaluating operational safety, combustion performance, or compliance with gas quality standards. Simplified models that rely solely on CH4 content may underestimate potential risks, such as failing to meet Wobbe index requirements or experiencing performance deviations in end-use equipment.
Author Contributions
Conceptualization, A.B.; methodology, A.B.; investigation, V.V., M.O. and A.B.; resources, A.B.; data curation, V.V., M.O. and A.B.; writing—original draft preparation, V.V. and M.O.; writing—review and editing, V.V., M.O. and A.B.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The study was developed under the project A-MoVeR—“Mobilizing Agenda for the Development of Products & Systems towards an Intelligent and Green Mobility”, operation no. 02/C05-i01.01/2022.PC646908627-00000069, approved under the terms of the call no. 02/C05-i01/2022—Mobilizing Agendas for Business Innovation, financed by European funds provided to Portugal by the Recovery and Resilience Plan (RRP), in the scope of the European Recovery and Resilience Facility (RRF), framed in the Next Generation UE, for the period from 2021–2026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Portgás. Manual de Especificações Técnicas—Projeto e Construção de Redes e Ramais de Distribuição de Gás Natural; Portgás: Porto, Portugal, 2018. [Google Scholar]
- EN 16723-1:2016; Natural Gas and Biomethane for Use in Transport and Biomethane for Injection in the Natural Gas Network—Part 1: Specifications for Biomethane for Injection in the Natural Gas Network. European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- EN 16726:2015; Gas Infrastructure—Quality of Gas—Group H. European Committee for Standardization (CEN): Brussels, Belgium, 2015.
- Perry, R.H.; Green, D.W. (Eds.) Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Li, H.; Xiao, Y.; Zhang, Y.; Li, Y. Effects of natural gas composition on the performance and emissions of gas turbine combined cycle power plants. Energy Convers. Manag. 2017, 148, 1076–1089. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).